Visual Abstract
Keywords: hemodialysis, exercise, intradialytic exercise, cardiovascular disease, meta-analysis
Abstract
Key Points
Individuals receiving hemodialysis have high rates of cardiovascular disease not explained by traditional cardiovascular risk factors.
Intradialytic exercise improves cardiovascular outcomes, including arterial resistance, BP, and heart rate variability.
Clinicians should consider including intradialytic aerobic exercise programs in hemodialysis care to supplement broader treatment plans.
Background
Cardiovascular disease is the leading cause of death among people with kidney failure on hemodialysis, for whom improving cardiovascular health is a research priority. Intradialytic myocardial stunning is common and associated with adverse cardiovascular events. Intradialytic exercise may mitigate intradialytic myocardial stunning and improve cardiovascular structure and function. This systematic review investigated the effect of intradialytic exercise on cardiovascular outcomes in adults undergoing maintenance hemodialysis (PROSPERO CRD42018103118).
Methods
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, we systematically searched MEDLINE, Embase, Cochrane CENTRAL, SportDiscus, and PEDro databases from 1960 until June 2022, for randomized and nonrandomized studies investigating the effect of intradialytic exercise programs on objective cardiovascular outcomes, prespecified as primary or secondary outcomes. The primary outcome was arterial resistance.
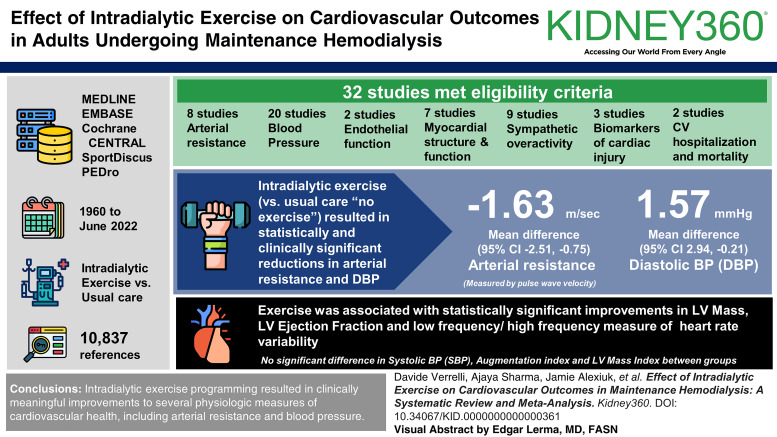
Results
Of 10,837 references identified, 32 met eligibility criteria. These studies investigated the effect of intradialytic exercise on arterial resistance (eight studies), BP (20 studies), myocardial structure and function (seven studies), endothelial function (two studies), sympathetic overactivity (nine studies), biomarkers of cardiac injury (three studies), and cardiovascular hospitalization and mortality (two studies). Most studies used aerobic exercise as the intervention and usual care (no exercise) controls. Meta-analysis of intradialytic exercise versus usual care resulted in a statistically significant reduction in arterial resistance measured by pulse wave velocity with mean difference −1.63 m/s (95% confidence interval, −2.51 to −0.75). Meta-analyses for diastolic BP, left ventricular ejection fraction, and low-frequency/high-frequency ratio measure of heart rate variability also showed statistically significant improvements with exercise. There was no significant difference in change in systolic BP, augmentation index, and left ventricular mass index between groups.
Conclusions
Intradialytic exercise programming resulted in a clinically meaningful improvement to pulse wave velocity, a component of arterial resistance. Improvements in several physiologic measures of cardiovascular health, including diastolic BP, left ventricular ejection fraction, and heart rate variability measured by the low-frequency/high-frequency ratio were also observed. The effects of intradialytic exercise on major adverse cardiovascular events remains uncertain.
