Abstract
Deep Brain Stimulation (DBS) has become a pivotal therapeutic approach for Parkinson's Disease (PD) and various neuropsychiatric conditions, impacting over 200,000 patients. Despite its widespread application, the intricate mechanisms behind DBS remain a subject of ongoing investigation. This article provides an overview of the current knowledge surrounding the local, circuit, and neurobiochemical effects of DBS, focusing on the subthalamic nucleus (STN) as a key target in PD management.
The local effects of DBS, once thought to mimic a reversible lesion, now reveal a more nuanced interplay with myelinated axons, neurotransmitter release, and the surrounding microenvironment. Circuit effects illuminate the modulation of oscillatory activities within the basal ganglia and emphasize communication between the STN and the primary motor cortex. Neurobiochemical effects, encompassing changes in dopamine levels and epigenetic modifications, add further complexity to the DBS landscape.
Finally, within the context of understanding the mechanisms of DBS in PD, the article highlights the controversial question of whether DBS exerts disease-modifying effects in PD. While preclinical evidence suggests neuroprotective potential, clinical trials such as EARLYSTIM face challenges in assessing long-term disease modification due to enrollment timing and methodology limitations. The discussion underscores the need for robust biomarkers and large-scale prospective trials to conclusively determine DBS's potential as a disease-modifying therapy in PD.
Keywords: Deep brain stimulation, Parkinson’s disease, Neuromodulation, Neuroprotection
Introduction
Present-day deep brain stimulation (DBS) evolved from the observation that high-frequency electrical current delivered to the ventral intermediate nucleus of the thalamus can elicit effects similar to those of ablative procedures [1]. In non-human primates (NHPs) treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), subthalamic nucleus (STN) lesions and high-frequency STN stimulation both ameliorated cardinal symptoms of Parkinson's disease (PD) [2,3], thus leading to human trials followed by regulatory approval of STN DBS [4]. Since its introduction in the late 1980's [5], DBS has been performed in over 200,000 patients, most commonly targeting the STN for PD, but also at other targets and for other disorders, including epilepsy, pain, and several psychiatric conditions [6]. Despite advancements in treatments, indications, and technology, the mechanisms underlying DBS are not fully understood, and are still under investigation [7,8]. This brief review will outline current understandings of local, circuit, and neurobiochemical mechanisms of DBS, focusing on STN DBS, and then discuss current controversies related to whether DBS may offer disease-modifying effects in PD.
Local Effects of DBS
To begin to characterize the local effects exerted by DBS at its target site, several key factors must be considered [9,10], including: (i) whether the target is grey matter (i.e., cell bodies), white matter (i.e., axons), or some combination [11]; (ii) the anatomical make-up of the stimulated structure (i.e., distribution of inputs/outputs) and the functional properties of engaged synapses [12,13]; (iii) the diameter and degree of myelination on any stimulated axons; (iv) the orientation of axons [14]; (v) the distance of stimulation to target [15]; and the local microenvironment around the electrode, including astrocytes and microglia [16].
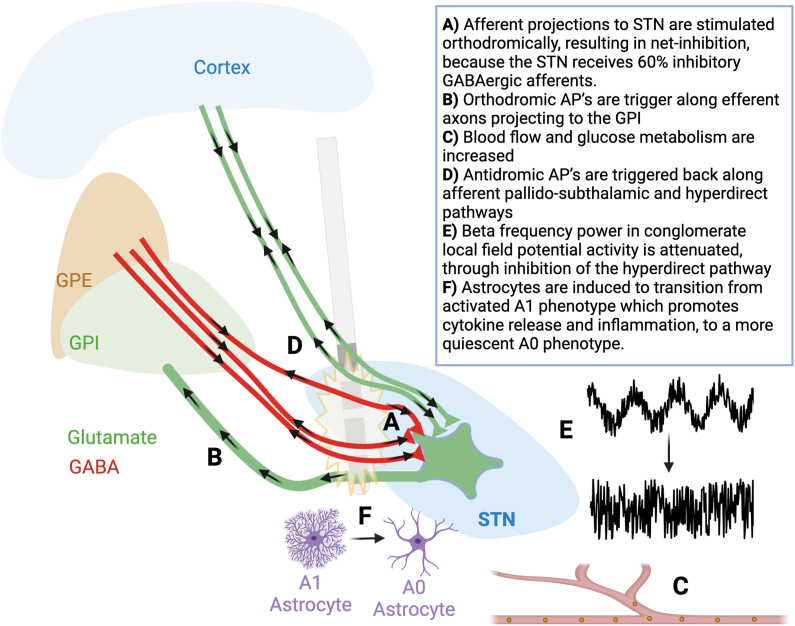
Due to similar clinical effects of stimulation and lesioning, the mechanism of STN DBS was initially thought to be that of a ‘titratable lesion’ [17]. In untreated PD, STN neurons fire with increased frequency, bursting, and synchrony [18,19], and DBS reduces intrinsic STN neuronal firing [[20], [21], [22]], largely through stimulation of afferent axons terminating within the STN (Fig. 1A).
Fig. 1.
Schematic illustration of the multiple and varied mechanisms by which DBS can act, shown in the context of STN DBS. AP: action potential; STN: subthalamic nucleus; GPi: globus pallidus interna; GPe: globus pallidus externa; GABAergic: gamma amino butyric acid releasing neurons. Figure inspired from figure 3 in ref 12, and figures 2 and 3 from ref 8.
Using concepts such as rheobase and chronaxie, which refer to the amplitude and duration of current required to elicit an action potential (AP), respectively, it has been shown that DBS acts preferentially on large myelinated axons [[23], [24], [25]]. In the case of high-frequency DBS (>100 Hz), with stimulation delivered at more than twice the intrinsic firing rate of a neuron, APs are generated on nearby axons, including DBS target afferent (input) and efferent (output) fibers. These APs can propagate both orthodromically (downstream, toward the terminal synapse) and antidromically (upstream, back towards the afferent cell body) (Fig. 1A and B) [26].
In the example of subthalamic nucleus (STN) DBS, both afferent and efferent axons are likely to be stimulated, triggering bidirectional APs in both axon populations [[27], [28], [29]]. Antidromic effects on STN afferent fibers can block the input of naturally generated information from upstream neurons (e.g., cortical or pallidal inputs), whereas antidromic effects on the STN efferent fibers can block the output of information from the STN. Optogenetic studies in pre-clinical PD models suggest that antidromic effects on the cortical-STN (hyperdirect) pathway may be sufficient to alleviate the cardinal features of PD (Fig. 1B) [30].
