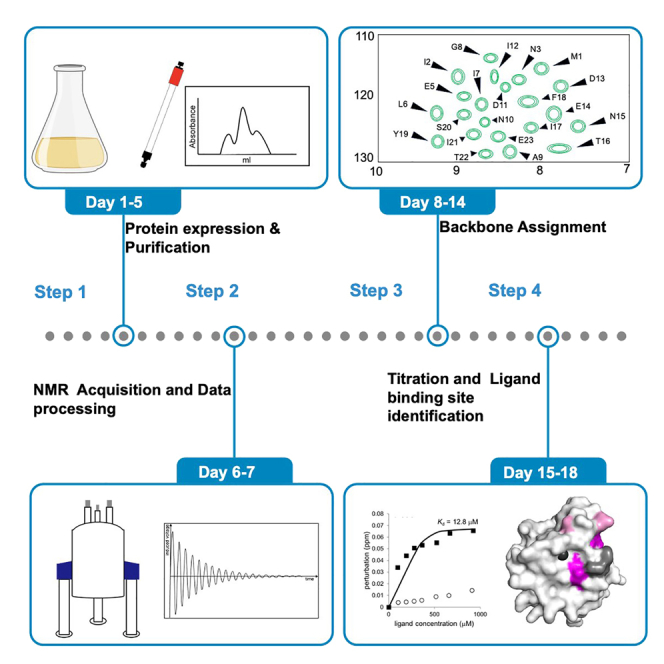
Summary
Mincle (macrophage-inducible C-type lectin, CLEC4E) is a C-type lectin immune-stimulatory receptor that can be targeted for inducing potent adjuvant effects. Mincle can recognize trehalose dimycolate and related glycolipids. Here, we present a protocol to identify the ligand binding mode of Mincle. We describe steps for preparing labeled Mincle ectodomain, data acquisition, and analysis of nuclear magnetic resonance experiments using non-detergent sulfobetaine-195. This protocol can be applied to other protein-ligand interactions that have aggregation problems for complex formation.
For complete details on the use and execution of this protocol, please refer to Furukawa et al.1
Subject areas: Surface plasmon resonance, SPR, Protein Biochemistry, Protein expression and purification, NMR
Graphical abstract
Highlights
-
•
Steps for preparing protein sample for NMR analysis
-
•
The protocol to prevent protein aggregation using NDSB-195
-
•
Steps for determining the dissociation constant between protein and ligands
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Mincle (macrophage-inducible C-type lectin, CLEC4E) is a C-type lectin immune-stimulatory receptor that can be targeted for inducing potent adjuvant effects. Mincle can recognize trehalose dimycolate and related glycolipids. Here, we present a protocol to identify the ligand binding mode of Mincle. We describe steps for preparing labeled Mincle ectodomain, data acquisition, and analysis of nuclear magnetic resonance experiments using non-detergent sulfobetaine-195. This protocol can be applied to other protein-ligand interactions that have aggregation problems for complex formation.
Before you begin
The protocol describes here is the detail steps for determining glycolipid binding sites of Mincle using solution NMR spectroscopy. Notably, this protocol using NDSB-195 can be used for the analysis of interactions between proteins and other small molecules, especially, if interactions cause aggregation.
