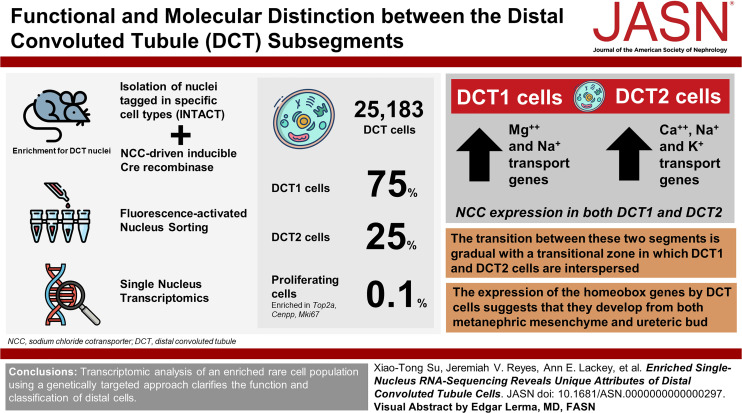
Visual Abstract
Keywords: distal tubule, ion transport, kidney tubule
Abstract
Significance Statement
High-resolution single-nucleus RNA-sequencing data indicate a clear separation between primary sites of calcium and magnesium handling within distal convoluted tubule (DCT). Both DCT1 and DCT2 express Slc12a3, but these subsegments serve distinctive functions, with more abundant magnesium-handling genes along DCT1 and more calcium-handling genes along DCT2. The data also provide insight into the plasticity of the distal nephron-collecting duct junction, formed from cells of separate embryonic origins. By focusing/changing gradients of gene expression, the DCT can morph into different physiological cell states on demand.
Background
The distal convoluted tubule (DCT) comprises two subsegments, DCT1 and DCT2, with different functional and molecular characteristics. The functional and molecular distinction between these segments, however, has been controversial.
Methods
To understand the heterogeneity within the DCT population with better clarity, we enriched for DCT nuclei by using a mouse line combining “Isolation of Nuclei Tagged in specific Cell Types” and sodium chloride cotransporter–driven inducible Cre recombinase. We sorted the fluorescently labeled DCT nuclei using Fluorescence-Activated Nucleus Sorting and performed single-nucleus transcriptomics.
Results
Among 25,183 DCT cells, 75% were from DCT1 and 25% were from DCT2. In addition, there was a small population (<1%) enriched in proliferation-related genes, such as Top2a, Cenpp, and Mki67. Although both DCT1 and DCT2 expressed sodium chloride cotransporter, magnesium transport genes were predominantly expressed along DCT1, whereas calcium, electrogenic sodium, and potassium transport genes were more abundant along DCT2. The transition between these two segments was gradual, with a transitional zone in which DCT1 and DCT2 cells were interspersed. The expression of the homeobox genes by DCT cells suggests that they develop along different trajectories.
Conclusions
Transcriptomic analysis of an enriched rare cell population using a genetically targeted approach clarifies the function and classification of distal cells. The DCT segment is short, can be separated into two subsegments that serve distinct functions, and is speculated to derive from different origins during development.
Introduction
The mammalian kidneys play a critical role in regulating electrolyte balance and maintaining BP. At least 14 kidney tubule segments have been described within the nephron, of which the distal convoluted tubule (DCT) is the shortest; yet this short segment plays a crucial role in various homeostatic processes, including sodium, potassium, calcium, and magnesium handling. The DCT comprised two subsegments commonly called DCT1 and DCT2 (or early and late DCT), with different functional and molecular characteristics.1–3 The functional and molecular distinction between these segments, however, has been controversial4 and inconsistently delineated within single-cell transcriptomic datasets from kidney tissue.5–8 This likely reflects the fact that DCT cells comprise only a small percentage of kidney cortical tissue. The best study to date by Chen and colleagues, therefore, enriched for DCT cells using cell surface markers, thus clearly identifying DCT1 and DCT2 cells 9; in that study, however, the proportions of DCT1 and DCT2 cells were not similar to proportions reported using more standard approaches.10,11
They also identified a cluster of DCT-derived cells with features consistent with a proliferative phenotype.9 The “distal tubule,” including the DCT, connecting tubule, and cortical collecting duct, is embryologically unique, as it develops from fusion of the nephrogenic blastema with the ureteric bud. Fate mapping experiments suggest that cells from a distal nephron lineage reprogram during development to resemble cells of ureteric lineage, through a process of mutual induction.12 This origin may explain the difficulty, despite decades of work, in establishing a single site at which nephron and bud join, but it also has been suggested as a reason that this segment is uniquely plastic, in response to physiological perturbations. To date, the failure to recapitulate fusion of nephron and bud has been a limitation toward kidney organoid development.13
As the DCT comprises only a small percentage of renal cortical cells, the information gained from single-cell analysis of whole kidney tissue is often limited. Prior efforts to characterize DCT cells have been limited by the rarity of these cells within the kidney. To address this, Chen et al. enriched for DCT cells using a cell surface marker–based fluorescence-activated cell sorting protocol and analyzed the enriched distal nephron population using the single-cell RNA-sequencing (scRNA-Seq); they not only confirmed the existence of DCT1 and DCT2 but also found a population of cells that were enriched in cell cycle–associated and cell proliferation–associated genes.9 Of note, however, the proportion of DCT1 to DCT2 was 13:1 in their study, which differs from the ratio observed in morphologic studies.11 Thus, we used a highly enriched population of DCT nuclei generated using the Isolation of Nuclei Tagged in specific Cell Types (INTACT) system for single-nucleus RNA-sequencing (snRNA-Seq) to understand the heterogeneity within the DCT population with better clarity.
Methods
Study Approval
Animal studies were approved by the Oregon Health and Science University IACUC (protocol IP00286) or by the German Animal Welfare Regulation Authorities (G0148/18).
Mouse Models
All mice were housed under standard conditions, under light/dark cycle (12:12 hours), with free access to food and drinking water. Sodium chloride cotransporter (NCC)-Cre-INTACT mice were used for snRNA-Seq experiments. The Cre/LoxP technology was used to label DCT nuclei with a nuclear green fluorescent protein (GFP), which allowed for subsequent fluorescence-activated nuclei sorting (FANS) enrichment. This system, called INTACT, fluorescently labels the nuclei from genetically targetable populations of cells. This is achieved by tagging the C-terminus of SUN1, a nuclear membrane protein, with two tandem copies of superfolder GFP (sfGFP) and six copies of the Myc epitope (SUN1-sfGFP-Myc).14,15 The INTACT mice were crossed with the NCC-CreERT2 mice16 to create a mouse line expressing sfGFP at the inner nuclear membrane in DCT cells on tamoxifen induction. Three female NCC-Cre-INTACT mice were i.p. injected with 1 mg tamoxifen dissolved in corn oil daily for 5 days followed by a 10-day induction period to induce the sfGFP expression. Water was provided ad libitum.
Nuclei Isolation from Mouse Kidney Cortex and FANS
Kidneys were removed after PBS washout via aortic perfusion, and the cortex was dissected and then snap-frozen in liquid nitrogen. Frozen tissue was stored at −80°C until subsequent tissue processing. We modified from the 0.03% Tween 20 detergent, salts, and Tris buffer (INNER cell) nuclei extraction17 and the kidney nuclei isolation protocol.5,18 The nuclei isolation buffer (NIB) contains 146 mM NaCl, 10 mM Tris-HCl (pH 7.5), 1 mM CaCl2, 21 mM MgCl2, 0.03% Tween-20, 0.01% BSA, and one tablet of cOmplete ULTRA per 10 ml NIB. The samples were ground for 30 times in 2 ml NIB1 (4 ml NIB+20µl RNasin Plus+20µl SUPERaseIN) with a 2 ml Dounce grinder and a loose pestle, and then, homogenate was passed through a 200 µm strainer. The homogenate was ground for 15 times with a tight pestle, and then, 2 ml NIB1 was added, and the homogenate was incubated for 5 minutes on ice. The homogenate was passed through a 40 µm strainer and then centrifuged at 500×g for 5 minutes at 4°C. The pellet was resuspended in 4 ml NIB2 (4 ml NIB+4 µl RNasin Plus+4 µl SUPERaseIN), and the suspension was incubated on ice for 5 minutes and then centrifuged at 500×g for 5 minutes at 4°C. The pellet was resuspended in 1.5 ml of nuclei resuspension buffer (10 ml Dulbecco's Phosphate-Buffered Saline +10 µl RNasin Plus) and then centrifuged at 500×g for 5 minutes at 4°C. Repeat this step, and then, the suspension was passed through a 5 µm strainer. The suspension was centrifuged at 500×g for 5 minutes at 4°C, and the pellet was resuspended in 10 ml NSB. The nuclei suspension was mixed with 5 µl Vybrant Ruby stain and incubated on ice for 15 minutes. The nuclei were sorted in 500 µl final resuspension buffer (1 ml Dulbecco's Phosphate-Buffered Saline with 1% BSA+5 µl Protector RNase inhibitor) with a low flow rate and pressure to ensure high viability. Two main gates, 488 (GFP, 530/40 filter) and 561+683 (Vybrant DyeCycle Ruby, 670/40 filter), were used. Low trigger pulse width was used as singlet discriminator. One hundred thousand nuclei were collected and centrifuged at 500×g for 5 minutes at 4°C. The top supernatant was carefully removed, and 50 µl volume was left to resuspend the nuclei pellet. Ten microliters of nuclei were mixed with 10 µl Trypan Blue and then loaded on the Fuchs-Rosenthal disposable hemocytometer for counting. Nuclei at 700–1200 nuclei/µl were directly loaded to 10× chips for gel bead-in emulsions generation.