The acute effects of stimulation on a grey matter target are dependent on the proportion of inhibitory/excitatory inputs [13]. The STN receives approximately 60% inhibitory GABAergic afferents (mostly from the globus pallidus), and 40% excitatory glutamatergic afferents (mostly from the cortex via the hyperdirect pathway) [31]. A single pulse of electrical stimulation, triggers release of neurotransmitters from these afferents, eliciting a weak net inhibition of the STN neuronal cell bodies (Fig. 1A) [13]. With prolonged high frequency stimulation, neural activity of the STN neurons continues to be silenced by mechanisms including decreased synaptic potentiation and synaptic depletion [12,13]; this phenomenon is also seen at other targets, such as the ventral intermedius (ViM), with chronic stimulation, despite having a high proportion of excitatory inputs [12,13]. Perhaps the surest indication that STN DBS does not act solely through local inhibition of STN neurons, is that stimulation drives downstream globus pallidus activity through activation of STN glutamatergic efferents (Fig. 1C) [32,33]. Further evidence that STN DBS acts through more complex mechanisms than simply local suppression of STN neuron firing, is the fact that blood flow and glucose metabolism are elevated by stimulation (Fig. 1D) [[34], [35], [36], [37]].
Demonstration of orthodromic and antidromic effects on STN afferent and efferent fibers gave rise to the so-called “axon-soma decoupling” theory of DBS [38]. Orthodromic effects on the STN afferent fibers however can result in the release of neurotransmitter at local STN synapses (i.e., gamma aminobutyric acid [GABA] from the globus pallidus externa [GPe], or glutamate from cortex), whereas orthodromic effects on the STN efferent fibers can produce downstream glutamate release at target structures. Thus, an important consideration is the reliability of information transfer, which depends on the functional properties of engaged synapses (i.e., short term plasticity). To this end, cortical inputs to STN have been shown to depress rapidly, whereas GPe inputs and STN outputs are able to maintain neurotransmitter release even with a high rate of activation, effectively uncoupling the cortex from basal ganglia circuitry [39,40].
Circuit Effects of DBS
In its early years, DBS was heralded as a method for producing a reversible and titratable lesion through a depolarization blockade [1,11]. Today, it is understood that DBS acts through far more complex and varied mechanisms, with the prevailing theory being that DBS disrupts aberrant oscillatory activities, or ‘circuitopathies’, allowing circuits to normalize to a less pathological state [41,42]. In 2004, Grill introduced the term “informational lesion” in describing how DBS delivers therapeutic effects in disease states [43], and subsequent research has strengthened this assertion of a neuro-restorative mechanism [44].
Deep brain structures, particularly those in the basal ganglia, are linked together as part of multi-nodal circuits, which transmit information through changes in temporal-alignment of neuronal oscillatory activity. Oscillations refer to rhythmic fluctuations in local field potential (LFP), driven by nested oscillators, which reflect a conglomerate of local neural activity [7]. Oscillations can occur across a range of frequencies (delta: 1–4 Hz, theta: 4–7 Hz, alpha: 7–13 Hz, beta: 13–35 Hz, gamma: 35–80 Hz), and are conserved across species [45]. Increased power in the theta and beta range LFP's is thought to be anti-kinetic, helping to maintain a physiologic status quo, whereas increased gamma-range power is pro-kinetic and seen during movement [11,46,47]. In PD (pre-clinical models and in humans), bradykinesia and rigidity are linked to pathological elevations in beta power throughout the basal ganglia, which persists through movements [48,49]. The extent of beta-power elevation scales directly with the severity of symptoms [50]. Levodopa-induced-dyskinesia is associated with heightened gamma power, and the oscillations contributing to tremor remain more difficult to parse out [48,49,51,52].
PD involves a state of exaggerated synchrony, and therefore it follows that high-frequency (i.e., 130 Hz) DBS could disrupt this synchrony to alleviate cardinal symptoms, while very-low frequency (i.e., 10 Hz) can actually increase pathological synchrony and worsen symptoms [53]. Intriguingly, stimulation at 60 Hz (which many refer to as ‘low frequency stimulation’) may offer superior relief of freezing of gait and other axial symptoms compared to traditional high-frequency stimulation [54,55]. Theories for the different therapeutic effect of 60 Hz stimulation include reduced current spread to the nearby pedunculopontine nucleus area, or alternatively, by boosting prokinetic gamma oscillations [56].
Early work in the field demonstrated that both levodopa and DBS reduce pathological beta activity in the acute setting [48,49]. More recently, by using dual recording/stimulating DBS systems, it has been demonstrated that clinically-effective stimulation lowers beta activity in a dose-dependent manner (Fig. 1E) [57]. Within the beta range, it appears that low-beta (13–20 Hz) frequency is most closely associated with motor symptoms in PD [[58], [59], [60]], whereas coherence between the STN and cortex is mediated by high-beta (20–35 Hz) [[61], [62], [63]] which is transmitted through the hyperdirect pathway [64]. Another process of information transfer through the cortical-striatal-thalamic motor circuit involves fluctuations in amplitude at a higher frequency band driving oscillations at a lower frequency, a phenomenon called phase-amplitude-coupling (PAC) [65]. Mounting evidence suggests that elevated beta power is linked to fluctuations in the amplitude of gamma oscillations in the primary motor cortex (M1) [65,66]. In addition to mitigating exaggerated low-beta power, STN DBS also reduces PAC between the STN and M1 [67,68]. It should be noted that treating beta oscillations is not a silver-bullet for PD, as not all patients show clear beta pathology, and furthermore, beta rhythms may fluctuate in consistency during various states, such as sleep [69]. Other neural signatures, such as evoked resonant neural activity (ERNA) show promise for being a complimentary biomarker in PD [70,71].
Studies examining the placement of electrodes in relation to clinical response support the notion that DBS acts through circuit-wide modulation. In STN DBS, the strongest response is seen in patients when stimulation is delivered to regions of the STN structurally connected to the supplementary motor area and functionally anti-correlated to M1 [72]. STN DBS acts on brain-wide circuits, and with chronic clinically-effective stimulation, circuit activity begins to more closely resemble healthy controls [44,73]. All told, DBS in PD exerts a multitude of local effects, which effectively isolate a nucleus from a structural and functional neurocircuit (i.e., the dorsolateral STN being isolated from the cortical-basal ganglia-thalamic-cortical motor circuit), and promotes re-emergence of normal circuit activity.
