Abstract
Objective:
To evaluate the longitudinal relationship in insomnia symptoms over time with incident memory problems and dementia diagnoses among U.S. adults aged 65 years and older.
Methods:
Secondary analyses were performed on 9,518 elderly participants (≥ 65 years) who completed the 2006 wave of the Health and Retirement Study (HRS) and were followed-up to determine if insomnia symptom scores (2006–2014) were associated with time-to-onset of [1] physician-diagnosed “memory-related disease”, “Alzheimer’s disease” and/or “dementia, senility or any other serious memory impairment” and [2] diagnosis of dementia based on HRS-specific criteria. Cox proportional hazards models were constructed adjusting for socio-demographic, lifestyle, and health characteristics.
Results:
In fully adjusted models, severe insomnia symptoms were associated with increased risk of physician-diagnosed memory problems. Individuals reporting any change (increase or decrease) in insomnia symptoms during the 2006–2010 period were more likely to be diagnosed with dementia based on HRS criteria. Finally, those who experienced an increase in the severity of insomnia symptoms over time exhibited 41–72% increased risks of physician-diagnosed memory problems and 45–58% increased risks of dementia diagnosis based on HRS criteria.
Conclusions:
When severe insomnia symptoms increased over time, physician-diagnosed memory problems and dementia diagnoses also increased among U.S. elderly people over a 10-year follow-up period. More studies are required to confirm these findings using large prospective cohort designs and validated tools.
Keywords: Aging, Alzheimer’s disease, Insomnia, Dementia, Neurodegenerative, Sleep
1. INTRODUCTION:
Poor sleep is recognized as a modifiable behavioral characteristic that is implicated in the onset, progression and exacerbation of a wide range of chronic conditions, including diabetes1–3, cardiovascular diseases4–6, cancers7–9, injuries10–12 and cognitive disorders.13–15 Research also suggests that sleep may affect immune, metabolic, thermoregulatory as well as cardio-respiratory functioning.16 A recent survey by the National Sleep Foundation indicated that nearly 53% of U.S. adults reported less than seven hours of sleep on workdays and that 37% of them reported their sleep quality as fair or poor.17 Although evidence remains inconclusive, population-based studies have found that suboptimal sleep duration (≤ 6 h or ≥ 9 h) may increase cardiovascular-, cancer- and all-cause mortality rates18, 19 whereas cardio-metabolic risk, social functioning and life expectancy may be influenced by indicators of sleep quality.20 Furthermore, a wide range of sleep disturbances that could impact sleep quality, including insomnia and obstructive sleep apnea, has been previously linked to detrimental physical, mental and cognitive health outcomes.16, 21 Although frequently undetected, symptoms of insomnia have been reported in 40–70% of older adults whereas the prevalence of moderate-to-severe obstructive sleep apnea (OSA) has been estimated at 9% among middle-aged men and at 4% among middle-aged women.22, 23
Aging is often accompanied by a decline in sleep duration and quality with nearly half of older adults experiencing long-term sleep disturbances.24 In particular, the tendency to wake up repeatedly during the night, to remain awake for longer periods of time, and to experience fewer hours of sleep increases with age.24 The burden of chronic disease attributable to poor sleep is expected to rise in response to changes in the age structure of the population, which will feature a near doubling of the number of adults aged 65 years and older in developed countries by 2050.16 Population aging is also expected to increase the burden of neurodegenerative disorders such Alzheimer’s (AD)25 and Parkinson’s26, 27 diseases. Together, these demographic and epidemiological trends underscore the importance of understanding sleep as a modifiable pathway to neurodegenerative disorders. To date, a limited number of prospective cohort studies have attempted to evaluate the temporal relationship between sleep and broadly defined neurodegenerative disorders28, 29, and fewer have assessed insomnia symptoms as a risk factor for diagnosed memory problems.30 Accordingly, we performed secondary analyses of existing data from the Health and Retirement Study (HRS) to evaluate the longitudinal relationship between insomnia symptoms and incident memory problems as diagnosed by a physician or HRS-specific criteria among U.S. elderly people, 65 years and older at baseline.
2. MATERIALS AND METHODS:
2.1. Data source:
Initiated in 1992, the HRS is an ongoing, nationally representative longitudinal study of community-dwelling U.S. adults over the age of 50 and their spouses of any age. The HRS was designed to study economic well-being, labor force participation, health and family composition among older adults through biennial surveys administered by telephone or face-to-face interviews. Although the HRS only interviews community-dwelling adults in their baseline surveys, respondents who enter long-term care facilities are also retained. Multistage probability sampling of U.S. households within geographical strata was performed whereby African Americans, Hispanics and residents of Florida were over-sampled. Response rates at baseline and follow-up waves were >80% for all HRS interviews. Written informed consent was provided by all participants and the University of Michigan’s Institutional Review Board approved study protocols. The HRS is sponsored by the National Institute on Aging (grant number U01AG009740) and the Social Security Administration. Because our study relied on de-identified, public-use data, it was considered research not involving human subjects by our institution. Details of HRS procedures were reported elsewhere.31, 32
2.2. Study population:
The original HRS study consists of participants from whom data were collected in 1992, 1994 and 1996, and the Study of Asset and Health Dynamics of the Oldest Old (AHEAD) consists of those from whom data were collected in 1993 and 1995. The two studies were merged and two new cohorts (the Children of the Depression (born 1924–1930) and the War Babies (1942–1947)) were added in 1998. Subsequently, the Early Baby Boomers (1948–1953) cohort was added in 2004, the Mid Baby Boomers (1954–1959) cohort was added in 2010 and the Late Baby Boomers (1960–1965) cohort was added in 2016. We restricted our analyses to a baseline cohort of 2006 HRS wave participants, 65 years and older, who were followed-up for up to 10 years (2006–2016) until they developed the outcome of interest, were lost to follow-up or were deceased. Mid Baby Boomers and Late Baby Boomers cohorts were enrolled after 2006 and therefore automatically excluded from this study. Starting in 2006, HRS began collecting data on psychosocial factors, whereby half of the sample completed detailed face-to-face interviews that included physical, biological and psychosocial measures, and the other half completed a core interview by telephone. To reduce study-related costs and burden on participants, enhanced interviewing was alternated between half-samples at each subsequent wave. Specifically, interviewers left behind a self-report psychological questionnaire at the end of each interview, and respondents were asked to return the completed questionnaire by mail to the University of Michigan. The response rate for the leave-behind questionnaire among interviewees was ~90%. Since psychosocial data were collected every 4 years, our sample was restricted to HRS participants with complete exposure data in 2006, 2010 and/or 2014 and complete follow-up wave data in 2008, 2010, 2012, 2014 and/or 2016. We further excluded participants with a history of physician-diagnosed “emotional, nervous or psychiatric problems” and/or “memory-related disease” by the 2006 HRS wave (when examining physician-diagnosed memory problems or HRS-specific dementia diagnosis) and additionally those diagnosed with dementia during the 2006 HRS wave using HRS-specific criteria (when examining HRS-specific dementia diagnosis). Finally, we excluded participants with missing data on key socio-demographic, lifestyle and health characteristics, the main exposure (insomnia symptoms) and/or outcome variables of interest.
