Abstract
Pancreatobiliary intraductal papillary neoplasms (IPNs) represent precursors of pancreatic cancer or bile duct cholangiocarcinoma that can be detected and treated. Despite advances in diagnostic methods, identifying these premalignant lesions is still challenging for treatment providers. Modern imaging, biomarkers and molecular tests for genomic alterations can be used for diagnosis and follow-up. Surgical intervention in combination with new chemotherapeutic agents is considered the optimal treatment for malignant cases. The balance between the risk of malignancy and any risk of resection guides management policy; therefore, treatment should be individualized based on a meticulous preoperative assessment of high-risk stigmata. IPN of the bile duct is more aggressive; thus, early diagnosis and surgery are crucial. The conservative management of low-risk pancreatic branch-duct lesions is safe and effective.
Keywords: Biliary tree diseases, Pancreatic cystic neoplasms, Biliary tract neoplasms, Extrahepatic cholangiocarcinoma, Pancreatic adenocarcinoma
Core Tip: The balance between overlooking a potential malignancy and the outcomes of a high-risk major operation should be accounted for in the decision-making process of the therapeutic plan. Despite the use of modern diagnostic modalities, overtreatment may occur in many patients; thus, the correct management of pancreatobiliary intraductal papillary neoplasms (IPNs) must be individualized. The proper management of pancreatobiliary IPNs is based on a precise preoperative diagnosis that correctly evaluates the defined high-risk stigmata and worrisome features.
TO THE EDITOR
We read the paper by Mocchegiani et al[1] with great interest, and we would like to congratulate the authors for their very nice work on intraductal papillary neoplasm of the bile duct (IPNB), which is an updated impressive approach. This neoplasm resembles the pancreatic intraductal papillary mucinous neoplasm (IPMN). Taking this opportunity, we will make some considerable comments on pancreatobiliary intraductal papillary neoplasms since both IPNB and pancreatic IPMN have a common genomic background, corresponding manifestations and several similarities; however, peculiarities and some differences exist in their biological behavior and subsequent management. IPMN was first described by Ohashi et al[2] in 1982 as a different entity from mucinous cystic neoplasms and cancer and is considered a premalignant lesion of pancreatic ductal adenocarcinoma[3]. However, IPNB is rare, less common than IPMN, and more aggressive since it can progress to cholangiocarcinoma[4]. Both IPNB and IPMN are characterized by intraductal overproduction of mucin and growth of the papillary epithelium, which results in similar imaging findings[4].
Panceatobiliary intraductal neoplasms include: (1) IPMN pancreatic, IPNB; (2) Intraductal oncocytic papillary neoplasm (IOPN); and (3) Intraductal tubulopapillary neoplasm[5].
IBNB, first described by Chen et al[6] in 2001, is a slow-growing precancerous lesion that evolves into carcinoma[1,7,8]. The other precursor lesion of invasive cholangiocarcinoma, an aggressive disease with poor outcomes, is biliary intraepithelial neoplasia[7,9]. The mucin produced may cause transient ductal obstruction manifested by recurrent episodes of acute cholangitis, obstructive jaundice and bile duct dilatation[8,10]. IPNB must be considered when a patient presents with such a clinical situation without common bile duct gallstones. Early diagnosis and proper management of this precancerous lesion are important for preventing a dismal disease course and improving long-term oncological outcomes[4].
IPNB has histopathological features and genetic substrates, i.e., gene mutations, similar to those of pancreatic IPMN. IPNB and IPMN usually constitute distinct entities with separate development. However, rare cases of simultaneous coexistence or even metachronic tract occurrences after initial surgical resection, which are rarer, have been reported[11]. Additionally, metachronic development of another new lesion may occur after curative intervention, but the development of a new lesion in the bile duct is less common than that in the pancreatic remnant[12].
The involved mutations included mutations in the Tp16, TP53, KRAS, GNAS, BRAF, SMAD4, STK11 CTNNB1, PIK3CA, RNF43, APC, CTNNB1, ZNRF3, CDKNZA, BRCA 1 and BRCA 2 genes[1,13,14]. There is an association between KRAS and GNAS gene mutations in IPNNs and between the PRKACA and PRKACB genes in IOPNs, which influences oncocytic tumorigenesis and morphology and may lead to therapeutic targets[13].
