Abstract
Polysaccharides from the red seaweed Gracilaria fisheri possess many functions, which include antioxidant, antiviral, and antibacterial activities. However, detailed data on their immunomodulatory activities are scarce. Here, we isolated sulfated galactans (SG) from G. fisheri. We found that the predominant SG from G. fisheri, termed SG-1, had an estimated molecular mass of 100 kDa and activated murine J774A.1 macrophages via the dectin-1 signaling pathway. Furthermore, we observed enhancement of nitric oxide (NO) secretion, increased expression of inducible nitric oxide synthase (iNOS) mRNA, and increased mRNA levels of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukins IL-1β and IL-6 by SG-1 in macrophages. Moreover, there was higher expression of intercellular adhesion molecule 1 (ICAM-1) and co-stimulatory molecules (B7-1 and B7-2) mRNA. Treatment with G. fisheri SG-1 at 50 μg/mL generally achieved or exceeded the pro-inflammatory activities of 100 ng/mL lipopolysaccharide. Our study demonstrates immune-stimulatory activities of G. fisheri SG that may be of value for immune-potentiating treatment in humans or livestock.
Keywords: immunomodulation, cytokine, macrophage, polysaccharide, red seaweed, Gracilaria fisheri
1. Introduction
In Asian countries, in particular in south-east Asia, seaweeds have been used for a long time as a food source and a component of traditional medicine preparations (1). Several seaweed phytochemicals have been extensively studied and are being developed into novel pharmaceutical products (2, 3). Among them, water-soluble polysaccharides have gained most attention, because they lack toxicity and exhibit a variety of bioactivities, such as anti-cancer, anti-diabetic and anti-oxidant properties (3, 4).
Polysaccharides from seaweed differ from those obtained from all other plants as they contain unique galactans, which are composed of repeating agarobiose units, alternating between 3-linked β-D-galactopyranose (G) and 4-linked α-galactopyranose (LA) moieties (5). They can be divided into agar (with L-series α-galactose) and carrageenan (with D-series α-galactose) (6). Substantial amounts of the α-galactose residues may be in the 3,6-anhydro-galactose form. The G unit can bear sulfate or methyl group substitutions (7). Sulfation can vary in carrageenans, with one, two, or three sulfate groups linked to the G unit. Sulfate groups can also be found in the α-galactose (LA) unit (7).
Gracilaria fisheri, a species of red seaweed (red algae, rhodophyta), is commonly used as a fresh vegetable and as a dried product. It has been reported that G. fisheri extracts have antibacterial properties (8), and sulfated galactans from G. fisheri have antioxidant activity (9) as well as immune-stimulatory and antiviral effects in shrimp (10). There are also studies that have shown immunostimulatory and anti-tumorigenic activities of sulfated polysaccharides from brown and green seaweeds (11–13). Finally, immunomodulatory activities of extracts from other red seaweeds have been reported in-vitro and in-vivo (14–17), but we lack information on immunomodulatory activities of purified sulfated polysaccharides from red seaweed.
Several studies have demonstrated that polysaccharides from edible plants stimulate macrophages (18). Macrophages play essential roles in both innate and adaptive immunity to pathogens and are central to the elimination of cancer cells and foreign particles (19). Generally, polysaccharides bind to receptors on the surface of macrophages such as Toll-like receptor (TLR) 4, CD14, CD11b/CD18, scavenger receptor, dectin-1, and mannose receptor, which leads to the stimulation of phagocytosis and increased production of nitric oxide (NO), cytokines, chemokines, and inducible nitric oxide synthase (iNOS) (18). Moreover, macrophages can also present digested antigen to helper T cells and are able to express the major histocompatibility complex (MHC) class II and co-stimulatory molecules such as B7-1 (CD80) and B7-2 (CD86), which are used to generate the second signal to trigger T lymphocytes (20).
Here, we investigated the immune-stimulating activity of sulfated polysaccharides isolated from the red seaweed, G. fisheri. Additionally, we analyzed the signaling pathways that are activated by G. fisheri extracts in J774A.1 murine macrophage cells.
2. Materials and methods
2.1. Red seaweed G. fisheri collection
The red seaweed G. fisheri was collected from a commercial seaweed pond in Ko Yo District, Songkhla Province, Thailand. The sample was washed thoroughly with seawater, tap water, and distilled water, and then air-dried and further dried at 35 – 40 °C overnight. The dried seaweed was cut into small pieces (0.5 – 1 cm), milled to powder, passed through a 100-mesh sieve, and stored in a refrigerator at 4 °C.
2.2. Extraction of the sulfated polysaccharides from G. fisheri
Polysaccharides from G. fisheri were extracted with hot water as follows. The G. fisheri powder was boiled in distilled water (1:5 w/v) at 85 °C for 2 h using a reflux condenser under reduced pressure. Separation of the residue from the extract was performed by centrifugation (10,000 × g for 7 min). The supernatant was collected, precipitated by adding three volumes of 99.5% ethanol (v/v), and stored at 4 °C. Stored samples were re-dissolved in distilled water, dialyzed against distilled water using a membrane with a molecular weight cut-off of 6 – 8 kDa, and freeze-dried to obtain the crude sulfated polysaccharide. The yield was calculated with the following equation: Sulfated polysaccharide yield (mg/g) = dry weight (mg)/ dry weight of seaweed material (g).
2.3. Purification of sulfated polysaccharide
The sulfated polysaccharide was purified using anion exchange chromatography. The crude extract (500 mg) was dissolved in 4 mL of 50 mM NaOAc (pH 5.5) and applied to a DEAE Sephadex® column (1 × 25 cm) equilibrated with the same buffer. The column was run with a stepwise gradient (0 M NaCl for 75 ml, 0.25 M NaCl for 195 ml, 0.5 M NaCl for 150 ml) in the same buffer at a flow rate of 1.0 mL/min. 3-ml fractions were collected, and purified sulfated polysaccharide was pooled from fractions 35 to 50, which were dialyzed against distilled water and freeze-dried.
2.4. Chemical component analysis
Determination of total sugar
The total sugar content was analyzed by the phenol sulfuric acid method as described by Dubois et al. (21) with slight modifications, using galactose as the standard. A sample solution of 0.2 mL was mixed with 0.2 mL of a phenol solution (5.0%, w/v) and 1.0 mL concentrated sulfuric acid was added to the reaction mixture. After incubating for 20 min at room temperature, the absorbance was measured at 490 nm with a microplate reader. Distilled water was used as blank. The calculations were based on a calibration curve obtained with galactose. The results were expressed as galactose equivalents in milligrams per gram of extract.
Monosaccharide composition analysis
The monosaccharide components of the sulfated polysaccharide extract was analyzed using high-performance liquid chromatography (HPLC). The purified crude polysaccharide (2 mg) was hydrolyzed with 0.5 ml of 2 N HCl at 100 °C for 2 h. The hydrolysate was washed with distilled water to remove the acid, evaporated using a rotary evaporator, and then washed again three times or until all the acid was removed. The hydrolysate was then kept in a brown-glass screw-cap vial before monosaccharide analysis using glucose, galactose, mannose and xylose as standards, and ribose as internal standard.