Neurobiochemical Effects of DBS
STN DBS increases striatal extracellular dopamine levels in rodent PD models both during active stimulation and for extended periods of time following cessation of stimulation [74,75]. Despite these reports from animal models, positron emission tomography (PET) studies of humans undergoing STN DBS have not consistently demonstrated increases in striatal dopamine [[76], [77], [78]]. Possible reasons for this discrepancy include the fact that humans with PD already have extensive loss of nigrostriatal dopaminergic neurons, the inability of PET to pick up on subtle changes, or the fact that the small unmyelinated dopaminergic neurons are more difficult to stimulate compared to large myelinated fibers [79].
As suggested above, the activation of inputs and outputs can give rise to neurotransmitter release. In STN, this results in persistent activation and release of GABA at the level of STN [39,40] and glutamate at downstream structures [32,80]. Activation of similar circuitry may also be associated with globus pallidus interna (GPi) DBS [81,82], which perhaps gives rise to similarities in the functional cortical network activation response [83]. With other targets, such as the nucleus accumbens or the ventromedial prefrontal cortex (rat homologue to the subcallosal cingulate cortex), other neurotransmitters such as noradrenaline and serotonin have been found to be increased at efferently-connected structures [84,85].
Even the mere insertion of an electrode triggers a cascade of neurocytochemical events, including microgliosis/astrocytosis, inflammation, trophic factor release (brain-derived neurotrophic factor [BDNF] and granulocyte-colony-stimulating factor), and depending on the target, possibly even neurogenesis [86]. These changes are most dynamic and pronounced in the first 2–4 weeks after implantation, which is part of the rationale for waiting several weeks after electrode placement before initiating stimulation in clinical settings [87].
Astrocytes, as part of the “tripartite synapse”, may also play a key role in driving DBS effects [88]. In response to electrical stimulation, astrocytes may release adenosine and glutamate, and through activation of calcium influx via voltage-gated calcium (Ca2+) channels, demonstrate waves of Ca2+ currents propagating away from the source of stimulation [89,90]. DBS can trigger astrocytes to release extracellular matrix proteins such as insulin growth factor 1 (IGF1), which may offer protection against excitotoxicity [91,92]. In PD, astrocytes are prone to adopt an activated ‘A1’ phenotype, to play a deleterious role in promoting cytokine release, inflammation, and microglial activation. High frequency DBS appears to shift astrocytes from an activated A1 to a more quiescent A0 phenotype, through inhibition of the transcriptional factors like nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Fig. 1F) [93]. The overall effects of astrocytes remain to be fully understood, as there is evidence that they can either counteract or perpetuate the inactivating effect of DBS on a grey matter structure [89,90,94].
DBS also appears to exert epigenetic effects. PD is noted to impair transcriptional regulatory processes, which can be quantified with DNA methylation markers and microRNA analyses on peripheral blood samples [[95], [96], [97]]. PD patients treated with STN DBS have marked reversals in these microRNA and DNA methylation markers to the point of more closely resembling healthy controls [[95], [96], [97]]. However, these results must be interpreted with caution due to small sample size and the possibility that analysis of peripheral blood may not be representative of what is occurring in the brain at the site of stimulation.
Does DBS Exert Disease-Modifying Effects in PD?
There are two powerful, symptomatic treatments available for PD: dopamine replacement and DBS [98]. Both treatments have revolutionized the care of patients with PD, dramatically improving motor performance, quality of life, and the natural history of PD. The positive effects on the natural history raise the question as to whether they in fact reduce the neurodegenerative process and thereby have disease-modifying properties. In the case of levodopa and dopamine agonists, randomized-controlled trials (RCTs) comparing early versus later initiation of pharmacotherapy demonstrated that overall disease progression is unchanged between groups, refuting their disease modifying effects [99,100]. DBS may be more challenging to evaluate for disease-modifying effects because it is offered when the disease is already quite advanced – on average at least 7 years following onset of symptoms, and after at least 1.5 years of motor fluctuations or levodopa-induced-dyskinesia [101,102] and thus when substantial loss of dopaminergic neurons has already occurred [103].
The question of whether DBS can alter the neurodegenerative process in PD is especially intriguing given the extent of pre-clinical evidence revealing that DBS promotes neurogenesis, synaptogenesis, and neuroprotection [104]. STN DBS results in increased survival of dopaminergic neurons in toxin-mediated PD models in rodents [[105], [106], [107]] and non-human primates [108]. Increased survival of dopaminergic neurons with active STN DBS was also shown in an α-synuclein overexpressing rodent model [109,110]. A leading theory to explain this neuroprotective effect is that DBS induces the release of BDNF, which binds to topomyosin-related kinase type 2 (trkB) receptors. BDNF and trkB promote survival and plasticity within the nigrostriatal pathway [[111], [112], [113]]. Other theories to explain the survival of dopaminergic cells in pre-clinical models with STN DBS include reduced excitotoxicity and increased dendritic spine density [114,115].
There were high hopes that the EARLYSTIM trial - which randomized 251 patients with PD and early motor complications to early DBS versus continued medical management - would shed light on whether STN DBS can alter the course of PD [101]. However, the active-stimulation group experienced significant motor and quality of life improvements, while the sham-stimulation arm remained stable over 24 months, making it impossible to assess for a disease-modifying effect (at least through clinical scores) [101]. Longer-term outcomes from the EARLYSTIM trial have not yet been published, and these may help uncover if earlier DBS can modify the trajectory of PD, but even for this trial, the average enrollment was 7.5 years after diagnosis and thus may prove inconclusive. A group at Vanderbilt went one step further than EARLYSTIM, performing a 30-patient RCT which implanted patients at a much earlier timepoint than EARLYSTIM - prior to the onset of motor complications [116]. The Vanderbilt group found that there may be some signal for improved control of rest tremor and reduction of medications [116]; however, due to small numbers and other limitations, it was not possible to assess for disease-modifying effects [[116], [117], [118]]. Furthermore, there are important concerns about considering patients with early PD, prior to onset of motor or medication-related complications, for a neurosurgical procedure [119], including but not limited to the need for an early biomarker for the diagnosis of PD.
