Abstract
Background
Significant errors of activated partial thromboplastin time (aPTT) ratio were frequently observed in blood sampling from central venous dialysis catheter (CVC) of hemodialysis (HD) patients. Following the draw-and-return methods, initial blood withdrawal from the catheter before sampling can reduce the error, but the optimal withdrawal volume remains undetermined.
Aim
The objective of this study is to determine the optimal blood withdrawal volume for the draw-and-return methods to improve aPTT ratio accuracy in hemodialysis patients with CVC.
Methods
A prospective study was conducted in patients receiving HD via CVC. Four blood samples were collected from each patient, involving a peripheral venipuncture and three draw-and-return samples (10 ml, 20 ml and 25 ml groups). The aPTT ratio of a peripheral sample was used as a reference to determine the aPTT ratio accuracy for each draw-and-return group. Subsequently, the agreement was illustrated using modified Bland-Altman plot.
Results
A total of 1,000 samples were obtained from 250 patients. The patients had a mean age of 59.6 ± 15.4 years, with 17.2% using citrate as the CVC's locking agent. The adjusted accuracies of the aPTT ratio varied significantly among the three withdrawal volumes (p-value <0.001). The 25 ml group demonstrated the highest accuracy (43.2%; 95%CI, 38.0–48.4), followed by the 20 ml group (30.0%; 95%CI, 24.9–35.2), and the 10 ml group (18.0%; 95%CI, 12.8–23.2). Additionally, using citrate as a locking agent provided more than 80.0% aPTT ratio accuracy, whereas heparin demonstrated inferior accuracy even in the 25 ml withdrawal group.
Conclusion
The optimal blood withdraw volume for the draw-and-return methods concluded at 20 ml for citrate locked-CVC and 25 ml for heparin which significantly improved aPTT ratio accuracies. Applying citrate as a locking agent provides clear benefits for aPTT ratio monitoring, while peripheral venipuncture is recommended in cases of heparin-locked CVC.
Keywords: Hemodialysis, Central venous dialysis catheter, APTT ratio, Blood sampling, Withdrawal volume
1. Introduction
Hemodialysis (HD) patients require a vascular access (VA) to facilitate the connection of a dialyzer to their circulatory system [1]. Various VA options are currently available including arteriovenous fistulas, arteriovenous grafts, and catheters. However, the selection of VA varies across countries and primarily depends on specific treatment plans of individual patients [2]. According to the 2020 report from the United States Renal Data System, over 80% of HD patients initiate their treatment with a catheter, specifically central venous dialysis catheter (CVC), as a preferred option [3,4]. Within the lumen of the catheter, some anticoagulants including heparin and citrate will be used to lock the catheter in order to prevent thrombosis [1]. Moreover, during the HD process, it is crucial to administer anticoagulants, such as low molecular weight heparin (LMWH) or unfractionated heparin (UFH), in appropriate doses to prevent circuit clot [5]. However, excessive usage of heparin can result in bleeding event, and its antithrombotic effect varies among patients, necessitating close monitoring in standard practice [5,6]. Heparin effect can be directly observed by using anti-activated factor X (anti-Xa) activity or activated partial thromboplastin time (aPTT) ratio which is more widely used and economically reasonable in our setting [7,8].
To minimize patient discomfort and failure of peripheral venipuncture, blood samples for coagulation testing are often collected from CVC access or dialysis circuit [9]. However, previous reports have highlighted a potential problem of sample contamination, as locking agents like heparin or citrate from CVC can introduce errors in the coagulation profile measurement [[10], [11], [12]]. Consequently, these inaccuracies can lead to improper adjustments in anticoagulation dosing [1]. To enhance the precision of coagulation monitoring, several methodologies have been proposed, including blood sampling through the dialysis circuit [13], pull-push method [14,15], and discard method which removes heparin-contaminated blood before sampling [16,17].
While removing contaminated blood prior to sampling is considered reliable and widely used, cumulative volume of waste blood may become clinically significant in critically ill patients [18]. Due to this consideration, the draw-and-return methods, which are also referred to as the reinfusion techniques, have become an alternative. These methods involve initial withdrawal of blood before sampling, returned into the patient's circulation afterward to maintain homeostasis and minimize blood loss [19]. Despite the methods being studied and implemented in our HD center for decades, the optimal volume of withdrawn blood remains uncertain. Therefore, the primary aim of this study is to determine the optimal withdrawal volume for the draw-and-return methods to improve the aPTT ratio accuracy by evaluating the accuracies of 10 ml, 20 ml, and 25 ml withdrawn volumes in patients receiving HD via CVC.
2. Materials and methods
2.1. Patients and data collection
This prospective study included a single group of patients who were recruited from Nopparat Rajathanee Hospital, Bangkok, Thailand during August 1, 2020 to November 19, 2021. Patients at any age groups who received (i) HD via CVC and (ii) blood coagulation test were included. We excluded patients who were unable to provide informed consent or undergo peripheral venipuncture.
