Abstract
There is increasing concern about the potential effects of anesthesia exposure on the developing brain. The effects of relatively brief anesthesia exposures used repeatedly to acquire serial magnetic resonance imaging scans could be examined prospectively in rhesus macaques. We analyzed MR diffusion tensor imaging (DTI) of 32 rhesus macaques (14 females, 18 males) aged two weeks to 36 months to assess postnatal white matter (WM) maturation. We investigated the longitudinal relationships between each DTI property and anesthesia exposure, taking age, sex, and weight of the monkeys into consideration. Quantification of anesthesia exposure was normalized to account for variation in exposures. Segmented linear regression with two knots provided the best model for quantifying WM DTI properties across brain development as well as the summative effect of anesthesia exposure. The resulting model revealed statistically significant age and anesthesia effects in most WM tracts. Our analysis indicated there were major effects on WM associated with low levels of anesthesia even when repeated as few as three times. Fractional anisotropy values were reduced across several WM tracts in the brain, indicating that anesthesia exposure may delay WM maturation, and highlight the potential clinical concerns with even a few exposures in young children.
Keywords: diffusion tensor imaging (DTI), anesthesia exposure, development, ketamine, isoflurane
Introduction:
There is increasing awareness of the potential effects of anesthesia exposure on the developing brain (Block et al., 2017). The most consistent findings involve learning and behavioral difficulties after pediatric anesthesia exposure used for required surgical procedures (Bartels et al., 2009; Block et al., 2012; DiMaggio et al., 2009, 2011; Flick et al., 2011; Hansen et al., 2011; Ing et al., 2012, 2022; Sprung et al., 2012; Wilder et al., 2009). While some studies have been conducted in humans, most are limited by employing retrospective analysis. In contrast, with animal modeling, one can take advantage of controlled anesthesia events and more definitively link the effects of exposure with later neurodevelopmental outcomes. Rhesus macaques (Macaca mulatta) are commonly used as a nonhuman primate (NHP) model because of their phylogenetic similarity to humans (Lacreuse, 2009), the capacity for reliably breeding in a standardized laboratory setting, and larger brains, which provide the potential to investigating more complex neurobehavioral effects of perturbation (Beck et al., 2020). The use of NHP models also allows for experimental control and imaging at young ages during critical periods of neurodevelopment, when it is difficult to recruit and image young children. These advantages make young monkeys well-suited for elucidating the effect of anesthesia on neurodevelopment. Employing rodent models, many previous studies showed that exposure to a wide range of anesthetics both before and soon after birth led to neuroapoptosis (Cattano et al., 2008; Jevtovic-Todorovic et al., 2003; Lu et al., 2006; Ma et al., 2007; Saito et al., 1993; Young et al., 2005). In addition, investigations with primates found similar effects with histological studies revealing widespread neuroapoptosis after exposure to ketamine and isoflurane (Brambrink et al., 2010, 2012; Creeley et al., 2014; Paule et al., 2011; Slikker et al., 2007; Zou et al., 2009). It was also shown that some neurobehavioral outcomes are affected, with exposed macaques having slower response times and inferior peformances on learning tasks even 3.5 years after exposure (Paule et al., 2011).
Further, macaques exposed to repeated anesthesia with sevoflurane during the first four weeks after birth had an increased frequency of anxiety-related behaviors in 5 months after exposure (Raper et al., 2015). Similarly, exposures to isoflurane for 5 hours during the first two weeks after birth were associated with decreased close social behavior in the group with multiple exposures and increased anxiety-related behaviors and behavioral inhibition in group with single exposure at 2 years old (Neudecker et al., 2021). In keeping with these findings, it was found that the use of repeated, but not single, surgical plane of anesthesia with isoflurane in infant monkeys increased the animals’ emotional reactivity (Coleman et al., 2017).
However, a major limitation of the previously published NHP studies is that most assessed exposure durations beyond the clinically relevant duration routinely used in pediatric practice. Bartels et al. (2018) analyzed records of more than 1.5 million children in the National Anesthesia Clinical Outcomes Registry. It was found that the duration of general anesthetic exposure for most pediatric patients is less than one hour. Children under 1 year have the longest median exposure duration - 79 minutes, and 13.7% were exposed to general anesthesia for more than 3 hours (Bartels et al., 2018). While the recent reports on rhesus macaques demonstrated detrimental behavioral and neural effects of isoflurane (Creeley et al., 2014; Raper et al., 2015; Schenning et al., 2017), the investigations used anesthesia exposure designed to replicate a repeated and comparably deep plane of anesthesia (achieved with 1.3 to 2.5% isoflurane), typically used only in surgical procedures for human infants that involve extended periods (4-5 hours or longer). No longitudinal study to date has looked at exposures that would correspond to the dosage experienced by the majority of children during most pediatric surgeries or during a standard MR imaging session done with anesthesia (Bartels et al., 2018).
We analyzed the effects of repeated exposure to ketamine and isoflurane on brain development from the longitudinal perspective with the first exposures as young as two weeks of postnatal age up to the first exposure at 24 months, which occurred within the context of MRI scanning. Ketamine and isoflurane are frequently used in both veterinary medicine and in clinical practice with humans (Cheung & Yew, 2019; Durrmeyer et al., 2010; Hall & Shbarou, 2009; Kohrs & Durieux, 1998; McPherson et al., 2021; Miyabe-Nishiwaki et al., 2010; Roelofse, 2010; Sahyoun et al., 2021). Ketamine acts as a noncompetitive antagonist of NMDA receptors and can disrupt corticocortical information transfer in a frontal-to-parietal distribution (Bergman, 1999; L. Li & Vlisides, 2016). NMDA receptors play a vital role in neurodevelopment, and modulation of these pathways can affect synaptic arrangement (Scheetz & Constantine-Paton, 1994). Isoflurane is a halogenated ether used as an inhalational anesthetic. Although all the precise mediators have not been completely delineated, isoflurane acts as a GABA agonist (Franks & Lieb, 1994; Klockgether et al., 1988; Westphalen & Hemmings, 2003). Dexmedetomidine is also a commonly used anesthetic in veterinary medicine and functions as a selective α2 adrenoceptor agonist (Gertler et al., 2001; He et al., 2013; Ibacache et al., 2004; Isik et al., 2006; Shukry et al., 2005). Dexmedetomidine was administered to some subjects ≥ 7 months but was not considered in the analysis because it was inconsistently employed only at low levels and, according to literature, has neuroprotective rather than toxic effects (Li et al., 2014; Sanders et al., 2009).
Materials and Methods
Subjects
Thirty-two subjects were selected from the Wisconsin Neurodevelopment Rhesus Database (Young et al., 2017), which was a prospective and longitudinal analysis study with a primary aim to generate a brain atlas and elucidate general developmental patterns. Anesthesia was administered only for this MR imaging or in the course of routine veterinary care. The staggered nature of the scanning schedule with this controlled experimental design resulted in a substantial range of cumulative anesthesia exposure across subjects. This variation enabled a secondary, quantitative analysis of the effect of repeated anesthesia exposure on brain development. A summary of descriptive information about the subjects is in Supplementary materials Table S1.