2.3. Variable definitions:
2.3.1. Insomnia symptoms:
Key symptoms of insomnia comprise difficulty initiating sleep, difficulty maintaining sleep, early morning awakening and nonrestorative sleep. As such, the HRS applied a modified version of the Jenkins Sleep Questionnaire, a validated and widely used screening tool that measures self-reported sleep complaints rather than diagnosed sleep disorders. Frequency of insomnia symptoms was determined among HRS participants at 2006, 2010 and 2014 waves using four questionnaire items (“How often do you have trouble falling asleep?”; “How often do you have trouble with waking up during the night?”; “How often do you have trouble with waking up too early and not being able to fall asleep again?”; “How often do you feel really rested when you wake up in the morning?”) with possible responses being “most of the time”, “sometimes” or “rarely or never”. Those reporting “most of the time” to any of the first three symptoms and those reporting “sometimes” or “rarely or never” to the fourth symptom were considered as having insomnia symptoms. Total insomnia symptoms score was computed that ranges between “0=no insomnia” and “8=severe insomnia” after reverse-coding responses to the first three items. We also evaluated insomnia symptoms severity at each wave (2006, 2010 and 2014) by identifying a symptom as positive among participants indicating having it “most of the time” for the first three items and “rarely or never” for the fourth item, and subsequently created an index for the number of insomnia symptoms ranging between 0 and 4. We used these insomnia symptom scores to create variables indicating between-wave (2006 to 2010, 2010 to 2014, 2006 to 2014) changes in insomnia symptoms whereby a positive number suggested an increase, a negative number suggested a decrease and a zero suggested no change over time.33, 34
2.3.2. Physician-diagnosed memory problems:
Cumulative incidence of self-reported physician-diagnosed memory problems was defined among participants using questionnaire items pertaining to “memory-related disease” (2008 HRS wave), “Alzheimer’s disease” (2010–2016 HRS waves) and/or “dementia, senility or any other serious memory impairment” (2010–2016 HRS waves). 35, 36
2.3.3. Health and Retirement Study Dementia diagnosis:
The Langa-Weir classification of cognitive function (1 = “Normal”, 2 = “Cognitively Impaired but not Demented”, 3 = “Demented”) is available at each HRS wave since 1995, with imputation of missing data. For self-responding HRS participants ≥ 65 years, a 35-point scale was used that combines immediate and delayed 10-noun free recall test to measure memory (0–20), a serial sevens subtraction test to measure working memory (0–5), a counting backwards test to measure speed of mental processing (0–2) and three mental status questions (date naming (0–4); object naming (0–2); naming the president and the vice president of the United States (0–2)). Since 2000, for proxy-responding HRS participants ≥ 65 years, an 11-point cognition scale (0–11) was generated based on proxy assessment of memory (0 = “excellent”, 1 = “very good”, 2 = “good”, 3 = “fair”, 4 = “poor”), Instrumental Activities of Daily Living limitations (0–5), and interviewer’s assessment of cognitive impairment (0 = “none”, 1 = “may have impairment”, 2 = “has impairment”). HRS-specific dementia diagnosis was defined as a dichotomous variable (0 = “Normal/Cognitively Impaired but not Demented”, 1 = “Demented”).37–41
2.3.4. Socio-demographic characteristics:
Baseline HRS data were extracted on sex (male, female), age (65–69, 70–74, 75–79, 80+ years), race (White/Caucasian, Black/African American, Other), ethnicity (Hispanic, non-Hispanic), marital status (married, not married), education (no degree, GED or high school diploma, college degree or higher), and total wealth (<25,000; 25,000–124,999; 125,000–299,999; 300,000–649,999; 650,000+).42
2.3.5. Lifestyle characteristics:
Baseline HRS data were extracted from the 2006 wave on smoking status (never, ever), frequency of alcohol consumption (abstinent, 1–3 days per month, 1–2 days per week, ≥3 days per week) and frequency of moderate and vigorous exercise reported as never, 1–4 times per month, or >1 times per week.16, 36
2.3.6. Health characteristics:
Baseline HRS data were extracted from the 2006 HRS on body mass index (BMI), presence of cardiovascular risk factors, self-rated health and depressive symptoms. BMI was defined as a continuous variable and categorized as <25, 25–29.9, ≥30 kg/m2. Cardiovascular risk factors were determined through physician-diagnosed hypertension, diabetes, stroke, heart attack, coronary heart disease, angina, congestive heart failure and/or other heart problems. Self-rated health was evaluated using a single item (“would you say your health is excellent, very good, good, fair, or poor?”) and dichotomized as “excellent/very good/good” versus “fair/poor”. Depressive symptoms were assessed using the modified 8-item Center for Epidemiological Studies Depression Scale (CES-D) and total CES-D score was calculated with higher scores indicating worse symptoms of depression.16, 32
2.4. Statistical analysis:
All statistical analyses were conducted using STATA version 15 (College Station, TX), taking complex sampling design into consideration. Cox proportional hazards regression models were constructed to examine longitudinal relationships between key exposure and outcome variables, as described in similarly conducted HRS-based studies.33, 43, 44 We used age at each follow-up time and calculated time-to-event by subtracting the age at baseline from the age at occurrence of an event. Specifically, we examined each insomnia indicator (insomnia status, insomnia score, severe insomnia status, severe insomnia score, change in insomnia score over time, change in insomnia severity over time) as a predictor of cumulative incidence or time-to-onset of memory problems or dementia diagnosis while sequentially controlling for socio-demographic, lifestyle and health characteristics. Model I controlled for socio-demographic characteristics alone, Model II controlled for socio-demographic and lifestyle characteristics and Model III controlled for socio-demographic, lifestyle and health characteristics. Complete subject analysis was performed using available data on variables of interest and two-tailed statistical tests were evaluated at an alpha level of 0.05.
Data Availability Statement:
The authors have access to de-identified HRS raw data through online registration on study’s website. Therefore, data are restricted and cannot be publicly shared for legal and ethical reasons.
3. RESULTS:
A total of 11,401 participants were ≥65 years at baseline and, of those, 9,550 did not report a history of “emotional, nervous or psychiatric problems” and/or “memory-related disease” as of the 2006 wave of HRS data collection. Furthermore, 9,518 participants remained after excluding missing data on smoking status (n=94), frequency of alcohol drinking (n=2), frequency of moderate exercise (n= 9), frequency of vigorous exercise (n=14), self-rated health (n=12) and CES-D (n=535). Of those, 9,469 had complete data on insomnia symptoms during the 2006 wave, 7,392 had complete data on insomnia symptoms during the 2010 wave and 5,679 had complete data on insomnia symptoms during the 2014 wave of data collection. After accounting for losses to follow-up and deaths, 4,548 had complete self-reported data on memory problems during the 2006–2016 HRS waves. The baseline sample of 9,518 HRS participants contributed 99,176 person-years of follow-up and a total of 1,146 failures in terms of physician-diagnosed memory problems at the end of the follow-up period (Figure 1). Sub-analyses involving a maximum of 6,907 HRS participants with no dementia diagnosed based on HRS criteria in 2006 and with complete data thereafter were performed to assess insomnia symptoms in relation to HRS-based dementia diagnosis.
Figure 1.