Data of baseline demographic (e.g., age, sex, underlying diseases), laboratory investigations (e.g., creatinine, hemoglobin, hematocrit), catheter usage and the specification of vascular access were collected from all eligible patients. The study protocol was registered on the Thai Clinical Trial Registry (TCTR20201126001) and has been approved by The Human Research Ethics Committee of Nopparat Rajathanee Hospital (42/2563).
2.2. Blood collection procedures
All eligible patients underwent four blood collection methods before HD, which were administered by the dialysis nursing staff at the hospital's dialysis unit. The reference sample for blood collection method was obtained from peripheral venipuncture, while the draw-and-return samples were acquired from the arterial line of the catheter, incorporating three withdraw volumes of 10 ml, 20 ml and 25 ml for each sample, respectively.
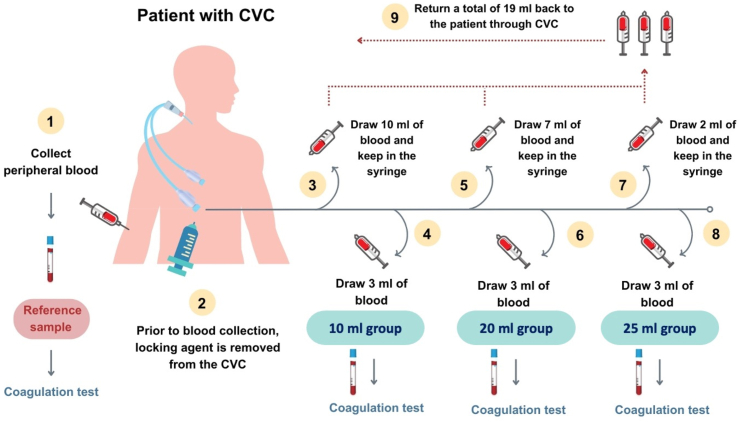
The procedure began with sterilizing the access site and removing the locking agent from both lumens of the catheter. The first draw-and-return method started by drawing 10 ml of blood from the CVC and retaining the withdrawn blood in the syringe. A new sterile syringe was replaced to collect the next 3 ml of blood from the CVC and labeled as the 10 ml method. Repeatedly, for the 20 ml method, 7 ml of blood was withdrawn using a new syringe and kept sterile. This process summed up the total withdrawn volume to 20 ml thus, the next 3 ml of blood was collected and labeled as the 20 ml method. Similarly, the last sample proceeded with 2 ml of blood removal using a new syringe and the collection of 3 ml of blood was subsequently done for the 25 ml method. It is important to note that a new sterile syringe was replaced at each withdrawal procedure and the total of 19 ml of blood that had been withdrawn (including 10 ml, 7 ml, and 2 ml) was gradually returned to the patients through the CVC. In total, four samples were obtained from each patient and subsequently subjected to analysis for aPTT ratio. Fig. 1 demonstrates blood collection procedures and experimental design.
Fig. 1.
Graphical scheme of experimental design. CVC, central venous dialysis catheter.
All samples were collected and transferred with 2% sodium citrate collection tubes (BD Vacutainer®, Becton, Dickinson and Company, New Jersey, United States) to the central laboratory of the hospital following routine practice. The samples were immediately analyzed upon arrival at the laboratory (delivery time ranged between 5 and 10 minutes). Samples were analyzed using CS-1600 System coagulation analyzer and Thromborel S reagent kit (Sysmex Corporation, Kobe, Japan) according to the manufacturer's protocol. The machine's detection limit for coagulation time was at 180 seconds, indicating that any results exceeding this threshold were considered as reaching the 180-seconds limit for coagulation time.
2.3. Definitions
-
1.
Percentage of error (% error): the error of the laboratory test results from the testing sample compared to the reference sample. The value was derived from ((value from the testing sample – value from the reference sample)/value from the reference sample) × 100.
-
2.Allowable total errors (%): acceptable performance described by the percentage of error defined according to the Clinical Laboratory Improvement Amendments (CLIA) [[20], [21], [22]]. The allowable total error of all laboratory tests in this study are as followed.
-
(i)Prothrombin time (PT): ±15%;
-
(ii)Activated partial thromboplastin time (aPTT) (second): ±15%;
-
(iii)aPTT ratio = ± 15%
-
(i)
-
3.
Crude accuracy: percentage of laboratory test results within the allowable total error range, before adjusting for extraneous effects (i.e., age, gender, underlying diseases, baseline laboratory results and catheter usage factors).
-
4.
Adjusted accuracy: percentage of laboratory test results within the allowable total error range, after adjusting for extraneous effects.