In this protocol, we first described the procedure for expressing and purifying 13C/15N uniformly labeled Mincle protein. We further described the data acquisition and analysis of NMR spectra. We also described the procedure to perform backbone assignments of Mincle, to trace cross-peak movements in ligand titration experiments for determining interacting surfaces, and to map the binding site onto 3D structure of Mincle.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Competent cells BL21-Gold (DE3) | Stratagene | CAT# 230132, Lot# 0770198 |
| Rosetta2 (DE3) | Novagen | Cat# 71397 |
| Chemicals, peptides, and recombinant proteins | ||
| Triton X-100 | Nacalai Tesque | Cat# 35501-15 |
| CHL medium (X10) (15N: −99%) | SI Science | N/A |
| CHL medium (X10) (13C,15N: −97%) | SI Science | N/A |
| Guanidine-HCl | FUJIFILM Wako | Product # 074-05005, Lot # CAL1442 |
| 2-(N-morpholino)ethanesulfonic acid (MES) | Dojindo Laboratories | Product # GB12, Lot # JU070 |
| NaCl | FUJIFILM Wako | Product # 197-01667, Lot# DLR1126 |
| EDTA | Dojindo Laboratories | Product #N001, Lot # LB153 |
| Dithiothreitol | FUJIFILM Wako | Product # 042-29222, Lot # LKQ2881 |
| 2-Amino-2-hydroxymethyl-1,3-propanediol (Tris-HCl) | FUJIFILM Wako | Product # 203-06277, Lot # SKF7764 |
| L-arginine | FUJIFILM Wako | Product # 280-56551, Lot # LEQ1994 |
| CaCl2 | FUJIFILM Wako | Product # 039-00475, Lot# HWH7483 |
| D(+)-Glucose | FUJIFILM Wako | Product # 049-31165, Lot# DCN6677 |
| 15NH4Cl | Sigma-Aldrich | Product # 299251 Lot# TA1296V |
| MnCl2·4H2O | Nacalai Tesque | Product # 21211-45, Lot# M9E4837 |
| MgSO4·7H2O | FUJIFILM Wako | Product # 131-00405, Lot# SER2697 |
| FeCl3·6H2O | FUJIFILM Wako | Product # 091-00872, Lot# WKH5575 |
| D-biotin | MP Biomedicals | Product # 101023, Lot# 3570J |
| Thiamine | FUJIFILM Wako | Product # 203-00851, Lot# PDN2668 |
| Cytidine | Tokyo Chemical Industry | Product # 2C0522, Lot # 5PVOG-K0 |
| Guanosine | FUJIFILM Wako | Product # 079-01111, Lot # DCE1994 |
| Adenosine | Nacalai Tesque | Product # 01994-54, Lot# M2K1414 |
| Thymidine | Nacalai Tesque | Product # 07147-61, Lot #M2E8546 |
| KH2PO4 | FUJIFILM Wako | Product # 169-04245, Lot# SAF5755 |
| Na2HPO4·12H2O | FUJIFILM Wako | Product # 196-02835, Lot# SKF4981 |
| Cystamine | FUJIFILM Wako | Product # 030-17632, Lot # PTF0078 |
| Cysteamine | FUJIFILM Wako | Product # 032-19392, Lot # CAH4591 |
| HEPES | Dojindo Laboratories | Product # GB10, Lot # JJ052 |
| NDSB-195 | FUJIFILM Wako | Product # 148-08831, Lot # WDM6790 |
| Tetracycline | FUJIFILM Wako | Product # 205-08591, Lot# TSF0373 |
| Ampicillin | FUJIFILM Wako | Product # 014-23302, Lot# PTG5411 |
| Trehalose-C10 | Dojindo Laboratories | Product #T460, Lot# EX006 |
| Trehalose | FUJIFILM Wako | Product # 209-14165, Lot# STH0357 |
| Glycerol | FUJIFILM Wako | Product # 075-00616, Lot# SKF4281 |
| D2O | ISOTEC | Product # 151882, Lot# TA2195V |
| Deposited data | ||
| Backbone resonances of I99K Mincle | This study | BMRB:50217 |
| Recombinant DNA | ||
| pET22b-Mincle I99K | Furukawa et al.2 | N/A |
| Software and algorithms | ||
| NMRpipe | Delaglio et al.3 | N/A |
| Sparky | N/A | https://www.cgl.ucsf.edu/home/sparky/ |
| PyMOL | N/A | http://www.pymol.org/pymol |
| Other | ||
| HiLoad 26/60 Superdex 75 pg | Cytiva (GE Healthcare) | Product # 17-1070-01 |
| Superdex 10/300 GL | Cytiva (GE Healthcare) | Product # 17-5174-01 |
| 5-mm microcell NMR tube | Shigemi | Product # BMS-005B |
| 5 mm thin wall precision NMR sample tube 8″ L, 600 MHz | Wilmad LabGlass | Product #1050-54611 |
| Pasteur pipette | Hilgenberg | Product # 31-501-66 |
| Diaphragm pump | Iwaki | Product # FTP-20A |
| Sonicator (SONIFIER 250 and SONIFIER 450) | Branson | N/A |
| Amicon Ultra Ultracel-3 regenerated cellulose membrane, 4 mL sample volume | Merck | Product # UFC800324 |
| 0.22 μm filter | Merck | Product # SLGVR33RB |
| Incubator shaker | Eppendorf | Product # M1335-0015 |
Materials and equipment
General stock solution
-
•
1 M Tris-HCl (pH 8.0) buffer: add 121.14 g of Tris-HCl in 900 mL ddH2O, adjust the pH to 8.0, and finally fill up to 1 L. The buffer can be stored at 25°C for 1 month.