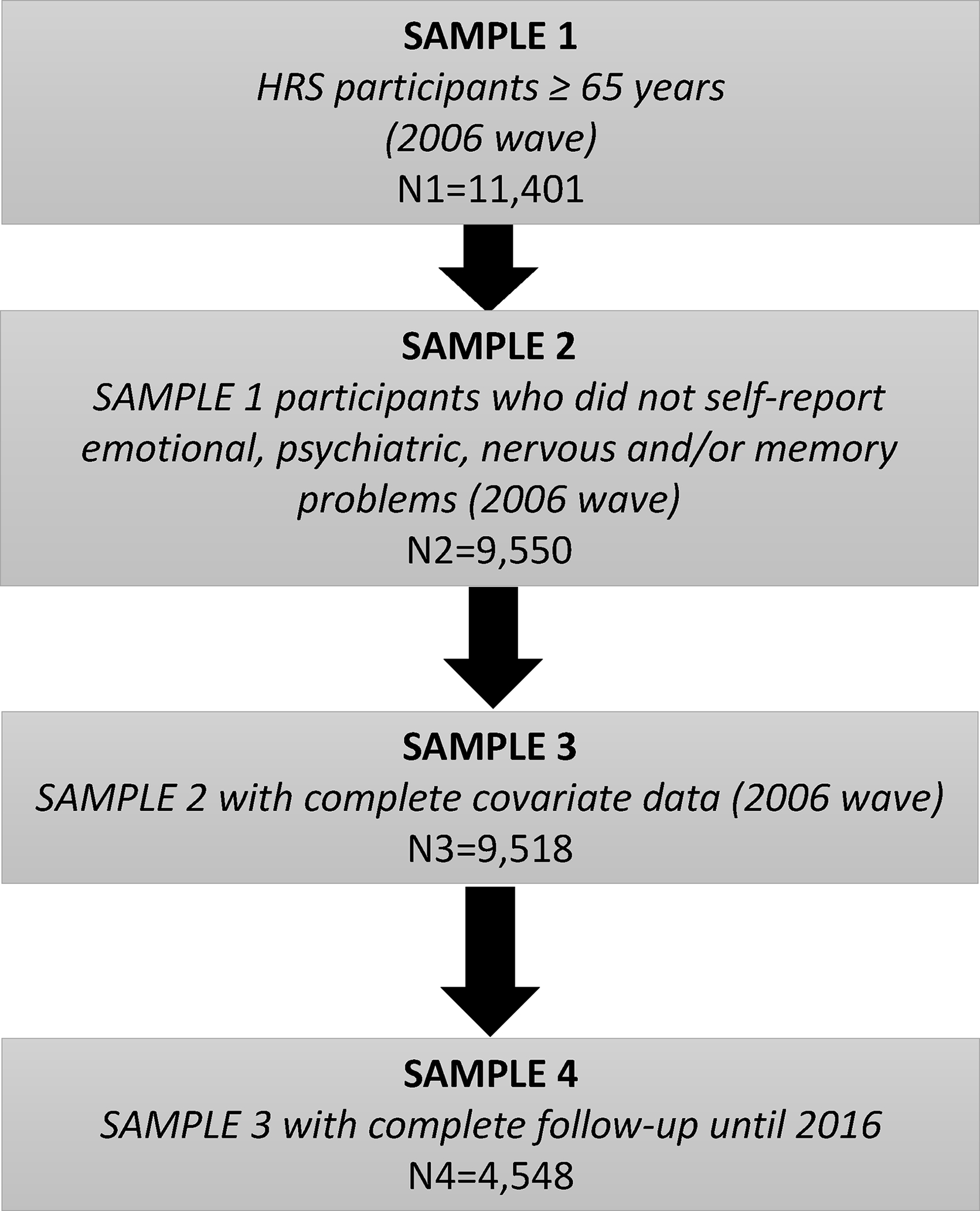
Study flowchart – 2006–2016 Health and Retirement Study
Table 1 presents the socio-demographic, lifestyle and health characteristics of the study population, which consists of 9,518 HRS participants (55% female) with mean age of 75 years at baseline. Eighty-nine percent of participants were of non-Hispanic white race, 6% self-reported Hispanic origin, 58% were married, 39% had ≥ college degree, and 24% reported total wealth ≥ $650,000. Furthermore, 57% were ever smokers, 50% abstained from alcohol consumption, 55% performed moderate exercise >1 time/week, and 66% did not perform any vigorous exercise. Nearly 72% had one or more cardiovascular risk factor and 28% reported self-rated health as fair or poor. The mean BMI of participants was 28.3 kg/m2, and their mean CES-D score was 1.2.
Table 1.
Socio-demographic, lifestyle and health characteristics of study sample at baseline – 2006–2016 Health and Retirement Study (n=9,518)
| % | Mean ± SEM | |
|---|---|---|
| SOCIO-DEMOGRAPHIC CHARACTERISTICS: | ||
| Sex: | ||
| Male | 44.9 | |
| Female | 55.1 | |
| Age (years): | ||
| Continuous | 74.9 ± .08 | |
| 65–69 | 28.9 | |
| 70–74 | 24.4 | |
| 75–79 | 20.2 | |
| 80+ | 26.6 | |
| Race: | ||
| White/Caucasian | 88.6 | |
| Black/African American | 8.3 | |
| Other | 3.2 | |
| Ethnicity: | ||
| Hispanic | 93.6 | |
| Non-Hispanic | 6.4 | |
| Marital status: | ||
| Married | 58.4 | |
| Not married | 41.6 | |
| Education: | ||
| No degree | 24.6 | |
| GED or high school diploma | 36.0 | |
| College degree or higher | 39.4 | |
| Total wealth ($): | ||
| <25,000 | 14.9 | |
| 25,000–124,999 | 17.7 | |
| 125,000–299,999 | 21.8 | |
| 300,000–649,999 | 21.4 | |
| 650,000+ | 24.1 | |
| LIFESTYLE CHARACTERISTICS: | ||
| Smoking status: | ||
| Never | 43.2 | |
| Ever | 56.8 | |
| Frequency of alcohol consumption: | ||
| Abstinent | 49.7 | |
| 1–3 days per month | 17.6 | |
| 1–2 days per week | 14.2 | |
| ≥ 3 days per week | 18.5 | |
| Frequency of moderate exercise: | ||
| Never | 23.5 | |
| 1–4 times per month | 21.6 | |
| >1 times per week | 54.9 | |
| Frequency of vigorous exercise: | ||
| Never | 65.9 | |
| 1–4 times per month | 12.0 | |
| >1 times per week | 22.1 | |
| HEALTH CHARACTERISTICS: | ||
| Body mass index (kg/m2): | ||
| Continuous | 28.3 ± 0.09 | |
| < 25 | 11.3 | |
| 25–29.9 | 15.7 | |
| ≥30 | 73.1 | |
| Cardiovascular risk factors: | ||
| Yes | 72.1 | |
| No | 27.9 | |
| Self-rated health: | ||
| Continuous | 2.8 ± 0.01 | |
| Excellent/Very good/Good | 72.5 | |
| Fair/Poor | 27.5 | |
| Center for Epidemiological Studies Depression score: | ||
| Continuous | 1.2 ± 0.02 |
Abbreviations: SEM=Standard error of the mean.