2.4. Sample size estimation
In a pilot data collection, the correlation of the aPTT ratios of the 10 ml and 20 ml groups was 0.7, and the 10 ml group demonstrated 18% accuracy. Based on our hypothesis, the draw-and-return methods were expected to exhibit a minimum 5% improvement in accuracies which were considered clinically significant. Hence, to obtain 80% statistical power, 5% alpha error for one-sided test, the minimum sample size estimated via a paired proportion method was 248 patients.
2.5. Statistical analysis
Data were presented as number and percentage for categorical data while mean and standard deviation (SD) or median and interquartile range (IQR) were used for numerical data as appropriate. The results of coagulation tests, including mean of aPTT ratio, mean difference and % error, were calculated based on the predefined-criteria, which were subsequently accounted for the percentage of accuracy. The accuracies were compared using multivariable mixed-effects regression, which adjusted for gender, age, catheter insert site, locking agent volume, interdialytic day, volume of coagulation applied during previous dialysis, underlying diseases (i.e., liver disease), current medication (i.e., peripheral heparin infusion, antiplatelet), laboratory data (i.e., hemoglobin, creatinine). Bonferroni adjustment was employed and p-value less than 0.05 was considered statistically significant.
The Modified Bland-Altman plot was applied to illustrate the agreement between the draw-and-return and reference methods. The percentage of error, representing the deviation from the reference value, was illustrated along with the allowable total error. All statistical analyses were performed using Stata 17 (StataCorp, College Station, Texas, United States).
3. Results
3.1. Participants
A total of 263 patients that met inclusion criteria were identified during the study period. Of these, 13 patients (4.9%) were excluded from the study due to their inability to undergo peripheral venipuncture. The remaining patients provided informed consent and agreed to participate in the study. Patient's characteristics are described in Table 1. Half of the patients were male (50.0%) with a mean age of 59.6 (±15.4) years. The majority of patients were diagnosed with hypertension (86.8%), followed by diabetes (59.6%) and liver disease (11.6%). None of them were prescribed warfarin, but almost one-third (32.0%) were prescribed antiplatelet drugs which included salicylate (n = 63) and clopidogrel (n = 41). Non-tunneled with non-cuffed catheter was predominantly applied (59.6%) among these patients, as well as the usage of heparin as a locking agent (82.8%). The median usage of CVC was 9 days, ranging from 1 to 2,568 days (IQR, 3–203 days). There was no report on catheter-related bloodstream infection (CRBSI).
Table 1.
Patient's characteristics.
| Characteristics (N = 250) | n | % |
|---|---|---|
| Male | 125 | 50.0 |
| Age (Years), mean ± SD | 59.6 | ±15.4 |
| Underlying diseases | ||
| At least one | 246 | 98.4 |
| Hypertension | 217 | 86.8 |
| Diabetes | 149 | 59.6 |
| Liver diseases | 29 | 11.6 |
| Systematic lupus erythematous | 9 | 3.6 |
| Hemophilia | 0 | 0 |
| Current medication | ||
| Warfarin | 0 | 0 |
| Intravenous unfractionated heparin (UFH) | 5 | 2.0 |
| Antiplatelet (Salicylate and clopidogrel) | 80 | 32.0 |
| Laboratory investigations | ||
| Blood Urea Nitrogen (mg/dL), median (IQR) | 60 | (40–87) |
| Creatinine (mg/dL), median (IQR) | 7.7 | (5.2–10.7) |
| Hemoglobin (gm/dL), mean ± SD | 8.7 | ±1.6 |
| Hematocrit (%), mean ± SD | 26.1 | ±4.8 |
| Platelet count (*103 cells/mm3), median (IQR) | 203.5 | (145.0–276.0) |
| Types of central venous dialysis catheter (CVC) | ||
| Non-tunneled, non-cuffed catheter | 149 | 59.6 |
| Tunneled, cuffed catheter | 101 | 40.4 |
| CVC site | ||
| Arterial catheter | 101 | 40.4 |
| Venous catheter | 149 | 59.6 |
| CVC usage (days), median (IQR) | 9 | (3–203) |
| Locking agent | ||
| Heparin | 207 | 82.8 |
| Citrate | 43 | 17.2 |
| Locking agent volume (ml), mean ± SD | 1.5 | ±0.3 |
| Previous anticoagulant used during hemodialysis | ||
| No | 149 | 59.6 |
| Heparin | 93 | 37.2 |
| Low molecular weight heparin | 8 | 3.2 |
Abbreviation: IQR, interquartile range; SD, standard deviation.