-
•
5 M NaCl: add 292.5 g of NaCl in 900 mL ddH2O, dissolve completely, and then, fill up to 1 L. The buffer can be stored at 25°C for one year.
-
•
1 M MES (pH 6.5) buffer: add 19.5 g of 2-(N-morpholino)ethanesulfonic acid (MES) in 90 mL ddH2O, adjust the pH to 6.5 and finally fill up to 100 mL. The buffer should be stored with the protection from the light and can be kept at 25°C for one year.
-
•
0.5 M EDTA (pH 8.0): add 93.06 g of Ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA) in 400 mL ddH2O, adjust the pH to 8.0 and finally fill up to 500 mL. The buffer can be kept at 25°C for one year.
-
•
1 M CaCl2 : add 110.98 g of NaCl in 900 mL ddH2O, dissolve completely, and then, fill up to 1 L. The buffer can be stored at 25°C for one year.
-
•
50 mM CaCl2: Mix 5 mL of 1 M CaCl2 buffer and 95 mL ddH2O. The buffer can be stored at 25°C for one year.
-
•
10 mM FeCl3: add 270 mg of FeCl3·6H2O in 90 mL ddH2O, dissolve completely, and then, fill up to 100 mL. The buffer can be stored at 25°C for one year.
-
•
1 M MgSO4: add 24.65 g of NaCl in 90 mL ddH2O, dissolve completely, and then, fill up to 100 L. The buffer can be stored at 25°C for one year.
-
•
50 mM MnCl2: add 990 mg of NaCl in 90 mL ddH2O, dissolve completely, and then, fill up to 100 mL. The buffer can be stored at 25°C for one year.
M9 medium
Prepare M9 medium by the mixture of s 100 mL of Solution A, 880 mL of Solution B, 20 mL of Solution C, 2 mL of 50 mM CaCl2 solution and 1 mL of glycerol. The M9 minimal medium can be stored at 4°C for 1 week. Tap water contains trace amounts of minerals, such as metal ions. Therefore, tap water in Solutions A and C can be used to enhance E. coli growth in minimum medium.
[Solution A] The buffer should be autoclaved after the preparation
| Reagent | Final concentration | Amount |
|---|---|---|
| KH2PO4 | 22 mM | 3 g |
| Na2HPO4 | 49 mM | 6 g |
| NaCl | 8.6 mM | 0.5 g |
| Tap water | Up to 100 mL | |
| Total | 100 mL |
[Solution B] The buffer should be autoclaved after the preparation
| Reagent | Final concentration | Amount |
|---|---|---|
| 10 mM FeCl3 | 56 μM | 5.0 mL |
| 1 M MgSO4 | 2.3 mM | 2.0 mL |
| 50 mM MnCl2 | 56 μM | 1.0 mL |
| Thiamine Hydrochloride | 270 μM | 80 mg |
| Adenosine | 8.5 μM | 2 mg |
| Thiamine | 8.6 μM | 2 mg |
| Guanosine | 8.0 μM | 2 mg |
| Cytosine | 20 μM | 2 mg |
| D-biotin | 9.3 μM | 2 mg |
| Deionized Water | 872 mL | |
| Total | 880 mL |
[Solution C] The buffer should be filtered with 0.2 μm filter after the preparation
| Reagent | Final concentration | Amount |
|---|---|---|
| 15NH4Cl or14NH4Cl | 1.8–1.9 M | 2 g |
| 13C Glucose or12C Glucose | 2.8 M | 10 g |
| Tap Water | Up to 20 mL | |
| Total | 20 mL |
[Suspension buffer] This buffer can be stored at 25°C for one month
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M Tris-buffer (pH8.0) | 50 mM | 50 mL |
| 5 M NaCl | 100 mM | 20 mL |
| Deionized Water | 930 mL | |
| Total | 1000 mL |
[Triton buffer] This buffer can be stored at 25°C for one month
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M Tris-buffer (pH 8.0) | 50 mM | 50 mL |
| 5 M NaCl | 100 mM | 20 mL |
| Triton X-100 | 0.5% | 5 mL |
| Deionized Water | 925 mL | |
| Total | 1000 mL |
[Solubilization buffer] The buffer should be stored with protection from light and kept at 25°C for one year
| Reagent | Final concentration | Amount |
|---|---|---|
| Guanidine | 6 M | 57.3 g |
| 1 M MES (pH 6.5) | 50 mM | 5 mL |
| 5 M NaCl | 100 mM | 2 mL |
| 0.5 M EDTA (pH 8.0) | 10 mM | 2 mL |
Adjust the pH of the buffer to 6.5 at 25°C. Add Milli-Q water up to 100 mL.