Table 2 presents summary statistics for key exposure and outcome variables over the 2006–2016 HRS waves. The rate of insomnia symptoms appeared to be stable over three data waves (2006, 2010, 2014), with nearly 40% having insomnia symptoms. By contrast, the rate of having at least one severe insomnia symptom decreased from 81.5% in 2006 to 75.4% in 2014. Whereas the average of insomnia symptom scores increased from 2.4 in 2006 to 2.7 in 2014, there was no substantial change in the average score for severe insomnia symptoms. Change over time (2006–2010, 2010–2014, 2006–2014) suggested a nearly equal proportion of HRS participants who experienced no change, an increase or a decrease in prevalent insomnia symptoms. Finally, cumulative incidence of physician-diagnosed memory problems increased from 3.5% in 2008 to 11.0% in 2016 and cumulative incidence of HRS-based dementia diagnosis among 6,907 study-eligible subjects increased from 1.6% in 2008 to 10.8% in 2016.
Table 2.
Summary statistics for the key exposure and outcome variables over the 2006–2016 Health and Retirement Study waves (n=9,518)
| 2006 | 2008 | 2010 | 2012 | 2014 | 2016 | |
|---|---|---|---|---|---|---|
| Insomnia symptoms: | ||||||
| N | 9,518 | 7,509 | 5,803 | |||
| % Yes | 40.3 | -- | 41.4 | -- | 41.8 | -- |
| Mean ± SEM | 2.4 ± 0.02 | -- | 2.6 ± 0.02 | 2.7 ± 0.03 | ||
| 2006–2010: | ||||||
| % No change | 25.8 | |||||
| % Increase | 41.1 | |||||
| % Decrease | 33.0 | |||||
| 2010–2014: | ||||||
| % No change | 28.2 | |||||
| % Increase | 37.8 | |||||
| %Decrease | 34.0 | |||||
| 2006–2014: | ||||||
| % No change | 28.2 | |||||
| % Increase | 37.8 | |||||
| %Decrease | 34.0 | |||||
| Severe insomnia symptoms: | ||||||
| % Yes | 81.5 | 76.4 | 75.4 | |||
| Mean ± SEM | 1.6 ± 0.01 | -- | 1.5 ± 0.01 | -- | 1.5 ± 0.02 | -- |
| 2006–2010: | ||||||
| % No change | 38.6 | |||||
| % Increase | 26.8 | |||||
| %Decrease | 34.7 | |||||
| 2010–2014: | ||||||
| % No change | 41.3 | |||||
| % Increase | 29.5 | |||||
| %Decrease | 29.2 | |||||
| 2006–2014: | ||||||
| % No change | 37.1 | |||||
| % Increase | 27.1 | |||||
| %Decrease | 35.8 | |||||
| Physician-diagnosed memory problems: | ||||||
| % Memory problems | -- | 3.5 | -- | -- | -- | -- |
| % Dementia | -- | -- | 2.2 | 3.9 | 5.5 | 6.9 |
| % Alzheimer’s disease | -- | -- | 1.9 | 3.1 | 4.2 | 5.1 |
| % Any | -- | 3.5 | 4.2 | 6.7 | 9.0 | 11.0 |
| Dementia diagnosis: | ||||||
| % Yes | -- | 1.6 | 4.3 | 7.1 | 9.7 | 10.8 |
Table 3 presents Cox proportional hazards models for any or severe insomnia symptoms as a predictor of time-to-onset of physician-diagnosed memory problems, before and after adjustment for baseline characteristics. In fully adjusted models, there was no significant association between insomnia symptoms and physician-diagnosed memory problems (hazard ratio (HR) = 0.96, 95% confidence interval (CI): 0.84, 1.12) whereas severe insomnia symptoms were associated with increased risk of physician-diagnosed memory problems (HR=1.21, 95% CI: 1.02, 1.44). Results also suggested that a unit increase in insomnia symptoms was associated with a slight decrease (HR=0.95, 95% CI: 0.92, 0.99) whereas a unit increase in severe insomnia symptoms was associated with a slight increase (HR=1.10, 95% CI: 1.04, 1.18) in the risk of physician-diagnosed memory problems, after controlling for socio-demographic, lifestyle and health characteristics. Participants who experienced decreased insomnia symptoms (any or severe) between different time periods had similar risks of physician-diagnosed memory problems when compared to those who did not experience a change in their insomnia symptoms, after controlling for confounders. By contrast, those who experienced an increase in the severity of insomnia symptoms over time (2006–2010, 2010–2014, 2006–2014) exhibited 41%–72% increased risks of physician-diagnosed memory problems in fully adjusted models.
Table 3.
Cox proportional hazards model for insomnia symptoms as a predictor of time-to-onset of physician-diagnosed memory problems – 2006–2016 Health and Retirement Study (n=9,518)
| Unadjusted | Model I a | Model II b | Model III c | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Insomnia symptoms: | ||||||||
| Any: | ||||||||
| Yes | 1.08 | 0.95, 1.23 | 1.03 | 0.90, 1.17 | 1.04 | 0.91, 1.19 | 0.96 | 0.84, 1.12 |
| No | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Continuous | 1.00 | 0.97, 1.03 | 0.98 | 0.95, 1.02 | 0.98 | 0.95, 1.02 | 0.95 | 0.92, 0.99 |
| Severe: | ||||||||
| Yes | 1.06 | 0.89, 1.26 | 1.12 | 0.95, 1.33 | 1.12 | 0.95, 1.33 | 1.21 | 1.02, 1.44 |
| No | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Continuous | 1.04 | 0.98, 1.11 | 1.07 | 1.00, 1.13 | 1.06 | 1.00, 1.13 | 1.10 | 1.04, 1.18 |
| Change in insomnia symptoms (Any): | ||||||||
| 2006–2010: | ||||||||
| Increase | 1.13 | 0.93, 1.36 | 1.04 | 0.87, 1.26 | 1.04 | 0.86, 1.26 | 1.02 | 0.84, 1.23 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.32 | 1.08, 1.61 | 1.23 | 1.01, 1.50 | 1.22 | 0.99, 1.48 | 1.16 | 0.95, 1.42 |
| 2010–2014: | ||||||||
| Increase | 1.06 | 0.84, 1.36 | 1.00 | 0.79, 1.26 | 0.99 | 0.79, 1.26 | 0.98 | 0.77, 1.24 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.23 | 0.97, 1.56 | 1.12 | 0.89, 1.41 | 1.12 | 0.88, 1.41 | 1.05 | 0.83, 1.33 |
| 2006–2014: | ||||||||
| Increase | 1.06 | 0.84, 1.36 | 1.00 | 0.79, 1.27 | 0.99 | 0.79, 1.26 | 0.97 | 0.77, 1.24 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.23 | 0.97, 1.56 | 1.12 | 0.89, 1.41 | 1.12 | 0.88, 1.41 | 1.05 | 0.83, 1.33 |
| Change in insomnia symptoms (Severe): | ||||||||
| 2006–2010: | ||||||||
| Increase | 1.51 | 1.27, 1.79 | 1.39 | 1.17, 1.65 | 1.38 | 1.16, 1.64 | 1.41 | 1.18, 1.68 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.18 | 0.99, 1.39 | 1.12 | 0.95, 1.33 | 1.12 | 0.94, 1.32 | 1.14 | 0.96, 1.36 |
| 2010–2014: | ||||||||
| Increase | 1.42 | 1.16, 1.74 | 1.34 | 1.09, 1.64 | 1.34 | 1.09, 1.64 | 1.34 | 1.09, 1.64 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.19 | 0.96, 1.48 | 1.13 | 0.91, 1.39 | 1.14 | 0.92, 1.41 | 1.13 | 0.91, 1.39 |
| 2006–2014: | ||||||||
| Increase | 1.87 | 1.53, 2.29 | 1.73 | 1.41, 2.10 | 1.70 | 1.39, 2.08 | 1.72 | 1.40, 2.11 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.22 | 0.99, 1.50 | 1.22 | 0.99, 1.49 | 1.20 | 0.98, 1.48 | 1.24 | 0.99, 1.54 |
Abbreviations: HR=hazard ratio; CI: confidence interval.