Arguing against a disease-modifying effect are the often-reported rapid DBS withdrawal effects from accidental cessation of stimulation, resulting in emergence of akinetic-rigid symptoms [120]. Compared to patients treated only with medications, PD symptoms seem to progress at a similar rate in patients with chronic STN DBS [121]. Also arguing against disease-modifying DBS effects is a PET biomarker study showing no change in 18F-fluorodopa between DBS and non-DBS patients [122], as well as 2 post-mortem studies [123,124]. Standard MRI sequences and even PET using dopamine markers may not be powered to detect subtle reductions in cell death. Similar arguments exist for treatments in Alzheimer disease [125] and as such much investigation is still needed in both the preclinical and clinical space to understand if there is a role for DBS to be a disease-modifying therapy. This will require development of robust and sensitive biomarkers that allow for the early diagnosis of PD and for tracking disease progression.
DBS is an effective symptomatic treatment in PD and growing list of other indications. Its mechanisms of action are still being studied, but several decades of research have converged on the theory that DBS can relatively inactivate and isolate a grey-matter target, blocking pathologic oscillations from engaging that structure. Relatedly, the fact that APs are more readily generated in large myelinated neurons results in DBS driving activity along white matter tracts to modulate widespread circuits. Based on animal literature, robust long-term improvements in human RCTs, and the circuit-modifying effects of DBS, there is a reason to suspect that DBS may have disease-modifying properties, but decisive evidence in humans is lacking. Addressing this question will require a large prospective trial, which includes careful preoperative stratification of PD patients, early DBS intervention compared with a large matched control group, and sensitive biomarkers including structural/metabolic imaging tools [9,126].
Authors’ Contributions
Benjamin Davidson made significant contributions to conceptualization, data curation, methodology, validation, visualization, writing (original draft) and writing (review/editing).
Luka Milosevic made significant contributions to conceptualization, data curation, methodology, validation, visualization, writing (original draft) and writing (review/editing).
Ghazaleh Darmani made significant contributions to conceptualization, data curation, methodology, validation, visualization, writing (original draft) and writing (review/editing).
Laura Kondrataviucute made significant contributions to conceptualization, data curation, methodology, validation, visualization, writing (original draft) and writing (review/editing).
Lorraine Kalia made significant contributions to conceptualization, methodology, project administration, resources, supervision, writing (original draft) and writing (review/editing).
Suneil Kalia made significant contributions to conceptualization, methodology, project administration, resources, supervision, writing (original draft) and writing (review/editing).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Benabid A.L., Pollak P., Louveau A., Henry S., de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 2.Bergman H., Wichmann T., DeLong M.R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 3.Benazzouz A., Gross C., Feger J., Boraud T., Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382–389. doi: 10.1111/j.1460-9568.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- 4.Limousin P., Pollak P., Benazzouz A., Hoffmann D., Le Bas J.F., Broussolle E., et al. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 5.Benabid A.L., Pollak P., Hommel M., Gaio J.M., de Rougemont J., Perret J. Treatment of Parkinson tremor by chronic stimulation of the ventral intermediate nucleus of the thalamus] Rev Neurol (Paris) 1989;145:320–323. [PubMed] [Google Scholar]
- 6.Lozano A.M., Lipsman N., Bergman H., Brown P., Chabardes S., Chang J.W., et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15:148–160. doi: 10.1038/s41582-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrington T.M., Cheng J.J., Eskandar E.N. Mechanisms of deep brain stimulation. J Neurophysiol. 2016;115:19–38. doi: 10.1152/jn.00281.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann W.J., Steiner L.A., Milosevic L. Neurophysiological mechanisms of deep brain stimulation across spatiotemporal resolutions. Brain. 2023 doi: 10.1093/brain/awad239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakobs M., Fomenko A., Lozano A.M., Kiening K.L. Cellular, molecular, and clinical mechanisms of action of deep brain stimulation-a systematic review on established indications and outlook on future developments. EMBO Mol Med. 2019;11 doi: 10.15252/emmm.201809575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert Z., Mason X., Sebastian R., Tang A.M., Martin Del Campo-Vera R., Chen K.H., et al. A review of neurophysiological effects and efficiency of waveform parameters in deep brain stimulation. Clin Neurophysiol. 2023;152:93–111. doi: 10.1016/j.clinph.2023.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Udupa K., Chen R. The mechanisms of action of deep brain stimulation and ideas for the future development. Prog Neurobiol. 2015;133:27–49. doi: 10.1016/j.pneurobio.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Milosevic L., Kalia S.K., Hodaie M., Lozano A.M., Popovic M.R., Hutchison W.D., et al. A theoretical framework for the site-specific and frequency-dependent neuronal effects of deep brain stimulation. Brain Stimul. 2021;14:807–821. doi: 10.1016/j.brs.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Milosevic L., Kalia S.K., Hodaie M., Lozano A.M., Fasano A., Popovic M.R., et al. Neuronal inhibition and synaptic plasticity of basal ganglia neurons in Parkinson's disease. Brain. 2018;141:177–190. doi: 10.1093/brain/awx296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rattay F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience. 1999;89:335–346. doi: 10.1016/s0306-4522(98)00330-3. [DOI] [PubMed] [Google Scholar]
- 15.Deniau J.M., Degos B., Bosch C., Maurice N. Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. Eur J Neurosci. 2010;32:1080–1091. doi: 10.1111/j.1460-9568.2010.07413.x. [DOI] [PubMed] [Google Scholar]
- 16.Reddy G.D., Lozano A.M. Postmortem studies of deep brain stimulation for Parkinson's disease: a systematic review of the literature. Cell Tissue Res. 2018;373:287–295. doi: 10.1007/s00441-017-2672-2. [DOI] [PubMed] [Google Scholar]
