Abstract
Purpose
To establish, validate, and clinically evaluate a nomogram for predicting the risk of sarcopenia in patients with peripheral arterial disease (PAD) based on clinical and lower extremity computed tomography angiography (LE-CTA) imaging characteristics.
Methods
Clinical data and CTA imaging features from 281 PAD patients treated between January 1, 2019, and May 1, 2023, at two hospitals were retrospectively analyzed using binary logistic regression to identify the independent risk factors for sarcopenia. These identified risk factors were used to develop a predictive nomogram. The nomogram's effectiveness was assessed through various metrics, including the receiver operating characteristic (ROC) curve, area under the curve (AUC), concordance index (C-index), Hosmer-Lemeshow (HL) test, and calibration curve. Its clinical utility was demonstrated using decision curve analysis (DCA).
Results
Several key independent risk factors for sarcopenia in PAD patients were identified, namely age, body mass index (BMI), history of coronary heart disease (CHD), and white blood cell (WBC) count, as well as the severity of luminal stenosis (P < 0.05). The discriminative ability of the nomogram was supported by the C-index and an AUC of 0.810 (95% confidence interval: 0.757–0.862). A robust concordance between predicted and observed outcomes was reflected by the calibration curve. The HL test further affirmed the model's calibration with a P-value of 0.40. The DCA curve validated the nomogram's favorable clinical utility. Lastly, the model underwent internal validation.
Conclusions
A simple nomogram based on five independent factors, namely age, BMI, history of CHD, WBC count, and the severity of luminal stenosis, was developed to assist clinicians in estimating sarcopenia risk among PAD patients. This tool boasts impressive predictive capabilities and broad utility, significantly aiding clinicians in identifying high-risk individuals and enhancing the prognosis of PAD patients.
Keywords: Peripheral artery disease, Sarcopenia, Computed tomography angiography, Risk factors, Nomogram
1. Introduction
Peripheral arterial disease (PAD) is a form of arterial atherosclerosis that primarily affects large arteries, excluding coronary arteries. The most common area of occurrence is the lower limbs [1]. PAD affects approximately 230 million people worldwide and is linked to poor prognosis and elevated disability and mortality rates [2,3]. PAD commonly coexists with other chronic and cardiovascular diseases, such as central vascular and coronary artery diseases, diabetes, chronic kidney disease, and sarcopenia. These comorbidities increase negative prognoses and mortality risks in patients [4,5]. Sarcopenia, a disease affecting skeletal muscles, manifests as a gradual reduction in muscle mass and associated functional capacity. It is correlated with severe consequences, including falls, functional deterioration, muscular weakness, and increased mortality rates [5,6]. The overall prevalence of sarcopenia in the general elderly population is approximately 10%. However, the risk of sarcopenia is significantly higher among individuals with PAD [7]. A systematic review of 17 studies, which included 2362 PAD patients averaging 72.42 years old, revealed that 34.63% had sarcopenia— a rate much higher than in the general elderly demographic [5]. Sarcopenia is not only prevalent in PAD patients but also serves as a critical risk factor for worse outcomes. PAD patients with sarcopenia are more likely to have increased mortality, cardiovascular events, and amputation risks compared to those without [[8], [9], [10]]. Thus, emphasis on the early detection of PAD patients with heightened sarcopenia risk becomes paramount for implementing timely and effective interventions.
The European Working Group on Sarcopenia in Older Persons (EWGSOP2) delineated three primary diagnostic criteria for sarcopenia: muscle weakness, low muscle mass, and poor physical performance [11]. A variety of assessment methods have been developed to evaluate skeletal muscle quality, including computed tomography (CT), magnetic resonance imaging, dual-energy X-ray absorptiometry, and bioelectrical impedance analysis (BIA). However, each of these technologies has its own set of limitations [12,13]. Notably, the SARC-F questionnaire recommended by the EWGSOP2 for sarcopenia screening often misses diagnoses due to its limited sensitivity [14]. Hence, the urgency for research into better screening and early diagnosis methods for sarcopenia in PAD patients is evident.
Lower extremity computed tomography angiography (LE-CTA) has become a key non-invasive diagnostic tool for PAD, providing high-quality imaging of lower extremity arteries. This technique employs advanced vascular analysis software to accurately detail arterial lesions, including the conditions of the lumen and vessel wall and allows for the precise grading of arterial stenosis [15]. These details are crucial for the formulation of therapeutic plans, enhancing the technique's clinical application. This study formulated a nomogram that combines CTA features with clinical data to address the challenge of predicting sarcopenia in patients with PAD. This holistic approach is anticipated to surpass the accuracy and efficacy of traditional methods, which primarily depend on singular clinical indicators, in forecasting sarcopenia risk among PAD patients. This innovative tool delineates a precise numerical correlation between risk factors and sarcopenia in the context of PAD [16]. It equips physicians with the capability to perform tailored risk assessments and initiate timely interventions, potentially enhancing patient outcomes and prognoses.
2. Materials and methods
2.1. Patient selection
Our study retrospectively analyzed the clinical data and CTA imaging features of all patients admitted to two hospitals between January 1, 2019, and May 1, 2023, who were diagnosed with PAD based on LE-CTA.
In our research, the diagnostic criteria for PAD were based on the American Heart Association guidelines of 2016 [17] which require patients to exhibit the corresponding clinical symptoms and CTA imaging features of the lower extremities. The inclusion criteria for our study were patients confirmed with PAD and those aged >40 years. The exclusion criteria were patients (1) with severe consciousness impairment or poor general condition; (2) a malignant tumor or heart, liver, or kidney failure; (3) in the active period of Takayasu's arteritis or having other infectious diseases; (4) with autoimmune diseases such as inflammatory bowel disease; (5) accompanying hereditary myopathy or other neuromuscular diseases; (6) on long-term bed rest or with muscle atrophy caused by other diseases; and (7) patients with incomplete clinical follow-up information. Accordingly, the study included 281 patients (201 males and 80 females). This retrospective study received authorization from the ethical committees of the aforementioned two hospitals. The research strictly adhered to the ethical guidelines outlined by the Declaration of Helsinki.