SnRNA-Seq
The snRNA-Seq was performed using a Chromium Next gel bead-in emulsion Single-Cell 3′ Reagent Kit v3.1 (10× Genomics). Single nuclei were partitioned in droplets with single gel beads, which contained primers with cell-tagging indexes. Single-nucleus suspensions with concentration of at least 300/µl were loaded targeting 10,000 nuclei per sample. The resulting cDNA was profiled on a Bioanalyzer NanoChip (Agilent) and then used as a template for library preparation according to the reagent kit protocol. The final libraries were profiled on a TapeStation D1000 tape (Agilent) and quantified using real-time PCR (Kapa Biosystems) on a StepOnePlus real-time PCR workstation (Thermo/ABI). Libraries were sequenced on a NovaSeq 6000 (Illumina). FASTQ files were prepared using bcl2fastq (Illumina) and then aligned to a reference genome using Cell Ranger v. 6.1.2 (10× Genomics). Reads were mapped to both exonic and intronic regions to include the pre-mRNA transcriptome. Library preparation and sequencing were done by the OHSU Integrated Genomics Laboratory, RRID:SCR_022651.
SnRNA-Seq Data Analysis
Ambient RNA, Doublet, and Mitochondrial Feature Removal
Ambient RNA contamination was estimated and removed using SoupX v. 1.6.1.19 Cells with cleaned-up reads were then subjected to doublet removal using DoubletFinder.20 Mitochondrial features were manually removed by excluding all features starting with mt−.21 These processing steps were performed separately for each sample. Count matrices now only containing singlets were loaded to Seurat v. 4.0 for merging and further filtering.22 Nuclei with the features >4000 or <500 and counts >10,000 were considered low-quality nuclei and were filtered out. The filtered Seurat object was then inputted to SCTransform for normalization and variance stabilization.23 The normalized count matrix was integrated using Seurat's built-in FindIntegrationAnchors. In total, 7466 (23%) nuclei were filtered out from a total of 32,649 nuclei within the snRNA-Seq dataset because they were suspected to be heterotypic doublets that contained one DCT nucleus and another cell type nucleus. This was supported by the decreased expression of NCC in these clusters, in addition to the clusters having increased genetic complexity and elevated nFeature_RNA.
Batch Effect Correction and Cell Clustering
Dimensional reduction analysis was performed using principal component analysis. Clusters were generated using the built-in FindNeighbors and FindClusters Seurat commands using the first 50 principal component analysis dimensions with a 0.15 clustering resolution. The uniform manifold approximation and projection technique was used for 2D projection and visualization. Clusters were annotated based on selected canonical gene markers and the top five genes in the cluster as defined using FindAllMarkers. DCT clusters, namely DCT1 (early DCT), DCT2 (late DCT), and ProLIF (proliferating cell population), were manually separated and reintegrated for downstream analyses. The final Seurat object from three animals had a total of 21,280 features from 25,183 nuclei.
SnRNA-Seq Differential Gene Analysis
Differential gene expression analysis was done with FindMarkers using the normalized and corrected values stored in the sct slot using the DESeq2 mode.24 The final differentially expressed gene (DEG) list was filtered based on minimum detection rate (min.pct) at 10%, average fold difference of 1.5 (log fold difference >0.3219), and adjusted P value of 0.05.
Cellular Identity and Functional Scores
Using Seurat's AddModuleScore function, we computed scores using the average expression levels of a set of genes on a single-cell level. DCT1 score was computed from the average expression of the top ten genes (Erbb4, Egf, Trpm7, Fgf13, Col5a2, Umod, Ptgfr, Stk32b, Rtl4, Abca13) distinguishing DCT1 from DCT2 based after running FindMarkers in our control dataset. The same was done to compute for the DCT2 score using the following gene set—Slc8a1, Arl15, Calb1, Slc2a9, Phactr1, Gls, S100g, Kl, Klk1, and Egfem1. Mg score was computed from the average expression of a curated list of known magnesiotropic genes expressed in the DCT that have reported magnesium derangement phenotype, namely Trpm6, Trpm7, Egf, Umod, Prox1, Fxyd2, Cnnm2, and Slc41a3.25–33 Ca score was computed from the average expression of a curated list of known calciotropic genes expressed in the DCT that have reported calcium derangement phenotype, namely Trpv5, Calb1, Slc8a1, S100g, Vdr, and Ryr2.34–40
Data Visualization
Dimension, feature, dot, and violin plots were generated from built-in Seurat visualization functions. Score density plots were generated using the density function of ggplot2.41 Kernel density estimation was performed to fit a smooth curve reflecting the histogram of the module score distribution.
Pseudotime Analysis
The Seurat object was converted to a Monocle cell dataset using SeuratWrappers.42–45 Pseudotime was computed using monocle3 and was used to plot nuclei into a normalized expression heatmap.
Pathway Analysis
The DEG list was subjected to overrepresentation analysis using the enrichGo function of ClusterProfiler.46 Gene symbols were converted to ENTREZIDs using the Biological ID translator feature. Upregulated pathways were defined as pathways corresponding to the genes with actual log fold difference of >1.25 and downregulated pathways with values <−1.25.
Immunofluorescence
Mice were anesthetized with a ketamine–xylazine–acepromazine cocktail (50:5:0.5 mg/kg). The right kidney was tied off, removed, and flash-frozen in liquid nitrogen for isolation of protein. The left kidney was perfusion fixed with retrograde abdominal aortic perfusion of 3% paraformaldehyde in PBS (pH 7.4). After perfusion, the left kidney was removed, dissected, and cryopreserved in 800 mOsm sucrose in PBS overnight before being embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek, Torrance, CA). Slides were prepared by cutting 5 µm sections and were stored at −80°C until use. Immunofluorescent staining was prepared as follows. Slides were incubated with 0.5% Triton X-100 in PBS for 30 minutes. Sections were then blocked with 5% milk in PBS for 30 minutes followed by incubation with primary antibody, diluted in blocking buffer, for 1 hour at room temperature or overnight at 4°C. Sections were washed with PBS three times and incubated with fluorescent dye-conjugated secondary antibody, diluted in blocking buffer, for 1 hour at room temperature. Sections were washed with PBS three times and stained with 4',6-diamidino-2-phenylindole before being mounted with ProLong Diamond Antifade Mountant (Thermo Fisher Scientific, Carlsbad, CA). For microdissected tubule immunofluorescence, kidneys were perfused and digested in L-15 medium with 1 unit/ml type II collagenase at 37°C for 30 minutes. The microdissected tubules were transferred to 5×5-mm cover slip coated with poly-D-lysine. The tubules were fixed with formalin for 30 minutes on ice and stained following the same protocol described above. Images were captured using a KEYENCE BZ-X800 microscope (Itasca, IL). To quantify the NCC-Cre recombination rate, cells were counted in three separate randomly selected images from each animal (n=3).