All infants were reared normally by their mothers until weaning at 6–7 months of age. Afterward, the older juveniles were housed in small, stable social groups to provide companionship. The rearing and housing strategies were designed to facilitate regular socialization and to ensure standardized environmental conditions. The research protocol was approved by the Institutional Animal Care and Use Committee (IACUC).
Anesthesia Exposure
The monkeys were initially administered a low dose of ketamine hydrochloride (10 mg/kg IM) for transport to the MRI facility and imaging. For infants younger than six months of age, additional immobilization for scanning was achieved with inhalant isoflurane (1.5%). For monkeys older than 7 months of age, the immobilization required for scanning was achieved by administering dexmedetomidine (0.015 mg/kg IM). That immobilizing effect was reversed rapidly at the end of the scanning session by administering atipamezole (0.15 mg/kg IV). The anesthesia plane was monitored with a pulse oximeter to track heart rate and oxygen saturation in both younger and older subjects. Overall exposure time was less than two hours. A few subjects received additional exposure to ketamine during the course of routine veterinary procedures. All exposures took body weight into account for the dosing and weight at the time of scanning was also considered in the analysis (for details, see Table S1).
MR Imaging Acquisition and Processing
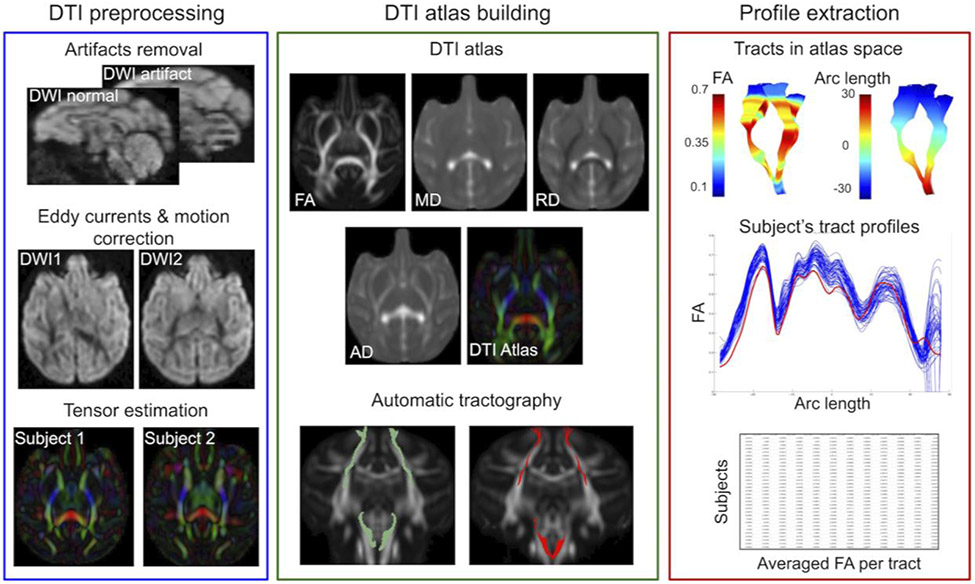
Scans were performed on a GE MR750 3.0T MRI scanner (General Electric Medical, Milwaukee WI) using a human 8-channel brain array coil at the Waisman Laboratory for Brain Imaging and Behavior at the University of Wisconsin-Madison. There were 120 unique diffusion gradient encoding directions acquired with b = 1000 s/mm2 and 10 non-diffusion-weighted baseline images with b = 0 s/mm2. A scan time of approximately 17.5 min was required to obtain the DWI data. Additionally, structural T1- and T2- weighted scans were acquired but are not analyzed in this study. The whole scanning protocol lasted approximately 50 minutes. The methods used for the DTI analysis are adapted from the NA-MIC DTI atlas-based analysis pipeline (Verde et al., 2014). A visual representation of the pipeline is presented in Figure 1. All preprocessing, including eddy current and motion correction and automatic removal of artifact-rich images, was performed using DTIPrep. We applied the following processing: 1. Automated and visual DTI QC, 2. Longitudinal DTI atlas building, 3. Definition of 44 white matter fiber tracts in atlas space. 4. Extraction of fiber profiles of DTI scalars using DTIAtlasFiberAnalyzer (Verde et al., 2014). DTI characterizes the properties of water diffusion in brain tissue. The water diffusion is anisotropic in white matter regions with axons that are aligned and densely packed. The following DTI scalars are widely used to describe the magnitude and direction of the water diffusivity in the brain. Fractional anisotropy (FA) is a measure of directional variation in diffusivities and is sensitive to changes in white matter micro-organization, but not specific to the type of the changes. FA should be interpreted along with the radial diffusivity (RD) and the axial diffusivity (AD) for more specific interpretations. RD describes the magnitude of diffusion perpendicular to white matter fibers and sensitive to myelination level, loss of neurons, and reduction of axonal density. AD describes diffusion parallel to the fibers and is sensitive to axon loss or injury. Mean diffusivity (MD) increases in response to edema and cell loss, and decreases with cell proliferation. The mean FA, AD, MD, and RD per tract were computed for the final analyses. Major tracts that were defined and analyzed are shown, along with their anatomical position, abbreviations, and other detailed information in the Supplementary materials (Table S2).
Figure 1:
The DWI data processing was based on DTI atlas building, automatic fiber tractography, and fiber profile extraction. In the statistical analysis, the averaged per tract FA was used.
Statistical Analysis
The analysis focused on the effect of anesthesia exposures on WM integrity as reflected by DTI properties, and included several steps. First, we chose the number of knots in the continuous, piecewise linear model of FA as a function of age. Then we optimized knot location via a grid search independently for each tract and each of the DTI scalars. Finally, we included in the model other variables of interest, such as normalized anesthesia exposure.
Model Choice
We chose the number of knots in a continuous, piecewise linear model as a function of age with a variable number of knots over the age covariates using only FA. Models with 1, 2, and 3 knots were compared visually using graphical displays. We chose the two-knot model, because the model with one knot yielded acceptable results but captured less variance, and the model with three knots led to overfitting on many tracts.
Choosing the knots
Optimization of the ages for knot positions was implemented individually for each tract in the brain and each DTI scalar. That is, each tract and DTI metric was allowed to have different knots. For the two-knot model, the first knot was restricted to be within the range of 50-to-400 days in 10-day increments. The second knot was restricted to be within 50 days beyond the first knot in ten-day increments up to the maximum age of 500 days. Every combination of knots was tested, and the model with the highest R2 value was chosen.