2.2. Definition of sarcopenia
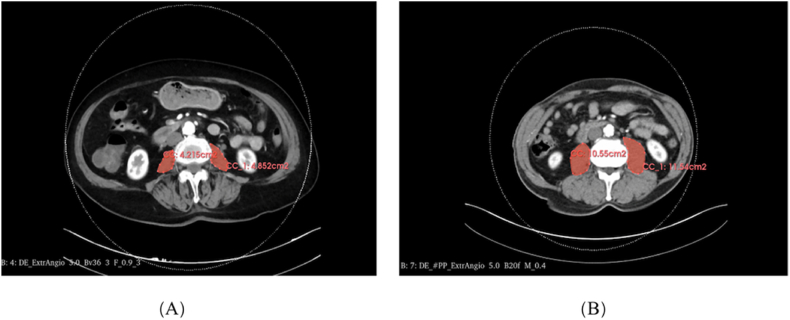
The correlation between CT images of the third lumbar vertebra (L3) and overall muscle mass has been well-established and extensively documented [11]. In this study, the CT scans of PAD patients were delineated using 3D Slicer software (version 5.1.0, American). The methodology enabled the semi-quantitative and semi-automatic assessment of the bilateral psoas muscle's cross-sectional area at the lower margin of the L3 vertebra (Fig. 1 A, B). The psoas muscle index (PMI, cm2/m2) was then determined using the formula:
Fig. 1.
Measurement of psoas muscle areas from computed tomography slices at the level of the third lumbar vertebra. The lumbar-muscle areas of peripheral arterial disease (PAD) patients with sarcopenia (A) and without sarcopenia (B).
PMI = (area of the left psoas muscle + area of the right psoas muscle)/height2.
Gender-specific PMI thresholds, as prescribed in established guidelines, were used to diagnose sarcopenia. Specifically, men with a PMI <5.5 cm2/m2 and women with a PMI <4.0 cm2/m2 were identified as having sarcopenia [8,18,19])
2.3. CTA assessment and clinical variables
LE-CTA was conducted using Siemens' third-generation dual-source CT system (SOMATOM Definition Force, Siemens AG, Munich, Germany). The scan spanned from the abdominal aorta's end to the dorsalis pedis artery. The scan settings included a layer thickness of 0.5 mm and an interlayer spacing of 0.6 mm. We used a trigger threshold of 150 Hounsfield units. The delays for the arterial and venous phase scans were 8 s and 25 s, respectively. The scan length varied based on each patient's specific conditions, averaging around 30 s. An iodixanol injection (100 mL: 32 g; Qing Liming, NJCTTQ, China) was delivered via the antecubital vein, with volumes ranging from 60 to 80 mL and an injection speed of 5–6 mL/s. Following this, we collected and uploaded data and images for volume reformation processing at a dedicated workstation. Curved planar reformation and maximum intensity projection were applied when necessary. Two independent radiologists analyzed the CTA images according to the Consensus Definitions from the Peripheral Academic Research Consortium (PARC) and the 2015 Trans-Atlantic Inter-Society Consensus (TASC) II revision classification standard [20,21]. They focused on various aspects: (1) lesion location, (2) lesion length, (3) severity of luminal stenosis, and (4) the TASC II classification. Additionally, they performed calcification grading, which included (5) the Peripheral Arterial Calcium Scoring System (PACSS) classification and (6) the PARC classification, (7) and evaluated the formation of collateral circulation (See Fig. 2 A-F and Table 1 for details).
Fig. 2.
Computed tomography angiography (CTA) imaging manifestations in peripheral arterial disease (PAD) patients.(A) The consensus definitions from the Peripheral Academic Research Consortium (PARC) were referenced to quantify vessel stenosis. (B) Vessel selection and measurement were conducted using CTA with axial and maximum intensity projection images. The severity of lumen stenosis was defined by the following rating system: mild stenosis was less than 50% (C); moderate stenosis was 50%–69% (D); severe stenosis was 70%–99% (E); and occluded vessels was 100% (F).
Table 1.
Lesion and vessel characteristics and definitions.
| Lesion or vessel | Term | Definition |
|---|---|---|
| Severity of luminal stenosis | Mild | <50% |
| Moderate | 50%–69% | |
| Severe | 70%–99% | |
| Occluded | 100% | |
| Lesion length | Focal | ≤1 cm |
| Short | >1 and < 5 cm | |
| Intermediate | ≥5 and < 15 cm | |
| Long | ≥15 cm | |
| PACSS classification | Grade 0 | The target vessel is not calcified |
| Grade 1 | Unilateral calcification of target vessel <5 cm | |
| Grade 2 | Unilateral calcification of target vessel ≥5 cm | |
| Grade 3 | Bilateral calcification of target vessel <5 cm | |
| Grade 4 | Bilateral calcification of target vessel ≥5 cm | |
| PARC classification | No calcification | No calcification |
| Focal calcification | <180° (1 side of the vessel) and less than one-half of the total lesion length | |
| Mild calcification | <180° and greater than one-half of the total lesion length | |
| Moderate calcification | ≥180° (both sides of the vessel at the same location) and less than one-half of the total lesion length | |
| Severe calcification | >180° (both sides of the vessel at the same location) and greater than one-half of the total lesion length |
PACSS, Peripheral Arterial Calcium Scoring System; PARC, Peripheral Academic Research Consortium.
The study undertook a comprehensive examination of various clinical parameters, including age, sex, and body mass index (BMI). We also evaluated the patients' medical histories, focusing on conditions such as diabetes, high blood pressure, coronary heart disease (CHD), stroke, smoking habits, and alcohol consumption. A meticulous assessment of biological markers was also conducted. This included the white blood cell count (WBC), platelet-to-lymphocyte ratio (PLR), glycated hemoglobin, fasting blood glucose, and albumin (ALB) levels. Liver enzymes, such as aspartate aminotransferase and alanine transaminase, were evaluated, as were renal function indicators like blood urea nitrogen and serum creatinine. The lipid profile assessed total cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels. Blood uric acid levels were also measured. Blood samples were collected after an 8-h fast following the admission of the patient to the hospital. Each variable was meticulously inspected by the research team to ensure data integrity and completeness.
2.4. Development and validation of the nomogram
A single-factor logistic regression analysis of the data obtained from PAD patients was performed. Any factors with a P-value <0.05 in this univariate analysis were subsequently included in a multivariate logistic regression analysis. Significant independent risk factors were identified by stepwise backward regression. We leveraged these factors to construct a nomogram to determine the risk of sarcopenia in PAD patients. The evaluation of this nomogram encompassed discrimination, calibration, and clinical utility. The area under the curve (AUC) and the C-index values served as indicators of its predictive value. Calibration curves complemented by the Hosmer-Lemeshow (HL) test were used to evaluate the calibration's accuracy. Decision curve analysis (DCA) was used to measure the nomogram's net clinical benefit. Internal validation was executed using a bootstrap technique with 1000 iterations to ascertain the nomogram's dependability. Throughout the study, P-values of <0.05 were considered to indicate statistical significance.
2.5. Statistical analysis
Data analysis was conducted using SPSS version 27.0 and R version 4.3.0. Continuous variables are presented as either the mean ± SD or median (interquartile range), whereas categorical variables are represented by counts (n) and percentages (%). Continuous variables were analyzed by the Student's t-test or the Mann-Whitney U test, depending on their distribution. For categorical data, comparisons were made using the chi-squared test. A P-value of less than 0.05 was deemed statistically significant.