For high-resolution images, 4 μm thick sections were cut, mounted on glass slides, and deparaffinized. Heat-induced epitope retrieval was performed for 6 minutes by cooking slides in citrate buffer (10 mM sodium citrate, pH 6.0) using a pressure cooker. Sections were blocked with 5% BSA in TBS for 30 minutes at room temperature and incubated with primary antibodies dissolved in 1% BSA/TBS overnight at 4°C. After washing in TBS, fluorescently labeled secondary antibodies were dissolved in 1% BSA/TBS and incubated for 1 hour at room temperature. Sections were washed in TBS and mounted in PBS-glycerol (1:9). Nuclei were stained with 4',6-diamidino-2-phenylindole. Images were acquired using a Zeiss laser scanning microscope 5 Exciter confocal microscope (laser scanning microscope) equipped with Imager.M1 and a NeoFluar objective lens (63×/Numerical Aperture 1.40). The laser lines used were 405, 488, 543, and 633 nm. Sections were examined in fluorescence microscopy under a Zeiss Axio Imager Z2 LM equipped with an ApoTome2 structured illumination acquisition system and a Plan-Apochromat 20x/0.8 objective using Zeiss ZEN 2012 software (blue edition). Image processing was completed using FIJI software (National Institutes of Health, Bethesda, MD) or BZ-X800 Analyzer (KEYENCE).
Antibodies and Chemicals
A list of antibodies and chemicals, including vendor and catalog numbers, is presented in Supplemental Table 1.
Data Availability
Sequencing data are deposited in Gene Expression Omnibus under accession number GSE228367. To ensure data accessibility to nonbioinformaticians, we made the DCT snRNA-Seq data available for further exploration via an interactive web tool generated using ShinyCell47 at https://ellisonlab.shinyapps.io/dct_shinycell/.
Code Availability
All analysis code is available on GitHub at https://github.com/OHSU-NHT/Su_dct_2023.
Results
Enriched snRNA-Seq Confirms Segmentation of the DCT
To determine the heterogeneity of DCT cells, we took advantage of single-cell transcriptomics and performed snRNA-Seq. To enable more robust enrichment of nuclei from DCT cells and introduce less processing artifacts, thereby increasing the cytologic resolution, we bred a DCT-specific inducible NCC-Cre recombinase mouse line16 with the INTACTflx/flx15 mouse line to generate DCT-INTACT mice. As reported previously, the NCC-Cre only drives recombination in DCT cells on induction.16 Confirmed with immunofluorescence, these mice express a GFP conjugated to the nuclear envelope protein Sun1 in cells that also express NCC (Figure 1A), but not in AQP2-expressing cells or AQP2-negative intercalated cells within connecting tubule/cortical collecting duct (Figure 1B). The Cre recombination rate, determined as the ratio of GFP+-NCC+ cells to total NCC+ cells after tamoxifen induction, is 99.8%±0.04% (n=3). A small proportion of GFP+/NCC− cells (1.1%±0.3%, n=3) may be attributed to sectioning artifacts, as most GFP+/NCC− cells are from NCC+ tubules (Figure 1A, Supplemental Figure 1). The nuclei are large subcellular structures, and it is possible that the section only contains the nuclei but not the apical membrane; the possibility of leakiness during early development, however, cannot be excluded. To determine the leakiness, three images were taken from two vehicle-treated DCT-INTACT mice (n=2). The amount of leakiness was determined as the ratio of GFP+/NCC+ cells to total NCC+ cells. There was minimal leakage of Cre-ERT2 activity with only 7.4%±1.4% (n=2) of DCT cells containing GFP in the absence of tamoxifen induction. Importantly, GFP+ nuclei are only found in NCC+ cells/tubules (Supplemental Figure 2). In addition, the expression of GFP is marginal when not induced with tamoxifen. We isolated nuclei from flash-frozen kidneys of the induced DCT-INTACT mice by combining approaches from Humphreys and Regev5,17,18 and then enriching for DCT nuclei using FANS of the signal from GFP and DNA (Figure 1, C and D and Supplemental Figure 3). We confirmed the integrity of the nuclei following FANS (Figure 1E) before processing through the 10× chromium controller for sequencing.
Figure 1.

Enriched DCT snRNA-Seq confirms the DCT1 and DCT2 segmentation. (A) Immunofluorescence image showing pNCC (red) and GFP (green) colocalization. A small proportion of GFP+/NCC− cells may be attributed to sectioning artifacts (arrow), although the possibility of leakiness during early development cannot be excluded. Nuclei are stained using DAPI in blue. (B) Immunofluorescence image showing AQP2 (red) and GFP (green) colocalization. GFP do not express in AQP2+ cells or intercalated cells within connecting tubule/cortical collecting duct (arrowhead). Nuclei are stained using DAPI in blue. (C and D) Example of GFP+-nuclei sort gating. (C) The y axis is Vybrant Ruby stain (DNA dye) emission; x axis is GFP emission. (D) All GFP+-singlet nuclei were collected (also shown in Supplemental Figure 3). (E) Image showing sorted GFP+-nuclei at high magnification revealing green INTACT (GFP) signals on the surface and blue DNA (DAPI) signals completely enclosed within the nuclei, indicating the healthy integrity of the sorted nuclei. (F) UMAP projection displaying nuclei from three 10× chromium control mouse DCT datasets. There are three populations within all DCT nuclei, DCT1, DCT2, and a proliferating population (Prolif). DAPI, 4',6-diamidino-2-phenylindole; DCT, distal convoluted tubule; GFP, green fluorescent protein; INTACT, Isolation of Nuclei TAgged in specific Cell Types; NCC, sodium chloride cotransporter; snRNA-Seq, single-nucleus RNA-sequencing; UMAP, uniform manifold approximation and projection.
Targeting sequencing 10,000 nuclei per animal led to average recovery of 12,513 nuclei/animal with an average of the median gene number per nucleus of 1843 and an average of mean reads per nucleus of 23,878 (Supplemental Table 2). Following analysis through the Seurat pipeline that included doublet removal with DoubletFinder, ambient RNA removal with SoupX, normalization with scTransform, and integration with Seurat, we generated a 2D reduction of the first 20 principal components using uniform manifold approximation and projection to represent cellular clustering. There were additional populations that expressed markers that define other segments of the nephron in addition to Slc12a3 (Supplemental Figure 4). On the basis of this incongruent marker expression and level of gene per nucleus (Supplemental Figure 5), we suspect that these were heterotypic doublet nuclei and manually filtered them before proceeding with the subsequent analysis. It is important to note that our experimental approach captured an uncommonly high number of doublets (23%). We suspect that the high number of doublets was the result of a liberal FANS sorting strategy and could be largely reduced with a more stringent sorting strategy.
After filtering suspected doublet populations, we observed three distinct populations of distal convoluted cells from DCT-INTACT mice that include DCT1, DCT2, and proliferating cells (Figure 1F and Supplemental Figure 6) similar to the distal tubule scRNA-Seq dataset that has previously been reported by Chen et al.9 We directly compared the list of DEGs that define DCT1, DCT2, and proliferating cells between our nuclei-based strategy from their cell-based strategy. The widely accepted DCT1-specific marker, parvalbumin (Pvalb), is highly expressed in DCT1 scRNA-Seq datasets9 but is very low in our snRNA-Seq dataset. This is consistent with other published snRNA-Seq datasets from both human6,48 and mouse5,49 (on the basis of our analysis on these datasets) and may reflect differences in the cytosolic and nuclear pool of Pvalb mRNA. The highly expressed genes that are common in both studies include Egf, Umod, Trpm7, Col5a2, and Ptgfr in DCT1; Slc8a1, Calb1, S100g, Klk1, and Trpv5 in DCT2; and Cenpf, Top2a, and Mki67 in proliferating cells. There is a strong correlation between the same populations in both the enriched DCT snRNA-Seq dataset and the targeted distal tubule scRNA-Seq dataset (Supplemental Figure 7, A and B) leading us to believe that these are the same populations of cells. Yet, in contrast to Chen et al., we found the proportion of DCT1 to DCT2 to be 3:1 rather than 14:1 as they have reported. One possible explanation for the different ratio between DCT1 and DCT2 cells in Chen et al. is that the positive selection marker (Emb) that they used for distal tubule enrichment has higher expression in DCT1 than DCT2 (Supplemental Figure 7C). The ratio of 3:1 is similar to that measured by Tahaei and colleagues11 using immunohistochemical techniques, suggesting that DCT-INTACT approach captures an accurate representation of the proportion of DCT1 and DCT2 within kidney tissue.
