Abstract
Mouse cathelin-related antimicrobial peptide (CRAMP) and its homologue human cathelicidin (LL-37) play active roles in innate immune responses, angiogenesis, and wound healing. In addition, LL-37/CRAMP fends off microbes and protects against infections in the colon, where the epithelium is exposed to myriad of enteric pathogens. It is increasingly recognized that LL-37/CRAMP maintains colon mucosal barrier integrity, shapes the composition of microbiota, and protects the host from tumorigenesis. In this review, we discuss the importance of LL-37/CRAMP in the homeostasis of the host, with novel findings derived from mice deficient in CRAMP that support the proposition for this natural antimicrobial peptide and an immune modulator as a drug lead for therapeutic development.
Keywords: CRAMP, homeostasis, colitis, cancer, microbiota
I. INTRODUCTION
LL-37 and CRAMP are cathelicidin-related antimicrobial peptides, which belong to a family of host-derived antibacterial polypeptides. The first cathelicidin, cecropin, was isolated in 1980 from tissues of the Hyalophora cecropia moth.1 The first mammalian cathelicidins (bactenecins) were isolated in the late 1980s from bovine neutrophils and were named Bac5 and 7.2 However, some investigators considered that the first mammalian cathelicidin should be rabbit CAP18.3 Until now, cathelicidins have been identified in a range of animals, including cattle, buffalo, horse, pig, sheep, goat, deer, chicken, fish, snake, rhesus monkey, guinea pig, mouse, rat, and human.2,4–17
One cathelicidin gene has been identified in humans which encodes LL-37 of 37 aa residues with a molecular weight of 18 kDa,18,19 also known as hCAP-18, FALL-39, or CAMP—human cationic antimicrobial peptide.18,19 In mice, the gene Cramp was mapped to chromosome 9 in a region of conserved synteny, homologous to the map locations of cathelicidins in human.15
LL-37 is expressed by various cells and tissues such as bone marrow (BM) myeloid cells, neutrophils, macrophages and epithelial cells. In human tissues, the expression of LL-37 is detected in the skin and gastrointestinal tract, including mouth, tongue, esophagus, and colon, as well as in the urinary tract and the lung (Table 1).20–23 CRAMP was expressed abundantly by mouse granulocytes and bone marrow cells of the myeloid lineage, which agrees with the sites of expression of cathelicidins in humans and during embryogenesis as early as E12, the earliest stage of blood development.15,18,24,25 CRAMP is detectable in adult mouse testis, spleen, stomach, and intestine but not in brain, liver, heart, or skeletal muscle.15 Both LL-37 in human and mouse CRAMP possess intrinsic antimicrobial activity to act as “natural antibiotics” in the host. However, they are also able to activate host cells by interacting with cellular receptors.
TABLE 1:
Distribution of LL-37/CRAMP in cells and tissues
| Leukocytes | Neutrophils |
| Macrophages | |
| B cells | |
| γδ T cells | |
| Epithelial cells | Mast cells |
| Lung | |
| Stomach | |
| Colon | |
| Urinary tract | |
| Cervix | |
| Body fluids | Inflamed skin |
| Bronchoalveolar lavage fluid | |
| Seminal plasma | |
| Cervicovaginal secretion | |
| Saliva | |
| Plasma |
II. RECEPTOR FOR LL-37/CRAMP
It has been demonstrated that LL-37 uses human formyl peptide receptor 2 (FPR2), a G-protein–coupled, seven-transmembrane domain receptor,26 as the receptor to mediate its chemotactic and angiogenic effects on myeloid cells.27,28 Fpr2 is the mouse homologue of FPR2. Mouse CRAMP utilizes Fpr2 to induce leukocyte chemotaxis and activation.29 There is a significantly reduced recruitment of Ly6C+ inflammatory dendritic cells (DC) into the bronchiolar area in the allergic inflammatory airway of Fpr2- or CRAMP-deficient mice.30 Injection of mouse CRAMP into skin air pouches results in the accumulation of neutrophils and monocytes, confirming the capacity of CRAMP to act as a chemoattractant in vivo via Fpr2.29
Interestingly, LL-37 is also reported to interact with a P2X7 receptor and epidermal growth factor receptor (EGFR).31 P2X7 receptors have been implicated in ATP-mediated cell death, in the regulation of receptor trafficking and inflammation.32–34 LL-37 promotes high glucose–attenuated epithelial wound healing in cultured corneas35 and activates innate immunity on airway epithelial surfaces by EGFR transactivation.36 Furthermore, LL-37 is able to activate insulin-like growth factor-1 receptor (IGF-1R) on cancer cells, which results in increased cell proliferation and the manifestation of a metastatic phenotype.37 Therefore, LL-37 appears to activate multiple cellular receptors to exert biological effects.