Model I was adjusted for socio-demographic characteristics;
Model II was adjusted for socio-demographic and lifestyle characteristics;
Model III was adjusted for socio-demographic, lifestyle and health characteristics.
Table 4 presents Cox proportional hazards models for any or severe insomnia symptoms as a predictor of time-to-onset of HRS-based dementia diagnosis, before and after adjustment for baseline characteristics. In fully-adjusted models, HRS-based dementia diagnosis was associated with any change in insomnia symptoms between 2006 and 2010 (Increase: HR=1.27, 95% CI: 1.03, 1.55; Decrease: HR=1.36, 95% CI: 1.09, 1.69). By contrast, HRS-based dementia diagnosis was only associated with increase in severity of insomnia symptoms between time periods (2006–2010: HR=1.58, 95% CI: 1.31, 1.90; 2010–2014: HR=1.47, 95% CI: 1.21, 1.80; 2006–2014: HR=1.45, 95% CI: 1.18, 1.79).
Table 4.
Cox proportional hazards model for insomnia symptoms as a predictor of time-to-onset of dementia diagnosis – 2006–2016 Health and Retirement Study (n=6,907)
| Unadjusted | Model I a | Model II b | Model III c | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Insomnia symptoms: | ||||||||
| Any: | ||||||||
| Yes | 1.12 | 0.97, 1.30 | 1.06 | 0.91, 1.24 | 1.04 | 0.90, 1.22 | 0.98 | 0.83, 1.15 |
| No | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Continuous | 1.04 | 1.00, 1.08 | 1.01 | 0.98, 1.05 | 1.01 | 0.98, 1.05 | 0.99 | 0.95, 1.03 |
| Severe: | ||||||||
| Yes | 0.92 | 0.77, 1.09 | 0.98 | 0.82, 1.19 | 0.98 | 0.81, 1.17 | 0.99 | 0.83, 1.20 |
| No | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Continuous | 0.95 | 0.88, 1.02 | 0.98 | 0.92, 1.05 | 0.98 | 0.92, 1.05 | 0.99 | 0.93, 1.07 |
| Change in insomnia symptoms (Any): | ||||||||
| 2006–2010: | ||||||||
| Increase | 1.34 | 1.10, 1,64 | 1.24 | 1.01, 1.52 | 1.26 | 1.03, 1.54 | 1.27 | 1.03, 1.55 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.51 | 1.23, 1.86 | 1.41 | 1,14, 1.74 | 1.41 | 1.14, 1.74 | 1.36 | 1.09, 1.69 |
| 2010–2014: | ||||||||
| Increase | 0.86 | 0.68, 1.10 | 0.82 | 0.64, 1.04 | 0.81 | 0.64, 1.04 | 0.78 | 0.61, 1.00 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.38 | 1.11, 1.73 | 1.25 | 1.00, 1.56 | 1.25 | 0.99, 1.57 | 1.20 | 0.96, 1.52 |
| 2006–2014: | ||||||||
| Increase | 0.86 | 0.67, 1.10 | 0.82 | 0.64, 1.04 | 0.81 | 0.64, 1.04 | 0.78 | 0.61, 1.00 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.38 | 1.11, 1.72 | 1.25 | 1.00, 1.56 | 1.25 | 0.99, 1.57 | 1.21 | 0.96, 1.52 |
| Change in insomnia symptoms (Severe): | ||||||||
| 2006–2010: | ||||||||
| Increase | 1.65 | 1.38, 1.99 | 1.59 | 1.33, 1.92 | 1.58 | 1.31, 1.90 | 1.58 | 1.31, 1.90 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 1.22 | 1.02, 1.46 | 1.16 | 0.97, 1.39 | 1.15 | 0.96, 1.37 | 1.15 | 0.95, 1.38 |
| 2010–2014: | ||||||||
| Increase | 1.54 | 1.27, 1.88 | 1.47 | 1.22, 1.79 | 1.48 | 1.21, 1.80 | 1.47 | 1.21, 1.80 |
| No change | 0.97 | 0.76, 1.22 | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 0.97 | 0.76, 1.22 | 0.96 | 0.75, 1.21 | 0.95 | 0.74, 1.21 | ||
| 2006–2014: | ||||||||
| Increase | 1.59 | 1.31, 1.96 | 1.48 | 1.21, 1.82 | 1.47 | 1.19, 1.80 | 1.45 | 1.18, 1.79 |
| No change | Ref. | -- | Ref. | -- | Ref. | -- | Ref. | -- |
| Decrease | 0.88 | 0.71, 1.09 | 0.88 | 0.70, 1.82 | 0.86 | 0.68, 1.07 | 0.85 | 0.68, 1.07 |
Abbreviations: HR=hazard ratio; CI: confidence interval.
Model I was adjusted for socio-demographic characteristics;
Model II was adjusted for socio-demographic and lifestyle characteristics;
Model III was adjusted for socio-demographic, lifestyle and health characteristics.