Additional covariates were subsequently added to the model once the extent of the age effect had been determined, including gender, weight, brain size, normalized anesthesia exposure, and the Finteraction terms. Besides age, the normalized anesthesia exposure was the other covariate with statistically significant p-values consistently less than 0.05. Thus, the other covariates were not included in the final model.
Normalized anesthesia
The rationale for employing normalized anesthesia exposure (NAE) has been described and documented previously (Young et al., 2021). Total prior exposures to ketamine and isoflurane were normalized across MRI scan-related exposures and then aggregated into a single measure as NAE = Total ketamine/10 + Total isoflurane/2 for each monkey (see Table S1). Mean inhalant isoflurane per session was 2.0% and mean ketamine dose per session was 10 mg/kg. The NAE reflected the cumulative amount of anesthesia to which a monkey had been exposed.
The final model for FA
The above model was fit separately for each tract in the brain (, , , ).
Because our final model choice used segmented regression over the age covariate with different knots for each tract, parameter estimates of , , and were carefully and systematically examined. The parameter represents the daily change of FA before the first knot is reached. The daily change of FA between the first and second knots is represented by . Similarly, the daily change after the second knot is . Comparing these three time periods across tracts was not possible because the knots are at different ages.
The same model was applied for RD and AD. FA, MD, RD, and AD were allowed to have different knots. That is, the optimization techniques were implemented individually for each tract separately for FA, MD, RD, and AD
Results:
Effect of Age
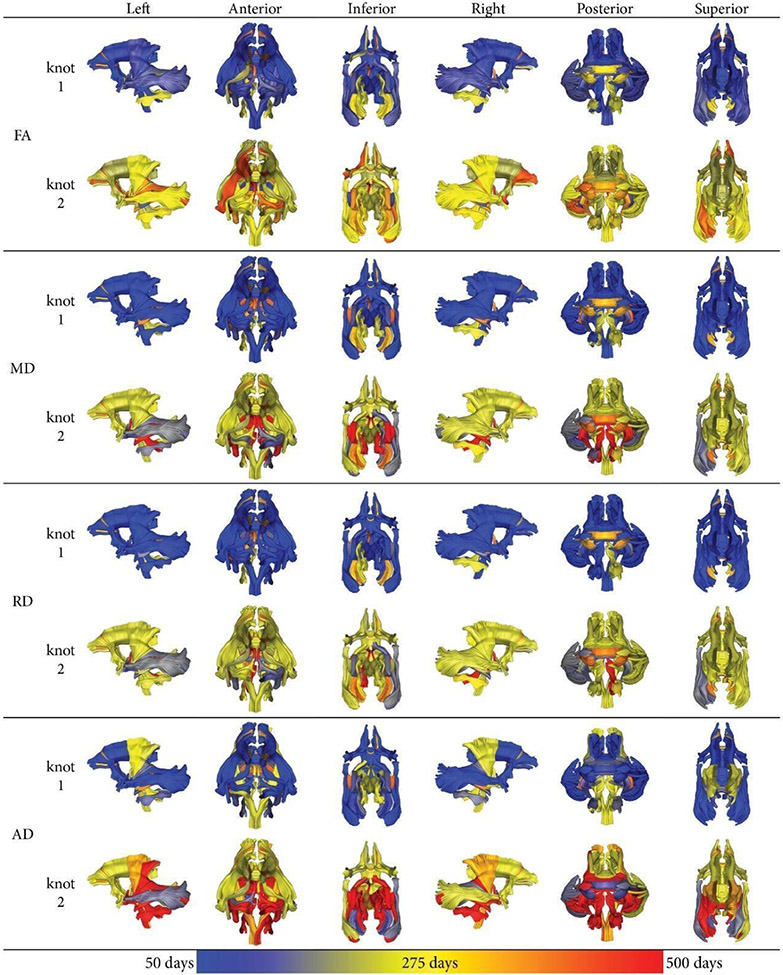
For FA, the age at the first knot was evinced at a young age (<200 days) across most of the brain, with the exception of the cerebellar tracts, splenium and genu of corpus callosum (Figure 2, Table S3). For MD and RD, a similar pattern was evident with regard to knot timing. For AD, the cerebrospinal tracts manifest their first knot later than 200 days (Figure 2). The trajectories for FA were similar to the ones observed in children, with rapid development occurring early in postnatal life followed by an asymptotic slowing (Figure 3). Tracts with the second knot at a later time point (> 400 days) included the uncinate fasciculi, optic radiations, and fornix. However, unlike the first knot, the timing for the second knot was more variable and thus should be interpreted with caution. For RD, there were fewer tracts with late second knots, while for MD and especially AD, more tracts with late second knots were observed.
Figure 2:
Age of the first and the second knots for all fiber tracts and DTI scalars after optimizing the segmented regression model in the two-knot model. The age is color coated, ranging from 50 days (blue) to 275 days (yellow) to maximally 500 days (red).
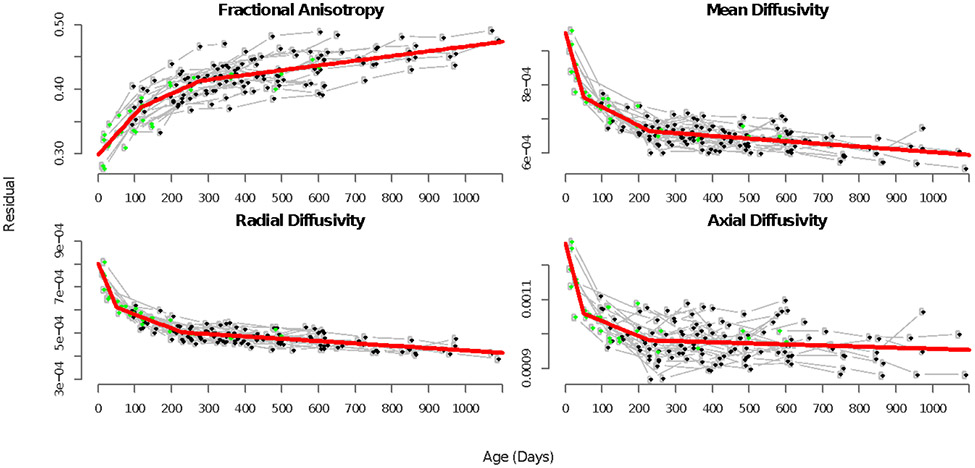
Figure 3:
The final model fit using the optimized knots for the inferior longitudinal fasciculus. The first scan for each individual is shown in green. Subsequent scans are shown in black and are traced with gray lines. Age (in days) is the x-axis, and FA, MD, RD, or AD on the y-axis. The final two-knot model (normalized anesthesia is ignored for this plot) is shown in red and captures developmental trajectories well. For all tracts see Figure S3
The two-knot model of WM metrics progression over age captured the developmental trajectories in FA, MD, RD, and AD trajectories (Figure 3, S3).