III. LL-37/CRAMP IS REQUIRED FOR COLON EPITHELIAL BARRIER INTEGRITY
The colon mucosal barrier consists of epithelial and immune cells with participation of a balanced microbiota. LL-37/CRAMP as a natural antimicrobial peptide, produced by colon epithelial cells and macrophages, plays an important role in maintaining colon microbiota balance and supports mucosal homeostasis.
A. Contribution of CRAMP to Intact Colon Crypt Structure
In the colon, LL-37/CRAMP is detectable in epithelial cells located on the luminal surface and in upper crypts with little or no expression in deeper crypts.38 The peptide is likely associated with the differentiation of colon epithelial cells because LL-37 mRNA and protein were upregulated in spontaneously differentiating Caco-2 human colon epithelial cells as well as in HCA-7 human colon epithelial cells treated with a differentiation-inducing agent, sodium butyrate.38 In CRAMP−/− mice, the length of colonic crypts was significantly shortened, implying a consequence of reduced proliferation of epithelial cells due to lack of CRAMP as a possible differentiation stimulant.39
B. Contribution of CRAMP to Colon Mucus Integrity
Human normally live in symbiosis with ~ 1013 bacteria present in the colon.40 Normal intestinal microbiota inhabits the colon mucus layer without penetrating an inner layer to trigger undesirable inflammatory responses.41 The inner layer of the mucus is densely packed and firmly attached to the epithelium normally “free” of bacteria. The outer layer of the mucus is movable with an expanded volume and colonized by bacteria.42 In a human colonic cell line, HT-29, LL-37/CRAMP directly stimulates mucus synthesis through MAP kinase activation and up-regulation of MUC gene transcription.43 In the colon of mice deficient in CRAMP, the mucus layer is thinner and discontinuous with severe disruptions,44,45 and therefore more easily colonized and penetrated by E. coli strain O157:H7.44 CRAMP−/− mice exhibit defects in re-epithelialization of injured colon tissues due to lack of CRAMP stimulation.46
C. Contribution of CRAMP to Microbicidal Function of Macrophages
Macrophages represent the first line of defense against invading bacterial pathogens. Tissueresident macrophages patrol the colon epithelial layer of barrier, putative entry, and colonization sites for pathogens, to control invaders in addition to their functions in removal of dying cells by efferocytosis.47,48 Our recent study revealed that myeloid cell–specific CRAMP−/− (LysMCre-CRAMPF/F KO) mice were more sensitive to DSS-induced colitis as compared with intestinal epithelial cell–specific CRAMP−/− (VillinMCre-CRAMPF/F KO) mice,39 indicating that macrophage-derived CRAMP plays an important role in maintaining microbicidal function in colon mucosa. CRAMP expression in mouse macrophages was increased after infection by an intracellular pathogen, Salmonella typhimur.49 Mouse macrophage cell line J774A.1 and bone marrow–derived macrophages (BMMs) infected by M. smegmatis showed increased Camp (CRAMP gene) mRNA levels, coinciding with increases in their killing activity.50 Macrophages infected with S. typhimur exhibited a punctate-patterned, yet increased, expression of CRAMP in the perinuclear region. CRAMP reduced Salmonella division in Wild type (WT) macrophages, but the bacteria showed enhanced survival within macrophages derived from CRAMP-deficient mice. Mechanistically, intracellular reactive oxygen intermediates and proteases in macrophages may be associated with CRAMP production and activity.49 Some studies also showed that human LL-37 is not only directly bactericidal but serves also as a mediator of vitamin D3-induced autophagy in macrophages which activates the transcription of autophagy-related genes Beclin-1 and Atg5, in association with killing of intracellular bacteria.51 Therefore, CRAMP/LL37 is critical for protecting the integrity of the colon mucosa, as illustrated in Fig. 1.