Functional and Developmental Heterogeneity along the DCT Is Revealed by Enriched snRNA-Seq
The embryologic origins of cells along the distal nephron have remained controversial, but single-cell data from Ransick and colleagues suggested the existence of transitional cells.12 To explore the molecular heterogeneity of DCT1 and DCT2 populations and to provide information about its functional implications, we determined the top ten DEGs by fold difference that distinguish DCT1 from DCT2 (Figure 2A). By contrast, the proliferating cell population has lower expression of these top ten DEGs, but higher expression of cell cycle machinery and proliferative genes, including Top2a, Cenpp, Cep128, Knl1, and Mki67 (Figure 2A and Supplemental Table 3). We amalgamated the expression of the top ten DEGs for DCT1 (Erbb4, Egf, Trpm7, Fgf13, Col5a2, Umod, Ptgfr, Stk32b, Rtl4, Abca13) and DCT2 (Slc8a1, Arl15, Calb1, Slc2a9, Phactr1, Gls, S100g, Kl, Klk1, Egfem1) using the “AddModuleScore” function in Seurat to generate a DCT1 or DCT2 score. The visualization of this score shows clear distinction between DCT1 and DCT2 cells (Figure 2, B and C). We also calculated the pseudotime using monocle 3; pseudotime is a measure of how much progress an individual cell has made through a process, such as cell differentiation.42–45 In many biologic processes, cells do not progress in perfect synchrony, but transition from one state to the next according to their progress along a learned trajectory.42–45 Put simplistically, pseudotime is the distance between a cell and the start of the trajectory. The trajectory within DCT is continuous starting within DCT1 and progressing to DCT2 (Figure 2D), suggesting that DCT2 is more differentiated than DCT1 or proliferating DCT cells. The expression of the DCT1 or DCT2 score driving genes is plotted against pseudotime as z-score (Figure 2, E and F). These results indicate that although DCT1 and DCT2 have separate functions, the transition between them is gradual.
Figure 2.

The transition between DCT1 and DCT2 is gradual. (A) Dot plot showing the expression of top ten genes in DCT1, DCT2, and proliferating cells across all clusters. DCT1 and DCT2 have different signatures in the top genes' expression; proliferating cells are enriched in cell cycle/proliferation-related genes. Data are normalized and scaled (z-score) to examine relative expression across the cell clusters; “average expression” is the z-score of the average gene expression of all cells within a cluster (scaled values); and “percent expressed” is the percentage of cells with nonzero gene expression. (B and C) UMAP projection for DCT1 (B) and DCT2 (C) score expression in all nuclei. The color indicates the expression of scores. DCT1 score is higher in DCT1 and there is a gradient of DCT1 score within DCT1 cells, which is similar in DCT2 score. (D) UMAP projection illustrating pseudotime. The labeled “1” within a circle represents the cell at time “0” or the “roots” of the trajectory. Black lines delineate the graph structure. Within DCT1 cells, there is a lower degree of transcriptional change from the initial to the final state, while DCT2 cells exhibit more significant changes. (E and F) Bar plots displaying the z-scores of the top ten genes' expression for DCT1 (E) and DCT2 (F) along the pseudotime (from DCT1 to DCT2). A gradient of gene expression is observed across all DCT cells rather than a discrete pattern. (G and H) Immunofluorescence images of microdissected tubules (G) and thin sections (H). In these images, pNCC (red) labels the entire DCT, parvalbumin (green) specifically marks DCT1, and calbindin (magenta) labels DCT2 and connecting tubule. Arrowheads indicate DCT2 cells in the DCT1 and DCT2 transition segment, while arrows point to DCT1 cells in that segment. Notably, there is not a clear border between DCT1 and DCT2; instead, a transitional segment exists where these cells are interspersed.
Although transcriptomics provides high-resolution information about cell properties, it does not provide information about spatial cell location in the tubule segments. To study the transition between DCT1 and DCT2, immunostaining in microdissected tubules and thin sections was applied. The more proximal thick ascending limb (TAL) and DCT have distinct morphologic and molecular features; immunostaining in microdissected tubules showed a sharp border between TAL and DCT (Figure 2G). By contrast, immunofluorescence in microdissected tubules (Figure 2G) and thin sections (Figure 2H) revealed that DCT1 and DCT2 do not have a clear border. Parvalbumin (Pvalb, DCT1 marker) expression is high in DCT1 and gets weaker and spotty toward DCT2. In the “transition zone” between DCT1 and DCT2, parvalbumin and calbindin (DCT2 and connecting tubule marker) expression is intermingled, confirming that the transition between DCT1 and DCT2 is gradual.
DCT2 (with the connecting tubule) is the major site for active transcellular calcium reabsorption and exhibits electrogenic Na and K transport.50,51 By contrast, magnesium transport has generally been described as occurring throughout the DCT.52 Our high-resolution transcriptomic data, however, indicate a separation between primary sites of calcium and magnesium handling, with more abundant magnesium-handling genes along DCT1 (Figure 3A and Supplemental Figure 8A) and more abundant for calcium-handling genes along DCT2 (Figure 3B and Supplemental Figure 8B). As previously suggested, NCC (Slc12a3) and its regulatory factors are more highly expressed by DCT1 cells, whereas both NCC and epithelial sodium channel (ENaC) as well as ENaC regulatory genes are expressed along DCT2 (Figure 3C and Supplemental Figure 8C). Consistent with others' reports,9,53,54 the alpha subunit of ENaC is widely expressed along the distal nephron, including DCT1 cells. Immunofluorescence of EGF, TRPM6, NCX1, and KLK1 showed a consistent expression pattern indicating that EGF and TRPM6 are more highly expressed in DCT1, whereas NCX1 and KLK1 are more highly expressed in DCT2 (Figure 3E).
Figure 3.

DCT1 and DCT2 have different expression patterns in electrolyte transport proteins. (A–C) Distributions of transcripts associated with magnesium (A), calcium (B), and sodium (C) transport. Genes related to magnesium transport and Slc12a3 (NCC) regulation exhibit higher expression in DCT1, whereas genes linked to calcium transport and ENaC regulation show higher expression in DCT2. Data are normalized and scaled (z-score) to assess relative expression across cell clusters; “average expression” is the z-score of the average gene expression of all cells within a cluster (scaled values); and “percent expressed” is the percentage of cells with nonzero gene expression. (D) Dot plot of Hoxd10 (nephron progenitor origin homeobox gene), Hoxd7 (ureteric bud origin homeobox gene), Emx1 (regulator of distal tubule development), and Sall3 (DCT-selective zinc-finger protein). Distinct expression patterns of these homeobox genes are observed in DCT1, DCT2, and proliferating cells, suggesting mixed origins of DCT cells. (E) Immunofluorescence images depicting EGF, TRPM6, NCX1, and KLK1 colocalization with NCC (DCT), parvalbumin (DCT1), or calbindin (DCT2/connecting tubule). The protein expression pattern corresponds to the gene expression pattern shown in (D). Arrowheads denote DCT2 cells, while arrows indicate DCT1 cells. EGF and TRPM6 exhibit higher expression in DCT1, whereas NCX1 and KLK1 show higher expression in DCT2. (F and G) UMAP projection illustrating magnesium (F) and calcium (G) score expression across all nuclei. Color gradient indicates the expression of the scores. The magnesium score is higher in DCT1 cells, displaying a gradient within DCT1 cells, while the calcium score is higher in DCT2 cells, showing a gradient within DCT2 cells. ENaC, epithelial sodium channel.
To better characterize the expression patterns for genes involved in divalent ion metabolism, we defined a magnesium cassette, with genes relevant to magnesium transport (Trpm6, Trpm7, Egf, Umod, Prox1, Fxyd2, Cnnm2, and Slc41a3),25–33 and a calcium cassette, with genes relevant to calcium transport (Slc8a1, Calb1, Vdr, S100g, Trpv5, and Ryr2).34–40 As we did with the DCT1 and DCT2 scores, we amalgamated the magnesium/calcium cassette gene expression into magnesium and calcium scores. As expected, the magnesium cassette is enriched in DCT1 cells, whereas the calcium cassette is enriched in DCT2 cells (Figure 3, F and G). The DCT1/DCT2/magnesium/calcium scores were shown to be consistent with scores derived for the enriched distal nephron scRNA-Seq dataset9 (Supplemental Figure 9).
