Abstract
Purpose
Our quality improvement study aimed to determine whether application of a neonatal early-onset sepsis calculator (NSC) among well-appearing infants born at ≥35 weeks’ gestation to mothers with chorioamnionitis decreases the number of lab evaluations (LEs) and antibiotic treatments (Abxs) without missing early-onset sepsis.
Methods
We compared 2 years (January 1, 2019–January 3, 2021) of data from a historical-control group before implementation of the NSC to 1 year (January 4, 2021–December 31, 2021) of data from a calculator group after implementation of the NSC to evaluate whether LE and Abx decreased following implementation of the NSC on January 4, 2021. A P-value of <0.05 was considered statistically significant for the chi-squared test, Fisher’s exact test, Student’s t-test, and Mann-Whitney U test used for the analyses.
Results
In the historical-control group, 94% of infants received LE and Abx. Retrospective application of the NSC in the historical-control group decreased LE from 94% to 21% and Abx from 94% to 13%. In the calculator group, 14% and 5% of infants received LE and Abx, respectively, and none of the blood culture was positive. Median time from birth to antibiotic initiation was significantly longer (14.5 vs 3.8 hours; P=0.0037) with no increase in median length of stay (2.3 vs 2.4 days; P=0.02) after NSC implementation. No significant difference in neonatal intensive care unit admission was identified between groups (4% vs 1%; P=0.15).
Conclusions
There was a significant decrease in LE and Abx among well-appearing infants born at ≥35 weeks’ gestation to mothers with chorioamnionitis after implementation of the NSC without missing early-onset sepsis. There was no increase in neonatal intensive care unit admission or length of hospital stay in infants who received antibiotics later after they appeared equivocal or clinically ill in the calculator group. Larger prospective studies that include follow ups are needed to confirm that early-onset sepsis is not missed.
Keywords: neonatal, early-onset sepsis, calculator, chorioamnionitis
Neonatal early-onset sepsis (EOS) is defined as an invasive bacterial infection of the blood and/or cerebrospinal fluid that occurs in the first 72 hours after birth. It is most often caused by Group B Streptococci (GBS) or Escherichia coli as an ascending infection from the maternal genitourinary or gastrointestinal tract.1–3 The incidence of EOS due to GBS infection has decreased from 1.7 cases per 1,000 births (1993) to 0.22 cases per 1,000 births (2016) as per Centers for Disease Control and Prevention (CDC) reports.4 The rate of GBS infection among newborns in the first week of life has declined by 80% since active prevention began in the mid-1990s.5
The CDC provided guidelines in 2010 for intrapartum antibiotic prophylaxis and algorithms for the evaluation and treatment of at-risk infants to prevent neonatal GBS infection.5–7 Studies have shown a high rate of evaluation (15%–20%) and treatment with empiric antibiotics (5%–8%) by using 2010 CDC guidelines in contrast to decreasing incidence of EOS (0.3–0.8 cases per 1000 births) with the use of intrapartum antibiotic prophylaxis.8–10 Overuse of antibiotics in an attempt to prevent infectious morbidity and mortality not only adds to the cost of hospitalization but also leads to inadvertent adverse events caused by the medication, an increase in antimicrobial resistance, dysbiosis, and the development of allergic and inflammatory bowel disease later in life.11,12