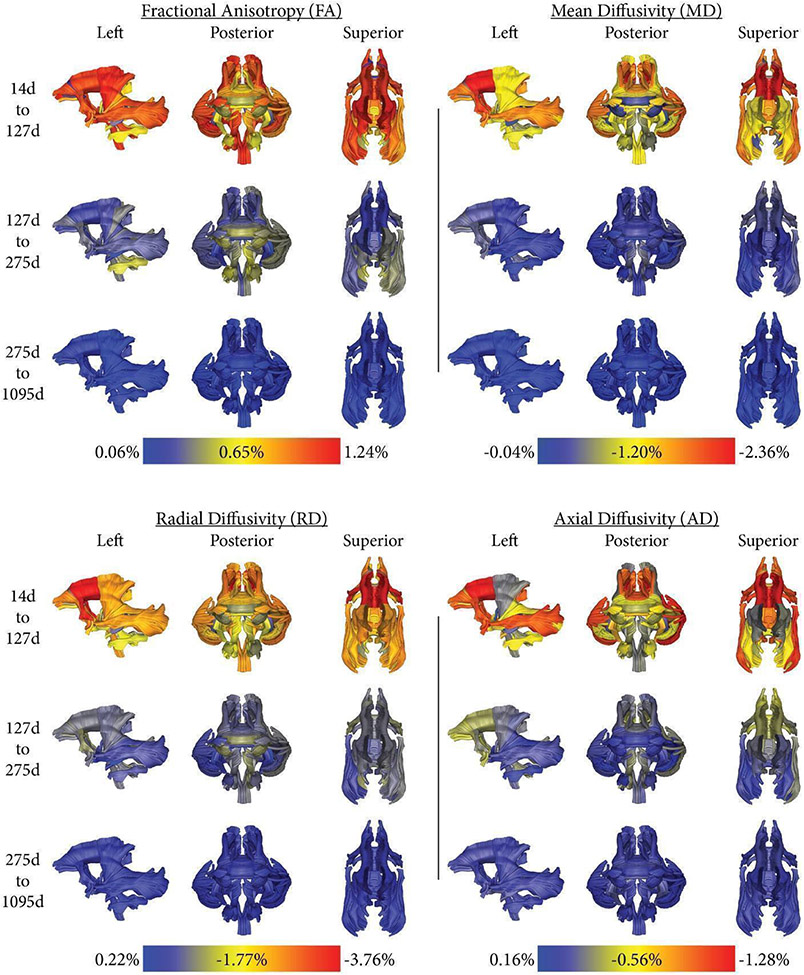
Figure 4 plots the average rate of change of FA, MD, RD, and AD for the three-time periods of 14-127 days, 127-275 days, and 275-1095 days. The average of the first knot for FA was 127 days, and the average of the second knot for FA was 275 days. As expected, we observed a much higher change rate in FA, MD, RD, and AD from 14 to 127 days than during the later periods. Figure 4 shows individual differences in FA change rates across the brain.
Figure 4:
The average rate of change of FA, MD, RD, or AD (per week), normalized as a percentage of the final model’s FA, MD, RD, or AD value at 1,095 days with no anesthesia exposures, is shown for various periods in days. 14d was chosen to be the start point as it was the earliest time scan, 1095d was the latest. 127 days was the average of the first knot for FA, 275d the average of the second knot for FA. Please note, that each diffusion property has a different color map scale to provide an equal visualization range, FA increases with age, and RD, AD, and MD decreases.
Effect of Normalized Anesthesia Exposure
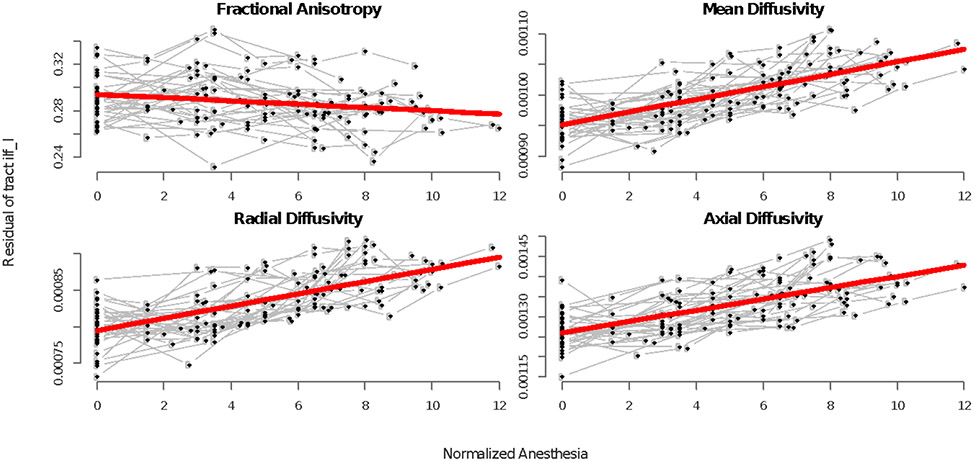
Our analysis also indicated that repeated exposure to anesthesia negatively affected the maturation of WM (Figure 5, 6, Figure S4). All parameter estimates of NAE were negatively associated with FA and positively linked with MD, RD, and AD. Monkeys who had experienced a higher cumulative exposure had a larger impact on their WM indices (Figure 5, S4, S5a, S5b).
Figure 5:
Scans for each individual are shown in black and traced with gray lines. Normalized anesthesia is on the x-axis and residuals are on the y-axis. The final longitudinal model for overall anesthesia exposure after removing the effects of age is shown in red and captures the cumulative effect of anesthesia on the inferior longitudinal fasciculus. See supplemental for all tracts (Figure S4).