3.2. Accuracy of draw-and-return methods on aPTT ratio
A total of 1,000 samples were obtained from 4 collection methods including peripheral venipuncture, 10 ml, 20 ml and, 25 ml of draw-and-return methods which contained 250 samples each. APTT ratios of the peripheral and draw-and-return samples (10 ml, 20 ml, 25 ml groups) were determined. Among three groups, 10 ml obtained the highest aPTT ratio, following by 20 ml and 25 ml groups, respectively. Therefore, when comparing to the reference, the resulting mean difference and percentage of error of the 25 ml group showed the least deviation (mean difference 0.74 ± 1.27; %error 67.55 ± 116.98). Consistently, adjusted accuracies from these methods were significantly different (p-value <0.001), where the 25 ml group revealed the highest adjusted accuracy (43.2%; 95%CI, 38.0–48.4) (Table 2). The results remained consistent when adjusted with Bonferroni correction.
Table 2.
Activated partial thromboplastin time ratios (aPTT ratios) from different techniques compared to a reference.
| Mean ± SD | Mean difference ±SD | % Error ±SD | % Crude accuracy (95% CI) | % Adjusted accuracya (95% CI) | P-value compared to 10 ml technique | P-value compared to 20 ml technique | |
|---|---|---|---|---|---|---|---|
| Both locking agent | |||||||
| Peripheral blood | 1.12 ± 0.25 | Reference | Reference | Reference | Reference | – | – |
| 10 ml group | 4.33 ± 2.10 | 3.21 ± 2.05 | 294.19 ± 200.35 | 18.00 (11.2–24.8) | 18.00 (12.8–23.2) | – | – |
| 20 ml group | 2.45 ± 1.75 | 1.34 ± 1.71 | 121.48 ± 159.14 | 30.00 (23.2–36.8) | 30.00 (24.9–35.2) | <0.001 | – |
| 25 ml group | 1.86 ± 1.30 | 0.74 ± 1.27 | 67.55 ± 116.98 | 43.20 (36.4–50.0) | 43.20 (38.0–48.4) | <0.001 | <0.001 |
| Heparin (n = 207, 82.8%) | |||||||
| Peripheral blood | 1.32 ± 0.25 | Reference | Reference | Reference | Reference | – | – |
| 10 ml group | 4.96 ± 1.68 | 3.83 ± 1.64 | 349.64 ± 166.77 | 3.86 (0–9.9) | 3.87 (1.0–9.1) | – | – |
| 20 ml group | 2.73 ± 1.80 | 1.60 ± 1.77 | 145.09 ± 165.08 | 17.39 (11.3–23.5) | 17.39 (12.2–22.6) | <0.001 | – |
| 25 ml group | 2.02 ± 1.37 | 0.88 ± 1.35 | 80.43 ± 124.50 | 33.33 (27.2–39.5) | 33.33 (28.1–38.5) | <0.001 | <0.001 |
| Citrate (n = 43, 17.2%) | |||||||
| Peripheral blood | 1.05 ± 0.21 | Reference | Reference | Reference | Reference | – | – |
| 10 ml group | 1.27 ± 0.82 | 0.22 ± 0.85 | 27.24 ± 116.03 | 86.05 (74.5–97.5) | 86.05 (77.7–94.4) | – | – |
| 20 ml group | 1.12 ± 0.24 | 0.07 ± 0.18 | 7.83 ± 22.40 | 90.70 (79.2–100) | 90.70 (82.3–99.1) | 0.276 | – |
| 25 ml group | 1.09 ± 0.22 | 0.04 ± 0.14 | 5.53 ± 18.85 | 90.70 (79.2–100) | 90.70 (82.3–99.1) | 0.276 | 1.000 |
Abbreviation: CI, confidence interval; SD, standard deviation.
Accuracy adjusted by gender, age, catheter position, priming volume, interdialytic day, hemoglobin, creatinine, anticoagulant of last HD, heparin peripheral infusion and antiplatelet usage.
3.3. Subgroup analysis by locking agents
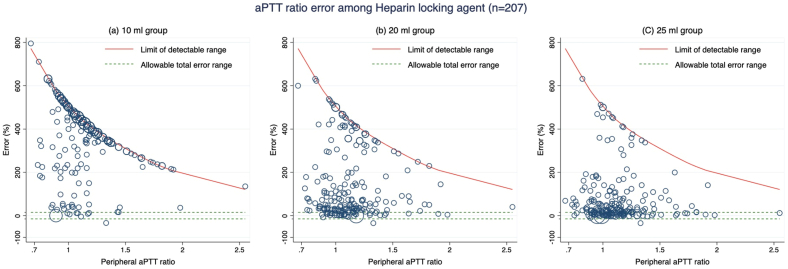
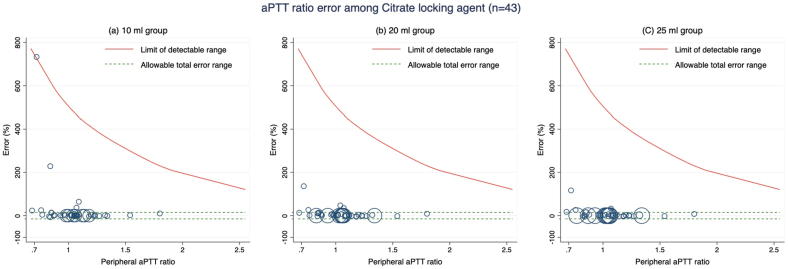
In order to evaluate the accuracy of the aPTT ratios in two different locking agents, we conducted a subgroup analysis using data from the heparin (n = 207) and citrate (n = 43) samples. Within the heparin locking agent samples, the 25 ml group displayed the highest accuracy, showing an adjusted accuracy of 33.3% (95% CI, 28.1–38.5) (Table 2 and Fig. 2a–c). In the citrate locking agent samples, both the 20 ml and 25 ml groups exhibited similar adjusted accuracies at 90.7% (95% CI, 82.3–99.1). However, these results did not differ significantly from those obtained from the 10 ml group (p-value 0.276; Bonferroni adjustment p-value 0.828) (Table 2 and Fig. 3a–c). All withdrawal volumes in the citrate locking agent samples demonstrated superior accuracies compared to the heparin samples (p-value <0.001). The differences in adjusted accuracies between both locking agents in each method were reported in Supplementary Table S1.