For pre-column derivatization, which was adapted from the report by Castells et al. (22), a solution of 4-(3-methyl-5-oxo-2-pyrazolin-l-yl) benzoic acid (PMPA) was prepared immediately before use by the following procedure. PMPA crystals were dissolved in 0.25 M NaOH in 50% methanol up to the point at which the pH stabilized at 7.8 – 8 and then completed to volume with 50% methanol to obtain a final concentration of 0.15 M PMPA. At this step an internal standard (ribose) was gravimetrically added to the aliquots of the hydrolyzed samples (300 μL). Then, the extracts were incubated with 1 mL 0.15 M PMPA/50% methanol at 70 °C for 2 h. After incubation, the samples were acidified by addition of 150 μL 2 M HCl and shaken to precipitate most of the excess derivatization agent. After a few minutes, the samples were centrifuged for 5 min at ~ 6,000 × g, and the obtained supernatants were filtered through 0.2-μm nylon membrane filters.
Chromatography
Chromatographic analysis was performed on a Waters liquid chromatography system, equipped with a Waters 2996 photodiode array detector, Waters 1525 binary HPLC pumps, a Waters in-line degasser AF and a Waters 717 plus autosampler. The system was controlled with Empower software. Chromatographic separations were performed using a Purospher®Star RP-18 end-capped C18 column (4.6 × 150 mm, 5 μm pore size, Merck KGaA, Damstadt, Germany) at ambient temperature. The monitoring wavelength was set to 271 nm. An elution gradient over 60 mL from 0 to 100% buffer B (Buffer A: 20 mM sodium dihydrogen phosphate/5 mm phosphoric acid pH 2.6 in water; buffer B: 100% acetonitrile) was used at a flow rate of 1 mL min−1. 20-μL samples were injected using a sample loop. At the end of each run, the column was rinsed by a cleanup procedure with pure acetonitrile.
Fourier-Transformed Infrared Spectroscopy (FT-IR spectroscopy)
The FT-IR spectra were recorded on a Perkin-Elmer spectrum GX FT-IR spectrometer. SG-1 was analyzed as KBr pellet. Baseline correction was applied in the 400 – 4,000 cm−1 range.
Nuclear magnetic resonance (NMR) spectra
SG was dissolved in deuterium oxide (D2O), and 1H and 13C nuclear magnetic resonance spectra were acquired on a Bruker (AVANCE 500) UltraShield-NMR spectrometer at 80°C. Sodium 2,2-dimethylsilapentane-5-sulphonate (DSS) was used as the internal standard (0.00 ppm for 1H). 1H and 13C NMR chemical shifts were measured in ppm relative to internal reference D2O at 4.7 ppm. A distortion-less enhancement by polarization transfer (DEPT 135) spectrum was recorded to determine each carbon’s hydrogenation; the acquisition and delay times were 1.0 s. 2D 1H and 13C Heteronuclear Single Quantum Coherence (HSQC) spectra were acquired using the pulse programs supplied with the apparatus.
Determination of protein contents
Protein content was measured as described by Lowry et al. using bovine serum albumin (BSA) as standard (23). 100-μL samples were mixed with 100 μL of biuret reagent. After incubation at room temperature for 10 min, 50 μL 50.0% Folin-Ciocalteu reagent was added and the mixtures were maintained at room temperature for 30 min. Absorbance was measured at 660 nm with a microplate reader, with distilled water used as blank.
Determination of sulfate content
Sulfate content was determined by a turbidimetric method (24). After acid hydrolysis of the sample (5 mg) with 0.2 mL of 1 M HCl for 8 h at 100 °C, the sulfate content was measured as follows. A standard curve was prepared with a solution of K2SO4. Then, 3.8 mL trichloroacetic acid (3.0% w/v) and 1.0 mL of a barium chloride-gelatin solution were added, mixed and incubated at room temperature for 15 min. Absorbance was measured at 360 nm with a microplate reader, with hydrochloric acid solution used as blank.
Molecular weight analysis
Molecular weights were determined using gel permeation (size exclusion) chromatography using a TSKgel G4000 PWXL column (7.8 mm × 300 mm) at a temperature of 30 °C and a flow rate of 1.0 mL min−1. Milli-Q water was used as the mobile phase. A sample of 50 μL was injected onto the column.
T-series standard dextrans (T-2000, 500, 70, 40, 20, 10) were used to compute a calibration curve to estimate the sample molecular weight (MW) by determining the respective Kav values using the formula Kav = Ve – V0/Vt – V0 (where Ve is the elution volume in mL, V0 the column void volume in mL, and Vt the total column volume in mL). The average molecular weights were automatically estimated by the Waters Empower software based on the linear relationship between the Kav of molecules and the logarithms of their molecular weights.
2.5. Macrophage cell culture
The J774A.1 murine macrophage cell line was used for the experiments. Cells were grown in completed Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen, Thailand) and 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT, USA) and cultured in a humidified incubator containing 5% CO2 at 37 °C. Adherent cells were removed from the culture surface using Gibco™ Trypsin-EDTA (0.25%) when the cells were at 80 % confluence. Cell viability was determined to be at least 90%. Afterwards, non-adherent cells were removed by washing with serum-free medium and seeded in 24-well plates at a density of 4 × 105 cells/well and incubated in 5% fetal calf serum-DMEM medium in the presence or absence of lipopolysaccharide (LPS, 100 ng/mL) or G. fisheri SG-1 (10, 20, 30, 40 or 50 μg/mL) for 24 h.
2.6. Determination of cell viability
Cell viability was measured using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Briefly, J774A.1 macrophage cells at 4 × 105 cells/ml were cultured in a 96-well plate for 24 h. The cells were subsequently treated with different concentrations of G. fisheri SG-1 (10 – 50 μg/mL) for 24 h and incubated with MTT for another 4 h at 37 °C and 5% CO2. The index of the cell viability was determined by measuring formazan production with an ELISA reader (Benchmark Plus, Bio-Rad, and Hercules, CA, USA) at an absorbance of 600 nm. Cell viability was determined relative to the untreated control cells.
2.7. Determination of NO production
J774A.1 cells were cultured in a 96-well plate at a density of 4 × 105 cells/ml at 37 °C for 24 h. Then, the cells were treated with different concentrations of G. fisheri SG-1 (10–50 μg/mL) for 24 h. Afterwards, NO in the culture supernatants was measured by the addition of 100 μL of Griess reagent [1% sulfanilamide and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride in 5% phosphoric acid] to 100 μL of each sample for 10 min. The absorbance at 540 nm was measured, and the concentration of nitrite was calculated using a calibration standard curve constructed using sodium nitrite dissolved in DMEM.
2.8. Determination of phagocytic activity
Phagocytic activity was determined with a zymosan-nitroblue tetrazolium (NBT) reduction assay (25). J774A.1 cells, at 4 × 105 cell/mL, were treated with 10 – 50 μg/ml of G. fisheri SG-1 at 37 °C for 24 h. Then the cells were washed twice with DMEM and treated with 600 μg/mL zymosan at 37 °C for 1 h. The cells were washed with methanol for three times and lysed using a 2 M KOH in dimethyl sulfoxide (DMSO) solution . The amount of formazan product was measured at 570 nm. The effect of the aqueous G. fisheri extract on phagocytic activity of J774A.1 was expressed in comparison to the solvent control as the percentage of phagocytic stimulation.
2.9. Determination of gene expression by quantitative real time-polymerase chain reaction (qPCR)
The mRNA expression levels of iNOS, dectin-1, lysozyme M, TNF-α, IL-1, IL-6, B7-1, B7-2 and ICAM-1 genes were determined by qPCR. To that end, J774A.1 cells (1.5 × 106 cells/well) were added to a 6-well plate, incubated for 1 h and further exposed to final concentrations of SG-1 at 30, 40, 50 μg/mL or 100 ng/mL LPS for 20 h at 37 °C under a humidified 5%-CO2 atmosphere. Subsequently, total RNA was extracted from treated cells using an RNA extraction kit (FAVORGEN Biotech, Taiwan). RNA concentration and purity were assessed by determining the 260/280 nm ratio using a spectrophotometer and only samples with a 260/280 nm ratio of 1.6 – 2.2 were used. The cDNA was synthesized from 500 ng of RNA template using Oligo(dT)18 primer and a reverse transcription system according to the instructions provided by the manufacturer (Solis BioDyne, Estonia).
