Abstract
The need for reproducible yet technically simple methods yielding high-quality cardiomyocytes is essential for research in cardiac biology. Performing cellular and molecular functional experiments (e.g., contraction, electrophysiology, calcium cycling, etc.) on cardiomyocytes are the gold standard for establishing mechanism(s) of disease. Mouse is the species of choice for functional experiments and the described technique is specifically for the isolation of mouse cardiomyocytes. Previous methods requiring a Langendorff apparatus require high levels of training and precision for aortic cannulation, often resulting in ischemia. The field is shifting towards Langendorff-free isolation methods which are simple, reproducible, and yield viable myocytes for physiological data acquisition and culture. These methods greatly diminish ischemia time compared to aortic cannulation and result in reliably obtained cardiomyocytes. Our adaptation to the Langendorff free method includes an initial perfusion with ice-cold clearing solution, use of stabilizing platform that ensures a steady needle during perfusion, and additional digestion steps to ensure reliably obtained cardiomyocytes for use in functional measurements and culture. This method is simple and quick to perform and requires little technical skill.
SUMMARY:
The gold standard in cardiology for cellular and molecular functional experiments are cardiomyocytes. This article describes adaptations to the non-Langendorff technique to isolate mouse cardiomyocytes.
INTRODUCTION:
For decades, an essential idea in cardiac biology literature is molecular mechanism of action. Mechanism of action must be established in order to publish reliable studies. A well-established strategy to determine molecular mechanism is isolated cardiomyocyte studies, which require high-quality cardiomyocytes for attaining trustable data. Cellular and molecular experiments performed on cardiomyocytes to determine mechanism of action are the gold standard for investigating contraction1, electrophysiology2, calcium (Ca2+) cycling3, myofilament Ca2+ sensitivity4, cytoskeletal5, metabolism6, effects of hormones7, signaling molecules8, drug studies9, etc. Mouse has become the species of choice for most cardiac biology experiments due to the ease of genetic manipulation, small size, relatively short lifespan, cost, etc10. However, the reliable isolation of high-quality mouse cardiomyocytes is not trivial with current techniques.
Labs have been isolating cardiomyocytes for almost 70 years11. Virtually all techniques to isolate cardiomyocytes rely on digestion of the heart via various enzymes (collagenase, protease, trypsin, etc). In the early periods (1950s-60s), the chunk method was employed, which involves removing the heart, cutting into much smaller pieces and incubating in solution with collagenase/protease/trypsin12. With updates to the “Langendorff apparatus”13, in the 1970s labs began isolating cardiomyocytes using the coronary artery perfusion-based isolation technique (retrograde perfusion with enzyme via the Langendorff apparatus), and remains the dominant method of myocyte isolation in the field today, ~50 years later14–16. Recent work has shifted to cannulating the heart in vivo to limit hypoxia time and ischemic damage resulting in superior cardiomyocyte isolations (better yields and higher quality).17. Recently, this has evolved into performing in vivo, Langendorff-free heart perfusions18–22. We have evolved the Langendorff-free cardiomyocyte isolation technique based on the Ackers-Johnson et al.18 technique and adapted various components from the many previous isolation techniques. These key adaptations include injection of an ice-cold clearing buffer, the incorporation of a supporting platform to stabilize the needle, allowing for decreased manipulation of the heart. Also detailed in this technique is a temperature control of injected buffers via a warming jacket (37°C), decreased time between in vivo injection and digestion due to less EDTA perfusion as previously published18. By decreasing manipulation of the heart and therefore minimizing puncture site size, thorough and constant perfusion of the coronary arteries is obtained. We also added additional digestions by using the chunk isolation method, decreased the amount of EDTA in the injected clearing buffer, and changed the pH. Our described technique is more reliable, more efficient, and does not require the extensive training/practice compared to the using the Langendorff apparatus (see Table 1).
Table 1.
A table of noted differences between our method, Langendorff methods, and previously published Langendorff-free methods of isolating mouse cardiomyocytes.
| Our adaptations | Langendorff technique | Langendorff-free method | |
|---|---|---|---|
| Prevention of blood clots | 3 mL ice-cold clearing solution (contains EDTA) | Heparin | 17 mL of EDTA solution |
| Non-perfusion time | Shortest | Longest | Short, but does not use ice-cold solution |
| Technical skill/training | Little | High | Little |
| Supporting platform to stabilize needle | Yes | No | |
| Temperature control of buffers (37⁰C) | Yes | Yes | No |
| Enzyme | Liberase | Lab-dependent | Collagenase |
| Post-digestion | Yes | Lab-dependent | No |
PROTOCOL:
All procedures performed in this study were approved by the Institutional Animal Care and Use Committee at the Ohio State University in accordance with NIH guidelines.
- SOLUTION PREPARATION
-
1.1Before Isolation (up to 2 weeks in advance of myocyte isolation)
-
1.1.1Perfusion Buffer Base: Prepare 1 L of perfusion buffer base (NaCl, KCl, NaH2PO4, HEPES, Glucose, BDM) in 1 L of ultrapure 18.2 MΩ cm H2O. Adjust to pH 7.4 prior to 0.22 um sterile filtration. Store at 4 °C until day of isolation.