IPNB represents 5%-15% of relatively rare bile duct neoplasms and is found mainly in East Asia, particularly in elderly individuals older than 67 years[8,10,14,15]. These tumors develop throughout the intrahepatic (type 1) and extrahepatic (type 2) biliary tree[8,14]. Type 2 tumors are more common than type 1 tumors and have a worse prognosis. Magnetic resonance imaging (MRI)-magnetic resonance cholangiopancreatography (MRCP) features may be valuable in distinguishing between the two types of lesions and evaluating the risk of malignancy[15]. These tumors may be adenomas, borderline neoplasias, in situ carcinomas with regular overgrowth, or tubular mucinous adenocarcinomas with irregular overgrowth[1]. High peritumoral and intratumoral budding may be prognostic factors for worse outcomes in patients with extrahepatic distal cholangiocarcinoma[16].
Extensive radical surgical resection is the management method of choice for surgically fit patients with IPNB. Depending on the location, hepatectomy, pancreatoduodenectomy or radical common bile duct resection can be performed[10].
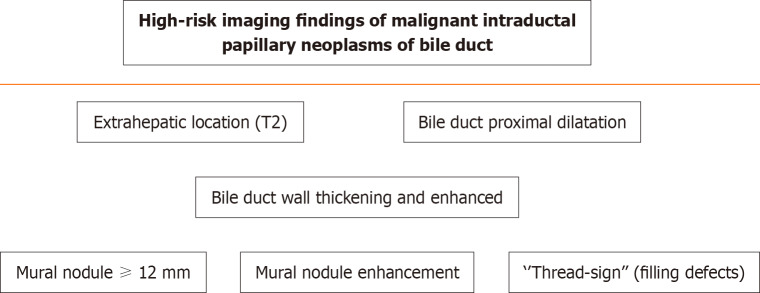
A recent European multicenter study showed a median postoperative survival of 5.7 years and a 5-year overall survival of 63%[17]. In unfit patients, novel endoscopic resection[1], endoscopic radiofrequency ablation or photodynamic therapy can be performed[8]. High-risk imaging findings and strong indications for surgery included a mural nodule more than 12 mm in length and mural nodule enhancement[1]. They are shown in Figure 1[1,4].
Figure 1.
Scheme of Magnetic resonance imaging-cholangiopancreatography indications for malignant intraductal papillary neoplasm of the bile duct (bile duct).
Pancreatic IPMNs represent approximately 1% of all pancreatic neoplasms and usually cause recurrent episodes of acute pancreatitis, which can lead to pancreatic dysfunction but may also be asymptomatic. The biological behavior of these tumors ranges from benign to malignant according to the type. The majority of these tumors do not progress to invasive pancreatic carcinoma. There are three types of lesions: Main-duct (MD)-IPMNs, branch-duct (BD)-IPMNs and mixed IPMNs[3]. Both age and metabolic syndrome increase the occurrence of IPMNs[18]. Acute pancreatitis predicts malignancy and constitutes an indication for pancreatectomy[19]. High-risk stigmata and worrisome features may predict malignant transformation in clinical practice and determine management policy, as shown in Table 1[3,20].
Table 1.
High-risk stigmata and worrisome features of malignant pancreatic intraductal papillary mucinous neoplasms
|
High-risk stigmata
|
Worrisome features
|
| Dilated main pancreatic duct ≥ 10 mm | Cyst size 3 ≥ cm |
| Enhanced solid mural nodule 5 ≥ mm | Thickened and enhanced cyst wall |
| Obstructive jaundice | Abrupt dilatation of the main pancreatic duct 5-9 mm |
| Distal atrophy of the pancreas | |
| Lymph node involvement |
Improvements in diagnostic modalities have led to a continual increase in the incidence of IPNB[6]. MRI is the main imaging tool used[4,8,15]. These lesions are intraductal masses accompanied by proximal dilatation and occasionally distal dilatation. The “thread sign” shown in MRCP corresponds to filling defects due to mucin hypersecretion[4].
The first-line modern imaging techniques include contrast-enhanced ultrasound (US), MRI-MRCP and multidetector helical computed tomography, followed by endoscopic US (EUS)[8,21,22]. Additionally, EUS may provide guided needle biopsy[21].