For qPCR, the ready-to-use PCR master-mix 5 × HOT FIREPol® Evagreen® qPCR Mix Plus (Solis BioDyne, Estonia) was used. The PCR reaction consisted of 2 μL of cDNA template (250 ng), 0.5 μL of forward and reverse primers (10 ng/μL) and 5 μL of PCR master-mix in a final volume of 25 μL. The amplification reaction was performed with activation of polymerase at 95 °C for 15 min followed by 40 cycles of denaturation at 95 °C for 15 sec, annealing at 54 °C for 20 sec, and elongation at 72 °C for 30 sec. The primer sequences are shown in Table 1. The relative gene expression level was quantitated from fluorescence signal emission using the comparative CT method. A melting point dissociation curve was generated to confirm the presence of a single product. For all samples, measurements were performed in triplicate. The relative gene expression was evaluated from the following equation: Fold change = 2−ΔΔCT
Table 1.
Composition of crude SG extract of Gracilaria fisheri and purified fractions of SG obtained after anion-exchange chromatography
| Compound | Yield (mg/g) | Protein (mg/g) | The sulfate content (mg/g) | Total sugar content (mg/g) | Sugar component (mg/g) | ||
|---|---|---|---|---|---|---|---|
| galactose | glucose | xylose | |||||
| Crude SG | 150.5±11.9* | 26.3±3.00a | 84.1±2.30a | 802.8±14.9a | 410.30±15.60a | 59.8±6.32a | 18.4±5.13a |
| F1 | 145.0±14.2#a | 15.2±5.40b | 21.6±14.00b | 602.0±14.90b | 880.30±10.2b | 84.8±6.90b | 20.4±3.37a |
| F2 | 69.01±8#b | 14.8±4.10b | 22.0±11.00c | 591.5±10.30b | 751.5±14.12c | 90.8±5.21b | 25.0±4.10a |
Values within columns with different superscript letter differ significantly (p£0.05).
Yield from milled seaweed step
Yield from crude SG
2.10. Statistical analysis
Statistical analysis was performed using GraphPad Prism version 8 with one-way ANOVAs with Tukey’s post-test. All data show the mean and standard deviation (S.D.). All replicates are biological.
3. Results
3.1. Isolation and characterization of G. fisheri SG
Crude polysaccharide from the red seaweed G. fisheri was extracted with hot water. The fractions to be further analyzed were selected based on the total carbohydrate elution profile of the anionic exchange chromatography purification step, which showed two main peaks (Fig. 1A), one of which eluted at the 0.25 M-NaCl step of the stepwise gradient, and the other at the 0.5 M NaCl step. The two polysaccharide fractions (SG-1 and SG-2) were freeze-dried and a yield of 145 mg and 69 mg for SG-1 and SG-2, respectively, per g of crude extract was achieved (Table 1).
Figure 1.
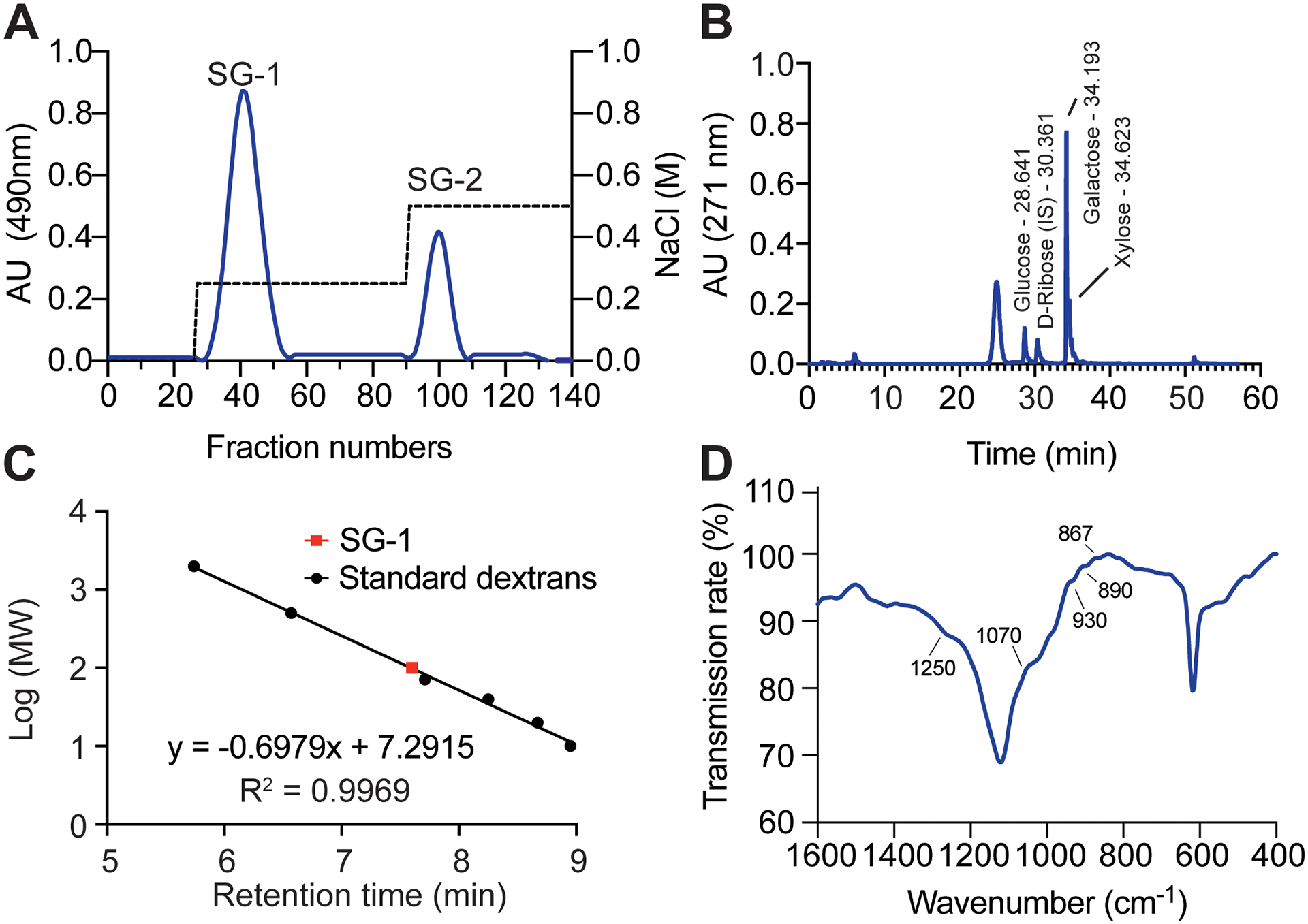
Purification and characterization of G. fisheri SG-1. (A) Separation of G. fisheri crude extract by anion exchange chromatography. (B) Monosaccharide analysis by HPLC. (C) Estimation of molecular weight by size exclusion chromatography. Calibration was performed with T-series standard dextrans and the molecular weight (MW) of SG-1 was calculated to be 97.15 kDa using linear regression. (D) FT-IR spectroscopy.
