Abstract
Multiple myeloma (MM) is a plasma cell malignancy that remains incurable and poses a significant threat to global public health. The multifunctional transcription factor c-Myc plays a crucial role in various cellular processes and is closely associated with MM progression. As part of the basic-helix-loop-helix-leucine zipper (bHLHZip) family, c-Myc forms heterodimers with its obligate partner Max, binds to the Enhancer-box (E-box) of DNA, and ultimately co-regulates gene expression. Therefore, impeding the capacity for heterodimerization to bind to DNA represents a favored strategy in thwarting c-Myc transcription. In this study, we first synthesized a series of novel 2-iminobenzimidazole derivatives and further estimated their potential anti-MM activity. Notably, among all the derivatives, 5b and 5d demonstrated remarkable inhibitory activity against RPMI-8226 and U266 cells, with IC50 values of 0.85 μM and 0.97 μM for compound 5b, and 0.96 μM and 0.89 μM for compound 5d. Western blot and dual-luciferase reporter assays demonstrated that compounds 5b and 5d effectively suppressed both c-Myc protein expression and transcriptional activity of the c-Myc promoter in RPMI-8226 and U266 cells. Furthermore, these compounds induced apoptosis and G1 cell cycle arrest in the aforementioned MM cells. Molecular docking studies revealed that 5b and 5d exhibited strong binding affinity to the interface between c-Myc/Max and E-box of DNA. Taken together, our findings suggest that further investigations are warranted for potential therapeutic applications of 5b and 5d for c-Myc-related diseases.
Keywords: Multiple myeloma, c-Myc, 2-Iminobenzimidazoles, Molecular docking
Graphical abstract
2-iminobenzimidazoles as potential c-Myc inhibitors for treating multiple myeloma.
1. Introduction
Multiple myeloma (MM), the second most common blood cancer, is a plasma cell malignancy frequently accompanied by hypercalcemia, immune dysfunction, and renal failure [[1], [2], [3]]. Despite the employment of various therapeutic methods, such as immunomodulatory agents, proteasome inhibitors, and autologous stem cell transplantation (ASCT), MM remains an incurable disease with nearly 160,000 new cases and 106,000 deaths annually [4,5]. Unfortunately, relapse and drug resistance continue to pose significant challenges in the management of MM [6,7]. Therefore, it is imperative to discover novel drugs that exhibit superior selectivity and potency in the treatment of MM.
c-Myc, a crucial transcription factor and member of the MYC family, is closely linked to oncogenesis and implicated in various cellular processes [[8], [9], [10]]. Increasing evidence suggests that it functions as a primary oncogene in MM [11]. Given its crucial role in regulating cellular function, c-Myc is a natural target for drug development. However, due to its short half-life of only 20–30 min and lack of a specific drug-binding pocket, direct interaction with drugs remains challenging [12,13]. In the basic helix-loop-helix leucine zipper (bHLHZip) family, c-Myc interacts with Max (Myc associated factor X), another bHLHZip protein that serves as the obligatory partner of c-Myc, to form the c-Myc/Max heterodimerization. This process is regulated by the coiling of their respective bHLHZip domains [[14], [15], [16]]. Upon the formation of the c-Myc/Max heterodimer, it recognizes and binds to the E-box, a DNA sequence 5′-CACGTG-3′, ultimately leading to transcriptional activation [17,18]. Therefore, c-Myc is widely regarded as one of the most valuable targets in cancer research.
A plethora of strategies, both direct and indirect, have been employed to target c-Myc by exploiting its multiple regulatory mechanisms, encompassing c-Myc transcription and mRNA stability, c-Myc protein stability and degradation, as well as c-Myc binding to its interactome Max [19]. In 2003, Yin et al. [99] discovered three compounds (10058-F4, 10074-G5, and 10074-A4) that block the c-Myc/Max interaction, specifically inhibit Myc transcriptional activity and reduce cell growth in Myc-transformed rat fibroblasts [20]. However, these chemicals have limited clinical applicability due to their low potency, lack of selectivity, and poor pharmacokinetic behavior in vivo [21,22]. Despite significant efforts to optimize the two compounds, namely their derivatives JY-3-094 and 3jc48-3, resulting in varying degrees of improved potency, animal models have yet to demonstrate satisfactory tumor reduction performance [[23], [24], [25]]. Inhibiting c-Myc transcriptional activity can be also achieved by blocking the direct binding of c-Myc/Max to DNA using natural compounds like celastrol and celastrol-inspired triterpenoids [26], synthetic mimetics such as JKY-2-169 [27], or small molecule inhibitors including MYRA-A [28], NSC308848 [29], and KSI-3716 [30]. To date, the US Food and Drug Administration (FDA) has not approved any c-Myc drugs, thus emphasizing the pressing need to cultivate increasingly innovative inhibitors targeting c-Myc.
Virtually, we have been dedicating ourselves to seeking and discovering newer and more potential c-Myc inhibitors [[31], [32], [33], [34], [35]]. Recently, we discovered a new 2-iminobenzimidazole compound, XYA1353 (Fig. 1), which exhibits anti-MM activity both in vitro and in vivo by disrupting the canonical NF-κB signaling pathway through reducing expression of P65/P50 and phosphorylation levels of p-IκBα [36]. To optimize the lead compound XYA1353, a range of novel 2-iminobenzimidazole derivatives (Fig. 1) were designed and further elevated their anti-MM activity. We discovered compounds 5b and 5d as the most potent anti-MM agents, with their IC50 values being below approximately 1 μM. Moreover, these compounds demonstrated promise as inhibitors of c-Myc by effectively reducing the expression of c-Myc at both the mRNA and protein levels, as well as inhibiting the transcriptional activity of the c-Myc promoter.
Fig. 1.

Chemical structures of XYA1353 and target compounds.
2. Materials and methods
2.1. Chemistry
All starting regents were commercially available and solvents were used without any purification process unless noted. Each step purification of synthesized compounds was carried out with silica gel (purchased from Qingdao Ocean Chemical Co. Ltd) column chromatography. 1H and 13C NMR spectra were determined on a JEOL spectrometer in CDCl3 or DMSO‑d6. HRMS was analyzed via the Agilent 6550 Q-TOF instrument. Target compounds’ melting points were attained by using a YRT-3 apparatus.
2.2. Synthesis and characterization of 5a-5d
2.2.1. Synthesis of 1 (2-nitro-N-(2-phenoxyethyl)aniline)
A mixture containing 10 mmol of 2-nitroaniline and 20 mmol of NaH in 10 mL of DMF was chilled to 0°C. Subsequently, the mixture was treated with 13 mmol of 2-phenoxyethyl bromide, followed by heating the resulting combination at a temperature of 120 °C for 1 h. Upon cooling the solution to room temperature, it was poured into water and extracted with EA. The organic phase was washed with brine, dried over anhydrous Na2SO4, and subsequently concentrated under vacuum. Compound 1 was obtained after purification of the residue through column chromatography using a mixture of PE and EA in a ratio of 30:1 (v/v).
2.2.2. Synthesis of 2 (N1-(2-phenoxyethyl)benzene-1,2-diamine)
The water solution (10 ml) of NH4Cl (35 ml) was gradually mixed with the THF (40 ml) solution of compound 1 (7 mmol), subsequently, the iron powder (35 mmol) was added and the mixture was stirred at 70 °C for 10h. After monitoring by TLC, the mixture above was filtered and the filtrate was collected, dried over anhydrous Na2SO4, and concentrated to get crude product, which was purified by column chromatography using PE/EA = 5/1 (v/v) to afford compound 2.
2.2.3. Synthesis of 3a-3d
Intermediate 2 (3.94 mmol) was with various substituted benzyl bromides (5.12 mmol) and K2CO3 (5.12 mmol) in DMF solution at 120 °C for 2 h. After monitoring by TLC, the reaction solution was cooled, poured into water, and extracted with EA. The organic phase was collected, washed with brine, and dried over anhydrous Na2SO4. By concentration under vacuum, the residue was purified via column chromatography using PE/EA = 10/1 (v/v) to give compounds 3a-3d.
2.2.4. Synthesis of 4a-4d
The methanol solution of compound 3a-3d (1.94 mmol) was treated with a dropwise addition of BrCN solution (2.33 mmol) in dichloromethane (8 mL). Following stirring at room temperature for 10 h, the solvent was evaporated and the resulting mixture was then adjusted to a pH value of 8–9 using saturated NaHCO3 aqueous solution before undergoing extraction with dichloromethane. The combined organic phase was subsequently washed with brine, dried using anhydrous Na2SO4, and concentrated under vacuum to obtain compounds 4a-4d.
2.2.5. Synthesis of 5a-5d
The ethyl ether solution of compounds 4a-4d (1.70 mmol) was combined with an aqueous solution of KOH (2.57 mmol), and the resulting mixture was stirred for 0.5 h. Upon completion of the reaction, the mixture was quenched with brine, extracted with ethyl ether, dried over anhydrous Na2SO4, and evaporated to afford final compounds 5a-5d.