In light of medical interventions for large numbers of uninfected infants using 2010 CDC guidelines for the management of EOS, Puopolo et al designed a multivariate risk assessment neonatal early-onset sepsis calculator (NSC) based on data such as gestational age at birth, highest maternal antepartum temperature, maternal GBS colonization status, duration of rupture of membrane, and type and duration of intrapartum antibiotic therapies at the time of birth.13–16 Puopolo et al selected an objective variable, the highest antepartum temperature, rather than a subjective variable, chorioamnionitis diagnosis, for ease of extraction from the electronic medical record.13 The NSC provides the risk per thousand live births along with clinical recommendations for specific levels of predicted risk based on the class determined by an infant’s clinical presentation: well-appearing, equivocal, or clinically ill. The perinatal research unit at Kaiser Permanente Northern California conducted a prospective study in a cohort of 204,485 infants born at 35 weeks’ gestation or later and reported that using the NSC decreased the blood culture rates from 14.5% to 4.9% and the empiric antibiotic treatment (Abx) in the first 24 hours from 5.0% to 0.4% without apparent adverse effects.16 Similarly, other prospective studies have shown that using the NSC has significantly reduced Abx with no increase in adverse events.17,18 AAP has endorsed using the NSC to assess the risk of EOS among infants born at ≥35 weeks’ gestation.19–21
Chorioamnionitis, also known as intraamniotic infection, is an infection with resultant inflammation of any combination of the amniotic fluid, placenta, fetus, fetal membranes or decidua.22 Infants born to mothers diagnosed with or suspected to have chorioamnionitis are at high risk of EOS.21 Most studies applying the NSC on these high-risk infants were performed by retrospective application of the NSC.23–27
This quality improvement project aims to compare lab evaluation (LE), which includes complete blood cell count and blood culture; empiric Abx; time interval of antibiotic initiation from birth; neonatal intensive care unit (NICU) admission prior to discharge; and length of hospital stay (LOS) among well-appearing infants born at ≥ 35 weeks’ gestation to mothers with chorioamnionitis after implementation of the NSC to a historical-control group in the same center.
METHODS
This quality improvement study was performed at a tertiary level academic birth center. The health system’s institutional review board (IRB) determined the study did not constitute human subjects research and waived IRB oversight.
Inclusion Criteria
Infants born at ≥35 weeks’ gestation to mothers with a diagnosis of chorioamnionitis and admitted directly to the Mother Baby Unit at birth from January 1, 2019, to December 31, 2021, were included in the study.
Exclusion Criteria
Infants who were admitted to the NICU prior to transfer to the Mother Baby Unit, who were clinically ill at birth, or who had major congenital malformations were excluded from the study.
Study Design
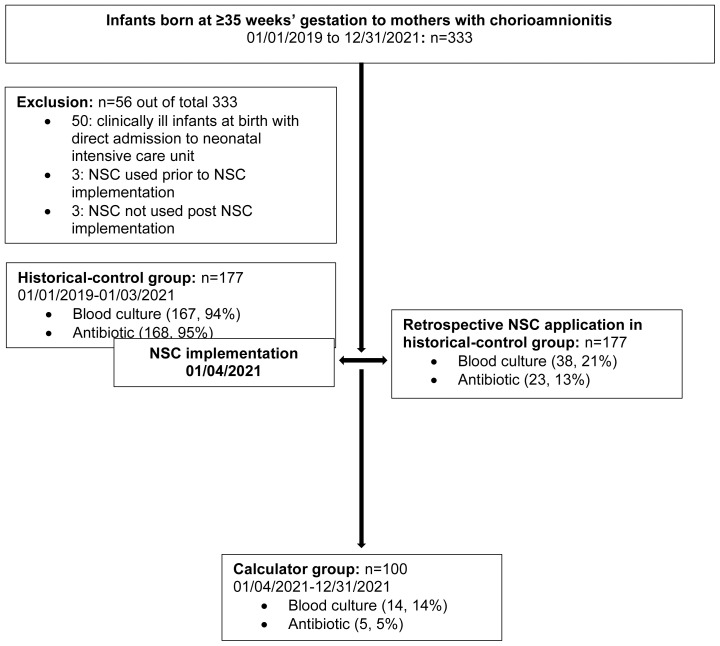
Our center followed 2010 CDC guidelines for GBS prevention until January 3, 2021, and all newborns born to mothers with chorioamnionitis routinely received limited LE and empiric Abx. The NSC was introduced and education provided to the health care providers (nurses and physicians) in the Mother Baby Unit. The NSC was then integrated into the electronic medical record and implemented in the Mother Baby Unit from January 4, 2021. Mother-infant dyads were identified using ICD code chorioamnionitis/intraamniotic infection and physician notes mentioning a diagnosis of chorioamnionitis/intraamniotic infection and intrapartum Abx. The obstetrical team diagnosed chorioamnionitis/intraamniotic infection based on 2017 American Congress of Obstetricians and Gynecologists criteria. Maternal and neonatal charts were reviewed. Maternal information collected included maternal age, type of delivery, duration of rupture of membrane, GBS colonization status, highest maternal antepartum temperature, placental pathology report, and intrapartum Abx. Neonatal information collected included date, time and gestational age at birth, birth weight, gender, LE, Abx, LOS, NICU admission before discharge, and readmission with sepsis within 7 days of birth. For comparison, the study population was stratified into 2 groups: the historical-control group and the calculator group. The historical-control