We then analyzed the monosaccharide composition of the two obtained fractions using HPLC. SG-1 was found to contain abundant amounts of galactose and small amounts of glucose and xylose (Fig. 1B). The galactose content of SG-1 (880 mg/g) was higher than that of SG-2 (751 mg/g). We selected SG-1 for further chemical analyses and determination of immunostimulatory activities on macrophages due to its higher abundance and higher galactose content.
Further analysis by size exclusion chromatography revealed that SG-1 has an estimated molecular mass of ~ 100 kDa (Fig. 1C) and chemical analysis showed a sulfate content of 21.16 ± 14 mg/g. The sulfate content we determined is higher than that previously reported of G. fisheri sulfated polysaccharides by other authors (9). Assuming that SG-1 predominantly represents one defined polysaccharide, we next performed FT-IR spectroscopy to identify the position of the sulfates in the SG-1 polysaccharide. Typical absorption bands corresponding to polysaccharides were identified (26–28). FT-IR spectra of SG-1 showed absorption bands at 1250, 1070, 930, 867, 850 and 827 cm−1 (Fig. 1D).
Finally, the composition and structure of SG-1 was analyzed by 2D 1H and 13C HSQC (Fig. 2). The HSQC spectra revealed the key features that are characteristic of a galactan subunit structure. Two major correlation signals at δ 5.56,98.2 and δ 4.99,102.4 and two minor signals in this region at δ 5.71,100.9 and δ 4.87,103.5 were detected. Moreover, we observed minor correlation signals at δ 4.29, 60.9, δ 4.21,61.3, and δ 4.10, 71.6.
Figure 2.

1H and 13C HSQC spectrum of SG-1 in D2O solution
3.2. Activation of macrophages by G. fisheri SG-1
Macrophages are major tissue phagocytes with the ability to engulf and destroy foreign particles (29). Activation of macrophages is a key event in innate and adaptive immunity and essential for the defense against invading microorganisms (30). Several polysaccharides from various plants have been documented to have immunostimulatory effects on macrophages. For example, polysaccharides isolated from the fruiting bodies of Hericium erinaceus (31) Inonotus obliquus (32) and Ganoderma atrum (33) activated NO production, increased phagocytic activity, and promoted the expression of pro-inflammatory cytokines and co-stimulatory molecules in macrophages. However, these polysaccharides are structurally different from those isolated from red seaweed. As stated above, we lack information on immune-stimulatory activities of purified red seaweed polysaccharides.
To investigate the effect of G. fisheri SG-1 on the phagocytic activity of macrophages, we performed zymosan-nitroblue tetrazolium (NBT) reduction assays. The phagocytosis of macrophages was significantly enhanced by G. fisheri SG-1 at concentrations of 10 (13.04% ± 2.65), 20 (23.49% ± 0.98), 30 (35.82% ± 1.31), 40 (57.34% ± 5.11) and 50 μg/ml (72.21% ± 3.94) in a dose-dependent manner (Fig. 3A). We next investigated macrophage function by assessment of expression of lysozyme M, which is the murine homolog of human lysozyme and has been associated with protection from bacterial infections (34–36). As shown in Figure 3B, G. fisheri SG-1 significantly increased the expression of lysozyme M mRNA at concentrations 30, 40 and 50 μg/mL (1.52 ± 0.06, 1.61 ± 0.05 and 1.76 ± 0.13 fold of control, respectively) in a dose-dependent manner. Together, these results indicate that G. fisheri SG-1 efficiently enhances macrophage activity.
Figure 3.

Effect of G. fisheri SG-1 on phagocytic activity in J774A.1 macrophage cells. (A) Percentage of phagocytosis stimulation as compared to solvent control. (B) Expression of lysozyme M mRNA. (A,B) Experiments were performed with n = 3 biologically independent samples. Error bars show the mean ± SD; *, p < 0.05; **, p<0.01, ***, p<0.001; ****, p<0.0001 (one-way ANOVA with Tukey’s post-test versus control values).
3.3. Signaling pathway involved in macrophage activation by G. fisheri SG-1
While polysaccharides extracted from seaweeds have been demonstrated here and in previous studies to exhibit immune-stimulating properties (10–17), the signaling pathway(s) involved in macrophage activation by seaweed polysaccharides, in particular those produced by G. fisheri, remain unclear. Dectin-1 is the major macrophage receptor for β-glucans and generates a proinflammatory response after recognition of carbohydrates from a variety of sources, including edible mushrooms, seaweeds, yeasts and pathogenic microorganisms (37, 38). We therefore hypothesized that G. fisheri SG-1 triggers macrophage activity via the activation of the dectin-1 signaling pathway. G. fisheri SG-1 at 30, 40 and 50 μg/mL significantly enhanced dectin-1 mRNA expression as 1.47 ± 0.17, 1.59 ± 0.22 and 1.74 ± 0.21 fold of control, respectively (Fig. 4A).
Figure 4.

Effect of G. fisheri SG-1 on expression of dectin-1 mRNA (A), production of NO (B), iNOS mRNA expression (C), and cell cytotoxicity (D). Experiments were performed with n = 3 biologically independent samples. Error bars show the mean ± SD; *, p < 0.05; **, p<0.01, ***, p<0.001; ****, p<0.0001 (one-way ANOVA with Tukey’s post-test versus control values).
3.4. Activation of nitric oxide and cytokine expression in macrophages by G. fisheri SG-1
Associated with phagocytosis and activation of macrophages is enhanced expression of inflammatory and cytotoxic molecules, such as nitric oxide (NO), and increased expression of a series of cytokines.
To test for NO expression, we incubated J774A.1 macrophage cells with various concentrations of G. fisheri SG-1, LPS, or vehicle for 24 h, and determined the NO metabolite nitrite by the Griess assay (39). A minimum amount of NO was released when J774A.1 macrophages were exposed to medium alone, whereas incubation of the cells with only 10 μg/ml of G. fisheri SG was sufficient to detect a significant increase in NO production (11.27 μM ± 2.62). Moreover, a dose-dependent enhancement of NO production was observed in macrophages treated with 20 – 50 μg/ml of G. fisheri SG-1, 18.81 μM ± 8.07, 29.42 μM ± 0.97, 30.16 μM ± 1.39 and 31.01 μM ± 1.23, respectively (Fig. 4B).
Generally, NO production requires nitric oxide synthase (NOS). Several inflammatory stimuli can induce the expression of inducible NOS (iNOS), one of the isoforms of NOS, in various cell types such as macrophages (40). Thus, we examined whether the observed increase in NO production in macrophages is due to up-regulation of iNOS expression by determining iNOS mRNA expression by qRT-PCR 24 h after addition of G. fisheri SG-1. G. fisheri SG treatment significantly promoted the expression of iNOS mRNA relative to the constitutively expressed β-actin in a dose-dependent fashion, for 30, 40 and 50 μg/ml of SG-1; 1.52 ± 0.04, 1.68 ± 0.07 and 1.91 ± 0.05 fold of control, respectively (Fig. 4C). Notably, to confirm that the effects of G. fisheri SG-1 on macrophage responses were not due to cytotoxic effects on the cells, we performed an MTT assay to measure cell viability. G. fisheri SG-1 at all concentrations tested had no cytotoxic effect on J774A.1 macrophage cells (Fig. 4D).
