Abstract

Experimental and theoretical studies were carried out to investigate the ozonolysis of trans-2-methyl-2-pentenal. The experiments were conducted in atmospheric simulation chambers coupled to a Fourier transform infrared (FTIR) spectrometer and a gas chromatograph–mass spectrometer at room temperature and atmospheric pressure in the presence of an excess of cyclohexane in dry conditions (RH < 1%). The ozonolysis reaction was investigated theoretically from the results of accurate density functional (M06-2X) and ab initio [CCSD(T)] computations, employing the AVTZ basis set. The sequence of reaction steps was established, and the system of kinetics equations was modeled using MESMER. In the first step, a primary ozonide is formed, which then decomposes along two pathways. The principal ozonolysis products are propanal, methylglyoxal, ethylformate, and a secondary ozonide. An interesting competition between sequential reaction steps and well-skipping is found, which leads to an inversion of the expected methylglyoxal/propanal product ratio at temperatures below 210 K. The mechanism of the “hot ester” reaction channel of the Criegee intermediate was revisited. The computed ozonolysis rate constant and product branching ratio are in excellent agreement with the experimental data that are also reported in the present work.
1. Introduction
Emission of various types of oxygenated volatile organic compounds influences atmospheric chemistry through the formation of tropospheric ozone and the production of secondary organic aerosol (SOA). Among the biogenic volatile organic compounds, C6 unsaturated aldehydes belong to the category of “volatile leaf substances”.1−3 These compounds are introduced into the atmosphere during the enzymatic oxidation of linoleic acids as a cellular and molecular response of plants to stress conditions like temperature and dryness.3 Their emission is influenced by various physical and meteorological parameters such as temperature, solar radiation intensity, or humidity.4 Although these unsaturated oxygenated compounds have been detected in the atmosphere at concentrations on the order of only a few parts per billion by volume (ppbv), their concentration may increase due to global warming. Furthermore, their use as flavoring agents in the food industry5 constitutes another source of their presence in the atmosphere.
The atmospheric behavior of the volatile organic compounds depends on their chemical structure, polarity, and solubility in water.6 Presence of an olefinic bond makes them highly reactive toward atmospheric photo-oxidants, and thus, their fate during atmospheric oxidation, particularly toward ozone, is crucial to assess. Ozonolysis leads to the formation of other oxygenated species and the production of SOA and can be a source of OH radicals. These compounds may therefore have an impact on the tropospheric composition, as well as on the climate.
In the present work, we have focused on the atmospheric ozonolysis of 2-methyl-2-pentenal (2M2P), an α,β-unsaturated aldehyde. The kinetics of this reaction has been studied experimentally by three of the present authors7 and also by Gaona Colmán and co-workers.8 The rate coefficients at room temperature obtained in these studies, k = 1.58 ± 0.20 × 10–18 cm3 molecule–1 s–1 and k = 7.1 ± 1.6 × 10–18 cm3 molecule–1 s–1, respectively, show a significant discrepancy. Gas-phase reactions of alkenes with ozone are known to produce a considerable amount of the hydroxy radical, OH, which then also interacts with the remaining alkene molecules. Such interference of the OH radical can be minimized by adding an OH scavenger to the simulation chamber, as was done by Kalalian et al.7 Gaona Colmán et al.8 did not use any OH radical scavenger, which could lead to overestimation of the rate constant.
To date, no mechanistic or theoretical studies exist regarding the ozonolysis of the title compound. Ozonolysis reactions are, in general, highly complex and can produce a plethora of chemical compounds, depending on the experimental conditions. The objective of the present work is to determine the mechanism of the primary reaction steps of the ozonolysis of 2M2P to identify the first-generation products and product branching ratios through both experimental and theoretical approaches.
The experiments were conducted in an atmospheric simulation chamber coupled with a Fourier transform infrared (FTIR) spectrometer and a gas chromatograph/mass spectrometer, while theoretical calculations were initially performed at the density functional theory (DFT) level and then refined by coupled-cluster singles-doubles and perturbative triples, CCSD(T), single-point computations. Finally, the system of chemical reactions was set up and modeled with the master equation solver MESMER.9
Though the fundamental aspects of the ozonolysis mechanism of alkenals are believed to be understood, see, for example, refs (10−12), and the two expected main products methylglyoxal and propanal are formed, among others, with a slight preference for methylglyoxal, our detailed analysis provides new insights. First of all, there is an interesting competition between a sequential mechanism and a well-skipping mechanism on the methylglyoxal pathway, which has never been described in the literature. This competition leads to a temperature dependence of the methylglyoxal/propanal product ratio. As a consequence, the established preference for the bicarbonyl compound, methylglyoxal,11 is inverted at lower temperature, which is demonstrated in Subsection 3.2. Another new finding is the mechanism of ester formation, which is produced with methylglyoxal. The hypothesis that the “hot ester” is formed from a Criegee intermediate in a unimolecular reaction passing through a cyclic dioxirane13−15 is not favorable in the present case. Instead, the “hot ester” is produced by catalytic conversion of the Criegee intermediate, as is shown in Subsection 3.3. The excellent agreement between the experimental and theoretical results indicates that the reaction pathways proposed here are indeed correct, providing insights into the atmospheric implications of this reaction.
