Abstract
Following exposure to drugs of abuse, long-term neuroadaptations underlie persistent risk to relapse. Endocannabinoid signaling has been associated with drug-induced neuroadaptations, but the role of lipases that mediate endocannabinoid biosynthesis and metabolism in regulating relapse behaviors following prolonged periods of drug abstinence has not been examined. Here, we investigated how pharmacological manipulation of lipases involved in regulating the expression of the endocannabinoid 2-AG in the nucleus accumbens (NAc) influence cocaine relapse via discrete neuroadaptations. At prolonged abstinence (30 days) from cocaine self-administration, there is an increase in the NAc levels of DAGL, the enzyme responsible for the synthesis of the endocannabinoid 2-AG, along with decreased levels of MAGL, which hydrolyzes 2-AG. Since endocannabinoid-mediated behavioral plasticity involves phosphatase dysregulation, we examined the phosphatase calcineurin after 30 days of abstinence and found decreased expression in the NAc, which we demonstrate is regulated through the transcription factor EGR1. Intra-NAc pharmacological manipulation of DAGL and MAGL with inhibitors DO-34 and URB-602, respectively, bidirectionally regulated cue-induced cocaine seeking and altered the phosphostatus of translational initiation factor, eIF2α. Finally, we found that cocaine-seeking 30 days after abstinence leads to decreased phosphorylation of eIF2α and reduced expression of its downstream target NPAS4, a protein involved in experience-dependent neuronal plasticity. Together, our findings demonstrate that lipases that regulate 2-AG expression influence transcriptional and translational changes in the NAc related to drug relapse vulnerability.
Introduction
Drug addiction is a neuropsychiatric disease in which individuals experience recurring episodes of relapse following prolonged periods of abstinence1. Escalated cocaine craving may contribute to persisting relapse vulnerability, which is triggered by drug-associated cues and mediated by neuroadaptations in brain regions that govern reward processing, including the nucleus accumbens (NAc)2, 3. Transcriptional and translational alterations in the NAc mediate long-term neuroplasticity and subsequent behavioral adaptations that occur following exposure to drugs of abuse4, 5. Although these neuroadaptations in the NAc have been thought to mediate perpetual relapse vulnerability, the precise mechanisms are not well understood2.
Endocannabinoid signaling within the neural “reward circuitry” influences motivated behaviors6, including following cocaine exposure7–9. Endocannabinoid signaling in the brain primarily comprises metabotropic receptors, endogenous ligands, and enzymes that synthesize and degrade the ligands10, 11. Endocannabinoids include anandamide and 2-arachidonoyl glycerol (2-AG)11, 12, the most predominant ligand for cannabinoid type 1 (CB1) receptors13, 14. 2-AG is produced through the metabolism of 1,2-diacylglycerol by sn1-specific diacylglycerol lipase (DAGL)15–17 and is hydrolyzed by monoacylglycerol lipase (MAGL)18. Endocannabinoid signaling mediates long-term depression (LTD) of glutamatergic synaptic transmission in the NAc19. This endocannabinoid-mediated LTD is altered and is part of the synaptic remodeling that occurs during prolonged abstinence from cocaine self-administration2, 20, 21. While previous studies demonstrate that drug seeking intensifies, or incubates, during abstinence22, the functional or behavioral significance of the changes induced by LTD or the role that the endocannabinoids may play in such plasticity remains undetermined. Moreover, it is unclear how endocannabinoid signaling in the NAc may underlie transcriptional and/or translational changes during abstinence.
The immediate early gene for early growth response 1 (EGR1) encodes a master transcriptional regulator that is essential for cocaine-induced cellular and behavioral plasticity23, 24. The regulation of EGR1 itself is mediated in part through endocannabinoid signaling as well as calcineurin, a neuron-enriched phosphatase25–27, suggesting that EGR1 may be an essential hub in the neurobiological adaptations induced by endocannabinoids. Furthermore, endocannabinoid-mediated acquisition and consolidation of memory are thought to involve altered regulation of downstream phosphatases28.
The phosphostatus of eukaryotic initiation factor 2 α-subunit (eIF2α), an integral component of protein synthetic machinery, is critical for the initiation of protein translation29. Typically, phosphorylation of eIF2α halts translational machinery whereas dephosphorylation of eIF2α leads to increased protein synthesis30. Endocannabinoid-dependent synaptic remodeling has been linked to transcriptional and translational changes31, and endocannabinoid-mediated presynaptic plasticity involves the phosphatase calcineurin32. Moreover, calcineurin promotes auto-phosphorylation of PKR-like ER kinase (PERK), which in turn increases the phosphorylation of eIF2α and attenuates protein translation33. The phosphostatus of eIF2α has been shown to regulate experience-dependent translational and behavioral plasticity34–40. Cue-induced cocaine seeking following prolonged abstinence reduces the levels of phosphorylated eIF2α (p-eIF2α) in the NAc, and pharmacologically inhibiting eIF2α dephosphorylation attenuates cue-induced cocaine seeking41. Although eIF2α dephosphorylation increases general translation, there is reduced translation of a subset of target mRNAs containing open reading frames such as those for Neuronal PAS domain protein 4 (NPAS4) and activating transcription factor 4 (ATF4) that are thought to facilitate behavioral adaptations34, 35, 38, 39, 41, 42.
In this study, we examined the roles of DAGL and MAGL in the NAc in mediating the expression of cocaine seeking during prolonged abstinence following cocaine self-administration. Additionally, we sought to determine how DAGL and MAGL may regulate transcriptional and translational mechanisms following prolonged abstinence. These findings provide novel insight into the long-lasting neural adaptations that lead to relapse vulnerability.
Materials and Methods
Subjects
Male Sprague-Dawley rats (250–275 g; Envigo, Indianapolis, IN) were habituated to a colony room with constant temperature and humidity levels for 5 to 7 days. Rats were singly housed and had ad libitum access to food and water. Behavioral testing was performed during the dark phase of the 12-h light-dark cycle. This study was conducted in accordance with the guidelines set up by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
Drugs
Cocaine hydrochloride, gifted by the National Institute on Drug Abuse (Bethesda, MD), was dissolved in sterile 0.9% saline (1.75 mg/mL). Pump durations/injection volumes were adjusted daily according to the body weights to deliver 0.5 mg/kg/infusion (inf). The DAGL inhibitor DO-34 (1 μg/0.3μL) (Aobious, Gloucester, MA) was dissolved in dimethyl sulfoxide (DMSO) as a stock solution and used at a final concentration of 10% DMSO and 90% saline. URB-602, a noncompetitive inhibitor that preferentially inhibits MAGL and potentiates 2-AG levels43 was used to inhibit MAGL. URB602 (300 pmol/0.3 μL) (Tocris, Bristol, UK) was dissolved in a solution containing 10% DMSO in saline. The pharmacological agents and doses were selected from previously published studies43, 44.
Jugular catheterization and patency testing
Rats were implanted with chronic indwelling jugular catheters as described previously45, 46 and given 4 days to recover from surgery. Catheters were flushed daily with 0.2 mL of a solution of enrofloxacin (4 mg mL−1) in heparinized saline (50 IU mL−1 in 0.9% sterile saline) to preserve catheter patency. One day prior to behavioral testing, each animal received an intravenous infusion of ketamine hydrochloride (0.5 mg/kg in 0.05 mL), and an appropriate behavioral response (loss of muscle tone and righting reflexes) was used to verify catheter patency. Only rats with patent catheters were included in data analyses.
Acquisition of extended access self-administration
After the rats recovered from catheter surgery, they were assigned to self-administer either saline or cocaine during the dark phase in test chambers (MED Associates, St. Albans, VT) that were equipped with two snout-poke holes. During the acquisition, rats were trained for 1 h/day for 5 days. A response in the active hole resulted in an intravenous injection of saline or cocaine (0.5 mg/kg/inf) using a fixed ratio of 1 (FR1) schedule of reinforcement that was increased daily to an FR5 schedule. Infusions were accompanied by a 5-s illumination of the stimulus light above the active snout-poke hole, with the house light being extinguished for the duration of the infusion and the following 30-s timeout period. Responses to the inactive hole resulted in no programmed consequences. The criterion for the acquisition of cocaine self-administration was an average of 10 cocaine infusions per day during the last 3 days of the 5-day acquisition phase24, 47.
Extended-access self-administration training
Following the acquisition phase, rats were allowed to self-administer either saline or cocaine (0.5 mg/kg/inf) in the training phase (6 h/day) using an FR5 schedule of reinforcement for 10 days. This procedure is commonly used in models of persistent and elevated drug seeking (i.e., incubation)48, 49. After each session of 6 h/day, the catheters were flushed and the rats returned to their home cages in the colony room. Only rats that responded for an average of 50 infusions or more in the last 5 sessions were used for further experiments24, 47.