group included mothers and infants managed per 2010 CDC guideline for GBS prevention prior to implementation of the NSC from January 1, 2019, to January 3, 2021. The calculator group included mothers and infants managed using the NSC prospectively by the treating team following implementation of the NSC from January 4, 2021, to December 31, 2021 (Figure 1). In the historical-control group, the NSC was applied retrospectively by the study team. Maternal and neonatal information was entered into the NSC website15 using a baseline EOS incidence of 0.5/1000 live births, and the NSC recommendation was obtained. We were unable to track the follow-up data in the historical-control group as the electronic medical record vendor in our hospital was switched in February 2020 and the medical record number for the same infant was different. We ran the readmission data on infants born in our hospital and indications for readmission from January 1, 2019, to January 10, 2021.
Figure 1.
Stratification of study population. NSC, neonatal early-onset sepsis calculator.
Statistical Analysis
This study was a quality improvement project with no hypothesis or power calculation. The data were analyzed using Statistical Analysis Software version 9.4 (SAS Institute Inc.). Chi-squared or Fisher’s exact test was used for categorical variables, and Student’s t-test or Mann-Whitney U test was used for continuous variables. A P-value of <0.05 was considered statistically significant for all analyses
RESULTS
Out of 10,090 live births from January 1, 2019, to December 31, 2021, 333 infants were born at ≥35 weeks’ gestation to mothers with chorioamnionitis (Figure 1). Of those 333 infants, 56 were excluded: 50 for direct admission to the NICU with clinical illness, 3 for the use of the NSC prior to NSC implementation, and 3 for not using the NSC after NSC implementation.
A comparison of demographics and clinical characteristics revealed no difference in gestational age at birth, maternal GBS colonization, duration of rupture of membrane, maternal intrapartum antibiotics, mode of delivery, and birth weight of the infants between the groups (Table 1). Racial diversity was similar between groups, apart from a higher number of Native Hawaiian or Pacific Islanders in the calculator group. Median highest maternal antepartum temperature was found to be higher in the historical-control group.
Table 1.
Comparison of Demographics and Clinical Characteristics
| Variables | Historical-control group (n=177) | Calculator group (n=100) | P | ||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | ||
| Race | |||||
| White | 107 | 60% | 64 | 64% | 0.5595 |
| Black | 3 | 2% | 3 | 3% | 0.6707 |
| Asian | 42 | 24% | 16 | 16% | 0.1289 |
| Native Hawaiian or Pacific Islander | 2 | 1% | 8 | 8% | 0.0052 |
| American Indian or Alaska Native | 6 | 3% | 2 | 2% | 0.7151 |
| Unknown | 17 | 10% | 8 | 8% | 0.6544 |
| Hispanic/Latino | 20 | 11% | 14 | 14% | 0.5106 |
| Gestational age in weeks, median (IQR) | 39 (39–40) | n/a | 39 (39–40) | n/a | 0.4782 |
| Maternal GBS colonization | |||||
| Positive | 23 | 13% | 16 | 16% | 0.457 |
| Negative | 152 | 86% | 84 | 84% | |
| Highest maternal antepartum temperature in °F, median (IQR) | 101.1 (100.8–101.5) | n/a | 100.9 (100.6–101.3) | n/a | 0.0171 |
| Duration of rupture of membranes in hours, median (IQR) | 13.5 (9.5–19.02) | n/a | 12.875 (8.615–17.21) | n/a | 0.2056 |
| Maternal intrapartum antibiotics | |||||
| BSA of ≥4 hours prior to birth | 27 | 15% | 12 | 12% | 0.57 |
| BSA of 2–3.9 hours prior to birth | 27 | 15% | 20 | 20% | 0.3986 |
| GBS specific antibiotics at >2 hours prior to birth | 24 | 14% | 18 | 18% | 0.4149 |
| None or any antibiotics at <2 hours prior to birth | 99 | 56% | 50 | 50% | 0.409 |
| Delivery by C-section | 76 | 43% | 34 | 34% | 0.1442 |
| Birth weight in grams, median (IQR) | 3495 (3240–3775) | 64.8% | 3425 (3167–3697) | n/a | 0.2746 |
BSA, broad spectrum antibiotics; GBS, Group B streptococcus; IQR, interquartile range; n/a: not available.