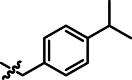
1-(4-isopropylbenzyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (5a). White solid, yield: 97%; M.p. 126.4–128.1 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.29–7.17 (m, 4H), 7.14 (d, J = 8.1 Hz, 2H), 7.05 (d, J = 7.4 Hz, 1H), 6.94–6.79 (m, 6H), 5.00 (s, 2H), 4.27–4.16 (m, 4H), 2.81 (dt, J = 13.8, 6.9 Hz, 1H), 1.19–1.08 (m, 6H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.22, 153.30, 147.34, 134.82, 131.95, 131.36, 129.55, 127.34, 126.41, 120.78, 120.03, 119.92, 114.41, 106.70, 106.39, 65.24, 43.53, 40.68, 33.13, 23.90. ESI-HRMS [M+H]+ m/z:386.2222, calcd for C25H27N3O, 386.2227.
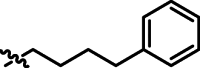
1-(2-phenoxyethyl)-3-(4-phenylbutyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (5b). White solid, yield: 90%; M.p. 114.1–116.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.28–7.19 (m, 4H), 7.19–7.10 (m, 3H), 7.07–6.97 (m, 1H), 6.94–6.82 (m, 6H), 4.22–4.12 (m, 4H), 3.81 (t, J = 6.5 Hz, 2H), 2.59 (t, J = 7.1 Hz, 2H), 1.65–1.54 (m, 4H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.18, 153.05, 142.05, 131.86, 131.47, 129.51, 128.35, 128.32, 128.26, 125.70, 120.74, 119.85, 119.71, 114.36, 106.56, 105.95, 65.21, 40.55, 40.33, 34.85, 28.23, 27.07. ESI-HRMS [M+H]+ m/z:386.2221 calcd for C25H27N3O, 386.2227.
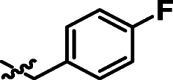
1-(4-fluorobenzyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (5c). White solid, yield: 91%; M.p. 145.1–146.9 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.36–7.27 (m, 2H), 7.25–7.17 (m, 2H), 7.08 (t, J = 8.9 Hz, 2H), 7.01 (s, 1H), 6.88 (d, J = 7.3 Hz, 1H), 6.86–6.77 (m, 5H), 5.00 (s, 2H), 4.23–4.13 (m, 4H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 162.58, 160.16, 158.20, 153.21, 133.69, 131.97, 131.18, 129.54, 129.45, 129.37, 120.78, 120.10, 119.94, 115.38, 115.16, 114.39, 106.73, 106.33, 65.25, 43.08, 40.68. ESI-HRMS [M+H]+ m/z:362.1668, calcd for C22H20FN3O, 362.1663.
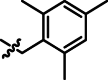
1-(2-phenoxyethyl)-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (5d). Yellow solid, yield: 88%; M.p. 125.4–127.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.35–7.21 (m, 3H), 7.01 (t, J = 7.6 Hz, 1H), 6.95–6.76 (m, 6H), 6.32 (d, J = 7.9 Hz, 1H), 5.16 (s, 2H), 4.44 (t, J = 5.1 Hz, 2H), 4.25 (t, J = 5.2 Hz, 2H), 2.28–2.09 (m, 9H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.03, 151.76, 137.10, 137.09, 131.03, 130.25, 129.56, 129.47, 128.14, 121.36, 121.25, 120.91, 114.31, 108.59, 108.25, 99.51, 65.03, 48.63, 41.74, 41.35, 20.54, 19.79. ESI-HRMS [M+H]+ m/z:386.2227, calcd for C25H27N3O, 386.2227.
2.3. Synthesis and characterization of compounds 8a-9b
2.3.1. Synthesis of 6a and 6b
The solution of 2-aminobenzimidazole (15 mmol) in DMF (15 mL) was treated with NaH (19 mmol) and either 2-phenoxyethyl bromide or 2-(bromomethyl)-1,3,5-trimethylbenzene (15 mmol) at 0 °C, then the ice water bath was removed and the reaction solution was stirred at room temperature for another 2h. By monitoring with TLC, the reaction solution was poured into ice water and extracted with EA, followed by collecting the organic phase and washing with brine. After concentration under vacuum, the residue was recrystallized in anhydrous ethanol to yield 6a and 6b.
1-(2-phenoxyethyl)-1H-benzo[d]imidazole-2-amine (6a). White solid, yield: 32%; M.p. 228.1–229.5 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.30–7.18 (m, 3H), 7.11 (dd, J = 7.7, 0.9 Hz, 1H), 6.97–6.80 (m, 5H), 6.51–6.37 (m, 2H), 4.38 (t, J = 5.5 Hz, 2H), 4.19 (t, J = 5.5 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.08, 154.98, 142.74, 134.64, 129.57, 120.86, 120.39, 118.09, 114.73, 114.35, 107.98, 65.87, 41.22. ESI-HRMS [M+H]+ m/z:254.1285, calcd for C15H15N3O, 254.1288.
1-(2,4,6-trimethylbenzyl)-1H-benzo[d]imidazole-2-amine (6b). White solid, yield: 30%; M.p. 269.1–271.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.10 (d, J = 7.8 Hz, 1H), 6.93–6.78 (m, 3H), 6.71–6.53 (m, 3H), 6.24 (d, J = 7.9 Hz, 1H), 5.13 (s, 2H), 2.22 (s, 3H), 2.20–2.11 (m, 6H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 155.22, 142.88, 137.02, 136.80, 133.71, 129.32, 128.77, 120.03, 117.82, 114.80, 108.06, 41.81, 20.52, 19.61. ESI-HRMS [M+H]+ m/z:266.1645, calcd for C17H19N3, 266.1652.
2.3.2. Synthesis of 7
0.4 mmol of various substituted benzyl bromides were added to a solution of compound 6a or 6b (0.4 mmol) in acetonitrile. The mixture was stirred at 70 °C for 24 h, and the solvent was then evaporated. The resulting residue was recrystallized with acetone to yield compound 7.
2.3.3. Synthesis of 8a-9b
Intermediate 7 was subjected to a reaction with a water solution of KOH in ethyl ether at room temperature for half an hour. After completion of the reaction, the mixture was combined with brine, extracted using diethyl ether, dried using anhydrous Na2SO4, and finally concentrated under the vacuum to obtain compound 8a-9b.
1-(2,4,6-trimethylbenzyl)-1H-benzo[d]imidazole-2-amine (8a). White solid, yield: 98%; M.p. 273.0–275.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.86 (dd, J = 9.1, 4.0 Hz, 2H), 7.81–7.73 (m, 2H), 7.50–7.42 (m, 3H), 7.29–7.22 (m, 2H), 7.18 (d, J = 7.6 Hz, 1H), 6.99–6.93 (m, 2H), 6.93–6.85 (m, 4H), 5.30 (s, 2H), 4.33 (t, J = 5.2 Hz, 2H), 4.26 (t, J = 5.1 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.18, 152.93, 134.65, 132.76, 132.28, 131.74, 131.10, 129.59, 128.34, 127.62, 126.41, 126.03, 125.69, 125.58, 120.85, 120.66, 120.52, 114.40, 107.46, 107.08, 65.22, 44.41, 41.00. ESI-HRMS [M+H]+ m/z:394.1908, calcd for C26H23N3O, 394.1914.
1-(2-phenoxyethyl)-3-(3-phenoxypropyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8b). White solid, yield: 95%; M.p. 110.2–112.2 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.30–7.16 (m, 4H), 7.10 (d, J = 6.8 Hz, 1H), 6.96 (td, J = 7.3, 1.0 Hz, 1H), 6.93–6.80 (m, 8H), 4.28–4.12 (m, 4H), 4.05–3.88 (m, 4H), 2.06 (t, J = 6.4 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.36, 158.35, 158.14, 158.13, 158.10, 152.74, 131.70, 131.29, 129.58, 129.54, 129.51, 120.81, 120.61, 120.34, 120.28, 114.44, 114.37, 107.16, 106.37, 65.22, 64.59, 40.84, 37.90, 27.25. ESI-HRMS [M+H]+ m/z:388.2016 calcd for C24H25N3O2, 388.2020.
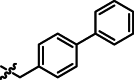
1-([1,1′-biphenyl]-4-ylmethyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8c). White solid, yield: 86%; M.p. 214.1–215.9 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.59 (dd, J = 11.7, 7.9 Hz, 4H), 7.43 (t, J = 7.5 Hz, 2H), 7.40–7.29 (m, 3H), 7.26 (dd, J = 11.7, 4.2 Hz, 2H), 7.07 (d, J = 7.9 Hz, 1H), 6.98–6.78 (m, 6H), 5.10 (s, 2H), 4.31–4.14 (m, 4H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.21, 153.30, 139.85, 139.14, 136.67, 132.01, 131.34, 129.54, 128.93, 127.91, 127.42, 126.83, 126.62, 120.77, 120.07, 119.93, 114.40, 106.72, 106.38, 65.26, 43.49, 40.70. ESI-HRMS [M+H]+ m/z:420.2067, calcd for C28H25N3O, 420.2070.