Cannulation and microinjection procedure
On abstinence day 22 (AD22), rats were implanted with bilateral guide cannulas as described previously45, 46. Briefly, animals were anesthetized with ketamine and xylazine (60 and 5 mg/kg, respectively, intraperitoneally) and positioned in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Cannulas (C235G-2.4; Plastics One, Roanoke, VA) were implanted in the NAc using the following coordinates: AP, +1.8 mm from bregma; ML, +1.2 mm; DV, −6.5 mm46, 50. Cannulas were fixed to the skull with an acrylic resin and stainless-steel screws. Animals were handled daily and sham injected during the recovery period (AD23 to AD27) to habituate them to the microinjection procedure. Microinjections were carried out on AD28, AD29, and AD30. Microinjections were infused at a rate of 0.5 μL/min using a microsyringe (Hamilton, Reno, NV) connected to an infusion pump. At the end of the procedure, the syringe remained connected to the cannula for 3 min to allow the diffusion of the drug. One hour after the final microinjection (AD30), rats were placed in the operant chambers and tested in the cue-induced cocaine-seeking test as described below. A chronic microinjection schedule for DAGL and MAGL inhibitors was performed to ensure changes in gene expression levels that typically cannot be achieved through an acute injection.
Abstinence and cue-induced seeking
After training, catheters were flushed, and rats were returned to their home cages to undergo abstinence for 1 (AD1) or 30 (AD30) days. For studies in which rats received intracranial injections, rats were returned to the operant chamber for a 60-min test to measure cue-induced seeking 1 h after the microinjection. Cue tests were performed following forced abstinence (AD1 or AD30) under extinction conditions (i.e., no drug availability when the cue was present) as previously described24. The total number of active responses was used as the operational measure of cocaine seeking. We report active responses for seeking tests in figures, and inactive responses are reported in Supplementary Table 1.
Food reinforcement
Food self-administration was conducted as previously described with minor modifications46. Briefly, rats were food restricted to 10% of their body weight. Behavioral training was conducted daily for 1 h over a 10-day period in 2-lever operant chambers placed within sound-attenuating ventilated boxes (Coulbourn Instruments, LLC, Allentown, PA) during the dark phase of the 12-h light/dark cycle. Rats were trained to obtain a reward of a maximum of 50 sucrose pellets (45-mg pellet; Bio-Serv, Flemington, NJ) following lever press responses under an FR1 schedule. The reinforcement schedule was progressively increased to FR5 over the 10-day period. Twenty-two days after training (AD22), rats underwent bilateral cannulation targeted at the NAc. After 5 days of recovery (AD23 to AD27), rats received bilateral intra-NAc injections of DO-34, URB-602, and vehicle on AD28, AD29, and AD30. One hour after microinjections on AD30, rats were tested for cue-induced food seeking under extinction conditions, where an active lever press delivered the cue light previously paired with food pellet reward without dispensing any food reward.
Locomotor activity
One hour after receiving microinjections of DO-34, URB-602, or vehicle on AD29, rats were placed inside transparent plastic cages (40 × 40 × 30 cm), and the locomotor activity was recorded by an infrared motion-sensor system (AccuScan Instruments, Inc., Columbus, OH). Versa Max animal activity software monitored the distance rats traveled during a 1-h test.
Immunoblotting
On AD1 and AD30, rats from different experimental groups were euthanized by rapid decapitation. Brains were removed and sliced into 1-mm-thick sections using a brain matrix (Braintree Scientific, Inc., Braintree, MA), and 2-mm-diameter tissue punches from the NAc shell regions were collected and rapidly frozen on dry ice. Western blotting was carried out as previously described45, 46. Briefly, punches were homogenized in 25 mmol/L Tris (pH 8.0) and 0.25 mol/L sucrose buffer. Total protein was extracted, and 30-μg samples were loaded onto 10% Tris-sodium dodecyl sulfate (SDS) polyacrylamide gels for electrophoretic separation. Bands were then transferred to nitrocellulose membranes and blocked with Rockland buffer (Rockland Immunochemicals, Inc., Limerick, PA). Membranes were incubated overnight at 4°C with primary antibodies diluted in blocking buffer (Rockland Immunochemicals, Inc.), including anti-DAGL (1:200, ab81984; Abcam, Cambridge, MA), anti-MAGL (1:200, ab24701; Abcam), anti-eIF2α (1:333, #9722; Cell Signaling Technologies, Inc., Danvers, MA), anti-phospho eIF2α (Ser51, 1:150, #9721; Cell Signaling Technologies, Inc.), anti-EGR1 (1:100, sc515830; Cell Signaling Technologies, Inc.), anti-NPAS4 (1:1,000; gifted by the Zhen Yan lab at the University at Buffalo and procured from Michael Greenberg at Harvard), anti-ATF4 (1:10, #118150; Cell Signaling Technologies Inc.), anti-calcineurin (1:2,000, ab3673; Abcam), and anti-β-actin (1:10,000, #3700; Cell Signaling Technologies, Inc.). Membranes were incubated with IRDye secondary antibodies (1:5,000; LI-COR, Inc., Lincoln, NE) for 1 h at room temperature. The blots were imaged using an Odyssey infrared imaging system (LI-COR, Inc.) and quantified by densitometry using ImageJ (National Institutes of Health, Bethesda, MD). β-Actin was used as a loading control.
RNA extraction and qPCR
For quantitative PCR (qPCR), NAc tissue punches were collected from animals at AD30 as previously described24. Total RNA was extracted with TRIzol reagent (Ambion, Austin, TX) and purified using the E.Z.N.A. MicroElute Total RNA kit (Omega Bio-Tek, Norcross, GA). RNA concentrations were determined with a NanoDrop One spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and reverse-transcribed using a SuperScript III first-strand synthesis system (Invitrogen of Thermo Fisher Scientific). mRNA expression was measured using IQ SYBR green SuperMix (Bio-Rad Laboratories, Hercules, CA), and quantification was conducted on an iQ5 system (Bio-Rad Laboratories). All reactions were run in triplicates, and quantifications were analyzed by a relative threshold cycle quantification method using Gapdh as a housekeeping gene. For EGR1 and calcineurin, the primer sequences used were 5′-TGGACAACTACCCCAAACTGG-3′ (forward) and 5′-AAAAGGACTTGGTCAGGTG-3′ (reverse) and 5′-TAACTTTCGAGCCAGCCCAG-3′ (forward) and 5′-CGGAAATGGAACGGCTTTCA-3’ respectively. For Gapdh, the primer sequences were 5′-AACGACCCCTTCATTGAC-3′ (forward) and 5′-TCCACGACATACTCAGCA-3′ (reverse).
Chromatin immunoprecipitation
Quantitative chromatin immunoprecipitation for EGR1 was performed as previously described24 by processing bilateral NAc punches obtained at AD30 following cocaine self-administration. Four NAc punches from 2 rats were pooled for each sample (n = 5–6 samples/group) and fixed with 1% formaldehyde followed by quenching with 2 M glycine. Chromatin was solubilized with cell and nuclear lysis buffers and sheared using a Bioruptor 300 (Diagenode, Liège, Belgium) at 4°C with high sonication intensity 3 times at 30s on/30s off for 10 min. Fragment sizes of 250 to 1,000 base pairs were verified on a 2% agarose gel. Magnetic sheep anti-rabbit beads (Invitrogen) were incubated with 7.5μg of EGR1 antibody (#4153; Cell Signaling Technology) at 4°C overnight on a rotator. Magnetic bead/antibody complexes were then washed, and 70 μL of the slurry was incubated with the sheared chromatin sample for 16 h at 4°C; 10% of each sample of sheared chromatin was used as an input control. Samples were washed with LiCl and Tris-EDTA buffers and reverse cross-linked at 65°C overnight, with proteins and RNA removed by proteinase K (Invitrogen) and ribonuclease (Roche, Basel, Switzerland), respectively. Finally, DNA was purified using a DNA purification kit (Qiagen, Hilden, Germany). Nonspecific binding was determined using an immunoglobulin G control.
qPCR was performed (iQ5 system; Bio-Rad Laboratories, Hercules, CA) to identify the binding of EGR1 to the proximal promoter region of the calcineurin gene using the primer sequences 5′-GCGGGACTGACTGGCACCC-3′ (forward) and 5′-GGGTGCCAGTCAGTCCCGC-3′ (reverse). All chromatin immunoprecipitation-qPCR primers were tested for efficiency before being used under experimental conditions. Amplification reactions were run in triplicates with iQ SYBR green (Bio-Rad Laboratories), and each sample was normalized to the immunoglobulin G control. Fold changes were calculated relative to the saline control.
Statistical analysis
Analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). Self-administration training was analyzed using within-subject two-way repeated measures analyses of variance (ANOVAs), followed by Bonferroni post hoc comparisons. Cue-induced relapse locomotor activity and immunoblotting were subjected to Student t tests. Significance was set at a P value of <0.05, and data are presented as the means ± SEMs.
Results
Dysregulation of DAGL and MAGL in the NAc following prolonged, but not acute, abstinence from cocaine self-administration.
After acute abstinence (AD1) (Figure 1A, B) following extended-access self-administration of cocaine or saline (two-way repeated-measures ANOVA, drug × session interaction effect F(9,144) = 2.874, P = 0.00043) protein levels of DAGL (t(15) = 0.9, P = 0.39) and MAGL (t(14) = 0.52, P = 0.60) (Figure 1C, D) were unaltered between cocaine and saline groups. On the contrary, after prolonged abstinence (AD30) from cocaine or saline self-administration (Figure 1A, B) (two-way repeated-measures ANOVA, drug × session interaction effect F(9,135) = 3.991, P = .00002), DAGL protein levels (Figure 1E) were significantly increased (t(15) = 2.56, P = 0.022) and MAGL protein levels (Figure 1F) were significantly decreased (t(15) = 2.81, P = 0.012) in the NAc of cocaine-treated rats compared to their respective saline controls.
Figure 1. Dysregulation of endocannabinoid lipases in the NAc following self-administration of cocaine.