Tumor metabolic activity was detected by positron emission tomography (PET) using 18FDG-PET[8] or the novel 68Ga-labeled fibroblast activation protein inhibitors-PET[23].
Peroral cholangioscopy[24] or pancreatoscopy[25] can directly visualize ducts to aid in diagnosing neoplastic lesions. Additionally, intraoperative pancreatoscopy[26] or even robotic pancreatectomy[27] can assist in determining the extent of pancreatectomy.
The serum elastase-1 concentration[28] and carbohydrate antigen 19-9 concentration or pancreatic juice cytology[29] may predict malignancy. Liquid biopsy may assist in determining malignancy by detecting cancer cells or molecular parts in the blood[30].
For the vast majority of MD-IPMNs and mixed IPMNs, surgery is needed. BD-IPMNs without high-risk stigmata have a low possibility of malignancy; thus, conservative management with long-term imaging surveillance is appropriate[31-34].
After curative resection, IPNB malignancies exhibit a better prognosis than original cholangiocarcinomas[8], and IPMNs exhibit a better prognosis than pancreatic ductal adenocarcinomas[35]; however, the recurrence rate is up to 27% for IPNB[15] and up to 43% for IPMN[36]. Thus, regular follow-up is mandatory for early recurrence detection and reoperation in the pancreatic remnant[37].
In conclusion, surgery is the cornerstone of management for patients at high risk for potential malignancies, particularly bile duct IPNB and pancreatic main duct IPMN. Long-term follow-up ensures early detection of recurrence. Conservative management and surveillance are indicated for patients with low-risk pancreatic branch duct IPMNs. However, management must be individualized to avoid overtreatment or overlooking a malignancy.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 2, 2024
First decision: January 17, 2024
Article in press: February 26, 2024
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Mikulic D, Croatia S-Editor: Li L L-Editor: A P-Editor: Zheng XM
Contributor Information
Efstathios T Pavlidis, 2nd Propedeutic Department of Surgery, Hippokration General Hospital, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece.
Ioannis N Galanis, 2nd Propedeutic Department of Surgery, Hippokration General Hospital, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece.
Theodoros E Pavlidis, 2nd Propedeutic Department of Surgery, Hippokration General Hospital, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki 54642, Greece. pavlidth@auth.gr.
References
- 1.Mocchegiani F, Vincenzi P, Conte G, Nicolini D, Rossi R, Cacciaguerra AB, Vivarelli M. Intraductal papillary neoplasm of the bile duct: The new frontier of biliary pathology. World J Gastroenterol. 2023;29:5361–5373. doi: 10.3748/wjg.v29.i38.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohashi K, Murakami Y, Murayama M, Takekoshi T, Ohta H, Ohashi I. Four cases of mucus secreting pancreatic cancer. Prog Dig Endosc. 1982;20:348–351. [Google Scholar]
- 3.Pavlidis ET, Sapalidis KG, Pavlidis TE. Modern aspects of the management of pancreatic intraductal papillary mucinous neoplasms: a narrative review. Rom J Morphol Embryol. 2022;63:491–502. doi: 10.47162/RJME.63.3.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraus M, Klang E, Soffer S, Inbar Y, Konen E, Sobeh T, Apter S. MRI features of intraductal papillary mucinous neoplasm of the bile ducts, "The myth about the cyst": A systematic review. Eur J Radiol Open. 2023;11:100515. doi: 10.1016/j.ejro.2023.100515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanuma Y, Sato Y, Kakuda Y, Naito Y, Fukumura Y, Fukushima M, Minato H, Aishima S, Ohike N, Furukawa T. Interobserver agreement of pathologic classification and grading of tumoral intraductal pre-invasive neoplasms of the bile duct. Ann Diagn Pathol. 2024;69:152247. doi: 10.1016/j.anndiagpath.2023.152247. [DOI] [PubMed] [Google Scholar]