3. Results
3.1. Basic characteristics and univariate analysis of the study population
This study finally included 281 PAD patients (201 males and 80 females; mean age, 73.0 years). Of them, 116 (41.3%) were diagnosed with sarcopenia, and 165 had no sarcopenia. The findings of the univariate logistic regression analysis indicated that age, BMI, ALB level, CHD, WBC, PLR, lesion length, the severity of luminal stenosis, TASC II, PACSS, and PARC classification were statistically significant risk factors (P < 0.05). Detailed patient characteristics are shown in Table 2.
Table 2.
Baseline characteristics and univariate analysis of patients with PAD.
| Variables | Total (n = 281) | Sarcopenia (n = 116) | No sarcopenia (n = 165) | P value |
|---|---|---|---|---|
| Age (years) | 73.0 ± 9.26 | 76.5 ± 8.67 | 70.6 ± 8.89 | <0.001 * |
| Sex, n (%) | ||||
| Male | 201 (71.5) | 80 (69.0) | 121 (73.3) | |
| Female | 80 (28.5) | 36 (31.0) | 44 (26.7) | 0.425 |
| BMI (kg/m2) | 22.5 (14.7, 46.3) | 20.8 (14.7, 46.3) | 23.4 (15.2, 32.5) | <0.001 * |
| Diabetes, n (%) | ||||
| No | 138 (49.1) | 62 (53.4) | 76 (46.1) | |
| Yes | 143 (50.9) | 54 (46.6) | 89 (53.9) | 0.223 |
| HBP, n (%) | ||||
| No | 69 (24.6) | 25 (21.6) | 44 (26.7) | |
| Yes | 212 (75.4) | 91 (78.4) | 121 (73.3) | 0.327 |
| CHD, n (%) | ||||
| No | 233 (82.9) | 90 (77.6) | 143 (86.7) | |
| Yes | 48 (17.1) | 26 (22.4) | 22 (13.3) | 0.049 * |
| Stroke, n (%) | ||||
| No | 217 (77.2) | 89 (76.7) | 128 (77.6) | |
| Yes | 64 (22.8) | 27 (23.3) | 37 (22.4) | 0.867 |
| Smoking, n (%) | ||||
| No | 233 (82.9) | 97 (83.6) | 136 (82.4) | |
| Yes | 48 (17.1) | 19 (16.4) | 29 (17.6) | 0.793 |
| Drinking, n (%) | ||||
| No | 261 (92.9) | 109 (94.0) | 152 (92.1) | |
| Yes | 20 (7.1) | 7 (6.0) | 13 (7.9) | 0.555 |
| HbA1c (%) | 6.80 (4.50, 13.7) | 6.75 (4.50, 12.8) | 6.80 (4.70, 13.7) | 0.502 |
| ALB (g/L) | 38.2 ± 5.45 | 36.3 ± 5.92 | 39.5 ± 4.65 | <0.001 * |
| ALT (U/L) | 15.0 (2.00, 80.0) | 14.0 (3.00, 80.0) | 16.0 (2.00, 74.0) | 0.949 |
| AST (U/L) | 19.0 (8.00, 90) | 19.0 (9.00, 90.0) | 18.0 (8.00, 88.0) | 0.137 |
| FBG (mmol/L) | 5.82 (2.28, 19.4) | 5.35 (2.28, 19.4) | 5.93 (2.86, 15.8) | 0.754 |
| BUN (mmol/L) | 6.30 (2.00, 31.5) | 6.65 (2.00, 29.5) | 6.20 (2.70, 31.5) | 0.535 |
| Scr (μmol/L) | 76.2 (36.7, 226) | 76.5 (36.7, 226) | 76.2 (39.7, 224) | 0.542 |
| BUA (μmol/L) | 337 ± 109 | 338 ± 122 | 337 ± 99.3 | 0.897 |
| TC (mmol/L) | 4.00 (1.99, 10.5) | 3.85 (2.14, 10.5) | 4.14 (1.99, 8.90) | 0.380 |
| TG (mmol/L) | 1.28 (0.39, 8.79) | 1.20 (0.50, 8.79) | 1.34 (0.39, 7.30) | 0.242 |
| HDL-C (mmol/L) | 1.00 (0.45, 2.21) | 1.00 (0.51, 2.07) | 1.00 (0.45, 2.21) | 0.904 |
| LDL-C (mmol/L) | 2.45 (0.75, 7.00) | 2.32 (0.75, 5.66) | 2.47 (0.98, 7.00) | 0.373 |
| WBC ( × 10^9/L) | 7.15 (2.23, 24.2) | 7.79 (2.67, 24.2) | 7.01 (2.23, 16.6) | 0.001 * |
| PLR | 146 (27.1, 685) | 155 (49.1, 685) | 144 (27.1, 604) | 0.002 * |
| Lesion involved site, n (%) | ||||
| Lliac artery | 277 (98.6%) | 115 (99.1%) | 162 (98.2%) | 0.515 |
| Femoropopliteal artery | 271 (96.4%) | 116 (100%) | 155 (93.9%) | 0.999 |
| Below knee triple arteries | 261 (92.9%) | 112 (96.6%) | 149 (90.3%) | 0.055 |
| Lesion length, n (%) | ||||
| Focal | 14 (5.0) | 1 (0.9) | 13 (7.9) | Ref. |
| Short | 57 (20.3) | 17 (14.7) | 40 (24.2) | 0.011 * |
| Intermediate | 81 (28.8) | 30 (25.9) | 51 (30.9) | 0.004 * |
| Long | 129 (45.9) | 68 (58.6) | 61 (37.0) | 0.027 * |
| Severity of luminal stenosis, n (%) | ||||
| Mild | 24 (8.5) | 1 (0.9) | 23 (13.9) | Ref. |
| Moderate | 37 (13.2) | 8 (6.9) | 29 (17.6) | 0.001 * |
| Severe | 96 (34.2) | 34 (29.3) | 62 (37.6) | <0.001 * |
| Occluded | 124 (44.1) | 73 (62.9) | 51 (30.9) | 0.001 * |
| TASC II classification, n (%) | ||||
| A | 18 (6.4) | 1 (0.9) | 17 (10.3) | Ref. |
| B | 81 (28.8) | 29 (25.0) | 52 (31.5) | 0.004 * |
| C | 133 (47.3) | 59 (50.9) | 74 (44.8) | 0.033 * |
| D | 49 (17.4) | 27 (23.3) | 22 (13.3) | 0.199 |
| PACSS classification, n (%) | ||||
| Grade 0 | 2 (0.7) | 1 (0.9) | 1 (0.6) | Ref. |
| Grade 1 | 9 (3.2) | 0 (0) | 9 (5.5) | 0.850 |
| Grade 2 | 7 (2.5) | 2 (1.7) | 5 (3.0) | 0.167 |
| Grade 3 | 159 (56.6) | 54 (46.6) | 105 (63.6) | 0.999 |
| Grade 4 | 104 (37.0) | 59 (50.9) | 45 (27.3) | <0.001 * |
| PARC classification, n (%) | ||||
| No calcification | 2 (0.7) | 1 (0.9) | 1 (0.6) | Ref. |
| Focal calcification | 17 (6.0) | 3 (2.6) | 14 (8.5) | 0.982 |
| Mild calcification | 47 (16.7) | 15 (12.9) | 32 (19.4) | 0.017 * |
| Moderate calcification | 93 (33.1) | 35 (30.2) | 58 (35.2) | 0.029 * |
| Severe calcification | 122 (43.4) | 62 (53.4) | 60 (36.4) | 0.055 |
| Collateral circulation formation, n (%) | ||||
| No | 275 (97.9) | 112 (96.6) | 163 (98.8) | |
| Yes | 6 (2.1) | 4 (3.4) | 2 (1.2) | 0.222 |
Data are the number of patients and percentage if not specified.