2. Experimental Part
2.1. Methodology
The ozonolysis of 2M2P was performed at room temperature (T = 298 ± 2 K) and at a pressure of 1 bar in a 63 L atmospheric simulation chamber in the presence of an excess of cyclohexane to trap the OH radicals that were likely to be formed during the ozonolysis reaction of the unsaturated compound. The experimental setup was described in detail in a previous study.16 The concentrations of the reagents and the products formed during the ozonolysis reaction were monitored in situ by infrared (IR) spectroscopy and by solid-phase microextraction coupled with gas chromatography/mass spectrometry (SPME–GC/MS). During each experiment, the unsaturated aldehyde (2M2P) was driven by an airflow into the simulation chamber, resulting in a concentration of 4 × 1014 molecule cm–3. An OH scavenger (cyclohexane) was introduced in the same manner as that of 2M2P, at a high concentration of about 1 × 1016 molecule cm–3 to ensure OH scavenging. Ozone was introduced, initiating the ozonolysis reaction. Sampling of the reaction medium was done by exposing a polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber previously covered with o-(2,3,4,5,6-pentafluorobenzyl)-hydroxylamine (PFBHA) for 5 min in the simulation chamber. The derivatization conditions were taken from Reisen et al.17 This implies an exposure of the PDMS/DVB fiber for 1 h in the head space of a vial containing 4 mL of the aqueous solution of PFBHA (0.4 g/L) under a magnetic stirrer. These conditions ensure a maximum coating of the fiber by the PFBHA and a sufficient quantity of oxime for the detection: the aldehydes present in the simulation chamber adsorb on the PFBHA-doped fiber and form the corresponding oxime. The SPME sampling was carried out every 15 min, and the analysis was performed by GC–MS using the total ion current mode. At the same time, IR spectra were recorded every 3 min. Each spectrum is an average of 100 scans to improve the signal-to-noise ratio. The 2M2P and product bands were then processed and integrated. The areas of the chromatographic peaks or IR band were related to the concentration of the products through calibration curves previously obtained for the different molecules.
The ozonolysis of 2M2P generated carbonyl, bicarbonyl, and hydroxyl–carbonyl compounds, of which pure samples were needed for reference. Propanal and ethanal were commercially available, while other compounds, such as methylglyoxal, were synthesized according to methods given by Horowitz et al.18 and Lockhart et al.19 By analogy with the standard spectrum of 2-hydroxybutanal, the quantification of 2-hydroxypropanal was carried out using FTIR by integrating the spectral band at 1043–1077 cm–1. Both FTIR and SPME/GC–MS techniques were used to monitor 2M2P. The integrated IR bands for 2M2P were 1615–1674 cm–1. For propanal and methylglyoxal, only SPME/GC–MS was used. Hydroxypropanal was followed by integrating the IR spectral band at 1040–1070 cm–1. The identification and quantification of the compounds mentioned above were carried out using calibration curves previously established by FTIR and SPME/GC–MS.
The obtained product yields correspond to the average of 6 repetitions performed by the two analytical techniques (FTIR and SPME–GC/MS). Uncertainties in yields are twice the standard deviation (2σ). Chemicals were provided by Sigma-Aldrich: 2M2P (≥97%), cyclohexane (≥99%), PFBHA (≥98%), propanal (≥97%), ethanal (≥99%), 2- and methylglyoxal (40% in H2O). Gases were provided by Air Liquide: air (>99.9999%), O2 (>99.999%), He (>99.999%).
2.2. Experimental Results
During the experiment, the temporal evolution of the reagent and products formed during the ozonolysis reaction of 2M2P was followed. In order to establish a detailed reaction mechanism, an in-depth analysis of the IR spectra and chromatograms recorded during the ozonolysis reaction was carried out, resulting in the identification of two principal compounds, methylglyoxal and propanal, and two other compounds, acetaldehyde and hydroxypropanal, formed in much smaller quantities. The spectrum subtraction method was used to analyze the observed products using FTIR spectroscopy. Figure S1 of the Supporting Information shows the approach used to quantify 2-hydroxypropanal. An example of chromatograms showing the peaks characterizing the reaction products and reagent during the ozonolysis of 2M2P is given in Figure S2 of the Supporting Information. Figure 1 shows an example of the temporal evolution of the molar fractions of these products. Their concentrations become relatively constant after a reaction time of 60 min.
Figure 1.

Time evolution of the concentration of 2M2P and four product molecules. Also shown is the carbon balance. Its difference to 100% measures further product molecules that have not been identified.
The carbon balance CB(t) was also determined at various times during the experiment according to
| 1 |
where χi denotes the mole fraction of species i and Ni denotes the number of carbon atoms it contains. As can be seen, there is a deficit of about 50% of the carbon budget when the consumption of 2M2P exceeds 50%. This is due to the formation of other products in the reactor that could not be identified with the available analytical techniques. The product yields
| 2 |
were determined by plotting their concentrations as a function of the concentration of the converted reactant, Δ[2M2P], during the course of the reaction, as shown in Figures 2 and S3 of the Supporting Information The formation yields correspond to the slopes of the plotted curves. They are summarized in Table 1.
Figure 2.

Product yields of propanal and methylglyoxal as a function of consumed 2M2P.
Table 1. Observed Ozonolysis Products and Their Yieldsa.
| molecule | yield (%) |
|---|---|
| methylglyoxal | 52 ± 10% |
| propanal | 45 ± 18% |
| 2-hydroxypropanal | 6 ± 4 |
| acetaldehyde | 9 ± 8 |
The yields of the first-generation products sum up to 97%. They were determined independently for each species and not normalized to 100%.
According to these results, the principal products are methylglyoxal and propanal, obtained with formation yields of 52 ± 10 and 45 ± 18%, respectively. The oximes of methylglyoxal and propanal were identified by GC–MS. The concentrations of methylglyoxal and propanal were also measured independently by FTIR in the spectral regions of 1331–1405 and 1662–1824 cm–1, respectively. The yields obtained with both techniques were in good agreement. Acetaldehyde and hydroxypropanal were also observed, but with low formation yields of 9 ± 8 and 8 ± 4%, respectively, as shown in Figure S3 of the Supporting Information. As described in some previous publications,20,21 the analysis of the residual FTIR spectra obtained after subtraction shows the appearance of a spectral band located between 1043 and 1077 cm–1, which may correspond to 2-hydroxypropanal. In addition, the production of this species was confirmed by SPME and SPME–GC/MS analysis following the formation of their corresponding oxime. No IR spectral band corresponding to acetaldehyde was identified. This is probably due to the fact that its concentration was below the detection limit of FTIR. However, this compound was detected and quantified using SPME–GC/MS analysis. The low production rates of both 2-hydroxypropanal and acetaldehyde indicate that these are not first-generation products of the ozonolysis.