Fig. 2.
Modified Bland-Altman plot of activated partial thromboplastin time (aPTT) ratio error of the heparin locking agent group determined by (a) 10 ml group, (b) 20 ml group and (c) 25 ml group.
Fig. 3.
Modified Bland-Altman plot of activated partial thromboplastin time (aPTT) ratio error of the citrate locking agent group determined by (a) 10 ml group, (b) 20 ml group and (c) 25 ml group.
4. Discussion
Our study demonstrated that in HD patients, blood sampling from the CVC using the draw-and-return methods significantly improved the accuracy of blood sampling on the aPTT ratios when an initial 25 ml of blood was withdrawn before sampling. Specifically, when different locking agents were employed, the results were considerably different, with citrate exhibiting notably higher accuracies of aPTT ratios compared to heparin.
When blood samples are obtained directly from the catheter, contamination of the CVC's locking agents, such as heparin, affects the coagulation profiles and consequently prolongs the prothrombin time and aPTT ratio. Several strategies were created to collect blood samples from the CVC [23,24]. When comparing the draw-and-return methods to other techniques such as the discard methods and the pull-push (mixing) methods, several advantages and limitations become evident. While both the discard and draw-and-return methods remove heparin-contaminated blood before sampling, the discard methods require the wastage of blood. On the other hand, the pull-push methods involve mixing the blood in the catheter before sampling. Althoughthe pull-push methods avoid blood wastage and reduce the risk of medical staff exposure to blood, the mixing process may lead to hemolysis [24]. Consequently, considering hemolysis and blood loss, our draw-and-return methods provided substantial benefits over the other two models. However, limited studies assess the accuracy of coagulation parameters, especially in terms of heparin monitoring in HD patients.
Though removal of heparin-contaminated blood is similar in both discard methods and draw-and-return methods, a consensus has not been established concerning the optimal discard volume for achieving an accurate coagulation value [25,26]. Previous studies have been conducted using the discard methods with several discarded volumes including 3 ml, 5 ml, 10 ml, 15 ml, 20 ml, and 25 ml [16,17,27]. The aPTT ratios mean difference ranged from 0.96 to 1.81 [17]. In our center, we have implemented the 10 ml draw-and-return method in practice, which also led to noticeable errors in coagulation profiles, especially aPTT ratio measurement, resulting in inappropriate management of anticoagulant administration. In the current study, we addressed this issue by increasing the volume of blood withdrawn to 20 ml and 25 ml and evaluating its accuracy on aPTT ratios. Our study exhibited comparable trends in which our aPTT ratios mean difference ranged around 0.74–3.21. The findings revealed a notable improvement in accuracies, aligning with previous studies in discard methods that have demonstrated a continuous reduction in errors when the amount of discarded blood increases [16,17]. Nonetheless, despite withdrawing 25 ml of blood, the coagulation panel still displayed significant deviations from the peripheral samples [16].
Although increasing the volume of discarded blood has been shown to improve aPTT ratio accuracy, it is crucial to consider the practicality in clinical aspects. A substantial amount of withdrawn blood may impact patient's hemodynamic status, especially in cases where anemia is present due to blood loss [18]. Considering these factors, we recommend 25 ml withdrawal for the draw-and-return methods since it simplifies the entire process by using only a single 25-ml syringe. Plus, returning the blood may help lessen blood wastage as well as mitigating potential adverse effects on the patient's hemodynamic stability [28].