Next, we measured the effects of G. fisheri SG-1 on the expression of the genes encoding the cytokines IL-1β, IL-6 and TNF-α in J774A.1 macrophage cells. To that end, macrophage cells were treated with different concentrations of G. fisheri SG-1 or LPS for 24 h. G. fisheri SG-treated J774A.1 macrophage cell at 30, 40 and 50 μg/mL significantly increased the expression of IL-1β (1.42 ± 0.11, 1.71 ± 0.07 and 1.91 ± 0.07 fold of control, respectively), IL-6 (1.67 ± 0.14, 1.86 ± 0.05 and 1.99 ± 0.10 fold of control, respectively) and TNF-α (1.53 ± 0.10, 1.72 ± 0.07 and 1.90 ± 0.09 fold of control, respectively) mRNA in dose-dependent manner (Fig. 5A–C).
Figure 5.

Effect of G. fisheri SG-1 on TNF-α, IL-6 and IL-1β mRNA expression of macrophage J774A.1 cells. Experiments were performed with n = 3 biologically independent samples. Error bars show the mean ± SD; *, p < 0.05; **, p<0.01, ***, p<0.001; ****, p<0.0001 (one-way ANOVA with Tukey’s post-test versus control values).
3.5. Effect of G. fisheri SG on J774A.1 macrophage cell intracellular adhesion molecule and co-stimulatory molecule mRNA expression
Activated macrophages not only have phagocyte activity but also function as antigen presenting cells (APCs) to present digested antigen to T cells. Antigen presentation reactions are dependent upon expression of the class II major histocompatibility antigens (MHC), the T-cell receptor, the presented antigen and also the presence of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) (41). Moreover, enhanced expression of co-stimulatory surface molecules such as B7-1 and B7-2 also favors antigen presentation (42). To investigate whether G. fisheri SG-1 can stimulate expression of ICAM-1 and co-stimulatory molecules, J774A.1 macrophage cells were treated with G. fisheri SG-1 at 30, 40 and 50 μg/mL 24 h. G. fisheri SG-1 significantly enhanced ICAM-1 (1.48 ± 0.18, 1.55 ± 0.26 and 1.76 ± 0.26 fold of control, respectively), B7-1 (1.42 ± 0.13, 1.74 ± 0.12 and 1.99 ± 0.09 fold of control, respectively) and B7-2 (1.43 ± 0.11, 1.56 ± 0.06 and 1.70 ± 0.12 fold of control, respectively) mRNA expression in a dose-dependent manner (Fig. 6A–C), which generally are only constitutively expressed in low levels on APCs (43).
Figure 6.

Effect of G. fisheri SG-1 on ICAM-1, B7-1 and B7-2 mRNA expression in macrophage J774A.1 cells. Experiments were performed with n = 3 biologically independent samples. Error bars show the mean ± SD; *, p < 0.05; **, p<0.01, ***, p<0.001; ****, p<0.0001 (one-way ANOVA with Tukey’s post-test versus control values).
4. Discussion
Sulfated polysaccharides from seaweeds are receiving growing scientific interest due to their immunostimulatory and anti-tumorigenic activities (10, 44, 45). The red seaweed G. fisheri is one of many seaweeds for which immunomodulatory effects have been reported. In this species, sulfated polysaccharides have been attributed stimulatory effects on immune responses in shrimp (10). Although polysaccharides extracted from G. fisheri are thus known to possess immune-stimulating properties (10), the signaling pathway that leads to the activation of macrophages has remained unclear. In the present study, immunomodulatory activities of purified sulfated polysaccharides from G. fisheri on J774A.1 murine macrophage cells were studied, and the signaling pathway involved in the macrophage-mediated immune response was analyzed (Fig. 7).
Figure 7.

Proposed signaling pathway for macrophage activation by sulfated galactan (SG). SG-1 from G. fisheri activates macrophages via the dectin-1 signaling pathway. SG-1 activates macrophage phagocytosis and increases iNOS and cytokine mRNA expression, and NO production. Moreover, SG-1 also enhances mRNA expression of intercellular adhesion molecule 1 (ICAM-1) and co-stimulatory molecules (B7-1 and B7-2).
Chemical analysis of the two polysaccharide fractions (SG-1 and SG-2) that we obtained showed a sulfate content higher than that previously reported for G. fisheri sulfated polysaccharides (10) and also other Gracilaria spp. (7, 46, 47) (Table 1). Moreover, we also found that SG-1 contains small amounts of glucose and xylose. This is in accordance with the notion that sulfated polysaccharides from seaweed can contain a variety of sugars such as L-fucose, D-xylose, D-galactose, D-glucuronic acid, D-mannose and D-glucose (48, 49). We further analyzed the size of SG-1 by size exclusion chromatography and found that its molecular weight is ~ 100 kDa (Fig. 1C), which is in agreement with a previous report (10). Next, we used FT-IR spectroscopy to identify where the sulfates are positioned. The FT-IR spectrum of SG-1 showed an absorption pattern that is similar to those described for other sulfated seaweed polysaccharides, including those isolated from red seaweed (10), inasmuch as it included signals at 1250, 1070, 930, 890, and 867 cm−1 (Fig. 1D). It has been reported that the broad absorption seen at 1250 cm−1 is due to S=O stretching and representative of sulfate groups (50), while signals at 1070 cm−1 are characteristic of the “skeletal mode” of red seaweed galactan (46). Furthermore, signals at 1070 cm−1 and 930 cm−1 are associated with the presence of a C3–O–C6 bridge of the anhydrogalactose residue (5), while signal at and 890 cm−1 is characteristic of a non-sulfated pyranosyl ring as found in agar {Matsuhiro, 1993 #81}. Finally, the spectra at 867 cm−1 is representative of the C–O–SO3 bridge at C6 of galactose and C2 of galactose, respectively (51).
The obtained HSQC spectrum had several key features characteristic of a galactan subunit structure. Two major correlation signals at δ 5.56,98.2 and δ 4.99,102.4 correspond to anomeric proton-carbon from 1,4-α-L-anhydrogalctose (LA-1) and 1,3-β-D-galactose (G-1), respectively (7, 52). Another two minor signals in this region at δ 5.71,100.9 and δ 4.87, 103.5 correspond to anomeric proton-carbon from 1,4-α-L-galactose-6-sulfated (L1–6S) and 1,3-β-D-galactose linked to 1,4-α-L-galactose-6-sulfated [G-1(L-6S)], respectively. The signal at δ 5.76,100.1 has been reported to be Floridian starch (53). Three distinguish inverse signal correlates to CH2 at the C6 positions of the galactan subunits. Correlation at δ 4.29, 60.9 attributes to C6 of 1,3-β-D-galactose (G-6) linked 1,4-α-Lanhydrogalactose (LA), while the signal at δ 4.21, 61.3 correlates to C6 of 1,3-β-D-galactose (G-6) linked 1,4-α-L-galactose-6-sulfated (L-6S), and δ 4.10, 71.6 attributes to O-methylation at C6 of 1,3-β-D-galactose (G-6M) (5, 54).
The combined results of the FT-IR and 2D HSQC spectra indicate that SG-1 of G. fisheri is agar that consists mostly of 1,3-β-D-galactose-linked 1,4-α-L-anhydrogalactose with partial sulfonation at C6 of 1,4-α-L-galactose and partial O-methylation at C6 of 1,3-β-D-galactose.
Macrophage activation and phagocytosis of pathogens is considered one of the most important events of the innate immune response to infection. During the phagocytic process, activated macrophages produce several inflammatory and cytotoxic molecules. In this study, we found that sulfated polysaccharide from the red seaweed G. fisheri promotes phagocytic activity of J774A.1 murine macrophage cells in a concentration-dependent manner.