[Refolding buffer] This buffer should be used immediately after the preparation
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M tris-buffer (pH8.0) | 100 mM | 20 mL |
| L-arginine | 1 M | 174.2 g |
| 1 M CaCl2 buffer | 20 mM | 20 mL |
| Cystamine | 3.73 mM | 840 mg |
| Cysteamine | 6.73 mM | 720 mg |
| Deionized Water | Mess up to 1000 mL | |
| Total | 1000 mL |
[Gel filtration buffer] This buffer can be stored at 25°C for one month
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 M tris-buffer (pH8.0) | 20 mM | 20 mL |
| 5 M NaCl | 100 mM | 20 mL |
| 1 M CaCl2 buffer | 20 mM | 20 mL |
| Deionized Water | 940 mL | |
| Total | 1000 mL |
Step-by-step method details
Production of the Mincle protein for NMR measurements
Timing: 1–5 days
-
1.Transform Escherichia coli using the pET22b plasmid encoding the human Mincle ectodomain (residues 74-219).
-
a.Grow Transformed Rosetta2 (DE3) strain chemically competent cells on LB agar plates containing both 100 μg/mL ampicillin and 20 μg/mL chloramphenicol.
-
b.Grow BL21-Gold (DE3) strain and grown on LB agar plates containing both 100 μg/mL ampicillin.
-
a.
-
2.
Inoculate some colonies into 10 mL of 2xYT medium containing 100 μg/mL ampicillin and 20 μg/mL chloramphenicol (only in case of Rosetta2 (DE3)) at 37°C with shaking at 200 rpm, 14–18 h.
-
3.
Pour Rosetta2 (DE3) strain culture medium into 1 L of CHL of 15N, or 15N and 13C labeled medium containing 100 μg/mL ampicillin and 20 μg/mL chloramphenicol or BL21-Gold (DE3) into M9 medium containing 100 μg/mL ampicillin in a 2 L plastic Flask and continue growing cells in an Incubator Shaker at 37°C with shaking at 200 rpm until the optical density of the culture medium reaches from 0.4 to 0.6 at an absorbance of 600 nm.
-
4.
Add 1 mL of 1.0 M Isopropyl β-d-1-thiogalactopyranoside (IPTG) and incubate at 37°C with shaking for 4–6 h.
-
5.
Harvest cells by centrifugation at 5,000 rpm (4,424 × g) at 4°C for 10 min and discard supernatant.
Pause point: The E. coli pellet can be stored at −80°C for at least one year.
-
6.Purification of inclusion body.
-
a.Suspend E. coli pellet with 30 mL of the suspension buffer and sonicate it using SONIFIER 250 (Branson) with output control as 5 and duty cycle of 50% for 20 min.
-
b.Centrifuge the sonicated solution with 8590 × g at 4°C for 15 min.
-
c.Wash the pellet with the triton buffer.
-
d.Further, wash the pellet with the Triton buffer three times.
-
a.
-
7.Solubilization of the denature Mincle protein.
-
a.Add 6 mL of the solubilization buffer to the pellet obtained at step 6 and rotate overnight.
-
b.On the following day, centrifuge the solution with 8590 ×g at 4°C for 15 min. Preserve the supernatant as the inclusion body solubilized solution.
-
a.
Pause point: The inclusion body can be stored at −80°C for at least one year.
-
8.Refolding and purification of Mincle protein.
-
a.One hour after the addition of dithiothreitol (10 mM), 100 mg of the solubilized proteins were slowly diluted into 1000 mL of a refolding buffer solution.
-
b.The refolding mixture was purified by gel filtration chromatography with HiLoad 26/60 Superdex 75 pg and subsequently with Superdex 10/300 GL (Cytiva, Tokyo, Japan). Finally, 9.6 mg of the Mincle protein was obtained.
-
a.
-
9.
Change the buffer of Mincle protein at a concentration to 10 mM HEPES-NaOH (pH 7.0) containing 20 mM CaCl2, 0.5 or 0 M NDSB-195 by amicon Ultra.