FIG. 1:
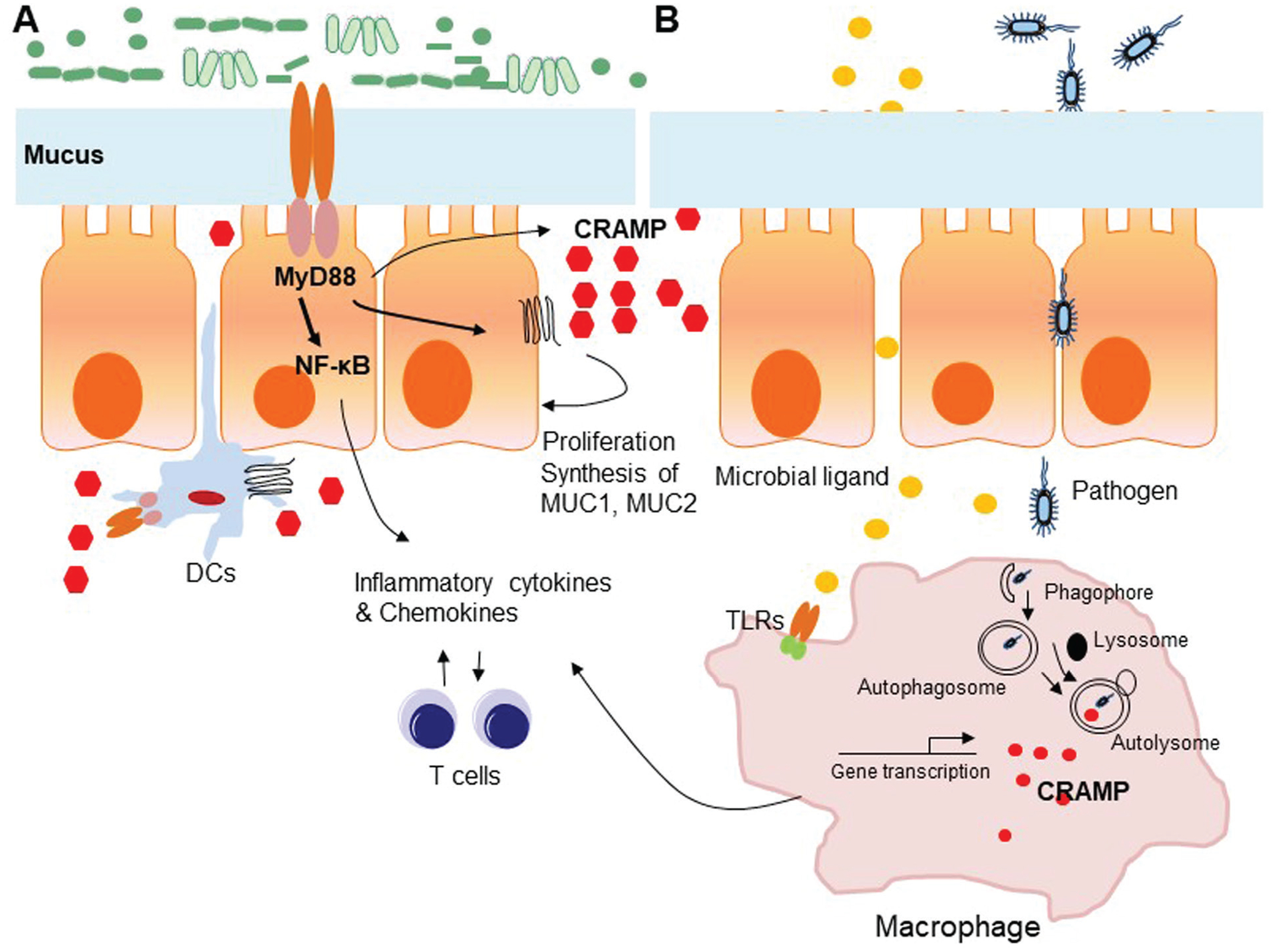
Contribution of CRAMP to colon epithelial barrier integrity. A. The contribution of CRAMP to colon crypt structure and mucus layer integrity–Colon mucosal barrier consists of epithelial and immune cells, which partner with resident microbiota to form a barrier fending off harmful substances. Epithelial cell–derived CRAMP is detectable in cells located on the mucosal surface and in upper crypts, with little or no expression seen within the deeper colon crypts. CRAMP stimulates the proliferation and differentiation of colon epithelial cells through Fpr2 espressed by epithelial cells. CRAMP also directly stimulates mucus synthesis through up-regulation of MUC1 and MUC2 and MAP kinase activation in colon epithelial cells. B. The contribution of CRAMP to the antimicrobicidal function of macrophages–resident macrophages in colon mucosa patrol and moniter pathogen invaders in the mucosal barrier. Upon stimulation by bacteria and their products, macrophages increase CRAMP production, which directly or in cooperation with autophagy kill and clear intracellular bacteria.
IV. CONTRIBUTION OF LL-37/CRAMP–COLON MICROBIOTA BALANCE
In the human gut, there are estimated to be > 1,000 species-level phylotypes of bacteria.52 Most of these phylotypes belong to only a few phyla. In general, Bacteroidetes and Firmicutes are dominant, whereas Actinobacteria, Proteobacteria, and Verrucomicrobia are frequent but generally minor constituents.53 A multitude of species of bacteria in the gut are in equilibrium because of control by many factors. Antimicrobial peptides (AMPs) are major players in maintaining microbiota balance in the gut.54 LL-37/CRAMP, as one of the AMPs, plays an important role in intestinal microbe ecosystems.