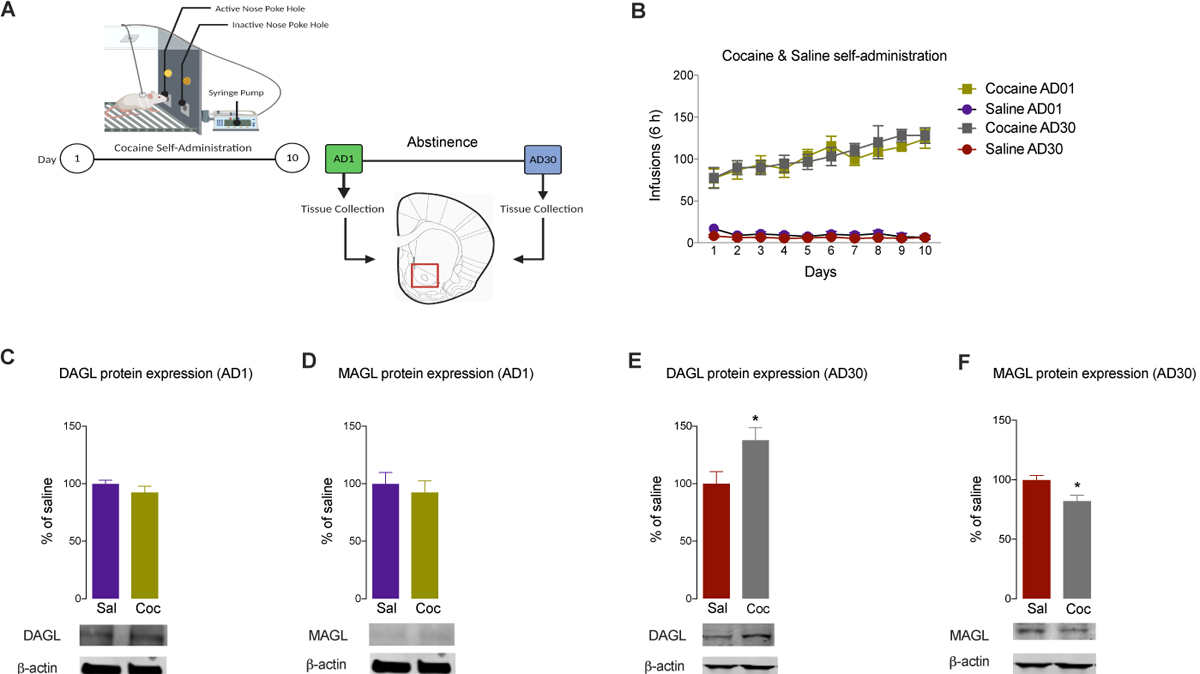
(A) Experimental timeline for 10-day self-administration training and withdrawal (AD1 and AD30). (B) Number of infusions during self-administration training for animals receiving saline (Sal) or cocaine (Coc) that underwent one of the two forced abstinence periods (AD1 or AD30; n = 8–10/group). DAGL and MAGL protein expression levels in the NAc after an acute (AD1) (C and D, respectively) or prolonged (E and F, respectively) abstinence period (AD30) (n = 8–10/group). All data are presented as means ± SEMs with statistical significance (*) at P < 0.05.
Reduced EGR1 transcriptional regulation in the NAc following prolonged abstinence.
Endocannabinoid signaling has been associated with changes in the expression of the phosphatase calcineurin and the transcription factor EGR125. Therefore, we analyzed EGR1 and calcineurin levels in the NAc of rats at AD30 following extended-access cocaine self-administration. We found that EGR1 mRNA (t(7) = 2.88, P = 0.02) (Figure 2A) and protein (t(9) = 2.28, P = 0.04) (Figure 2B) levels were attenuated at AD30 in cocaine-treated rats compared to levels in their respective saline controls. Concomitant to the reduced expression of EGR1, we found a decrease in the binding of EGR1 to the gene encoding calcineurin (t(8) = 1.99, P = 0.04) (Figure 2C), demonstrating that the transcriptional regulation of calcineurin by EGR1 may be altered. Based on the decreased binding of EGR1 to the calcineurin promoter, we probed the NAc expression of calcineurin at AD30. We found that mRNA (t(10) = 3.092, P = 0.005) (Figure 2D) and protein (t(12) = 2.73, P = 0.01) (Figure 2E) expression levels of calcineurin were also attenuated in the NAc at AD30 in cocaine-treated rats compared to that in saline controls. In contrast, mRNA levels of ERG1 (t(10) = 0.6938, P = 0.50) (Supplementary Figure 1A) and calcineurin (t(11) = 0.09, P = 0.46) (Supplementary Figure 1B) in the NAc were unaltered at AD1.
Figure 2. Reduced EGR1 transcriptional regulation in the NAc following prolonged abstinence.
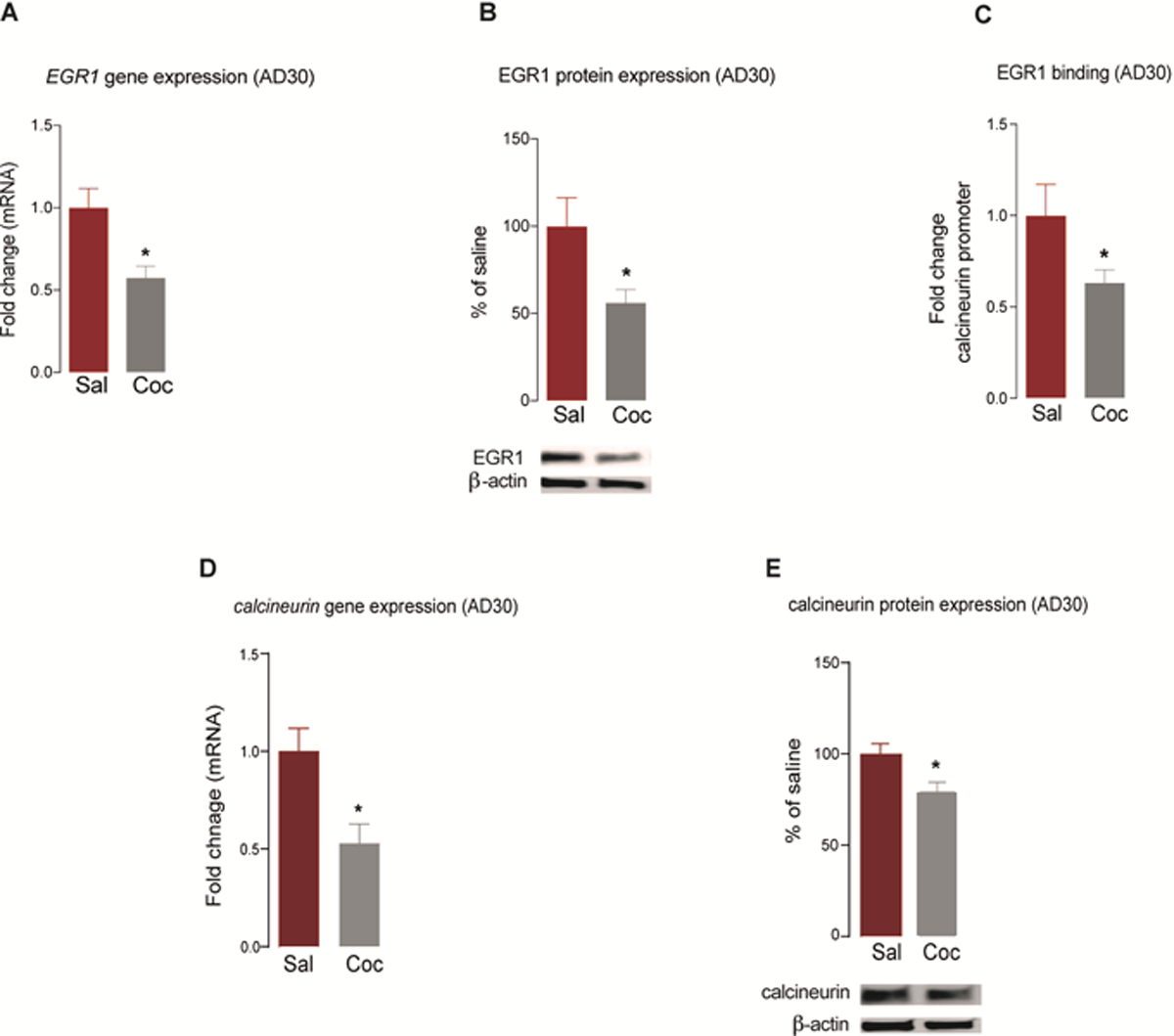
Gene (A) and protein (B) expression of EGR1 at AD30 (n = 5–7/group). (C) Fold change in EGR1 binding to calcineurin gene promoter (n = 5/group). Gene (D) and protein (E) expression of calcineurin at AD30 (n = 5–7/group). All data are presented as means ± SEMs with statistical significance (*) at P < 0.05.
Decreased p-eIF2α and downstream target NPAS4 following prolonged abstinence.