-
1.1.2Clearing buffer: Prepare 1 L of Clearing buffer (NaCl, KCl, NaH2PO4, HEPES, Glucose, EDTA, BDM) in 1 L of ultrapure 18.2 MΩ cm H2O. Adjust to pH 7.4 prior to 0.22 um sterile filtration. Store at 4 °C until day of isolation.
-
1.1.3100 mM CaCl2 Stock Solution: Weigh out 1.11 g of CaCl2 and dissolve into 100 mL of ultrapure 18.2 MΩ cm H2O. Store at room temperature until day of isolation.
-
1.1.4Liberase Aliquots: Dissolve 5 mg of digestive enzymes in 5 mL of sterile, RNase-free water. Prepare 0.8 mL aliquots and store at −20 °C until day of isolation.
-
1.1.5Laminate Dishes: Apply 100 μL of 40 μL/mL mouse laminin to sterile, glass bottom 30 mL petri dishes. Allow to sit for 30 min and remove excess laminin. Set laminin on petri dish with UV incubation at room temperature for 30 min. Store at 4 °C until day of isolation (laminated petri dishes can be used up to 2 weeks).
-
1.1.6DMEM Media: In a biosafety cabinet, add 2.5 mL FBS, 0.5 mL Penicillin-Streptomycin (50 U/mL – 50 μg), 1mL 500 mM BDM to 45.5 mL DMEM. Store at 4 °C until day of isolation (media can be used up to 2 weeks).
-
1.1.7M199 Media: Prepare 1 L of M199 Media (NaHCO3, HEPES, L-glutathione, M199, BDM, BSA) in 1 L of ultrapure 18.2 MΩ cm H2O. Adjust to pH 7.4 prior to sterile filtration (0.22 μm filter). Store at 4 °C until day of isolation.
-
1.1.8500 mM BDM Stock: Add 0.5 g BDM to 10 mL ultrapure 18.2 MΩ cm H2O. Store at 4 °C until day of isolation.
-
1.1.9Stabilizing platform creation: Mold play dough around the base of the needle screwed into the luer stopcock. Mold to the petri dish that will contain the heart and ensure that the needle is at proper height for ventricle injection (~5 mm above bottom of dish).
-
1.1.1
-
1.2The Day of Isolation
-
1.2.1Perfusion Buffer: The day of the isolation, add 9.5 mg of MgCl2 to 100 mL perfusion buffer base. Allow to mix completely before removing 30 mL of completed perfusion buffer and place in a clean, wide-mouth 100 mL glass bottle with a screw cap lid (Digestion Buffer). These additions can be made the day of isolation without additional pH adjustments as their addition negligibly alters the pH. Remove 12 mL of perfusion buffer and set to the side (Stop Buffer). Pour the remaining 58 mL of perfusion buffer into another clean, wide-mouth 100 mL glass bottle with a screw cap lid. Store the remaining perfusion buffer at 37 °C throughout isolation.
-
1.2.2Digestion Buffer: Add 7.5 μL of 100 mM CaCl2 to 30 mL of perfusion buffer for a final concentration of 25 μM. Immediately prior to the isolation, add 770 μL of Liberase TH to the digestion buffer for a final Liberase concentration of 26 μg/mL.
-
1.2.3Stop Buffer: Dissolve 240 mg of BSA in 12 mL of perfusion buffer. Store at 37 °C throughout isolation.
-
1.2.4Clearing Buffer: Prepare a 3 mL syringe of Clearing Buffer and clear the needle of bubbles. Store on ice until isolation.
-
1.2.1
-
1.1
- MANIFOLD PREPARATION
-
2.1Clear the temperature-controlled manifold with freshly prepared perfusion buffer, careful to ensure no bubbles remain. Using a luer lock connector, screw in a 26 G needle and clear of bubbles. A cleared manifold is essential for a successful isolation.
-
2.1
- ANIMAL PREPARATION
-
3.1Inject the mouse with 100 mg/kg ketamine and 20 mg/kg xylazine by body weight immediately prior to the isolation. This procedure used a male, 4-month-old C57Bl/6 mouse.
-
3.2It may be helpful to place the mouse on a heated surgical pad to encourage sedation. Tent the arms of the mouse and tape the base of the tail to a blue lab diaper. Ensure that the animal is fully sedated by toe-pinch reflex.
-
3.1
- CARDIOMYOCYTE ISOLATION PROCEDURE
-
4.1Expose the sternum of the mouse and, lateral to the midline, cut proximally through the ribs and to the axilla. Gently cut fully through the diaphragm, ensuring shallow cuts to avoid the heart. Clamp the sternum with a hemostat and fold the ribs backwards to expose the thoracic cavity.