-
10.
Adjust the volume of protein to 360 μL and add 40 μL of D2O (final D2O concentration becomes to 10%).
-
11.
Transfer protein solution to a 5-mm microcell NMR tube (Shigemi, Tokyo, Japan).
-
12.
Degas dissolved air in the tube using the diaphragm pump (IWAKI GLASS, FTP-20A).
-
13.
Insert the inner tube and remove air bubbles from the sample portion.
NMR measurement using topspin
Timing: 6–7 days
-
14.
Here, a Bruker 800 MHz AVANCE NEO NMR system equipped with a cryogenic triple resonance probe, CPTCI was used.
-
15.
Click topspin icon on NMR instrument and open the topspin-gui.
-
16.
Enter ‘ej’ command in topspin-gui terminal to float the NMR sample tube with sample-holder and enter the ‘ij’ to load the NMR sample tube on to the probe.
-
17.
Create a new experimental directory using the command ‘new’ and load the experimental parameter, ZGPR. Set up the sample temperature of the sample to 298 K using edte panel (command: edte) on topspin-gui and equilibrate the temperature of the sample for 5 min.
-
18.
Use the commands “lock H2O + D2O”, “atma” and “topshim rga shigemi full” for frequency lock, tune and shimming, respectively. Notes: Details are described in the TopSpin manuals.
-
19.
Calibrate the 90˚ pulse width for 1 H (90˚ hard pulse) for the sample using a command ‘pulsecal’.
-
20.
Start the measurement by command ‘zgefp’. The obtained spectrum confirms the suppression of the water signal.
-
21.
Create a new experiment directory to be carried over for this calibrated experiment. (using the command ‘new’ with check the ‘Use current experiment’).
-
22.
Load the Bruker pulse sequences for 2D sofast HN-HMQC experiments using the following commands, ‘sofast’.
-
23.
Notes: The NMR instrument used introduced the sofast pulse program packages described in the following paper. Therefore, The ‘sofast’ command automatically optimizes the parameters required for a sofast experiment from the calibrated experiment.4
-
24.
Set the number of integration times (Number of scan) by command ‘ns’.
-
25.
Execute the measurement by command ‘zg’.
-
26.
After the measurement is completed, use the ‘xfb’ command to perform a 2D Fourier transform to check the spectrum.
NMR data processing
Timing: 6–7 days
Prerequisite: NMRPipe and NMRdraw,3 and Sparky.5
-
27.
Perform Fourier transform by command ‘convft.com’.
-
28.
Open the NMRdraw program by command ‘nmrDaw’.
-
29.
Open file. File name is should be “xxx.ft”.
-
30.
Command ‘h’ and one dimensional spectrum will be appeared. Change the value of p0 to make the downward signal disappear. Check the p0 value and close NMRdraw.
-
31.
Command ‘gedit convft.com’ under the directory where convft.com locates.
-
32.
Change the -p0 value of -fn sol (For example, if p0 value above is −65 and original p0 value is −153, it should be changed to −218).
-
33.
Command ‘wq’.
-
34.
Perform Fourier transform again by command ‘convft.com’.
-
35.
Execute the NMRdraw by command ‘nmrdraw’.
-
36.
Open file.
-
37.
Check the phase.
-
38.
Close NMRdRaw.
-
39.
Command ‘pipe2ucsf xxx.ft yyy.ucsf’ to convert from NMRdraw format spectrum file to Sparky format file.
Backbone assignment
Timing: 8–14 days
-
40.
Execute the Sparky by command ‘sparky’.
-
41.
The basic Sparky commands were referred to the following web page https://www.cgl.ucsf.edu/home/sparky/manual/index.html.
-
42.
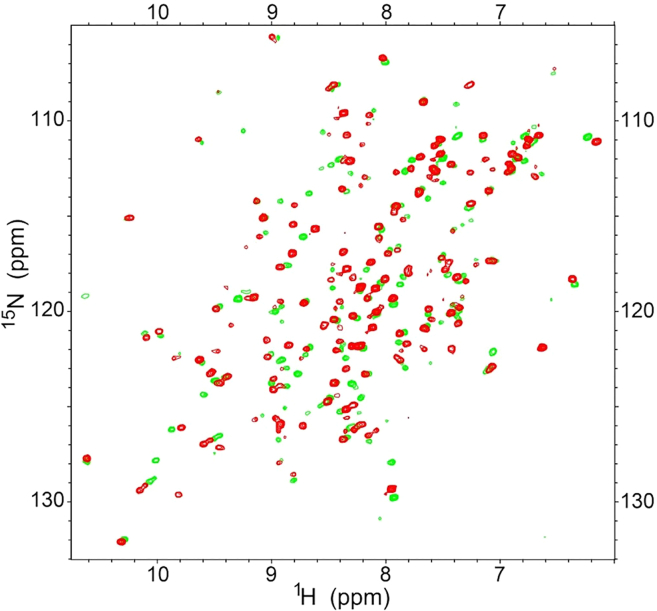
Open all ucsf files (Figure 1).