*P < 0.05.
BMI, body mass index; HBP, high blood pressure; CHD, coronary heart disease; WBC, white blood cell count; PLR, platelet-to-lymphocyte ratio; HbA1c, glycated hemoglobin; FBG, fasting blood glucose; ALB, albumin; AST, aspartate aminotransferase; ALT, alanine transaminase; BUN, blood urea nitrogen; Scr, serum creatinine; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BUA, blood uric acid; PACSS, Peripheral Arterial Calcium Scoring System; PARC, Peripheral Academic Research Consortium.
3.2. Screening for risk factors
The above risk factors were included in the multivariate analysis. Multivariate logistic regression analysis revealed that age, BMI, CHD, WBC, and the severity of luminal stenosis were independent risk factors for sarcopenia among PAD patients (P < 0.05, Table 3).
Table 3.
Multivariate logistic regression analyses for patients with PAD.
| Variables | OR | 95%CI | P value |
|---|---|---|---|
| Age (years) | 1.053 | 1.019–1.088 | 0.002 * |
| BMI (kg/m2) | 0.837 | 0.765–0.917 | <0.001 * |
| CHD, n (%) | 0.401 | 0.184–0.871 | 0.021 * |
| WBC ( × 10^9/L) | 1.172 | 1.061–1.295 | 0.002 * |
| Severity of luminal stenosis, n (%) | |||
| Mild | Ref. | ||
| Moderate | 0.063 | 0.008~0.506 | 0.009 * |
| Severe | 0.246 | 0.097~0.628 | 0.003 * |
| Occluded | 0.498 | 0.270~0.920 | 0.026 * |
*P < 0.05.
BMI, body mass index; HBP, high blood pressure; CHD, coronary heart disease; WBC, white blood cell count; OR, odds ratio; CI, confidence interval.
3.3. Development and validation of the nomogram
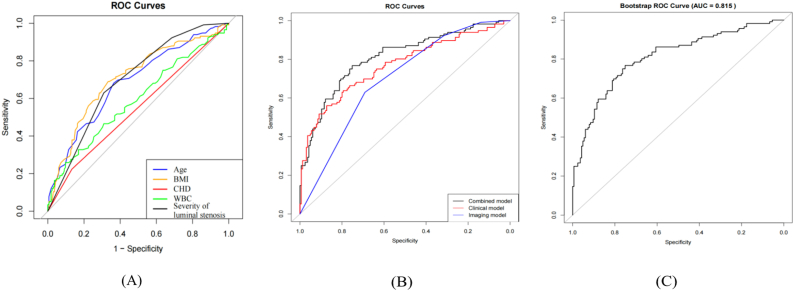
A predictive nomogram was formulated to estimate sarcopenia risk in individuals with PAD based on the identified independent risk variables (Fig. 3). The receiver operating characteristics (ROC) curves of individual predictors were compared to determine their predictive accuracy for sarcopenia in PAD patients (Fig. 4A). Specifically, the AUC values for age, BMI, CHD, WBC, and the severity of luminal stenosis were 0.685, 0.707, 0.545, 0.590, and 0.696, respectively. A comparison of the ROC curves of clinical models, imaging models, and their combination (Fig. 4B) showed that the AUC was higher for the model combining clinical and CTA imaging features, reaching 0.810 (95% confidence interval (CI): 0.757–0.862). In contrast, the AUCs for the clinical model alone and the imaging model alone were 0.768 and 0.696 (95% CI: 0.709–0.826 and 0.635–0.757), respectively. Thus, the nomogram that integrated clinical and CTA features demonstrated superior predictive performance. Bootstrap resampling (1,000 iterations) was employed for internal validation, resulting in an AUC of 0.815 (95% CI: 0.757–0.862) for the nomogram model (Fig. 4C). The model's C-index exceeded 0.80, denoting a robust predictive capability.
Fig. 3.
Nomogram model predicting sarcopenia in peripheral arterial disease (PAD) patients with scores. Scores ranging from 0 to 100 were assigned for each variable of age, body mass index (BMI), coronary heart disease (CHD), white blood cell (WBC) count, and the severity of luminal stenosis. The sum of these scores provided the total points score, which subsequently predicted the likelihood of sarcopenia.
Fig. 4.
Receiver operating characteristics (ROC) curves for predicting sarcopenia in patients with peripheral arterial disease (PAD). (A) The ROC curves depict sensitivity versus specificity for individual sarcopenia risk factors: age, body mass index (BMI), coronary heart disease (CHD), white blood cell (WBC) count, and the severity of luminal stenosis. The light gray line signifies an area under the curve (AUC) value of 50%. (B) The ROC curves of clinical models, imaging models, and their combination. (C) The ROC curve represents the AUC obtained through bootstrap resampling (1,000 iterations) for internal validation of the nomogram model.
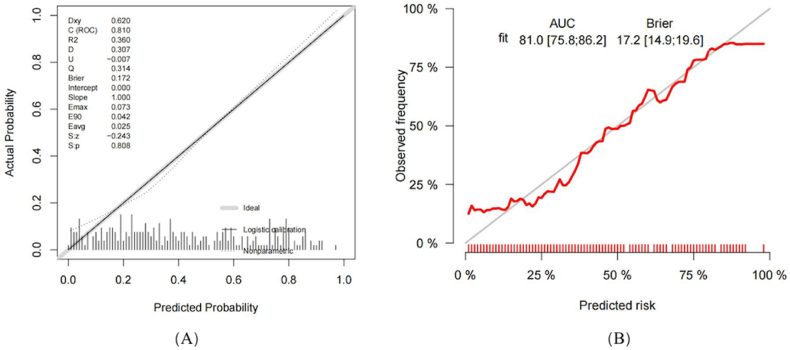
The calibration curve for the nomogram (Fig. 5A) demonstrated an alignment between the forecasted and observed probabilities. The model's Brier value stood at 0.172, and the Hosmer-Lemeshow (HL) test produced a non-significant χ2 value of 9.40 (P = 0.40), indicating a reliable fit. A subsequent calibration curve after internal validation (Fig. 5B) corroborated the primary calibration findings, suggesting consistent performance. The AUC and the HL test indicated that the model could aptly predict sarcopenia in PAD patients.