To determine the potential role of reduced EGR1 and calcineurin expression, we measured phosphorylation levels of a known downstream target, eIF2α, in a new group of animals that underwent a cue-induced cocaine-seeking test on AD30 after they had learned to self-administer the drug (Figure 3A) (two-way repeated-measures ANOVA, drug × session interaction effect F(9,110) = 3.428, P = .0009). Following the cue-induced cocaine-seeking test, AD30 cocaine rats showed significantly more total active responses than saline controls (t(11) = 4.94, p = 0.002) (Figure 3B), and NAc tissues collected from these animals 1 h later had reduced phosphorylated p-eIF2α (t(11) = 1.938, P = 0.0393) (Figure 3C) but no changes in total eIF2α protein expression levels (t(11) = 0.9362, P = 0.3693) (Figure 3D) compared to that in saline controls. We then investigated NPAS4 and ATF4 protein levels, which are targets modulated by eIF2α53, 41 and mediate activity-dependent translational changes37, 51. We found reduced NPAS4 protein expression (t(10) = 2.23, P = 0.04) in cocaine-treated rats compared to that in saline controls (Figure 3E). However, no changes in ATF4 levels were observed following cocaine seeking (t(11) = 1.19, P = 0.12) (Figure 3F), which aligns with previous findings41.
Figure 3. eIF2α phosphorylation is reduced after cue-induced cocaine seeking on AD30.
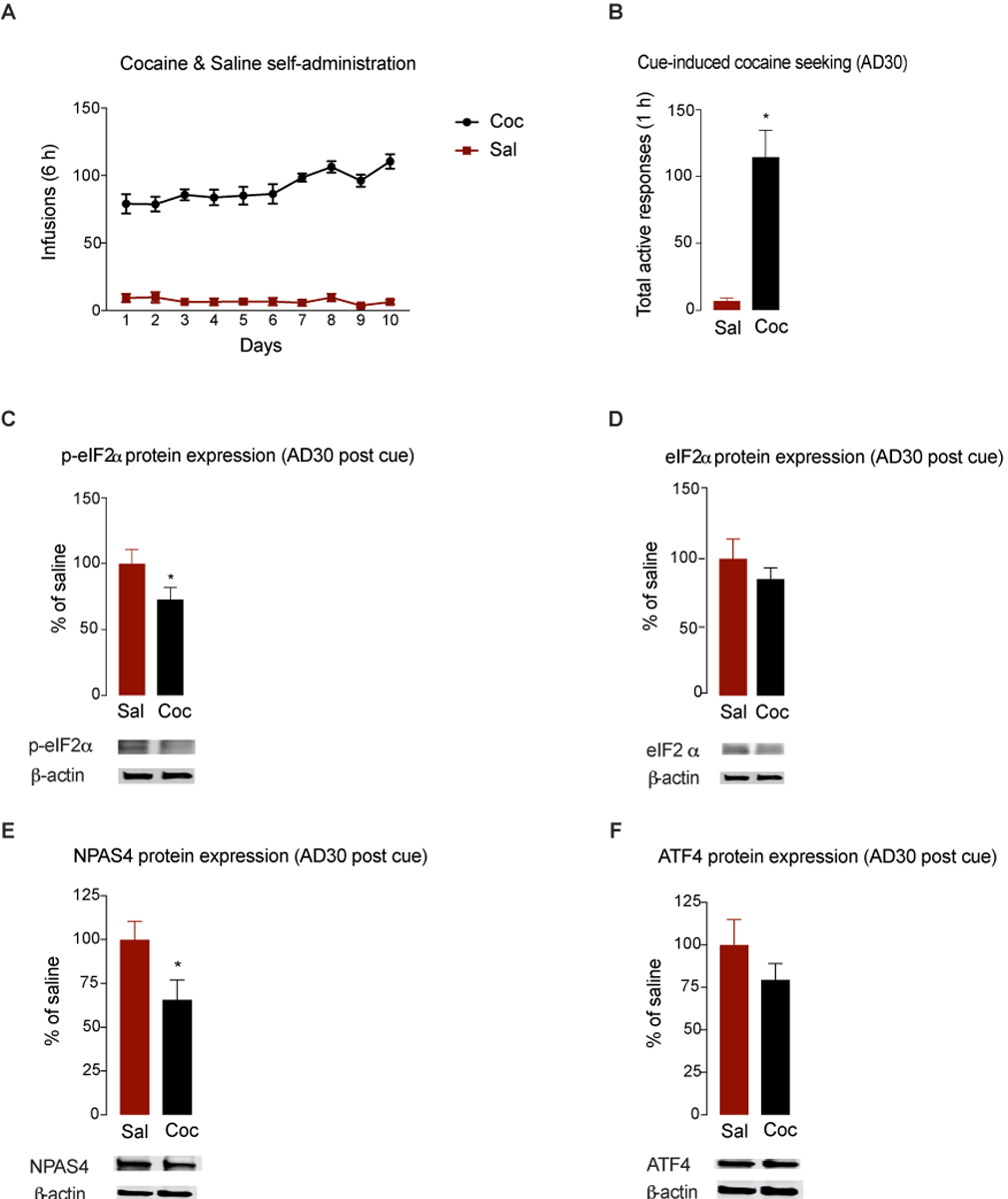
(A) Number of infusions during self-administration training for animals receiving saline (Sal) or cocaine (Coc). (B) Total active responses to a previously drug-paired cue on AD30 by animals trained to administer saline or cocaine. Phosphorylation (C) and total protein (D) levels of eIF2α in the NAc on AD30 following cue exposure. Protein levels of NPAS4 (E) and ATF4 (F) in the NAc on AD30 following cue exposure (n = 6–7/group). All data are presented as means ± SEMs with statistical significance (*) at P < 0.05.
Pharmacological manipulation of DAGL and MAGL bidirectionally regulate cue-induced cocaine seeking and eIF2α phosphorylation following prolonged abstinence.
2-AG influences reward-related behaviors7, 40, and we found that the enzymes that regulate 2-AG, MAGL, and DAGL are altered during prolonged abstinence from extended-access cocaine self-administration. Therefore, we used pharmacological tools to examine the role of these enzymes in cocaine craving following prolonged abstinence (Figure 4A). In one experiment, a group of rats was pseudorandomly divided into vehicle and DAGL groups following extended-access cocaine self-administration based on drug infusions (two-way repeated-measures ANOVA, treatment effect, F(1,19) = 0.02, P = 0.9646 [Figure 4B]). Rats then received intra-NAc microinjections of the DAGL inhibitor DO-34 or vehicle on AD28, AD29, and AD30. One hour after the microinjections on AD30, rats were returned to operant chambers for a cue-induced cocaine-seeking test. Inhibition of DAGL reduced total active responses compared to the vehicle group (t(16) = 2.22, P = 0.0411) (Figure 4C). Interestingly, DAGL inhibition also increased ERG1 mRNA expression (t(9) = 1.86, P = 0.04) (Figure 4D) and p-eIF2α protein levels (t(9) = 2.71, P = 0.02) (Figure 4E) compared to that in vehicle controls.
Figure 4. Pharmacological inhibition of DAGL and MAGL in the NAc bidirectionally alters cue-induced cocaine seeking and translational regulation.
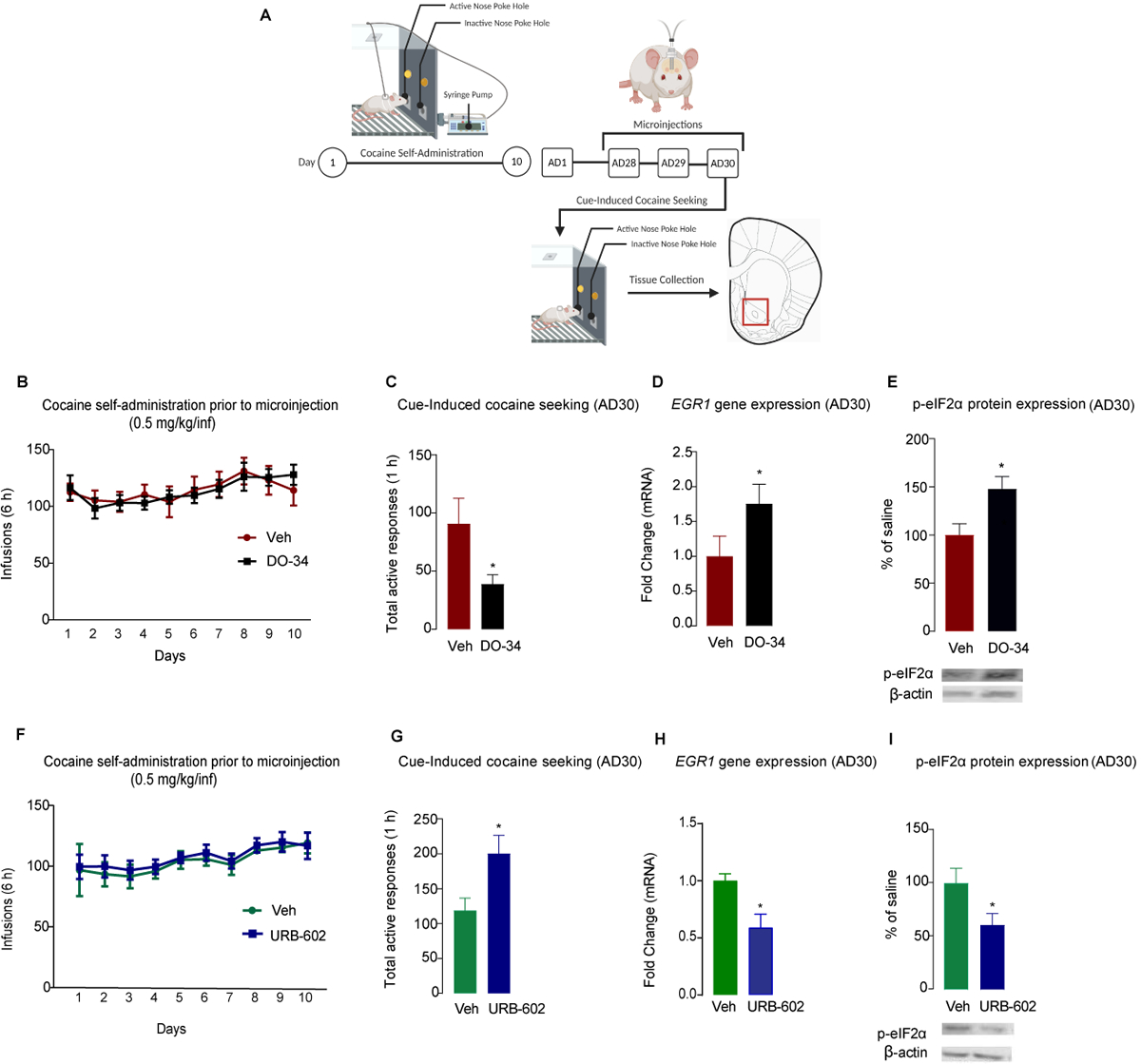
(A) Experimental timeline for 10-day self-administration training, abstinence periods, intra-NAc microinjections, cue-induced seeking test, and tissue collection. (B) Number of infusions during self-administration training for animals that later received DO-34 or vehicle (Veh). (C) Total active responses to a previously drug-paired cue on AD30 by animals receiving DO-34 or vehicle. (D) EGR1 gene expression in the NAc at AD30 following cue exposure in animals receiving DO-34 or vehicle microinjections. (E) eIF2α phosphorylation levels in the NAc at AD30 following cue exposure in animals receiving DO-34 or vehicle. (F) Numbers of infusions during self-administration training for animals that later received URB-602 or vehicle (Veh). (G) Total active responses to a previously drug-paired cue on AD30 by animals receiving URB-602 or vehicle. (H) EGR1 gene expression in the NAc at AD30 following cue exposure in animals receiving URB-602 or vehicle microinjections. (I) eIF2α phosphorylation levels in the NAc at AD30 following cue exposure in animals receiving URB-602 or vehicle. All data are presented as means ± SEMs (n = 10–11/group) with statistical significance (*) at P < 0.05.