The human distal nephron has not been characterized in the detail we have provided here. To compare mouse and human DCT cell transcriptomic profiles, we acquired the human snRNA-Seq data from the Kidney Precision Medicine Project (KPMP).48 Data were downloaded on November 29, 2022. This dataset includes 13 healthy reference, ten patients with CKD, and six patients with AKI. We separated DCT cells (DCT1, DCT2, cycling DCT, and degenerative DCT as in the original KPMP nomenclature) from all cells (Figure 4A and Supplemental Figure 10) and applied the DCT1/DCT2/magnesium/calcium scores to the human DCT cells. Like in mice, DCT1 and magnesium scores are higher in human DCT1 cells than in DCT2 cells, and DCT2 and calcium scores are higher in DCT2 cells than in DCT1 cells (Figure 4, B–E). We then compared the list of DEGs that define DCT1, DCT2, and proliferating cells between mouse and human DCT nuclei and found a significant correlation between DCT1, DCT2, and the proliferating cell (cycling DCT in KPMP human DCT cells) markers (Figure 4, F and G), which confirms that human and mouse DCT segmental functions are organized similarly.
Figure 4.
Human DCT cells from KPMP dataset have similar signatures to mouse DCT cells. (A) UMAP projection displaying all DCT cells from the KPMP snRNA-Seq dataset. The annotations are originally from KPMP. The dDCT stands for degenerative DCT; cysDCT stands for cycling DCT, which is similar to the proliferating DCT cells in our dataset. (B–E) UMAP projection illustrating DCT1 (B), DCT2 (C), magnesium (D), and calcium (E) score expression across all DCT nuclei. The color gradient represents the expression levels of the scores. DCT1 and magnesium scores exhibit higher expression in DCT1 cells, while DCT2 and calcium scores are higher in DCT2 cells, consistent with the mouse data. (F and G) Correlation analysis of cluster-defining DEGs between mouse and human DCT1 versus DCT2 (F) and DCT versus proliferating cells (G). Similarity in DCT1 and DCT2 defining genes between human and mouse indicates a comparable organization of their DCT segmental functions. The x axis displays the average log2 fold change of human DEGs, while the y axis shows the average log2 fold change of mouse DEGs. R2 represents the coefficient of determination. DEGs with an adjusted P value < 0.01 in both mouse and human datasets are highlighted. DEG, differentially expressed gene; KPMP, Kidney Precision Medicine Project.
The origin of cell types along the distal nephron has been controversial. Recent lineage tracing approaches suggest that DCT2 cells may derive from the ureteric bud, although they express canonical markers of more proximal cells.12,55 To explore this, we applied pathway analysis, which is a computational method that determines whether a priori defined set of genes show statistically significant differences.46 The pathway analysis revealed enrichment for well-known DCT1-related pathways, including metal ion transport, Na transport, protein autophosphorylation, and nutrient level response (Figure 5A and Supplemental Figure 11). Interestingly, and in stark contrast with DCT1, transcript enrichment in DCT2 cells predominantly involves renal system development, urogenital system development, and branching morphogenesis of epithelial tubules (Figure 5B and Supplemental Figure 12). The functional importance of these differences will be discussed below.
Figure 5.

Transcripts enriched in DCT1 or DCT2 cells involve distinct pathways. (A and B) DEG pathway analysis performed separately for DCT1 (A) and DCT2 (B). Significant cell-specific DEGs from DCT1 or DCT2 were used for pathway analysis. The displayed number of genes involved in each pathway highlights the top 36 pathways for DCT1 and top 30 pathways for DCT2 (the complete list is available in Supplemental Figures 11 and 12). Noteworthy pathways for DCT1 include ion transport (metal, sodium, etc.), response to nutrient levels, and protein autophosphorylation. Conversely, pathways identified for DCT2 involve kidney/nephron development, calcium homeostasis, and cell proliferation, among others.
Cell Type Transitions along the DCT Are Gradual
Given the postulated dichotomous origins of the DCT and connecting tubule, cell types in these segments have been inferred to be discrete and, at segment junctions, mosaic.12 Yet, transcriptomic work in the brain and other tissues has shown that cell types may transition gradually from one to the other, complicating classification.56 To unmask additional layers of cell type heterogeneity within each population, we separated DCT1 and DCT2 from the mouse DCT dataset (Figure 1D) and reclustered at higher resolution. This analysis confirmed as many as four populations (DCT1 A–D)9 of DCT1 cells can be identified (Figure 6A and Supplemental Figures 13 and 15A). Yet, our high-fidelity transcriptomics showed that, instead of being distinct cell types, these populations express the same core set of genes and are discriminated based on gene expression gradients (Figure 6B). The cells, therefore, transition from more strictly DCT1-like in character to more strictly DCT2-like in character, with DCT1-A having the greatest abundance of DCT1 markers (Egf and Erbb4), and DCT1-B having the greatest abundance of DCT2 markers (Slc8a1 and Calb1). Both magnesium-handling and sodium-handling genes were robustly expressed by DCT1-A through DCT1-D, while calcium-handling genes were expressed predominantly by DCT1-B cells, making DCT1-B cells the most similar to DCT2 cells (Figure 6C and Supplemental Figure 15, B–D).
Figure 6.

DCT1 cells are one cell type, and DCT2 cells have two subtypes. (A) UMPA projection illustrating DCT1 subtypes within all DCT1 nuclei. Four DCT1 subtypes, labeled as DCT1A–D, were identified based on gene expression patterns. (B) Dot plot of top five genes expressed in DCT1A–D. These subpopulations express the same set of genes and are discriminated based on the gene expression gradients. (C) Distributions of transcripts associated with magnesium, calcium, and sodium transport. The cells display a transition from more strictly DCT1-like pattern in gene expression pattern to more strictly DCT2-like pattern. DCT1A–D show similar expression patterns for magnesium and sodium handling, while DCT1-B exhibits greater expression of calcium-handling genes. (D) Dot plot of Hoxd10, Hoxd7, Emx1, and Sall3 in DCT1A–D. These subtypes exhibit varying expression patterns of the homeobox genes, suggesting mixed origins of DCT1 cells. (E) UMPA projection displaying DCT2 subtypes within all DCT2 nuclei. Two DCT2 subtypes, labeled as DCT2-X and DCT2-Y, were identified based on gene expression patterns. (F) Dot plot of top five genes expressed in DCT2-X and DCT2-Y. These populations exhibit different gene expression patterns. (G) Distributions of transcripts associated with magnesium, calcium, and sodium transport. DCT2-X exhibits higher expression of genes related to magnesium and calcium transport, along with Slc12a3 (NCC) regulation; while DCT2-Y displays higher expression in electrogenic sodium transport (ENaC) genes. (H) Dot plot of Hoxd10, Hoxd7, Emx1, and Sall3 in DCT2-X and DCT2-Y. These subtypes exhibit differing expression patterns of the homeobox genes, suggesting mixed origins of DCT2 cells. Data are normalized and scaled (z-score) to examine relative expression across the cell clusters; “average expression” is the z-score of the average gene expression of all cells within a cluster (scaled values); and “percent expressed” is the percentage of cells with nonzero gene expression.
In contrast to the cell type gradients in DCT1, DCT2 cells comprised two patterns, DCT2-X and DCT2-Y (Figure 6, E and F and Supplemental Figures 14 and 15A). Compared with DCT2-Y, DCT-X has a higher expression of Slc12a3 (NCC), NCC regulation-associated genes (including Slc12a3, Wnk1, and Klhl3), magnesium transport-related genes (including Trpm6, Cnnm2, and Egf), and calcium transport–related genes (including Calb1, Slc8a1, and Vdr); whereas DCT2-Y has a higher expression of genes supporting electrogenic sodium transport (including Klk1, Scnn1g, and Hsd11b2) (Figure 6G and Supplemental Figure 15, B–D). These patterns indicate that DCT2-X is involved in divalent cation transport, whereas DCT2-Y, which is enriched in ENaC (Scnn1a), 11-β-dehydrogenase isozyme 2 (Hsd11b2), mineralocorticoid receptor (Nr3c2), and Kallikrein-1 (Klk1), mediates electrogenic Na transport. As snRNA-Seq does not provide spatial data and our immunohistochemistry cannot distinguish cell subtypes, we cannot conclude whether the DCT2-X and DCT2-Y cells are intermingled or anatomically sequential.