Of the 277 included infants, 177 were in the historical-control group and 100 were in the calculator group. Of the 177 infants in the historical-control group, 167 (94%) had blood culture performed and 168 (95%) received Abx. Retrospective use of the NSC in the historical-control group recommended blood culture among 38 (21%) infants and Abx among 23 (13%) infants. Retrospective application of the NSC in the historical-control group decreased LE from 94% to 21% and Abx from 94% to 13%. Of the 100 infants in the calculator group, 14 (14%) infants received blood culture and 5 (5%) infants received Abx (Figure 1).
The numbers of complete blood cell count, blood culture, and Abx in the calculator group were significantly less than those in the historical-control group (P≤0.001) (Table 2). There were two blood cultures positive for Escherichia Coli in the historical-control group, and all blood cultures were negative in the calculator group. Of the two blood cultures positive for EOS in the historical-control group, the NSC recommended Abx on one and monitoring vital signs every 4 hours on the other. The median time interval of antibiotic initiation from birth was significantly longer in the calculator group, specifically 14.5 hours compared to 3.8 hours in the historical-control group (P=0.0037). There was no significant difference in NICU admission prior to discharge between the two groups with 4% NICU admission in the historical-control group and 1% in the calculator group (P=0.15). There was no increase in median LOS in the calculator group with a median LOS of 2.3 days in the calculator group and 2.4 days in the historical-control group (P=0.02).
Table 2.
Comparison of Clinical Outcomes
| Variables | Historical-control group (n=177) | Calculator group (n=100) | P | ||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | ||
| CBC count and blood culture | 167 | 94% | 14 | 14% | <0.0001 |
| Positive blood culture | 2 | 1% | 0 | 0% | n/a |
| Antibiotic treatment | 168 | 95% | 5 | 5% | <0.0001 |
| Birth to antibiotic treatment interval in hours, median (IQR) | 3.833 (3.25–4.39) | n/a | 14.53 (11.53–14.9) | n/a | 0.0037 |
| NICU admission | 7 | 4% | 1 | 1% | 0.1584 |
| Birth to discharge interval in days, median (IQR) | 2.35 (2.0–2.73) | n/a | 2.25 (1.82–2.60) | n/a | 0.0288 |
CBC, complete blood count; IQR, interquartile range; NICU, neonatal intensive care unit; n/a: not available.
There were 14 (8%) postpartum chorioamnionitis cases in the historical-control group and 11 (11%) in the calculator group. All postpartum chorioamnionitis cases had normal antepartum temperatures of less than 100.4°F. All infants born to mothers with postpartum chorioamnionitis were well-appearing, and the NSC did not recommend blood culture or Abx in either group. None of the infants born to mothers with postpartum chorioamnionitis required NICU admission prior to discharge.