In vitro, synthetic CRAMP exhibits antimicrobial activity against the murine enteric pathogen Citrobacter rodentium, which, like the clinically related human pathogens enteropathogenic Escherichia coli and enterohemorrhagic E. coli, adheres to the apical membrane of intestinal epithelial cells.55 Synthetic CRAMP and LL-37 also kill E. coli O157:H7 in vitro.44 CRAMP−/− mice infected with C. rodentium by oral inoculation suffer from increased bacterial colonization in the colon and developed significantly higher fecal counts of C. rodentium.55 Those inoculated with E. coli O157:H7 also exhibited higher fecal counts of this strain, and the bacteria penetrated the mucus layer, forming a higher number of attaching and effacing lesions.44 Therefore, LL-37/CRAMP mediates innate intestinal defense against colonization by epithelium-adherent bacteria to maintain gut microbiota balance. Recent studies revealed that CRAMP acts as a limiting factor on dysbiosis to maintain ecologic balance in the colon.39 Single-housed CRAMP−/− mice showed a significantly different microbiota composition in feces as compared to single-housed WT mice. However, after 4-wk cohousing, microbiota composition in the feces of WT mice shifted markedly toward that of CRAMP−/− mice. Meanwhile, WT mice cohoused with CRAMP−/− mice exhibited a phenotype similar to CRAMP−/− mice, indicating that the phenotype of CRAMP−/− mice is transferable to WT mice by cohousing, presumably through the transfer of pathogenic bacteria species that overgrow in the absence of CRAMP.39 Sequencing of microbiota DNA in fecal pellets of mice revealed significantly different microbiota composition in non-cohoused WT and CRAMP−/− mice, in particular after DSS treatment, with increased Mucispirillum schaedleri, Clostridium populeti, and Acetivibrio cellulosolvens in WT mice, but increased Odoribacter laneus, Ruminococcus lactaris, Desulfovibrio piger, Desulfomicrobium orale, Mogibacterium neglectum, and Bacteroides acidifaciens in CRAMP−/− mice. It is notable that some species, such as M. neglectum, D. piger, and D. orale, which are typically found in oral microbiota, were detected in CRAMP−/− mice after DSS treatment. The frequencies of O. laneus, D. piger, and D. orale were significantly increased in WT mouse feces after cohousing with CRAMP−/− mice.39 Therefore, CRAMP deficiency was associated with severe dysbiosis in mice, in particular in chemically induced colitis. The role of CRAMP in colon microbiota balance is depicted in Fig. 2.
FIG. 2:

Contribution of CRAMP to colon microbiota balance. CRAMP-deficient mice show severe dysbiosis in the colon. This is verified by cohousing and single housing of WT mice (A) and CRAMP−/− mice (B). Single-housed CRAMP−/− mice show a significantly altered phenotype, including shortened colon crypts, thinner mucus layer, and dysbiosis with a different microbiota composition in the feces from that of single housed WT mice. WT mice cohoused with CRAMP−/− mice adopted a similar phenotype and microbiota composition from CRAMP−/− mice (C).
V. ANTI-INFLAMMATORY EFFECT OF LL-37/CRAMP IN COLITIS
Disturbance in colon homeostasis results in altered composition of the colon microbiota, or dysbiosis. Crohn’s disease (CD) and ulcerative colitis (UC), the two major forms of IBD, are characterized by chronic relapsing inflammation of the digestive tract. IBD is caused by complex interaction of genetic, microbial, and immunological factors. Several risk genes identified for IBD are linked to innate immune recognition of bacteria such as NOD2 and NLRP3 or processing and elimination of bacteria.
Colon bacteria have a potentially pathogenic role in intestinal inflammation,56 as shown by evidence that germ-free animals do not develop intestinal inflammation. It is well-established that interaction between the intestinal microbiome and colon mucosa initiates inflammatory bowel disease and impaired healing. Our recent investigation revealed that CRAMP−/− mice are highly sensitive to DSS-induced colitis associated with more extensive mucosal injury, higher-level production of proinflammatory cytokines, and increased infiltration of inflammatory cells in the gut, culminating in decreased mouse survival. As stated earlier, CRAMP deficiency also alters the composition of microbiota in the colon, as shown by the observation that antibiotics alleviated the severity of DSS-induced colitis in CRAMP−/− mice. In addition, the colon phenotype found in CRAMP−/− mice was transferable to WT mice after cohousing (Fig. 2). Furthermore, administration of synthetic CRAMP significantly reduced the development of DSS-induced ulcerative colitis in mice with a reduction in the number of fecal bacteria.57 Administration of plasmid58 or Lactococcus lactis encoding CRAMP gene59 alleviated DSS-induced colitis in mice, emphasizing the protective role of CRAMP in chemically induced colitis via its antimicrobial activity.