3. Computational Part: The Mechanism of the Ozonolysis of 2M2P
3.1. General Considerations
Computations were performed at the unrestricted U-M06-2X/AVTZ level of theory using Gaussian G16.22 The M06-2X functional has been chosen for its reliability in the calculation of thermochemical and kinetics data of main-group compounds.23 The character of stationary points was confirmed by frequency analysis, and intrinsic reaction coordinate pathways were computed at the two sides of the transition state barriers to confirm the mechanism. The energy values of the stationary points were then refined by coupled-cluster, UCCSD(T)/AVTZ, single-point calculations using Molpro.24 The augmented correlation-consistent polarized valence triple-ζ basis set, AVTZ, was used throughout.
The general mechanism of the reaction of unsaturated organic compounds with ozone was identified by Criegee.10,25 Ozone undergoes a 1,3-cycloaddition to an unsaturated carbon–carbon bond, forming an unstable primary ozonide, which then breaks up into a carbonyl compound and a carbonyl oxide, known as the Criegee intermediate, which in turn may rearrange to form a secondary ozonide. The precise mechanism depends on the substituents at the two carbon atoms that form the unsaturated bond and the reaction conditions.
An alternative mechanism was proposed by O’Neal and Blumstein,26 by which the primary ozonide is converted into a ketohydroperoxide, which then decomposes into OH and alkyl radicals. Such a mechanism can be excluded for the present system because the barrier of formation is too high, which is elaborated further in the discussion in Section 4.
3.2. Sequential Pathways
We have studied here the ozonolysis of trans-2M2P in the gas phase. The mechanism proceeds according to Criegee and is presented in Figure 3, while Figure 4 shows the corresponding energy diagram. The computed energies of all species are collected in Table 2. Ozone is a challenging system for single-reference methods, but only far from its equilibrium configuration.27 For the Criegee intermediates, Vereecken and Francisco and Vereecken et al.13,28 have demonstrated that such methods are sufficiently accurate. This is confirmed by the present study as all T1 values are reasonable. Furthermore, no spin contamination was found. The Cartesian coordinates of all species are available in Supporting Information.
Figure 3.
Mechanism of the ozonolysis of trans-2M2P up to first-generation products. The unstable Criegee intermediate will undergo further reactions.
Figure 4.
Schematic potential energy diagram. CCSD(T) electronic energy values are given with respect to the most stable products and are in units of kJ mol–1. Vibrational zero-point corrections are not included.
Table 2. Energies and Thermodynamic Functions of the Chemical Speciesa.
| molecule | M06-2X/AVTZ |
CCSD(T)/AVTZ |
||||
|---|---|---|---|---|---|---|
| E (Eh) | ZPE (Eh) | ΔG0 (Eh) | S0 (cal K–1 mol–1) | E (Eh) | T1 | |
| 2M2P | –309.836 | 0.147 | –309.72 | 91.0 | –309.3495 | 0.012 |
| O3 | –225.406 | 0.008 | –225.42 | 58.0 | –225.1499 | 0.024 |
| primary ozonide | –535.352 | 0.161 | –535.23 | 100.8 | –534.5929 | 0.015 |
| TS1 | –535.311 | 0.158 | –535.19 | 100.3 | –534.5638 | 0.017 |
| Criegee intermediate | –342.218 | 0.069 | –342.18 | 80.3 | –341.7469 | 0.034 |
| propanal | –193.127 | 0.085 | –193.07 | 70.8 | –192.8398 | 0.013 |
| TS2 | –535.317 | 0.159 | –535.20 | 99.8 | –534.5685 | 0.016 |
| intermediate I0 | –535.371 | 0.158 | –535.25 | 112.7 | –534.6111 | 0.014 |
| secondary ozonide | –535.443 | 0.163 | –535.32 | 101.9 | –534.6772 | 0.013 |
| TS3 | –535.357 | 0.157 | –535.24 | 104.4 | –534.5929 | 0.015 |
| methylglyoxal | –267.144 | 0.066 | –267.11 | 74.5 | –266.7607 | 0.015 |
| ethylformate | –268.376 | 0.091 | –268.31 | 74.7 | –267.9920 | 0.014 |
Data were computed at the M06-2X/AVTZ level of theory, except for the values in the second last column, which correspond to a single-point UCCSD(T)/AVTZ calculation at the M06-2X-optimized geometry. ZPE is the vibrational zero-point energy. The T1 diagnostics are reported in the last column.
Following the formation of the primary ozonide, the reaction proceeds via two distinctive pathways. The first leads to the formation of propanal and the reactive Criegee intermediate methylglyoxal oxide passing through the transition state TS1.
On the second pathway, through the transition state TS2, methylglyoxal and the Criegee intermediate propanal oxide, CH3CH2CHOO, are formed. However, the fate of the Criegee intermediate is different from that obtained in the first pathway. The transition state is a high-energy adduct of methylglyoxal and the Criegee intermediate propanal oxide. On the reaction path at the product side, these two species separate slightly, releasing energy, to arrive at a configuration denoted I0. This low-energy adduct, though characterized by all-positive harmonic frequencies, is not stable. There is no energy barrier, and therefore, the reaction does not stop here. It seems that energy is deposited in modes mostly orthogonal to those associated with prompt dissociation. This hypothesis would explain why the complex does not fall apart but instead rearranges internally to reach a more stable configuration, that of the secondary ozonide, becoming vibrationally “hot” as a consequence and eventually leading to dissociation. To be sure, propanal and the Criegee intermediate from the first pathway also form an adduct complex, stabilized by 41 kJ/mol, but it will not rearrange. Rather, the Criegee intermediate will engage in secondary reactions.
Rupture of the O–O bond of the hot secondary ozonide produces the transition state TS3 shown in Figure 5 together with the numbering of atoms for further discussion.
Figure 5.