Furthermore, the administration of different locking agents had a considerable impact on the coagulation profile. Our subgroup analyses demonstrated that samples collected from CVCs locked with a sodium citrate solution exhibited remarkably high accuracy of the aPTT ratio, outperforming the samples from CVCs locked with heparin. In the blood coagulation cascade, the formation of a blood clot involves intrinsic and extrinsic pathways that are influenced by several coagulation factors. Heparin is a polysaccharide that possesses anticoagulant activity, interfering with the intrinsic pathway through the acceleration of antithrombin (AT) activity. The AT will inactive coagulation enzymes; therefore, coagulation factors, especially factor Xa and thrombin, were inhibited [29]. Besides, the exogenous pathway also involves binding between tissue factor and factor VII, which is facilitated by calcium, thereby activating factor Xa and thrombin. For this mechanism, citrate exerts its anticoagulation activity by chelating to calcium ions, also producing an anticoagulant effect [7]. Subsequently, the chelated complex will be immediately metabolized by the liver, reducing the further risk of bleeding effects. Some studies suggested using sodium citrate as an alternative to heparin and considered it to provide advantages over heparin in terms of functional assessment [30]. Furthermore, there was no significant evidence of the rate of thrombosis, catheter dysfunction, and CRBSI in randomized controlled trials using sodium citrate [[30], [31], [32]].
4.1. Clinical implication
The indication for choosing locking agents depends primarily on the patients' background and underlying diseases. Considering thrombosis monitoring, although citrate is favored for its accuracy in coagulation measurement, vigilance is necessary due to its ability to chelate calcium ions, which may affect metabolite levels owing to the blood infusion [33]. Therefore, caution is needed when employing citrate as a locking agent in patients with hypocalcemia or liver disease [34]. Instead, those patients should use heparin as an alternative, with peripheral venipuncture being the preferred sampling method. If not possible, withdrawing the initial 25 ml of contaminated blood from the central venous catheter is recommended. Besides, although there was initial concern regarding the clotting of the withdrawn blood and the risk of infection when the blood was reinfused, the procedure has been demonstrated to be safe as no infection or clotting was observed [[35], [36], [37]]. Also, there was no CRBSI incidence in our experiments.
4.2. Strength and limitation
This study highlights the use of draw-and-return methods for collecting blood samples from CVCs for the measurement of the aPTT ratio. Our study possesses several strengths, including a prospective data collection, well-powered statistical analyses, and single patient control, which helps minimize interpersonal variation. However, certain limitations should be acknowledged. First, it is important to note that all our samples from these four collection techniques were obtained simultaneously, in a consecutive manner. This experimental procedure does not reflect real-world practice, and further investigation based on a 3 × 3 crossover trial is necessary to reinforce these findings. Secondly, the machine's limit of detection hinders the accurate determination of coagulation time. This technical limitation, however, provided minimal impact on the percentage of accuracy estimation because PTT exceeding 180 seconds were extremely high, accounting for more than 200% of the %error and certainly surpassing the allowable total error limit (which was defined at 15% cut-off). Enhancing the precision of coagulation measurements can mitigate the issue of prolonged PTT values which can be confined within the detection range.
5. Conclusion
Our study demonstrated that, for the draw-and-return methods, the optimal blood withdrawal volume of 25 ml for heparin and equal to or more than 20 ml for citrate locking agents improved aPTT ratio accuracy in HD patients with CVC. Though the results were notably superior in citrate-locked samples for monitoring blood coagulation, heparin usage may be unavoidable in certain cases, and it is advised to prioritize peripheral venipuncture for blood sampling. Additional validation in other settings and study designs is necessary to support these findings.
Ethical approval
The study received approval from the Human Research Ethics Committee of Nopparat Rajathanee Hospital (42/2563).
Data availability
Data will be made available upon reasonable request to the corresponding author.
