Abstract
Rationale & Objective:
Dialysis-requiring acute kidney injury (AKI-D) has increased substantially in the United States. We examined trends in and comorbid conditions associated with hospitalizations and in-hospital mortality in the setting of AKI-D among people with versus without diabetes.
Study Design:
Cross-sectional study.
Setting & Participants:
Nationally representative data from the National Inpatient Sample and National Health Interview Survey were used to generate 16 cross-sectional samples of US adults (aged ≥18 years) between 2000 and 2015.
Exposure:
Diabetes, defined using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes.
Outcome:
AKI-D, defined using ICD-9-CM diagnosis and procedure codes.
Analytical Approach:
Annual age-standardized rates of AKI-D and AKI-D mortality were calculated for adults with and without diabetes, by age and sex. Data were weighted to be representative of the US noninstitutionalized population. Trends were assessed using join point regression with annual percent change (Δ/y) reported.
Results:
In adults with diabetes, AKI-D increased between 2000 and 2015 (from 26.4 to 41.1 per 100,000 persons; Δ/y, 3.3%; P < 0.001), with relative increases greater in younger versus older adults. In adults without diabetes, AKI-D increased between 2000 and 2009 (from 4.8 to 8.7; Δ/y, 6.5%; P < 0.001) and then plateaued. AKI-D mortality significantly declined in people with and without diabetes. In adults with and without diabetes, the proportion of AKI-D hospitalizations with liver, rheumatic, and kidney disease comorbid conditions increased between 2000 and 2015, while the proportion of most cardiovascular comorbid conditions decreased.
Limitations:
Lack of laboratory data to corroborate AKI diagnosis; National Inpatient Sample data are hospital-level rather than person-level data; no data for type of diabetes; residual unmeasured confounding.
Conclusions:
Hospitalization rates for AKI-D have increased considerably while mortality has decreased in adults with and without diabetes. Hospitalization rates for AKI-D remain substantially higher in adults with diabetes. Greater AKI risk-factor mitigation is needed, especially in young adults with diabetes.
Acute kidney injury (AKI) is a common and serious complication requiring hospitalization.1 Patients with the most severe form of AKI—those requiring dialysis (AKI-D)—have higher rates of adverse outcomes, incidence of kidney failure, in-hospital mortality, and high health care costs.2–5 Worldwide, the incidence of AKI-D has increased significantly in recent years,6 in large part due to the increasing age of the population and the increasing burden of acute and chronic medical illnesses.7 Despite this, some evidence suggests that mortality rates associated with AKI-D are declining.8,9
Diabetes is the most common cause of kidney failure, accounting for 46% of all new cases in the United States,10 and it has also been linked to higher risk for AKI and AKI mortality.11 In 2017, it was estimated that 425 million adults were living with diabetes, and this number is expected to reach 629 million by 2045.12 Furthermore, an increasing number of young adults are being diagnosed with type 2 diabetes, a disease traditionally considered a problem of middle age,12 exposing them to a longer diabetes duration and increased risk for developing complications.
Using national US data, we recently reported a significant increase in AKI-D hospitalization rates among people with and without diabetes between 2000 and 2014, with absolute increases greater in adults with diabetes.13 In that brief report, we did not explore trends in AKI-D by age and sex, AKI-D–associated in-hospital mortality, or co-morbid conditions. Quantifying the burden of AKI-D and comorbid conditions in diabetes by key demographic subgroups is an important step in the prevention and management of this life-threatening complication.
Therefore, in this study we examined 3 key questions. (1) What are the current trends in the annual incidence of AKI-D hospitalizations among adults with versus without diabetes by age and sex? (2) Do trends in AKI-D–associated in-hospital mortality differ in people with and without diabetes by age and sex? (3) Which comorbid conditions are associated with the increase in AKI-D in adults with and without diabetes?
Methods
Data Sources
We estimated the number of adults 18 years and older with and without diabetes per year (2000-2015) using the National Health Interview Survey (NHIS). The NHIS is a multistage probability survey that samples an average of 35,000 adults per year to estimate the health of the US population, the prevalence and incidence of disease, the extent of disability, and the use of health care services.14 We defined adults with diabetes as persons who responded yes to the question “Other than during pregnancy, have you ever been told by a doctor or other health professional that you have diabetes or sugar diabetes?” Data from the NHIS were weighted to make estimates representative of the demographic characteristics of the US civilian noninstitutionalized population.
To estimate the number of AKI-D hospitalizations, AKI-D in-hospital mortality, and comorbid conditions among AKI-D hospitalizations, we used the National Inpatient Sample (NIS), the largest all-payer hospital inpatient care database in the United States.15 The NIS provides information on primary and secondary diagnoses and procedures, admission and discharge status, and payments, along with hospital and discharge weights for producing nationally representative estimates. The NIS is drawn from the states participating in the Healthcare Cost and Utilization Project, 33 to 46 states with 7 to 8 million unweighted inpatient records per year.15 The current study was based on hospitalizations from January 1, 2000, through September 30, 2015, during which a person was admitted to the hospital with AKI-D.
AKI-D hospitalizations were defined using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code for AKI (584) and at least 1 procedure code of hemodialysis (39.95) or peritoneal dialysis (54.98), excluding chronic renal failure (V45.1, V56.0, V56.31, V56.32, and V56.8). Comorbid conditions were classified using the Charlson Comorbidity Index, excluding diabetes, defined using ICD-9-CM diagnosis codes16,17 (Table S1). The Charlson Comorbidity Index was categorized as follows: low morbidity (score 0-2), moderate morbidity (score 3-5), moderate to high morbidity (score 6-8), and high morbidity (score ≥9). In addition, we included cardiac procedures most likely to be associated with kidney failure, identified using ICD-9-CM procedure codes that are listed in Table S1. AKI-D, AKI-D mortality, comorbid conditions, and cardiac procedures were considered to be related to diabetes if the hospitalization also included a diabetes diagnosis code (ICD-9-CM codes 250, 357.2, and 366.41).
Statistical Analysis
Using NIS and NHIS data, we constructed 16 cross-sectional populations of adults with and without diabetes per year between 2000 and 2015. Annual rates per 100,000 persons were estimated as the number of AKI-D hospitalizations with and without diabetes (as determined from NIS), divided by the number of persons with and without diabetes in the population (as determined from NHIS) (Fig S1). Age- (grouped into 18-44, 45-64, 65-74, and ≥75 years) and sex-specific rates were also estimated by diabetes status. Total and sex-specific rates were age-standardized using the 2000 US standard population and the age groups listed. Excess risk between diabetic and nondiabetic populations was estimated as the rate ratio (RR; age-standardized diabetes rate/age-standardized nondiabetes rate).