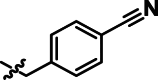
4-((2-imino-3-(2-phenoxyethyl)-2,3-dihydro-1H-benzo[d]imidazole-1-yl)methyl)benzonitrile (8d). White solid, yield: 88%; M.p. 135.2–137.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.82–7.69 (m, 2H), 7.44 (d, J = 8.2 Hz, 2H), 7.31–7.20 (m, 2H), 7.11 (d, J = 7.7 Hz, 1H), 6.98–6.90 (m, 2H), 6.90–6.83 (m, 4H), 5.18 (s, 2H), 4.30–4.18 (m, 4H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.18, 152.99, 143.20, 132.50, 131.90, 131.00, 129.55, 128.11, 120.81, 120.50, 120.24, 118.78, 114.39, 110.06, 107.11, 106.50, 65.25, 43.65, 40.85. ESI-HRMS [M+H]+ m/z:369.1704, calcd for C23H20N4O, 369.1710, found.
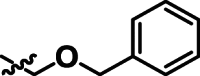
1-((benzyloxy)methyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8e). White solid, yield: 81%; M.p. 149.5–151.2 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.30 (dd, J = 7.0, 1.6 Hz, 1H), 7.28–7.19 (m, 6H), 7.07 (d, J = 7.3 Hz, 1H), 7.04–7.00 (m, 1H), 6.96 (td, J = 7.5, 1.1 Hz, 1H), 6.92–6.82 (m, 4H), 5.39 (s, 2H), 4.55 (s, 2H), 4.25–4.14 (m, 4H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.18, 153.07, 137.91, 132.07, 130.88, 129.53, 128.22, 127.48, 120.84, 120.77, 120.09, 114.37, 106.94, 70.75, 69.63, 65.19, 40.58. ESI-HRMS [M+H]+ m/z:374.1865, calcd for C23H23N3O2, 374.1863.
1-(2-phenoxyethyl)-3-(quinolin-8-ylmethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8f). White solid, yield: 91%; M.p. 130.2–132.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 9.04 (dd, J = 4.3, 1.8 Hz, 1H), 8.46 (dd, J = 8.3, 1.8 Hz, 1H), 7.96 (d, J = 7.1 Hz, 1H), 7.66 (dd, J = 8.3, 4.2 Hz, 1H), 7.53–7.42 (m, 2H), 7.32 (d, J = 6.5 Hz, 1H), 7.29–7.22 (m, 2H), 7.22–7.12 (m, 2H), 7.06 (t, J = 7.2 Hz, 1H), 6.92 (t, J = 7.2 Hz, 1H), 6.84 (d, J = 7.8 Hz, 2H), 5.95–5.84 (m, 2H), 4.54 (t, J = 5.3 Hz, 2H), 4.32 (t, J = 5.4 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.06, 152.09, 150.16, 145.34, 136.78, 133.22, 131.16, 130.68, 129.61, 128.05, 127.94, 126.90, 126.36, 121.99, 121.86, 121.82, 120.97, 114.38, 108.94, 108.43, 65.16, 41.79, 41.72. ESI-HRMS [M+H]+ m/z:395.1865, calcd for C25H22N4O, 395.1866.
1-(anthracen-9-ylmethyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8g). Yellow solid, yield: 92%; M.p. 186.1–188.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 8.77–8.59 (m, 3H), 8.19–8.04 (m, 2H), 7.60–7.47 (m, 4H), 7.26 (dd, J = 8.5, 7.4 Hz, 2H), 7.02–6.84 (m, 4H), 6.70 (t, J = 7.6 Hz, 1H), 6.39 (t, J = 7.7 Hz, 1H), 6.15–6.03 (m, 2H), 5.94 (d, J = 7.8 Hz, 1H), 4.32 (t, J = 5.0 Hz, 2H), 4.23 (t, J = 5.1 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.15, 155.09, 151.93, 131.05, 130.74, 130.35, 130.21, 129.85, 129.76, 129.72, 129.41, 128.74, 127.42, 126.25, 125.61, 125.55, 123.84, 121.66, 121.56, 121.17, 121.04, 120.65, 118.43, 114.50, 109.18, 108.92, 108.24, 66.14, 65.49, 48.79, 41.91, 41.46, 14.56. ESI-HRMS [M+H]+ m/z:444.2070, calcd for C30H25N3O, 444.2070.
1-(2-phenoxyethyl)-3-(4-(trifluoromethyl)benzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine(8h). White solid, yield: 90%; M.p. 105.1–107.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 8.24 (s, 1H), 8.12 (dd, J = 8.1, 1.9 Hz, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.60 (t, J = 7.9 Hz, 1H), 7.24 (dd, J = 8.3, 7.5 Hz, 2H), 7.13 (d, J = 7.5 Hz, 1H), 7.01–6.84 (m, 6H), 5.23 (s, 2H), 4.32–4.18 (m, 4H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.15, 152.90, 147.89, 139.71, 134.04, 131.80, 130.91, 130.18, 129.57, 122.44, 122.26, 120.85, 120.70, 120.46, 114.38, 107.33, 106.73, 65.25, 43.36, 40.91. ESI-HRMS [M+H]+ m/z:412.1634, calcd for C23H20F3N3O, 412.1631.
1-(3-nitrobenzyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8i). Yellow solid, yield: 91%; M.p. 107.1–109.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.74 (s, 1H), 7.61 (d, J = 7.1 Hz, 1H), 7.57–7.42 (m, 2H), 7.29–7.19 (m, 2H), 7.07 (d, J = 7.6 Hz, 1H), 6.93–6.82 (m, 6H), 5.16 (s, 2H), 4.23 (s, 4H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.20, 153.23, 139.10, 131.96, 131.33, 131.15, 129.67, 129.63, 129.52, 129.36, 129.04, 128.73, 128.24, 125.53, 124.10, 124.06, 124.03, 123.99, 122.82, 120.78, 120.26, 120.12, 120.05, 114.37, 106.85, 106.27, 65.26, 43.39, 40.69. ESI-HRMS [M+H]+ m/z:389.1609, calcd for C22H20N4O3, 389.1608.
4'-((2-imino-3-(2-phenoxyethyl)-2,3-dihydro-1H-benzo[d]imidazole-1-yl) methyl)-[1,1′-biphenyl]-2-carbonitrile (8j). White solid, yield: 88%; M.p. 251.1–253.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.94 (d, J = 8.2 Hz, 1H), 7.81–7.72 (m, 1H), 7.60–7.51 (m, 4H), 7.42 (d, J = 8.2 Hz, 2H), 7.34–7.21 (m, 3H), 7.14 (d, J = 7.4 Hz, 1H), 7.10–6.98 (m, 2H), 6.94–6.85 (m, 3H), 5.30 (s, 2H), 4.40 (t, J = 5.3 Hz, 2H), 4.28 (t, J = 5.3 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.07, 152.12, 144.08, 137.02, 133.92, 133.62, 131.25, 130.61, 130.13, 129.59, 129.03, 128.32, 127.42, 121.58, 121.46, 120.92, 118.59, 114.37, 110.12, 108.50, 107.99, 65.11, 44.13, 41.49. ESI-HRMS [M+H]+ m/z:445.2016, calcd for C29H24N4O, 445.2023.
1-(2-(2-ethoxyphenoxy)ethyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine(8k). White solid, yield: 84%; M.p. 170.1–171.9 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.23 (dd, J = 8.5, 7.4 Hz, 2H), 7.18 (dd, J = 6.0, 2.7 Hz, 1H), 7.10 (dd, J = 6.2, 2.5 Hz, 1H), 6.99–6.77 (m, 9H), 4.30–4.12 (m, 8H), 3.89 (q, J = 7.0 Hz, 2H), 1.25 (t, J = 7.0 Hz, 3H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.11, 152.77, 148.16, 147.95, 131.84, 131.66, 129.52, 121.29, 120.79, 120.72, 120.24, 120.21, 114.36, 113.41, 113.28, 107.48, 106.83, 66.49, 65.24, 63.60, 41.29, 40.80, 14.78. ESI-HRMS [M+H]+ m/z428.2122 calcd for C25H27N3O3, 418.2125.
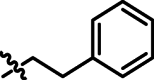
1-phenethyl-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8l). White solid, yield: 81%; M.p. 117.0–119.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.33–7.18 (m, 7H), 7.18–7.13 (m, 2H), 7.10–7.00 (m, 2H), 6.92 (t, J = 7.4 Hz, 1H), 6.88–6.79 (m, 2H), 4.37 (t, J = 5.3 Hz, 2H), 4.25–4.15 (m, 4H), 2.92 (t, J = 8 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.02, 151.26, 137.92, 130.91, 130.36, 129.60, 129.07, 128.33, 126.52, 121.53, 121.48, 120.94, 114.34, 108.61, 108.11, 65.08, 42.93, 41.38, 33.14. ESI-HRMS [M+H]+ m/z:358.1918, calcd for C23H23N3O, 358.1914.
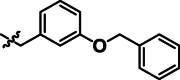
1-(3-(benzyloxy)benzyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8m). White solid, yield: 30.0%. M.p. 145.0–147.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.41–7.35 (m, 4H), 7.34–7.30 (m, 1H), 7.27–7.18 (m, 4H), 7.06–6.95 (m, 4H), 6.94–6.89 (m, 2H), 6.88–6.82 (m, 3H), 5.14 (s, 2H), 5.03 (s, 2H), 4.36 (t, J = 5.2 Hz, 2H), 4.26 (t, J = 5.3 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.50, 158.07, 152.35, 138.20, 136.87, 131.31, 130.73, 129.79, 129.55, 128.46, 127.90, 127.83, 121.19, 121.09, 120.88, 119.52, 114.37, 114.13, 113.36, 108.05, 107.69, 69.17, 65.15, 48.16, 44.28, 41.29. ESI-HRMS [M+H]+ m/z:450.2184, calcd for C29H27N3O2, 450.2176.