Transition state TS3 and numbering of the relevant atoms, which is CH3–CO–C(1)HO(2)···O(1)–C(2)HO(3)···C(3)H2–CH3. The dotted bonds are those to be broken.
The reaction through transition state TS3 proceeds in
a rather interesting way to form methylglyoxal and ethylformate (ethylmethanoate).
The peculiar product findings can be explained by the singlet biradical
character of transition state TS3. Inspection of the imaginary
mode of TS3 reveals concerted breaking of the C(1)–O(1) and C(2)–C(3) bonds, forming three fragments. The two oxygen bearing fragments
show biradical,  , structures that rearrange on the downhill
reaction path to form C=O double bonds. One of the fragments
gives the origin to methylglyoxal. The second oxygen-bearing fragment,
, structures that rearrange on the downhill
reaction path to form C=O double bonds. One of the fragments
gives the origin to methylglyoxal. The second oxygen-bearing fragment, 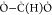 , which has now become O=C(H)
, which has now become O=C(H)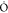 , then combines with the ethyl radical to
form ethylformate. This novel mechanism of catalyzed conversion of
a Criegee intermediate into an ester has not been described before.
It is commonly assumed that the ester is produced by the unimolecular
rearrangement of the Criegee intermediate, passing through a cyclic
dioxirane structure. In the case of 2M2P, the dioxirane would decompose
into ethane and CO2.
, then combines with the ethyl radical to
form ethylformate. This novel mechanism of catalyzed conversion of
a Criegee intermediate into an ester has not been described before.
It is commonly assumed that the ester is produced by the unimolecular
rearrangement of the Criegee intermediate, passing through a cyclic
dioxirane structure. In the case of 2M2P, the dioxirane would decompose
into ethane and CO2.
The mechanism is supported by an analysis of the spin densities in Table 3. The two unpaired electrons are located on O(2) and O(3) and have α and β spins, respectively, demonstrating the singlet biradical character of TS3. The α and β spin distributions of the remaining atoms indicate homolytic bond breaking. The calculations were performed with the MP2 method since DFT spin densities may not be accurate.29
Table 3. Spin Densities, SD, of the Transition State TS3 Computed at the MP2/AVQZ Level of Theory Using the M06-2X/AVTZ-Optimized Geometrya.
| atom | C(1) | C(2) | C(3) | O(1) | O(2) | O(3) |
|---|---|---|---|---|---|---|
| SD | –0.28 | 0.10 | –0.21 | 0.26 | 0.92 | –0.78 |
To close this section, we mention that we have also examined a third pathway, similar to that passing through the transition state TS2, but with the cleavage of a different C–C bond, by which propanal would be produced. Such a reaction is highly endothermic, by 240 kJ/mol, and does not take place.
3.3. Master Equation Modeling: Well-Skipping Pathways
The system of kinetic equations originating from the reaction mechanism established in the present work, shown in Figure 3, was then modeled with MESMER, a free master equation solver.9 In brief, the MESMER model used the RRKM method to calculate the microcanonical rate coefficients for all reactions except the initial association reaction of ozone and 2M2P
| 3 |
which, because it is almost barrierless, was treated using the inverse Laplace transform (ILT) method.30 The collision energy transfer was modeled using an exponential down model with a ⟨ΔE⟩d value of 125 cm–1 and a temperature dependence of ⟨ΔE⟩d of T0.66. The Arrhenius parameters were obtained by fitting the experimental association rate coefficients using the Levenberg–Marquardt algorithm within MESMER. The experimental and fitted high-pressure association rate coefficients are compared in Table 4 and show very good agreement, which is consistent with this part of the system being effective at a high pressure. It also indicates that the ILT is functioning correctly. The decay of 2M2P reacting with ozone was studied with the MESMER code under pseudo-first-order conditions at T = 298 K and p = 1 bar just as in the experiment. The loss rate constant is ktheor = 1.53 × 10–18 cm3 molecule–1 s–1, from which the tropospheric lifetime of 2M2P is estimated to be τ = 1/k[O3] ≈ 7 days, with a typical tropospheric ozone concentration of [O3] = 1012 molecule cm–3.
Table 4. Comparison of Experimental and Theoretical Rate Constants for the Reaction 2M2P + O3 → Products, in Units of 10–18 cm3 molecule–1 s–1a.
| T (K) | kexp | ktheor |
|---|---|---|
| 273 | 1.24 ± 0.10 | 1.25 |
| 298 | 1.58 ± 0.20 | 1.53 |
| 333 | 1.91 ± 0.13 | 1.93 |
| 353 | 2.18 ± 0.17 | 2.16 |
The uncertainty of the experimental temperatures is ±2 K.
The temperature dependence of the rate constant is shown in Figure 6. The Arrhenius expression, ktheor(T) = 1.42 × 10–17 e–663.63/T cm3 molecule–1 s–1, is identical to that found experimentally, kexp(T) = (1.42 ± 0.56) × 10–17 e–(664±124)/T cm3 molecule–1 s–1.
Figure 6.
Arrhenius plot of the overall rate constant. k(T) = 1.417 × 10–17 e–663.63/T cm3 molecule–1 s–1, the activation energy is Ea = 5517.75 J mol–1.
Excellent agreement is obtained not only for the high-pressure rate constants but also for the product ratios in Table 5, which supports the above-suggested mechanism. It also demonstrates that the quantum chemical approach, CCSD(T)/AVTZ//M06-2X/AVTZ, is accurate. The CCSD(T) energy values are expected to have chemical accuracy, that is, an error not exceeding 1 kcal/mol = 1.5 mEh.