Fig. 5.
The calibration curve for predicting sarcopenia in patients with peripheral arterial disease (PAD). (A) The calibration curve from the nomogram model illustrates the predicted versus observed probability of sarcopenia. Optimal model performance is indicated by the proximity of the decision curve to the ideal (prominently hatched) line. The horizontal axis denotes the predictive probability of sarcopenia occurrence, while the vertical axis reflects the actual probability of sarcopenia. (B) The chart showcases a comparison between observed and predicted sarcopenia risks derived from 1,000 internal validations using the bootstrap resampling technique.
DCA revealed that the nomogram outperformed other models in terms of patient net benefit within a probability range of 1–99% (Fig. 6A). Thus, the nomogram offers significant clinical advantages within this bracket. Internal validation using bootstrap resampling confirmed the model's clinical utility (Fig. 6B). In conclusion, this prediction model stands out for its precise discrimination and wide-ranging relevance.
Fig. 6.
Decision curve analysis (DCA) for predicting sarcopenia in patients with peripheral arterial disease (PAD). (A) DCA for predicting sarcopenia based on the nomogram model (red line). The graph plots the standardized net benefit against the net cost benefit across varying probability thresholds. The light gray line (All) signifies patients with sarcopenia who have been treated, while the darker horizontal line (None) designates the absence of sarcopenia in the patients. A pronounced risk threshold was correlated with an elevated likelihood of a sarcopenia diagnosis. (B) This graph encapsulates the findings derived from 1,000 internal validations conducted using the bootstrap resampling technique. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Nomograms are valuable tools for predicting the likelihood of disease occurrence by analyzing and incorporating identified risk factors. They play a pivotal role in clinical decision-making, particularly in the fields of oncology and chronic diseases [22] These prediction models also offer clear quantitative indicators, enabling accurate therapeutic and prognostic evaluations [23]. Sun et al. [24] elucidated the prognostic ramifications of sarcopenia in patients with acute myeloid leukemia and introduced an innovative prognostic model. Similarly, Mo et al. [25] employed a nomogram to estimate sarcopenia risk in community-dwelling elderly individuals. However, despite the significance of nomograms, no studies have yet constructed a nomogram specifically for forecasting the risk of sarcopenia in PAD patients.
Our study devised and substantiated a straightforward nomogram for predicting the likelihood of sarcopenia in PAD patients to address this need. The model utilizes only five readily available independent risk factors for sarcopenia, namely age, BMI, history of CHD, WBC count, and the severity of luminal stenosis, to identify PAD patients with an elevated risk of sarcopenia (Table 4). Our preliminary results suggest that this nomogram has satisfactory accuracy and can predict sarcopenia in PAD patients. Evidence from internal samples underscored its robust stability, indicating its extensive applicability.
Table 4.
Overview of independent risk factors and mechanisms for sarcopenia in PAD patients.
| Independent Risk Factor | Impact | Mechanism |
|---|---|---|
| Age | Positively correlates with sarcopenia | Age-related factors including (1) decreased physical activity, (2) reduction in muscle fibers and hormonal levels, (3) elevated inflammatory markers, and (4) diminished mitochondrial function, synergistically contribute to sarcopenia. |
| BMI | Negatively correlates with sarcopenia | A low BMI indicates malnutrition, highlighting a risk for reduced muscle mass, which is a precursor to sarcopenia. |
| CHD | Positively correlates with sarcopenia | CHD affects sarcopenia through (1) reduced cardiac output leading to lower skeletal muscle blood supply, and (2) activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, disrupting protein metabolism and causing muscle atrophy. |
| WBC | Positively correlates with sarcopenia | Elevated WBC levels (1) indicate chronic inflammation, facilitating muscle protein catabolism and apoptosis, and (2) release of pro-inflammatory cytokines such as IL-6 and TNF-α, reducing muscle mass and function. |
| Severity of luminal stenosis | Positively correlates with sarcopenia | Narrowed peripheral arteries (1) reduce blood flow and muscle oxygen supply, impairing muscle function, and (2) induce symptoms like pain and fatigue, limiting physical activity and exacerbating sarcopenia risk. |
BMI, body mass index; CHD, coronary heart disease; WBC, white blood cell count.
This study confirmed an association between age and the prevalence of sarcopenia in PAD patients, aligning with the findings of earlier studies [26,27]. Research conducted by Selçuk et al. [18], encompassing 217 PAD patients with an average age of 61.5 years, showed a 37.8% incidence of sarcopenia among these patients. Similarly, Taniguchi et al. [8] found a 66.7% incidence among 75 PAD patients with a mean age of 72.9 years. These data imply a progressive increase in sarcopenia incidence with advancing age. Several factors contribute to this age-related rise in sarcopenia. One factor commonly associated with aging is the decline in physical activity [28]. Aging is also linked to a reduction in skeletal muscle fibers and hormone levels, such as insulin, estrogen, testosterone, and growth hormone, accompanied by the upregulation of inflammatory markers. These alterations could cause protein synthesis to decrease and muscle breakdown to increase. The contribution of other factors, including malnutrition, osteoporosis, neuromuscular functional deterioration, and potential genetic factors, cannot be overlooked [26]. Emerging studies also suggest age-induced mitochondrial reactive-oxygen-species clusters, mitochondrial DNA mutations, and mitochondrial dysfunction as probable causes of sarcopenia [29]. The compromised physical function and limited mobility of PAD patients, as well as the presence of underlying diseases and comorbidities, puts these patients at high risk for sarcopenia. Therefore, it is crucial to pay attention to the muscular condition of PAD patients, providing early preventive measures through nutritional education and guidance on physical activity, with the ultimate aim of mitigating sarcopenia incidence.
This study established a negative correlation between BMI and the incidence of sarcopenia in PAD patients. Previous studies have produced conflicting conclusions regarding the relationship between BMI and sarcopenia. Some studies identified low BMI as a risk factor for sarcopenia in PAD patients [30,31]. Matsubara et al. [32]documented an elevated occurrence of sarcopenia in PAD patients possessing a BMI <22.0 kg/m2. Conversely, other studies suggested a positive correlation between high BMI and increased sarcopenia incidence [33]. The root of this discrepancy likely lies in the divergent health issues linked to both low and high BMI values. Whereas a low BMI often signals malnutrition—a precursor to sarcopenia—a high BMI can lead to sarcopenic obesity. The dynamic between obesity and sarcopenia is reciprocal, contributing to a complex interplay of pathophysiological mechanisms, such as elevated levels of pro-inflammatory cytokines, oxidative stress, insulin resistance, hormonal changes, and diminished physical activity [34]. These findings highlight the need to emphasize muscle-status evaluation in the management of PAD patients with low BMI without overlooking sarcopenic obesity in this population. Therefore, personalized and targeted interventions should be implemented to increase the long-term prognosis of patients with PAD.