- 17.Levy R., Lang A.E., Dostrovsky J.O., Pahapill P., Romas J., Saint-Cyr J., et al. Lidocaine and muscimol microinjections in subthalamic nucleus reverse Parkinsonian symptoms. Brain. 2001;124:2105–2118. doi: 10.1093/brain/124.10.2105. [DOI] [PubMed] [Google Scholar]
- 18.Bergman H., Wichmann T., Karmon B., DeLong M.R. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 19.Benazzouz A., Breit S., Koudsie A., Pollak P., Krack P., Benabid A.L. Intraoperative microrecordings of the subthalamic nucleus in Parkinson's disease. Mov Disord. 2002;17(Suppl 3):S145–S149. doi: 10.1002/mds.10156. [DOI] [PubMed] [Google Scholar]
- 20.Salin P., Manrique C., Forni C., Kerkerian-Le Goff L. High-frequency stimulation of the subthalamic nucleus selectively reverses dopamine denervation-induced cellular defects in the output structures of the basal ganglia in the rat. J Neurosci. 2002;22:5137–5148. doi: 10.1523/JNEUROSCI.22-12-05137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magarinos-Ascone C., Pazo J.H., Macadar O., Buno W. High-frequency stimulation of the subthalamic nucleus silences subthalamic neurons: a possible cellular mechanism in Parkinson's disease. Neuroscience. 2002;115:1109–1117. doi: 10.1016/s0306-4522(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 22.Meissner W., Leblois A., Hansel D., Bioulac B., Gross C.E., Benazzouz A., et al. Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain. 2005;128:2372–2382. doi: 10.1093/brain/awh616. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre C.C., Anderson R.W. Deep brain stimulation mechanisms: the control of network activity via neurochemistry modulation. J Neurochem. 2016;139(Suppl 1):338–345. doi: 10.1111/jnc.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holsheimer J., Dijkstra E.A., Demeulemeester H., Nuttin B. Chronaxie calculated from current-duration and voltage-duration data. J Neurosci Methods. 2000;97:45–50. doi: 10.1016/s0165-0270(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 25.Nowak L.G., Bullier J. Axons, but not cell bodies, are activated by electrical stimulation in cortical gray matter. II. Evidence from selective inactivation of cell bodies and axon initial segments. Exp Brain Res. 1998;118:489–500. doi: 10.1007/s002210050305. [DOI] [PubMed] [Google Scholar]
- 26.Li S., Arbuthnott G.W., Jutras M.J., Goldberg J.A., Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- 27.Dostrovsky J.O., Levy R., Wu J.P., Hutchison W.D., Tasker R.R., Lozano A.M. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol. 2000;84:570–574. doi: 10.1152/jn.2000.84.1.570. [DOI] [PubMed] [Google Scholar]
- 28.Anderson T., Hu B., Pittman Q., Kiss Z.H. Mechanisms of deep brain stimulation: an intracellular study in rat thalamus. J Physiol. 2004;559:301–313. doi: 10.1113/jphysiol.2004.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gradinaru V., Mogri M., Thompson K.R., Henderson J.M., Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders T.H., Jaeger D. Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiol Dis. 2016;95:225–237. doi: 10.1016/j.nbd.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parent A., Hazrati L.N. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto T., Elder C.M., Okun M.S., Patrick S.K., Vitek J.L. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefani A., Fedele E., Pierantozzi M., Galati S., Marzetti F., Peppe A., et al. Reduced GABA content in the motor thalamus during effective deep brain stimulation of the subthalamic nucleus. Front Syst Neurosci. 2011;5:17. doi: 10.3389/fnsys.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hershey T., Revilla F.J., Wernle A.R., McGee-Minnich L., Antenor J.V., Videen T.O., et al. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- 35.Payoux P., Remy P., Damier P., Miloudi M., Loubinoux I., Pidoux B., et al. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol. 2004;61:1307–1313. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- 36.Hilker R., Voges J., Weisenbach S., Kalbe E., Burghaus L., Ghaemi M., et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson's disease. J Cerebr Blood Flow Metabol. 2004;24:7–16. doi: 10.1097/01.WCB.0000092831.44769.09. [DOI] [PubMed] [Google Scholar]
- 37.Volonte M.A., Garibotto V., Spagnolo F., Panzacchi A., Picozzi P., Franzin A., et al. Changes in brain glucose metabolism in subthalamic nucleus deep brain stimulation for advanced Parkinson's disease. Parkinsonism Relat Disorders. 2012;18:770–774. doi: 10.1016/j.parkreldis.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 38.McIntyre C.C., Savasta M., Kerkerian-Le Goff L., Vitek J.L. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Steiner L.A., Barreda Tomas F.J., Planert H., Alle H., Vida I., Geiger J.R.P. Connectivity and dynamics underlying synaptic control of the subthalamic nucleus. J Neurosci. 2019;39:2470–2481. doi: 10.1523/JNEUROSCI.1642-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner L.A., Kuhn A.A., Geiger J.R., Alle H., Popovic M.R., Kalia S.K., et al. Persistent synaptic inhibition of the subthalamic nucleus by high frequency stimulation. Brain Stimul. 2022;15:1223–1232. doi: 10.1016/j.brs.2022.08.020. [DOI] [PubMed] [Google Scholar]
- 41.Dostrovsky J.O., Lozano A.M. Mechanisms of deep brain stimulation. Mov Disord. 2002;17(Suppl 3):S63–S68. doi: 10.1002/mds.10143. [DOI] [PubMed] [Google Scholar]
- 42.Lozano A.M., Lipsman N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 2013;77:406–424. doi: 10.1016/j.neuron.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Grill W.M., Snyder A.N., Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 44.Horn A., Wenzel G., Irmen F., Huebl J., Li N., Neumann W.J., et al. Deep brain stimulation induced normalization of the human functional connectome in Parkinson's disease. Brain. 2019 doi: 10.1093/brain/awz239. [DOI] [PubMed] [Google Scholar]
- 45.Buzsaki G., Logothetis N., Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80:751–764. doi: 10.1016/j.neuron.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown P., Williams D. Basal ganglia local field potential activity: character and functional significance in the human. Clin Neurophysiol. 2005;116:2510–2519. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Johnson L., Vitek J. In: 6th ed. Winn R., editor. Vol. 91. Elsevier; New York, NY: 2011. Deep brain stimulation: mechanisms of action; pp. 635–646. (Youmans and Winn neurological surgery). [Google Scholar]
- 48.Kuhn A.A., Doyle L., Pogosyan A., Yarrow K., Kupsch A., Schneider G.H., et al. Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson's disease. Brain. 2006;129:695–706. doi: 10.1093/brain/awh715. [DOI] [PubMed] [Google Scholar]