Table 5. Mole Fractions of the Products Formed by the Ozonization Reaction of 2-Methyl-pentenal at the Four Experimental Temperatures. P, MG, and SO Denote Propanal, Methylglyoxal, and the Secondary Ozonide, Respectivelya.
| T (K) | mole fractions χ |
ratio | ||
|---|---|---|---|---|
| P | MG | SO | P/MG | |
| 273 | 0.254 | 0.340 | 0.406 | 0.75 |
| 0.252 | 0.331 | 0.423 | 0.76 | |
| 298 | 0.256 | 0.368 | 0.377 | 0.70 |
| 0.254 | 0.359 | 0.391 | 0.71 | |
| 333 | 0.262 | 0.414 | 0.325 | 0.63 |
| 0.264 | 0.380 | 0.358 | 0.69 | |
| 353 | 0.264 | 0.439 | 0.297 | 0.60 |
| 0.265 | 0.403 | 0.330 | 0.66 | |
The P/MG ratio is presented in the last column. The first line for each temperature is computed with the harmonic frequencies, while in the second line, anharmonicity is simulated through the scale factor f = 0.985.
For the temperature of 298 K, the experimental product ratio propanal/methylglyoxal is P/MG = 0.87 ± 0.38, while the theoretical value is found to be 0.70, which is safely within the error bounds. The theoretical value was computed using the harmonic frequencies to estimate the zero-point vibrational energy. The effect of vibrational anharmonicity was studied by scaling the harmonic frequencies by a factor of f = 0.985. This scaling thus affects the barrier heights at the milli-Hartree level, consistent with the accuracy of the energy computations. The P/MG ‘branching ratio is practically unchanged for the temperature of the experiment. At higher temperatures, the ratio augments slightly in favor of propanal formation. As Figure 1 shows, the experimentally identified products of the ozonolysis account for about 50% of the carbon balance. This may be compared with the carbon contained in the secondary ozonide and the ester, computed as
| 4 |
where the factors of six or three denote the number of carbon atoms in the respective molecules and the division by six reflects the number of carbon atoms of 2M2P. For the temperature of T = 298 K, this yields, using the mole factions χ from Table 5, a carbon balance of 56%, which compares well with the 50% of carbon unaccounted for in the experiment. For the other three temperatures, experimental product ratios are not available.
The results of Table 5 can be fully explained with the reaction mechanism of Figure 3. The first step of the ozonolysis, 2M2P + O3 → primary ozonide, is highly exothermic, and hence, the primary ozonide is formed with such high internal or kinetic energy that it easily overcomes the TS1 and TS2 barriers. The reaction along pathway 1 then continues readily toward a Criegee intermediate and propanal. As a result of the large excess energy of the primary ozonide, the mole fraction of propanal depends only weakly on the temperature. The second reaction, along pathway 2, involves three steps. In the first step, the intermediate I0 is formed, which is a low-energy adduct of the same two species of the excited transition state TS2. In the second step, the secondary ozonide is formed, and in the third step, it is converted into methylglyoxal and ethylformate. The stabilization of the secondary ozonide must be relatively slow so that there is a considerable fraction of hot secondary ozonide that passes over the large barrier of 0.07873 Eh = 206.7 kJ/mol formed by transition state TS3. Under low-temperature conditions, the barrier would hardly be overcome as the rate constant is only 5.18 × 10–19 s–1 at T = 298 K. Hence, methylglyoxal and ethylformate would not be formed in large quantities. The appearance of either of these species is thus evidence of a well-skipping mechanism overcoming the two ozonide wells, i.e., they are not formed in a sequential series of reactions. Both products are hot, chemically activated. The hot ester will readily disintegrate.15
In contrast to the formation of propanal following a one-well-skipping mechanism, with almost temperature-independent product mole fractions, there is a certain temperature dependence of the pathway 2 product mole fractions. The product mole fraction of methylglyoxal increases with temperature, while that of the secondary ozonide diminishes, indicating that its collisional stabilization becomes less effective. The secondary ozonide might be observable by Raman spectroscopy31 or by atmospheric sampling Townsend discharge ionization mass spectrometry.32
Neither the ester nor the secondary ozonide was detectable in the experiment. This is further discussed in Section 4.
Having validated our data in this way, we may now compute the second-order loss rate constant at temperatures not accessible in the experiment. These values could be interesting in view of the typical conditions in the troposphere.
The rate constants for the individual conversion reactions are reported in Table 6. Their numerical values are consistent with the product mole fractions given in Table 5. We note in particular that propanal is produced in a lower mole fraction than that of methylglyoxal as transition state TS1 is higher in energy than TS2. This preference is expected from the literature11 from conventional analysis. However, this changes as the temperature is decreased, as demonstrated in Figure 7. Below a temperature of about 210 K, the production of propanal is larger than that of 2-methylglyoxal. At low temperatures, collisions are relatively less effective at activating than deactivating a molecule, and therefore, more of the secondary ozonide is deactivated below the reaction threshold of TS3. Hence 2-methylglyoxal production will decline in favor of the formation of the secondary ozonide.
Table 6. Conversion Rate Constants of the Ozonization Reaction at p = 1 bar and the Four Temperatures of the Experimenta.
| T (K) |
k (10–7 s–1) |
||
|---|---|---|---|
| R1 | R2 | R3 | |
| 273 | 3.24 | 5.12 | 4.33 |
| 298 | 3.99 | 5.75 | 5.73 |
| 333 | 5.07 | 6.30 | 8.02 |
| 353 | 5.71 | 6.43 | 9.49 |
The reactions are denoted as follows: reaction R1: 2M2P → Criegee intermediate + propanal, reaction R2: 2M2P → secondary ozonide, and reaction R3: 2M2P → methylglyoxal + ethylformate. The rate constants are represented by the following Arrhenius expressions: k1(T) = 3.94 × 10–6 e–681.7/T s–1, k2(T) = 1.43 × 10–6 e–276.9/T s–1, and k3(T) = 1.38 × 10–5 e–946.8/T s–1.
Figure 7.
Relative yields of propanal and methylglyoxal as a function of temperature. The points represent the MESMER results from Table 5. The curves were computed from the Arrhenius representations of the rate constants reported in the caption of Table 6.