Figure 6:
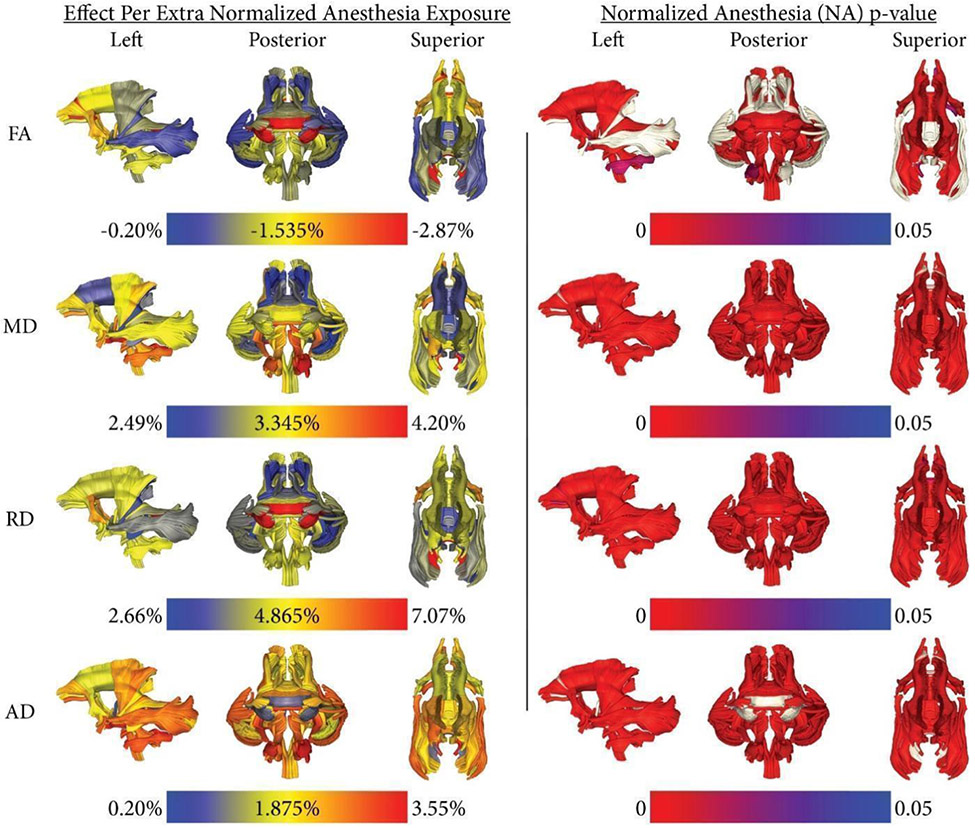
Normalized anesthesia estimates: The estimated difference in FA, MD, RD, or AD per single anesthesia exposure (the average magnitude of a singular anesthesia dose was used). The estimated difference in FA, MD, RD, or AD is normalized as a percentage of the final model’s respective FA, MD, RD, or AD value at 1,095 days (3 years of age) with no anesthesia exposures. Noteworthy, each diffusion property has its range for the colormap in the left column, FA decreases, and MD, RD, and AD increase. Normalized anesthesia p-values: The p-values of the normalized anesthesia covariate. Tracts that do not meet the α = 0.05 significance level appear in light gray
When compared to FA, all of the diffusivity measures revealed stronger associations with anesthesia exposure, with RD showing the larger vulnerability: up to a 7% increment was associated with each additional exposure to anesthesia (Figure 6).
Unlike the consistent developmental trajectories across the diffusion measures illustrated in Figures 2 and 4, the associations with anesthesia exposure varied across the WM indices (Figure 6) with the clearest concordance between FA and RD. For MD and AD, the clearest associations were seen in ILF and the splenium of the corpus callosum when compared with the FA and RD findings.
Discussion
The longitudinal database of MRI scans acquired from typically developing rhesus macaques allowed us to model the normal developmental trajectory of WM between 14 and 1095 postnatal days in animals without any other experimental manipulations. In addition, because acute anesthesia was required to immobilize the monkeys for each scan, it also permitted an evaluation of the potential effect of repeated exposure to short bouts of anesthesia. To determine the effect of anesthesia, the two pharmacological agents – ketamine used as the preanesthetic and isoflurane employed for inhalant anesthesia – were considered as a single covariate and all exposures were normalized to an average dose per imaging session. While this analytic approach did not permit us to compare the separate effects of different anesthetics, it enabled us to consider the summative effect of the cumulative exposure to anesthesia across scan sessions.
Multiple exposures to the commonly used anesthetics for MRI scanning had a surprisingly large overall effect on WM integrity across the brain. The current findings replicate and extend a previous report documenting a similar widespread reduction in the micro-organization of WM in monkeys after anesthesia exposure during the first year of life (Young et al., 2021). However, that analysis was not able to verify sustained effects on WM that persisted through the older juvenile and peri-pubertal stages in the monkeys. In addition, the current analysis was conducted on scans acquired from a different cohort of monkeys, indicating that the findings on the effects of anesthesia generalize across studies. There was widespread decrease in FA and an increase in RD and AD present, and thus no sign of recovery with age as the monkeys approached the pubertal transition in brain maturation. Given the extent of the effects on WM integrity, it is likely that the exposure to repeated episodes of anesthesia would also have functional consequences, which would also be in keeping with reports of effects long-lasting cognitive impairments in monkeys up to 36 months of age which would be comparable to pubertal transition in children (Clancy et al., 2007). Others have also documented a reduction in cognitive ability (Alvarado et al., 2017; Lee et al., 2014; Paule et al., 2011) and alterations in motor activity (Coleman et al., 2017), and social behavior (Neudecker et al., 2021) due to exposure to anesthesia.
The extent of the effects of anesthesia exposure did vary across the diffusion measures. RD revealed the most substantial effects, accounting for a considerable contribution to the anesthesia effect on FA (i.e., FA is a composite of RD, MD, and AD values). RD is sensitive to the amount of myelination (Alexander et al., 2007; Winklewski et al., 2018). Loss of oligodendrocytes could be the likely mediating process, which accounted for the altered RD in the monkeys because oligodendrocytes have a major role in myelin synthesis, and histological studies of brain tissue have demonstrated that oligodendrocytes are susceptible to anesthesia exposure (Brambrink et al., 2012; Creeley et al., 2014).
Unlike the majority of reports that detected WM alterations after anesthesia exposure (Block et al., 2017; Creeley et al., 2014; Schenning et al., 2017; Young et al., 2021), there have been some inconsistent findings, including the lack of a significant effect of anesthesia on WM tracts when using ex vivo DTI to examine 3-month old rabbits exposed to repetitive isoflurane anesthesia at 8, 11, 14 postnatal days, but showed an increase in FA in hippocampal gray matter (Aksenov et al., 2020). In this study WM integrity may not have been compromised because of the young age of exposure in the rabbit kits and the study N was small (4 control and 5 exposed kits). Nevertheless, they were still able to detect an increase in FA in hippocampal GM after repeated exposure to anesthesia (Aksenov et al., 2020). Another important methodological consideration is the possibility of an influence on diffusivity indices when applied to fixed tissue (Zhang et al., 2012). One advantage of the primate model was the possibility of acquiring the MR scans noninvasively from monkeys that were otherwise not manipulated with other experimental procedures.
The identification of a potentially critical period when the brain is especially susceptible to anesthetics is another important question to resolve. Our results did not reveal a sensitive developmental stage, but that could be due to the summing of anesthesia dose across sessions or being statistically underpowered when subdividing the monkeys in this way. However, a general vulnerability rather than a particular stage of brain maturation would be in keeping a recent review of human studies. The authors concluded that younger children were not necessarily more vulnerable (Davidson & Sun, 2018). It is also conceptual challenging to define a sensitive postnatal period because of the non-uniform heterochronic nature of neurodevelopment, which would likely result in specific sensitive periods for different brain regions and systems. Our analysis also did not indicate that there was a lingering consequence of a single exposure. Instead, subsequent exposures seemed to increase the summative effect on the maturational trajectory. These issues need better resolution in the clinical literature, which has reached conflicting conclusions (DiMaggio et al., 2009, 2011; Flick et al., 2011; Sprung et al., 2012). Nonetheless, the evidence from the current primate model indicates that repeated exposure to anesthesia at clinically relevant durations can result in a surprisingly large reduction in overall WM integrity.