CRediT authorship contribution statement
Chitrada Thongdee: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Lalita Lumkul: Writing – review & editing, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Pakpoom Wongyikul: Writing – review & editing, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Nantana Spilles: Writing – review & editing, Resources, Investigation, Data curation, Conceptualization. Boonruksa Laonapaporn: Writing – review & editing, Resources, Investigation, Data curation, Conceptualization. Jayanton Patumanond: Writing – review & editing, Validation, Methodology, Formal analysis, Data curation, Conceptualization. Phichayut Phinyo: Writing – review & editing, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was partially supported by Chiang Mai University and Faculty of Medicine, Chiang Mai University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28651.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Clinical practice guidelines for vascular access. Am. J. Kidney Dis. 2006;48:S248–S273. doi: 10.1053/j.ajkd.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Pisoni R.L., Zepel L., Fluck R., Lok C.E., Kawanishi H., Süleymanlar G., et al. International differences in the location and use of arteriovenous accesses created for hemodialysis: results from the dialysis outcomes and practice patterns study (DOPPS) Am. J. Kidney Dis. 2018;71(4):469–478. doi: 10.1053/j.ajkd.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Silverstein D.M., Trerotola S.O., Clark T., James G., Ng W., Dwyer A., et al. Clinical and regulatory considerations for central venous catheters for hemodialysis. Clin. J. Am. Soc. Nephrol. 2018;13(12):1924–1932. doi: 10.2215/CJN.14251217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2022. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. [Google Scholar]
- 5.Kessler M., Moureau F., Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin. Dial. 2015;28(5):474–489. doi: 10.1111/sdi.12380. [DOI] [PubMed] [Google Scholar]
- 6.McGee D.C., Gould M.K. Preventing complications of central venous catheterization. N. Engl. J. Med. 2003;348(12):1123–1133. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 7.Suranyi M., Chow J.S. Review: anticoagulation for haemodialysis. Nephrology. 2010;15(4):386–392. doi: 10.1111/j.1440-1797.2010.01298.x. [DOI] [PubMed] [Google Scholar]
- 8.Toulon P., Smahi M., De Pooter N. APTT therapeutic range for monitoring unfractionated heparin therapy. Significant impact of the anti-Xa reagent used for correlation: response from original authors Toulon et al. J. Thromb. Haemostasis. 2021;19(8):2090–2091. doi: 10.1111/jth.15350. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar T., Wong J., Harvey M., Weidersehn L., Surmon L., Makris A. Comparison of sampling methods for international normalized ratio monitoring in haemodialysis patients (INRHaemo study) Am. J. Nephrol. 2021;52(1):17–25. doi: 10.1159/000513094. [DOI] [PubMed] [Google Scholar]
- 10.Pinto K.M. Accuracy of coagulation values obtained from a heparinized central venous catheter. Oncol. Nurs. Forum. 1994;21(3):573–575. [PubMed] [Google Scholar]
- 11.Seghers C.B., Ver Elst K., Claessens J., Weekx S., Vermeiren S., Henckes M. A new method for the measurement of international normalized ratio in hemodialysis patients with heparin-locked tunneled dialysis catheters. Internet J. Nephrol. 2020;2020 doi: 10.1155/2020/7586437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thongdee C., Phinyo P., Patumanond J., Satirapoj B., Spilles N., Laonapaporn B., et al. Ultrafiltration rates and intradialytic hypotension: a case-control sampling of pooled haemodialysis data. J. Ren. Care. 2021;47(1):34–42. doi: 10.1111/jorc.12340. [DOI] [PubMed] [Google Scholar]
- 13.Rioux J.P., De Bortoli B., Quérin S., Déziel C., Troyanov S., Madore F. Measurement of the international normalized ratio (INR) in hemodialysis patients with heparin-locked central venous catheters: evaluation of a novel blood sampling method. J. Vasc. Access. 2009;10(3):180–182. doi: 10.1177/112972980901000308. [DOI] [PubMed] [Google Scholar]
- 14.Lokeskrawee T., Muengtaweepongsa S., Patumanond J., Sawaengrat C. Accuracy of laboratory tests drawn by pull-push method from central venous catheterization after routine flushing with 10 ml normal saline in patients with sepsis at the emergency department. Heliyon. 2021;7(6) doi: 10.1016/j.heliyon.2021.e07355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., Zhang X., Qin Y., Wang F., Cui M., Shi Y., et al. A comparative study on coagulation and hematologic laboratory techniques for blood sampling using the push-pull method from a CVC versus venipuncture. J. Vasc. Access. 2022 doi: 10.1177/11297298221118742. [DOI] [PubMed] [Google Scholar]
- 16.Mayo D.J., Dimond E.P., Kramer W., Horne M.K., 3rd Discard volumes necessary for clinically useful coagulation studies from heparinized Hickman catheters. Oncol. Nurs. Forum. 1996;23(4):671–675. [PubMed] [Google Scholar]
- 17.Sombolos K.I., Fragia T.K., Bamichas G.I., Christidou F.P., Stangou M.I., Karagianni A.C., et al. Heparin solution locked in acute hemodialysis catheters: impact on activated partial thromboplastin time. ASAIO J. 2003;49(3):287–289. doi: 10.1097/01.mat.0000065466.80851.1c. [DOI] [PubMed] [Google Scholar]