Interestingly, LL-37/CRAMP also has antifibrogenic effects on murine colitis-associated fibrosis by directly inhibiting collagen synthesis in colonic fibroblasts. Chronic colitis induced by trinitrobenzene sulphonic acid (TNBS) was associated with increased colonic collagen (col1a2) mRNA expression. Intracolonic CRAMP administration or intravenous delivery of the lentivirus-overexpressing CRAMP gene significantly reduced colonic collagen (col1a2) mRNA expression in TNBS-exposed mice. Cecal inflammation associated with increased collagen (col1a2) mRNA expression is also caused by Salmonella infection, which was prevented by intravenous delivery of the Camp (CRAMP-) –expressing lentivirus. A mechanism study revealed that LL-37/CRAMP inhibited TGF-β1– and/or IGF-1–induced collagen synthesis in colon fibroblasts.60 Thus, LL-37/CRAMP attenuates colitis associated with acute and chronic inflammation.
VI. PROTECTION AGAINST COLON TUMORIGENESIS BY LL-37/CRAMP
LL-37/CRAMP is a double-edged sword in promoting and inhibiting tumor growth. On the one hand, LL-37/CRAMP acts as a ligand for different cell membrane receptors whose expression on cancer cells varies. Overexpression of LL-37/CRAMP was found to promote the development and progression of ovarian,28,61–64 lung,31,65,66 and breast cancers67,68 but to suppress gastric69 and colon cancer.70
On the other hand, LL-37 is highly expressed in normal colon mucosa but is down-regulated in colon cancer tissues.70,71 Therefore, some investigators suggest that low levels of LL-37 may serve as a biomarker for colon cancer. In mice, CRAMP protects the animals from carcinogenesis with AOM-DSS–induced colitis, which may be caused by increased epithelial cell turnover and leukocyte infiltration in colon mucosa following more severe damage and slower recovery.39
The mechanisms by which LL-37/CRAMP suppresses the development of colon cancer is not completely clear. But lines of evidence suggest that (1) LL-37 induces the death of colon cancer cells by activation of caspase-independent apoptosis and autophagy72; (2) LL-37 induces apoptotic death of colon cancer cells by regulating metabolic profile, especially purine metabolism, glycolysis, and the tricarboxylic acid cycle73; (3) LL-37/CRAMP inhibits colon cancer development by interfering with epithelial mesenchymal transition (EMT) and fibroblast-supported cancer cell proliferation.74 These observations support the notion that CRAMP deficiency confers on mice increased susceptibility to chemically induced colitis and cancer. Therefore, LL-37/CRAMP may constitute a plausible candidate of therapeutics agent(s).
VII. PERSPECTIVES
LL-37/CRAMP as important host defense peptides are multifunctional, exhibiting broad-range antimicrobial and immune regulatory activity. Currently, the possibility of applying LL-37/CRAMP as therapeutic agents has been explored. For example, CRAMP encoded by plasmid and Lactococcus lactis have been used for treating colitis.58,59 PG-1 (pig peptide protegrin) offers 100% protection of rats against infections caused by intraperitoneal injection of P. aeruginosa, S. aureus, and methicillin-resistant S. aureusin.75 Ovine cathelicidins SMAP29 and SMAP34 are potential candidates for human therapy against bacterial infection and immune suppression.76 However, the significance of LL-37/CAMP and related peptides in human immune responses and cancer development remains to be fully recognized. Further understanding of the biological activity of LL-37 and CRAMP as well as other related host-derived microbicidal peptides will be beneficial for development of novel therapeutic agents combating infectious, inflammatory, and cancerous diseases.
ACKNOWLEDGMENTS
The authors thank Dr. Joost J. Oppenheim for reviewing the manuscript and Ms. Cheri Rhoderick for secretarial assistance. This study was supported in part by federal funds from the National Cancer Institute (NCI), National Institutes of Health (NIH), under Contract No. HHSN261200800001E and was supported in part by the Intramural Research Program of the NCI, NIH. M.Z. was also supported in part by a fund from the National Natural Science Foundation, Project 81873842.