Some differences in vulnerability between females and males have been reported previously (De Bellis et al., 2001; Giedd et al., 1999; Lenroot et al., 2007; Schmithorst et al., 2002), but concurring with our previous work in another cohort of monkeys (Kubicki et al., 2018), the monkey’s sex was not a significant factor until after the pubertal transition. After puberty, which occurs between 3-4 years of age in the rhesus monkey, there are marked sex differences because the female brain reaches maturity at a younger age, and the male brain continues to grow in size for several more years (Knickmeyer et al., 2010). In the current analysis, the magnitude of the effect of anesthesia exposure was similar in both sexes. Further, neither the monkeys’ body weight nor total brain volume had a large influence on the progressive maturation of WM microstructure.
Beyond documenting the effects of anesthesia effects on WM, this DWI study is the first to systematically characterize the developmental WM trajectories of rhesus monkeys from 2 weeks to 36 months of age. A previous study characterized WM changes starting from 4 years (Kubicki et al., 2018). The FA trajectories were similar to those observed in children, with rapid change during the first months followed by an asymptotic slowing (Shi et al., 2013). Interestingly, the slope of the developmental trajectories was not necessarily indicative of the sensitivity to the effects of anesthesia. For example, the splenium of the corpus callosum evinced only a modest age-related change in FA but was found to be very sensitive to anesthesia (Figure 6). In contrast, the tapetum of the corpus callosum also exhibited only a small maturational change in FA but was relatively sensitive to anesthesia across all diffusion properties (Figure 6).
Limitations
Because the original aim of this MR scanning project was to generate a developmental brain atlas, it should be acknowledged that the DTI information was not ideally suited to differentiate the effect of a single discrete exposure versus multiple episodes, other than by generating a metric of cumulative exposure. All anesthesia sessions were weighted equally in the analysis, even though it is possible that early exposures could potentially have a more substantial effect than later exposures. In addition, this experimental design did include a control condition with monkeys receiving repeat sedations without a MR scan. However, current animal welfare guidelines that emphasize the ethical importance of not using more animals than needed would not support the inclusion of that type of additional control conditions. Although unlikely, some effects on brain development could be partially due to other required aspects of the protocol, including the periodic relocation of the younger monkeys for 2-3 hours away from the mother, which was necessary to acquire the scan. However, after the older infants were weaned from their mothers by 7 months of age, this concern about acute separation would not have been a factor. Finally, because the endpoint of the scanning occurred at 3 years of age, we cannot conclude that there wasn’t a subsequent recovery. However, that would assume the compensatory processes occurred after puberty when at least the female brain does not increase further in size, and a maturational phase of cortical thinning has already begun. It seems more parsimonious that the impact of a change in maturational trajectories would continue to manifest in adulthood, especially if there were behavioral and emotional sequelae of the neural alterations.
Conclusions
Our study revealed that there is a significant decrease in WM diffusivity associated with repeated exposure to relatively low levels of anesthesia, which had occurred in the context of MRI scanning sessions. Decreased FA values were evident after as few as 3 exposures, highlighting a translational concern when even a few cumulative exposures may be required for medical procedures in young children. The findings raise general concerns about the repeated use of anesthesia in pediatric practice and also in veterinary medicine with young animals.
Supplementary Material
Acknowledgments:
This research was supported by grants from the NIMH (MH901645, MH091645-S1, MH100031) and the NICHD (HD003352, HD003110, and HD079124).
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability Statement
The neuroimaging data that support the findings of this study are openly available in UNC-Wisconsin Neurodevelopment Rhesus Database at https://data.kitware.com/#collection/54b582c38d777f4362aa9cb3, reference number (Young et al, 2017). The normalized anesthesia exposure data are available in the supplementary material of this article.
References
- Aksenov DP, Venkatasubramanian PN, Miller MJ, Dixon CJ, Li L, & Wyrwicz AM (2020). Effects of neonatal isoflurane anesthesia exposure on learning-specific and sensory systems in adults. Scientific Reports, 10(1), 13832. 10.1038/s41598-020-70818-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, & Field AS (2007). Diffusion tensor imaging of the brain. Neurotherapeutics : The Journal of the American Society for Experimental NeuroTherapeutics, 4(3), 316–329. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado MC, Murphy KL, & Baxter MG (2017). Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. British Journal of Anaesthesia, 119(3), 517–523. 10.1093/bja/aew473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels DD, McCann ME, Davidson AJ, Polaner DM, Whitlock EL, & Bateman BT (2018). Estimating pediatric general anesthesia exposure: Quantifying duration and risk. Paediatric Anaesthesia, 28(6), 520–527. 10.1111/pan.13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, Althoff RR, & Boomsma DI (2009). Anesthesia and cognitive performance in children: No evidence for a causal relationship. Twin Research and Human Genetics : The Official Journal of the International Society for Twin Studies, 12(3), 246–253. 10.1375/twin.12.3.246 [DOI] [PubMed] [Google Scholar]
- Beck RT, Lubach GR, & Coe CL (2020). Feasibility of successfully breeding rhesus macaques (Macaca mulatta) to obtain healthy infants year-round. American Journal of Primatology, 82(1), e23085. 10.1002/ajp.23085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman SA (1999). Ketamine: Review of its pharmacology and its use in pediatric anesthesia. Anesthesia Progress, 46(1), 10–20. [PMC free article] [PubMed] [Google Scholar]
- Block RI, Magnotta VA, Bayman EO, Choi JY, Thomas JJ, & Kimble KK (2017). Are Anesthesia and Surgery during Infancy Associated with Decreased White Matter Integrity and Volume during Childhood? Anesthesiology, 127(5), 788–799. 10.1097/ALN.0000000000001808 [DOI] [PubMed] [Google Scholar]
- Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, & Todd MM (2012). Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology, 117(3), 494–503. 10.1097/ALN.0b013e3182644684 [DOI] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, & Olney JW (2012). Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology, 116(2), 372–384. 10.1097/ALN.0b013e318242b2cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, & Olney JW (2010). Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology, 112(4), 834–841. 10.1097/ALN.0b013e3181d049cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattano D, Young C, Straiko MMW, & Olney JW (2008). Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesthesia and Analgesia, 106(6), 1712–1714. 10.1213/ane.0b013e318172ba0a [DOI] [PubMed] [Google Scholar]