Using NIS data, annual in-hospital mortality was estimated as the number of deaths among patients with AKI-D per 100 persons and age-standardized as described. Co-morbid conditions among AKI-D hospitalizations are reported as proportion or median, when appropriate, with 95% confidence interval (CI) or interquartile range reported. The delta method was used to compute standard errors and 95% CIs for AKI-D rates, proportions, and RRs, accounting for the weighted design of both the NIS and NHIS.18 Stata, version 14.1 (StataCorp), was used to estimate all rates, RRs, and proportions, as specified.
Join point regression was used to estimate the annual percentage change (Δ/y) in previously calculated rates of AKI-D, AKI-D mortality, RRs, and proportion of AKI-D hospitalizations with comorbid conditions over time.19 The Joinpoint Trend Analysis software uses permutation tests to identify points at which linear trends change significantly in either direction or magnitude and calculates the annual percentage change for each time period identified. A maximum of 2 join points was specified and included an offset of log-person-years. P < 0.05 was established as statistical significance.
In 2012, the NIS sampling design was changed, which had implications for trend analyses. Per NIS guidelines, we used NIS-provided trend weights for the years preceding 2012 and the discharge weights beginning in 2012 to make the discharge outcome consistent with the new sampling design.20,21
On October 1 2015, ICD-10-CM was implemented in the United States. Therefore, 2015 annual administrative data include both ICD-9-CM (January 1, 2015, to September 30, 2015) and ICD-10-CM (October 1, 2015, to December 31, 2015). Due to the discontinuity across the 2 coding systems, this study uses ICD-9-CM data in the first 9 months of 2015 only. Therefore, 2015 population data (from NHIS) were weighted by 0.75 to reflect that only three-quarters of the numerator data were used.22
NHIS is approved by the Research Ethics Review Board of the National Center for Health Statistics and the US Office of Management and Budget. All NHIS respondents provided oral consent before participation. The NIS is a publicly available data set, does not contain direct personal identifiers, and is therefore exempt from review by the Institutional Review Board of the Centers for Disease Control and Prevention.
Results
AKI-D Incidence
In adults with diabetes, rates of AKI-D increased 55.7% between 2000 and 2015 (from 26.4 to 41.1 per 100,000 persons; Δ/y, 3.3%; P < 0.001) (Fig 1 and Table 1). Among adults without diabetes, rates increased 71.8% between 2000 and 2009 (from 4.8 to 8.3 per 100,000 persons; Δ/y, 6.5%; P < 0.001) and then plateaued. In 2015, rates of hospitalizations for AKI-D remained almost 5 times as high in adults with diabetes compared with adults without diabetes (RR, 5.0; 95% CI, 4.8-5.1), and this excess risk did not change significantly between 2000 and 2015 (P = 0.3).
Figure 1.

Trends in age-standardized rates of hospitalizations for dialysis-requiring acute kidney injury (AKI-D) in people with and without diabetes, United States, 2000-2015. *A join point at which linear trends change in either direction or magnitude.
Table 1.
Trends in Hospitalization Rates for Dialysis-Requiring AKI in People With Versus Without Diabetes, by Age and Sex, United States 2000-2015
| DM | AKI Hospitalization Rate, per 100,000 persons (95% CI) |
Trend 1a |
Trend 2a |
Trend 3a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2005 | 2010 | 2015 | Year | Δ/y | P | Year | Δ/y | P | Year | Δ/y | P | ||
| Total b | ||||||||||||||
|
| ||||||||||||||
| Y | 26.4 (25.4-27.4) | 30.3 (29.5-31.2) | 41.76 (41.0-42.6) | 41.1 (40.1-42.1) | 2000-2015 | 3.3% | <0.001 | NA | ||||||
|
| ||||||||||||||
| N | 4.8 (4.8-4.9) | 70 (6.9-7.1) | 9.1 (9.0-9.2) | 8.3 (8.2-8.4) | 2000-2009 | 6.5% | <0.001 | 2009-2012 | −5.8% | 0.3 | 2012-2015 | 4.9% | 0.09 | |
|
| ||||||||||||||
| Men | ||||||||||||||
|
| ||||||||||||||
| 18-44 y | Y | 21.0 (19.9-22.2) | 22.3 (21.2-23.3) | 37.4 (36.4-38.3) | 43.9 (42.2-45.5) | 2000-2015 | 4.3% | <0.001 | NA | |||||
|
| ||||||||||||||
| N | 2.1 (2.1-2.1) | 2.4 (2.4-2.4) | 2.7 (2.7-2.8) | 2.8 (2.7-2.8) | 2000-2009 | 4.3% | <0.001 | 2009-2012 | −6.9% | 0.4 | 2012-2015 | 8.0% | 0.08 | |
|
| ||||||||||||||
| 45-64 y | Y | 27.6 (27.0-28.2) | 371 (36.6-37.6) | 52.7 (52.2-53.2) | 50.9 (50.4-51.4) | 2000-2009 | 4.1% | <0.001 | NA | |||||
|
| ||||||||||||||
| N | 4.7 (4.6-4.8) | 8.1 (8.0-8.2) | 10.8 (10.7-10.9) | 11.0 (11.0-11.1) | 2000-2015 | 4.2% | <0.001 | NA | ||||||
|
| ||||||||||||||
| 65-74 y | Y | 42.4 (41.3-43.5) | 54.1 (53.1-55.1) | 63.3 (62.5-64.1) | 66.2 (65.4-66.9) | 2000-2015 | 3.2% | <0.001 | NA | |||||
|
| ||||||||||||||
| N | 15.1 (14.8-15.3) | 22.9 (22.6-23.2) | 29.6 (29.3-29.9) | 24.7 (24.4-25.0) | 2000-2015 | 2.1% | 0.02 | NA | ||||||
|
| ||||||||||||||
| ≥75 y | Y | 65.1 (63.3-66.8) | 64.4 (62.9-66.0) | 81.8 (80.7-83.0) | 83.6 (82.3-84.8) | 2000-2015 | 1.7% | 0.02 | NA | |||||
|
| ||||||||||||||
| N | 28.8 (28.4-29.2) | 38.7 (38.2-39.1) | 53.2 (52.7-53.7) | 46.2 (45.8-46.7) | 2000-2009 | 5.8% | <0.001 | 2009-2015 | −3.0% | 0.2 | NA | |||
|
| ||||||||||||||
| Women | ||||||||||||||
|
| ||||||||||||||
| 18-44 y | Y | 14.4 (13.4-15.4) | 18 (17.2-18.9) | 21.9 (21.1-22.8) | 18.1 (17.1-19.1) | 2000-2015 | 4.2% | 0.01 | NA | |||||
|
| ||||||||||||||
| N | 1.0 (1.0-1.1) | 1.5 (1.5-1.6) | 1.9 (1.8-1.9) | 1.5 (1.5-1.5) | 2000-2015 | 2.7% | <0.001 | NA | ||||||
|
| ||||||||||||||
| 45-64 y | Y | 29.2 (28.5-29.9) | 29.9 (29.3-30.5) | 41.7 (41.2-42.1) | 38.6 (38.2-39.1) | 2000-2015 | 2.2% | 0.01 | NA | |||||
|
| ||||||||||||||
| N | 3.4 (3.3-3.5) | 4.5 (4.4-4.5) | 6.4 (6.3-6.4) | 6.3 (6.3-6.4) | 2000-2007 | 7.1% | <0.001 | 2009-2015 | −0.1% | 0.9 | ||||
|
| ||||||||||||||
| 65-74 y | Y | 47.7 (46.6-48.8) | 48.4 (47.4-49.3) | 64.4 (63.5-65.3) | 58.8 (58-59.6) | 2000-2015 | 1.7% | 0.01 | NA | |||||
|
| ||||||||||||||
| N | 8.7 (8.5-8.9) | 13.1 (12.9-13.3) | 18.6 (18.4-18.8) | 15.0 (14.8-15.2) | 2000-2009 | 7.1% | <0.001 | 2009-2015 | −2.8% | 0.2 | ||||
|
| ||||||||||||||
| ≥75 y | Y | 45.4 (44.2-46.7) | 59.8 (58.7-61.0) | 71.5 (70.4-72.5) | 56.6 (55.6-57.6) | 2000-2015 | 1.3% | 0.2 | NA | |||||
|
| ||||||||||||||
| N | 13.7 (13.4-13.9) | 21.4 (21.1-21.6) | 25.5 (25.2-25.8) | 22.8 (22.5-23.1) | 2000-2008 | 6.3% | <0.001 | 2008-2015 | −1.6% | 0.2 | ||||
Abbreviations: Δ/y, annual change; AKI, acute kidney injury; CI, confidence interval; DM, diabetes mellitus; N, no; NA, no second/third trend identified; Y, yes.