4. Discussion and Conclusions
We conducted an experimental and theoretical study to investigate the ozonolysis of 2M2P. No other mechanistic studies have been reported in the literature to the best of our knowledge. The present results may be compared to those obtained for other 2-alkenals with C3 to C9.8,11,12,20,21,33 These studies were carried out in the presence of an OH radical scavenger except for that by Gaona Colmán et al.8 All of these studies show that the ozonolysis of asymmetric alkyl-substituted alkenes leads to the preferential formation of bicarbonyl compounds such as glyoxal and methylglyoxal, at least at room temperature. We have shown here that this preference is inverted at a lower temperature.
The primary steps of the reaction mechanism of ozonolysis of 2M2P were established computationally. Ozonolysis of 2M2P proceeds via electrophilic addition of ozone to the olefinic bond to give a primary ozonide, which then breaks up in two different ways through which, eventually, 2-methylglyoxal and propanal are formed. Experimentally, the sum of the yields of these two products is 97 ± 21%, which indicates that these two species are formed in the first reaction steps. The calculations fully support the experimental findings.
The basic steps of the ozonolysis mechanism of alkenals are well-established. However, what we have shown here is that owing to its high exothermicity, the reaction does not proceed in the assumed sequential way. Only the formation of propanal proceeds straightforwardly following the decomposition of the primary ozonide. A Criegee intermediate, CHOC(CH3)OO, is formed at the same time, which undergoes further reactions,34 possibly leading to acetaldehyde, which has been observed in very small mole fractions (9 ± 8%). The formation of 2-methylglyoxal, in contrast, involves a three-step process. As the calculations show, the breakup of the primary ozonide on this second pathway produces an activated adduct of methylglyoxal and the Criegee intermediate propanaloxide, CH3CH2CHOO. One part of the adduct is collisionally stabilized and transformed into a secondary ozonide, hereby passing through an intermediate, I0. It is well-known10 that aldehyde oxides have a greater affinity toward cycloaddition than ketone oxides; thus, they form secondary ozonides more easily, which explains the mechanistic differences between the two pathways. The formation of a secondary ozonide might be surprising at first sight. Secondary ozonides play a role in the ozonolysis of terpenes, where the Criegee intermediate and the carbonyl compound are connected by a ring bridge and, hence, cannot separate. But other examples of secondary ozonide formation from “unbridged” molecules are known, and some of them were presented in the work by Jalan et al.35
As just described, one part of adduct I0 is transformed into a secondary ozonide. Another part of the activated adduct skips the potential energy well of the secondary ozonide and passes through a high-energy transition state, TS3, derived from the secondary ozonide by the oxygen–oxygen bond breaking to finally yield methylglyoxal and ethylformate. The latter is formed through catalytic conversion of the Criegee intermediate, which is a novel mechanism proposed in the present work. The so-called “hot ester” channel itself is well-established.15
The ester disintegrates readily at the high temperature of 600 K, at which the GS-MS system operates, and for this reason, it could not be detected in the experiment. Methylglyoxal, produced by the same reaction, would also decompose if it were not derivatized to form an oxime. In other words, the experimental setup used here permits detection of aldehydes and ketones but not of esters or secondary ozonides. Such products were not expected when the experiment was carried out, some years before the theoretical simulations.
The carbon contents of the ester and that of the secondary ozonide account for the experimental carbon loss. The experimental and theoretical production yields of methylglyoxal, 52 ± 10 and 58.9%, respectively, compare well. A small amount, 6 ± 4%, of 2-hydroxypropanal was found in the experiment, which must have been produced in a secondary reaction step. It is possible that a small portion of the I0 adduct complex separates and the liberated propanal oxide, CH3CH2CHOO, reacts further to form 2-hydroxypropanal. The principal transformation pathway of the I0 complex, however, is the formation of the secondary ozonide, demonstrated by energy minimization. It is vibrationally hot and dissociates.
In the present work, we have modeled the ozonolysis of 2M2P under the experimental conditions. The presence of a scavenger suppresses possible reactions of 2M2P with OH radicals. The experimental rate constant therefore is that of the pure ozonolysis reaction, eq 3. The fact that the observed and computed rate constants for the decomposition of 2M2P are in excellent agreement, as are the primary product yields, demonstrates that the ozonolysis reaction proceeds by the proposed mechanism. This also excludes alternative decomposition schemes of the primary ozonide such as sequential O–O and C–C bond breaking, by which a ketohydroperoxide would be formed, which is in line with published results on other unsaturated compounds. The studies by Nguyen et al.36 and other authors demonstrated that the ketohydroperoxide channel competes with the Criegee channel for the ozonolysis of ethene. The relative importance of the ketohydroperoxide channel is likely due to the fact that there are four hydrogen atoms available to undergo the α-hydrogen shift that leads to the ketohydroperoxide. Recent work by Fan et al.37 on the ozonolysis of ethyl vinyl ether, a molecule of the type RHC=CH2, found a marginal importance (0.65%) of the ketohydroperoxide channel. The shifted hydrogen came exclusively from the unsubstituted carbon atom. Our molecule, 2M2P, is of the type R1R2C=CR3H, and hence, the ketohydroperoxide channel seems even less important. Furthermore, the energy barrier for its formation is very high, 221 kJ/mol higher than TS2, see Supporting Information.
We have demonstrated that a master equation approach is necessary to describe correctly the complex system of reactions in which sequential steps compete with well-skipping. Theoretical investigation of the possible secondary reaction processes is beyond the scope of the present work.
Acknowledgments
Computer time was provided by the ROMEO HPC Center at the University of Reims Champagne-Ardenne and the CRIANN HPC Center at the University of Rouen. The authors gratefully acknowledge financing from the CNRS, project IEA no. 317871 and from the “PHC-Utique” program of the French Ministry of Foreign Affairs and Ministry of Higher Education and Research and the Tunisian Ministry of Higher Education and Scientific Research in the CMCU project number PC24G1301. N.D. acknowledges support from the French Embassy in Tunis. The experimental work was funded by the INSU-LEFE-CHAT program.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpca.3c04965.