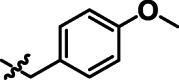
1-(4-methoxybenzyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8n). White solid, yield: 85%; M.p. 151.4–152.9 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.32–7.21 (m, 5H), 7.12 (d, J = 7.5 Hz, 1H), 7.06 (t, J = 7.4 Hz, 1H), 7.00 (t, J = 7.5 Hz, 1H), 6.91 (t, J = 7.3 Hz, 1H), 6.88–6.79 (m, 4H), 5.15 (s, 2H), 4.39 (t, J = 5.2 Hz, 2H), 4.25 (t, J = 5.2 Hz, 2H), 3.69 (s, 3H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.67, 158.05, 152.05, 131.21, 130.54, 129.55, 128.86, 128.82, 128.79, 128.78, 128.29, 121.35, 121.29, 120.89, 114.36, 113.98, 108.36, 108.07, 65.16, 55.07, 43.98, 41.40. ESI-HRMS [M+H]+ m/z:374.1869, calcd for C23H23N3O2, 374.1863.
1-cinnamyl-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8o). White solid, yield: 83%; M.p. 160.1–162.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.34 (d, J = 7.3 Hz, 2H), 7.31–7.19 (m, 5H), 7.16 (d, J = 7.1 Hz, 1H), 7.08–7.03 (m, 1H), 7.01–6.93 (m, 2H), 6.93–6.80 (m, 3H), 6.55 (d, J = 15.9 Hz, 1H), 6.36–6.22 (m, 1H), 4.74–4.60 (m, 2H), 4.29 (t, J = 4.9 Hz, 2H), 4.23 (t, J = 4.8 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.24, 158.22, 152.59, 136.09, 131.81, 131.79, 131.05, 129.66, 128.77, 127.89, 126.42, 123.96, 120.93, 120.72, 120.66, 114.45, 107.57, 107.17, 65.36, 42.90, 41.07. ESI-HRMS [M+H]+ m/z:370.1915, calcd for C24H23N3O, 370.1914.
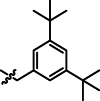
1-(3,5-di-tert-butylbenzyl)-3-(2-phenoxyethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8p). White solid, yield: 91%; M.p. 105.1–107.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.23 (t, J = 8.0 Hz, 3H), 7.14 (dd, J = 11.5, 4.5 Hz, 3H), 7.01 (d, J = 7.0 Hz, 1H), 6.97–6.87 (m, 3H), 6.83 (d, J = 7.9 Hz, 2H), 5.07 (s, 2H), 4.29 (t, J = 5.2 Hz, 2H), 4.22 (t, J = 5.1 Hz, 2H), 1.17 (s, 18H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.16, 153.00, 150.38, 136.24, 131.74, 131.25, 129.52, 121.58, 120.79, 120.74, 120.44, 120.30, 114.32, 107.23, 107.00, 65.25, 44.53, 40.82, 34.40, 31.19. ESI-HRMS [M+H]+ m/z:456.3010, calcd for C30H37N3O, 456.3009.
1-(2-phenoxyethyl)-3-(quinolin-2-ylmethyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8q). White solid, yield: 87%; M.p. 136.1–138.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 8.26 (d, J = 8.6 Hz, 1H), 7.94 (dd, J = 13.0, 7.7 Hz, 2H), 7.80–7.69 (m, 1H), 7.64–7.51 (m, 1H), 7.33–7.20 (m, 3H), 7.10 (d, J = 7.6 Hz, 1H), 6.98–6.75 (m, 6H), 5.32 (s, 2H), 4.33–4.19 (m, 4H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.20, 157.50, 153.28, 146.99, 137.10, 132.04, 131.48, 129.82, 129.51, 128.50, 127.87, 126.97, 126.43, 120.76, 120.28, 120.06, 119.34, 114.39, 106.88, 106.45, 65.26, 46.95, 40.79. ESI-HRMS [M+H]+ m/z:395.1869 calcd for C25H22N4O, 395.1866.
5-((2-imino-3-(2-phenoxyethyl)-2,3-dihydro-1H-benzo[d]imidazole-1-yl)methyl)picolinonitrile (8r). White solid, yield: 90%; M.p. 184.2–186.2 °C. 1H NMR (400 MHz, DMSO‑d6): δ 8.84–8.68 (m, 1H), 8.00 (d, J = 7.9 Hz, 1H), 7.87 (dd, J = 8.1, 2.2 Hz, 1H), 7.40–7.33 (m, 1H), 7.31–7.22 (m, 2H), 7.21 (d, J = 7.4 Hz, 1H), 7.13 (dd, J = 10.9, 4.2 Hz, 1H), 7.05 (dd, J = 7.7, 7.1 Hz, 1H), 6.92 (t, J = 7.3 Hz, 1H), 6.84 (d, J = 7.9 Hz, 2H), 5.41 (s, 2H), 4.42 (t, J = 5.2 Hz, 2H), 4.27 (t, J = 5.2 Hz, 2H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.01, 151.81, 150.38, 136.72, 136.44, 131.66, 131.24, 130.16, 129.58, 129.00, 122.02, 121.75, 120.94, 117.46, 114.33, 108.91, 108.12, 65.16, 42.35, 41.68. ESI-HRMS [M+H]+ m/z:370.1660, calcd for C22H19N5O, 370.1662.
1-(3-phenoxypropyl)-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8s). White solid, yield: 91%; M.p. 184.5–186.4 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.27 (dd, J = 8.5, 7.4 Hz, 1H), 7.08 (d, J = 7.8 Hz, 1H), 6.94–6.85 (m, 4H), 6.85–6.72 (m, 2H), 6.67–6.54 (m, 1H), 6.49 (s, 2H), 6.23 (d, J = 7.8 Hz, 1H), 5.12 (s, 2H), 3.97 (dd, J = 14.4, 6.6 Hz, 2H), 2.22 (s, 3H), 2.20 (d, J = 2.4 Hz, 4H), 2.16 (s, 6H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.26, 155.22, 151.75, 142.86, 137.06, 137.02, 136.80, 133.70, 130.83, 130.39, 129.46, 129.42, 129.32, 128.77, 128.23, 121.10, 120.62, 120.03, 117.83, 114.80, 114.39, 108.06, 107.63, 64.41, 41.83, 41.59, 38.45, 27.13, 20.53, 19.77, 19.62. ESI-HRMS [M+H]+ m/z:400.2383, calcd for C26H29N3O, 400.2383.
1-(quinolin-8-ylmethyl)-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8t). White solid, yield: 80%; M.p. 130.1–132.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 9.12–9.00 (m, 1H), 8.43 (d, J = 8.2 Hz, 1H), 7.91 (d, J = 8.1 Hz, 1H), 7.64 (dd, J = 8.3, 4.2 Hz, 1H), 7.51 (t, J = 7.5 Hz, 1H), 7.28 (d, J = 7.0 Hz, 1H), 6.87 (s, 2H), 6.83–6.42 (m, 3H), 6.22 (d, J = 7.8 Hz, 1H), 5.72 (s, 2H), 5.05 (s, 2H), 2.27 (s, 6H), 2.22 (s, 3H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 153.51, 150.08, 145.55, 137.08, 136.65, 136.55, 134.63, 131.67, 131.43, 129.36, 128.00, 127.43, 126.52, 126.34, 121.81, 119.95, 119.79, 106.60, 106.32, 40.75, 20.59, 20.03. ESI-HRMS [M+H]+ m/z:407.2229, calcd for C27H26N4, 407.2230.
1-(3-nitrobenzyl)-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8u). Yellow solid, yield: 82%; M.p. 193.0–194.8 °C. 1H NMR (400 MHz, DMSO‑d6): δ 8.25–8.06 (m, 2H), 7.87–7.59 (m, 2H), 6.95–6.82 (m, 3H), 6.80–6.62 (m, 2H), 6.35–6.08 (m, 1H), 5.20 (s, 2H), 5.04 (s, 2H), 2.27–2.19 (m, 9H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 153.11, 147.87, 140.07, 136.95, 136.54, 134.00, 131.25, 130.99, 130.08, 129.33, 122.28, 121.85, 120.17, 119.84, 106.67, 106.27, 64.95, 43.09, 40.70, 20.50, 19.90. ESI- ESI-HRMS [M+H]+ m/z:401.1976, calcd for C24H24N4O2, 401.1972.
1-([1,1′-biphenyl]-4-ylmethyl)-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8v). White solid, yield: 84%; M.p. 164.0–166.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.65 (t, J = 8.0 Hz, 4H), 7.45 (t, J = 7.6 Hz, 2H), 7.42–7.31 (m, 3H), 7.24 (d, J = 7.8 Hz, 1H), 7.01 (t, J = 7.7 Hz, 1H), 6.96–6.84 (m, 3H), 6.37 (d, J = 8.0 Hz, 1H), 5.39 (s, 2H), 5.25 (s, 2H), 2.29–2.18 (m, 9H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 151.42, 139.62, 137.36, 137.20, 135.12, 130.45, 130.21, 129.57, 129.01, 127.84, 127.80, 127.62, 127.03, 126.71, 122.07, 121.86, 108.95, 44.53, 42.22, 20.60, 19.86. ESI-HRMS [M+H]+ m/z:432.2441, calcd for C30H29N3, 432.2434.