Homeobox genes have been considered to be major drivers of morphologic development in animals.57,58 The expression patterns of the 39 mammalian homeobox genes are distinct in DCT1, DCT2, and proliferating cells (Supplemental Figure 16), suggesting that they develop along different trajectories. Within the homeobox gene family, Hoxb7 is specific to the ureteric bud derivatives and Hoxd10 is specific to the cells of nephron progenitor origin.12 The expression of Hoxb7 in proliferating cells is the highest, and the expression of Hoxd10 is highest in DCT1 (Figure 3D). Emx1 has been reported to be an important regulator of distal tubule development in zebrafish,59 which is not selective for DCT1 compared with DCT2, whereas Sall3 is a DCT-selective zinc-finger protein,60 which is higher in proliferating cells and DCT1 compared with DCT2 (Figure 3D). Among the subpopulations, the expression of Hoxb7 in DCT1-B is the highest (only lower than the proliferating cells) and the expression of Hoxd10 is higher in DCT1-D (Figure 6, D and H and Supplemental Figure 16C). Collectively, these results suggest that DCT1 and DCT2 cells have mixed origins, some originating from the ureteric bud and some from the nephron progenitor. Yet, the DCT proliferating cells originate from ureteric buds.
Discussion
The DCT-Cre-INTACT approach allowed us to examine the distal nephron in unprecedented detail with respect to cell types and cell states. In rodents and humans, the transition from DCT1 to connecting tubule is gradual at the microscopic level61; this is in contrast to the abrupt change from TAL to DCT. We observed this gradual transition in immunofluorescent experiments but also noted that DCT1 and DCT2 cells are sometimes intermingled. Yang and colleagues4 used similar techniques to categorize transitions between segments and suggested that the overlap between NCC and ENaC was minimal. To obtain more information about the nature of these transitional segments, we used snRNA-Seq. Zeng56 recently described a comprehensive functional–transcriptomic analysis of brain cells, providing a useful definition of cells types and cell states. One feature that was noted was the coexistence of both discrete and continuous variations between cell types revealed by transcriptomic studies. Continuous variations tend to manifest among closely related transcriptomic clusters or subtypes situated at lower branches. Cells situated at the extremes of the continuum display markedly different transcriptomic profiles, while the transition between these ends occurs gradually among the constituent cells forming the continuum. Because we achieved a highly enriched population of DCT cells using the INTACT approach, we were able to determine that DCT1 cells and DCT2 cells are discrete but are connected by a continuum of cell states (Figure 2, E and F). This revealed heterogeneity of segments that are difficult to study using electrophysiological approaches for technical reasons and suggested the existence of multiple cell states along this segment. This complexity poses challenges in categorizing cells into types using statistical criteria and can make it difficult to determine an exact number of cell types.56 Yet, the continuum of cell states between cell types retains biologic significance in that cell states may change in response to environmental or endogenous stimuli. Future experiments in which stimuli, such as dietary sodium or potassium and diuretic treatment, which are known to alter the function of the DCT, can be applied to investigate how the distal nephron remodels and how cell states and perhaps cell types change. The transition from DCT to connecting tubule could not be evaluated here, as we used the slc12a3 promoter to drive expression of the INTACT protein.
Nephrons develop from mesenchymal progenitors and connect to the ureteric epithelial collecting system, which is developed from the ureteric bud.62 While most studies conclude that the connecting tubule derives from the ureteric bud and DCT cells from mesenchymal progenitors, the nature of the transitional segment, comprising the DCT2 and “early connecting tubule” in rodents and humans, has been obscure.63 McMahon and colleagues combined fate mapping and transcriptomics to clarify this issue. They reported that both principal-like cells and intercalated cells in the connecting tubule had both nephron and ureteric features.62
Our work shows that cells gradually transition from DCT1 to DCT2 and likely connecting tubule at the transcript level, supporting McMahon's observation that these segments do not derive from one or the other embryological origin. Yet, both DCT1 and DCT2 express more Hoxd10 (nephron) progenitors. The pseudotime analysis discussed above suggests that DCT2 cells are more differentiated than DCT1 cells. DCT2 cells are rich in transcripts related to kidney development, whereas DCT1 cells are rich in transcripts related to transport. Interestingly, despite being intermingled among DCT1 and DCT2 cells (immunofluorescence data not shown), the proliferating cells have more Hoxb7, suggesting that they develop from ureteric bud. Our work is consistent with the DCT as the site at which nephron and bud fuse, but it suggests that mutual induction leads to a continuum of cell types that manifest dual embryologic origins.
One limitation of our study is we used only female mice, and there is known sexual dimorphism in DCT.11,64 For initial cell characterization, we therefore decided that including both male and female mice for the transcriptomic experiments would increase the variability of the results, limiting statistical power. We have previously shown that the female DCT is more plastic than in males.11 As one of our long-term goals is to study the effects of dietary perturbation on DCT plasticity, we selected female mice for the initial studies. Further studies are needed to directly compare male and female datasets. A second limitation derives from the NCC-Cre-INTACT mouse model. In this model, the reporter expression is induced by a 5-day tamoxifen injection, which may result in altered patterns of gene expression. To limit these artifactual effects, we performed a 10-day washout to eliminate residual tamoxifen before collecting tissue for nuclei isolation, but we cannot rule out persistent effects of tamoxifen. In addition, the INTACT approach artificially expresses a fluorescent protein on the surface of the nuclei, which may alter gene expression. Another limitation of this approach is that it only captures cells that are actively expressing NCC-CreERT2 at the time of tamoxifen injection and therefore may exclude cells of the DCT that are transiently expressing NCC.
In summary, the nucleus isolation approach eliminates differences in recovery efficiency across cell types, thereby providing a more accurate representation of a small population of kidney cells. With the enriched cell type analysis, we will have the ability to generate high-fidelity resolution data with better clarity. Moreover, our high-resolution DCT transcriptome dataset can be used as a reference atlas for future work to map and identify DCT1, DCT2, and proliferating cells from nonenriched kidney nuclei datasets.
Supplementary Material
Acknowledgments
We thank the OHSU Flow Cytometry, Gene Profiling, and Massively Parallel Sequencing core facilities for technical support. Because Dr. David H. Ellison is a Deputy Editor of JASN, he was not involved in the peer-review process for this manuscript. Another editor oversaw the peer-review and decision-making process for this manuscript.
Footnotes
J.W.N. and D.H.E. contributed equally to this work.
See related editorial, “Heterogeneity of Distal Convoluted Tubule Cells,” on pages 389–391.
Disclosures
D.H. Ellison reports consultancy for Boehringer Ingelheim; honoraria from Renaissance School of Medicine, Boston University School of Medicine; royalties as an author for UpToDate; advisory or leadership role as a Consulting Editor for Hypertension and Deputy Editor of JASN; and role on the Editorial Board of American Journal of Physiology-Renal Physiology. J.A. McCormick reports role on the Editorial Boards of American Journal of Physiology-Renal Physiology, Frontiers in Physiology: Renal and Epithelial Physiology, and Kidney360. P.A. Welling reports consultancy for Boehringer Ingelheim and advisory or leadership role as an American Journal of Physiology-Renal Physiology Editorial Board member and Chair of Finance Committee and Council Member for American Society of Physiology. All remaining authors have nothing to disclose.
Funding
X.-T. Su: ASN Foundation for Kidney Research (Ben J. Lipps Fellowship) and National Institute of Diabetes and Digestive and Kidney Diseases. D.H. Ellison: National Institute of Diabetes and Digestive and Kidney Diseases (DK133220 and DK51496) and Fondation Leducq (17CVD05). C.-L. Yang: National Institute of Diabetes and Digestive and Kidney Diseases (DK51496). J.A. McCormick: National Institute of Diabetes and Digestive and Kidney Diseases (DK098141 and DK132066). J.W. Nelson: American Heart Association (20CDA35320169) and National Institute of Diabetes and Digestive and Kidney Diseases (K01DK121737). R.J. Cornelius: National Institute of Diabetes and Digestive and Kidney Diseases (K01DK120790). P.A. Welling: Fondation Leducq (17CVD05).
Author Contributions
Conceptualization: David H. Ellison, James A. McCormick, Jonathan W. Nelson, Jeremiah V. Reyes, Xiao-Tong Su, Paul A. Welling, Chao-Ling Yang.
Data curation: Sebastian Bachmann, Ryan J. Cornelius, Hasan Demirci, Yujiro Maeoka, Xiao-Tong Su.
Formal analysis: Sebastian Bachmann, Hasan Demirci, Jonathan W. Nelson, Xiao-Tong Su.