- Cheung HM, & Yew DTW (2019). Effects of Perinatal Exposure to Ketamine on the Developing Brain. Frontiers in Neuroscience, 13. 10.3389/fnins.2019.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, & Anand KJS (2007). Extrapolating brain development from experimental species to humans. Neurotoxicology, 28(5), 931–937. 10.1016/j.neuro.2007.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K, Robertson ND, Dissen GA, Neuringer MD, Martin LD, Cuzon Carlson VC, Kroenke C, Fair D, & Brambrink AM (2017). Isoflurane Anesthesia Has Long-term Consequences on Motor and Behavioral Development in Infant Rhesus Macaques. Anesthesiology, 126(1), 74–84. 10.1097/ALN.0000000000001383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, & Brambrink AM (2014). Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology, 120(3), 626–638. 10.1097/ALN.0000000000000037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, & Sun LS (2018). Clinical Evidence for Any Effect of Anesthesia on the Developing Brain. Anesthesiology, 128(4), 840–853. 10.1097/ALN.0000000000001972 [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, & Boring AM (2001). Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex (New York, N.Y. : 1991), 11(6), 552–557. [DOI] [PubMed] [Google Scholar]
- DiMaggio C, Sun LS, Kakavouli A, Byrne MW, & Li G (2009). A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. Journal of Neurosurgical Anesthesiology, 21(4), 286–291. 10.1097/ANA.0b013e3181a71f11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaggio C, Sun LS, & Li G (2011). Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesthesia and Analgesia, 113(5), 1143–1151. 10.1213/ANE.0b013e3182147f42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrmeyer X, Vutskits L, Anand KJS, & Rimensberger PC (2010). Use of analgesic and sedative drugs in the NICU: integrating clinical trials and laboratory data. Pediatric Research, 67(2), 117–127. 10.1203/PDR.0b013e3181c8eef3 [DOI] [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, & Warner DO (2011). Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics, 128(5), e1053–61. 10.1542/peds.2011-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP, & Lieb WR (1994). Molecular and cellular mechanisms of general anaesthesia. Nature, 367(6464), 607–614. 10.1038/367607a0 [DOI] [PubMed] [Google Scholar]
- Gertler R, Brown HC, Mitchell DH, & Silvius EN (2001). Dexmedetomidine: A novel sedative-analgesic agent. Proceedings (Baylor University. Medical Center), 14(1), 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, & Rapoport JL (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2(10), 861–863. 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Hall RW, & Shbarou RM (2009). Drugs of choice for sedation and analgesia in the neonatal ICU. Clinics in Perinatology, 36(2), 215–226, vii. 10.1016/j.clp.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Hansen TG, Pedersen JK, Henneberg SW, Pedersen D. a, Murray JC, Morton NS, & Christensen K (2011). Academic performance in adolescence after inguinal hernia repair in infancy: A nationwide cohort study. Anesthesiology, 114(5), 1076–1085. 10.1097/ALN.0b013e31820e77a0 [DOI] [PubMed] [Google Scholar]
- He L, Wang X, Zheng S, & Shi Y (2013). Effects of dexmedetomidine infusion on laryngeal mask airway removal and postoperative recovery in children anaesthetised with sevoflurane. Anaesthesia and Intensive Care, 41(3), 328–333. [DOI] [PubMed] [Google Scholar]
- Ibacache ME, Munoz HR, Brandes V, & Morales AL (2004). Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesthesia and Analgesia, 98(1), 60–63, table of contents. [DOI] [PubMed] [Google Scholar]
- Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJJ, Li G, & Sun LS (2012). Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. PEDIATRICS, 130(3), e476–e485. 10.1542/peds.2011-3822 [DOI] [PubMed] [Google Scholar]
- Ing C, Warner DO, Sun LS, Flick RP, Davidson AJ, Vutskits L, McCann ME, O’Leary J, Bellinger DC, Rauh V, Orser BA, Suresh S, & Andropoulos DB (2022). Anesthesia and Developing Brains: Unanswered Questions and Proposed Paths Forward. Anesthesiology, 136(3), 500–512. 10.1097/ALN.0000000000004116 [DOI] [PubMed] [Google Scholar]
- Isik B, Arslan M, Tunga AD, & Kurtipek O (2006). Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatric Anaesthesia, 16(7), 748–753. 10.1111/j.1460-9592.2006.01845.x [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, & Wozniak DF (2003). Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 23(3), 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockgether T, Turski L, Schwarz M, Sontag KH, & Lehmann J (1988). Paradoxical convulsant action of a novel non-competitive N-methyl-D-aspartate (NMDA) antagonist, tiletamine. Brain Research, 461(2), 343–348. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Styner M, Short SJ, Lubach GR, Kang C, Hamer R, Coe CL, & Gilmore JH (2010). Maturational trajectories of cortical brain development through the pubertal transition: Unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cerebral Cortex (New York, N.Y. : 1991), 20(5), 1053–1063. 10.1093/cercor/bhp166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrs R, & Durieux ME (1998). Ketamine. Anesthesia & Analgesia, 87(5), 1186–1193. 10.1213/00000539-199811000-00039 [DOI] [PubMed] [Google Scholar]
- Kubicki M, Baxi M, Pasternak O, Tang Y, Karmacharya S, Chunga N, Lyall AE, Rathi Y, Eckbo R, Bouix S, Mortazavi F, Papadimitriou G, Shenton ME, Westin CF, Killiany R, Makris N, & Rosene DL (2018). Lifespan Trajectories of White Matter Changes in Rhesus Monkeys. Cerebral Cortex, 29(4), 1584–1593. 10.1093/cercor/bhy056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse H (2009). Nonhuman Primate Models of Cognitive Aging. In Bizon JL & Woods A (Eds.), Animal Models of Human Cognitive Aging (pp. 29–58). Humana Press. 10.1007/978-1-59745-422-3 [DOI] [Google Scholar]
- Lee BH, Chan JT, Hazarika O, Vutskits L, & Sall JW (2014). Early exposure to volatile anesthetics impairs long-term associative learning and recognition memory. PloS One, 9(8), e105340. 10.1371/journal.pone.0105340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, & Giedd JN (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage, 36(4), 1065–1073. 10.1016/J.NEUROIMAGE.2007.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, & Vlisides PE (2016). Ketamine: 50 Years of Modulating the Mind. Frontiers in Human Neuroscience, 10, 612. 10.3389/fnhum.2016.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zeng M, Chen W, Liu C, Wang F, Han X, Zuo Z, & Peng S (2014). Dexmedetomidine reduces isoflurane-induced neuroapoptosis partly by preserving PI3K/Akt pathway in the hippocampus of neonatal rats. PloS One, 9(4), e93639–e93639. 10.1371/journal.pone.0093639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LX, Yon J-H, Carter LB, & Jevtovic-Todorovic V (2006). General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis : An International Journal on Programmed Cell Death, 11(9), 1603–1615. 10.1007/s10495-006-8762-3 [DOI] [PubMed] [Google Scholar]
- Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP, & Maze M (2007). Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology, 106(4), 746–753. 10.1097/01.anes.0000264762.48920.80 [DOI] [PubMed] [Google Scholar]
- McPherson C, Ortinau CM, & Vesoulis Z (2021). Practical approaches to sedation and analgesia in the newborn. Journal of Perinatology, 41(3), 383–395. 10.1038/s41372-020-00878-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabe-Nishiwaki T, Masui K, Kaneko A, Nishiwaki K, Shimbo E, & Kanazawa H (2010). Hypnotic effects and pharmacokinetics of a single bolus dose of propofol in Japanese macaques (Macaca fsucata fsucata). Veterinary Anaesthesia and Analgesia, 37(6), 501–510. 10.1111/j.1467-2995.2010.00564.x [DOI] [PubMed] [Google Scholar]
- Neudecker V, Perez-Zoghbi JF, Coleman K, Neuringer M, Robertson N, Bemis A, Glickman B, Schenning KJ, Fair DA, Martin LD, Dissen GA, & Brambrink AM (2021). Infant isoflurane exposure affects social behaviours, but does not impair specific cognitive domains in juvenile non-human primates. British Journal of Anaesthesia, 126(2), 486–499. 10.1016/j.bja.2020.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker WJ, & Wang C (2011). Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicology and Teratology, 33(2), 220–230. 10.1016/j.ntt.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Alvarado MC, Murphy KL, & Baxter MG (2015). Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology, 123(5), 1084–1092. 10.1097/ALN.0000000000000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofse JA (2010). The evolution of ketamine applications in children. Paediatric Anaesthesia, 20(3), 240–245. 10.1111/j.1460-9592.2009.03145.x [DOI] [PubMed] [Google Scholar]
- Sahyoun C, Cantais A, Gervaix A, Bressan S, Löllgen R, Krauss B, de Jaeger A, Frederiksen MS, Chéron G, Röher K, Hoffmann F, Fodor L, Sforzi I, Shavit I, Pucuka Z, Masilionis V, Farrugia R, Borensztajn D, Garrido A, … on behalf of the Pediatric Emergency Medicine Comfort and Analgesia Research in Europe (PemCARE) group of the Research in European Pediatric Emergency Medicine. (2021). Pediatric procedural sedation and analgesia in the emergency department: Surveying the current European practice. European Journal of Pediatrics, 180(6), 1799–1813. 10.1007/s00431-021-03930-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Fujita T, & Igarashi M (1993). Effects of inhalational anesthetics on biochemical events in growing neuronal tips. Anesthesiology, 79(6), 1338–1347; discussion 28A-29A. [PubMed] [Google Scholar]
- Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, & Maze M (2009). Dexmedetomidine Attenuates Isoflurane-induced Neurocognitive Impairment in Neonatal Rats. Anesthesiology: The Journal of the American Society of Anesthesiologists, 110(5), 1077–1085. 10.1097/ALN.0b013e31819daedd [DOI] [PubMed] [Google Scholar]
- Scheetz AJ, & Constantine-Paton M (1994). Modulation of NMDA receptor function: Implications for vertebrate neural development. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 8(10), 745–752. [DOI] [PubMed] [Google Scholar]
- Schenning KJ, Noguchi KK, Martin LD, Manzella FM, Cabrera OH, Dissen GA, & Brambrink AM (2017). Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicology and Teratology, 60, 63–68. 10.1016/j.ntt.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, & Holland SK (2002). Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology, 222(1), 212–218. 10.1148/radiol.2221010626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Short SJ, Knickmeyer RC, Wang J, Coe CL, Niethammer M, Gilmore JH, Zhu H, & Styner MA (2013). Diffusion tensor imaging-based characterization of brain neurodevelopment in primates. Cerebral Cortex. 10.1093/cercor/bhr372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukry M, Clyde MC, Kalarickal PL, & Ramadhyani U (2005). Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatric Anaesthesia, 15(12), 1098–1104. 10.1111/j.1460-9592.2005.01660.x [DOI] [PubMed] [Google Scholar]
- Slikker WJ, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, & Wang C (2007). Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicological Sciences : An Official Journal of the Society of Toxicology, 98(1), 145–158. 10.1093/toxsci/kfm084 [DOI] [PubMed] [Google Scholar]
- Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, & Warner DO (2012). Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clinic Proceedings, 87(2), 120–129. 10.1016/j.mayocp.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde AR, Budin F, Berger J-B, Gupta A, Farzinfar M, Kaiser A, Ahn M, Johnson H, Matsui J, Hazlett HC, Sharma A, Goodlett C, Shi Y, Gouttard S, Vachet C, Piven J, Zhu H, Gerig G, & Styner M (2014). UNC-Utah NA-MIC framework for DTI fiber tract analysis. Frontiers in Neuroinformatics, 7, 51. 10.3389/fninf.2013.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, & Hemmings HCJ (2003). Effects of isoflurane and propofol on glutamate and GABA transporters in isolated cortical nerve terminals. Anesthesiology, 98(2), 364–372. [DOI] [PubMed] [Google Scholar]
- Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, & Warner DO (2009). Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology, 110(4), 796–804. 10.1097/01.anes.0000344728.34332.5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, & Szarmach A (2018). Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Frontiers in Neurology, 9, 92. 10.3389/fneur.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Jevtovic-Todorovic V, Qin Y-Q, Tenkova T, Wang H, Labruyere J, & Olney JW (2005). Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. British Journal of Pharmacology, 146(2), 189–197. 10.1038/sj.bjp.0706301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JT, Shi Y, Niethammer M, Grauer M, Coe CL, Lubach GR, Davis B, Budin F, Knickmeyer RC, Alexander AL, & Styner MA (2017). The UNC-Wisconsin rhesus macaque neurodevelopment database: A structural MRI and DTI database of early postnatal development. Frontiers in Neuroscience, 11(FEB). 10.3389/fnins.2017.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JT, Vlasova RM, Howell BR, Knickmeyer RC, Morin E, Kuitchoua KI, Lubach GR, Noel J, Hu X, Shi Y, Caudill G, Alexander AL, Niethammer M, Paule MG, Coe CL, Sanchez M, & Styner M (2021). General anaesthesia during infancy reduces white matter micro-organisation in developing rhesus monkeys. British Journal of Anaesthesia, 126(4), 845–853. 10.1016/j.bja.2020.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jones MV, McMahon MT, Mori S, & Calabresi PA (2012). In vivo and ex vivo diffusion tensor imaging of cuprizone-induced demyelination in the mouse corpus callosum. Magnetic Resonance in Medicine, 67(3), 750–759. 10.1002/mrm.23032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker WJ, & Wang C (2009). Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. International Journal of Developmental Neuroscience : The Official Journal of the International Society for Developmental Neuroscience, 27(7), 727–731. 10.1016/j.ijdevneu.2009.06.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The neuroimaging data that support the findings of this study are openly available in UNC-Wisconsin Neurodevelopment Rhesus Database at https://data.kitware.com/#collection/54b582c38d777f4362aa9cb3, reference number (Young et al, 2017). The normalized anesthesia exposure data are available in the supplementary material of this article.