This study identified a positive correlation between the occurrence of CHD and sarcopenia in PAD patients, consistent with prior studies [35,36]. Although the precise mechanisms linking CHD and sarcopenia must be thoroughly examined, numerous intersecting pathways may account for this relationship. These include reduced daily physical activity, insulin resistance, oxidative stress, chronic inflammation, nutritional deficiencies, hormonal imbalances, and aging [37,38]. Notably, the decline in the cardiac output of CHD patients can lead to a decrease in peripheral skeletal muscle perfusion. Accompanied by diminished physical strength and exercise capacity, the physical activity levels of these patients may decline, accelerating sarcopenia onset and progression. Importantly, activation of the renin-angiotensin-aldosterone system and the sympathetic nervous system, which are linked to CHD, can disrupt protein metabolism, leading to reduced muscle mass and accelerated skeletal muscle damage, thus promoting sarcopenia [39]. Given that skeletal muscle serves as the main depot for glycogen storage, a decrease in muscle mass can directly affect the body's capacity to store glycogen. This impairment may prevent insulin from transforming excess blood glucose into glycogen, leading to insulin resistance, which is a critical factor in CHD pathogenesis. Sarcopenic patients also exhibit significantly elevated levels of interleukin-6 and angiotensin II, inflammatory factors that can promote CHD progression [39]. This mutual causality between CHD and sarcopenia, extending beyond mere comorbidity, necessitates heightened awareness of the robust correlation between these two conditions in clinical practice.
This study found a positive correlation between the WBC count and sarcopenia risk in PAD patients, aligning with the findings of previous studies [40,41]. Chronic inflammation is considered to be the basis of the pathophysiology of sarcopenia [42] and is instrumental in reducing skeletal muscle mass by triggering muscle-protein hydrolysis and muscle-cell apoptosis and impairing muscle regeneration [43]. Several inflammatory markers, such as the WBC count, cytokines (e.g., interleukin-1b, −6, −8, −10, and tumor necrosis factor-α), and acute-phase reactants (e.g., fibrinogen and C-reactive protein) are often used to evaluate systemic inflammation status [44]. Among these inflammatory markers, WBC count is a commonly measured systemic inflammatory indicator, routinely measured in clinical practice. WBCs are known to trigger the release of specific pro-inflammatory cytokines, such as IL-6 and TNF-α, among others [45]. Visser et al. [46]established a connection between increased plasma concentrations of these pro-inflammatory cytokines and reduced muscle mass and vigor. Therefore, we believe that the WBC count, as part of a complete blood cell count, is not only simple and economical but also a reliable inflammatory marker. Given its widespread use in clinical practice, we propose using WBC counts to identify individuals predisposed to PAD and sarcopenia. However, further exploration is warranted to elucidate the relationship between WBC counts and the convergence of PAD and sarcopenia.
This study established a positive correlation between the severity of lower limb arterial stenosis and sarcopenia, especially in cases of severe or rapidly progressing arterial constriction, consistent with previous studies [31]. Lower limb arterial stenosis impedes blood flow, particularly during physical activities or exercises, leading to an inadequate supply of blood to the muscles and tissues in the lower limbs [31,47]. This chronic insufficiency can cause muscle tissue atrophy and impair function, potentially resulting in sarcopenia. Stenosis may induce symptoms such as pain, fatigue, weakness, and cramping in the lower limbs, which can further limit patients' daily activities and exercise, increasing the risk of sarcopenia. These insights emphasize the important connection between lower limb arterial stenosis and sarcopenia, underscoring the need for further research to identify the physiological mechanisms of this association and to inform more effective prevention and treatment strategies.
In this study, we successfully developed a predictive nomogram based on clinical and lower limb CTA imaging features that accurately predicts the risk of sarcopenia in patients with PAD. This nomogram demonstrated exceptional predictive capabilities, effectively addressing the deficiencies of existing methods in predicting sarcopenia. As an economical and non-invasive tool, the nomogram accurately identifies early-stage sarcopenia in PAD patients. Its straightforward parameter settings and high practical utility offer clinicians an effective means to continuously monitor and assess patient conditions. This facilitates the timely identification of high-risk patients and the implementation of appropriate preventative measures, significantly improving patient outcomes. DCA showed that our nomogram provides additional net benefits to clinical decision-making across a range of threshold probabilities, indicating that it can significantly enhance the diagnosis and treatment outcomes of sarcopenia in PAD patients. Another innovation of our study is the integration of clinical and imaging indicators, a combined approach whose predictive value significantly surpassed that of using clinical or imaging indicators alone. Unlike other single-center studies, our research collected data from two hospitals, enhancing the reliability and general applicability of our findings. Collectively, these characteristics ensure the scientific integrity, rationality, and logical consistency of our study.
Despite these strengths, this study had the following limitations. (1) As a retrospective study, data availability was restricted, and not all potential pathological data could be incorporated. (2) The nomogram underwent only internal validation, and external validation is necessary to enhance its predictive accuracy. (3) Cases with missing data were omitted from the study, possibly leading to selection bias. (4) While BMI is a straightforward assessment tool, it does not accurately measure muscle mass, a factor crucial for diagnosing sarcopenia. This study did not use specialized methods like BIA phase angle measurements, which could offer a more precise sarcopenia assessment. (5) Although we excluded some diseases that could affect the WBC, the WBC may still be influenced by factors beyond sarcopenia. Therefore, caution is necessary when using the WBC directly as a diagnostic or assessment tool to ensure the accuracy of evaluations. This study did not thoroughly investigate participants' dietary and exercise habits, which are crucial for managing sarcopenia. Future research should gather detailed information on these aspects to fully understand their impact on sarcopenia. Recent studies have highlighted the role of specific microRNAs, including miR-133b, miR-206, and miR-146a, in the progression of sarcopenia, especially in patients with PAD [[48], [49], [50]]. These findings reveal how malnutrition, physical activity, and acupuncture can influence muscle function by modulating these microRNAs, suggesting their potential as soluble biomarkers for monitoring sarcopenia in PAD patients. In summary, this research and recent discoveries encourage further exploration of the roles of these microRNAs in PAD-associated sarcopenia and call for targeted therapeutic strategies to improve muscle health and enhance the quality of life for patients, underscoring the importance of a comprehensive approach in future sarcopenia research.