Macrophages respond to foreign particles and immunostimulatory agents such as lipopolysaccharide (LPS) with the activation of various genes, including the gene for lysozyme, which is a major contributor to the oxygen-independent pathway of bacterial killing in macrophage phagosomes and has been used to monitor macrophage activation. During macrophage differentiation, expression of the lysozyme M gene is continuously increased, resulting in a high level of expression in mature macrophages (35, 55–57). Furthermore, it has been shown that expression of the lysozyme M gene is increased in LPS-activated macrophages (36). Moreover, many previous studies suggested that lysozyme M has an important role in protecting the host from bacterial infections (34). In this study, we found that G. fisheri SG-1 significantly increased the expression of lysozyme M mRNA in a dose-dependent manner, indicating that SG-1 enhances macrophage activity.
In addition to eliminating ingested pathogens, the phagocytosis process activates signaling cascades that trigger expression of molecules involved in both innate and adaptive immune responses, such as cytokines and their receptors, enzymes, reactive oxygen/nitrogen species, cell surface molecules and other mediators (58). Our results demonstrate that G. fisheri SG-1 modulates host immune function by stimulating the release of various cytokines as well as NO, which is reminiscent of effects reported by Pérez-Recalde et al., who showed that sulfated polysaccharides from the red seaweed Nemalion helminthoides activated NO production and increased phagocytic activity (59). IL-1β, IL-6 and TNF-α, similarly to NO, not only act as major immune mediators but also induce tumoricidal macrophage activity (45, 60–62). Several sulfated polysaccharides have been shown to induce an increase in NO production. For example, the sulfated polysaccharides obtained from Codium fragile have been shown to promote NO production in RAW 264.7 macrophages (63). Sulfated polysaccharides from Dictyopteris divaricate (64), Porphyra haitanensis (65), and Ecklonia cava (66) revealed similar immunostimulatory effects in RAW 264.7 macrophages. The sulfated polysaccharides in those reports had stronger effects on NO production than SG-1. Differences in NO production capacity promoted by different sulfated polysaccharide may be related to their chemical characteristics, such as polysaccharide type, molecular weight, monosaccharide composition, conformation and branching of the molecule, and sulfate content (32–34).
During the development of adaptive immunity, macrophages present digested antigen to T cell receptors (TCR) and upregulate the expression of co-stimulatory molecules such as B7-1 and B7-2 (20). It has been shown that B7-1 and B7-2 are constitutively expressed on mouse peritoneal macrophages, and LPS predominantly regulates their expression at the level of transcription (43). Here, we demonstrate that G. fisheri SG-1 also enhances expression of both B7-1 and B7-2 mRNAs. These data indicate that G. fisheri SG-1 may be able to increase antigen presentation ability of macrophages. Because transcription of B7-1 and B7-2 genes requires the activation of NF-kB (67), it is possible that G. fisheri SG-1 upregulates B7 gene expression via an NF-kB-dependent intracellular signaling pathway, as has been shown for Nori seaweed extracts that potentially also contained SG (17).
The carboxymethyl and sulfate groups of sulfated polysaccharides are necessary for binding to β-glucan receptors in the macrophage membrane, such as complement receptor 3 (CR3), scavenger receptors, dectin-1, and toll-like receptors, which leads to increased proliferation and differentiation of macrophages (68). It has been shown that carbohydrates from a variety of sources, including edible mushrooms, seaweeds, yeasts and pathogenic microorganisms can be recognized by dectin-1, the major macrophage receptor for β-glucans (40, 41). Similarly, we found that high concentration of G. fisheri SG-1 significantly enhanced dectin-1 mRNA expression. Moreover, it has been postulated that the movement across the cell membrane was restricted due to the large size of sulfated galactan from G. fisheri (10). Thus, it is possible that the immunomodulatory activity of SG-1 may be mediated through an interaction between substituted groups of SG-1 and surface receptors on the macrophage which lead to the activation of downstream signaling cascades.
The expression of dectin-1 can be influenced by various cytokines, steroids and microbial stimuli. For example, Willment et al. showed that the level of dectin-1 mRNA was markedly influenced by cytokines and biological response modifiers (69). IL-4 and IL-13, for example, which are associated with the alternative activation of macrophages (M2 macrophages), increase the expression of dectin-1 at the cell surface, whereas dexamethasone represses dectin-1 expression (69). Nevertheless, the dectin-1 regulation pathway remains mostly unclear.
Dectin-1 is composed of two ligand-binding sites: one that recognizes an endogenous ligand on T cells, and another recognizing exogenous carbohydrates (70). In accordance with its capacity to recognize carbohydrates, dectin-1 has been implicated in the recognition and phagocytosis of microorganisms with intact β-glucans as well as β(1–3)-linked glucose oligomers of 10 or 11-unit length (71). For example, fungal cell wall composed of β-(1,3)- and β-(1,6)-glucans can be recognized by the dectin-1 receptor. In addition, dectin-1-induced-signaling leads to the production of cytokines and non-opsonic phagocytosis of yeast by murine macrophages (37, 72). Importantly, it has been demonstrated that seaweed-derived polysaccharides can activate cells expressing human dectin-1 (73). In the light of these previous reports, our results thus suggest that the dectin-1 pathway plays a fundamental role in coordinating the immunomodulatory effects on macrophages we show here for G. fisheri SG-1.
Finally, various seaweed extract have been shown to possess anticancer properties against numerous forms of cancer (74–76). For example, fucoidan, sulfated polysaccharide from brown seaweed, is naturally effective against bile duct cancer (74), human breast cancer (77), and reduced chemotherapy toxicities in colorectal cancer patients (78). Recently, Lins et al. have shown that sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer) exhibited antitumor effect without toxicity in a murine model (44). Thus, it may be possible that sulfated polysaccharides of the red seaweed G. fisheri also have anti-angiogenic and antitumor activities, which is in accordance with the immune-stimulating activities we show and remains to be addressed in detail.
5. Conclusions
In this study, we isolated the sulfated galactan (SG-1) of G. fisheri, which consists mostly of 1,3-β-D-galactose-linked 1,4-α-L-anhydrogalactose with partial sulfonation at C6 of 1,4-α-L-galactose and partial O-methylation at C6 of 1,3-β-D-galactose. Furthermore, we describe immune-stimulatory activities of G. fisheri SG-1 on murine macrophages that include activation, enhancement of phagocytosis, and increased expression of a series of cytokines and other molecules, of which some have been associated with beneficial immune functions and tumor-inhibiting properties. These findings suggest potential applicability of G. fisheri SG-1 as an immune-potentiator in humans and livestock. Further studies regarding the detailed structures and biological activities of G. fisheri SG, including the less abundant SG-2, are now in progress.
Funding
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), U.S. National Institutes of Health (NIH) and the National Research Council Thailand (NRCT).