-
4.2Gently remove the pericardium from the heart and cut fully through the inferior vena cava, immediately distal to the heart. Use a 3mL syringe with a 26G needle to quickly inject 3 mL ice-cold Clearing Buffer into the right ventricle of the heart over 1 minute
-
4.3Gently hold the heart using tweezers and pull away from the body, exposing as much of the aorta as possible. Using a hemostat, clamp the ascending aorta, being careful not to clamp the atria, and excise the heart from the chest.
-
4.4Quickly transfer the clamped heart to the lid of a polypropylene petri dish with approximately 10 mL of warm perfusion buffer. Place the clamped heart into the supporting platform and use a stabilized 26G needle attached to the manifold to inject 10 mL of temperature-controlled 37 °C perfusion buffer into the left ventricle over 5 minutes.
-
4.5Transfer the clamped heart and supporting platform to a petri dish with approximately 5 mL of digestion buffer. Exchange input syringes for a 50 mL syringe with 25 mL digestion buffer.
-
4.6Clear the manifold of any bubbles and remaining perfusion buffer before injection. Carefully replace needle into same needle position in left ventricular apex.
-
4.7Use a perfusion pump to inject temperature-controlled 37 °C digestion buffer into the left ventricle for 15 minutes using a 50 mL syringe. The solution should be temperature-controlled by a water jacket previously calibrated to eject 37 °C solution at the end of the needle.
-
4.8Remove the ventricles from the atria and transfer to a 10 mL beaker. Add 3 mL digestion buffer to the beaker and, using sharp scissors, cut the ventricles into large chunks. Cover the beaker with aluminum foil and place in a shaking water bath preheated to 37 °C for 5 minutes.
-
4.9Discard the supernatant, careful to avoid removing any chunks of tissue. Resuspend the tissue chunks in 3 mL digestion buffer and triturate for approximately 4 minutes or until a homogenous mixture is achieved.
-
4.10Filter cells through 70 um nylon cell strainer into a 50 mL polypropylene tube.
-
4.11Return filtrate to a 14 mL round-bottom polypropylene falcon tube and centrifuge for 1 minute at 100 rpm. Discard the supernatant and resuspend the pellet in 3 mL of stop buffer.
-
4.12Add 54 μL of 100 mM CaCl2 stock to the resuspended cells for a final CaCl2 concentration of 1.8 mM. This step can also be performed stepwise to increase cell yield.
-
4.13Allow live cells to gravity settle for 10 minutes before removing supernatant containing dead cells. Resuspend pellet in storage solution (perfusion solution with 200 μM Ca). These cells can now be used for functional experiments (i.e., Ca Imaging/contraction, patch clamp, etc.), culture, etc.
-
4.14Cells may also be resuspended in lab and experiment dependent solutions.
-
4.1
- CELL CULTURE
-
5.1Count cells using a hemocytometer or by field view count using a gridded cover slip. As myocytes are very large, counting using a hemocytometer is not always accurate. This is used to get an idea of approximately how many cells were obtained from the isolation.
-
5.2Dilute cells to ~25,000 cells/mL with DMEM Media. Add 1 mL of cell solution to each well.
-
5.3Incubate at 37 °C, 95% O2 5% CO2 for 2 hrs.
-
5.4Gently aspirate DMEM media from each well and add 2 mL of M199 media.
-
5.5Incubate at 37 °C, 95% O2 5% CO2 for up to 24 hrs.
-
5.1
REPRESENTATIVE RESULTS:
There are a few elements to examine when determining the success of an isolation. First, the cardiomyocytes must be rod-shaped with no membrane blebs, such as the cells isolated in figures 1A and 1B. A typical isolation will yield ~80% of the myocytes being rod-shaped. If the isolation yields anything less than 50% rod shaped cells, then it is considered an unsuccessful isolation and cardiomyocytes are not used. Lastly, the cardiomyocytes should be quiescent. The myocytes spontaneously contracting demonstrates that they are not Ca2+ tolerant and will produce unreliable data. Of all the isolations performed using the above technique, nearly all isolations are considered successful. Cultured cardiomyocytes are considered successful if they are rod-shaped with no membrane blebs or rounded edges with high survival rates (figure 2).
Figure 1.