Only 33 (19%) cases in the historical-control group and 31 (31%) cases in the calculator group had placental histopathological reports available. Of those cases with placental histopathological reports, 16 (48%) cases in the historical-control group and 17 (55%) cases in the calculator group had a histopathological diagnosis of chorioamnionitis. Only 42% of the cases in the calculator group had follow-up data within 7 days of birth after discharge. None of the infants from either group was readmitted with sepsis to our hospital within 7 days of birth.
DISCUSSION
This study primarily assessed LE and Abx among high-risk infants born to mothers with chorioamnionitis by retrospective NSC application in a historical-control group and prospective application of the NSC following a change in practice in the same birth center. The study demonstrated a reduction in recommendation for both LE and Abx. Several prospective studies on infants born at ≥35 weeks’ gestation demonstrated that using the NSC can significantly reduce Abx with no increase in adverse events.17,18 Few studies to date have retrospectively applied the NSC to infants born to mothers with chorioamnionitis, and none have compared the management after NSC implementation in the same center.23–27 Adherence to a recommended EOS management guideline before and after the change in practice made comparison of the outcomes between the two different management strategies possible. The reduction in the recommendations for blood culture and Abx by retrospective NSC application in this study was similar compared to previous studies.24–27 Prospective use of the NSC decreased Abx more than projected by retrospective usage. Additionally, prospective use of the NSC meant more accuracy, given the availability of the clinical information required for the NSC in real time, compared to retrospective application, which used data required for the NSC through chart review.
The NSC recommended Abx only in one of the two culture-positive infants in the historical-control group. The other infant with culture-positive EOS, for whom the NSC suggested monitoring of vitals every 4 hours without empiric Abx, would have later become equivocal or clinically ill, and application of the NSC later would have recommended Abx. Thus, it cannot be assumed that the NSC missed Abx in this culture-positive infant.
In addition to a decrease in expense associated with LE and Abx, there were intangible benefits such as decrease in pain infliction, separation from the mother for LE and intravenous catheter placement, and side effects from antibiotics in the calculator group. Infants in the historical-control group received antibiotics soon after birth (median time interval to antibiotic initiation was 3.8 hours). One of 5 infants treated with antibiotics in the calculator group received them at 3.3 hours of life. The remaining 4 received antibiotics after the clinical classification of the infants progressed to equivocal, later resulting in a median time interval to antibiotic initiation from birth of 14.5 hours. This increase in time interval for antibiotic initiation did not translate to an increase in NICU admission or an increase in LOS in the calculator group. Of the 7 admissions to the NICU prior to discharge in the historical-control group, 2 admissions were for culture-positive EOS, 1 was for respiratory distress at 22 hours of life, and 4 were for hypoglycemia. One admission to the NICU prior to discharge in the calculator group was for hypoglycemia. The admission of a greater number of infants to the NICU prior to discharge in the historical-control group explains the longer LOS in the historical-control group compared to the calculator group, despite a delay in the initiation of antibiotics in the calculator group.
The NSC was developed using only antepartum temperature, and postpartum fever was not evaluated specifically in the risk prediction model.15 Therefore, we used antepartum temperature for the risk calculation for infants born to mothers with postpartum chorioamnionitis. Although this study had a small number of infants born to mothers with postpartum chorioamnionitis, we explored the NSC recommendations and outcomes for these infants, which has not been done in previous studies. However, we found that all the infants born to mothers with postpartum chorioamnionitis were well-appearing, and the NSC did not recommend blood culture or antibiotics in both the historical-control group and the calculator group. Furthermore, there were no NICU admissions in infants born to mothers with postpartum chorioamnionitis in either group. Larger studies on infants born to mothers with postpartum chorioamnionitis are required to ascertain whether the NSC, which uses antepartum temperature, is reliable to screen EOS among such infants.
This study has several limitations. This was a quality improvement study, and as such, stringent statistical analysis was not performed, thus limiting the ability to address type I and II errors. Histopathological confirmation of chorioamnionitis could not be performed, as placental pathology was not uniformly obtained in all cases with chorioamnionitis. The relative rarity of positive blood cultures after implementation of the NSC limits validation of the NSC. Changing the electronic medical record vendor in the historical-control group and the low percentage of follow-up data in the calculator group impaired our ability to ascertain follow-up data on all the cases. Finally, this study was performed in a single center with a relatively small number of infants.