- 18.Napolitano L.M. Scope of the problem: epidemiology of anemia and use of blood transfusions in critical care. Crit. Care. 2004;8(Suppl 2):S1–S8. doi: 10.1186/cc2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dech Z.F., Szaflarski N.L. Nursing strategies to minimize blood loss associated with phlebotomy. AACN Clin. Issues. 1996;7(2):277–287. doi: 10.1097/00044067-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Ehrmeyer S.S.W.J., Westgard S.A. The New Poor Lab's Guide to the Regulations: (CLIA, The Joint Commission, CAP & COLA) Westgard QC; Madison, WI: 2017. Successful strategies & specific applications of the regulations. [Google Scholar]
- 21.Innovations D. 2018. Allowable Total Error Table.https://datainnovations.com/allowable-total-error-table [2018 Aug 27]. Available from: [Google Scholar]
- 22.Westgard J.O., Westgard S.A. The quality of laboratory testing today: an assessment of sigma metrics for analytic quality using performance data from proficiency testing surveys and the CLIA criteria for acceptable performance. Am. J. Clin. Pathol. 2006;125(3):343–354. [PubMed] [Google Scholar]
- 23.Frey A.M. Drawing blood samples from vascular access devices: evidence-based practice. J. Infusion Nurs. 2003;26(5):285–293. doi: 10.1097/00129804-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Wyant S., Crickman R. Determining the minimum discard volume for central venous catheter blood draws. Clin. J. Oncol. Nurs. 2012;16(5):454–458. doi: 10.1188/12.CJON.454-458. [DOI] [PubMed] [Google Scholar]
- 25.Dalton K.A., Aucoin J., Meyer B. Obtaining coagulation blood samples from central venous access devices: a review of the literature. Clin. J. Oncol. Nurs. 2015;19(4):418–423. doi: 10.1188/15.CJON.19-04AP. [DOI] [PubMed] [Google Scholar]
- 26.Humphries L., Baldwin K.M., Clark K.L., Tenuta V., Brumley K. A comparison of coagulation study results between heparinized peripherally inserted central catheters and venipunctures. Clin. Nurse Spec. 2012;26(6):310–316. doi: 10.1097/NUR.0b013e31826e3efb. [DOI] [PubMed] [Google Scholar]
- 27.Lalthanthuami H.T., Kumari M.J., Venkateswaran R., Lakshmi P.R., Ramamoorthy L. Performance of 3 mL versus 5 mL discarded volume for blood sampling from central venous access device. J. Lab. Physicians. 2021;13(2):112–117. doi: 10.1055/s-0041-1726669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hess S., Decker M. Comparison of the single-syringe push-pull technique with the discard technique for obtaining blood samples from pediatric central venous access devices. J. Pediatr. Oncol. Nurs. 2017;34(6):381–386. doi: 10.1177/1043454217713453. [DOI] [PubMed] [Google Scholar]
- 29.Hirsh J., Anand S.S., Halperin J.L., Fuster V. Guide to anticoagulant therapy: heparin : a statement for healthcare professionals from the American Heart Association. Circulation. 2001;103(24):2994–3018. doi: 10.1161/01.cir.103.24.2994. [DOI] [PubMed] [Google Scholar]
- 30.Grudzinski A., Agarwal A., Bhatnagar N., Nesrallah G. Benefits and harms of citrate locking solutions for hemodialysis catheters: a systematic review and meta-analysis. Can. J. Kidney Health Dis. 2015;2:13. doi: 10.1186/s40697-015-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Correa Barcellos F., Pereira Nunes B., Jorge Valle L., Lopes T., Orlando B., Scherer C., et al. Comparative effectiveness of 30 % trisodium citrate and heparin lock solution in preventing infection and dysfunction of hemodialysis catheters: a randomized controlled trial (CITRIM trial) Infection. 2017;45(2):139–145. doi: 10.1007/s15010-016-0929-4. [DOI] [PubMed] [Google Scholar]
- 32.Quenot J.P., Helms J., Bourredjem A., Dargent A., Meziani F., Badie J., et al. Trisodium citrate 4% versus heparin as a catheter lock for non-tunneled hemodialysis catheters in critically ill patients: a multicenter, randomized clinical trial. Ann. Intensive Care. 2019;9(1):75. doi: 10.1186/s13613-019-0553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardinal P., Allan J., Pham B., Hindmarsh T., Jones G., Delisle S. The effect of sodium citrate in arterial catheters on acid-base and electrolyte measurements. Crit. Care Med. 2000;28(5):1388–1392. doi: 10.1097/00003246-200005000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Polaschegg H.D., Sodemann K. Risks related to catheter locking solutions containing concentrated citrate. Nephrol. Dial. Transplant. 2003;18(12):2688–2690. doi: 10.1093/ndt/gfg481. [DOI] [PubMed] [Google Scholar]
- 35.Cosca P.A., Smith S., Chatfield S., Meleason A., Muir C.A., Nerantzis S., et al. Reinfusion of discard blood from venous access devices. Oncol. Nurs. Forum. 1998;25(6):1073–1076. [PubMed] [Google Scholar]
- 36.Hinds P.S., Wentz T., Hughes W., Pearson T., Sims A., Mason B., et al. An investigation of the safety of the blood reinfusion step used with tunneled venous access devices in children with cancer. J. Pediatr. Oncol. Nurs. 1991;8(4):159–164. doi: 10.1177/104345429100800403. [DOI] [PubMed] [Google Scholar]
- 37.McBride C., Miller-Hoover S., Proudfoot J.A. A standard push-pull protocol for waste-free sampling in the pediatric intensive care unit. J. Infusion Nurs. 2018;41(3):189–197. doi: 10.1097/NAN.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request to the corresponding author.