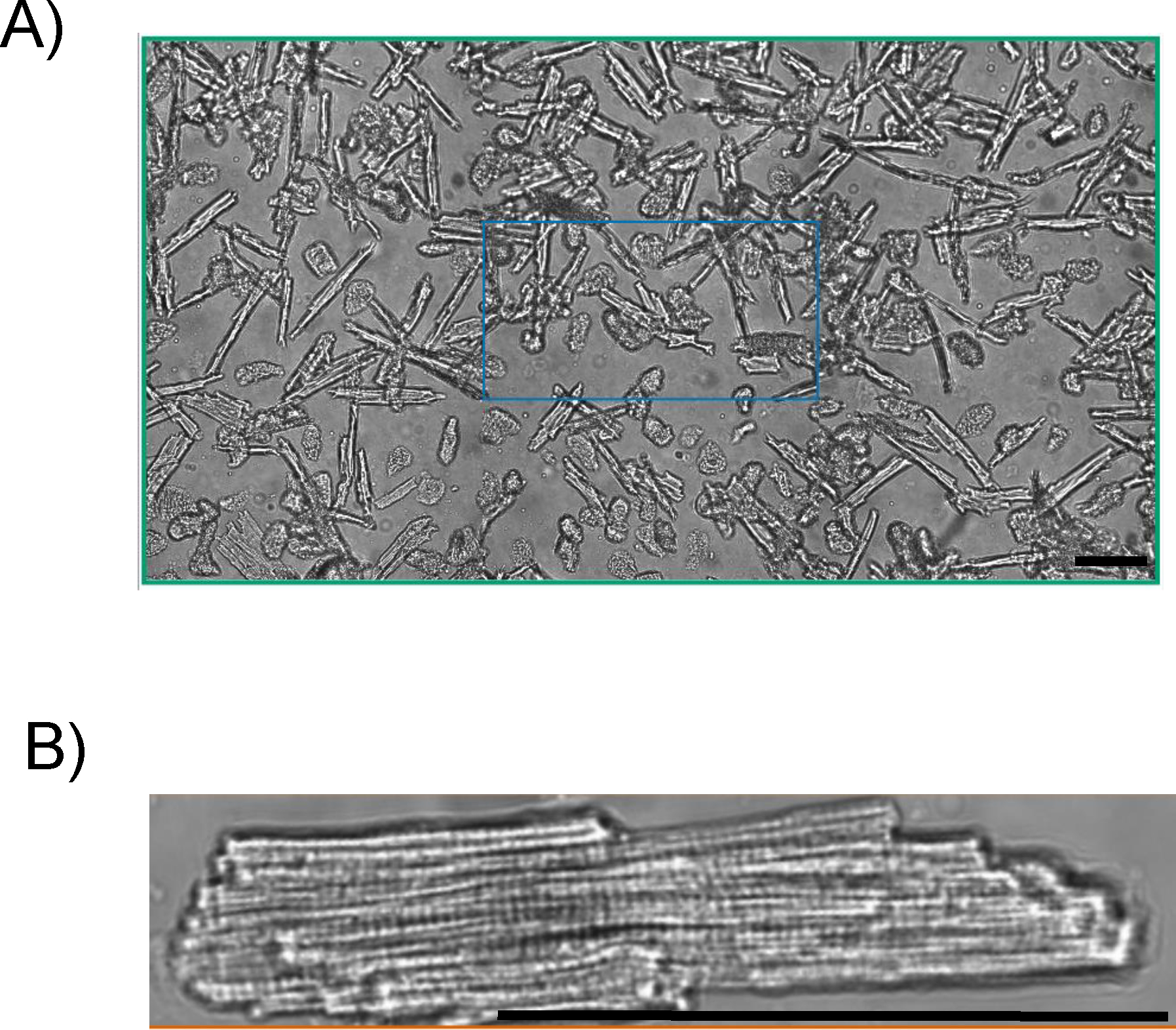
A) 4-month-old C57Bl/6 mouse cardiomyocytes imaged using Ionoptix Multicell bright field. Yield ~80%, 20X magnification. B) Ionoptix Multicell bright field image of a 4-month-old C57Bl/6 mouse cardiomyocyte. Cardiomyocyte are quiescent, exhibiting rod-shape with no membrane blebs. Scale bar is 100 μm.
Figure 2.
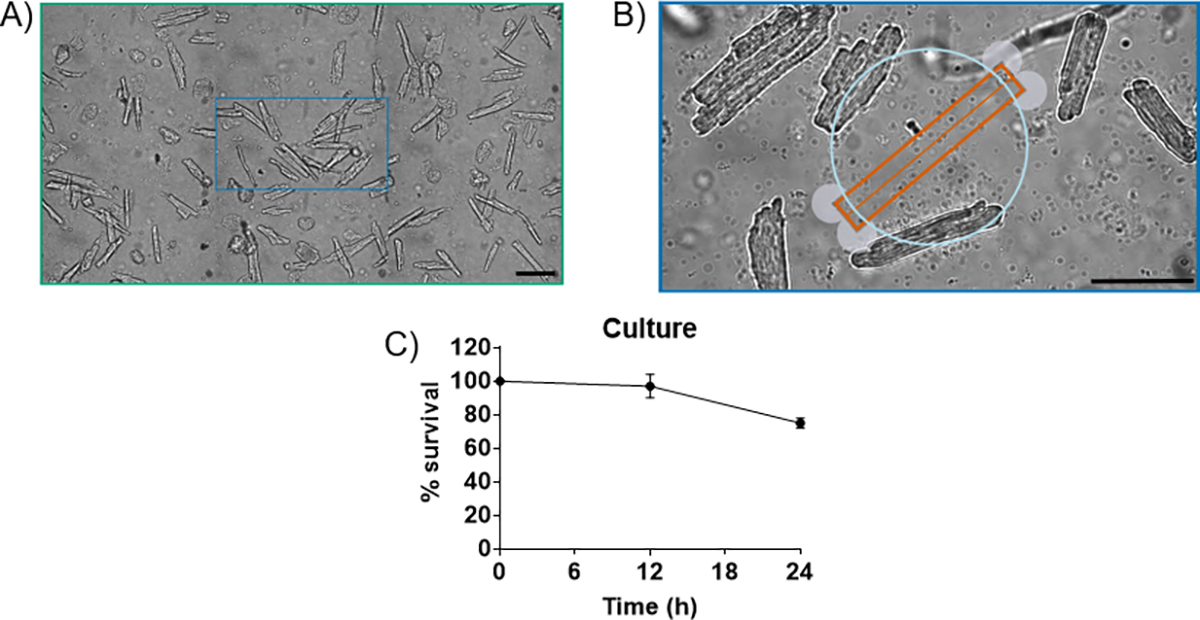
A) 4-month-old C57Bl/6 mouse cardiomyocytes cultured for 24 hrs in M199 media. ~80% viability, 20X magnification. B) Myocytes cultured for 24h. At 24h, cardiomyocytes exhibit rod-shape with no membrane blebs. C) % survival curve for cardiomycoytes cultured for 24h using the described method. Scale bar is 100 μm.