Introduction
Although considered life-sustaining therapy for people with kidney failure, many individuals on maintenance hemodialysis experience severe disease-related complications, including adverse cardiovascular events.1 Half of all patients die within the first 3 years of hemodialysis initiation, and half of all deaths are related to cardiovascular diseases.2 Individuals receiving hemodialysis have strikingly low physical activity levels and poor physical function, which decline over time and are associated with higher risks of cardiovascular and all-cause hospitalizations and death.3–13
Intradialytic hypotension is common. Present in 20%–30% of hemodialysis treatments, it can result from abnormal underlying cardiovascular physiology, volume removal and the hemodialysis procedure itself, or the compounded effects of both these factors.14 Regardless of its cause, intradialytic hypotension can lead to decreased cardiac output and decreased coronary perfusion, which contributes to temporary decreases in regional contraction of the myocardium during dialysis, known as myocardial stunning.14–18 Hemodialysis-related myocardial stunning is associated with decreased organ perfusion19–21 and contributes to the high rates of heart failure, cardiac events, and mortality observed in people receiving hemodialysis.17,22–25
In the general population, the cardioprotective benefits of exercise have been noted to exceed those gained from pharmacological interventions.26–28 Increased physical activity through exercise programming improves physical function in individuals receiving hemodialysis.29 Exercise during hemodialysis (intradialytic exercise) is convenient and has been associated with the highest exercise program adherence rates.30 Recent studies suggest such programs decrease hemodialysis-related cardiac stunning and improve aspects of cardiovascular structure and function. This may be through an ischemic preconditioning effect, in which minor ischemic insults provide cardioprotection by enhancing the ability of the tissue to respond to larger ischemic events.31–34
To summarize the evidence and identify knowledge gaps, we conducted a systematic review and meta-analysis to evaluate the effect of intradialytic aerobic and/or resistance exercise on objective measures of cardiovascular health in adults with kidney failure receiving maintenance hemodialysis.
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and registered the protocol with PROSPERO International Prospective Register of Systematic Reviews (CRD42018103118).35 Our search strategy aimed to identify randomized controlled trials (RCTs) and quasiexperimental studies that measured the effect of intradialytic exercise programs (aerobic, resistance, or both) on objectively measured cardiovascular outcomes associated with major adverse cardiovascular events as compared with a nonexercise/sham exercise control group in adults (18 years and older) on maintenance hemodialysis. Studies in which the intervention consisted of exercise sessions that occurred two or more times per week for at least 20 minutes of duration per session and for 2 weeks or longer were eligible for inclusion. Exclusion criteria were publication before 1960, inclusion of individuals who were younger than 18 years, no intradialytic exercise intervention, and no prespecified cardiovascular outcomes (Table 1).
Table 1.
Inclusion and exclusion criteria
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population/participants | Adults (18 yr or older) on chronic hemodialysis | Children (younger than 18 yr) and animals |
| Intervention | Exercise interventions performed during hemodialysis (intradialytic exercise) that include aerobic exercise, resistance exercise, or both | Studies including exercise interventions that do not include intradialytic aerobic exercise, resistance exercise, or both |
| Comparison | Nonexposed control group receiving standard/usual care or sham exercise (e.g., breathing exercises) | — |
| Outcome | Studies that report changes in relevant prespecified cardiovascular outcomes, including arterial resistance; BP; myocardial function as measured by changes in LVEF, shortening fraction, number of regional wall motion abnormalities, or myocardial blood flow; endothelial function; sympathetic overactivity as measured by change in baroreflex sensitivity and/or HRV; biomarkers of cardiac injury (e.g., troponin, brain-natriuretic peptide); cardiac structure as measured by changes in myocardial thickness and/or LVM; hospitalization and death for cardiovascular causes | Studies that do not include cardiovascular outcomes as mentioned in inclusion criteria |
| Other | Study design: randomized controlled or quasiexperimental design | Date: studies before 1960 |
HRV, heart rate variability; LVEF, left ventricular ejection fraction; LVM, left ventricular mass.