References
- 1.Rajapakse N, Kim SK. Nutritional and digestive health benefits of seaweed. Adv Food Nutr Res. 2011;64:17–28. [DOI] [PubMed] [Google Scholar]
- 2.Ely R, Supriya T, Naik CG. Antimicrobial activity of marine organisms collected off the coast of South East India. Journal of Experimental Marine Biology and Ecology. 2004;309(1):121–7. [Google Scholar]
- 3.Misurcova L, Skrovankova S, Samek D, Ambrozova J, Machu L. Health benefits of algal polysaccharides in human nutrition. Adv Food Nutr Res. 2012;66:75–145. [DOI] [PubMed] [Google Scholar]
- 4.Ngo DH, Kim SK. Sulfated polysaccharides as bioactive agents from marine algae. Int J Biol Macromol. 2013;62:70–5. [DOI] [PubMed] [Google Scholar]
- 5.Jantana Praiboon AC, Akakabe Yoshihiko, Bhumibhamond Orapinand, Kajiwara T. Physical and Chemical Characterization of Agar Polysaccharides Extracted from the Thai and Japanese Species of Gracilaria. ScienceAsia. 2006;32:11. [Google Scholar]
- 6.Usov AI. Polysaccharides of the red algae. Adv Carbohydr Chem Biochem. 2011;65:115–217. [DOI] [PubMed] [Google Scholar]
- 7.Maciel J, Chaves L, Souza B, Teixeira D, Freitas A, Feitosa J, et al. Structural characterization of cold extracted fraction of soluble sulfated polysaccharide from red seaweed Gracilaria birdiae. Carbohydrate Polymers. 2008;71(4):559–65. [Google Scholar]
- 8.Karnjana K, Soowannayan C, Wongprasert K. Ethanolic extract of red seaweed Gracilaria fisheri and furanone eradicate Vibrio harveyi and Vibrio parahaemolyticus biofilms and ameliorate the bacterial infection in shrimp. Fish Shellfish Immunol. 2019;88:91–101. [DOI] [PubMed] [Google Scholar]
- 9.Imjongjairak S, Ratanakhanokchai K, Laohakunjit N, Tachaapaikoon C, Pason P, Waeonukul R. Biochemical characteristics and antioxidant activity of crude and purified sulfated polysaccharides from Gracilaria fisheri. Biosci Biotechnol Biochem. 2016;80(3):524–32. [DOI] [PubMed] [Google Scholar]
- 10.Wongprasert K, Rudtanatip T, Praiboon J. Immunostimulatory activity of sulfated galactans isolated from the red seaweed Gracilaria fisheri and development of resistance against white spot syndrome virus (WSSV) in shrimp. Fish Shellfish Immunol. 2014;36(1):52–60. [DOI] [PubMed] [Google Scholar]
- 11.Kim JK, Cho ML, Karnjanapratum S, Shin IS, You SG. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int J Biol Macromol. 2011;49(5):1051–8. [DOI] [PubMed] [Google Scholar]
- 12.Vishchuk OS, Ermakova SP, Zvyagintseva TN. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: isolation, structural characteristics, and antitumor activity. Carbohydr Res. 2011;346(17):2769–76. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa JDS, Sabry DA, Silva CHF, Gomes DL, Santana-Filho AP, Sassaki GL, et al. Immunostimulatory Effect of Sulfated Galactans from the Green Seaweed Caulerpa cupressoides var. flabellata. Mar Drugs. 2020;18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Gomez F, Korbee N, Casas-Arrojo V, Abdala-Diaz RT, Figueroa FL. UV Photoprotection, Cytotoxicity and Immunology Capacity of Red Algae Extracts. Molecules. 2019;24(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicinskas E, Kalitnik AA, Karetin YA, Mohan Ram MSG, Achary A, Kravchenko AO. Immunomodulating Properties of Carrageenan from Tichocarpus crinitus. Inflammation. 2020;43(4):1387–96. [DOI] [PubMed] [Google Scholar]
- 16.Fu L, Qian Y, Wang C, Xie M, Huang J, Wang Y. Two polysaccharides from Porphyra modulate immune homeostasis by NF-kappaB-dependent immunocyte differentiation. Food Funct. 2019;10(4):2083–93. [DOI] [PubMed] [Google Scholar]
- 17.Song JH, Kang HB, Park SH, Jeong JH, Park J, You Y, et al. Extracts of Porphyra tenera (Nori Seaweed) Activate the Immune Response in Mouse RAW264.7 Macrophages via NF-kappaB Signaling. J Med Food. 2017;20(12):1152–9. [DOI] [PubMed] [Google Scholar]
- 18.Yin M, Zhang Y, Li H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front Immunol. 2019;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight SC, Stagg AJ. Antigen-presenting cell types. Curr Opin Immunol. 1993;5(3):374–82. [DOI] [PubMed] [Google Scholar]
- 21.DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry. 1956;28(3):350–6. [Google Scholar]
- 22.Castells CB, Arias VC, Castells RC. Precolumn derivatization of reducing carbohydrates with 4-(3-Methyl-5-oxo-2-pyrazolin-1-yl) benzoic acid. Study of reaction, high-performance liquid chromatographic separation and quantitative performance of method. Chromatographia. 2002;56(3–4):153–60. [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75. [PubMed] [Google Scholar]
- 24.Dodgson KS, Price RG. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem J. 1962;84:106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manosroi A, Saraphanchotiwitthaya A, Manosroi J. Immunomodulatory activities of fractions from hot aqueous extract of wood from Clausena excavata. Fitoterapia. 2004;75(3–4):302–8. [DOI] [PubMed] [Google Scholar]
- 26.Rochas C, Lahaye M, Yaphe W. Sulfate Content of Carrageenan and Agar Determined by Infrared Spectroscopy. Botanica Marina. 1986;29(4):335–40. [Google Scholar]
- 27.Gómez-Ordóñez E, Rupérez P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocolloids. 2011;25(6):1514–20. [Google Scholar]
- 28.Xu RB, Yang X, Wang J, Zhao HT, Lu WH, Cui J, et al. Chemical composition and antioxidant activities of three polysaccharide fractions from pine cones. Int J Mol Sci. 2012;13(11):14262–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17(1):9–17. [DOI] [PubMed] [Google Scholar]
- 30.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Son CG, Shin JW, Cho JH, Cho CK, Yun CH, Chung W, et al. Macrophage activation and nitric oxide production by water soluble components of Hericium erinaceum. Int Immunopharmacol. 2006;6(8):1363–9. [DOI] [PubMed] [Google Scholar]
- 32.Kim YO, Park HW, Kim JH, Lee JY, Moon SH, Shin CS. Anti-cancer effect and structural characterization of endo-polysaccharide from cultivated mycelia of Inonotus obliquus. Life Sci. 2006;79(1):72–80. [DOI] [PubMed] [Google Scholar]
- 33.Yu Q, Nie SP, Li WJ, Zheng WY, Yin PF, Gong DM, et al. Macrophage immunomodulatory activity of a purified polysaccharide isolated from Ganoderma atrum. Phytother Res. 2013;27(2):186–91. [DOI] [PubMed] [Google Scholar]
- 34.Goethe R, Phi-van L. Posttranscriptional lipopolysaccharide regulation of the lysozyme gene at processing of the primary transcript in myelomonocytic HD11 cells. Journal of Immunology. 1998;160(10):4970–8. [PubMed] [Google Scholar]
- 35.Rietschel ET, Brade H. Bacterial endotoxins. Sci Am. 1992;267(2):54–61. [DOI] [PubMed] [Google Scholar]
- 36.Markart P, Faust N, Graf T, Na CL, Weaver TE, Akinbi HT. Comparison of the microbicidal and muramidase activities of mouse lysozyme M and P. Biochem J. 2004;380(Pt 2):385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116(6):1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, Wolf AJ, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472(7344):471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43(5):645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–37, 37a–37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wingren AG, Parra E, Varga M, Kalland T, Sjogren HO, Hedlund G, et al. T cell activation pathways: B7, LFA-3, and ICAM-1 shape unique T cell profiles. Crit Rev Immunol. 1995;15(3–4):235–53. [DOI] [PubMed] [Google Scholar]