Data sources: National Center for Health Statistics, National Health Interview Survey, and National Inpatient Sample.
Indicates the year in which linear trends change in either direction or magnitude.
Total rates are age-standardized.
Similar trends in AKI-D rates were observed in men and women with and without diabetes. However, absolute rates remained higher in men versus women across all age groups (Table 1 and Fig S2). By age, among adults with diabetes, absolute rates remained highest among older age groups, though the greatest relative increases were observed in the younger age groups (18-44 and 45-64 years). In adults without diabetes, most sex-specific age groups saw an increase in AKI-D between 2000 and 2008/2009, followed by no statistically significant change, with the greatest relative increases observed in the 45 to 64 and 75 year and older age groups.
AKI-D–Associated In-Hospital Mortality
In-hospital mortality associated with AKI-D decreased during the study period, from 23.1 per 100 persons to 15.2 in adults with diabetes (Δ/y, −3.9%; P = 0.01) and from 34.4 to 28.7 in adults without diabetes (Δ/y, −2.0%; P < 0.001) (Table 2 and Fig 2). Similar trends were observed for men and women, though the decline among women with diabetes was not significant (Table 2 and Fig S3). All age groups experienced a significant decline, excluding age group 18 to 44 years, with no significant change observed in patients with or without diabetes.
Table 2.
Trends in Dialysis-Requiring AKI–Associated In-Hospital Mortality in People With Versus Without Diabetes, by Age and Sex, United States 2000-2015
| DM | AKI Hospitalization Rate, per 100 persons (95% CI) |
Trend 1a |
||||||
|---|---|---|---|---|---|---|---|---|
| 2000 | 2005 | 2010 | 2015 | Year | Δ/y | P | ||
| Total b | ||||||||
|
| ||||||||
| Y | 23.1 (18.2-28.0) | 14.7 (11.7-17.7) | 12.0 (9.9-14.0) | 15.2 (12.0-18.4) | 2000-2015 | −3.9% | 0.01 | |
|
| ||||||||
| N | 34.4 (32.0-36.8) | 33.4 (31.6-35.2) | 28.7 (27.3-30.1) | 28.4 (26.6-30.2) | 2000-2015 | −2.0% | <0.001 | |
|
| ||||||||
| Sex b | ||||||||
|
| ||||||||
| Male | Y | 13.5 (11.0-16.0) | 9.4 (7.7-11.0) | 5.3 (4.5-6.1) | 5.9 (4.7-71) | 2000-2015 | −5.4% | <0.001 |
|
| ||||||||
| N | 20.0 (18.9-21.0) | 18.5 (17.7-19.3) | 14.7 (14.1-15.3) | 14.9 (14.1-15.6) | 2000-2015 | −1.6% | <0.001 | |
|
| ||||||||
| Female | Y | 9.6 (7.2-12.0) | 5.3 (3.9-6.6) | 6.7 (5.4-8.0) | 9.3 (7.2-11.3) | 2000-2015 | −2.1% | 0.2 |
|
| ||||||||
| N | 14.4 (13.1-15.8) | 15.0 (14.0-16.0) | 14.0 (13.2-14.8) | 13.5 (12.4-14.6) | 2000-2015 | −2.4% | 0.004 | |
|
| ||||||||
| Age Group | ||||||||
|
| ||||||||
| 18-44 y | Y | 8.9 (5.5-12.2) | 5.8 (3.7-7.8) | 4.1 (2.6-5.5) | 7.3 (4.9-9.8) | 2000-2015 | −3.2% | 0.2 |
|
| ||||||||
| N | 9.4 (8.2-10.6) | 12.8 (11.8-13.7) | 8.2 (7.4-9.0) | 9.7 (8.6-10.8) | 2000-2015 | −1.5% | 0.2 | |
|
| ||||||||
| 45-64 y | Y | 11.0 (9.8-12.2) | 6.5 (5.8-7.2) | 6.9 (6.4-7.4) | 7.3 (6.7-7.9) | 2000-2015 | −3.8% | <0.001 |
|
| ||||||||
| N | 23.3 (22.2-24.5) | 19.7 (18.9-20.4) | 21.3 (20.7-21.9) | 18.3 (17.7-19.0) | 2000-2015 | −1.8% | 0.002 | |
|
| ||||||||
| 65-74 y | Y | 20.4 (18.8-22.1) | 9.7 (8.6-10.7) | 10.7 (10.0-11.5) | 6.1 (5.5-6.8) | 2000-2015 | −6.2% | <0.001 |
|
| ||||||||
| N | 31.6 (30.1-33.2) | 22.6 (21.6-23.6) | 20.8 (20.0-21.5) | 22.0 (21.1-22.9) | 2000-2015 | −2.1% | <0.001 | |
|
| ||||||||
| ≥75 y | Y | 21.3 (19.4-23.2) | 18.1 (16.8-19.5) | 10.0 (9.3-10.7) | 12.0 (11.1-13.0) | 2000-2015 | −6.3% | <0.001 |
|
| ||||||||
| N | 29.6 (28.5-30.8) | 25.3 (24.5-26.1) | 21.9 (21.3-22.5) | 19.6 (18.8-20.3) | 2000-2015 | −3.6% | <0.001 | |
Abbreviations: Δ/y, annual change; AKI, acute kidney injury; CI, confidence interval; DM, diabetes mellitus; N, no; Y, yes.