- 6.Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, Chiu CT, Kuo TT, Kamiya J, Oda K, Hamaguchi M, Ohno Y, Hsieh LL, Nimura Y. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651–658. doi: 10.1053/jhep.2001.28199. [DOI] [PubMed] [Google Scholar]
- 7.Nagashima D, Esaki M, Nara S, Ban D, Takamoto T, Mizui T, Shimada K, Hiraoka N. Novel insights into the intraepithelial spread of extrahepatic cholangiocarcinoma: clinicopathological study of 382 cases on extrahepatic cholangiocarcinoma. Front Oncol. 2023;13:1216097. doi: 10.3389/fonc.2023.1216097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JR, Jang KT, Jang JY. Intraductal papillary neoplasm of the bile duct: review of updated clinicopathological and imaging characteristics. Br J Surg. 2023;110:1229–1240. doi: 10.1093/bjs/znad202. [DOI] [PubMed] [Google Scholar]
- 9.Nakanuma Y, Sugino T, Kakuda Y, Nomura Y, Watanabe H, Terada T, Sato Y, Ohnishi Y, Fukumura Y. Pathological survey of precursor lesions in cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2023;30:893–903. doi: 10.1002/jhbp.1308. [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Li B, Zheng C. Clinicopathologic characteristics and long-term prognosis of intraductal papillary neoplasm of the bile duct: a retrospective study. Eur J Med Res. 2023;28:132. doi: 10.1186/s40001-023-01102-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao G, Xia T, Mou YP, Zhou YC. Reoperation for heterochronic intraductal papillary mucinous neoplasm of the pancreas after bile duct neoplasm resection: A case report. World J Gastrointest Surg. 2023;15:1542–1548. doi: 10.4240/wjgs.v15.i7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito T, Hisa T, Ito Y, Kudo A, Yamada T, Osera S, Tomori A, Fukushima H, Aoyagi D, Shiozawa S. Intraductal papillary neoplasm of the bile duct with metachronous development in the downstream bile duct after radical resection. Clin J Gastroenterol. 2024;17:155–163. doi: 10.1007/s12328-023-01867-x. [DOI] [PubMed] [Google Scholar]
- 13.Itoh T, Omori Y, Seino M, Hirose K, Date F, Ono Y, Mizukami Y, Aoki S, Ishida M, Mizuma M, Morikawa T, Higuchi R, Honda G, Okamura Y, Kinoshita K, Unno M, Furukawa T. Gene Rearrangement and Expression of PRKACA and PRKACB Govern Morphobiology of Pancreatobiliary Oncocytic Neoplasms. Mod Pathol. 2024;37:100358. doi: 10.1016/j.modpat.2023.100358. [DOI] [PubMed] [Google Scholar]
- 14.Wu RS, Liao WJ, Ma JS, Wang JK, Wu LQ, Hou P. Epidemiology and outcome of individuals with intraductal papillary neoplasms of the bile duct. World J Gastrointest Oncol. 2023;15:843–858. doi: 10.4251/wjgo.v15.i5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon SK, Lee JM, Yoo J, Park S, Joo I, Yoon JH, Lee KB. Intraductal papillary neoplasm of the bile duct: diagnostic value of MRI features in differentiating pathologic subclassifications-type 1 vs type 2. Eur Radiol. 2023 doi: 10.1007/s00330-023-10491-9. [DOI] [PubMed] [Google Scholar]
- 16.Jun SY, Hong SM, An S. Prognostic Significance of Intratumoral and Peritumoral Budding in Distal Extrahepatic Bile Duct Carcinoma. Pathobiology. 2023 doi: 10.1159/000535847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lluνs N, Serradilla-Martνn M, Achalandabaso M, Jehaes F, Dasari BVM, Mambrilla-Herrero S, Sparrelid E, Balakrishnan A, Hoogwater FJH, Amaral MJ, Andersson B, Berrevoet F, Doussot A, Lσpez-Lσpez V, Alsammani M, Detry O, Domingo-Del Pozo C, Machairas N, Pekli D, Alcαzar-Lσpez CF, Asbun H, Bjφrnsson B, Christophides T, Dνez-Caballero A, Francart D, Noel CB, Sousa-Silva D, Toledo-Martνnez E, Tzimas GN, Yaqub S, Cauchy F, Prieto-Calvo M, D'Souza MA, Spiers HVM, van den Heuvel MC, Charco R, Lesurtel M, Ramia JM. Intraductal papillary neoplasms of the bile duct: a European retrospective multicenter observational study (EUR-IPNB study) Int J Surg. 2023;109:760–771. doi: 10.1097/JS9.