- 49.Brown P. Bad oscillations in Parkinson's disease. J Neural Transm Suppl. 2006:27–30. doi: 10.1007/978-3-211-45295-0_6. [DOI] [PubMed] [Google Scholar]
- 50.Lofredi R., Okudzhava L., Irmen F., Brucke C., Huebl J., Krauss J.K., et al. Subthalamic beta bursts correlate with dopamine-dependent motor symptoms in 106 Parkinson's patients. NPJ Parkinsons Dis. 2023;9:2. doi: 10.1038/s41531-022-00443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swann N.C., de Hemptinne C., Miocinovic S., Qasim S., Wang S.S., Ziman N., et al. Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in Parkinson's disease. J Neurosci. 2016;36:6445–6458. doi: 10.1523/JNEUROSCI.1128-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherer M., Steiner L.A., Kalia S.K., Hodaie M., Kuhn A.A., Lozano A.M., et al. Single-neuron bursts encode pathological oscillations in subcortical nuclei of patients with Parkinson's disease and essential tremor. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2205881119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timmermann L., Wojtecki L., Gross J., Lehrke R., Voges J., Maarouf M., et al. Ten-Hertz stimulation of subthalamic nucleus deteriorates motor symptoms in Parkinson's disease. Mov Disord. 2004;19:1328–1333. doi: 10.1002/mds.20198. [DOI] [PubMed] [Google Scholar]
- 54.Vijiaratnam N., Girges C., Wirth T., Grover T., Preda F., Tripoliti E., et al. Long-term success of low-frequency subthalamic nucleus stimulation for Parkinson's disease depends on tremor severity and symptom duration. Brain Commun. 2021;3 doi: 10.1093/braincomms/fcab165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie T., Vigil J., MacCracken E., Gasparaitis A., Young J., Kang W., et al. Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology. 2015;84:415–420. doi: 10.1212/WNL.0000000000001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie T., Padmanaban M., Bloom L., MacCracken E., Bertacchi B., Dachman A., et al. Effect of low versus high frequency stimulation on freezing of gait and other axial symptoms in Parkinson patients with bilateral STN DBS: a mini-review. Transl Neurodegener. 2017;6:13. doi: 10.1186/s40035-017-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feldmann L.K., Neumann W.J., Krause P., Lofredi R., Schneider G.H., Kuhn A.A. Subthalamic beta band suppression reflects effective neuromodulation in chronic recordings. Eur J Neurol. 2021;28:2372–2377. doi: 10.1111/ene.14801. [DOI] [PubMed] [Google Scholar]
- 58.Fogelson N., Williams D., Tijssen M., van Bruggen G., Speelman H., Brown P. Different functional loops between cerebral cortex and the subthalmic area in Parkinson's disease. Cerebr Cortex. 2006;16:64–75. doi: 10.1093/cercor/bhi084. [DOI] [PubMed] [Google Scholar]
- 59.Litvak V., Jha A., Eusebio A., Oostenveld R., Foltynie T., Limousin P., et al. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- 60.Little S., Tan H., Anzak A., Pogosyan A., Kuhn A., Brown P. Bilateral functional connectivity of the basal ganglia in patients with Parkinson's disease and its modulation by dopaminergic treatment. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Wijk B.C., Beudel M., Jha A., Oswal A., Foltynie T., Hariz M.I., et al. Subthalamic nucleus phase-amplitude coupling correlates with motor impairment in Parkinson's disease. Clin Neurophysiol. 2016;127:2010–2019. doi: 10.1016/j.clinph.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oswal A., Brown P., Litvak V. Synchronized neural oscillations and the pathophysiology of Parkinson's disease. Curr Opin Neurol. 2013;26:662–670. doi: 10.1097/WCO.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 63.Oswal A., Beudel M., Zrinzo L., Limousin P., Hariz M., Foltynie T., et al. Deep brain stimulation modulates synchrony within spatially and spectrally distinct resting state networks in Parkinson's disease. Brain. 2016;139:1482–1496. doi: 10.1093/brain/aww048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oswal A., Cao C., Yeh C.H., Neumann W.J., Gratwicke J., Akram H., et al. Neural signatures of hyperdirect pathway activity in Parkinson's disease. Nat Commun. 2021;12:5185. doi: 10.1038/s41467-021-25366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Hemptinne C., Ryapolova-Webb E.S., Air E.L., Garcia P.A., Miller K.J., Ojemann J.G., et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimamoto S.A., Ryapolova-Webb E.S., Ostrem J.L., Galifianakis N.B., Miller K.J., Starr P.A. Subthalamic nucleus neurons are synchronized to primary motor cortex local field potentials in Parkinson's disease. J Neurosci. 2013;33:7220–7233. doi: 10.1523/JNEUROSCI.4676-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang A.I., Vanegas N., Lungu C., Zaghloul K.A. Beta-coupled high-frequency activity and beta-locked neuronal spiking in the subthalamic nucleus of Parkinson's disease. J Neurosci. 2014;34:12816–12827. doi: 10.1523/JNEUROSCI.1895-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Hemptinne C., Swann N.C., Ostrem J.L., Ryapolova-Webb E.S., San Luciano M., Galifianakis N.B., et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat Neurosci. 2015;18:779–786. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darcy N., Lofredi R., Al-Fatly B., Neumann W.J., Hubl J., Brucke C., et al. Spectral and spatial distribution of subthalamic beta peak activity in Parkinson's disease patients. Exp Neurol. 2022;356 doi: 10.1016/j.expneurol.2022.114150. [DOI] [PubMed] [Google Scholar]
- 70.Wiest C., He S., Duchet B., Pogosyan A., Benjaber M., Denison T., et al. Evoked resonant neural activity in subthalamic local field potentials reflects basal ganglia network dynamics. Neurobiol Dis. 2023;178 doi: 10.1016/j.nbd.2023.106019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neumann W.J., Horn A., Kuhn A.A. Insights and opportunities for deep brain stimulation as a brain circuit intervention. Trends Neurosci. 2023;46:472–487. doi: 10.1016/j.tins.2023.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Horn A., Reich M., Vorwerk J., Li N., Wenzel G., Fang Q., et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. 2017;82:67–78. doi: 10.1002/ana.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saenger V.M., Kahan J., Foltynie T., Friston K., Aziz T.Z., Green A.L., et al. Uncovering the underlying mechanisms and whole-brain dynamics of deep brain stimulation for Parkinson's disease. Sci Rep. 2017;7:9882. doi: 10.1038/s41598-017-10003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meissner W., Harnack D., Paul G., Reum T., Sohr R., Morgenstern R., et al. Deep brain stimulation of subthalamic neurons increases striatal dopamine metabolism and induces contralateral circling in freely moving 6-hydroxydopamine-lesioned rats. Neurosci Lett. 2002;328:105–108. doi: 10.1016/s0304-3940(02)00463-9. [DOI] [PubMed] [Google Scholar]