4'-((2-imino-3-(2,4,6-trimethylbenzyl)-2,3-dihydro-1H-benzo[d]imidazole-1-yl)methyl)-[1,1′-biphenyl]-2-carbonitrile (8w). White solid, yield: 88%; M.p. 135.0–136.8 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.90 (dd, J = 7.8, 1.1 Hz, 1H), 7.73 (td, J = 7.7, 1.3 Hz, 1H), 7.58–7.48 (m, 4H), 7.40 (d, J = 8.2 Hz, 2H), 6.88 (t, J = 6.0 Hz, 1H), 6.82 (s, 2H), 6.74 (td, J = 7.7, 0.9 Hz, 1H), 6.63 (td, J = 7.7, 1.0 Hz, 1H), 6.22 (d, J = 7.8 Hz, 1H), 5.14 (s, 2H), 5.01 (s, 2H), 2.23–2.15 (m, 9H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 153.71, 144.68, 138.66, 137.52, 137.23, 137.02, 134.39, 134.07, 131.81, 131.75, 130.64, 129.81, 129.76, 129.39, 128.73, 128.00, 120.53, 120.28, 119.11, 110.62, 107.16, 106.93, 65.47, 43.95, 41.22, 21.04, 20.47. ESI-HRMS [M+H]+ m/z:457.2386, calcd for C31H28N4, 457.2387.
1-(3,5-di-tert-butylbenzyl)-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8x). White solid, yield: 89%; M.p. 177.0–179.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.26 (t, J = 1.7 Hz, 1H), 7.23–7.11 (m, 2H), 6.96–6.75 (m, 4H), 6.67 (t, J = 7.7 Hz, 1H), 6.23 (d, J = 7.6 Hz, 1H), 5.07 (s, 2H), 5.05 (s, 2H), 2.26–2.17 (m, 9H), 1.22 (s, 18H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 153.20, 150.38, 136.91, 136.64, 136.55, 131.41, 131.05, 129.37, 129.24, 121.43, 120.74, 119.96, 119.76, 106.58, 44.35, 40.72, 34.46, 31.24, 20.54, 20.04. ESI-HRMS [M+H]+ m/z:468.3369, calcd for C32H41N3, 468.3373.
1-(naphthalen-1-ylmethyl)-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8y). White solid, yield: 90%; M.p. 281.7–283.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 8.26 (d, J = 7.9 Hz, 1H), 8.05–7.96 (m, 1H), 7.88 (d, J = 8.2 Hz, 1H), 7.69–7.56 (m, 2H), 7.46–7.34 (m, 1H), 7.05–6.91 (m, 4H), 6.90–6.77 (m, 2H), 6.41–6.31 (m, 1H), 5.76 (s, 2H), 5.23 (s, 2H), 2.34–2.21 (m, 9H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 151.84, 137.24, 137.20, 133.45, 131.22, 130.93, 130.54, 130.49, 129.56, 128.68, 128.16, 127.96, 126.46, 126.27, 125.40, 123.63, 123.02, 121.61, 121.39, 119.00, 108.49, 108.44, 99.53, 65.01, 43.42, 41.94, 20.61, 19.94. ESI-HRMS [M+H]+ m/z:406.2283, calcd for C28H27N3, 406.2278.
1-cinnamyl-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (8z). White solid, yield: 91%; M.p. 260.1–262.0 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.39 (dd, J = 7.6, 2.6 Hz, 3H), 7.33 (t, J = 7.5 Hz, 2H), 7.25 (t, J = 7.2 Hz, 1H), 7.09 (t, J = 7.7 Hz, 1H), 6.98–6.88 (m, 3H), 6.61 (d, J = 16.0 Hz, 1H), 6.42–6.30 (m, 2H), 5.30 (s, 2H), 4.96 (d, J = 5.4 Hz, 2H), 2.27–2.19 (m, 9H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.51, 150.64, 137.54, 137.42, 137.19, 137.09, 136.83, 135.78, 132.12, 130.20, 129.97, 129.53, 128.70, 128.43, 127.97, 127.88, 127.74, 127.53, 126.35, 122.74, 122.34, 122.21, 109.37, 43.67, 42.56, 20.53, 20.51, 19.77, 19.73. ESI-HRMS [M+H]+ m/z:382.2286 calcd for C26H27N3, 382.2278.
1-(naphthalen-2-ylmethyl)-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (9a). White solid, yield: 85%; M.p. 140.5–141.9 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.89 (dd, J = 9.0, 4.4 Hz, 2H), 7.85–7.76 (m, 2H), 7.56–7.48 (m, 2H), 7.44 (dd, J = 8.5, 1.6 Hz, 1H), 7.03 (d, J = 7.7 Hz, 1H), 6.89 (s, 2H), 6.84 (t, J = 7.7 Hz, 1H), 6.78–6.70 (m, 1H), 6.30 (d, J = 7.8 Hz, 1H), 5.36 (s, 2H), 5.14 (s, 2H), 2.30–2.17 (m, 9H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 152.46, 137.02, 136.86, 134.29, 132.72, 132.28, 130.91, 130.75, 129.41, 128.55, 128.32, 127.60, 127.52, 126.46, 126.04, 125.55, 125.35, 120.89, 120.67, 107.65, 107.53, 64.92, 44.52, 41.39, 20.51, 19.86, 15.17. ESI-HRMS [M+H]+ m/z:406.2275, calcd for C28H27N3, 406.2278.
1-(3-(benzyloxy)benzyl)-3-(2,4,6-trimethylbenzyl)-1,3-dihydro-2H-benzo[d]imidazole-2-imine (9b). White solid, yield: 87%; M.p. 117.9–119.9 °C. 1H NMR (400 MHz, DMSO‑d6): δ 7.44–7.29 (m, 5H), 7.24 (t, J = 7.9 Hz, 1H), 6.97–6.76 (m, 7H), 6.71 (t, J = 7.7 Hz, 1H), 6.27 (d, J = 7.7 Hz, 1H), 5.16–4.96 (m, 6H), 2.27–2.13 (m, 9H). 13C NMR (100 MHz, DMSO‑d6): δ ppm 158.48, 153.25, 139.11, 136.98, 136.51, 131.29, 131.20, 129.63, 129.31, 128.46, 127.89, 127.77, 119.94, 119.72, 119.57, 114.02, 113.13, 106.57, 106.44, 69.13, 43.71, 40.70, 20.52, 19.95. ESI-HRMS [M+H]+ m/z:462.2536, calcd for C31H31N3O, 462.2540.
2.4. Cell culture
In this study, human myeloma cells (RPMI-8226 and U266) and human renal epithelial cells (293T) were obtained from the Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy. All the cells were cultured in RPMI-1640 medium (KeyGEN BioTECH) containing 10% fetal bovine serum (ZETA LIFE) at 37 °C with 5% CO2 in the incubator.
2.5. Cell viability assay
Human myeloma cells (RPMI-8226 and U266) and human renal epithelial cells (293T) were seeded in a 96-well plate at a density of 1 × 104 cells per well, respectively. Following incubation with synthesized compounds at varying concentrations, the cell survival rate was determined using a CCK-8 kit (KeyGEN BioTECH, China) by monitoring absorbance at 450 nm according to the manufacturer's instructions.
2.6. Western blot and Q-PCR assay
Following the cultivation of RPMI-8226 and U266 cells under specific conditions, treatment with varying concentrations of compounds 5b, 5d, and 8g was administered. The resulting cells were first collected, and treated with a mixture of proteasome inhibitor and RIPA lysis solution to prepare protein samples. The samples were electrophoresed by SDS-PAGE, which was transferred to the PVDF membrane. After the block with 5% skimmed milk, the membrane was treated with corresponding primary as well as secondary antibodies, successively. The final result was recorded with a chemiluminescence system. Total RNA was extracted using Trizol reagent (Takara) and reverse-transcribed to complementary DNA using a Reverse Transcription Kit (Vazyme). Q-PCR assays were performed on a Roche 480 instrument, and the results were analyzed using the 2-△△CT method.
2.7. Dual-luciferase reporter assay
After seeding 293T cells in a 6-well plate and cultivating them at 5% CO2 and 37 °C overnight, the cells were transfected with luciferase plasmid when their growth density reached 80%. Six hours later, compounds 5b and 5d were added to the cells followed by another incubation for 24 h. The luciferase activity was measured using the Dual-Lumi™ II Luciferase Reporter Gene Assay Kit (Beyotime Biotechnology).
2.8. Cell apoptosis assay
The RPMI-8226 and U266 cells were seeded in a 12-well plate with 15,000 cells per well. After treatment with compounds 5b and 5d for 48 h, the cells were resuspended in PBS and Binding-Buffer before being stained with APC and 7AAD. Apoptotic cells were then quantified using flow cytometry (BD Biosciences) and analyzed using Flowjo-10 software.