DISCUSSION:
The principal advantage of our Langendorff-free cardiomyocyte isolation technique is that it limits hypoxia and ischemic time by not requiring cannulation to a Langendorff apparatus. Alternatively to classical Langendorff techniques which require minutes to remove, clean, and hang the heart, often resulting in ischemic damage to the myocyte, our method includes an in vivo clearing of blood via ice-cold clearing solution. The ice-cold clearing buffer contains ethylenediaminetetraacetic acid (EDTA), which irreversibly chelates divalent cations that efficiently removes calcium, making it an excellent anticoagulant, and stops contraction23. Using ice-cold solutions will inhibit contraction, which will slow the metabolic rate preventing damage caused to ischemia24. By removing the need for aortic cannulation, Langendorff-free techniques produces robust myocytes resulting in a more reliable preparation to yield trustworthy data, which is not always the case for Langendorff methods of isolation.
This is a variation of a previously published Langendorff-free isolation technique18 with adaptations as highlighted in Table 1. Most importantly, this technique introduces a stabilizing platform which limits the number of times the needle must be removed and replaced from the heart. Any unnecessary movement introduced to the needle while inserted into the ventricle widens the puncture site. A wider puncture site results in backflow out of the ventricle from the puncture site, decreased pressure in the heart, and decreased flow to the coronaries. This issue is fixed by using the stabilizing platform. By limiting the needle movement and the number of times the needle is removed and replaced (one compared to three times as previously described18), we maintain consistent flow to the coronaries and consistently achieve digestion. Another key adaptation was the addition of a temperature controlled jacket to maintain enzyme solutions at 37°C upon entering the heart (temperature bath was calibrated to eject at 37°C from the needle). The digestion enzyme used has optimum reaction activity at 37°C, and the addition of temperature control increases enzyme activity, therefore decreasing digestion time. Liberase also has minimal lot-to-lot variability and has higher reproducibility compared to other enzymes. Another adaptation of previous techniques is decreased amount of injected clearing solution. Decreasing the amount of clearing buffer to enter the heart and allowing for more blood to be cleared by perfusion buffer decreases inactivation of the digestion enzyme by EDTA. Another reason that our method consistently achieves successful myocyte isolations is by usage of BDM. BDM is a myosin inhibitor and inhibits the power stroke mechanism of the sarcomere, thereby inhibiting contraction and limiting reoxygenation injury during digestion. By limiting contraction, we prevent calcium cycling and inherent reactive oxygen species production in contracting myocytes in culture. Since our culture media contains calcium, it is possible that the myocytes could contract and decrease subsequent yield after culture. We elect to culture our myocytes in BDM to prevent contraction and increase yield25,26. BDM can always be washed out of myocytes for functional measurements after culture with great success. Alternatively, all steps of this procedure can be performed without the addition of BDM, but isolations may not be considered as “successful” on BDM-free isolated myocytes.
Besides ischemic time, there are many other factors that will determine if an isolation will produce robust myocytes. Since there are different conditions in the individual labs, the persons performing the isolation may need to modify the amount of enzyme and/or calcium, the perfusion time and/or pump speed, and the rocking water bath speed and/or time. These parameters will be dependent upon the mouse model used, such as healthy or diseased (e.g., aging, etc.).
While this technique does not require surgical skill needed for the Langendorff based techniques, there are still critical steps to make the technique successful. The most common issue that yields poor myocytes with this technique is due to bubbles entering the perfusion system and blocking the myocardial tissue from access to perfusing buffer. The solution to this problem is careful practice to avoid bubbles (via proper syringe clearance technique, proper needle clearance technique, burping the manifold of bubbles when changing syringes, etc), as well as multiple exit points from the perfusion system in case a bubble is lodged in the manifold. Without bubbles, in our experience, we get near 100% successful myocyte isolations (defined as above 50% rod shaped myocytes, average is ~80%).