- 75.Lee K.H., Blaha C.D., Harris B.T., Cooper S., Hitti F.L., Leiter J.C., et al. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson's disease. Eur J Neurosci. 2006;23:1005–1014. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- 76.Strafella A.P., Sadikot A.F., Dagher A. Subthalamic deep brain stimulation does not induce striatal dopamine release in Parkinson's disease. Neuroreport. 2003;14:1287–1289. doi: 10.1097/00001756-200307010-00020. [DOI] [PubMed] [Google Scholar]
- 77.Thobois S., Fraix V., Savasta M., Costes N., Pollak P., Mertens P., et al. Chronic subthalamic nucleus stimulation and striatal D2 dopamine receptors in Parkinson's disease--A [(11)C]-raclopride PET study. J Neurol. 2003;250:1219–1223. doi: 10.1007/s00415-003-0188-z. [DOI] [PubMed] [Google Scholar]
- 78.Hilker R., Voges J., Ghaemi M., Lehrke R., Rudolf J., Koulousakis A., et al. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord. 2003;18:41–48. doi: 10.1002/mds.10297. [DOI] [PubMed] [Google Scholar]
- 79.Kringelbach M.L., Jenkinson N., Owen S.L., Aziz T.Z. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 80.Galati S., Mazzone P., Fedele E., Pisani A., Peppe A., Pierantozzi M., et al. Biochemical and electrophysiological changes of substantia nigra pars reticulata driven by subthalamic stimulation in patients with Parkinson's disease. Eur J Neurosci. 2006;23:2923–2928. doi: 10.1111/j.1460-9568.2006.04816.x. [DOI] [PubMed] [Google Scholar]
- 81.Johnson K.A., Cagle J.N., Lopes J.L., Wong J.K., Okun M.S., Gunduz A., et al. Globus pallidus internus deep brain stimulation evokes resonant neural activity in Parkinson's disease. Brain Commun. 2023;5 doi: 10.1093/braincomms/fcad025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steiner L.A., Milosevic L. A convergent subcortical signature to explain the common efficacy of subthalamic and pallidal deep brain stimulation. Brain Commun. 2023;5 doi: 10.1093/braincomms/fcad033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sobesky L., Goede L., Odekerken V.J.J., Wang Q., Li N., Neudorfer C., et al. Subthalamic and pallidal deep brain stimulation: are we modulating the same network? Brain. 2022;145:251–262. doi: 10.1093/brain/awab258. [DOI] [PubMed] [Google Scholar]
- 84.van Dijk A., Klompmakers A.A., Feenstra M.G., Denys D. Deep brain stimulation of the accumbens increases dopamine, serotonin, and noradrenaline in the prefrontal cortex. J Neurochem. 2012;123:897–903. doi: 10.1111/jnc.12054. [DOI] [PubMed] [Google Scholar]
- 85.Bregman T., Nona C., Volle J., Diwan M., Raymond R., Fletcher P.J., et al. Deep brain stimulation induces antidepressant-like effects in serotonin transporter knockout mice. Brain Stimul. 2018;11:423–425. doi: 10.1016/j.brs.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 86.Song S., Song S., Cao C., Lin X., Li K., Sava V., et al. Hippocampal neurogenesis and the brain repair response to brief stereotaxic insertion of a microneedle. Stem Cell Int. 2013;2013 doi: 10.1155/2013/205878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Picillo M., Lozano A.M., Kou N., Puppi Munhoz R., Fasano A. Programming deep brain stimulation for Parkinson's disease: the Toronto western hospital algorithms. Brain Stimul. 2016;9:425–437. doi: 10.1016/j.brs.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Fenoy A.J., Goetz L., Chabardes S., Xia Y. Deep brain stimulation: are astrocytes a key driver behind the scene? CNS Neurosci Ther. 2014;20:191–201. doi: 10.1111/cns.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sasaki T., Matsuki N., Ikegaya Y. Action-potential modulation during axonal conduction. Science. 2011;331:599–601. doi: 10.1126/science.1197598. [DOI] [PubMed] [Google Scholar]
- 90.Tawfik V.L., Chang S.Y., Hitti F.L., Roberts D.W., Leiter J.C., Jovanovic S., et al. Deep brain stimulation results in local glutamate and adenosine release: investigation into the role of astrocytes. Neurosurgery. 2010;67:367–375. doi: 10.1227/01.NEU.0000371988.73620.4C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jang J.S., Choi C.I., Yi J., Butters K., Kim I., Bhagwate A., et al. High frequency electrical stimulation promotes expression of extracellular matrix proteins from human astrocytes. Mol Biol Rep. 2019;46:4369–4375. doi: 10.1007/s11033-019-04890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen W., He B., Tong W., Zeng J., Zheng P. Astrocytic insulin-like growth factor-1 protects neurons against excitotoxicity. Front Cell Neurosci. 2019;13:298. doi: 10.3389/fncel.2019.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campos A.C.P., Kikuchi D.S., Paschoa A.F.N., Kuroki M.A., Fonoff E.T., Hamani C., et al. Unraveling the role of astrocytes in subthalamic nucleus deep brain stimulation in a Parkinson's disease rat model. Cell Mol Neurobiol. 2020;40:939–954. doi: 10.1007/s10571-019-00784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barat E., Boisseau S., Bouyssieres C., Appaix F., Savasta M., Albrieux M. Subthalamic nucleus electrical stimulation modulates calcium activity of nigral astrocytes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Soreq L., Bergman H., Israel Z., Soreq H. Deep brain stimulation modulates nonsense-mediated RNA decay in Parkinson's patients leukocytes. BMC Genom. 2013;14:478. doi: 10.1186/1471-2164-14-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soreq L., Salomonis N., Bronstein M., Greenberg D.S., Israel Z., Bergman H., et al. Small RNA sequencing-microarray analyses in Parkinson leukocytes reveal deep brain stimulation-induced splicing changes that classify brain region transcriptomes. Front Mol Neurosci. 2013;6:10. doi: 10.3389/fnmol.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Consales C., Merla C., Marino C., Benassi B. The epigenetic component of the brain response to electromagnetic stimulation in Parkinson's Disease patients: a literature overview. Bioelectromagnetics. 2018;39:3–14. doi: 10.1002/bem.22083. [DOI] [PubMed] [Google Scholar]
- 98.Mahlknecht P., Foltynie T., Limousin P., Poewe W. How does deep brain stimulation change the course of Parkinson's disease? Mov Disord. 2022;37:1581–1592. doi: 10.1002/mds.29052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schapira A.H., McDermott M.P., Barone P., Comella C.L., Albrecht S., Hsu H.H., et al. Pramipexole in patients with early Parkinson's disease (PROUD): a randomised delayed-start trial. Lancet Neurol. 2013;12:747–755. doi: 10.1016/S1474-4422(13)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verschuur C.V.M., Suwijn S.R., Boel J.A., Post B., Bloem B.R., van Hilten J.J., et al. Randomized delayed-start trial of levodopa in Parkinson's disease. N Engl J Med. 2019;380:315–324. doi: 10.1056/NEJMoa1809983. [DOI] [PubMed] [Google Scholar]