Data sources: National Center for Health Statistics, National Health Interview Survey, and National Inpatient Sample.
Indicates the year in which linear trends change in either direction or magnitude.
Total and sex-specific rates are age-standardized.
Figure 2.
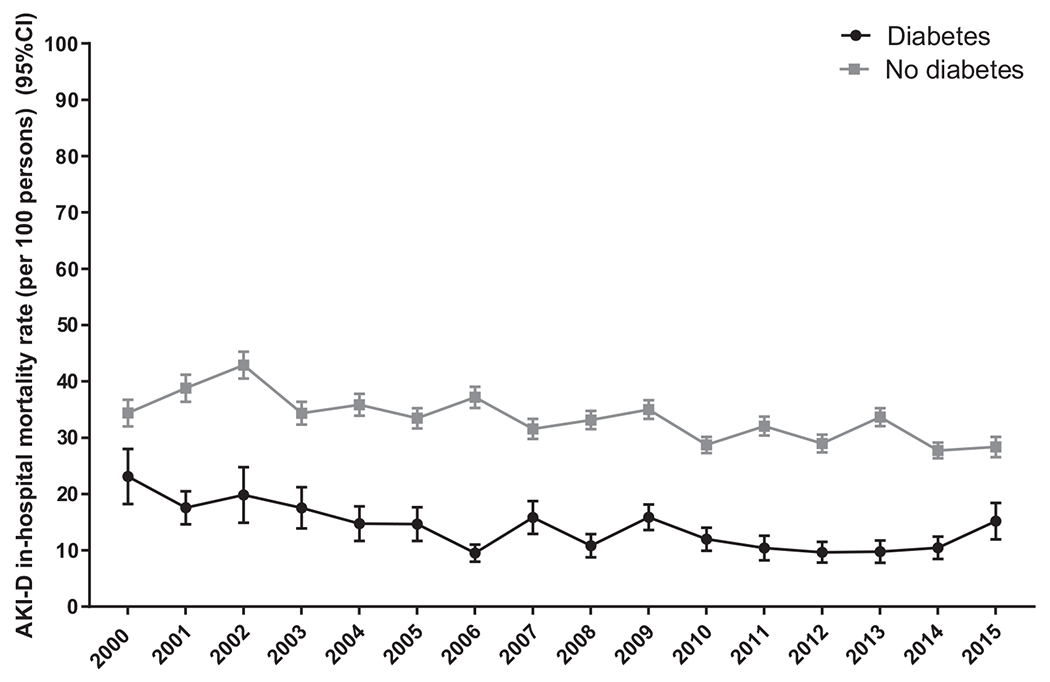
Trends in age-standardized in-hospital mortality associated with dialysis-requiring acute kidney injury (AKI-D) in people with versus without diabetes, United States 2000-2015. Abbreviation: CI, confidence interval.
Comorbid Conditions
Among AKI-D hospitalizations in patients with diabetes, the proportion of hospitalizations with low to moderate comorbid conditions did not change over time, while the proportion of high-comorbid hospitalizations increased between 2000 and 2009 (Δ/y, 4.4%; P = 0.02) and then plateaued (Table 3). In patients without diabetes, the proportion of hospitalizations with low morbidity decreased between 2000 and 2015 (Δ/y, −0.8%; P < 0.001), coinciding with an increase in the proportion of high-morbidity hospitalizations (Δ/y, 1.7%; P = 0.007). Patterns of specific comorbid conditions also changed over time. Notably, among patients with diabetes, the proportion of chronic pulmonary disease, liver disease (mild and moderate/severe), rheumatic disease, and kidney disease increased. In contrast, hypertension, myocardial infarction, congestive heart failure, and peripheral vascular disease declined between 2000 and 2015 (Table 3 and Fig S4). In addition, throughout the period, the proportion of men increased (from 50.6% to 57.1%; P < 0.001), and following an initial period of decline, the proportion of cardiac procedures increased beginning in 2009.
Table 3.
Time-Stratified Characteristics of Patients With Dialysis-Requiring AKI, by Diabetes Status, in the United States 2000-2015
| DM | Value (95% CI)a |
Trend 1b |
Trend 2b |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2005 | 2010 | 2015 | Year | Δ/yd | Year | Δ/yd | ||
| Characteristics | |||||||||
|
| |||||||||
| Male sex | Y | 50.6 (47.1-54.0) | 51.9 (48.9-55.0) | 55.3 (53.0-57.5) | 57.1 (54.3-59.3) | 2000-2015 | 0.7%d | NA | |
|
| |||||||||
| N | 58.9 (56.4-61.3) | 58.6 (56.6-60.6) | 58.3 (56.5-60.0) | 60.1 (58.2-61.0) | 2000-2015 | 0.0% | |||
|
| |||||||||
| Age, y | Y | 64.7 (63.8-65.7) | 64.2 (63.4-65.0) | 64.3 (63.7-65.0) | 64.9 (64.4-65.1) | 2000-2006 | −0.4% | 2006-2015 | 0.1% |
|
| |||||||||
| N | 62.3 (61.1-63.4) | 63.0 (61.9-64.0) | 63.1 (62.4-63.9) | 63.3 (62.7-64.0) | 2000-2015 | 0.0% | |||
|
| |||||||||
| Hospitalization duration, d | Y | 11.0 (6.0-17.0) | 9.0 (5.0-15.0) | 9.0 (5.0-14.0) | 9.0 (6.0-14.0) | —e | |||
|
| |||||||||
| N | 10.0 (6.0-18.0) | 11.0 (7.0-18.0) | 11.0 (6.0-18.0) | 11.0 (7.0-19.0) | —e | ||||
|
| |||||||||
| Cardiac procedures | Y | 16.3 (13.6-19.3) | 13.2 (11.0-15.9) | 10.3 (8.5-12.4) | 13.8 (12.2-15.7) | 2000-2009 | −5.0%d | 2009-2015 | 5.2%d |
| N | 13.3 (11.1-15.9) | 11.8 (10.3-13.6) | 10.7 (9.2-12.4) | 14.3 (12.8-15.9) | 2000-2009 | −1.3%d | 2009-2015 | 4.2%d | |
|
| |||||||||
| Comorbid Conditions | |||||||||
|
| |||||||||
| CCI score 0-2 | Y | 39.3 (35.7-43.0) | 39.7 (36.4-43.1) | 36.8 (34.7-39.0) | 38.9 (36.6-41.2) | 2000-2015 | −0.0% | NA | |
|
| |||||||||
| N | 48.5 (45.9-51.2) | 45.0 (43.0-47.1) | 41.7 (40.0-43.7) | 41.6 (39.7-43.5) | 2000-2015 | −0.8%d | NA | ||
|
| |||||||||
| CCI score 3-5 | Y | 55.5 (51.5-59.4) | 54.7 (51.4-58.0) | 57.4 (55.3-59.6) | 56.6 (54.2-58.9) | 2000-2015 | −0.1% | NA | |
|
| |||||||||
| N | 42.1 (39.5-44.7) | 45.1 (42.9-47.2) | 48.3 (46.5-50.1) | 47.4 (45.5-49.4) | 2000-2010 | 1.0%d | 2010-2015 | −0.3% | |
|
| |||||||||
| CCI score 6-8 | Y | 3.6 (2.6-5.1) | 4.2 (3.3-5.3) | 4.2 (3.5-5.1) | 3.1 (2.4-4.0) | 2000-2015 | −0.1% | NA | |
|
| |||||||||
| N | 6.6 (5.5-79) | 6.5 (5.6-7.8) | 6.9 (6.0-7.8) | 7.2 (6.3-8.3) | 2000-2015 | 0.5% | NA | ||
|
| |||||||||
| CCI score ≥9 | Y | 1.6 (0.9-2.8) | 1.4 (0.9-2.2) | 1.5 (1.1-2.1) | 1.5 (1.0-2.2) | 2000-2009 | 4.4%d | 2009-2015 | −4.7% |
|
| |||||||||
| N | 2.8 (2.0-3.7) | 3.4 (2.8-4.2) | 3.1 (2.6-3.8) | 3.7 (3.1-4.5) | 2000-2015 | 1.7%d | NA | ||
|
| |||||||||
| HTN | Y | 18.8 (16.2-21.7) | 14.7 (12.5-17.1) | 9.8 (8.7-11.2) | 9.1 (7.8-10.6) | 2000-2009 | −7.2%d | 2009-2015 | −1.4% |
|
| |||||||||