While the method has been simplified by removal of the surgical skill required for the Langendorff method, this adapted method has only been tried in mouse. Another advantage of the Langendorff-free method is that it can be used for many murine models (i.e., from neonate to senescence), as previously described.19 Unfortunately, larger mammals (i.e., dog, pig, etc.) will not be suitable for this technique due to the size of their hearts. It would be impossible to perfuse the heart with enough solution to acquire reliable myocytes.
Our adaption of the Langendorff-free method is a dependable method for successful isolation of mouse cardiomyocytes. Compared to the Langendorff method, this alternative approach requires little technical skill to consistently obtain cardiomyocytes.
table-materials.
| Name of Material/Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| 10 cc Bd Luer-Lok Syringe | Fisher Sci | 14-827-52 | |
| 10 mL Pyrex Low-Form Beaker | Cole-Palmer | UX-34502-01 | |
| 100 mL polypropylene cap glass media storage bottle | DWK Life Sciences | UX-34523-00 | |
| 14 mL Round-Bottom Polypropylene Test Tubes With Cap | Fisher Sci | 14-959-11B | |
| 2,3-Butanedione Monoxime | Sigma | B0753 | >98% |
| 3 cc BD Luer-Lok Syringe | Fisher Sci | 14-823-435 | |
| 35 mm glass bottom dishes | MatTek Corporation | P35G-1.0-20-C | |
| 50 mL BD Syringe without Needle | Fisher Sci | 13-689-8 | |
| 50 mL Conical Centrifuge Tubes | Cole-Palmer | EW-22999-84 | |
| 95% O2 5% CO2 | |||
| AIMS Space Gel Heating Pad | Fisher Sci | 14-370-223 | |
| BD PRECISIONGLIDE™ 27 G X 1/2” HYPODERMIC NEEDLES | Becton Dickinson | 305109 | |
| Bovine Serum Albumin | Sigma | A3803 | Heat shock fraction, lyophilized powder, essentially fatty acid free, >98% |
| Calcium Chloride dihydrate | Sigma | C7902 | >99% |
| D-(+)-Glucose | Sigma | G7021 | Suitable for cell culture, >99.5% |
| DMEM | Fisher Sci | 11965092 | |
| EDTA | Fisher Sci | AAA1071336 | |
| Falcon® 100 mm TC-treated Cell Culture Dish | Corning | 353003 | |
| FBS | R&D Systems (Bio-techne) | S11195 | |
| Fisherbrand Isotemp Heated Immersion Circulators | Fisher Sci | 13-874-432 | |
| Hartman Mosquito Hemostatic Forceps | World Precision Instruments | 15921 | |
| Hausser Scientific™ Hy-Lite™ Counting Chamber Set | Fisher Sci | 02-671-11 | |
| HEPES | Sigma | H4034 | >99.5% |
| Labeling Tape | Fisher Sci | 15-901-10R | |
| Legato 100 Syringe Pump | kdScientific | 788100 | |
| L-glutathione | Fisher Sci | ICN19467980 | |
| Liberase TH Research Grade | Sigma | 5401135001 | High thermolysin concentration |
| M199 | Fisher Sci | MT10060CV | |
| Magnesium Chloride | Invitrogen | AM9530G | |
| Mouse Laminin | Corning | 354232 | |
| Pen/Strep | Fisher Sci | ||
| Potassium Chloride | Sigma | P5405 | >99% |
| Precision Digital Reciprocating Water Bath | ThermoFisher Scientific | TSCIR19 | |
| Sodium Bicarbonate | Sigma | S5761 | Suitable for cell culture |
| Sodium Chloride | Sigma | S5886 | >99% |
| Sodium phosphate monobasic | Sigma | S5011 | >99% |
| Sterile Cell Strainer 70 um | Fisher Sci | 22-363-548 | |