4. DISCUSSION:
In this prospective cohort study of HRS participants aged ≥65 years at baseline, we evaluated distinct ways of defining insomnia symptoms as predictors of future diagnosis of memory problems or dementia over a 10-year period. Our results indicated that severe rather than any insomnia symptoms was positively associated with future physician-diagnosed memory problems, including AD and dementias, after adjusting for socio-demographic, lifestyle and health confounders. We also observed that in fully adjusted models an increase in insomnia symptom severity over time was predictive of physician-diagnosed memory problems as well as dementia diagnosis based on HRS criteria. These findings are consistent with a previously published study.45
The primary focus of recent studies has been on sleep duration rather than insomnia symptoms in relation to neurodegenerative disorders. Several of these studies found that longer rather than shorter sleep duration was associated with worse cognitive function and increased risks of AD and dementias. For instance, Gildner and colleagues conducted a longitudinal study of Mexican adults from the World Health Organization’s Study on global AGEing, who were 50 years and older and healthy sleepers at baseline, to examine if changes in sleep duration may impact rate of cognitive decline as determined by immediate and delayed verbal recall, forward and backward digit span, and verbal fluency.46 Their study suggested that declines in overall cognitive function, attention/working memory and executive function were associated with longer sleep duration among individuals who slept 6–9 hours at baseline.46 Low and colleagues analyzed cross-sectional data on 1,496 adults aged ≥60 years who participated in the 2013–2014 National Health and Nutrition Examination Surveys to evaluate weekday (or workday) nighttime sleep duration in relation to Consortium to Establish a Registry for AD Word Learning (CERAD-WL) immediate recall, CERAD-WL delayed recall, Animal Fluency Test (AFT), Digital Symbol Substitution Test (DSST) and subjective cognitive problems (SCP).47 Their study indicated no significant association of shorter sleep duration with cognition.47 By contrast, individuals who slept 10 hours or longer had lower scores on CERAD-WL immediate recall, CERAD-WL delayed recall, AFT, and DSST, and were more likely to report SCP and those who slept ≥8 hours had lower scores on CERAD-WL delayed recall.47 A longitudinal study involving 214 Swedish adults, ≥75 years, who were dementia-free at baseline and had three years of follow-up, was conducted by Hahn and colleagues48 whereby self-reported (reduced duration and/or depth) in sleep pattern was evaluated in relation to incident all-cause dementia and AD over 9 years of follow-up. After controlling for age, gender and education, reduced sleep duration was associated with increased all-cause dementia (HR=1.75; 95% CI: 1.04–2.93) and AD (HR=2.01; 95% CI: 1.12–3.61).48 These results persisted after controlling for lifestyle and vascular factors but not after controlling for depressive symptoms, implying that depressive symptoms may explain these observed relationships.48 In this study, adjustment for health-related characteristics, including depressive symptoms, did not alter the direction or magnitude of the hypothesized relationships between changes in insomnia symptoms over time and memory problems, despite established comorbidity of sleep disorders with psychiatric conditions.49
Classified as insomnia, circadian rhythm (sleep-wake schedule) disorders, hypersomnia, sleep-related breathing disorders (SBD), motor disturbances in sleep, and parasomnias, sleep disorders are associated with a wide range of neurologic conditions, including neurodegenerative disorders such as AD and dementias.50 However, it remains unclear whether memory problems resulting from AD and/or dementias may be associated with specific types of sleep disorders but not others. Whereas evidence that links insomnia to memory problems has been scarce, several studies have assessed SBD, especially OSA, as a potential marker of AD and/or dementias. In a cross-sectional study of 127 community-dwelling older adults from the Age-Well randomized clinical trial who were cognitively unimpaired at baseline and had completed detailed neuropsychological assessment, polysomnography, magnetic resonance imaging, florbetapir and fluorodeoxyglucose positron emission tomography scans, Andre and colleagues examined polysomnography-based SBD parameters as risk factors for brain changes (amyloid deposition, gray matter volume, perfusion, and glucose metabolism).25 Although participants with SBD showed greater amyloid burden (Cohen d=0.83), gray matter volume (Cohen d=0.75), perfusion (Cohen d=0.86), and metabolism (Cohen d=1.04), no association was found with cognition, self-reported cognitive and sleep difficulties, or excessive daytime sleepiness symptoms.25 Gronewold and colleagues examined the relationship between SBD severity measured using a portable sleep apnea examination device with severity of cognitive, emotional, and mobility impairment using a clinical sample of 82 geriatric patients with mild dementia.51 Investigators found low to moderate associations between SBD and dementia severities.51 In a systematic review of the literature, Bubu and colleagues highlighted evidence linking OSA and Continuous Positive Airway Pressure treatment to mild cognitive impairment and AD across different age groups.13
Our finding that increasingly severe insomnia symptoms may lead to worse cognition and subsequently a diagnosis of dementia is biologically plausible. Based on animal studies, sleep may be critical for metabolic homeostasis with evidence for increased β-amyloid clearance during sleep.52 Spira and colleagues analyzed data on 70 Baltimore Longitudinal Study on Aging participants and found that shorter sleep duration as well as lower quality of sleep was associated with greater β-amyloid burden in specific brain regions.53 Nevertheless, the temporal relationship between sleep disorders and memory problems remains inconclusive.25, 51, 54 In fact, evidence suggests that this relationship may be bi-directional and that distinct outcomes may occur when evaluating individuals who experienced sleep disorders across distinct time windows. According to Ju and colleagues, experimental evidence indicates that sleep deprivation may increase soluble beta-amyloid concentrations with chronic accumulation of beta-amyloids potentially leading to more wakefulness and altered sleep patterns, whereas early deposition of beta-amyloid in the context of normal cognition, mild dementia, and AD is associated with sleep abnormalities, and as such sleep and neurodegenerative disease may influence each other.55 According to Musiek and colleagues sleep-wake cycle and circadian rhythm disruption is frequently observed in the context of AD and has been considered as a late outcome of neurodegeneration, although recent evidence suggests it can occur earlier and could precede onset of cognitive symptoms, affecting disease process through biological mechanisms involving beta-amyloid deposition among others.56 A study by Choe and colleagues involving 202 cognitively normal older adults who participated in the Korean Brain Aging Study for the Early Diagnosis and Prediction of AD examined the relationship between sleep experiences during the young adulthood, midlife, and late-life periods with in vivo beta-amyloid deposition and AD signature regional neurodegeneration.57 Sleep duration and quality were repeatedly evaluated (20–30s, 40–50s, and most recent month), and outcomes were determined using comprehensive clinical assessment, Pittsburgh Compound B positron emission tomography, Fluorodeoxyglucose-PET, and magnetic resonance imaging.57 After controlling for age, gender, education, APOE epsilon 4 status, vascular risk score, Hamilton Depression Rating Scale score, and use of sleep medication, poor sleep quality, and short sleep duration during midlife were significantly associated with increased beta-amyloid deposition and AD signature regional hypometabolism.57 Although current poor sleep quality appeared to be associated with increased beta-amyloid deposition, this association disappeared after controlling for the effects of mid-life sleep quality.57 Neither quality nor duration of sleep during young adulthood was found to be related to beta-amyloid deposition or neurodegeneration.57
To our knowledge, this study is among the largest population-based, longitudinal studies to have examined the hypothesized relationships of interest. Whereas numerous studies have analyzed sleep indicators from the HRS33, 58, none of them have evaluated their association with diagnosed memory problems, AD, and/or dementias among elderly participants over a 10-year period of time. Nevertheless, study findings should be interpreted with caution in light of several limitations. First, a substantial proportion of HRS subjects identified at baseline had died or were lost to follow-up over the 10-year follow-up period potentially leading to selection bias. Second, measures of insomnia symptoms, memory problems and dementia were self- or proxy-reported, and memory problems were determined based on interactions with medical professionals. In addition to differences in criteria used to establish AD and/or dementia among medical professionals, there is notable variation in the frequency with which HRS respondents may seek medical care and with potential for disparities by race, ethnicity and socioeconomic status. Ascertainment bias may be an issue since HRS participants with sleep difficulties may be diagnosed with dementia because they are more likely to seek healthcare services. Unlike previously conducted studies based on the Study of Osteoporotic Fractures or the MrOS sleep study59–67, insomnia symptoms were evaluated using a validated 4-item questionnaire instead of well-established tools such as the Epworth sleepiness scale or Pittsburgh Sleep Quality Index, and no objective measurements such as polysomnography or actigraphy were available from the selected HRS respondents. Self-reported data are frequently obtained in large studies, and given the longitudinal HRS design, it is unlikely that misclassification of insomnia symptoms is dependent on diagnosis with memory problems or dementia. Therefore, it is safe to assume that non-differential misclassification may have occurred with measures of association biased towards the null value. Third, sleep duration was not evaluated in the 2006–2016 HRS waves, precluding our ability to simultaneously evaluate it with insomnia symptoms. Fourth, residual confounding may be a concern given that several risk or protective factors for AD and/or dementia were either not measured or inadequately measured. For comparability with previously conducted HRS studies, we controlled for baseline socio-demographic, lifestyle and health characteristics, although repeated measures for some of these characteristics were available for the generation of time-dependent covariates. Fifth, reverse causality could not be eliminated as a potential explanation for the observed associations, since sub-clinical neurodegenerative disease may have been responsible for patterns of insomnia symptoms. Finally, our study findings are only generalizable to adults ≥ 65 years in the United States, and should, therefore, be replicated in a wider range of populations.