-
43.
Synchronize the N axis of [1H-15N] HSQC with the N axis of CBCA(CO)NH, HNCACB, and HNCA by command yt. Similarly, the 1H axis is also synchronized.
-
44.
Tentative numbers are assigned to the signals observed in [1H-15N] HSQC. Here, MCn (n is a unique number) is used.
-
45.
Peak pick for the entire [1H-15N] HSQC spectrum.
-
46.
Select each peak from the peak list and assign a tentative assignment, where 'Group' is MCn for both the 15N and 1H axes, and 'Atom' is N and HN, respectively.
-
47.
Perform assignment on the signals observed in the HNCA that are derived from each of the tentatively assigned [1H-15N] HSQC signals.
-
48.
The 'Group' name on the 13C axis is MCn and the Atom name is CA. If a signal derived from one previous residue is observed, the group name is attributed as MCn-1.
-
49.
Next, we perform the attribution operation for CBCA(CO)NH, where the Group name for the 13C axis is MCn-1, and if the signal is from Cβ, the Atom name is CB.
-
50.
Finally, the attribution operation is performed for HNCACB, where the group name on the 13C axis is MCn, but if it is from one residue earlier, MCn-1 is used.
-
51.
Search for the combination of MCn and MCn-1 linked by the obtained chemical shift value information of CA.
-
52.
If there are multiple candidate linkages, the chemical shift values of CB are also used to determine the linkage combination.
-
53.
Combining multiple chain combinations, a cluster of chains is constructed.
-
54.
For each signal that constitutes a cluster, search the amino acid sequence for the part of the sequence that is consistent with the chemical shift values of CA and CB derived from each residue.
-
55.
If the corresponding amino acid sequence part is the only one, change the tentative assignment to the original assignment. For example, if it is Lys131, the Group is renamed to K131.
Figure 1.
The HSQC spectrum of Mincle without (green peaks) and with NDSB-195 (red peaks)
Titration analysis of ligands and observation of chemical shift perturbation (CSP)
Timing: 15–18 days
-
56.
Change the buffer of Mincle protein to 10 mM HEPES-NaOH (pH 7.0) containing 20 mM CaCl2, 0.5 or 0 M NDSB-195 by amicon Ultra.
-
57.
Adjust the volume of protein to 360 μL.
-
58.
Add 40 μL of D2O (final D2O concentration becomes to 10%).
-
59.
Transfer protein solution to 5 mm Thin Wall Precision NMR Sample Tube 8″ L, 600 MHz (Wilmad LabGlass).
-
60.
Prepare a 10-fold higher concentration of ligands, such as trehalose C10 and trehalose against Mincle protein. Here, we used trehalose C10 and trehalose as the examples. If the protein concentration is 0.45 mM, the concentration of trehalose C10 should be 4.5 mM.
-
61.
Measure NMR spectrum in the same procedure as the section of “NMR measurement using top spin” (This spectrum is the spectrum of the ligand concentration of zero equivalent).
-
62.
Transfer the protein solution to a 1.5 mL tube using a Pasteur pipette. Add 10 μL of trehalose C10 and trehalose to the protein solution and mix well (This solution is equivalent to 0.25 equivalent). Return the mixture to NMR tube and start the acquisition.
-
63.
After the acquisition, transfer the protein solution to a 1.5 mL tube using a Pasteur pipette. Add 10 μL of trehalose C10 and trehalose to the protein solution and mix well (This solution is equivalent to 0.5 equivalent). Return the mixture to NMR tube and start the acquisition.
-
64.
Continue step 56 until the chemical shift perturbation is not observed.
-
65.
Apply each step of the “NMR Data processing” section to obtained HSQC spectrum.
-
66.
In Sparky, open the obtained spectrums.
-
67.