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka S, Tsujimae M, Masuda A, Inoue J, Inomata N, Uemura H, Kohashi S, Nagao K, Masuda S, Abe S, Gonda M, Yamakawa K, Ashina S, Nakano R, Tanaka T, Yamada Y, Sakai A, Kobayashi T, Shiomi H, Fujita K, Anami T, Fujita T, Watanabe A, Kodama Y. Metabolic Syndrome Accelerates the Age-Related Increase of Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas. 2024;53:e9–e15. doi: 10.1097/MPA.0000000000002267. [DOI] [PubMed] [Google Scholar]
- 19.Xu JH, Ni CY, Zhuang YY, Li L, Lin Y, Xia ZS, Wu WR, Chen QK, Zhong W. Acute pancreatitis in intraductal papillary mucinous neoplasm: a single-center retrospective cohort study with systematic review and meta-analysis. BMC Gastroenterol. 2023;23:424. doi: 10.1186/s12876-023-02972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazami Y, Arita J, Nishioka Y, Kawaguchi Y, Ichida A, Ishizawa T, Akamatsu N, Kaneko J, Nakai Y, Koike K, Hasegawa K. Preoperative Predictive Features of Invasive Carcinoma Among Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas. 2022;51:642–648. doi: 10.1097/MPA.0000000000002078. [DOI] [PubMed] [Google Scholar]
- 21.Conti Bellocchi MC, Manfrin E, Brillo A, Bernardoni L, Lisotti A, Fusaroli P, Parisi A, Sina S, Facciorusso A, Gabbrielli A, Crinς SF. Rare Pancreatic/Peripancreatic Cystic Lesions Can Be Accurately Characterized by EUS with Through-the-Needle Biopsy-A Unique Pictorial Essay with Clinical and Histopathological Correlations. Diagnostics (Basel) 2023;13 doi: 10.3390/diagnostics13243663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minelli C, Balducci F, Cavalleri C, Milanetto AC, Ferrara F, Crimμ F, Quaia E, Vernuccio F. Intraductal papillary mucinous neoplasms of the pancreas: Uncommon imaging presentation, evolution and comparison of guidelines. Eur J Radiol Open. 2023;11:100531. doi: 10.1016/j.ejro.2023.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang M, Spektor AM, Hielscher T, Hoppner J, Glatting FM, Bicu F, Hackert T, Heger U, Pausch T, Gutjahr E, Rathke H, Giesel FL, Kratochwil C, Tjaden C, Haberkorn U, Rφhrich M. Static and Dynamic (68)Ga-FAPI PET/CT for the Detection of Malignant Transformation of Intraductal Papillary Mucinous Neoplasia of the Pancreas. J Nucl Med. 2023;64:244–251. doi: 10.2967/jnumed.122.264361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koiwai A, Hirota M, Murakami K, Katayama T, Kin R, Endo K, Kogure T, Takasu A, Sakurai H, Kondo N, Takami K, Yamamoto K, Katayose Y, Satoh K. Direct peroral cholangioscopy with red dichromatic imaging 3 detected the perihilar margin of superficial papillary extension in a patient with intraductal papillary neoplasm of the bile duct. DEN Open. 2023;3:e228. doi: 10.1002/deo2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarita MAT, Sakai A, Tsujimae M, Kobayashi T, Masuda A, Kanzawa M, Toyama H, Kodama Y. Use of Peroral Pancreatoscopy in the Diagnosis of Elusive Intraductal Papillary Mucinous Neoplasm With High-Grade Dysplasia. ACG Case Rep J. 2023;10:e01165. doi: 10.14309/crj.0000000000001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciprani D, Frampton A, Amar H, Oppong K, Pandanaboyana S, Aroori S. The role of intraoperative pancreatoscopy in the surgical management of intraductal papillary mucinous neoplasms of the pancreas: a systematic scoping review. Surg Endosc. 2023;37:9043–9051. doi: 10.1007/s00464-023-10518-8. [DOI] [PubMed] [Google Scholar]