- 101.Schuepbach W.M., Rau J., Knudsen K., Volkmann J., Krack P., Timmermann L., et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- 102.Merola A., Romagnolo A., Bernardini A., Rizzi L., Artusi C.A., Lanotte M., et al. Earlier versus later subthalamic deep brain stimulation in Parkinson's disease. Parkinsonism Relat Disorders. 2015;21:972–975. doi: 10.1016/j.parkreldis.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 103.Kordower J.H., Olanow C.W., Dodiya H.B., Chu Y., Beach T.G., Adler C.H., et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McKinnon C., Gros P., Lee D.J., Hamani C., Lozano A.M., Kalia L.V., et al. Deep brain stimulation: potential for neuroprotection. Ann Clin Transl Neurol. 2019;6:174–185. doi: 10.1002/acn3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spieles-Engemann A.L., Behbehani M.M., Collier T.J., Wohlgenant S.L., Steece-Collier K., Paumier K., et al. Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol Dis. 2010;39:105–115. doi: 10.1016/j.nbd.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Temel Y., Visser-Vandewalle V., Kaplan S., Kozan R., Daemen M.A., Blokland A., et al. Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res. 2006;1120:100–105. doi: 10.1016/j.brainres.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 107.Maesawa S., Kaneoke Y., Kajita Y., Usui N., Misawa N., Nakayama A., et al. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J Neurosurg. 2004;100:679–687. doi: 10.3171/jns.2004.100.4.0679. [DOI] [PubMed] [Google Scholar]
- 108.Wallace B.A., Ashkan K., Heise C.E., Foote K.D., Torres N., Mitrofanis J., et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129–2145. doi: 10.1093/brain/awm137. [DOI] [PubMed] [Google Scholar]
- 109.Fischer D.L., Manfredsson F.P., Kemp C.J., Cole-Strauss A., Lipton J.W., Duffy M.F., et al. Subthalamic nucleus deep brain stimulation does not modify the functional deficits or axonopathy induced by nigrostriatal alpha-synuclein overexpression. Sci Rep. 2017;7 doi: 10.1038/s41598-017-16690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Musacchio T., Rebenstorff M., Fluri F., Brotchie J.M., Volkmann J., Koprich J.B., et al. Subthalamic nucleus deep brain stimulation is neuroprotective in the A53T alpha-synuclein Parkinson's disease rat model. Ann Neurol. 2017;81:825–836. doi: 10.1002/ana.24947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baydyuk M., Nguyen M.T., Xu B. Chronic deprivation of TrkB signaling leads to selective late-onset nigrostriatal dopaminergic degeneration. Exp Neurol. 2011;228:118–125. doi: 10.1016/j.expneurol.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fischer D.L., Kemp C.J., Cole-Strauss A., Polinski N.K., Paumier K.L., Lipton J.W., et al. Subthalamic nucleus deep brain stimulation employs trkB signaling for neuroprotection and functional restoration. J Neurosci. 2017;37:6786–6796. doi: 10.1523/JNEUROSCI.2060-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leal G., Comprido D., Duarte C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76(Pt C):639–656. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 114.Tsukahara T., Takeda M., Shimohama S., Ohara O., Hashimoto N. Effects of brain-derived neurotrophic factor on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in monkeys. Neurosurgery. 1995;37:733–739. doi: 10.1227/00006123-199510000-00018. discussion 739-741. [DOI] [PubMed] [Google Scholar]
- 115.Guo W., Nagappan G., Lu B. Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev Neurobiol. 2018;78:647–659. doi: 10.1002/dneu.22592. [DOI] [PubMed] [Google Scholar]
- 116.Hacker M.L., Turchan M., Heusinkveld L.E., Currie A.D., Millan S.H., Molinari A.L., et al. Deep brain stimulation in early-stage Parkinson disease: five-year outcomes. Neurology. 2020;95:e393–e401. doi: 10.1212/WNL.0000000000009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Charles D., Konrad P.E., Neimat J.S., Molinari A.L., Tramontana M.G., Finder S.G., et al. Subthalamic nucleus deep brain stimulation in early stage Parkinson's disease. Parkinsonism Relat Disorders. 2014;20:731–737. doi: 10.1016/j.parkreldis.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hacker M.L., Meystedt J.C., Turchan M., Cannard K.R., Harper K., Fan R., et al. Eleven-Year outcomes of deep brain stimulation in early-stage Parkinson disease. Neuromodulation. 2023;26:451–458. doi: 10.1016/j.neurom.2022.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hariz M. There is no credible rational for deep brain stimulation in very early Parkinson's disease. Parkinsonism Relat Disorders. 2015;21:345–346. doi: 10.1016/j.parkreldis.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 120.Reuter S., Deuschl G., Falk D., Mehdorn M., Witt K. Uncoupling of dopaminergic and subthalamic stimulation: life-threatening DBS withdrawal syndrome. Mov Disord. 2015;30:1407–1413. doi: 10.1002/mds.26324. [DOI] [PubMed] [Google Scholar]
- 121.Fasano A., Romito L.M., Daniele A., Piano C., Zinno M., Bentivoglio A.R., et al. Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain. 2010;133:2664–2676. doi: 10.1093/brain/awq221. [DOI] [PubMed] [Google Scholar]
- 122.Hilker R., Portman A.T., Voges J., Staal M.J., Burghaus L., van Laar T., et al. Disease progression continues in patients with advanced Parkinson's disease and effective subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry. 2005;76:1217–1221. doi: 10.1136/jnnp.2004.057893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pal G.D., Ouyang B., Serrano G., Shill H.A., Goetz C., Stebbins G., et al. Comparison of neuropathology in Parkinson's disease subjects with and without deep brain stimulation. Mov Disord. 2017;32:274–277. doi: 10.1002/mds.26882. [DOI] [PubMed] [Google Scholar]
- 124.Pal G., Ouyang B., Verhagen L., Serrano G., Shill H.A., Adler C.H., et al. Probing the striatal dopamine system for a putative neuroprotective effect of deep brain stimulation in Parkinson's disease. Mov Disord. 2018;33:652–654. doi: 10.1002/mds.27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fischer D.L., Sortwell C.E. BDNF provides many routes toward STN DBS-mediated disease modification. Mov Disord. 2019;34:22–34. doi: 10.1002/mds.27535. [DOI] [PMC free article] [PubMed] [Google Scholar]