In another experiment, a separate group of animals was pseudorandomly divided following cocaine self-administration (two-way repeated-measures ANOVA, treatment effect, F(1,16) = 0.1624, P = 0.65 [Figure 4F]). In contrast to that with DAGL, inhibition of MAGL via URB-602 microinjections on AD28, AD29, and AD30 significantly increased responding in a cue-induced cocaine-seeking test at AD30 compared to the vehicle-treated rats (t(16) = 2.57, P = 0.0204) (Figure 4G). MAGL inhibition also downregulated EGR1 mRNA (t8 = 3.082, P = 0.01) (Figure 4H) and p-eIF2α protein levels (t14 = 2.19, P = 0.040) (Figure 4I). Overall, locomotor activity was not affected by intra-NAc microinjections of DO-34 (t(16) = 0.37, P = 0.71) (Supplementary Figure 2B) or URB-602 (t(16) = 0.389, P = 0.70) (Supplementary Figure 2D).
To investigate whether NAc endocannabinoid signaling contributes to non-drug rewards, we trained a separate group of rats to self-administer food and then pseudorandomly divided the rats into vehicle, DO-34, and URB-602 groups (Supplementary Figure 3A) based on their lever press responses (F2,18 = 0.88, P = 0.43) (Supplementary Figure 3B). As in previous experiments, we administered microinjections on AD28, AD29, and AD30. One hour after the microinjection on AD30, rats were returned to operant chambers for a seeking test under forced abstinence conditions. Total active lever presses did not differ between vehicle, DO-34, and URB-602 (F2,16= 0.03, P = 0.96) groups (Supplementary Figure 3C). Additionally, overall locomotion did not differ between the treatment groups (F2,16 = 0.57, P = 0.57) (Supplementary Figure 3D). These data suggest that the effect of endocannabinoid signaling in the NAc after prolonged abstinence is specific to drug-seeking behaviors.
Discussion
Here, we present evidence that pharmacological manipulation of DAGL and MAGL in the NAc regulates cue-induced cocaine seeking during prolonged abstinence, which may occur via transcriptional and translational changes. Our data demonstrate a temporally dependent change in DAGL and MAGL protein expression after 30 days, but not 1 day, of forced abstinence from extended-access cocaine self-administration. Furthermore, levels of p-eIF2α and its translational target NPAS4 were attenuated following cue-induced cocaine seeking on AD30, suggesting p-eIF2α-mediated dysregulated protein translation may underlie relapse vulnerability. At AD30, cocaine-treated rats also had lower calcineurin and EGR1 expression and EGR1-mediated transcriptional control of calcineurin, a modulator of the phosphostatus of eIF2α, suggesting altered transcriptional programming of EGR1. Finally, we demonstrate that regulating 2-AG synthesis and degradation through pharmacological manipulation of DAGL and MAGL, respectively, governs expression of cue-induced cocaine seeking bidirectionally. Overall, our findings demonstrate a pivotal role of the 2-AG lipases in influencing dysregulated transcriptional and translational mechanisms governing neuroadaptations underlying relapse vulnerability following exposure to and long-term abstinence from cocaine (Figure 5).
Figure 5. Proposed working model of endocannabinoid lipase-mediated neuroadaptations following prolonged abstinence from cocaine self-administration.
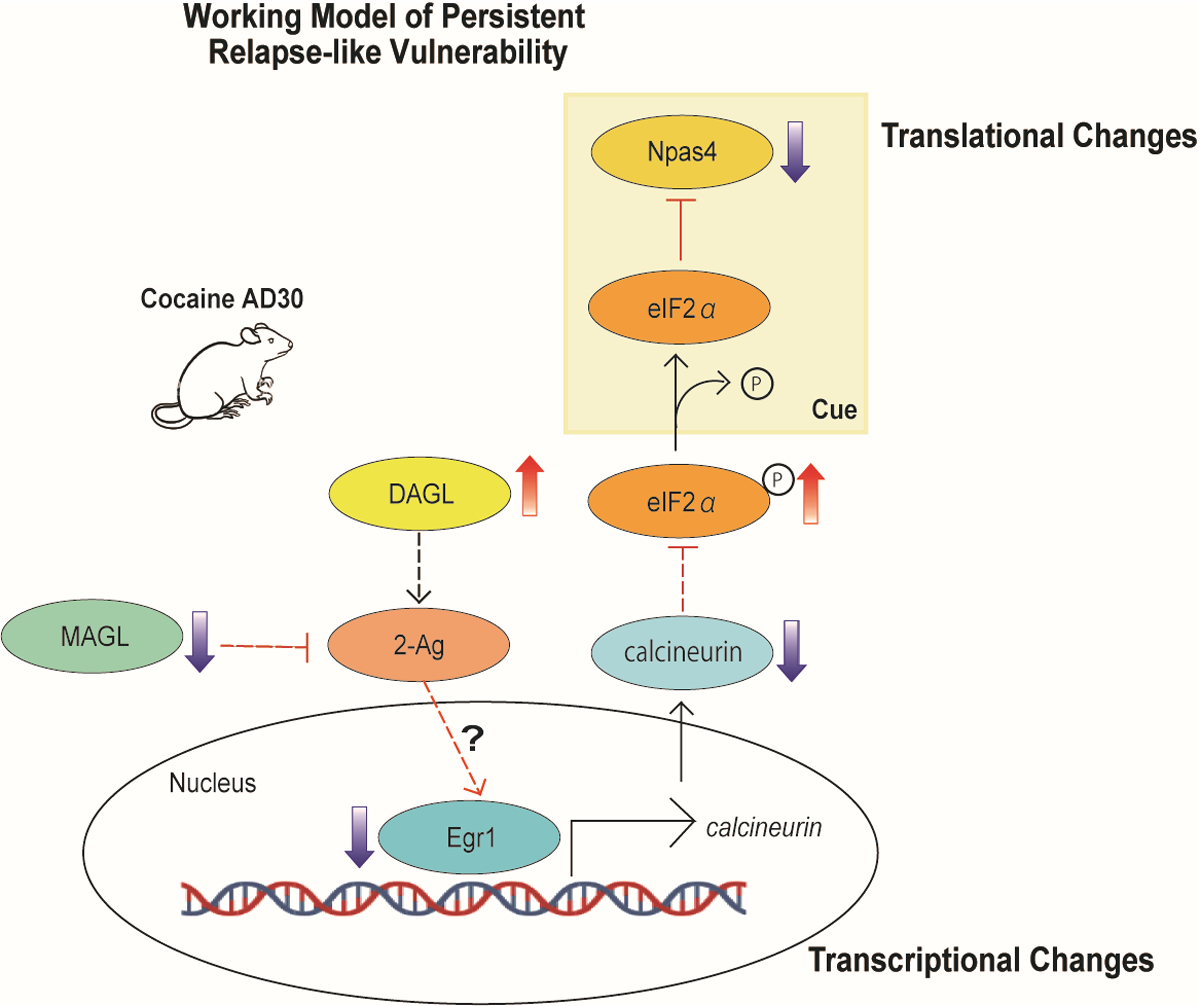
Prolonged abstinence from cocaine self-administration results in an imbalance in the endocannabinoid lipases characterized by an upregulation of DAGL and downregulation of MAGL in the NAc. Furthermore, EGR1 expression, EGR1 binding to the promoter of calcineurin, and calcineurin expression are reduced at AD30. This dysregulation of NAc endocannabinoid lipases coupled with dampened EGR1 transcriptional regulation on calcineurin facilitates reduced expression of translational initiator p-eIF2α and its downstream target NPAS4 upon exposure to drug-associated cues. eIF2α phosphorylation regulates activity-dependent protein synthesis of select targets underlying behavioral adaptations, and our findings elucidate a mechanism of endocannabinoid-mediated translational changes underlying cue-induced escalated cocaine craving. Red dashed lines indicate probable, but yet untested, cellular mechanisms.