- 27.Fong ZV, Zwart MJW, Gorris M, Voermans RP, van Wanrooij RLJ, Wielenga T, Del Chiaro M, Arnelo U, Daams F, Busch OR, Besselink MG. Intraoperative Pancreatoscopy During Robotic Pancreatoduodenectomy and Robotic Distal Pancreatectomy for Intraductal Papillary Mucinous Neoplasm with Involvement of the Main Pancreatic Duct. Ann Surg Open. 2023;4:e283. doi: 10.1097/AS9.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishima T, Takano S, Takayashiki T, Kuboki S, Suzuki D, Sakai N, Hosokawa I, Konishi T, Nishino H, Nakada S, Kouchi Y, Kishimoto T, Ohtsuka M. Serum elastase-1 predicts malignancy in intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2024;24:93–99. doi: 10.1016/j.pan.2023.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Nagayama R, Ueki T, Shimizu Y, Hijioka S, Nakamura M, Kitano M, Hara K, Masamune A, Kin T, Hanada K, Koshita S, Yamada R, Takenaka M, Itoi T, Yanagisawa A, Otuka T, Hirono S, Kanno A, Ideno N, Kuwahara T, Shimizu A, Kamata K, Asai Y, Takeyama Y. Is preoperative pancreatic juice cytology useful for determining therapeutic strategies for patients with intraductal papillary mucinous neoplasm of the pancreas? J Hepatobiliary Pancreat Sci. 2023 doi: 10.1002/jhbp.1394. [DOI] [PubMed] [Google Scholar]
- 30.Kuvendjiska J, Mόller F, Bronsert P, Timme-Bronsert S, Fichtner-Feigl S, Kulemann B. Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasm of the Pancreas. Life (Basel) 2023;13 doi: 10.3390/life13071570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferronato M, Lizzio CE, Berardinelli D, Marini D, Elia E, Andreetto L, Trentini A, Potenza MC, Serra C, Mazzotta E, Ricci C, Casadei R, Migliori M. Abdominal ultrasound in the characterization of branch-duct intraductal papillary mucinous neoplasms: A new tool for surveillance of low-risk patients? Dig Liver Dis. 2023 doi: 10.1016/j.dld.2023.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Hesse F, Ritter J, Hapfelmeier A, Braren R, Phillip V. Comparison of Magnetic Resonance Imaging and Endoscopic Ultrasound in the Sizing of Intraductal Papillary Mucinous Neoplasia of the Pancreas. Pancreas. 2023;52:e315–e320. doi: 10.1097/MPA.0000000000002264. [DOI] [PubMed] [Google Scholar]
- 33.Lattimore CM, Kane WJ, Subbarao S, Venitti C, Cramer CL, Turkheimer LM, Bauer TW, Turrentine FE, Zaydfudim VM. Long-term surveillance of branch-duct intraductal papillary mucinous neoplasms without worrisome or high-risk features. J Surg Oncol. 2023;128:1087–1094. doi: 10.1002/jso.27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng H, Dou W, Pan Y. The Surveillance for Presumed BD-IPMN of the Pancreas. Gastroenterology. 2023 doi: 10.1053/j.gastro.2023.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Holmberg M, Linder S, Kordes M, Liljefors M, Ghorbani P, Lφhr JM, Sparrelid E. Impact of spatio-temporal recurrence pattern on overall survival for invasive intraductal papillary mucinous neoplasia - A comparison with pancreatic ductal adenocarcinoma. Pancreatology. 2022;22:598–607. doi: 10.1016/j.pan.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Habib JR, Kinny-Kφster B, Amini N, Shoucair S, Cameron JL, Thompson ED, Fishman EK, Hruban RH, Javed AA, He J, Wolfgang CL. Predictors, Patterns, and Timing of Recurrence Provide Insight into the Disease Biology of Invasive Carcinomas Arising in Association with Intraductal Papillary Mucinous Neoplasms. J Gastrointest Surg. 2022;26:2311–2320. doi: 10.1007/s11605-022-05428-4. [DOI] [PubMed] [Google Scholar]
- 37.Fuji T, Umeda Y, Takagi K, Yoshida R, Yoshida K, Yasui K, Matsumoto K, Kato H, Yagi T, Fujiwara T. Optimal surveillance of intraductal papillary mucinous neoplasms of the pancreas focusing on remnant pancreas recurrence after surgical resection. BMC Cancer. 2022;22:588. doi: 10.1186/s12885-022-09650-w. [DOI] [PMC free article] [PubMed] [Google Scholar]