| N | 14.6 (12.7-16.6) | 11.8 (10.5-13.3) | 10.6 (9.4-11.8) | 10.4 (9.3-11.7) | 2000-2015 | −2.0%d | |||
|
| |||||||||
| MI | Y | 13.3 (10.7-16.3) | 9.6 (8.0-11.4) | 10.8 (9.6-12.2) | 8.6 (7.4-9.9) | 2000-2015 | −2.6%d | NA | |
|
| |||||||||
| N | 9.6 (8.3-11.2) | 9.6 (8.4-10.8) | 10.3 (9.3-11.5) | 8.9 (7.8-10.1) | 2000-2015 | −0.9%d | |||
|
| |||||||||
| CHF | Y | 55.4 (51.6-59.1) | 47.5 (44.1-50.9) | 45.1 (43.0-472) | 46.3 (43.9-48.6) | 2000-2015 | −1.3%d | NA | |
|
| |||||||||
| N | 39.3 (36.7-41.9) | 39.2 (37.0-41.5) | 35.4 (33.7-37.1) | 36.2 (34.3-38.3) | 2000-2015 | −0.7%d | |||
|
| |||||||||
| PVD | Y | 12.7 (10.6-15.2) | 10.3 (8.7-12.2) | 11.3 (9.9-12.8) | 9.9 (8.6-11.4) | 2000-2015 | −1.1%d | NA | |
|
| |||||||||
| N | 6.8 (5.7-8.2) | 7.0 (6.0-8.2) | 8.5 (7.6-9.6) | 8.3 (7.3-9.4) | 2000-2015 | 0.0% | |||
|
| |||||||||
| CBVD | Y | 6.5 (5.0-8.4) | 4.2 (3.3-5.5) | 6.5 (5.6-7.5) | 6.0 (5.0-7.2) | 2000-2015 | 0.3% | NA | |
|
| |||||||||
| N | 6.7 (5.5-8.1) | 4.1 (3.4-5.0) | 5.9 (5.2-6.7) | 6.8 (5.9-7.9) | 2000-2015 | 1.2% | |||
|
| |||||||||
| Dementia | Y | 0.5 (0.2-1.3) | 0.8 (0.4-1.4) | 1.4 (0.9-1.9) | 0.6 (0.3-1.1) | 2000-2015 | −1.9% | NA | |
|
| |||||||||
| N | 1.2 (0.8-1.9) | 0.8 (0.5-1.2) | 1.0 (0.7-1.4) | 1.4 (1.0-1.9) | 2000-2015 | 1.2% | |||
|
| |||||||||
| Chronic pulmonary disease | Y | 17.6 (15.2-20.3) | 21.1 (18.7-23.6) | 23.9 (22.1-25.9) | 23.9 (22.0-26.0) | 2000-2015 | 1.5%d | NA | |
|
| |||||||||
| N | 19.3 (17.4-21.5) | 22.1 (20.3-24.0) | 19.1 (17.5-20.9) | 20.2 (18.7-21.9) | 2000-2015 | 0.0% | |||
|
| |||||||||
| Peptic ulcer disease | Y | 2.2 (1.4-3.5) | 1.8 (1.2-2.7) | 1.7 (1.3-2.4) | 1.5 (1.0-2.2) | 2000-2015 | −1.9% | NA | |
|
| |||||||||
| N | 2.0 (1.5-2.8) | 1.6 (1.2-2.3) | 1.9 (1.5-2.5) | 2.0 (1.6-2.6) | 2000-2015 | 0.9% | |||
|
| |||||||||
| Rheumatic disease | Y | 1.3 (0.7-2.3) | 1.6 (1.0-2.4) | 1.4 (1.0-1.9) | 1.4 (0.9-2.0) | 2000-2015 | 3.5%d | NA | |
|
| |||||||||
| N | 3.8 (2.9-4.9) | 3.1 (2.4-3.8) | 3.4 (2.9-4.1) | 2.7 (2.1-3.4) | 2000-2015 | −0.2% | |||
|
| |||||||||
| Mild liver disease | Y | 5.6 (4.2-7.3) | 6.4 (5.2-7.8) | 7.6 (6.6-8.7) | 7.5 (6.3-8.9) | 2000-2015 | 3.2%d | NA | |
|
| |||||||||
| N | 11.0 (9.5-12.6) | 12.4 (11.0-14.0) | 15.6 (14.0-17.3) | 18.3 (16.9-19.8) | 2000-2015 | 3.3%d | |||
|
| |||||||||
| Moderate or severe liver disease | Y | 2.0 (1.2-3.2) | 2.0 (1.3-3.0) | 2.9 (2.3-3.6) | 2.7 (2.0-3.5) | 2000-2015 | 3.8%d | NA | |
|
| |||||||||
| N | 4.0 (3.1-5.2) | 4.0 (3.1-5.1) | 7.2 (6.2-8.4) | 8.0 (7.0-9.2) | 2000-2015 | 5.4%d | |||
|
| |||||||||
| Hemiplegia or paraplegia | Y | 1.0 (0.6-2.0) | 0.4 (0.2-1.0) | 1.3 (0.9-1.8) | 1.0 (0.6-1.6) | 2000-2015 | 1.7% | NA | |
|
| |||||||||
| N | 0.9 (0.5-1.4) | 0.7 (0.4-1.1) | 1.7 (1.3-2.1) | 1.8 (1.3-2.3) | 2000-2015 | 7.4%d | |||
|
| |||||||||
| Kidney disease | Y | 63.3 (61.5-68.9) | 73.4 (70.2-76.3) | 79.3 (77.6-81.0) | 76.6 (74.5-78.5) | 2000-2008 | 2.5%d | 2008-2015 | −0.5% |
|
| |||||||||
| N | 48.4 (45.1-51.7) | 55.6 (53.3-57.9) | 60.7 (58.5-62.8) | 57.8 (55.9-59.8) | 2000-2015 | 1.0%d | |||
|
| |||||||||
| Malignancyc | Y | 5.6 (4.2-7.5) | 4.5 (3.4-5.8) | 6.0 (5.1-6.9) | 5.0 (4.1-6.1) | 2000-2015 | 0.4% | NA | |
|
| |||||||||
| N | 10.5 (9.1-12.0) | 12.0 (10.6-13.6) | 10.3 (9.2-11.5) | 12.4 (11.2-13.7) | 2000-2015 | 0.9%d | |||
|
| |||||||||
| Metastatic solid tumor | Y | 2.0 (1.2-3.4) | 1.9 (1.3-2.7) | 1.7 (1.2-2.4) | 1.5 (1.0-2.2) | 2000-2015 | 1.1% | NA | |
|
| |||||||||
| N | 3.4 (2.6-4.5) | 4.5 (3.8-5.4) | 3.9 (3.3-4.6) | 4.8 (4.0-5.7) | 2000-2015 | 0.9% | |||
|
| |||||||||
| HIV/AIDS | Y | 0.5 (0.2-1.2) | 0.6 (0.3-1.1) | 0.4 (0.2-0.8) | 0.4 (0.2-0.9) | 2000-2015 | −1.1% | NA | |
|
| |||||||||
| N | 3.4 (2.6-4.5) | 2.4 (1.9-3.1) | 1.3 (1.2-2.3) | 1.3 (1.0-1.8) | 2000-2015 | −5.7%d | |||
Abbreviations: Δ/y, annual change; AKI, acute kidney injury; CBVD, cerebrovascular disease; CCI, Charlson Comorbidity Index; CHF, congestive heart failure; CI, confidence interval; DM, diabetes mellitus; HIV, human immunodeficiency virus; HTN, hypertension; MI, myocardial infarction; N, no; NA, no second/third trend identified; PVD, peripheral vascular disease; Y, yes.
Data sources: National Center for Health Statistics and National Inpatient Sample.
Data are reported as proportions, except age (mean) and hospitalization duration (median); values in parentheses are 95% CIs.