ABBREVIATIONS:
- AMPs
antimicrobial peptides
- BM
bone marrow
- CRAMP
cathelin-related antimicrobial peptide
- DC
dendritic cells
- EGFR
epidermal growth factor receptor
- EMT
epithelial mesenchymal transition
- FPR2
formyl peptide receptor 2
- Fpr2
mouse FPR2
- hCAP
human cathelicidin protein
- IGF-1R
insulin-like growth factor-1 receptor
- Ly6C
lymphocyte antigen 6 complex
- TNF-α
tumor necrosis factor-α
- UC
ulcerative colitis
- WT
wild type
REFERENCES
- 1.Hultmark D, Steiner H, Rasmuson T, Boman HG. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980;106:7–16. [DOI] [PubMed] [Google Scholar]
- 2.Gennaro R, Skerlavaj B, Romeo D. Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect Immun. 1989;57:3142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirata M, Yoshida M, Inada K, Kirikae T. Investigation of endotoxin binding cationic proteins from granulocytes: agglutination of erythrocytes sensitized with Re-LPS. Adv Exp Med Biol. 1990;256:287–99. [DOI] [PubMed] [Google Scholar]
- 4.Das H, Sharma B, Kumar A. Cloning and characterization of novel cathelicidin cDNA sequence of Bubalus bub-alis homologous to Bos taurus cathelicidin-4. DNA Seq. 2006;17:407–14. [DOI] [PubMed] [Google Scholar]
- 5.Scocchi M, Bontempo D, Boscolo S, Tomasinsig L, Giulotto E, Zanetti M. Novel cathelicidins in horse leukocytes (1). FEBS Lett. 1999;457:459–64. [DOI] [PubMed] [Google Scholar]
- 6.Kokryakov VN, Harwig SS, Panyutich EA, Shevchenko AA, Aleshina GM, Shamova OV, Korneva HA, Lehrer RI. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–6. [DOI] [PubMed] [Google Scholar]
- 7.Brogden KA, Kalfa VC, Ackermann MR, Palmquist DE, McCray PB, Tack BF. The ovine cathelicidin SMAP29 kills ovine respiratory pathogens in vitro and in an ovine model of pulmonary infection. Antimicrob Agents Chemother. 2001;45:331–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamova O, Brogden KA, Zhao C, Nguyen T, Kokryakov VN, Lehrer RI. Purification and properties of proline-rich antimicrobial peptides from sheep and goat leukocytes. Infect Immun. 1999;67:4106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez de Mera IG, Perez de la Lastra JM, Ayoubi P, Naranjo V, Kocan KM, Gortazar C, de la Fuente J. Differential expression of inflammatory and immune response genes in mesenteric lymph nodes of Iberian red deer (Cervus elaphus hispanicus) naturally infected with Mycobacterium bovis. Dev Comp Immunol. 2008;32:85–91. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y, Cai Y, Bommineni YR, Fernando SC, Prakash O, Gilliland SE, Zhang G. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J Biol Chem. 2006;281:2858–67. [DOI] [PubMed] [Google Scholar]
- 11.Uzzell T, Stolzenberg ED, Shinnar AE, Zasloff M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides. 2003;24:1655–67. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Hong J, Liu X, Yang H, Liu R, Wu J, Wang A, Lin D, Lai R. Snake cathelicidin from Bungarus fasciatus is a potent peptide antibiotic. PLoS One. 2008;3:e3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bals R, Lang C, Weiner DJ, Vogelmeier C, Welsch U, Wilson JM. Rhesus monkey (Macaca mulatta) mucosal antimicrobial peptides are close homologues of human molecules. Clin Diagn Lab Immunol. 2001;8:370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaoka I, Tsutsumi-Ishii Y, Yomogida S, Yamashita T. Isolation of cDNA encoding guinea pig neutrophil cationic antibacterial polypeptide of 11 kDa (CAP11) and evaluation of CAP11 mRNA expression during neutrophil maturation. J Biol Chem. 1997;272:22742–50. [DOI] [PubMed] [Google Scholar]
- 15.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Bio Chem. 1997;272:13088–93. [DOI] [PubMed] [Google Scholar]
- 16.Termen S, Tollin M, Olsson B, Svenberg T, Agerberth B, Gudmundsson GH. Phylogeny, processing and expression of the rat cathelicidin rCRAMP: a model for innate antimicrobial peptides. Cell Mol Life Sci. 2003;60:536–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–25. [DOI] [PubMed] [Google Scholar]
- 18.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, Gallo RL. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–7. [DOI] [PubMed] [Google Scholar]
- 19.Tomasinsig L, Zanetti M. The cathelicidins—structure, function and evolution. Curr Protein Peptide Sci. 2005;6:23–34. [DOI] [PubMed] [Google Scholar]
- 20.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A. 1998;95:9541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson GH. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Bio Chem. 1997;272:15258–63. [DOI] [PubMed] [Google Scholar]
- 22.Menard S, Forster V, Lotz M, Gutle D, Duerr CU, Gallo RL, Henriques-Normark B, Putsep K, Andersson M, Glocker EO, Hornef MW. Developmental switch of intestinal antimicrobial peptide expression. J Exp Med. 2008;205:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nature Med. 2006;12:63641. [DOI] [PubMed] [Google Scholar]