CONCLUSIONS
There was a significant decrease in LE and Abx among well-appearing infants born at ≥35 weeks’ gestation to mothers with chorioamnionitis after implementation of the NSC without missing EOS. It would seem that prospective application of the NSC decreased both LE and Abx more than projected by retrospective application in the historical-control group in the same birth center. There was no increase in neonatal intensive care unit admission or length of hospital stay in those infants who received antibiotics later after they appeared equivocal or clinically ill in the calculator group. Larger prospective studies including follow up after discharge are needed to confirm that EOS is not missed.
Patient-Friendly Recap.
Infants born to mothers with chorioamnioitis are at high risk of early-onset sepsis, yet overuse of antibiotics to prevent infection may lead to potential adverse effects such as the development of inflammatory bowel disease later in life, an increase in antimicrobial resistance, etc.
The authors investigated whether application of a neonatal early-onset sepsis calculator among well-appearing infants born at ≥35 weeks’ gestation to mothers with chorioamnionitis decreases lab evaluations and antibiotic treatments without missing early-onset sepsis.
There was a significant decrease in lab evaluations and antibiotic treatments, but larger studies that include follow ups are needed to corroborate these results.
Acknowledgments
I would like to thank our biostatistician, Kritika Garg, for statistical help, Christopher Kabir and Jessica Kram for their help with the scientific writing, and all the pediatricians and registered nurse practitioners from the Advocate Aurora Health ACH PR mother-baby unit for putting the neonatal early-onset sepsis calculator into practice.
Footnotes
Author Contributions: Study design: Bajracharya & Prazad. Data acquisition or analysis: all authors. Manuscript drafting: Bajracharya. Critical revision: Bajracharya, Prazad, & Bennett.
Conflicts of Interest: None.
References
- 1.Weston EJ, Pondo T, Lewis MM, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30:937–41. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–26. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polin RA Committee on F Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129:1006–15. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health, Panel C-TG; 2020. https://www.ncbi.nlm.nih.gov/books/NBK570371/ [PubMed] [Google Scholar]
- 5.Verani JR, McGee L, Schrag SJ, et al. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1–36. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5910a1.htm . [PubMed] [Google Scholar]
- 6.ACOG Committee Opinion No. 485: Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2011;117:1019–27. doi: 10.1097/AOG.0b013e318219229b. [DOI] [PubMed] [Google Scholar]
- 7.Brady MT, Polin RA. Prevention and management of infants with suspected or proven neonatal sepsis. Pediatrics. 2013;132:166–8. doi: 10.1542/peds.2013-1310. [DOI] [PubMed] [Google Scholar]
- 8.Mukhopadhyay S, Eichenwald EC, Puopolo KM. Neonatal early-onset sepsis evaluations among well-appearing infants: projected impact of changes in CDC GBS guidelines. J Perinatol. 2013;33:198–205. doi: 10.1038/jp.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuzniewicz MW, Walsh EM, Li S, et al. Development and implementation of an early-onset sepsis calculator to guide antibiotic management in late preterm and term neonates. Jt Comm J Qual Patient Saf. 2016;42:232–9. doi: 10.1016/s1553-7250(16)42030-1. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay S, Dukhovny D, Mao W, et al. 2010 perinatal GBS prevention guideline and resource utilization. Pediatrics. 2014;133:196–203. doi: 10.1542/peds.2013-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitre E, Susi A, Kropp LE, et al. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. 2018;172:e180315. doi: 10.1001/jamapediatrics.2018.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shouval DS, Rufo PA. The role of environmental factors in the pathogenesis of inflammatory bowel diseases: a review. JAMA Pediatr. 2017;171:999–1005. doi: 10.1001/jamapediatrics.2017.2571. [DOI] [PubMed] [Google Scholar]