Command ‘Ct’ to set the counter level. Set the counter level (such as 4.0e+05) to decrease noises and clear peaks from amino acid residues. Set the level as “20” and click the “Apply” button.
-
68.
Overlay spectrums of each titration.
-
69.
Produce the peak lists containing the 1H and 15N coordinates of individual peaks of the spectra.
-
70.
Calculate the observed CSPs in Mincle at each protein/ligand ratio relative to free state using the following equation,
where ΔδNH and ΔδN represent the chemical shift perturbations in the 1H and 15N dimensions, respectively.
-
71.
To determine the dissociation constant (KD), titration curves were fitted to the following equation, valid for 1:1 complex formation in fast exchange on the NMR chemical shift time-scale:
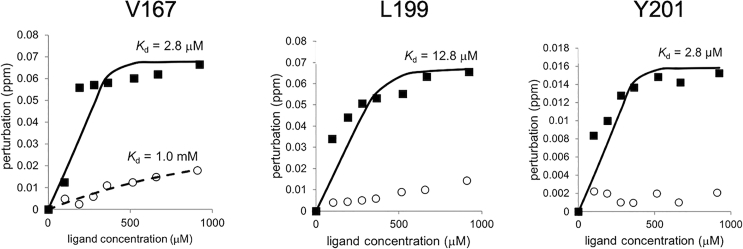
where [L] and [P] represent the ligand and protein concentrations, respectively. Δδ(obs) is the observed chemical shift perturbation at the ligand concentration [L]. Δδmax is the chemical shift perturbation at the saturation of ligand.6 The non-linear curve fitting using the Microsoft Excel 2010 Solver (Washington, USA) was applied for the determination of Δδmax and KD (Figure 2).7
-
72.
In detail, Δδmax and KD are variable parameters. In solver, Δδmax and KD are changed and finally determined to decrease the difference between the calculated and observed Δδobs and Δδobs . Map amino acids that showed chemical shift perturbation to Mincle protein structure using Pymol.
Figure 2.
The chemical perturbation change of Q149, L199, and Y201 of human Mincle by the titration of trehalose C10 and trehalose
The chemical shift perturbation of Q149, L199, and Y201 upon the titration of trehalose C10 (black-filled squares) and trehalose (open circles) were shown. KD was determined by fitting the equation in step 65 against the titration curve of trehalose C10 and trehalose titration to Mincle.
Expected outcomes
By the whole experiment, it is expected to identify the ligand binding site in Mincle and determine the KD value of the interaction with ligands, such as glycolipid1 and glycerolipid.8 This method is also suitable for the identification of the binding site of a drug without the co-crystallization of a protein and a drug. In our study, 1 L cell culture grown in M9 media yields more than 80 mg of Mincle.
Limitations
In Mincle protein, NMR signals of some amino acids near the presumable glycolipids binding site were unobserved due to their flexibility. These unobserved peaks prevented the complete understanding of the ligand binding mechanism. However, in some proteins, peaks are appeared after the ligand binding because the flexibility is decreased.
Troubleshooting
Problem 1
The yield of the inclusion body of the labeled protein is low (step 7).
Potential solution
-
•
Test other E. coli strains. For example, when we used BL21(DE3) with M9 medium, the expression of Mincle from 1 L culture was 3.1 mg, however, using BL21-Gold (DE3) with M9 medium, the expression of Mincle protein was 83.1 mg.
-
•
Use a codon-optimized expression vector.
Problem 2
The final yield (refolding efficiency) is low (step 8).
Potential solution
-
•
Check the purity of inclusion bodies.
-
•
Modify the ratio of cytamine and cysteamine.
-
•
Add EDTA (such as 0.5 mM), dithiothreitol (such as 1 mM) and/or CHAPS (such as 10 mM)
Problem 3
Observable peaks are less than the number of amino acid residues (step 51).
Potential solution
-
•
Decrease the temperature to suppress the flexibility of a protein.
-
•
Add the ligand to form the inflexible conformation of a protein.
-
•
Change the pH of the buffer and the concentration of salt.
Problem 4
Doublet peaks are observed (step 51).
Potential solution
-
•
Check if the dimer is formed by gel filtration chromatography.
-
•
Add triton (such as 0.05%) and/or dithiothreitol (such as 1 mM) to prevent aggregation.