2.9. Cell arrest assay
After incubating RPMI-8226 and U266 cells (15,000 cells per well) in a 12-well plate and treating them with compounds 5b and 5d, the cells were resuspended in PBS, fixed with 75% alcohol, and then resuspended again in 1000 μL of PBS. Subsequently, the cells were treated with Binding Buffer (100 μL), RNase-A (1 μL), and incubated at final concentrations of 100 μg/mL and 50 μg/mL respectively. The cell cycle distribution was analyzed using a flow cytometer (BD Biosciences), and the final results were obtained through MODFIT Software.
2.10. Molecular docking studies
The Surflex Dock of Sybyl-X2.1 software was employed to investigate the binding modes between compounds 5b and 5d with c-Myc/Max based on the crystal structure of the c-Myc/Max complex (PDB ID: 1NKP). The binding site for ligands was identified as a well-defined pocket formed by specific residues in both c-Myc (Leu917, Phe921, and Lys939) and Max (Arg 212, Arg215, Asp 216, Ile218, Lys 219, Phe222, and Arg 239). Before docking simulations, hydrogen atoms missing from the protein structures were added using the biopolymer module while all water molecules were excluded. The default docking parameters were configured, and the binding affinities of two compounds were assessed based on the docking score expressed in -log (Kd) units.
2.11. Drug-likeness and ADME profiling
The resulting compounds 5b and 5d underwent In silico drug-likeness and pharmacokinetic evaluation using the online tool "SwissADME" (http://www.swissadme.ch/) to determine their endpoint parameters.
2.12. Statistical analysis
The experimental data were presented as mean ± SD, and statistical analysis was performed using GraphPad Prism 5.0 with the student's t-test and ANOVA. A p-value <0.05 was considered statistically significant.
3. Results and discussion
3.1. Synthesis of 5a-d, 8a-9b series derivatives
The synthetic procedures for compounds 5a-d and 8a-9b are depicted in Scheme 1 and Scheme 2, respectively. Initially, 2-nitroaniline was reacted with 2-phenoxyethyl bromide under the alkaline condition using NaH via nucleophilic substitution reaction to yield compound 1, which was subsequently reduced by means of iron powder to produce compound 2. Compound 3 was obtained by nucleophilic substitution reaction using K2CO3 of compound 2 with various substituted benzyl bromides, respectively. After treatment of compound 3 with BrCN, the ring closed and formed the key structure of 2-iminobenzimidazoles. Finally, compound 4 was combined with aqueous solution of KOH to give compound 5a-d.
Scheme 1.
Synthetic process of compounds 5a-d. Reagents and conditions: (a) 2-bromoethoxy benzene, NaH, DMF, reflux; (b) NH4Cl, Fe, THF, 60 °C; (c) various benzyl bromide, K2CO3, DMF, 120 °C; (d) BrCN, MeOH, CH2Cl2, rf; (e) KOH, Ethyl ether, rt.
Scheme 2.
Synthetic process of compounds 8a-9b. Reagents and conditions: (a) (2-bromoethoxy) benzene/2-(bromomethyl)-1,3,5-trimethylbenzene, NaH, DMF, rt; (b) various benzyl bromide, CH3CN, 70 °C; (c) KOH, Ethyl ether, rt.
Afterward, we changed the synthetic routes, and the most of target compounds followed Scheme 2. Firstly, the starting material 2-aminobenzimidazole was reacted with 2-phenoxyethyl bromide or 2-(bromomethyl)-1,3,5-trimethylbenzene via nucleophilic substitution reaction using NaH as catalyst to afford compound 6a-b. Then, the monosubstituted compound 6a-b were treated with various substituted benzyl bromides via another nucleophilic substitution reaction in the CH3CN solution at 70 °C to form disubstituted compound 7, respectively, which constructed the core structure of 2-iminobenzimidazoles. Ultimately, compound 7 was stirred with KOH water solution to attain compound 8a-9d.
3.2. Cytotoxicity of the compounds 5a-d and 8a-9b
The cytotoxicity of the target compounds against human myeloma cells (RPMI-8226 and U266) and human renal epithelial cells (293T), was assessed using CCK-8 assay. As presented in Table 1, most of the tested compounds demonstrated significant inhibitory effects with IC50 values below 5 μM, which further confirms that the core structure of 2-iminobenzimidazole is crucial for exerting anti-MM activity. Moreover, a preliminary analysis of the structure-activity relationship suggested that when R1 undergoes single displacement and R2 is H, compounds 6a and 6b exhibited weaker activity (IC50 above 5 μM) compared to other compounds. When R1 was ethoxybenzene and R2 was varied to 5a-d and 8a-r, the IC50 values ranged from approximately 1 μM–5 μM. Notably, compounds 5b, 5d, and 8g exhibited remarkable activity with IC50 values below 1 μM (R2 = propylbenzene for compound 5b; R2 = 2-methyl-1,3,5-trimethylbenzene for compound 5d; and R2 = 9-methylanthracene for compound 8g), which were more potent than the lead compound XYA1353 (IC50 about 2 μM [36]).
Table 1.
Inhibitory effect of 5a-d and 8a-9b on RPMI-8226 and U266 cell lines.
| Compounds | R1 | R2 | IC50(μM)a RPMI-8226 | IC50 (μM)a U266 |
|---|---|---|---|---|
| 5a |  |
 |
2.22 ± 0.21 | 2.45 ± 0.08 |
| 5b |  |
 |
0.85 ± 0.14 | 0.97 ± 0.11 |
| 5c |  |
 |
4.35 ± 0.48 | 4.15 ± 0.24 |
| 5d |  |
 |
0.96 ± 0.15 | 0.89 ± 0.09 |
| 6a |  |
H | 5.14 ± 0.24 | 4.96 ± 0.58 |
| 6d |  |
H | 4.93 ± 0.34 | 5.24 ± 0.33 |
| 8a |  |
 |
2.18 ± 0.41 | 2.44 ± 0.89 |
| 8b |  |
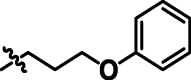 |
4.98 ± 0.56 | 4.26 ± 1.56 |
| 8c |  |
 |
4.95 ± 0.12 | 4.70 ± 1.11 |
| 8d |  |
 |
4.56 ± 0.11 | 4.23 ± 1.35 |
| 8e |  |
 |
2.85 ± 0.44 | 2.65 ± 0.88 |
| 8f |  |
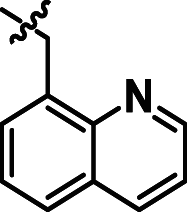 |
1.32 ± 0.55 | 1.45 ± 0.12 |
| 8g |  |
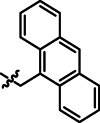 |
0.98 ± 0.10 | 1.01 ± 0.09 |
| 8h |  |
 |
2.92 ± 0.48 | 2.66 ± 0.17 |
| 8i |  |
 |
2.22 ± 0.17 | 1.98 ± 0.28 |
| 8j |  |
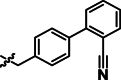 |
2.56 ± 0.29 | 2.77 ± 0.46 |
| 8k |  |
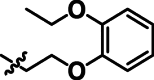 |
1.99 ± 0.07 | 1.76 ± 0.77 |
| 8l |  |
 |
5.13 ± 0.44 | 5.44 ± 0.59 |
| 8m |  |
 |
2.40 ± 0.59 | 2.67 ± 0.88 |
| 8n |  |
 |
2.03 ± 0.27 | 1.88 ± 0.44 |
| 8o |  |
 |
1.99 ± 0.39 | 2.66 ± 0.98 |
| 8p |  |
 |
1.04 ± 0.11 | 1.44 ± 0.44 |
| 8q |  |
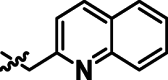 |
1.18 ± 0.79 | 1.05 ± 0.89 |
| 8r |  |
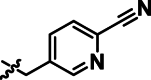 |
1.11 ± 0.26 | 1.55 ± 0.16 |
| 8s |  |
 |
1.87 ± 0.36 | 1.66 ± 0.05 |
| 8t |  |
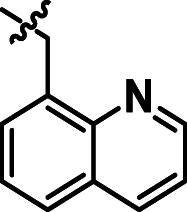 |
2.05 ± 0.58 | 1.99 ± 0.49 |
| 8u |  |
 |
2.45 ± 0.84 | 2.63 ± 0.33 |
| 8v |  |
 |
2.32 ± 0.54 | 2.20 ± 0.29 |
| 8w |  |
 |
2.21 ± 0.28 | 2.46 ± 0.37 |
| 8x |  |
 |
1.99 ± 0.07 | 2.23 ± 0.24 |
| 8y |  |
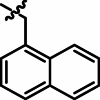 |
1.27 ± 0.53 | 1.34 ± 0.57 |
| 8z |  |
 |
1.02 ± 0.07 | 1.24 ± 0.48 |
| 9a |  |
 |
1.88 ± 0.12 | 1.59 ± 0.19 |
| 9b |  |
 |
1.76 ± 0.19 | 1.98 ± 0.42 |

IC50, the mean ± SD of 3 independent experiments.