In summary, we constructed a nomogram based on age, BMI, history of CHD, WBC count, and the severity of luminal stenosis to predict sarcopenia in PAD patients. This nomogram has been validated and demonstrates excellent predictive accuracy, reliable calibration curves, and clinical applicability. Our study contributes a straightforward and practical strategy for the early detection and prevention of sarcopenia in PAD patients, thereby maximizing the potential for early intervention and treatment.
Data availability statement
The data associated with our study was not deposited into a publicly available repository. Data will be made available on request.
Ethical approval
This study received ethical approval from the ethics committees of Wujin Hospital Affiliated with Jiangsu University and Changzhou No. 2 People's Hospital Affiliated with Nanjing Medical University (approval no. 2023-SR-095). The ethics committees granted an exemption from the requirement for informed consent, and we have provided the necessary documentation for this exemption.
CRediT authorship contribution statement
Lu Nie: Writing – original draft, Data curation. Qifan Yang: Methodology, Data curation. Qian Song: Investigation, Funding acquisition. Yu Zhou: Supervision, Software. Weimiao Zheng: Project administration, Methodology. Qiang Xu: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28732.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Criqui M.H., Matsushita K., Aboyans V., Hess C.N., Hicks C.W., Kwan T.W., et al. Lower extremity peripheral artery disease: Contemporary epidemiology, management gaps, and future directions: a scientific statement from the American heart association. Circulation. 2021;144(9):e171–e191. doi: 10.1161/cir.0000000000001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G. Inter-society consensus for the management of peripheral arterial disease (TASC II) J. Vasc. Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Perl L., Bental T., Vaknin-Assa H., Assali A., Codner P., Talmor-Barkan Y., et al. Independent impact of peripheral artery disease on percutaneous coronary intervention. J. Am. Heart Assoc. 2020;9(24) doi: 10.1161/jaha.120.017655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita K., Ballew S.H., Coresh J., Arima H., Ärnlöv J., Cirillo M., et al. Measures of chronic kidney disease and risk of incident peripheral artery disease: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2017;5(9):718–728. doi: 10.1016/s2213-8587(17)30183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pizzimenti M., Meyer A., Charles A.L., Giannini M., Chakfé N., Lejay A., et al. Sarcopenia and peripheral arterial disease: a systematic review. Journal of cachexia, sarcopenia and muscle. 2020;11(4):866–886. doi: 10.1002/jcsm.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cevei M., Onofrei R.R., Cioara F., Stoicanescu D. Correlations between the quality of life domains and clinical variables in sarcopenic osteoporotic postmenopausal women. J. Clin. Med. 2020;9(2) doi: 10.3390/jcm9020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shafiee G., Keshtkar A., Soltani A., Ahadi Z., Larijani B., Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taniguchi R., Deguchi J., Hashimoto T., Sato O. Sarcopenia as a possible negative predictor of limb salvage in patients with chronic limb-threatening ischemia. Annals of vascular diseases. 2019;12(2):194–199. doi: 10.3400/avd.oa.18-00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsubara Y., Matsumoto T., Inoue K., Matsuda D., Yoshiga R., Yoshiya K., et al. Sarcopenia is a risk factor for cardiovascular events experienced by patients with critical limb ischemia. J. Vasc. Surg. 2017;65(5):1390–1397. doi: 10.1016/j.jvs.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Sugai T., Watanabe T., Otaki Y., Goto J., Watanabe K., Toshima T., et al. Decreased psoas muscle computed tomography value predicts poor outcome in peripheral artery disease. Circ. J. : official journal of the Japanese Circulation Society. 2018;82(12):3069–3075. doi: 10.1253/circj.CJ-18-0726. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlandson M.C., Lorbergs A.L., Mathur S., Cheung A.M. Muscle analysis using pQCT, DXA and MRI. Eur. J. Radiol. 2016;85(8):1505–1511. doi: 10.1016/j.ejrad.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Buckinx F., Landi F., Cesari M., Fielding R.A., Visser M., Engelke K., et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. Journal of cachexia, sarcopenia and muscle. 2018;9(2):269–278. doi: 10.1002/jcsm.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo J., Leung J., Morley J.E. Validating the SARC-F: a suitable community screening tool for sarcopenia? J. Am. Med. Dir. Assoc. 2014;15(9):630–634. doi: 10.1016/j.jamda.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Shwaiki O., Rashwan B., Fink M.A., Kirksey L., Gadani S., Karuppasamy K., et al. Lower extremity CT angiography in peripheral arterial disease: from the established approach to evolving technical developments. Int. J. Cardiovasc. Imag. 2021;37(10):3101–3114. doi: 10.1007/s10554-021-02277-1. [DOI] [PubMed] [Google Scholar]
- 16.Peng B., Min R., Liao Y., Yu A. Development of predictive nomograms for clinical use to quantify the risk of amputation in patients with diabetic foot ulcer. J. Diabetes Res. 2021;2021 doi: 10.1155/2021/6621035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerhard-Herman M.D., Gornik H.L., Barrett C., Barshes N.R., Corriere M.A., Drachman D.E., et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American college of cardiology/American heart association task Force on clinical practice guidelines. Circulation. 2017;135(12):e726–e779. doi: 10.1161/cir.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selçuk N., Albeyoğlu Ş., Bastopcu M., Selçuk İ., Barutca H., Şahan H. Sarcopenia is a risk factor for major adverse cardiac events after surgical revascularization for critical limb ischemia. Vascular. 2023;31(1):64–71. doi: 10.1177/17085381211059383. [DOI] [PubMed] [Google Scholar]
- 19.Pizzimenti M., Charles A.L., Riou M., Thaveau F., Chakfé N., Geny B., et al. Usefulness of platelet-to-lymphocyte ratio as a marker of sarcopenia for critical limb threatening ischemia. Ann. Vasc. Surg. 2021;72:72–78. doi: 10.1016/j.avsg.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Patel M.R., Conte M.S., Cutlip D.E., Dib N., Geraghty P., Gray W., et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC) J. Am. Coll. Cardiol. 2015;65(9):931–941. doi: 10.1016/j.jacc.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaff M.R., White C.J., Hiatt W.R., Fowkes G.R., Dormandy J., Razavi M., et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the inter-society consensus for the management of peripheral arterial disease (TASC II) Vasc. Med. 2015;20(5):465–478. doi: 10.1177/1358863x15597877. [DOI] [PubMed] [Google Scholar]