In conclusion, more severe insomnia symptoms over time may be predictive of physician-diagnosed memory problems as well as dementia diagnosis based on pre-specified criteria over a 10-year follow-up period among U.S. elderly people. Additional studies are required to confirm these findings using large prospective cohort designs as well as validated tools for measuring insomnia symptoms, AD and dementias.
Supplementary Material
Acknowledgements:
The manuscript was supported in part by the Intramural Research Program of the National Institute on Aging in Baltimore, Maryland. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- AD
Alzheimer’s disease
- AHEAD
Study of Asset and Health Dynamics of the Oldest Old
- AFT
Animal Fluency Test
- BMI
Body mass index
- CERAD-WL
Consortium to Establish a Registry for AD Word Learning
- CES-D
Center for Epidemiological Studies Depression Scale
- CI
Confidence interval
- DSST
Digital Symbol Substitution Test
- HR
Hazard ratio
- HRS
Health and Retirement Study
- OSA
Obstructive sleep apnea
- SCP
Subjective cognitive problems
- SBD
Sleep-related breathing disorders
Footnotes
Declarations of interest: none.
Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy of Fort Belvoir Community Hospital, the Defense Health Agency, Department of Defense, or the U.S. Government. Any discussion or mention of commercial products or brand names does not imply or support any endorsement by the Federal Government.
References:
- 1.Aurora RN, Punjabi NM. Obstructive Sleep Apnea, Sleepiness, and Glycemic Control in Type 2 Diabetes. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 2019;15:749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang F, Chow IHI, Li L, et al. Sleep duration and patterns in Chinese patients with diabetes: A meta-analysis of comparative studies and epidemiological surveys. Perspectives in psychiatric care 2019;55:344–353. [DOI] [PubMed] [Google Scholar]
- 3.Yan B, Zhao B, Fan Y, et al. The association between sleep efficiency and diabetes mellitus in community-dwelling individuals with or without sleep-disordered breathing. Journal of diabetes 2020;12:215–223. [DOI] [PubMed] [Google Scholar]
- 4.Kuehn BM. Sleep Duration Linked to Cardiovascular Disease. Circulation 2019;139:2483–2484. [DOI] [PubMed] [Google Scholar]
- 5.Ludka O Sleep apnea and cardiovascular disease. Casopis lekaru ceskych 2019;158:178–184. [PubMed] [Google Scholar]
- 6.Tall AR, Jelic S. How broken sleep promotes cardiovascular disease. Nature 2019;566:329–330. [DOI] [PubMed] [Google Scholar]
- 7.Brenner R, Kivity S, Peker M, et al. Increased Risk for Cancer in Young Patients with Severe Obstructive Sleep Apnea. Respiration; international review of thoracic diseases 2019;97:15–23. [DOI] [PubMed] [Google Scholar]
- 8.Seijo LM, Perez-Warnisher MT, Giraldo-Cadavid LF, et al. Obstructive sleep apnea and nocturnal hypoxemia are associated with an increased risk of lung cancer. Sleep medicine 2019;63:41–45. [DOI] [PubMed] [Google Scholar]
- 9.Sillah A, Watson NF, Gozal D, Phipps AI. Obstructive sleep apnea severity and subsequent risk for cancer incidence. Preventive medicine reports 2019;15:100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoun R, Rawal H, Attarian H, Sahni A. Impact of traumatic brain injury on sleep: an overview. Nature and science of sleep 2019;11:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeon AB, Stocker RPJ, Germain A. Traumatic brain injury and sleep disturbances in combat-exposed service members and veterans: Where to go next? NeuroRehabilitation 2019;45:163–185. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Muresanu DF, Ozkizilcik A, et al. Sleep deprivation exacerbates concussive head injury induced brain pathology: Neuroprotective effects of nanowired delivery of cerebrolysin with alpha-melanocyte-stimulating hormone. Progress in brain research 2019;245:1–55. [DOI] [PubMed] [Google Scholar]
- 13.Bubu OM, Andrade AG, Umasabor-Bubu OQ, et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research. Sleep medicine reviews 2020;50:101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamble N, Yadav R, Lenka A, Kumar K, Nagaraju BC, Pal PK. Impaired sleep quality and cognition in patients of Parkinson’s disease with REM sleep behavior disorder: a comparative study. Sleep medicine 2019;62:1–5. [DOI] [PubMed] [Google Scholar]
- 15.Short MA, Chee MWL. Adolescent sleep restriction effects on cognition and mood. Progress in brain research 2019;246:55–71. [DOI] [PubMed] [Google Scholar]
- 16.Kim ES, Hershner SD, Strecher VJ. Purpose in life and incidence of sleep disturbances. Journal of behavioral medicine 2015;38:590–597. [DOI] [PubMed] [Google Scholar]
- 17.Ojile J National Sleep Foundation sets the standard for sleep as a vital sign of health. Sleep health 2017;3:226. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Cai S, Ling Y, et al. Association between total sleep time and all cancer mortality: non-linear dose-response meta-analysis of cohort studies. Sleep medicine 2019;60:211–218. [DOI] [PubMed] [Google Scholar]
- 19.Stone CR, Haig TR, Fiest KM, McNeil J, Brenner DR, Friedenreich CM. The association between sleep duration and cancer-specific mortality: a systematic review and meta-analysis. Cancer causes & control : CCC 2019;30:501–525. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Chopik WJ, Schiamberg LB. Longitudinal associations between marital quality and sleep quality in older adulthood. Journal of behavioral medicine 2017;40:821–831. [DOI] [PubMed] [Google Scholar]
- 21.Beverly Hery CM, Hale L, Naughton MJ. Contributions of the Women’s Health Initiative to understanding associations between sleep duration, insomnia symptoms, and sleep-disordered breathing across a range of health outcomes in postmenopausal women. Sleep health 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann CN, Canham SL, Mojtabai R, et al. Insomnia and health services utilization in middle-aged and older adults: results from the Health and Retirement Study. The journals of gerontology Series A, Biological sciences and medical sciences 2013;68:1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capobianco DM, Batilana A, Gandhi M, et al. Surgical treatment of sleep apnea: association between surgeon/hospital volume with outcomes. The Laryngoscope 2014;124:320–328. [DOI] [PubMed] [Google Scholar]
- 24.Vaghela P, Sutin AR. Discrimination and sleep quality among older US adults: the mediating role of psychological distress. Sleep health 2016;2:100–108. [DOI] [PubMed] [Google Scholar]
- 25.Andre C, Rehel S, Kuhn E, et al. Association of Sleep-Disordered Breathing With Alzheimer Disease Biomarkers in Community-Dwelling Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA neurology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Qassabi A, Fereshtehnejad SM, Postuma RB. Sleep Disturbances in the Prodromal Stage of Parkinson Disease. Current treatment options in neurology 2017;19:22. [DOI] [PubMed] [Google Scholar]
- 27.Targa AD, Lima MM. A circuitry for sleep in Parkinson s disease. Oncotarget 2017;8:5654–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santoro JD, Frankovich J, Bhargava S. Continued Presence of Period Limb Movements During REM Sleep in Patients With Chronic Static Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 2018;14:1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkelman JW, Lecea L. Sleep and neuropsychiatric illness. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2020;45:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herring WJ, Roth T, Krystal AD, Michelson D. Orexin receptor antagonists for the treatment of insomnia and potential treatment of other neuropsychiatric indications. Journal of sleep research 2019;28:e12782. [DOI] [PubMed] [Google Scholar]