Experimental part: examples of experimental IR spectra, chromatograms, and experimental analysis and theory part: Cartesian coordinates and harmonic frequencies of all compounds, further information on the decay pathways of the primary ozonide, and information on excluded pathways (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hatanaka A. The biogeneration of green odour by green leaves. Phytochemistry 1993, 34, 1201–1218. 10.1016/0031-9422(91)80003-J. [DOI] [Google Scholar]; The International Journal of Plant Biochemistry
- Nakamura S.; Hatanaka A. Green-Leaf-Derived C6-Aroma Compounds with Potent Antibacterial Action That Act on Both Gram-Negative and Gram-Positive Bacteria. J. Agric. Food Chem. 2002, 50, 7639–7644. 10.1021/jf025808c. [DOI] [PubMed] [Google Scholar]
- Davis M. E.; Burkholder J. B. Rate coefficients for the gas-phase reaction of OH with (<i&gt;Z&lt;/i&gt;)-3-hexen-1-ol, 1-penten-3-ol, (<i&gt;E&lt;/i&gt;)-2-penten-1-ol, and (<i&gt;E&lt;/i&gt;)-2-hexen-1-ol between 243 and 404 K. Atmos. Chem. Phys. 2011, 11, 3347–3358. 10.5194/acp-11-3347-2011. [DOI] [Google Scholar]
- Karl T.; Guenther A.; Lindinger C.; Jordan A.; Fall R.; Lindinger W. Eddy covariance measurements of oxygenated volatile organic compound fluxes from crop harvesting using a redesigned proton-transfer-reaction mass spectrometer. J. Geophys. Res.: Atmos. 2001, 106, 24157–24167. 10.1029/2000JD000112. [DOI] [Google Scholar]
- Younes M.; Aquilina G.; Castle L.; Engel K.-H.; Fowler P.; Frutos Fernandez M. J.; Fürst P.; Gundert-Remy U.; Husøy T.; et al. Scientific Opinion on Flavouring Group Evaluation 201 Revision 2 (FGE.201Rev2): 2-alkylated, aliphatic, acyclic alpha,beta-unsaturated aldehydes and precursors, with or without additional double-bonds, from chemical subgroup 1.1.2 of FGE.19. EFSA J. 2018, 16, e05423 10.2903/j.efsa.2018.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther A.; Hewitt C. N.; Erickson D.; Fall R.; Geron C.; Graedel T.; Harley P.; Klinger L.; Lerdau M.; Mckay W. A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res.: Atmos. 1995, 100, 8873–8892. 10.1029/94JD02950. [DOI] [Google Scholar]
- Kalalian C.; Roth E.; Chakir A. Rate Coefficients for the Gas-Phase Reaction of Ozone with C5 and C6 Unsaturated Aldehydes. Int. J. Chem. Kinet. 2018, 50, 47–56. 10.1002/kin.21139. [DOI] [Google Scholar]
- Gaona Colmán E.; Blanco M. B.; Barnes I.; Teruel M. A. Kinetics of the gas-phase reaction between ozone and three unsaturated oxygenated compounds: Ethyl 3,3-dimethyl acrylate, 2-methyl-2-pentenal and 6-methyl-5-hepten-2-one at atmospheric pressure. Atmos. Environ. 2015, 109, 272–278. 10.1016/j.atmosenv.2015.03.011. [DOI] [Google Scholar]
- Glowacki D. R.; Liang C.-H.; Morley C.; Pilling M. J.; Robertson S. H. MESMER: An Open-Source Master Equation Solver for Multi-Energy Well Reactions. J. Phys. Chem. A 2012, 116, 9545–9560. 10.1021/jp3051033. [DOI] [PubMed] [Google Scholar]
- Criegee R. Mechanism of Ozonolysis. Angew Chem. Int. Ed. 1975, 14, 745–752. 10.1002/anie.197507451. [DOI] [Google Scholar]
- Grosjean D.; Williams E. L. I.; Grosjean E. Atmospheric chemistry of isoprene and of its carbonyl products. Environ. Sci. Technol. 1993, 27, 830–840. 10.1021/es00042a004. [DOI] [Google Scholar]
- Grosjean E.; Grosjean D. Rate constants for the gas-phase reaction of ozone with unsaturated oxygenates. Int. J. Chem. Kinet. 1998, 30, 21–29. . [DOI] [Google Scholar]
- Vereecken L.; Francisco J. S. Theoretical studies of atmospheric reaction mechanisms in the troposphere. Chem. Soc. Rev. 2012, 41, 6259–6293. 10.1039/c2cs35070j. [DOI] [PubMed] [Google Scholar]
- Gutbrod R.; Schindler R. N.; Kraka E.; Cremer D. Formation of OH radicals in the gas phase ozonolysis of alkenes: the unexpected role of carbonyl oxides. Chem. Phys. Lett. 1996, 252, 221–229. 10.1016/0009-2614(96)00126-1. [DOI] [Google Scholar]
- Finlayson-Pitts B.; James Pitts J.. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications; Elsevier, 2000. [Google Scholar]
- Al Rashidi M.; El Masri A.; Roth E.; Chakir A. UV spectra and OH-oxidation kinetics of gaseous phase morpholinic compounds. Atmos. Environ. 2014, 88, 261–268. 10.1016/j.atmosenv.2014.01.057. [DOI] [Google Scholar]
- Reisen F.; Aschmann S. M.; Atkinson R.; Arey J. Hydroxyaldehyde Products from Hydroxyl Radical Reactions of Z-3-Hexen-1-ol and 2-Methyl-3-buten-2-ol Quantified by SPME and API-MS. Environ. Sci. Technol. 2003, 37, 4664–4671. 10.1021/es034142f. [DOI] [PubMed] [Google Scholar]
- Horowitz A.; Meller R.; Moortgat G. K. The UV–VIS absorption cross sections of the α-dicarbonyl compounds: pyruvic acid, biacetyl and glyoxal. J. Photochem. Photobiol., A 2001, 146, 19–27. 10.1016/S1010-6030(01)00601-3. [DOI] [Google Scholar]