Indicates the year in which linear trends change in either direction or magnitude.
Except malignant neoplasm of skin.
P < 0.05.
Linear trend not assessed because data are not normally distributed.
Similar patterns were noted in patients without diabetes with some exceptions: (1) the proportion of men did not increase, (2) malignancies and hemiplegia or paraplegia increased and human immunodeficiency virus infection/AIDS declined (compared with no change among patients with diabetes), and (3) peripheral vascular disease showed no change (compared with declines in patients with diabetes).
Discussion
In this nationally representative study, we report several key findings in the trends of AKI-D hospitalization among US adults with and without diabetes. First, absolute rates for AKI-D within the hospital setting remain almost 5 times as high in adults with versus without diabetes, and this excess risk has not improved over time. Second, AKI-D increased throughout the period in adults with diabetes but increased between 2000 and 2009 and then plateaued in adults without diabetes. Third, relative increases in AKI-D were greatest in younger versus older adults with diabetes. Fourth, absolute declines in in-hospital mortality associated with AKI-D were greater in adults with versus without diabetes. Last, the proportion of AKI-D hospitalizations with liver, rheumatic, and renal comorbid conditions has increased, while the proportion of most cardiovascular comorbid conditions has decreased. Collectively, our findings illustrate the clinical and public health importance of AKI-D in the United States and highlight the opportunity to prevent and manage its life-threatening complications, particularly among young adults with diabetes.
Our results are consistent with prior studies in the United States showing an increase in secular trends in the incidence of AKI-D in the general population.13,23–26 Similar increases have also been shown in general populations in England,27 Scotland,28 Denmark,29 and Lithuania.30 There are several possible reasons for the global increases in AKI-D. First, increasing patient age is a commonly cited reason for the increase in AKI-D.31 However, the greatest relative increases in AKI-D in this study were observed in younger adults. Further, increases in age-standardized rates suggest that age cannot explain all of this observed increase.
Second, increasing awareness of AKI may result in a change in coding practices. However, procedure codes for AKI-D have superior validity versus codes for non–dialysis-requiring AKI and are unlikely to be affected by changes in diagnostic coding practice.32
Third, it is possible that the shift in AKI-D may also represent a shift in clinical paradigm whereby clinicians are more willing to offer dialysis therapy to patients with AKI. However, parallel increases in both AKI-D and non–dialysis-requiring AKI suggest that this cannot explain all the observed increases.13
Last, better detection and awareness of AKI may lead to an increase in referral rates of patients with AKI to nephrologists.33,34 At the same time, lack of referral or delayed referrals may result in an increased incidence of AKI-D because of progression in AKI stages.33 However, collectively, these reasons fail to explain differential trends in adults with and without diabetes.
The increasing rates of AKI-D among adults with diabetes seen in this study are consistent with a recent resurgence of other diabetes-related complications in the United States.35 Between 2010 and 2015, national statistics have reported increases in lower-extremity amputations and hyperglycemic crises, while long-term improvements in rates of kidney failure, acute myocardial infarction, and stroke have stalled.35 Further, the recent increase in complication rates is occurring in young (aged 18-44 years) and middle-aged (aged 45-64 years) adults; in these individuals, the risk for hyperglycemic crisis, acute myocardial infarction, stroke, and lower-extremity amputations each increased in excess of 25% during just 5 years.35 We add to this growing body of literature that increases in AKI-D also disproportionally affect young adults with diabetes at or around the same time.