Furthermore, the substitution of R1 with 2-methyl-1,3,5-trimethylbenzene and variation of R2 resulted in an average IC50 value of approximately 2 μM for compound 8s-9b. This suggests that compounds containing the 2-methyl-1,3,5-trimethylbenzene moiety on R1 exhibit superior activity compared to those with ethoxybenzene on R1. More importantly, compounds 5b, 5d, and 8g showed negligible intrinsic cytotoxicity (IC50 > 90 μM), thus indicating their favorable safety profiles (Table S1). Therefore, compounds 5b, 5d, and 8g (all exhibiting IC50 values below 1 μM) were selected for further evaluation of their anti-MM activity.
3.3. Compounds 5b and 5d exerted inhibitory effects on the expression and transcriptional activity of c-Myc
Compounds 5b, 5d, and 8g were selected for Western blot analysis due to their apparent ability to suppress two MM cells. As one of the earliest inhibitors of c-Myc, 10074-G5 has been extensively utilized in investigating anti-tumor mechanisms and developing its derivatives. In this study, 10074-G5 was employed as a positive control. As depicted in Fig. 2A and B, compared to the control group, treatment with compounds 5b, 5d, and 10074-G5 resulted in decreased c-Myc protein expression in RPMI-8226 and U266 cells; however, compound 8g exhibited poor efficacy against U266 cells. Notably, both cell lines treated with compounds 5b and 5d demonstrated a more pronounced reduction in c-Myc protein expression than those treated with the positive control. Meanwhile, our Q-PCR assay revealed a significant reduction in the mRNA level of c-Myc gene upon treatment with compounds 5b and 5d in RPMI-8226 and U266 cells. (Fig. 2C and D).
Fig. 2.
(A–B) Western blot analysis of RPMI-8226 and U266 cells treated with 1 μM 8g, 5b, and 5d. The positive control used was 10074-G5 (The uncropped versions were shown in Fig. S1). (C–D) Q-PCR analysis of c-Myc mRNA levels in RPMI-8226 and U266 cells treated with 1 μM of compounds 8g, 5b, and 5d, as well as with 15 μM of compound 10074-G5. (E) Dual-luciferase reporter assay analysis of c-Myc's promoter activity affected by 5b and 5d. Error bars represent mean ± SD (n = 3). *P < 0.05, **P < 0.01.
Subsequently, we further evaluated the impact of compounds 5b and 5d on c-Myc transcriptional activity based on dual-luciferase reporter assay. It has been reported that c-Myc acts as a positive master regulator by binding to the conserved DNA sequence CACGTG (E-box motif) and further transcriptionally activating downstream target genes. In this study, we constructed a luciferase reporter gene plasmid by cloning multiple copies of the E-box motif. As illustrated in Fig. 2E, c-Myc significantly enhanced the transcriptional activity of its target genes. Conversely, compounds 5b and 5d exhibited inhibitory effects on c-Myc-mediated transcriptional activities, with compound 5d demonstrating superior efficacy. In summary, both compounds selectively targeted the c-Myc protein and exerted potent anti-MM effects.
3.4. Molecular modeling analysis
To predict the potential binding mode of compounds 5b and 5d with c-Myc, we proceeded to conduct a molecular docking study utilizing Sybyl-X2.1 software (Tripos Inc., USA). Considering the inhibitory effect of compounds 5b and 5d on c-Myc-mediated transcriptional activity, as demonstrated by dual-luciferase reporter assay, these two compounds were subjected to docking analysis at the interface between c-Myc/Max and E-box of DNA. As shown in Fig. 3A and B, both 5b and 5d were snugly embedded within the hydrophobic pocket, which is composed of residues Arg 214, Arg215, Ile218, and Phe222 in Max as well as Arg 911, Leu917, Phe921, and Lys939 in c-Myc. Additionally, 5b formed one hydrogen bond with the main chain of Arg914 while 5d had two hydrogen bonds with the main chain of Arg914 and the side chain of Lys 918. Thus, the stronger hydrophobic and hydrogen bond interactions established between c-Myc/Max and compounds 5b and 5d would disrupt the binding of c-Myc/Max to the E-box of DNA.
Fig. 3.
Predicted binding modes of 5b (A) and 5d (B) in the interface of c-Myc/Max to E-box of DNA. Proteins c-Myc and Max are represented as a cartoon, colored green and blue, respectively. Compounds 5b (A) and 5d (B) are displayed as sticks with yellow and cyan carbon atoms, respectively. Hydrogen bonds are indicated by a red dashed line. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.5. Compounds 5b and 5d induced apoptosis and G1 cell cycle arrest
In light of the fact that lead compound XYA1353 has been shown to induce apoptosis in MM cells by activating the caspase-dependent endogenous pathway [36], flow cytometry was performed on compounds 5b and 5d at a concentration of 1 μM to evaluate their effects on cell apoptosis in RPMI-8226 and U266 cells. The results depicted in Fig. 4A and B indicate that, compared to the control group, compounds 5b and 5d significantly induced apoptosis in two MM cell lines. Specifically, RPMI-8226 cells treated with 5b and 5d exhibited an apoptotic rate of approximately 80% and 50%, respectively, while U266 cells showed rates exceeding 20% and 35%. Next, we proceeded to evaluate the impact of 5b and 5d on cell cycle distribution. Our findings revealed that both compounds significantly increased the percentage of cells in the G1 phase. In detail, RPMI-8226 cells treated with 5b exhibited a more pronounced increase while U266 cells treated with 5d displayed a similar trend (as shown in Fig. 4C and D). Similar to the lead compound XYA1353, it will be necessary to assess the impact of compounds 5b and 5d on apoptosis-associated markers such as Bak, Bax, and PARP1 in future evaluations.
Fig. 4.
Effects of 5b and 5d on apoptosis (A and B) and cell cycle distribution (C and D) in RPMI-8226 and U266 cells. Error bars represent mean ± SD (n = 3). *P < 0.05, **P < 0.01.
3.6. Drug likeness and ADME analysis
It is well known that knowledge of the physiochemical properties and ADME profiles of hit/lead compounds is valuable in early drug discovery, reducing the risk of failure in later stages of development. The drug likeness and ADME analysis of compounds 5b and 5d were carried out by the free web tool “SwissADME” (http://www.swissadme.ch/) and the results were shown in Table 2. The table presents crucial data, including the count of heavy atoms, H-bond donors, and acceptors. Moreover, essential molecular properties such as the number of rotatable bonds, molar refractivity (MR), and Topological Polar Surface Area (TPSA) were computed.
Table 2.
Calculated physicochemical and ADME properties of compounds 5b and 5d.
| Property | 5b | 5d |
|---|---|---|
| Molecular weight | 385.50 g/mol | 385.50 g/mol |
| Num. heavy atoms | 29 | 29 |
| Num. arom. heavy atoms | 21 | 21 |
| Fraction Csp 3 | 0.24 | 0.24 |
| Num. rotatable bonds | 9 | 6 |
| Num. H-bond acceptors | 2 | 2 |
| Num. H-bond donors | 1 | 1 |
| Molar Refractivity | 118.71 | 119.18 |
| TPSA | 42.94 Å2 | 42.94 Å2 |
| GI absorption | High | High |
| BBB permeant | Yes | Yes |
| P-gp substrate | Yes | Yes |
| CYP1A2 inhibitor | Yes | Yes |
| CYP2C19 inhibitor | Yes | Yes |
| CYP2C9 inhibitor | Yes | Yes |
| CYP2D6 inhibitor | Yes | Yes |
| CYP3A4 inhibitor | Yes | Yes |
| Log Kp (skin permeation) | −5.02 cm/s | −4.91 cm/s |
| Lipinski | Yes; 1 violation: MLOGP>4.15 | Yes; 1 violation: MLOGP>4.15 |
| Ghose | Yes | Yes |
| Veber | Yes | Yes |
| Egan | Yes | Yes |
| Muegge | No; 1 violation: XLOGP3>5 | No; 1 violation: XLOGP3>5 |
| Bioavailability Score | 0.55 | 0.55 |
The physicochemical properties of compounds 5b and 5d were identical, except for the difference in the number of rotatable bonds. Additionally, both compounds exhibited high gastrointestinal (GI) absorption (as per the Boiled egg), and they also demonstrated BBB permeability. In terms of drug likeness, compounds 5b and 5d were found to be compliant with Lipinski, Ghose, Veber, and Egan criteria, with a bioavailability score of 0.55.
4. Conclusion
The present study involves the synthesis and evaluation of a series of novel 2-iminobenzimidazole derivatives as potential anti-multiple myeloma (MM) agents, building upon the lead compound XYA1353. According to the CCK-8 results, compounds 5b, 5d, and 8g demonstrated superior cytotoxicity against RPMI-8226 and U266 cells among the 34 tested compounds. Moreover, upon substitution of ethoxybenzene with 2-methyl-1,3,5-trimethylbenzene on the R1 side chain (compound 8s-9b), their inhibitory activity surpassed that of aforementioned compounds with an average IC50 value of approximately 2 μM.
The Western blot and Dual-luciferase reporter assay results demonstrated that compounds 5b and 5d exhibited the ability to reduce c-Myc protein expression, which was likely due to their suppressive effects on c-Myc promoter transcriptional activity. Furthermore, compounds 5b and 5d were capable of inducing apoptosis and G1 phase cell cycle arrest in RPMI-8226 and U266 cells. Therefore, owing to their groundbreaking chemical structure and extraordinary anti-MM activity, 2-iminobenzimidazoles (such as compounds 5b and 5d) can be identified as novel potent c-Myc inhibitors for the treatment of multiple myeloma. Future research should prioritize the investigation of the underlying molecular mechanism against c-Myc-related diseases, both in vitro and in vivo.