Funding acquisition: David H. Ellison, Jonathan W. Nelson, Xiao-Tong Su.
Investigation: Hasan Demirci, Jonathan W. Nelson, Xiao-Tong Su.
Methodology: David H. Ellison, Jonathan W. Nelson, Xiao-Tong Su.
Project administration: Jonathan W. Nelson, Xiao-Tong Su.
Resources: David H. Ellison, Xiao-Tong Su.
Software: Hyun Jun Jung, Anne E. Lackey, Jonathan W. Nelson, Jeremiah V. Reyes, Xiao-Tong Su.
Supervision: Sebastian Bachmann, David H. Ellison, Jonathan W. Nelson, Xiao-Tong Su.
Validation: Xiao-Tong Su.
Visualization: Xiao-Tong Su.
Writing – original draft: Xiao-Tong Su.
Writing – review & editing: Sebastian Bachmann, Ryan J. Cornelius, David H. Ellison, Hyun Jun Jung, Anne E. Lackey, Yujiro Maeoka, James A. McCormick, Jonathan W. Nelson, Jeremiah V. Reyes, Xiao-Tong Su, Paul A. Welling, Chao-Ling Yang.
Data Sharing Statement
Sequencing data are deposited in Gene Expression Omnibus under accession number GSE228367.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E577 and http://links.lww.com/JSN/E578.
Supplemental Table 1. Key resources.
Supplemental Table 2. Summary of cellranger outputs.
Supplemental Table 3. Differentially expressed genes.
Supplemental Figure 1. Immunofluorescence of NCC or AQP2 and GFP colocalization in NCC-INTACT continuous kidney sections post tamoxifen induction.
Supplemental Figure 2. Immunofluorescence of GFP costaining with NCC or AQP2 demonstrating minimal leakiness of NCC-CreERT2 in the OCT-INTACT reporter mouse line.
Supplemental Figure 3. Gating strategy for DCT nuclei sorting.
Supplemental Figure 4. Canonical marker expression for all cell types.
Supplemental Figure 5. Quality control of all nuclei.
Supplemental Figure 6. Clustering of DCT nuclei in three control samples.
Supplemental Figure 7. Comparing snRNA-Seq data and previously published scRNA-Seq data of DCT cells.
Supplemental Figure 8. Comparing snRNA-Seq data with previously published scRNA-Seq data of DCT cells.
Supplemental Figure 9. Validating the scores in a previously published mouse scRNA-Seq dataset.
Supplemental Figure 10. The Kidney Precision Medicine Project (KPMP) snRNA-Seq dataset.
Supplemental Figure 11. DCTl DEG pathway analysis.
Supplemental Figure 12. DCT2 DEG pathway analysis.
Supplemental Figure 13. DCTl subpopulation.
Supplemental Figure 14. DCT2 subpopulation.
Supplemental Figure 15. Distributions of transcripts associated with magnesium, calcium, and sodium transport.
Supplemental Figure 16. Homeobox genes distribution.
References
- 1.Bachmann S, Velazquez H, Obermuller N, Reilly RF, Moser D, Ellison DH. Expression of the thiazide-sensitive Na-Cl cotransporter by rabbit distal convoluted tubule cells. J Clin Invest. 1995;96(5):2510–2514. doi: 10.1172/JCI118311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obermuller N Bernstein P Velazquez H, et al. Expression of the thiazide-sensitive Na-Cl cotransporter in rat and human kidney. Am J Physiol. 1995;269(6 Pt 2):F900–F910. doi: 10.1152/ajprenal.1995.269.6.F900 [DOI] [PubMed] [Google Scholar]
- 3.Loffing J Loffing-Cueni D Valderrabano V, et al. Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol. 2001;281(6):F1021–F1027. doi: 10.1152/ajprenal.0085.2001 [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Xu Y, Gravotta D, Frindt G, Weinstein AM, Palmer LG. ENaC and ROMK channels in the connecting tubule regulate renal K+ secretion. J Gen Physiol. 2021;153(8):e202112902. doi: 10.1085/jgp.202112902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A. 2020;117(27):15874–15883. doi: 10.1073/pnas.2005477117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muto Y Wilson PC Ledru N, et al. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat Commun. 2021;12(1):2190. doi: 10.1038/s41467-021-22368-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H Gonzalez Villalobos R Yao X, et al. Mapping the single-cell transcriptomic response of murine diabetic kidney disease to therapies. Cell Metab. 2022;34(7):1064–1078.e6. doi: 10.1016/j.cmet.2022.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhardt L Koppitch K van Gestel J, et al. Lineage tracing and single-nucleus multiomics reveal novel features of adaptive and maladaptive repair after acute kidney injury. J Am Soc Nephrol. 2023;34(4):554–571. doi: 10.1681/ASN.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Chou CL, Knepper MA. Targeted single-cell RNA-seq identifies minority cell types of kidney distal nephron. J Am Soc Nephrol. 2021;32(4):886–896. doi: 10.1681/ASN.2020101407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm PR, Coleman R, Delpire E, Welling PA. Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol. 2017;28(9):2597–2606. doi: 10.1681/ASN.2016090948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahaei E, Coleman R, Saritas T, Ellison DH, Welling PA. Distal convoluted tubule sexual dimorphism revealed by advanced 3D imaging. Am J Physiol Renal Physiol. 2020;319(5):F754–F764. doi: 10.1152/ajprenal.00441.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ransick A Lindstrom NO Liu J, et al. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell. 2019;51(3):399–413.e7. doi: 10.1016/j.devcel.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oxburgh L Carroll TJ Cleaver O, et al. (Re)Building a kidney. J Am Soc Nephrol. 2017;28(5):1370–1378. doi: 10.1681/ASN.2016101077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deal RB, Henikoff S. A simple method for gene expression and chromatin profiling of individual cell types within a tissue. Dev Cell. 2010;18(6):1030–1040. doi: 10.1016/j.devcel.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mo A Mukamel EA Davis FP, et al. Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron. 2015;86(6):1369–1384. doi: 10.1016/j.neuron.2015.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelius RJ Sharma A Su XT, et al. A novel distal convoluted tubule-specific Cre-recombinase driven by the NaCl cotransporter gene. Am J Physiol Renal Physiol. 2020;319(3):F423–F435. doi: 10.1152/ajprenal.00101.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drokhlyansky E Smillie CS Van Wittenberghe N, et al. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182(6):1606–1622.e23. doi: 10.1016/j.cell.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson PC Wu H Kirita Y, et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci U S A. 2019;116(39):19619–19625. doi: 10.1073/pnas.1908706116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young MD, Behjati S. SoupX removes ambient RNA contamination from droplet-based single-cell RNA sequencing data. Gigascience. 2020;9(12):giaa151. doi: 10.1093/gigascience/giaa151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGinnis CS, Murrow LM, Gartner ZJ. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 2019;8(4):329–337.e4. doi: 10.1016/j.cels.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schirmer L Velmeshev D Holmqvist S, et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature. 2019;573(7772):75–82. doi: 10.1038/s41586-019-1404-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao Y Hao S Andersen-Nissen E, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–3587.e29. doi: 10.1016/j.cell.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20(1):296. doi: 10.1186/s13059-019-1874-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meij IC Koenderink JB van Bokhoven H, et al. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+),K(+)-ATPase gamma-subunit. Nat Genet. 2000;26(3):265–266. doi: 10.1038/81543 [DOI] [PubMed] [Google Scholar]
- 26.Schlingmann KP Weber S Peters M, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31(2):166–170. doi: 10.1038/ng889 [DOI] [PubMed] [Google Scholar]