Gregg et al35 offered several key plausible reasons that may explain these trends. First, the profile of newly identified diabetes cases may be changing. Higher levels of obesity prevalence, smoking, and poor management of blood pressure and lipid levels are observed among younger versus older adults with diabetes.36 This combined with chronic kidney disease may lead to earlier onset of complications such as AKI. Second, decreasing mortality among those with diabetes is lengthening the average duration of diabetes in the population and this shift may be affecting the risk for complications.35 Third, there may be stagnation in preventive care, evidenced by a decline in the proportion of young adults with diabetes meeting individualized haemoglobin A1c targets.37 Fourth, the introduction of high-deductible health care plans may have contributed to reductions in early preventive care, including nephrologist referral.38 In addition, the increasing cost of insulin and other diabetes medications could lead to rationing by patients to minimize costs, thus exposing them to increased risk for complications.39
On a more positive note, we show that AKI-D–associated in-hospital mortality has significantly declined between 2000 and 2015, except for those aged 18 to 44 years, for whom absolute rates are low, and this decline was greater in adults with versus without diabetes. Declines in AKI-D–associated mortality have been shown in several other studies.24,40,41 Reasons for the decline are not clear, though it is hypothesized that this may be due to improved dialysis and intensive care unit care, including intensive insulin therapy, which may benefit patients with diabetes more.42 In addition, some studies have suggested an association between earlier nephrology consultation and lower mortality after AKI.43 Reasons for the higher absolute in-hospital mortality rates among adults without versus with diabetes are unclear. Previous studies have shown diabetes and hyperglycemia to be independent risk factors for in-hospital mortality.44,45 However, more recent studies have failed to show such an association.46,47 It is hypothesized that relative to what was seen in older studies, diabetes is less of a risk factor for in-hospital mortality because insulin analogues have mostly replaced human insulins and sliding scales have been abandoned, changes that have lowered the incidence of hypoglycemia.47 In addition, in this study we showed that the proportion of hospitalizations with high severity, according to the Charlson Comorbidity Index, were consistently higher for adults without versus with diabetes (Table 3), which may also have contributed to the higher absolute in-hospital mortality rates in adults without diabetes. In addition, NIS data are hospital-level rather than person-level data. It therefore is possible that the in-hospital mortality among adults with diabetes is under-estimated because a person with diabetes is more likely to have several readmissions, which will inflate the denominator.
The results of this study have some implications for public health and health care practice. First, in this study we show that diabetes confers a 5-fold increased risk for AKI-D. The increasing prevalence of diabetes can be expected to increase the number of persons with AKI. Better awareness on the part of health care providers that diabetes is an important risk factor for AKI may help reduce its occurrence. For example, assessment of diabetes at hospital admission may assist physicians in better recognizing those at high risk for AKI who may warrant preventive therapeutic strategies such as closer monitoring of glucose levels and kidney function. In the intensive care unit setting, the KDIGO (Kidney Disease: Improving Global Outcomes) guideline recommends target glucose levels of 110 to 149 mg/dL to reduce AKI incidence without further aggravating mortality.48 Better assessment of comorbid conditions, in particular liver, renal, rheumatic, and chronic pulmonary comorbid conditions, may also improve the treatment of underlying conditions and prevent or mitigate additional damage to the kidneys.
To our knowledge, this is the largest nationally representative study to explore rates of AKI-D and AKI-D–associated mortality over time in adults with versus without diabetes. Nonetheless, there are limitations to be considered. First, we lack laboratory data to corroborate the diagnosis of AKI. However, previous studies have shown high sensitivity (90.4%) and specificity (93.8%) when using ICD-9-CM to diagnose AKI-D.32 Second, this analysis is unable to distinguish whether AKI was acquired within the hospital or in a community setting. In addition, the NIS data represent hospital discharges, not individual persons and therefore may include multiple hospital stays for some people. Third, all types of diabetes are included in the current analysis with the assumption that the vast majority (90%-95%) have type 2.49 In addition, the NHIS does not include undiagnosed diabetes and thus misclassification may have occurred for a number of patients. Last, we were unable to adjust for a number of possible confounders, including race or ethnicity, body mass index, prior chronic kidney disease, smoking, and socioeconomic position.
In conclusion, in the United States between 2000 and 2015, hospitalization rates of AKI-D increased in adults with and without diabetes while AKI-D–associated mortality declined. However, AKI-D remained substantially higher in adults with versus without diabetes and this excess risk has not improved over time. Most alarmingly, relative increases in AKI-D were greatest among young adults with diabetes. Increases in AKI-D may be explained in part by increases in liver, renal, and rheumatic, but not cardiovascular, comorbid conditions. This study highlights the need for greater AKI risk factor mitigation above and beyond traditional cardiovascular prevention and management, especially in young adults with diabetes.
Supplementary Material
Figure S1: Flow chart depicting use of NHIS and NIS to estimate national AKI-D in people with and without diabetes.
Figure S2: Trends in sex- and age-specific hospitalization rates for AKI-D in people with vs without diabetes, by sex, United States 2000-2015.
Figure S3: Trends in sex- and age-specific in-hospital mortality rates associated with AKI-D in people with vs without diabetes, United States 2000-2015.
Figure S4: Time-stratified characteristics of patients with AKI-D by diabetes status in the United States 2000-2015.
Table S1: ICD-9-CM codes for comorbid conditions and cardiac procedures.
Acknowledgements:
The authors thank the women and men who participated in the study and all staff involved at the National Center for Health Statistics for the study design, data collection, and data dissemination.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Supplementary Material
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Lameire N, Biesen WV, Vanholder R. Acute kidney injury. Lancet. 2008;372(9653):1863–1865. [DOI] [PubMed] [Google Scholar]
- 2.Moore BJ,Torio CM. Acute Renal Failure Hospitalizations, 2005-2014: Statistical Brief #231. Rockville, MD: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; 2006. [Google Scholar]
- 3.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1(1):43–51. [DOI] [PubMed] [Google Scholar]
- 4.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76(8):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr M, Bedford M, Matthews B, O’Donoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014;29(7):1362–1368. [DOI] [PubMed] [Google Scholar]
- 6.Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald R, McArthur E, Adhikari NK, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015;65(6):870–877. [DOI] [PubMed] [Google Scholar]
- 8.Brown JR, Rezaee ME, Hisey WM, Cox KC, Matheny ME, Sarnak MJ. Reduced mortality associated with acute kidney injury requiring dialysis in the United States. Am J Nephrol. 2016;43(4):261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng X, McMahon GM, Brunelli SM, Bates DW, Bates SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3)(suppl 1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patschan D, Muller GA. Acute kidney injury in diabetes mellitus. Int J Nephrol. 2016;2016:6232909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Diabetes Federation. IDF Diabetes Atlas. 8th ed. International Diabetes Federation; 2017. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/13-diabetes-atlas-seventh-edition.html. Accessed April 19, 2019. [Google Scholar]
- 13.Pavkov ME, Harding JL, Burrows NR. Trends in hospitalizations for acute kidney injury - United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2018;67(10):289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics. National Health Interview Survey, 2015. Public-use data file and documentation. http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm2016. Accessed April 19, 2019. [Google Scholar]
- 15.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. NIS database documentation. Rockville, MD: Agency for Healthcare Research and Quality; 2017. http://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. Accessed April 19, 2019. [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 18.Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J Clin Epidemiol. 2007;60(9):874–882. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Joinpoint Trend Analysis Software. https://surveillance.cancer.gov/joinpoint/. Accessed April 19, 2019.