- 13.Puopolo KM, Draper D, Wi S, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011;128:e1155–63. doi: 10.1542/peds.2010-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escobar GJ, Puopolo KM, Wi S, et al. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks’ gestation. Pediatrics. 2014;133:30–6. doi: 10.1542/peds.2013-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neonatal Early-Onset Sepsis Calculator. [Accessed July 30, 2022];Kaiser Permanente Research website. https://neonatalsepsiscalculator.kaiserpermanente.org/InfectionProbabilityCalculator.aspx . [Google Scholar]
- 16.Kuzniewicz MW, Puopolo KM, Fischer A, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr. 2017;171:365–71. doi: 10.1001/jamapediatrics.2016.4678. [DOI] [PubMed] [Google Scholar]
- 17.Stipelman CH, Smith ER, Diaz-Ochu M, et al. Early-onset sepsis risk calculator integration into an electronic health record in the nursery. Pediatrics. 2019;144:e20183464. doi: 10.1542/peds.2018-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson E, Akangire G, Bohning J, et al. Neonatal sepsis calculator in reduction of antibiotics use in EOS (early onset sepsis) Pediatrics. 2018;142(1_MeetingAbstract):401. doi: 10.1542/peds.142.1MA5.401. [DOI] [Google Scholar]
- 19.Puopolo KM, Benitz WE, Zaoutis TE, et al. Management of Neonates Born at ≥35 0/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics. 2018;142:e20182894. doi: 10.1542/peds.2018-2894. [DOI] [PubMed] [Google Scholar]
- 20.Puopolo KM, Lynfield R, Cummings JJ, et al. Management of infants at risk for group B streptococcal disease. Pediatrics. 2019;144:e20191881. doi: 10.1542/peds.2019-1881. [DOI] [PubMed] [Google Scholar]
- 21.Dhudasia MB, Flannery DD, Pfeifer MR, et al. Updated guidance: prevention and management of perinatal group B streptococcus infection. Neoreviews. 2021;22:e177–e88. doi: 10.1542/neo.22-3-e177. [DOI] [PubMed] [Google Scholar]
- 22.Committee Opinion No. 712: Intrapartum Management of Intraamniotic Infection. Obstet Gynecol. 2017;130:e95–e101. doi: 10.1097/AOG.0000000000002236. [DOI] [PubMed] [Google Scholar]
- 23.Money N, Newman J, Demissie S, et al. Anti-microbial stewardship: antibiotic use in well-appearing term neonates born to mothers with chorioamnionitis. J Perinatol. 2017;37:1304–09. doi: 10.1038/jp.2017.137. [DOI] [PubMed] [Google Scholar]
- 24.Carola D, Vasconcellos M, Sloane A, et al. Utility of early-onset sepsis risk calculator for neonates born to mothers with chorioamnionitis. J Pediatr. 2018;195(e1):48–52. doi: 10.1016/j.jpeds.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 25.Coleman C, Carola DL, Sloane AJ, et al. A comparison of triple I classification with neonatal early-onset sepsis calculator recommendations in neonates born to mothers with clinical chorioamnionitis. J Perinatol. 2020;40:1308–14. doi: 10.1038/s41372-020-0727-9. [DOI] [PubMed] [Google Scholar]
- 26.Hershkovich-Shporen C, Ujirauli N, Oren S, et al. Not all newborns born to mothers with clinical chorioamnionitis need to be treated. J Matern Fetal Neonatal Med. 2021;34:1949–54. doi: 10.1080/14767058.2019.1651281. [DOI] [PubMed] [Google Scholar]
- 27.Sloane AJ, Carola DL, Lafferty MA, et al. Management of infants born to mothers with chorioamnionitis: a retrospective comparison of the three approaches recommended by the committee on fetus and newborn. J Neonatal Perinatal Med. 2021;14:383–90. doi: 10.3233/NPM-200531. [DOI] [PubMed] [Google Scholar]