- 31.McGrath R, Vincent BM, Hackney KJ, Robinson-Lane SG, Downer B, Clark BC. The Longitudinal Associations of Handgrip Strength and Cognitive Function in Aging Americans. Journal of the American Medical Directors Association 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter JC, Handing EP, Casanova R, et al. Neighborhoods, sleep quality, and cognitive decline: Does where you live and how well you sleep matter? Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2018;14:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leggett AN, Sonnega AJ, Lohman MC. The association of insomnia and depressive symptoms with all-cause mortality among middle-aged and old adults. International journal of geriatric psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih YC, Han SH, Burr JA. Are Spouses’ Sleep Problems a Mechanism Through Which Health is Compromised? Evidence Regarding Insomnia and Heart Disease. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine 2019;53:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min Y, Nadpara PA, Slattum PW. The Association between Sleep Problems, Sleep Medication Use, and Falls in Community-Dwelling Older Adults: Results from the Health and Retirement Study 2010. Journal of aging research 2016;2016:3685789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leggett A, Pepin R, Sonnega A, Assari S. Predictors of New Onset Sleep Medication and Treatment Utilization Among Older Adults in the United States. The journals of gerontology Series A, Biological sciences and medical sciences 2016;71:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. The journals of gerontology Series B, Psychological sciences and social sciences 2011;66 Suppl 1:i162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davydow DS, Levine DA, Zivin K, Katon WJ, Langa KM. The association of depression, cognitive impairment without dementia, and dementia with risk of ischemic stroke: a cohort study. Psychosomatic medicine 2015;77:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langa KM, Larson EB, Crimmins EM, et al. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA internal medicine 2017;177:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei MY, Kabeto MU, Langa KM, Mukamal KJ. Multimorbidity and Physical and Cognitive Function: Performance of a New Multimorbidity-Weighted Index. The journals of gerontology Series A, Biological sciences and medical sciences 2018;73:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weir D, Faul J, Langa K. Proxy interviews and bias in the distribution of cognitive abilities due to non-response in longitudinal studies: a comparison of HRS and ELSA. Longitudinal and life course studies 2011;2:170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufmann CN, Mojtabai R, Hock RS, et al. Racial/Ethnic Differences in Insomnia Trajectories Among U.S. Older Adults. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 2016;24:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao J, Muniz-Terrera G, Scholes S, Hao Y, Chen YM. Lifestyle index for mortality prediction using multiple ageing cohorts in the USA, UK and Europe. Scientific reports 2018;8:6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGrath RP, Clark BC, Erlandson KM, et al. Impairments in Individual Autonomous Living Tasks and Time to Self-Care Disability in Middle-Aged and Older Adults. Journal of the American Medical Directors Association 2019;20:730–735 e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ownby RL, Peruyera G, Acevedo A, Loewenstein D, Sevush S. Subtypes of sleep problems in patients with Alzheimer disease. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 2014;22:148–156. [DOI] [PubMed] [Google Scholar]
- 46.Gildner TE, Salinas-Rodriguez A, Manrique-Espinoza B, Moreno-Tamayo K, Kowal P. Does poor sleep impair cognition during aging? Longitudinal associations between changes in sleep duration and cognitive performance among older Mexican adults. Archives of gerontology and geriatrics 2019;83:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Low DV, Wu MN, Spira AP. Sleep Duration and Cognition in a Nationally Representative Sample of U.S. Older Adults. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 2019;27:1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 2014;22:1262–1271. [DOI] [PubMed] [Google Scholar]
- 49.Spiegelhalder K, Regen W, Nanovska S, Baglioni C, Riemann D. Comorbid sleep disorders in neuropsychiatric disorders across the life cycle. Current psychiatry reports 2013;15:364. [DOI] [PubMed] [Google Scholar]
- 50.Sateia MJ, Greenough G, Nowell P. Sleep in neuropsychiatric disorders. Seminars in clinical neuropsychiatry 2000;5:227–237. [DOI] [PubMed] [Google Scholar]
- 51.Gronewold J, Haensel R, Kleinschnitz C, Frohnhofen H, Hermann DM. Sleep-Disordered Breathing in Hospitalized Geriatric Patients with Mild Dementia and Its Association with Cognition, Emotion and Mobility. International journal of environmental research and public health 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA neurology 2013;70:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou G, Liu S, Yu X, Zhao X, Ma L, Shan P. High prevalence of sleep disorders and behavioral and psychological symptoms of dementia in late-onset Alzheimer disease: A study in Eastern China. Medicine 2019;98:e18405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nature reviews Neurology 2014;10:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Experimental & molecular medicine 2015;47:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choe YM, Byun MS, Yi D, et al. Sleep experiences during different lifetime periods and in vivo Alzheimer pathologies. Alzheimer’s research & therapy 2019;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergmans RS, Zivin K, Mezuk B. Perceived sleep quality, coping behavior, and associations with major depression among older adults. Journal of health psychology 2019:1359105319891650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. Journal of the American Geriatrics Society 2011;59:2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep 2011;34:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. The journals of gerontology Series A, Biological sciences and medical sciences 2006;61:405–410. [DOI] [PubMed] [Google Scholar]
- 62.Blackwell T, Yaffe K, Laffan A, et al. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep 2014;37:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blackwell T, Yaffe K, Laffan A, et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. Journal of the American Geriatrics Society 2015;63:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song Y, Blackwell T, Yaffe K, et al. Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. Sleep 2015;38:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tranah GJ, Yaffe K, Nievergelt CM, et al. APOEepsilon4 and slow wave sleep in older adults. PloS one 2018;13:e0191281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yaffe K, Blackwell T, Barnes DE, Ancoli-Israel S, Stone KL, Study of Osteoporotic Fractures G. Preclinical cognitive decline and subsequent sleep disturbance in older women. Neurology 2007;69:237–242. [DOI] [PubMed] [Google Scholar]
- 67.Zeitzer JM, Blackwell T, Hoffman AR, et al. Daily Patterns of Accelerometer Activity Predict Changes in Sleep, Cognition, and Mortality in Older Men. The journals of gerontology Series A, Biological sciences and medical sciences 2018;73:682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors have access to de-identified HRS raw data through online registration on study’s website. Therefore, data are restricted and cannot be publicly shared for legal and ethical reasons.