- 42.Ye G, Barrera C, Fan X, Gourley WK, Crowe SE, Ernst PB, et al. Expression of B7-1 and B7-2 costimulatory molecules by human gastric epithelial cells: potential role in CD4+ T cell activation during Helicobacter pylori infection. J Clin Invest. 1997;99(7):1628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180(2):631–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lins KO, Bezerra DP, Alves AP, Alencar NM, Lima MW, Torres VM, et al. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J Appl Toxicol. 2009;29(1):20–6. [DOI] [PubMed] [Google Scholar]
- 45.Marinho-Soriano E, Bourret E. Polysaccharides from the red seaweed Gracilaria dura (Gracilariales, Rhodophyta). Bioresour Technol. 2005;96(3):379–82. [DOI] [PubMed] [Google Scholar]
- 46.Melo M. Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydrate Polymers. 2002;49(4):491–8. [Google Scholar]
- 47.Mazumder S, Ghosal PK, Pujol CA, Carlucci MJ, Damonte EB, Ray B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int J Biol Macromol. 2002;31(1–3):87–95. [DOI] [PubMed] [Google Scholar]
- 48.Duarte ME, Cardoso MA, Noseda MD, Cerezo AS. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr Res. 2001;333(4):281–93. [DOI] [PubMed] [Google Scholar]
- 49.Jiao G, Yu G, Zhang J, Ewart HS. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9(2):196–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernando IPS, Sanjeewa KKA, Samarakoon KW, Lee WW, Kim H-S, Kim E-A, et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae. 2017;32(1):75–86. [Google Scholar]
- 51.Pereira L, Amado AM, Critchley AT, van de Velde F, Ribeiro-Claro PJA. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocolloids. 2009;23(7):1903–9. [Google Scholar]
- 52.Barros FC, da Silva DC, Sombra VG, Maciel JS, Feitosa JP, Freitas AL, et al. Structural characterization of polysaccharide obtained from red seaweed Gracilaria caudata (J Agardh). Carbohydr Polym. 2013;92(1):598–603. [DOI] [PubMed] [Google Scholar]
- 53.Lahaye M, Rochas C, Yaphe W. A New Procedure for Determining the Heterogeneity of Agar Polymers in the Cell-Walls of Gracilaria Spp (Gracilariaceae, Rhodophyta). Can J Bot. 1986;64(3):579–85. [Google Scholar]
- 54.Falshawa RHF R, Pickeringb TD and Stevensona DE. Agars from Three Fijian Gracilaria Species. Botanica Marina. 1999;42:51–9. [Google Scholar]
- 55.di Luzio NR. Lysozyme activity: an index of macrophage functional status. Front Biol. 1979;48:447–62. [PubMed] [Google Scholar]
- 56.Cohn ZA, Wiener E. The Particulate Hydrolases of Macrophages. Ii. Biochemical and Morphological Response to Particle Ingestion. J Exp Med. 1963;118:1009–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goethe R, Loc PV. The far upstream chicken lysozyme enhancer at −6.1 kilobase, by interacting with NF-M, mediates lipopolysaccharide-induced expression of the chicken lysozyme gene in chicken myelomonocytic cells. J Biol Chem. 1994;269(49):31302–9. [PubMed] [Google Scholar]
- 58.Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol. 2002;14(1):136–45. [DOI] [PubMed] [Google Scholar]
- 59.Usov AI, Yarotsky SV, Shashkov AS. 13C-nmr spectroscopy of red algal galactans. Biopolymers. 1980;19(5):977–90. [Google Scholar]
- 60.Craigie JS, Wen ZC, editors. Effects of temperature and tissue age on gel strength and composition of agar from Gracilaria tikvahiae (Rhodophyceae) 1984. [Google Scholar]
- 61.Marinho-Soriano E Agar polysaccharides from Gracilaria species (Rhodophyta, Gracilariaceae). J Biotechnol. 2001;89(1):81–4. [DOI] [PubMed] [Google Scholar]
- 62.Murano E, Toffanin R, Zanetti F, Knutsen S, Paoletti S, Rizzo R. Chemical and macromolecular characterisation of agar polymers from Gracilaria dura (C. Agardh) J. Agardh (Gracilariaceae, Rhodophyta). Carbohydrate Polymers 1992;18:171–8. [Google Scholar]
- 63.Lee JB, Ohta Y, Hayashi K, Hayashi T. Immunostimulating effects of a sulfated galactan from Codium fragile. Carbohydr Res. 2010;345(10):1452–4. [DOI] [PubMed] [Google Scholar]
- 64.Cui Y, Liu X, Li S, Hao L, Du J, Gao D, et al. Extraction, characterization and biological activity of sulfated polysaccharides from seaweed Dictyopteris divaricata. Int J Biol Macromol. 2018;117:256–63. [DOI] [PubMed] [Google Scholar]
- 65.Liu QM, Xu SS, Li L, Pan TM, Shi CL, Liu H, et al. In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis. Carbohydr Polym. 2017;165:189–96. [DOI] [PubMed] [Google Scholar]
- 66.Cao RA, Lee Y, You S. Water soluble sulfated-fucans with immune-enhancing properties from Ecklonia cava. Int J Biol Macromol. 2014;67:303–11. [DOI] [PubMed] [Google Scholar]
- 67.Li J, Liu Z, Jiang S, Cortesini R, Lederman S, Suciu-Foca N. T suppressor lymphocytes inhibit NF-kappa B-mediated transcription of CD86 gene in APC. J Immunol. 1999;163(12):6386–92. [PubMed] [Google Scholar]
- 68.Meena DK, Das P, Kumar S, Mandal SC, Prusty AK, Singh SK, et al. Beta-glucan: an ideal immunostimulant in aquaculture (a review). Fish Physiol Biochem. 2013;39(3):431–57. [DOI] [PubMed] [Google Scholar]
- 69.Willment JA, Lin HH, Reid DM, Taylor PR, Williams DL, Wong SY, et al. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol. 2003;171(9):4569–73. [DOI] [PubMed] [Google Scholar]
- 70.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413(6851):36–7. [DOI] [PubMed] [Google Scholar]
- 71.Palma AS, Feizi T, Zhang Y, Stoll MS, Lawson AM, Diaz-Rodriguez E, et al. Ligands for the beta-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J Biol Chem. 2006;281(9):5771–9. [DOI] [PubMed] [Google Scholar]
- 72.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, et al. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196(3):407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takano T, Motozono C, Imai T, Sonoda KH, Nakanishi Y, Yamasaki S. Dectin-1 intracellular domain determines species-specific ligand spectrum by modulating receptor sensitivity. J Biol Chem. 2017;292(41):16933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fukahori S, Yano H, Akiba J, Ogasawara S, Momosaki S, Sanada S, et al. Fucoidan, a major component of brown seaweed, prohibits the growth of human cancer cell lines in vitro. Mol Med Rep. 2008;1(4):537–42. [PubMed] [Google Scholar]
- 75.Namvar F, Mohamed S, Fard SG, Behravan J, Mustapha NM, Alitheen NBM, et al. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chemistry. 2012;130(2):376–82. [Google Scholar]
- 76.Funahashi H, Imai T, Mase T, Sekiya M, Yokoi K, Hayashi H, et al. Seaweed prevents breast cancer? Jpn J Cancer Res. 2001;92(5):483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamasaki-Miyamoto Y, Yamasaki M, Tachibana H, Yamada K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J Agric Food Chem. 2009;57(18):8677–82. [DOI] [PubMed] [Google Scholar]
- 78.Ikeguchi M, Yamamoto M, Arai Y, Maeta Y, Ashida K, Katano K, et al. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol Lett. 2011;2(2):319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