- Lockhart J.; Blitz M.; Heard D.; Seakins P.; Shannon R. Kinetic Study of the OH + Glyoxal Reaction: Experimental Evidence and Quantification of Direct OH Recycling. J. Phys. Chem. A 2013, 117, 11027–11037. 10.1021/jp4076806. [DOI] [PubMed] [Google Scholar]
- Grira A.; Kalalian C.; Illmann J.; Patroescu-Klotz I.; El Dib G.; Coddeville P.; Canosa A.; Coeur C.; Wiesen P.; Roth E.; et al. Gas-phase ozonolysis of trans-2-hexenal: Kinetics, products, mechanism and SOA formation. Atmos. Environ. 2021, 253, 118344. 10.1016/j.atmosenv.2021.118344. [DOI] [Google Scholar]
- Kalalian C.; Grira A.; Illmann J. N.; Patroescu-Klotz I.; El Dib G.; Coddeville P.; Canosa A.; Wiesen P.; Aazaad B.; Senthilkumar L.; et al. Experimental and Theoretical Studies of Trans-2-Pentenal Atmospheric Ozonolysis. Atmosphere 2022, 13, 291. 10.3390/atmos13020291. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; et al. Gaussian 16, Revision C.01; Gaussian Inc: Wallingford CT, 2016. [Google Scholar]
- Hohenstein E. G.; Chill S. T.; Sherrill C. D. Assessment of the Performance of the M05-2X and M06-2X Exchange-Correlation Functionals for Noncovalent Interactions in Biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. 10.1021/ct800308k. [DOI] [PubMed] [Google Scholar]
- Werner H.-J.; Knowles P. J.; Knizia G.; Manby F. R.; Schütz M.; et al. MOLPRO, Version 2021.1, a Package of Ab Initio Programs, 2021; see http://www.molpro.net.
- Criegee R.; Wenner G. Die Ozonisierung des 9,10-Oktalins. Justus Liebigs Ann. Chem. 1949, 564, 9–15. 10.1002/jlac.19495640103. [DOI] [Google Scholar]
- O’Neal H. E.; Blumstein C. A new mechanism for gas phase ozone–olefin reactions. Int. J. Chem. Kinet. 1973, 5, 397–413. 10.1002/kin.550050310. [DOI] [Google Scholar]
- Sur S.; Quintas-Sánchez E.; Ndengué S. A.; Dawes R. Development of a potential energy surface for the O3–Ar system: rovibrational states of the complex. Phys. Chem. Chem. Phys. 2019, 21, 9168–9180. 10.1039/C9CP01044K. [DOI] [PubMed] [Google Scholar]
- Vereecken L.; Harder H.; Novelli A. The reactions of Criegee intermediates with alkenes, ozone, and carbonyl oxides. Phys. Chem. Chem. Phys. 2014, 16, 4039–4049. 10.1039/c3cp54514h. [DOI] [PubMed] [Google Scholar]
- Boguslawski K.; Jacob C. R.; Reiher M. Can DFT Accurately Predict Spin Densities? Analysis of Discrepancies in Iron Nitrosyl Complexes. J. Chem. Theory Comput. 2011, 7, 2740–2752. 10.1021/ct1006218. [DOI] [PubMed] [Google Scholar]
- Davies J. W.; Green N. J.; Pilling M. J. The testing of models for unimolecular decomposition via inverse laplace transformation of experimental recombination rate data. Chem. Phys. Lett. 1986, 126, 373–379. 10.1016/S0009-2614(86)80101-4. [DOI] [Google Scholar]
- Cataldo F. Thermal stability, decomposition enthalpy, and Raman spectroscopy of 1-alkene secondary ozonides. Tetrahedron Lett. 2015, 56, 994–998. 10.1016/j.tetlet.2015.01.056. [DOI] [Google Scholar]
- Nøjgaard J. K.; Nørgaard A.; Wolkoff P. Secondary ozonides of endo-cyclic alkenes analyzed by atmospheric sampling Townsend discharge ionization mass spectrometry. Int. J. Mass Spectrom. 2007, 263, 88–93. 10.1016/j.ijms.2007.01.004. [DOI] [Google Scholar]
- Atkinson R.; Arey J.; Aschmann S. M.; Corchnoy S. B.; Shu Y. Rate constants for the gas-phase reactions of cis-3-Hexen-1-ol, cis-3-Hexenylacetate, trans-2-Hexenal, and Linalool with OH and NO3 radicals and O3 at 296 ± 2 K, and OH radical formation yields from the O3 reactions. Int. J. Chem. Kinet. 1995, 27, 941–955. 10.1002/kin.550271002. [DOI] [Google Scholar]
- Atkinson R.; Arey J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. 10.1021/cr0206420. [DOI] [PubMed] [Google Scholar]
- Jalan A.; Allen J. W.; Green W. H. Chemically activated formation of organic acids in reactions of the Criegee intermediate with aldehydes and ketones. Phys. Chem. Chem. Phys. 2013, 15, 16841–16852. 10.1039/c3cp52598h. [DOI] [PubMed] [Google Scholar]
- Nguyen T. L.; Lee H.; Matthews D. A.; McCarthy M. C.; Stanton J. F. Stabilization of the simplest Criegee intermediate from the reaction between ozone and ethylene: A high-level quantum chemical and kinetic analysis of ozonolysis. J. Phys. Chem. A 2015, 119, 5524–5533. 10.1021/acs.jpca.5b02088. [DOI] [PubMed] [Google Scholar]
- Fan H.; Ma J.; Zhu L.; Liu B.; Liu F.; Shan X.; Wang Z.; Wang L. Unusual diradical intermediates in ozonolysis of alkenes: A combined theoretical and synchrotron radiation photoionization mass spectrometric study on ozonolysis of alkyl vinyl ethers. J. Phys. Chem. A 2022, 126, 8021–8027. 10.1021/acs.jpca.2c04382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