2-AG-mediated endocannabinoid signaling in the NAc is known to influence behavior related to drugs of abuse and natural rewards7. As the direct measurement and manipulation of 2-AG in vivo can be challenging and often not feasible52, targeting the enzymes that regulate 2-AG levels is a suitable alternative. Our findings are consistent with previous studies showing dysregulation of MAGL and DAGL levels in multiple brain regions after cocaine exposure53–55. However, we found that the dysregulation of DAGL and MAGL in the NAc is specific to prolonged and not acute abstinence from cocaine self-administration and was associated with altered transcriptional and translational processes, which are known to be key mediators of the neurobiology of addiction-like behaviors. Intra-NAc inhibition of DAGL at AD30 attenuated cue-induced cocaine seeking. Conversely, intra-NAc microinjections of URB-602 potentiated cue-induced cocaine seeking at AD30 and may have even exacerbated abstinence-dependent cocaine craving. Pharmacological inhibition of DAGL reversed (increased) the reduced levels of EGR1 and p-eIF2α observed in the NAc of cocaine-treated rats at AD30 (Figure 1). This relative directional change needs to be viewed in the context of both global neuroadaptations and abstinence in the rats that administered cocaine in comparison to their saline controls (Figure 1), whereas the effects of DAGL were observed by comparing rats that self-administered cocaine and were treated with or without the inhibitor at AD30 (Figure 4).
Numerous studies have established roles of transcriptional and epigenetic mechanisms in neuroadaptations associated with addiction phenotypes56. We validated findings from our previous study24 showing that EGR1 expression is attenuated in the NAc in cocaine-treated rats on AD30, which may regulate cocaine seeking through a ubiquitin mechanism involving chromatin remodeler INO8024. Calcineurin is known to mediate endocannabinoid actions, and EGR1 is modulated by the calcineurin pathway. However, it is unknown if EGR1 could act in a negative feedback loop that regulates calcineurin activity. Here, we found decreased expression of calcineurin and EGR1 in the NAc and reduced binding of EGR1 to the calcineurin gene promoter in cocaine-treated rats on AD30. Interestingly, calcineurin expression decreases with repetitive noncontingent cocaine administration57, and activation of calcineurin reduces cue-induced reinstatement in the amygdala58, thereby implicating calcineurin as a possible target of chronic cocaine-elicited neuroadaptations. Our data show that following prolonged abstinence, there is reduced transcriptional control of calcineurin expression, which was likely responsible for reduced eIF2α regulation, perhaps through a PERK mechanism, as described previously59.
eIF2α is involved in the regulation and expression of cocaine-seeking behaviors41. Accordingly, we observed a reduction of eIF2α phosphorylation in the NAc following re-exposure to a drug-paired cue in rats that had undergone prolonged abstinence. This reduction was accompanied by diminished levels of NPAS4, a protein regulated by eIF2α that undergoes rapid experience-dependent translation and affects synaptic mechanisms underlying memory formation42, 60, 61. However, the expression levels of another target of eIF2α, ATF4, which has been shown to mediate the reconsolidation of drug-induced memory in the amygdala62, was not altered, suggesting that there are cell- and region-specific eIF2α targets mediating behavioral adaptations.
Previous studies have implicated both CB1 and CB2 (endocannabinoid type 2) receptors in altering behavioral and neurobiological mechanisms associated with psychostimulant exposure63–64. For example, studies have reported loss of endocannabinoid-mediated LTD following cocaine exposure, which may also regulate the seeking of drugs of abuse65–70. Interestingly, altered endocannabinoid-mediated LTD is thought to occur due to a loss of MAGL activity, 2-AG elevations, and partial CB1 receptor desensitization/downregulation in MAGL knockout mice71. It is, therefore, possible that the increase in cocaine-seeking behavior at AD30 induced by inhibition of 2-AG degradation observed here is occurring through the CB1 receptors; however, further studies are required to define the precise cellular mechanisms.
Desensitization and downregulation of CB1 receptor levels have been shown to alter immediate early genes, such as those encoding EGR1 and c-Fos, which vary across brain regions and exposures to diverse environmental stimuli72, 73. EGR1 is sensitive to such activity-dependent synaptic remodeling74–79 and could explain, at least in part, the cocaine-induced temporal changes in the NAc due to DAGL and MAGL manipulations. The endocannabinoid pathway has, moreover, been linked to protein translation and memory formation40, 80, 81 that relies on alterations in both pre-81 and post-synaptic protein translational machinery. Because endocannabinoid mechanisms also play a critical role in non-neuronal cell types and interneurons in the NAc82, 83, future causal studies that examine cell-type and compartmentalization specificity are needed to parse out the intricate role of endocannabinoid signaling following exposure to drugs of abuse. Furthermore, it is important to note that our findings are based on the use of male subjects. This is particularly germane, as there are reported sex differences in endocannabinoid signaling that are thought to play a vital role in behavioral responding84, 85.
In summary, this study reveals that DAGL and MAGL act as modulators of cue-induced cocaine seeking during prolonged abstinence, which may involve EGR1 and p-eIF2α. This proposed mechanism incorporates DAGL and MAGL regulation of EGR1 transcriptional control of calcineurin activity, which presumably alters eIF2α-mediated translational control of NPAS4. This study is the first to report evidence of the role of manipulating endocannabinoid lipases to influence cocaine-induced behavioral plasticity following prolonged abstinence from extended-access cocaine self-administration. In the current context of the investigation, it cannot be claimed that the dysregulated endocannabinoid lipases and the resulting molecular changes are specific to cocaine incubation, per se. However, the molecular alterations we observed are more specific to prolonged abstinence from cocaine self-administration, which appears to foster maladaptive plasticity that regulates relapse vulnerability. Due to the role of DAGL and MAGL in mediating the biosynthesis of 2-AG, these findings provide evidence of the importance of 2-AG turnover by drugs of abuse as one of the many regulating factors modulating relapse vulnerability, which has implications for the development of new pharmacotherapies for treating drug addiction.
Supplementary Material
Supplementary Figure 1. EGR1 and calcineurin gene expression is unaltered at early abstinence timepoints. (A) EGR1 mRNA levels between saline and cocaine animals at AD1 following extended access cocaine self-administration. (B) calcineurin mRNA levels between saline and cocaine animals at AD1 following extended access cocaine self-administration. All data are presented as means ± SEMs (n = 5–7/group).
Supplementary Figure 2. Inhibition of DAGL and MAGL does not alter total eIF2α or general locomotor activity. Repeated intra-NAc microinjections of DO-34 did not change eIF2α protein expression (A) or locomotor activity (B) at AD30. Similarly, repeated intra-NAc injections of URB-602 did not result in changes to eIF2α protein expression (C) and locomotor activity (D) at AD30. All data are presented as means ± SEMs.
Supplementary Figure 3. Cue-induced food seeking following food self-administration is not regulated by accumbal DAGL and MAGL inhibition. (A) Experimental timeline for 10-day food self-administration training, abstinence periods, intra-NAc microinjections, and cue-induced seeking test. (B) Total active responses during self-administration training by animals that later received microinjections of DO-34, URB-602, or vehicle (Veh). (C) Total active responses during a 1-h cue-induced food-seeking test by animals receiving microinjections of DO-34, URB-602, or vehicle (Veh). (D) Total locomotor activity following microinjections of DO-34, URB-602, or vehicle (Veh). All data are presented as means ± SEMs (n = 6–8/group).