- 24.Gallo RL, Ono M, Povsic T, Page C, Eriksson E, Klagsbrun M, Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A. 1994;91:11035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J, Ross CR, Chengappa MM, Blecha F. Identification of a proline-arginine-rich antibacterial peptide from neutrophils that is analogous to PR-39, an antibacterial peptide from the small intestine. J Leukocyte Biol. 1994;56:807–11. [DOI] [PubMed] [Google Scholar]
- 26.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–8. [DOI] [PubMed] [Google Scholar]
- 27.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra PS, Vogelmeier C, Gallo RL, Clauss M, Bals R. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exper Med. 2000;192:1069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol. 2005;174:6257–65. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, Liu M, Liu Y, Wang C, Yoshimura T, Gong W, Le Y, Tessarollo L, Wang JM. Signal relay by CC chemo-kine receptor 2 (CCR2) and formylpeptide receptor 2 (Fpr2) in the recruitment of monocyte-derived dendritic cells in allergic airway inflammation. J Bio Chem. 2013;288:16262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Haussen J, Koczulla R, Shaykhiev R, Herr C, Pinkenburg O, Reimer D, Wiewrodt R, Biesterfeld S, Aigner A, Czubayko F, Bals R. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. [DOI] [PubMed] [Google Scholar]
- 32.Kawano A, Tsukimoto M, Noguchi T, Hotta N, Harada H, Takenouchi T, Kitani H, Kojima S. Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages. Biochem Biophys Res Commun. 2012;419:374–80. [DOI] [PubMed] [Google Scholar]
- 33.Qu Y, Dubyak GR. P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal. 2009;5:163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BH, Hwang DM, Palaniyar N, Grinstein S, Philpott DJ, Hu J. Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PLoS One. 2012;7:e35812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin J, Yu FS. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Invest Ophthalmol Visual Sci. 2010;51:1891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6. [DOI] [PubMed] [Google Scholar]
- 37.Girnita A, Zheng H, Gronberg A, Girnita L, Stahle M. Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene. 2012;31:352–65. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infection Immunity. 2002;70:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura T, McLean MH, Dzutsev AK, Yao X, Chen K, Huang J, Gong W, Zhou J, Xiang Y, O’Huigin C, Thovarai V, Tessarollo L, Durum SK, Trinchieri G, Bian XW, Wang JM. The antimicrobial peptide CRAMP is essential for colon homeostasis by maintaining microbiota balance. J Immunol. 2018;200:2174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansson P, Wiltschi B, Kumari P, Kessler B, Vonrhein C, Vonck J, Oesterhelt D, Grininger M. Inhibition of the fungal fatty acid synthase type I multienzyme complex. Proc Natl Acad Sci U S A. 2008;105:12803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tai EK, Wong HP, Lam EK, Wu WK, Yu L, Koo MW, Cho CH. Cathelicidin stimulates colonic mucus synthesis by up-regulating MUC1 and MUC2 expression through a mitogen-activated protein kinase pathway. J Cell Biochem. 2008;104:251–8. [DOI] [PubMed] [Google Scholar]
- 44.Chromek M, Arvidsson I, Karpman D. The antimicrobial peptide cathelicidin protects mice from Escherichia coli O157:H7-mediated disease. PLoS One. 2012;7:e46476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu WK, Wong CC, Li ZJ, Zhang L, Ren SX, Cho CH. Cathelicidins in inflammation and tissue repair: potential therapeutic applications for gastrointestinal disorders. Acta Pharmacol Sin. 2010;31:1118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264:182–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci U S A. 2004;101:2422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonawane A, Santos JC, Mishra BB, Jena P, Progida C, Sorensen OE, Gallo R, Appelberg R, Griffiths G. Cathelicidin is involved in the intracellular killing of mycobacteria in macrophages. Cell Microbiol. 2011; 13(10):1601–17. [DOI] [PubMed] [Google Scholar]
- 51.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43. [DOI] [PubMed] [Google Scholar]
- 52.Claesson MJ, O’Sullivan O, Wang Q, Nikkila J, Marchesi JR, Smidt H, de Vos WM, Ross RP, O’Toole PW. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One. 2009;4:e6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5:1465–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol. 2005, 174:4901–7. [DOI] [PubMed] [Google Scholar]