- 22.Iasonos A., Schrag D., Raj G.V., Panageas K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 2008;26(8):1364–1370. doi: 10.1200/jco.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 23.Lv J., Liu Y.Y., Jia Y.T., He J.L., Dai G.Y., Guo P., et al. A nomogram model for predicting prognosis of obstructive colorectal cancer. World J. Surg. Oncol. 2021;19(1):337. doi: 10.1186/s12957-021-02445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Q., Cui J., Liu W., Li J., Hong M., Qian S. The prognostic value of sarcopenia in acute myeloid leukemia patients and the development and validation of a novel nomogram for predicting survival. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo Y.H., Su Y.D., Dong X., Zhong J., Yang C., Deng W.Y., et al. Development and validation of a nomogram for predicting sarcopenia in community-dwelling older adults. J. Am. Med. Dir. Assoc. 2022;23(5) doi: 10.1016/j.jamda.2021.11.023. 715-21.e5. [DOI] [PubMed] [Google Scholar]
- 26.Beaudart C., Rizzoli R., Bruyère O., Reginster J.Y., Biver E. Sarcopenia: burden and challenges for public health. Archives of public health = Archives belges de sante publique. 2014;72(1):45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volpato S., Bianchi L., Cherubini A., Landi F., Maggio M., Savino E., et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(4):438–446. doi: 10.1093/gerona/glt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolland Y., Czerwinski S., Abellan Van Kan G., Morley J.E., Cesari M., Onder G., et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging. 2008;12(7):433–450. doi: 10.1007/bf02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo S.Z., No M.H., Heo J.W., Park D.H., Kang J.H., Kim S.H., et al. Role of exercise in age-related sarcopenia. Journal of exercise rehabilitation. 2018;14(4):551–558. doi: 10.12965/jer.1836268.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Addison O., Prior S.J., Kundi R., Serra M.C., Katzel L.I., Gardner A.W., et al. Sarcopenia in peripheral arterial disease: prevalence and effect on functional status. Arch. Phys. Med. Rehabil. 2018;99(4):623–628. doi: 10.1016/j.apmr.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai P.S., Lin D.C., Jan Y.T., Liu Y.P., Wu T.H., Huang S.C. Lower-extremity muscle wasting in patients with peripheral arterial disease: quantitative measurement and evaluation with CT. Eur. Radiol. 2023;33(6):4063–4072. doi: 10.1007/s00330-022-09356-4. [DOI] [PubMed] [Google Scholar]
- 32.Matsubara Y., Matsumoto T., Aoyagi Y., Tanaka S., Okadome J., Morisaki K., et al. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J. Vasc. Surg. 2015;61(4):945–950. doi: 10.1016/j.jvs.2014.10.094. [DOI] [PubMed] [Google Scholar]
- 33.Zamboni M., Mazzali G., Fantin F., Rossi A., Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr. Metabol. Cardiovasc. Dis. : Nutr. Metabol. Cardiovasc. Dis. 2008;18(5):388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Choi K.M. Sarcopenia and sarcopenic obesity. Kor. J. Intern. Med. 2016;31(6):1054–1060. doi: 10.3904/kjim.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida S., Kamiya K., Hamazaki N., Matsuzawa R., Nozaki K., Ichikawa T., et al. Association between sarcopenia and atherosclerosis in elderly patients with ischemic heart disease. Heart Ves. 2020;35(6):769–775. doi: 10.1007/s00380-020-01554-8. [DOI] [PubMed] [Google Scholar]
- 36.Zhang N., Zhu W.L., Liu X.H., Chen W., Zhu M.L., Kang L., et al. Prevalence and prognostic implications of sarcopenia in older patients with coronary heart disease. Journal of geriatric cardiology : JGC. 2019;16(10):756–763. doi: 10.11909/j.issn.1671-5411.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Haehling S., Ebner N., Dos Santos M.R., Springer J., Anker S.D. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat. Rev. Cardiol. 2017;14(6):323–341. doi: 10.1038/nrcardio.2017.51. [DOI] [PubMed] [Google Scholar]
- 38.Stephens J.W., Khanolkar M.P., Bain S.C. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202(2):321–329. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Cannataro R., Carbone L., Petro J.L., Cione E., Vargas S., Angulo H., et al. Sarcopenia: etiology, nutritional approaches, and miRNAs. Int. J. Mol. Sci. 2021;22(18) doi: 10.3390/ijms22189724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H.S., Koh I.H., Kim H.S., Kwon Y.J. Platelet and white blood cell count are independently associated with sarcopenia: a nationwide population-based study. Thromb. Res. 2019;183:36–44. doi: 10.1016/j.thromres.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Kim S.H., Kwon H.S., Hwang H.J. White blood cell counts, insulin resistance, vitamin D levels and sarcopenia in Korean elderly men. Scand. J. Clin. Lab. Invest. 2017;77(3):228–233. doi: 10.1080/00365513.2017.1293286. [DOI] [PubMed] [Google Scholar]
- 42.Schaap L.A., Pluijm S.M., Deeg D.J., Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006;119(6) doi: 10.1016/j.amjmed.2005.10.049. 526.e9-17. [DOI] [PubMed] [Google Scholar]
- 43.Argilés J.M., Busquets S., Stemmler B., López-Soriano F.J. Cancer cachexia: understanding the molecular basis. Nat. Rev. Cancer. 2014;14(11):754–762. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 44.Pearson T.A., Mensah G.A., Alexander R.W., Anderson J.L., Cannon R.O., 3rd, Criqui M., et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 45.Melani C., Mattia G.F., Silvani A., Carè A., Rivoltini L., Parmiani G., et al. Interleukin-6 expression in human neutrophil and eosinophil peripheral blood granulocytes. Blood. 1993;81(10):2744–2749. [PubMed] [Google Scholar]
- 46.Visser M., Pahor M., Taaffe D.R., Goodpaster B.H., Simonsick E.M., Newman A.B., et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2002;57(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 47.McDermott M.M. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ. Res. 2015;116(9):1540–1550. doi: 10.1161/circresaha.114.303517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long Y.F., Chow S.K., Cui C., Wong R.M.Y., Zhang N., Qin L., et al. Does exercise influence skeletal muscle by modulating mitochondrial functions via regulating MicroRNAs? A systematic review. Ageing Res. Rev. 2023;91 doi: 10.1016/j.arr.2023.102048. [DOI] [PubMed] [Google Scholar]
- 49.Jin J., Yang Z., Liu H., Guo M., Chen B., Zhu H., et al. Effects of acupuncture on the miR-146a-mediated IRAK1/TRAF6/NF-κB signaling pathway in rats with sarcopenia induced by D-galactose. Ann. Transl. Med. 2023;11(2):47. doi: 10.21037/atm-22-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iannone F., Montesanto A., Cione E., Crocco P., Caroleo M.C., Dato S., et al. Expression patterns of muscle-specific miR-133b and miR-206 correlate with nutritional status and sarcopenia. Nutrients. 2020;12(2) doi: 10.3390/nu12020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with our study was not deposited into a publicly available repository. Data will be made available on request.