Acknowledgments
This work was supported by the National Institutes of Health (National Institute on Drug Abuse [NIDA] grant no. R01DA037257 and R21DA044486 [to DMD]; National Institute of Neurological Disorders and Stroke [NINDS] grant no. F99NS108543 [to JAM]; and National Institute of General Medical Sciences [NIGMS] grant no. R25GM09545902 [to University at Buffalo]). Pedro Henrique Gobira was funded by a FAPESP grant (process no. 2017/19284-0). The NIDA Drug Supply Program generously gifted the cocaine used in these studies. The authors declare no biomedical financial interests or potential conflicts of interest.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005/November/01 2005;8(11):1442–1444. doi: 10.1038/nn1105-1442 [DOI] [PubMed] [Google Scholar]
- 2.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. Jun 2016;17(6):351–65. doi: 10.1038/nrn.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47 Suppl 1:214–226. doi: 10.1016/j.neuropharm.2004.06.027 [DOI] [PubMed] [Google Scholar]
- 4.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. Oct 12 2011;12(11):623–37. doi: 10.1038/nrn3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nestler EJ. Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci. Dec 2012;10(3):136–43. doi: 10.9758/cpn.2012.10.3.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattore L, Spano MS, Deiana S, et al. An endocannabinoid mechanism in relapse to drug seeking: A review of animal studies and clinical perspectives. Brain Research Reviews. 2007/January/01/ 2007;53(1):1–16. doi: 10.1016/j.brainresrev.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 7.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. Oct 2015;16(10):579–94. doi: 10.1038/nrn4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidhpura N, Parsons LH. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology. Dec 2011;61(7):1070–87. doi: 10.1016/j.neuropharm.2011.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sagheddu C, Muntoni AL, Pistis M, Melis M. Endocannabinoid Signaling in Motivation, Reward, and Addiction: Influences on Mesocorticolimbic Dopamine Function. Int Rev Neurobiol. 2015;125:257–302. doi: 10.1016/bs.irn.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 10.Hillard CJ. The Endocannabinoid Signaling System in the CNS: A Primer. Int Rev Neurobiol. 2015;125:1–47. doi: 10.1016/bs.irn.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu HC, Mackie K. An Introduction to the Endogenous Cannabinoid System. Biol Psychiatry. Apr 1 2016;79(7):516–25. doi: 10.1016/j.biopsych.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. Jun 2008;13(2):147–59. doi: 10.1111/j.1369-1600.2008.00108.x [DOI] [PubMed] [Google Scholar]
- 13.Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. Oct 4 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437 [DOI] [PubMed] [Google Scholar]
- 14.Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem. 2010;17(14):1360–81. doi: 10.2174/092986710790980050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reisenberg M, Singh PK, Williams G, Doherty P. The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling. Philos Trans R Soc. Dec 5 2012;367(1607):3264–75. doi: 10.1098/rstb.2011.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murataeva N, Straiker A, Mackie K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol. Mar 2014;171(6):1379–91. doi: 10.1111/bph.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida T, Fukaya M, Uchigashima M, et al. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. May 3 2006;26(18):4740–51. doi: 10.1523/JNEUROSCI.0054-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinh TP, Carpenter D, Leslie FM, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. Aug 6 2002;99(16):10819–24. doi: 10.1073/pnas.152334899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99(12):8384. doi: 10.1073/pnas.122149199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. Oct 12 2011;31(41):14536–41. doi: 10.1523/jneurosci.3625-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan B, Hillard CJ, Liu Q-s. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008;28(6):1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. Aug 2011;34(8):411–20. doi: 10.1016/j.tins.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman WM, Patel KM, Brucklacher RM, et al. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33(8):1807–1817. doi: 10.1038/sj.npp.1301577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner CT, Mitra S, Martin JA, et al. Ubiquitin-proteasomal regulation of chromatin remodeler INO80 in the nucleus accumbens mediates persistent cocaine craving. Sci Adv. 2019;5(10):eaay0351. doi: 10.1126/sciadv.aay0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olango WM, Geranton SM, Roche M, Hunt SP, Finn DP. Novel molecular correlates of endocannabinoid-mediated fear-conditioned analgesia in rats. Eur J Pain. Feb 2014;18(2):182–91. doi: 10.1002/j.1532-2149.2013.00369.x [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Li G, Xue Q, et al. Calcineurin/P-ERK/Egr-1 Pathway is Involved in Fear Memory Impairment after Isoflurane Exposure in Mice. Sci Rep. 2017/October/24 2017;7(1):13947. doi: 10.1038/s41598-017-13975-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, Li Y, MacNeil AJ, Junkins RD, Berman JN, Lin T-J. Calcineurin–Rcan1 Interaction Contributes to Stem Cell Factor–Mediated Mast Cell Activation. J Immunol. 2013:1301271. doi: 10.4049/jimmunol.1301271 [DOI] [PubMed] [Google Scholar]
- 28.Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem. Sep-Oct 2004;11(5):625–32. doi: 10.1101/lm.77904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34: 7–11. doi: 10.1042/BST20060007 [DOI] [PubMed] [Google Scholar]
- 30.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. Apr 2005;6(4):318–27. doi: 10.1038/nrm1618 [DOI] [PubMed] [Google Scholar]
- 31.Yuan S, Burrell BD. Endocannabinoid-dependent long-term depression in a nociceptive synapse requires coordinated presynaptic and postsynaptic transcription and translation. J Neurosci. 2013;33(10):4349–4358. doi: 10.1523/JNEUROSCI.3922-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci U S A. 2008;105(29):10250–10255. doi: 10.1073/pnas.0711880105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bollo M, Paredes RM, Holstein D, Zheleznova N, Camacho P, Lechleiter JD. Calcineurin Interacts with PERK and Dephosphorylates Calnexin to Relieve ER Stress in Mammals and Frogs. PLOS ONE. 2010;5(8):e11925. doi: 10.1371/journal.pone.0011925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol. 2007/July/01 2007;8(7):519–529. doi: 10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- 35.Costa-Mattioli M, Gobert D, Harding H, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2 alpha kinase GCN2. Nature. 2005/August/01 2005;436(7054):1166–1170. doi: 10.1038/nature03897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang W, Placzek AN, Viana Di Prisco G, et al. Translational control by eIF2α phosphorylation regulates vulnerability to the synaptic and behavioral effects of cocaine. Elife. 2016;5:e12052. doi: 10.7554/eLife.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Z, Belforte JE, Lu Y, et al. eIF2α Phosphorylation-Dependent Translation in CA1 Pyramidal Cells Impairs Hippocampal Memory Consolidation without Affecting General Translation. J Neurosci. 2010;30(7):2582–2594. doi: 10.1523/jneurosci.3971-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Prisco GV, Huang W, Buffington SA, et al. Translational control of mGluR-dependent long-term depression and object-place learning by eIF2alpha. Nat Neurosci. Aug 2014;17(8):1073–82. doi: 10.1038/nn.3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa-Mattioli M, Gobert D, Stern E, et al. eIF2 alpha Phosphorylation Bidirectionally Regulates the Switch from Short- to Long-Term Synaptic Plasticity and Memory. Cell. 2007;129(1):195–206. doi: 10.1016/j.cell.2007.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melas PA, Qvist JS, Deidda M, et al. Cannabinoid Modulation of Eukaryotic Initiation Factors (eIF2alpha and eIF2B1) and Behavioral Cross-Sensitization to Cocaine in Adolescent Rats. Cell Rep. Mar 13 2018;22(11):2909–2923. doi: 10.1016/j.celrep.2018.02.065 [DOI] [PubMed] [Google Scholar]
- 41.Werner CT, Stefanik MT, Milovanovic M, Caccamise A, Wolf ME. Protein Translation in the Nucleus Accumbens Is Dysregulated during Cocaine Withdrawal and Required for Expression of Incubation of Cocaine Craving. J Neurosci. Mar 14 2018;38(11):2683–2697. doi: 10.1523/JNEUROSCI.2412-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eacker SM, Crawford K, Brichta L, et al. Experience-dependent translational state defined by cell type-specific ribosome profiling. bioRxiv. 2017:169425. doi: 10.1101/169425 [DOI] [Google Scholar]
- 43.King AR, Duranti A, Tontini A, et al. URB602 Inhibits Monoacylglycerol Lipase and Selectively Blocks 2-Arachidonoylglycerol Degradation in Intact Brain Slices. Chemistry & Biology. 2007/December/26/ 2007;14(12):1357–1365. doi: 10.1016/j.chembiol.2007.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McReynolds JR, Doncheck EM, Li Y, et al. Stress Promotes Drug Seeking Through Glucocorticoid-Dependent Endocannabinoid Mobilization in the Prelimbic Cortex. Biol Psychiatry. Jul 15 2018;84(2):85–94. doi: 10.1016/j.biopsych.2017.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ZJ, Martin JA, Mueller LE, et al. BRG1 in the Nucleus Accumbens Regulates Cocaine-Seeking Behavior. Biol Psychiatry. Nov 1 2016;80(9):652–660. doi: 10.1016/j.biopsych.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner CT, Viswanathan R, Martin JA, et al. E3 Ubiquitin-Protein Ligase SMURF1 in the Nucleus Accumbens Mediates Cocaine Seeking. Biol Psychiatry. Dec 15 2018;84(12):881–892. doi: 10.1016/j.biopsych.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werner CT, Mitra S, Auerbach BD, et al. Neuroadaptations in the dorsal hippocampus underlie cocaine seeking during prolonged abstinence. Proc Natl Acad Sci U S A. 2020;117(42):26460–26469. doi: 10.1073/pnas.2006133117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed SH, Koob GF. Transition from Moderate to Excessive Drug Intake: Change in Hedonic Set Point. Science. 1998;282(5387):298. doi: 10.1126/science.282.5387.298 [DOI] [PubMed] [Google Scholar]