- 20.Agency for Healthcare Research and Quality. CUP NIS Trend Weights. Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality; 2015. www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Accessed April 19, 2019. [Google Scholar]
- 21.Houchens RL, Ross D, Elixhauser A. Using the HCUP National Inpatient Sample to Estimate Trends. 2015. HCUP Methods Series Report # 2006-05 ONLINE. U.S. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp. Accessed April 19, 2019. [Google Scholar]
- 22.Elixhauser A, Heslin KC, Owens PL. Healthcare cost and utilization project (HCUP) recommendations for reporting trends using ICD-9-CM and ICD-10-CM/PCS data. Agency for Healthcare Research and Quality; 2017. https://www.hcup-us.ahrq.gov/datainnovations/icd10_resources.jsp. Accessed April 19, 2019. [Google Scholar]
- 23.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol. 2013;24(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu RK, McCulloch CE, Heung M, et al. Exploring potential reasons for the temporal trend in dialysis-requiring AKI in the United States. Clin J Am Soc Nephrol. 2016;11(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siew ED, Davenport A. The growth of acute kidney injury: a rising tide or just closer attention to detail? Kidney Int. 2015;87(1):46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolhe NV, Muirhead AW, Wilkes SR, Fluck RJ, Taal MW. National trends in acute kidney injury requiring dialysis in England between 1998 and 2013. Kidney Int. 2015;88(5):1161–1169. [DOI] [PubMed] [Google Scholar]
- 28.Rennie TJ, Patton A, Dreischulte T, Bell S. Incidence and outcomes of acute kidney injury requiring renal replacement therapy: a retrospective cohort study. Nephron. 2016;133(4):239–246. [DOI] [PubMed] [Google Scholar]
- 29.Carlson N, Hommel K, Olesen JB, et al. Dialysis-requiring acute kidney injury in Denmark 2000-2012: time trends of incidence and prevalence of risk factors—a nationwide study. PLoS One. 2016;11(2):e0148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skarupskiene I, Balciuviene V, Ziginskiene E, Kuzminskis V, Vaiciuniene R, Bumblyte IA. Changes of etiology, incidence and outcomes of severe acute kidney injury during a 12-year period (2001-2012) in large university hospital. Nephrol Ther. 2016;12(6):448–453. [DOI] [PubMed] [Google Scholar]
- 31.Anderson S, Eldadah B, Halter JB, et al. Acute kidney injury in older adults. J Am Soc Nephrol. 2011;22(1):28–38. [DOI] [PubMed] [Google Scholar]
- 32.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688–1694. [DOI] [PubMed] [Google Scholar]
- 33.Ali T, Tachibana A, Khan I, et al. The changing pattern of referral in acute kidney injury. QJM. 2011;104(6):497–503. [DOI] [PubMed] [Google Scholar]
- 34.Healthy People 2020. Kidney Disease in the United States 2018. https://www.usrds.org/2018/download/v3_c01_HP2020_18_usrds.pdf. Accessed April 19, 2019.
- 35.Gregg EW, Hora I, Benoit SR. Resurgence in diabetes-related complications. JAMA. 2019;321(19):1867–1868. [DOI] [PubMed] [Google Scholar]
- 36.Ali MK, Bullard KM, Saaddine JB, et al. Achievement of goals in US diabetes care, 1999-2010. N Engl J Med. 2013;368(17):1613–1624. [DOI] [PubMed] [Google Scholar]
- 37.Carls G, Huynh J, Tuttle E, et al. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017;8(4):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wharam JF, Zhang F, Eggleston EM, et al. Effect of high-deductible insurance on high-acuity outcomes in diabetes. Diabetes Care. 2018;41(5):940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riddle MC, Herman WH. The cost of diabetes care. Diabetes Care. 2018;41(5):929–932. [DOI] [PubMed] [Google Scholar]
- 40.Brown JR, Rezaee ME, Hisey WM, Cox KC, Matheny ME, Sarnak MJ. Reduced mortality associated with acute kidney injury requiring dialysis in the United States. Am J Nephrol. 2016;43(4):261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenihan CR, Montez-Rath ME, More Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patient. N Engl J Med. 2001;345:1359–1367. [DOI] [PubMed] [Google Scholar]
- 43.Ponce D, de Zorzenon CP, dos Santos NY, Balbi AL. Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrol Dial Transplant. 2011;26:3202–3206. [DOI] [PubMed] [Google Scholar]
- 44.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. [DOI] [PubMed] [Google Scholar]
- 45.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478. [DOI] [PubMed] [Google Scholar]
- 46.Cei M, Mumoli N, Mantellassi M, Marrucci E. Glucose levels at admission and mortality in admitted medical patients: a not so strict association. Eur J Intern Med. 2011;22:e151. [DOI] [PubMed] [Google Scholar]
- 47.Cei F, Cannistraro D, Mumoli N, Cei M. In-hospital mortality and diabetes: absence of association. Eur J Intern Med. 2017;40:e17–e18. [DOI] [PubMed] [Google Scholar]
- 48.Zarbock A, John A, Jorres A, Kindgen-Milles D. New KIDIGO guidelines on acute kidney injury. Practical recommendations. Anaesthetist. 2014;63(7):578–588. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Flow chart depicting use of NHIS and NIS to estimate national AKI-D in people with and without diabetes.
Figure S2: Trends in sex- and age-specific hospitalization rates for AKI-D in people with vs without diabetes, by sex, United States 2000-2015.
Figure S3: Trends in sex- and age-specific in-hospital mortality rates associated with AKI-D in people with vs without diabetes, United States 2000-2015.
Figure S4: Time-stratified characteristics of patients with AKI-D by diabetes status in the United States 2000-2015.
Table S1: ICD-9-CM codes for comorbid conditions and cardiac procedures.