Funding
This research was funded by the Natural Science Research Project of Anhui Educational Committee (grant number: 2023AH051226); Anhui Provincial Natural Science Foundation (grant number: 2308085Y11); Scientific Research Foundation for High-level Talents of Anhui University of Science and Technology (grant number: 2023yjrc02).
CRediT authorship contribution statement
Shihao Li: Writing – review & editing, Writing – original draft, Visualization, Methodology, Data curation. Yinchuan Wang: Visualization, Data curation. Jiacheng Yin: Formal analysis. Kaihang Li: Formal analysis. Linlin Liu: Validation, Conceptualization. Jian Gao: Writing – review & editing, Validation, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28411.
Contributor Information
Linlin Liu, Email: liulinlin0103@163.com.
Jian Gao, Email: gaojian@aust.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Mikhael J., Bhutani M., Cole C.E. Multiple myeloma for the primary Care provider: a practical review to promote earlier diagnosis among diverse populations. Am. J. Med. 2023;136(1):33–41. doi: 10.1016/j.amjmed.2022.08.030. [DOI] [PubMed] [Google Scholar]
- 2.de Arriba de la Fuente F., Montes Gaisan C., de la Rubia Comos J. How to manage patients with lenalidomide-refractory multiple myeloma. Cancers. 2022;15(1) doi: 10.3390/cancers15010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Odat O.S., et al. Autophagy and apoptosis: current challenges of treatment and drug resistance in multiple myeloma. Int. J. Mol. Sci. 2022;24(1) doi: 10.3390/ijms24010644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mian H., et al. The prevalence and outcomes of frail older adults in clinical trials in multiple myeloma: a systematic review. Blood Cancer J. 2023;13(1):6. doi: 10.1038/s41408-022-00779-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chahin M., et al. Clinical considerations for immunoparesis in multiple myeloma. Cancers. 2022;14(9) doi: 10.3390/cancers14092278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazarbachi A.H., et al. Relapsed refractory multiple myeloma: a comprehensive overview. Leukemia. 2019;33(10):2343–2357. doi: 10.1038/s41375-019-0561-2. [DOI] [PubMed] [Google Scholar]
- 7.Joseph N.S., et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J. Clin. Oncol. 2020;38(17):1928–1937. doi: 10.1200/JCO.19.02515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhanasekaran R., et al. The MYC oncogene - the grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 2022;19(1):23–36. doi: 10.1038/s41571-021-00549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llombart V., Mansour M.R. Therapeutic targeting of "undruggable" MYC. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lourenco C., et al. MYC protein interactors in gene transcription and cancer. Nat. Rev. Cancer. 2021;21(9):579–591. doi: 10.1038/s41568-021-00367-9. [DOI] [PubMed] [Google Scholar]
- 11.Holien T., et al. Addiction to c-MYC in multiple myeloma. Blood. 2012;120(12):2450–2453. doi: 10.1182/blood-2011-08-371567. [DOI] [PubMed] [Google Scholar]
- 12.Dang C.V., et al. Drugging the 'undruggable' cancer targets. Nat. Rev. Cancer. 2017;17(8):502–508. doi: 10.1038/nrc.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han H., et al. Small-molecule MYC inhibitors suppress tumor growth and enhance immunotherapy. Cancer Cell. 2019;36(5):483–497 e15. doi: 10.1016/j.ccell.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prochownik E.V., Wang H. Normal and neoplastic growth suppression by the extended Myc network. Cells. 2022;11(4) doi: 10.3390/cells11040747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caforio M., et al. Recent advances in searching c-Myc transcriptional cofactors during tumorigenesis. J. Exp. Clin. Cancer Res. 2018;37(1):239. doi: 10.1186/s13046-018-0912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schutz S., et al. The disordered MAX N-terminus modulates DNA binding of the transcription factor MYC:MAX. J. Mol. Biol. 2022;434(22) doi: 10.1016/j.jmb.2022.167833. [DOI] [PubMed] [Google Scholar]
- 17.Pellanda P., et al. Integrated requirement of non-specific and sequence-specific DNA binding in Myc-driven transcription. EMBO J. 2021;40(10) doi: 10.15252/embj.2020105464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madden S.K., et al. Taking the Myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc. Mol. Cancer. 2021;20(1):3. doi: 10.1186/s12943-020-01291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carabet L.A., Rennie P.S., Cherkasov A. Therapeutic inhibition of Myc in cancer. Structural bases and computer-aided drug discovery approaches. Int. J. Mol. Sci. 2018;20(1) doi: 10.3390/ijms20010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin X., et al. Low molecular weight inhibitors of Myc-Max interaction and function. Oncogene. 2003;22(40):6151–6159. doi: 10.1038/sj.onc.1206641. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher S., Prochownik E.V. Small-molecule inhibitors of the Myc oncoprotein. Biochim. Biophys. Acta. 2015;1849(5):525–543. doi: 10.1016/j.bbagrm.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J., et al. Efficacy, pharmacokinetics, tisssue distribution, and metabolism of the Myc-Max disruptor, 10058-F4 [Z,E]-5-[4-ethylbenzylidine]-2-thioxothiazolidin-4-one, in mice. Cancer Chemother. Pharmacol. 2009;63(4):615–625. doi: 10.1007/s00280-008-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H., et al. Improved low molecular weight Myc-Max inhibitors. Mol Cancer Ther. 2007;6(9):2399–2408. doi: 10.1158/1535-7163.MCT-07-0005. [DOI] [PubMed] [Google Scholar]
- 24.Yap J.L., et al. Pharmacophore identification of c-Myc inhibitor 10074-G5. Bioorg Med Chem Lett. 2013;23(1):370–374. doi: 10.1016/j.bmcl.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shukla S., et al. 3JC48-3 (methyl 4'-methyl-5-(7-nitrobenzo[c][1,2,5]oxadiazol-4-yl)-[1,1'-biphenyl]-3-carboxylate): a novel MYC/MAX dimerization inhibitor reduces prostate cancer growth. Cancer Gene Ther. 2022;29(11):1550–1557. doi: 10.1038/s41417-022-00455-4. [DOI] [PubMed] [Google Scholar]
- 26.Wang H., et al. Direct inhibition of c-Myc-Max heterodimers by celastrol and celastrol-inspired triterpenoids. Oncotarget. 2015;6(32):32380–32395. doi: 10.18632/oncotarget.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung K.Y., et al. Perturbation of the c-Myc-Max protein-protein interaction via synthetic α-helix mimetics. J. Med. Chem. 2015;58(7):3002–3024. doi: 10.1021/jm501440q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo H., Henriksson M. Identification of small molecules that induce apoptosis in a Myc-dependent manner and inhibit Myc-driven transformation. Proc Natl Acad Sci U S A. 2006;103(16):6344–6349. doi: 10.1073/pnas.0601418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo H., et al. Myc overexpression enhances apoptosis induced by small molecules. Cell Cycle. 2006;5(19):2191–2194. doi: 10.4161/cc.5.19.3320. [DOI] [PubMed] [Google Scholar]
- 30.Jeong K.C., et al. Intravesical instillation of c-MYC inhibitor KSI-3716 suppresses orthotopic bladder tumor growth. J. Urol. 2014;191(2):510–518. doi: 10.1016/j.juro.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Yao R., et al. Identification of a novel c-myc inhibitor 7594-0037 by structure-based virtual screening and investigation of its anti-cancer effect on multiple myeloma. Drug Des Devel Ther. 2020;14:3983–3993. doi: 10.2147/DDDT.S264077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L., et al. Structure-based discovery of Licoflavone B and Ginkgetin targeting c-Myc G-quadruplex to suppress c-Myc transcription and myeloma growth. Chem. Biol. Drug Des. 2022;100(4):525–533. doi: 10.1111/cbdd.14064. [DOI] [PubMed] [Google Scholar]
- 33.Yao R., et al. Novel dual-targeting c-Myc inhibitor D347-2761 represses myeloma growth via blocking c-Myc/Max heterodimerization and disturbing its stability. Cell Commun. Signal. 2022;20(1):73. doi: 10.1186/s12964-022-00868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J., et al. Identification of trovafloxacin, ozanimod, and ozenoxacin as potent c-myc G-quadruplex stabilizers to suppress c-myc transcription and myeloma growth. Mol Inform. 2022;41(10) doi: 10.1002/minf.202200011. [DOI] [PubMed] [Google Scholar]
- 35.Geng X., et al. Design, synthesis, and biological evaluation of novel benzimidazolyl isoxazole derivatives as potential c-Myc G4 stabilizers to suppress c-Myc transcription and myeloma growth. J. Mol. Struct. 2023;1275 [Google Scholar]
- 36.Gao J., et al. A novel 2-iminobenzimidazole compound, XYA1353, displays in vitro and in vivo anti-myeloma activity via targeting NF-κB signaling. Mol. Cell. Biochem. 2023 doi: 10.1007/s11010-023-04764-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