| Student Fine Scissors | Fine Science Tools | 91460-11 | |
| VWR Absorbent Underpads | Fisher Sci | NC9481815 |
| Perfusion Pump Settings | ||||
| Volume | 49.99 mL | |||
| Rate | 120.6 mL/h | |||
| Diameter | 26.60 mm | |||
| Clearing Solution | ||||
| Compound | MW (g/mol) | Concentration (mM) | g/L | |
| NaCl | 58.44 | 130 | 7.5972 | |
| KCl | 74.55 | 5 | 0.37275 | |
| NaH2PO4 | 119.98 | 0.5 | 0.05999 | |
| HEPES | 238.3 | 10 | 2.383 | |
| Glucose | 180.16 | 10 | 1.8016 | |
| BDM | 101.1 | 10 | 0.0101 | |
| EDTA | 292.24 | 5 | 1.4612 | |
| Make in 1 L ultrapure 18.2 MΩ*cm H2O. Sterile Filter, pH 7.4 | ||||
| Perfusion Buffer | ||||
| Compound | MW (g/mol) | Concentration (mM) | g/L | |
| NaCl | 58.44 | 130 | 7.5972 | |
| KCl | 74.55 | 5 | 0.37275 | |
| NaH2PO4 | 119.98 | 0.5 | 0.05999 | |
| HEPES | 238.3 | 10 | 2.383 | |
| Glucose | 180.16 | 10 | 1.8016 | |
| BDM | 101.1 | 10 | 0.0101 | |
| MgCl2 | 95.2 | 1 | 0.095 | |
| Make in 1 L ultrapure 18.2 MΩ*cm H2O. Sterile Filter, pH 7.4 | ||||
| Digestion Buffer | ||||
| Compound | MW (g/mol) | Concentration (mM) | g/L | |
| NaCl | 58.44 | 130 | 7.5972 | |
| KCl | 74.55 | 5 | 0.37275 | |
| NaH2PO4 | 119.98 | 0.5 | 0.05999 | |
| HEPES | 238.3 | 10 | 2.383 | |
| Glucose | 180.16 | 10 | 1.8016 | |
| BDM | 101.1 | 10 | 0.0101 | |
| MgCl2 | 95.2 | 1 | 0.095 | |
| CaCl2 | 110.98 | 0.025 | 2.7745 | |
| Liberase TH | 770 ul | |||
| Make in 1 L ultrapure 18.2 MΩ*cm H2O. Sterile Filter, pH 7.4 | ||||
| Stop Buffer | ||||
| Stop Buffer = Perfusion Buffer + 2% BSA | ||||
| Compound | MW (g/mol) | Concentration (mM) | g/L | |
| NaCl | 58.44 | 130 | 7.5972 | |
| KCl | 74.55 | 5 | 0.37275 | |
| NaH2PO4 | 119.98 | 0.5 | 0.05999 | |
| HEPES | 238.3 | 10 | 2.383 | |
| Glucose | 180.16 | 10 | 1.8016 | |
| BDM | 101.1 | 10 | 0.0101 | |
| MgCl2 | 95.2 | 1 | 0.095 | |
| Bovine Serum Albumin | 66463 | |||
| Make in 1 L ultrapure 18.2 MΩ*cm H2O. Sterile Filter, pH 7.4 | ||||
| M199 Media | ||||
| Compound | MW (g/mol) | Concentration (mM) | g/L | |
| NaHCO3 | 84.007 | 2.2 | ||
| HEPES | 238.3 | 2.6 | ||
| L-glutathione | 307.3235 | 3.073 | ||
| BDM | 101.1 | |||
| BSA | 66463 | 0.2 | ||
| M199 Media | ||||
| Pen/Strep | 10 ml | |||
| Make in 1 L ultrapure 18.2 MΩ*cm H2O. Sterile Filter, pH 7.4 | ||||
| DMEM Media | ||||
| Compound | Volume (mL) | |||
| DMEM | 45.5 | |||
| FBS | 2.5 | |||
| Pen/Strep | 0.5 | |||
| BDM | 1 | |||
ACKNOWLEDGMENTS:
This work was supported by National Institutes of Health Grants R01 HL114940 (Biesiadecki) and R01 AG060542 (Ziolo), and T32 HL134616 (Sturgill and Salyer).
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/63056.
DISCLOSURES:
No conflicts of interest to disclose.
Contributor Information
Sarah L Sturgill, Department of Physiology and Cell Biology, The Ohio State University.
Lorien G Salyer, Department of Physiology and Cell Biology, The Ohio State University.
Brandon J Biesiadecki, Department of Physiology and Cell Biology, The Ohio State University.
Mark T Ziolo, Department of Physiology and Cell Biology, The Ohio State University.