- 49.Gancarz-Kausch AM, Adank DN, Dietz DM. Prolonged withdrawal following cocaine self-administration increases resistance to punishment in a cocaine binge. Scientific Reports. 2014/November/03 2014;4(1):6876. doi: 10.1038/srep06876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gancarz AM, Wang Z-J, Schroeder GL, et al. Activin receptor signaling regulates cocaine-primed behavioral and morphological plasticity. Nat Neurosci. 2015;18(7):959–961. doi: 10.1038/nn.4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Y, Bloodgood BL, Hauser JL, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008/October/01 2008;455(7217):1198–1204. doi: 10.1038/nature07319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanz C, Mattsson J, Stickel F, Dufour JF, Brenneisen R. Determination of the Endocannabinoids Anandamide and 2-Arachidonoyl Glycerol with Gas Chromatography-Mass Spectrometry: Analytical and Preanalytical Challenges and Pitfalls. Medical Cannabis and Cannabinoids. 2018;1(1):9–18. doi: 10.1159/000489032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palomino A, Pavon FJ, Blanco-Calvo E, et al. Effects of acute versus repeated cocaine exposure on the expression of endocannabinoid signaling-related proteins in the mouse cerebellum. Frontiers in integrative neuroscience. 2014;8:22. doi: 10.3389/fnint.2014.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanco E, Pavon FJ, Palomino A, et al. Cocaine-induced behavioral sensitization is associated with changes in the expression of endocannabinoid and glutamatergic signaling systems in the mouse prefrontal cortex. The international journal of neuropsychopharmacology. Oct 31 2014;18(1)doi: 10.1093/ijnp/pyu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eipper-Mains JE, Kiraly DD, Duff MO, et al. Effects of cocaine and withdrawal on the mouse nucleus accumbens transcriptome. Genes, brain, and behavior. Feb 2013;12(1):21–33. doi: 10.1111/j.1601-183X.2012.00873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker DM, Nestler EJ. Chapter 48 - Neuroepigenetics and addiction. In: Geschwind DH, Paulson HL, Klein C, eds. Handbook of Clinical Neurology. Elsevier; 2018:747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu X-T, Ford K, White FJ. Repeated Cocaine Administration Decreases Calcineurin (PP2B) but Enhances DARPP-32 Modulation of Sodium Currents in Rat Nucleus Accumbens Neurons. Neuropsychopharmacology. 2005/May/01 2005;30(5):916–926. doi: 10.1038/sj.npp.1300654 [DOI] [PubMed] [Google Scholar]
- 58.Rich MT, Huang YH, Torregrossa MM. Calcineurin Promotes Neuroplastic Changes in the Amygdala Associated with Weakened Cocaine-Cue Memories. J Neurosci. Dec 18 2019;doi: 10.1523/JNEUROSCI.0453-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma V, Ounallah-Saad H, Chakraborty D, et al. Local Inhibition of PERK Enhances Memory and Reverses Age-Related Deterioration of Cognitive and Neuronal Properties. J Neurosci. 2018;38(3):648. doi: 10.1523/JNEUROSCI.0628-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poo M-m, Pignatelli M, Ryan TJ, et al. What is memory? The present state of the engram. BMC Biology. 2016/May/19 2016;14(1):40. doi: 10.1186/s12915-016-0261-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun X, Lin Y. Npas4: Linking Neuronal Activity to Memory. Trends in neurosciences. 2016;39(4):264–275. doi: 10.1016/j.tins.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jian M, Luo Y-X, Xue Y-X, et al. eIF2α Dephosphorylation in Basolateral Amygdala Mediates Reconsolidation of Drug Memory. J Neurosci. 2014;34(30):10010–10021. doi: 10.1523/jneurosci.0934-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xi ZX, Peng XQ, Li X, et al. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci. Jul 24 2011;14(9):1160–6. doi: 10.1038/nn.2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. Aug 16 2006;26(33):8531–6. doi: 10.1523/jneurosci.0726-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. Jul 2006;9(7):868–9. doi: 10.1038/nn1713 [DOI] [PubMed] [Google Scholar]
- 66.Bilbao A, Neuhofer D, Sepers M, et al. Endocannabinoid LTD in Accumbal D1 Neurons Mediates Reward-Seeking Behavior. iScience. 2020;23(3):100951–100951. doi: 10.1016/j.isci.2020.100951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zlebnik NE, Cheer JF. Drug-Induced Alterations of Endocannabinoid-Mediated Plasticity in Brain Reward Regions. J Neurosci. 2016;36(40):10230. doi: 10.1523/JNEUROSCI.1712-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci. Jun 2004;7(6):585–6. doi: 10.1038/nn1251 [DOI] [PubMed] [Google Scholar]
- 69.Soares-Cunha C, Coimbra B, Sousa N, Rodrigues AJ. Reappraising striatal D1- and D2-neurons in reward and aversion. Neuroscience & Biobehavioral Reviews. 2016/September/01/ 2016;68:370–386. doi: 10.1016/j.neubiorev.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 70.Fourgeaud L, Mato S, Bouchet D, Hémar A, Worley PF, Manzoni OJ. A Single In Vivo Exposure to Cocaine Abolishes Endocannabinoid-Mediated Long-Term Depression in the Nucleus Accumbens. J Neurosci. 2004;24(31):6939. doi: 10.1523/JNEUROSCI.0671-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu Q-s. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31(38):13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Higginbotham JA, Wang R, Richardson BD, et al. CB1 receptor signaling modulates amygdalar plasticity during context-cocaine memory reconsolidation to promote subsequent cocaine seeking. bioRxiv. 2020:2020.06.02.130419. doi: 10.1101/2020.06.02.130419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazenka MF, Selley DE, Sim-Selley LJ. Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci. 2013;92(8–9):446–452. doi: 10.1016/j.lfs.2012.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89(1):121–145. doi: 10.1152/physrev.00017.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.French PJ, O’Connor V, Jones MW, et al. Subfield-specific immediate early gene expression associated with hippocampal long-term potentiation in vivo. Eur J Neurosci. Mar 2001;13(5):968–76. doi: 10.1046/j.0953-816x.2001.01467.x [DOI] [PubMed] [Google Scholar]
- 76.Matsuo R, Murayama A, Saitoh Y, Sakaki Y, Inokuchi K. Identification and cataloging of genes induced by long-lasting long-term potentiation in awake rats. J Neurochem. Jun 2000;74(6):2239–49. doi: 10.1046/j.1471-4159.2000.0742239.x [DOI] [PubMed] [Google Scholar]
- 77.Richardson CL, Tate WP, Mason SE, Lawlor PA, Dragunow M, Abraham WC. Correlation between the induction of an immediate early gene, zif/268, and long-term potentiation in the dentate gyrus. Brain Res. May 15 1992;580(1–2):147–54. doi: 10.1016/0006-8993(92)90938-6 [DOI] [PubMed] [Google Scholar]
- 78.Jones MW, Errington ML, French PJ, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. Mar 2001;4(3):289–96. doi: 10.1038/85138 [DOI] [PubMed] [Google Scholar]
- 79.Abraham WC, Mason SE, Demmer J, et al. Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience. Oct 1993;56(3):717–27. doi: 10.1016/0306-4522(93)90369-q [DOI] [PubMed] [Google Scholar]
- 80.Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. Sep 2009;12(9):1152–8. doi: 10.1038/nn.2369 [DOI] [PubMed] [Google Scholar]
- 81.Younts Thomas J, Monday Hannah R, Dudok B, et al. Presynaptic Protein Synthesis Is Required for Long-Term Plasticity of GABA Release. Neuron. 2016/October/19/ 2016;92(2):479–492. doi: 10.1016/j.neuron.2016.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winters BD, Kruger JM, Huang X, et al. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proc Natl Acad Sci U S A. Oct 2 2012;109(40):E2717–25. doi: 10.1073/pnas.1206303109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pickel VM, Chan J, Kash TL, Rodríguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and μ-opioid receptors in rat nucleus accumbens. Neuroscience. 2004/January/01/ 2004;127(1):101–112. doi: 10.1016/j.neuroscience.2004.05.015 [DOI] [PubMed] [Google Scholar]
- 84.Bara A, Manduca A, Bernabeu A, et al. Sex-dependent effects of in utero cannabinoid exposure on cortical function. Elife. 2018/September/11 2018;7:e36234. doi: 10.7554/eLife.36234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burgdorf CE, Jing D, Yang R, et al. Endocannabinoid genetic variation enhances vulnerability to THC reward in adolescent female mice. Sci Adv. 2020;6(7):eaay1502. doi: 10.1126/sciadv.aay1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. EGR1 and calcineurin gene expression is unaltered at early abstinence timepoints. (A) EGR1 mRNA levels between saline and cocaine animals at AD1 following extended access cocaine self-administration. (B) calcineurin mRNA levels between saline and cocaine animals at AD1 following extended access cocaine self-administration. All data are presented as means ± SEMs (n = 5–7/group).
Supplementary Figure 2. Inhibition of DAGL and MAGL does not alter total eIF2α or general locomotor activity. Repeated intra-NAc microinjections of DO-34 did not change eIF2α protein expression (A) or locomotor activity (B) at AD30. Similarly, repeated intra-NAc injections of URB-602 did not result in changes to eIF2α protein expression (C) and locomotor activity (D) at AD30. All data are presented as means ± SEMs.
Supplementary Figure 3. Cue-induced food seeking following food self-administration is not regulated by accumbal DAGL and MAGL inhibition. (A) Experimental timeline for 10-day food self-administration training, abstinence periods, intra-NAc microinjections, and cue-induced seeking test. (B) Total active responses during self-administration training by animals that later received microinjections of DO-34, URB-602, or vehicle (Veh). (C) Total active responses during a 1-h cue-induced food-seeking test by animals receiving microinjections of DO-34, URB-602, or vehicle (Veh). (D) Total locomotor activity following microinjections of DO-34, URB-602, or vehicle (Veh). All data are presented as means ± SEMs (n = 6–8/group).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