- 56.Kucharzik T, Maaser C, Lugering A, Kagnoff M, Mayer L, Targan S, Domschke W. Recent understanding of IBD pathogenesis: implications for future therapies. Inflam Bowel Dis. 2006;12:1068–83. [DOI] [PubMed] [Google Scholar]
- 57.Ahluwalia A, Tarnawski AS. Cathelicidin gene therapy: a new therapeutic option in ulcerative colitis and beyond? Gene Ther. 2013;20:119–20. [DOI] [PubMed] [Google Scholar]
- 58.Tai EK, Wu WK, Wang XJ, Wong HP, Yu L, Li ZJ, Lee CW, Wong CC, Yu J, Sung JJ, Gallo RL, Cho CH. Intrarectal administration of mCRAMP-encoding plasmid reverses exacerbated colitis in Cnlp(−/−) mice. Gene Ther. 2013;20:187–3. [DOI] [PubMed] [Google Scholar]
- 59.Wong CC, Zhang L, Li ZJ, Wu WK, Ren SX, Chen YC, Ng TB, Cho CH. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J Gastroenterol Hepatol. 2012;27:1205–12. [DOI] [PubMed] [Google Scholar]
- 60.Yoo JH, Ho S, Tran DH, Cheng M, Bakirtzi K, Kukota Y, Ichikawa R, Su B, Tran DH, Hing TC, Chang I, Shih DQ, Issacson RE, Gallo RL, Fiocchi C, Pothoulakis C, Koon HW. Anti-fibrogenic effects of the anti-microbial peptide cathelicidin in murine colitis-associated fibrosis. Cell Mol Gastroenterol Hepatol. 2015;1:55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 62.Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, Tomchuck SL, Honer zu Bentrup K, Danka ES, Henkle SL, Scandurro AB. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci U S A. 2009;106:3806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viswanathan A, Painter RG, Lanson NA, Wang G. Functional expression of N-formyl peptide receptors in human bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25:1263–9. [DOI] [PubMed] [Google Scholar]
- 64.Lim R, Lappas M, Riley C, Borregaard N, Moller HJ, Ahmed N, Rice GE. Investigation of human cationic antimicrobial protein-18 (hCAP-18), lactoferrin and CD163 as potential biomarkers for ovarian cancer. J Ovarian Res. 2013;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piktel E, Niemirowicz K, Wnorowska U, Watek M, Wollny T, Gluszek K, Gozdz S, Levental I, Bucki R. The role of cathelicidin ll-37 in cancer development. Arch Immunol Ther Exper. 2016;64:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li D, Beisswenger C, Herr C, Schmid RM, Gallo RL, Han G, Zakharkina T, Bals R. Expression of the antimicrobial peptide cathelicidin in myeloid cells is required for lung tumor growth. Oncogene. 2014;33:2709–16. [DOI] [PubMed] [Google Scholar]
- 67.Heilborn JD, Nilsson MF, Jimenez CI, Sandstedt B, Borregaard N, Tham E, Sorensen OE, Weber G, Stahle M. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114:713–9. [DOI] [PubMed] [Google Scholar]
- 68.Weber G, Chamorro CI, Granath F, Liljegren A, Zreika S, Saidak Z, Sandstedt B, Rotstein S, Mentaverri R, Sanchez F, Pivarcsi A, Stahle M. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res. 2009;11:R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu WK, Sung JJ, To KF, Yu L, Li HT, Li ZJ, Chu KM, Yu J, Cho CH. The host defense peptide LL-37 activates the tumor-suppressing bone morphogenetic protein signaling via inhibition of proteasome in gastric cancer cells. J Cell Physiol. 2010;223:178–86. [DOI] [PubMed] [Google Scholar]
- 70.Ren SX, Cheng AS, To KF, Tong JH, Li MS, Shen J, Wong CC, Zhang L, Chan RL, Wang XJ, Ng SS, Chiu LC, Marquez VE, Gallo RL, Chan FK, Yu J, Sung JJ, Wu WK, Cho CH. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012;72:6512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen X, Zou X, Qi G, Tang Y, Guo Y, Si J, Liang L. Roles and mechanisms of human cathelicidin LL-37 in cancer. Cell Physiol Biochem. 2018;47:1060–73. [DOI] [PubMed] [Google Scholar]
- 72.Ren SX, Shen J, Cheng AS, Lu L, Chan RL, Li ZJ, Wang XJ, Wong CC, Zhang L, Ng SS, Chan FL, Chan FK, Yu J, Sung JJ, Wu WK, Cho CH. FK-16 derived from the anti-cancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS One. 2013;8:e63641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuroda K, Fukuda T, Isogai H, Okumura K, Krstic-Demonacos M, Isogai E. Antimicrobial peptide FF/CAP18 induces apoptotic cell death in HCT116 colon cancer cells via changes in the metabolic profile. Int J Oncol. 2015;46:1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng M, Ho S, Yoo JH, Tran DH, Bakirtzi K, Su B, Tran DH, Kubota Y, Ichikawa R, Koon HW. Cathelicidin suppresses colon cancer development by inhibition of cancer associated fibroblasts. Clin Exp Gastroenterol. 2015;8:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reddy KV, Yedery RD, Aranha, C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004;24:536–47. [DOI] [PubMed] [Google Scholar]
- 76.Travis SM, Anderson NN, Forsyth WR, Espiritu C, Conway BD, Greenberg EP, McCray PB, Lehrer RI, Welsh MJ, Tack BF. Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun. 2000;68:2748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