-
•
The mixture of Cis and Trans formation of prolines might cause the appearance of double peaks. Make proline to alanine mutant and perform steps from 1 to 38 and check the HSQC spectrum if the mixture of Cis and Trans formation produced the duplication.
Problem 5
Line broadening and disappearance of the peaks were observed during titration. (step 53).
Potential solution
Perform experiments in NDSB-195 containing buffer or detergent-containing buffer, such as 0.1% Triton X-100 or 1% tween-20.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Katsumi Maenaka (maenaka@pharm.hokudai.ac.jp).
Technical contact
Questions about the technical specifics of performing the protocol should be directed to and will be answered by the technical contact, Katsumi Maenaka (maenaka@pharm.hokudai.ac.jp).
Materials availability
All reagents generated in this study will be made available on reasonable request.
Data and code availability
In this protocol, original data and code were not provided.
Acknowledgments
This research was supported by JSPS KAKENHI grant numbers JP18K14633 (to A.F.) and 20H05873 (to K.M.), Akiyama Foundation, Japan Allergy Association (to A.F.), “Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers” (no. S2701) (to K.M.), the Japan Agency for Medical Research and Development (AMED) under grant numbers JP17am0101093 and JP22ama121037 (to K.M.), Hokkaido University, Global Facility Center (GFC), Pharma Science Open Unit (PSOU) (to K.M.), funded by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) under “Support Program for Implementation of New Equipment Sharing System” (to K.M.), and the Collaborative Research Program of the Institute for Protein Research, Osaka University, NMRCR-20-02 (to K.M.).
This work was performed in part using NMR spectrometers with ultra-high magnetic fields at the Institute for Protein Research, Osaka University, and Global Facility Center (GFC) of Hokkaido University. Some related experiments were conducted using facilities of Hokkaido University, Global Facility Center (GFC), and Pharma Science Open Unit (PSOU).
Author contributions
Conceptualization, A.F. and K.M.; methodology, A.F., H.K., T.S., and K.M.; investigation, A.F., H.K., T.S., and K.M.; writing – original draft, A.F., H.K., T.S., and K.M.; writing – review and editing, A.F., H.K., T.S., and K.M.; funding acquisition, A.F. and K.M.; resources, A.F., H.K., T.S., and K.M.; supervision, K.M.
Declaration of interests
The authors declare no competing interests.
References
- 1.Furukawa A., Shuchi Y., Wang J., Guillen-Poza P.A., Ishizuka S., Kagoshima M., Ikeno R., Kumeta H., Yamasaki S., Matsumaru T., et al. Structural basis for plastic glycolipid recognition of the C-type lectin Mincle. Structure. 2023;31:1077–1085.e5. doi: 10.1016/j.str.2023.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa A., Kamishikiryo J., Mori D., Toyonaga K., Okabe Y., Toji A., Kanda R., Miyake Y., Ose T., Yamasaki S., Maenaka K. Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc. Natl. Acad. Sci. USA. 2013;110:17438–17443. doi: 10.1073/pnas.1312649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaglio F., Grzesiek S., Vuister G.W., Zhu G., Pfeifer J., Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 4.Rossi P., Xia Y., Khanra N., Veglia G., Kalodimos C.G. (15)N and (13)C- SOFAST-HMQC editing enhances 3D-NOESY sensitivity in highly deuterated, selectively [(1)H,(13)C]-labeled proteins. J. Biomol. NMR. 2016;66:259–271. doi: 10.1007/s10858-016-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goddard T.D., Kneller D.G. SPARKY3, University of California, San Francisco.
- 6.Fielding L. NMR methods for the determination of protein–ligand dissociation constants. Prog. Nucl. Magn. Reson. Spectrosc. 2007;51:219–242. doi: 10.1016/j.pnmrs.2007.04.001. [DOI] [Google Scholar]
- 7.Kemmer G., Keller S. Nonlinear least-squares data fitting in Excel spreadsheets. Nat. Protoc. 2010;5:267–281. doi: 10.1038/nprot.2009.182. [DOI] [PubMed] [Google Scholar]
- 8.Matsumaru T., Ikeno R., Shuchi Y., Iwamatsu T., Tadokoro T., Yamasaki S., Fujimoto Y., Furukawa A., Maenaka K. Synthesis of glycerolipids containing simple linear acyl chains or aromatic rings and evaluation of their Mincle signaling activity. Chem. Commun. 2019;55:711–714. doi: 10.1039/c8cc07322h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In this protocol, original data and code were not provided.