- 27.Groenestege WM Thebault S van der Wijst J, et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest. 2007;117(8):2260–2267. doi: 10.1172/JCI31680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thebault S, Alexander RT, Tiel Groenestege WM, Hoenderop JG, Bindels RJ. EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol. 2009;20(1):78–85. doi: 10.1681/ASN.2008030327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Baaij JH Arjona FJ van den Brand M, et al. Identification of SLC41A3 as a novel player in magnesium homeostasis. Sci Rep. 2016;6(1):28565. doi: 10.1038/srep28565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferioli S, Zierler S, Zaisserer J, Schredelseker J, Gudermann T, Chubanov V. TRPM6 and TRPM7 differentially contribute to the relief of heteromeric TRPM6/7 channels from inhibition by cytosolic Mg2+ and Mg·ATP. Sci Rep. 2017;7(1):8806. doi: 10.1038/s41598-017-08144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie M Bal MS Liu J, et al. Uromodulin regulates renal magnesium homeostasis through the ion channel transient receptor potential melastatin 6 (TRPM6). J Biol Chem. 2018;293(42):16488–16502. doi: 10.1074/jbc.RA118.003950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franken GAC Seker M Bos C, et al. Cyclin M2 (CNNM2) knockout mice show mild hypomagnesaemia and developmental defects. Sci Rep. 2021;11(1):8217. doi: 10.1038/s41598-021-87548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnoz C, Moser S, Kratschmar DV, Odermatt A, Loffing-Cueni D, Loffing J. Deletion of the transcription factor Prox-1 specifically in the renal distal convoluted tubule causes hypomagnesemia via reduced expression of TRPM6 and NCC. Pflugers Arch. 2021;473(1):79–93. doi: 10.1007/s00424-020-02491-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bindels RJ, Ramakers PL, Dempster JA, Hartog A, van Os CH. Role of Na+/Ca2+ exchange in transcellular Ca2+ transport across primary cultures of rabbit kidney collecting system. Pflugers Arch. 1992;420(5-6):566–572. doi: 10.1007/BF00374634 [DOI] [PubMed] [Google Scholar]
- 35.Koster HP, Hartog A, Van Os CH, Bindels RJ. Calbindin-D28K facilitates cytosolic calcium diffusion without interfering with calcium signaling. Cell Calcium. 1995;18(3):187–196. doi: 10.1016/0143-4160(95)90063-2 [DOI] [PubMed] [Google Scholar]
- 36.Magyar CE, White KE, Rojas R, Apodaca G, Friedman PA. Plasma membrane Ca2+-ATPase and NCX1 Na+/Ca2+ exchanger expression in distal convoluted tubule cells. Am J Physiol Renal Physiol. 2002;283(1):F29–F40. doi: 10.1152/ajprenal.00252.2000 [DOI] [PubMed] [Google Scholar]
- 37.Hoenderop JG van Leeuwen JP van der Eerden BC, et al. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest. 2003;112(12):1906–1914. doi: 10.1172/JCI19826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gkika D, Hsu YJ, van der Kemp AW, Christakos S, Bindels RJ, Hoenderop JG. Critical role of the epithelial Ca2+ channel TRPV5 in active Ca2+ reabsorption as revealed by TRPV5/calbindin-D28K knockout mice. J Am Soc Nephrol. 2006;17(11):3020–3027. doi: 10.1681/ASN.2006060676 [DOI] [PubMed] [Google Scholar]
- 39.Bindels RJ, Timmermans JA, Hartog A, Coers W, van Os CH. Calbindin-D9k and parvalbumin are exclusively located along basolateral membranes in rat distal nephron. J Am Soc Nephrol. 1991;2(6):1122–1129. doi: 10.1681/ASN.V261122 [DOI] [PubMed] [Google Scholar]
- 40.Zheng W, Xie Y, Li G, Kong J, Feng JQ, Li YC. Critical role of calbindin-D28k in calcium homeostasis revealed by mice lacking both vitamin D receptor and calbindin-D28k. J Biol Chem. 2004;279(50):52406–52413. doi: 10.1074/jbc.M405562200 [DOI] [PubMed] [Google Scholar]
- 41.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; 2016. [Google Scholar]
- 42.Trapnell C Cacchiarelli D Grimsby J, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32(4):381–386. doi: 10.1038/nbt.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu X, Hill A, Packer J, Lin D, Ma YA, Trapnell C. Single-cell mRNA quantification and differential analysis with Census. Nat Methods. 2017;14(3):309–315. doi: 10.1038/nmeth.4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu X Mao Q Tang Y, et al. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14(10):979–982. doi: 10.1038/nmeth.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao J Spielmann M Qiu X, et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature. 2019;566(7745):496–502. doi: 10.1038/s41586-019-0969-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouyang JF, Kamaraj US, Cao EY, Rackham OJL. ShinyCell: simple and sharable visualization of single-cell gene expression data. Bioinformatics. 2021;37(19):3374–3376. doi: 10.1093/bioinformatics/btab209 [DOI] [PubMed] [Google Scholar]
- 48.Lake BB Menon R Winfree S, et al.; KPMP Consortium. An atlas of healthy and injured cell states and niches in the human kidney. Nature. 2023;619(7970):585–594. doi: 10.1038/s41586-023-05769-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H, Kirita Y, Donnelly EL, Humphreys BD. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J Am Soc Nephrol. 2019;30(1):23–32. doi: 10.1681/ASN.2018090912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van de Graaf SF, Bindels RJ, Hoenderop JG. Physiology of epithelial Ca2+ and Mg2+ transport. Rev Physiol Biochem Pharmacol. 2007;158:77–160. doi: 10.1007/112_2006_0607 [DOI] [PubMed] [Google Scholar]
- 51.de Groot T, Bindels RJ, Hoenderop JG. TRPV5: an ingeniously controlled calcium channel. Kidney Int. 2008;74(10):1241–1246. doi: 10.1038/ki.2008.320 [DOI] [PubMed] [Google Scholar]
- 52.van der Wijst J, Bindels RJ, Hoenderop JG. Mg2+ homeostasis: the balancing act of TRPM6. Curr Opin Nephrol Hypertens. 2014;23(4):361–369. doi: 10.1097/01.mnh.0000447023.59346.ab [DOI] [PubMed] [Google Scholar]
- 53.Loffing J Pietri L Aregger F, et al. Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol. 2000;279(2):F252–F258. doi: 10.1152/ajprenal.2000.279.2.F252 [DOI] [PubMed] [Google Scholar]
- 54.Maeoka Y Su XT Wang WH, et al. Mineralocorticoid receptor antagonists cause natriuresis in the absence of aldosterone. Hypertension. 2022;79(7):1423–1434. doi: 10.1161/HYPERTENSIONAHA.122.19159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao C, Chen L, Chen E, Tsilosani A, Xia Y, Zhang W. Generation of distal renal segments involves a unique population of Aqp2+ progenitor cells. J Am Soc Nephrol. 2021;32(12):3035–3049. doi: 10.1681/ASN.2021030399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng H. What is a cell type and how to define it. Cell. 2022;185(15):2739–2755. doi: 10.1016/j.cell.2022.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6(12):893–904. doi: 10.1038/nrg1726 [DOI] [PubMed] [Google Scholar]
- 58.Mallo M. Reassessing the role of hox genes during vertebrate development and evolution. Trends Genet. 2018;34(3):209–217. doi: 10.1016/j.tig.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 59.Morales EE Handa N Drummond BE, et al. Homeogene emx1 is required for nephron distal segment development in zebrafish. Sci Rep. 2018;8(1):18038. doi: 10.1038/s41598-018-36061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen L, Chou CL, Knepper MA. A comprehensive map of mRNAs and their isoforms across all 14 renal tubule segments of mouse. J Am Soc Nephrol. 2021;32(4):897–912. doi: 10.1681/ASN.2020101406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L, Clark JZ, Nelson JW, Kaissling B, Ellison DH, Knepper MA. Renal-tubule epithelial cell nomenclature for single-cell RNA-sequencing studies. J Am Soc Nephrol. 2019;30(8):1358–1364. doi: 10.1681/ASN.2019040415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McMahon AP. Development of the mammalian kidney. Curr Top Dev Biol. 2016;117:31–64. doi: 10.1016/bs.ctdb.2015.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castaneda-Bueno M, Ellison DH. Blood pressure effects of sodium transport along the distal nephron. Kidney Int. 2022;102(6):1247–1258. doi: 10.1016/j.kint.2022.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torres-Pinzon DL, Ralph DL, Veiras LC, McDonough AA. Sex-specific adaptations to high-salt diet preserve electrolyte homeostasis with distinct sodium transporter profiles. Am J Physiol Cell Physiol. 2021;321(5):C897–C909. doi: 10.1152/ajpcell.00282.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequencing data are deposited in Gene Expression Omnibus under accession number GSE228367. To ensure data accessibility to nonbioinformaticians, we made the DCT snRNA-Seq data available for further exploration via an interactive web tool generated using ShinyCell47 at https://ellisonlab.shinyapps.io/dct_shinycell/.
Sequencing data are deposited in Gene Expression Omnibus under accession number GSE228367.