REFERENCES:
- 1.Ziolo MT, Dollinger SJ & Wahler GM Myocytes isolated from rejecting transplanted rat hearts exhibit reduced basal shortening which is reversible by aminoguanidine. J Mol Cell Cardiol. 30, 1009–1017. (1998). [DOI] [PubMed] [Google Scholar]
- 2.Ziolo MT et al. Myocytes isolated from rejecting transplanted rat hearts exhibit a nitric oxide-mediated reduction in the calcium current. J Mol Cell Cardiol. 33, 1691–1699. (2001). [DOI] [PubMed] [Google Scholar]
- 3.Traynham CJ et al. Diesterified Nitrone Rescues Nitroso-Redox Levels and Increases Myocyte Contraction Via Increased SR Ca(2+) Handling. PLoS One 7, e52005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nixon BR et al. Combined troponin I Ser-150 and Ser-23/24 phosphorylation sustains thin filament Ca(2+) sensitivity and accelerates deactivation in an acidic environment. J Mol Cell Cardiol 72, 177–185 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swager SA et al. Claudin-5 levels are reduced from multiple cell types in human failing hearts and are associated with mislocalization of ephrin-B1. Cardiovasc Pathol 24, 160–167, doi: 10.1016/j.carpath.2014.10.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinckard KM et al. A Novel Endocrine Role for the BAT-Released Lipokine 12,13-diHOME to Mediate Cardiac Function. Circulation 143, 145–159, doi: 10.1161/circulationaha.120.049813 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roof SR, Shannon TR, Janssen PM & Ziolo MT Effects of increased systolic Ca2+ and phospholamban phosphorylation during beta-adrenergic stimulation on Ca2+ transient kinetics in cardiac myocytes. Am J Physiol Heart Circ Physiol 301, H1570–1578 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris JE et al. Exercise-induced 3’-sialyllactose in breast milk is a critical mediator to improve metabolic health and cardiac function in mouse offspring. Nature metabolism 2, 678–687, doi: 10.1038/s42255-020-0223-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roof SR et al. CXL-1020, a Novel Nitroxyl (HNO) Prodrug, Is More Effective than Milrinone in Models of Diastolic Dysfunction-A Cardiovascular Therapeutic: An Efficacy and Safety Study in the Rat. Frontiers in physiology 8, 894, doi: 10.3389/fphys.2017.00894 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milani-Nejad NJ, P. M. . Small and large animal models in cardiac contraction research: advantages and disadvantages. . Pharmacol Ther 141, 235–249, doi:doi: 10.1016/j.pharmthera.2013.10.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harary I & Farley B In vitro studies of single isolated beating heart cells. Science 131, 1674–1675, doi: 10.1126/science.131.3414.1674 (1960). [DOI] [PubMed] [Google Scholar]
- 12.Kono T Roles of collagenases and other proteolytic enzymes in the dispersal of animal tissues. Biochim Biophys Acta 178, 397–400, doi: 10.1016/0005-2744(69)90410-0 (1969). [DOI] [PubMed] [Google Scholar]
- 13.Baker JB An improved apparatus for mammalian heart perfusion. J Physiol 115, 30–32p (1951). [PubMed] [Google Scholar]
- 14.Powell T & Twist VW A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochemical and biophysical research communications 72, 327–333, doi: 10.1016/0006-291x(76)90997-9 (1976). [DOI] [PubMed] [Google Scholar]
- 15.Motayagheni N Modified Langendorff technique for mouse heart cannulation: Improved heart quality and decreased risk of ischemia. MethodsX 4, 508–512, doi: 10.1016/j.mex.2017.11.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z et al. An improved procedure for isolating adult mouse cardiomyocytes for epicardial activation mapping. Journal of cellular and molecular medicine 25, 11257–11263, doi: 10.1111/jcmm.17049 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jian Z et al. In Vivo Cannulation Methods for Cardiomyocytes Isolation from Heart Disease Models. PLoS One 11, e0160605, doi: 10.1371/journal.pone.0160605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackers-Johnson M et al. A Simplified, Langendorff-Free Method for Concomitant Isolation of Viable Cardiac Myocytes and Nonmyocytes From the Adult Mouse Heart. Circ Res 119, 909–920, doi: 10.1161/circresaha.116.309202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weldrick JJ, Abdul-Ghani M, Megeney LA & Burgon PG A rapid and efficient method for the isolation of postnatal murine cardiac myocyte and fibroblast cells. Canadian journal of physiology and pharmacology 96, 535–539, doi: 10.1139/cjpp-2017-0742 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Myachina TA, Butova XA & Khohlova AD A modified Langendorff-free method for isolation of cadiomyocytes from adult rat heart. AIP Conference Proceedings 2174, 020140, doi: 10.1063/1.5134291 (2019). [DOI] [Google Scholar]
- 21.Omatsu-Kanbe M, Yoshioka K, Fukunaga R, Sagawa H & Matsuura H A simple antegrade perfusion method for isolating viable single cardiomyocytes from neonatal to aged mice. Physiological reports 6, e13688, doi: 10.14814/phy2.13688 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omatsu-Kanbe M, Fukunaga R, Mi X & Matsuura H An Antegrade Perfusion Method for Cardiomyocyte Isolation from Mice. J Vis Exp, doi: 10.3791/61866 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Bers DM, Patton CW & Nuccitelli R A practical guide to the preparation of Ca2+ buffers. Methods in cell biology 40, 3–29, doi: 10.1016/s0091-679x(08)61108-5 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Grosso DS, Frangakis CJ, Carlson EC & Bressler R Isolation and characterization of myocytes from the adult rat heart. Preparative biochemistry 7, 383–401, doi: 10.1080/00327487708061656 (1977). [DOI] [PubMed] [Google Scholar]
- 25.T Thum JB Butadione Monoxime increases the viability and yield of adult cardiomyocytes in primary cultures. Cardiovasc Toxicol 1, 61–72, doi:doi: 10.1385/ct:1:1:61 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Wolska BM & Solaro RJ Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am J Physiol 271, H1250–1255, doi: 10.1152/ajpheart.1996.271.3.H1250 (1996). [DOI] [PubMed] [Google Scholar]