We prespecified arterial resistance as the primary outcome of this review given that increased arterial resistance is associated with vascular calcification in people receiving dialysis,36 and both vascular calcifications and increased arterial resistance are associated with episodes of intradialytic hypotension.37,38
Secondary outcomes included systolic BP (SBP) and diastolic BP (DBP), cardiac structure and function, endothelial function, sympathetic overactivity, biomarkers of cardiac injury, and cardiovascular-related hospitalization and mortality rates. For inclusion in this review, studies needed to include at least one objective measure of a cardiovascular outcome as a prespecified primary or secondary outcome (Table 1).
The systematic search strategy was designed in collaboration with a medical librarian (A. Iansavitchene) and conducted using MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL) electronic databases (all through Ovid interface), SportDiscus (through EBSCO platform), and PEDro databases from 1960 or inception using a combination of medical subject headings (i.e., MeSH in MEDLINE) and free-text terms, such as “intradialytic,” “kinesiotherapy,” “exercise,” “aerobic,” “physical training,” and “resistance training.” Optimized high-sensitivity filters were used to refine search results for dialysis and chronic kidney disease content.39,40 Search strategies were modified using appropriate thesaurus terms and fields as indicated for each database (Supplemental Table 1). No language restrictions were applied. We reviewed reference lists of key review articles and the studies selected for inclusion. Ongoing trials, conference proceedings, and other gray literature were not searched separately. The initial database search was conducted on June 18, 2018, with the last updated search on June 14, 2022.
Study Screening and Selection
We used Covidence (Melbourne, Australia) software to upload and deduplicate citations, for abstract screening, full-text review, and data abstraction.41 Pairs of two blinded independent reviewers screened abstracts and reviewed full-text articles for inclusion, meeting after their independent review to obtain consensus (A. Sharma/D. Verrelli, K. Rossum/E. Ford, M. Sharma/J. Alexiuk, Q. Tays/D. Verrelli). A third reviewer (C. Bohm) adjudicated discrepancies when necessary. Data extraction from studies selected for inclusion was performed by the same paired reviewers in duplicate, with discrepancies resolved similarly.
Data extracted included characteristics of each study (first author, year, contact information, and country of publication), study design, sample size (if applicable), type of exercise (aerobic, resistance, or both), duration and frequency of intervention, target exercise dose/intensity, inclusion/exclusion criteria, study dropouts, reasons for attrition, patient characteristics (age, sex, time on dialysis, exercise adherence), and review-relevant outcomes.
Pairs of independent reviewers (A. Sharma/D. Verrelli, J. Alexiuk/D. Verrelli) evaluated studies for individual and cumulative risk of bias using the Cochrane Collaboration's Risk of Bias Assessment Tool for RCTs and Newcastle Ottawa Scale for non-RCTs.42 Discrepancies were resolved by consensus or third-party adjudication (C. Bohm).
Data Synthesis
For summative review, studies were grouped by outcome categories.43 We performed a thematic analysis using study and intervention characteristics, such as study design, type of exercise, and duration of intervention to identify patterns and similarities in outcome results within the studies.44 Although results reporting was variable, we used mean change of the outcome within the intervention and control groups over the study period as our principal summary measure and when available, also incorporated between group difference.
We proceeded with meta-analysis for outcomes for which more than two randomized controlled studies with required data were available for the same outcome measure. We performed meta-analysis for arterial resistance, DBP and SBP, left ventricular ejection fraction (LVEF), left ventricular mass, and heart rate variability (HRV) using random effects models. We did not impute missing data but attempted to contact corresponding authors for missing data as needed. In addition, when required, methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions were used to calculate mean difference (MD), SD, and SEM difference.45 Statistical heterogeneity was quantified using the I2 statistic and considered unimportant for I2 <40%, with statistical significance assessed using the chi-squared test. Review Manager (version 5.3) was used for the analysis.46
Results
Our search strategy identified 10,837 unique citations, of which 192 were selected for full-text review, and 32 studies published between 1992 and 2022 were included in this review (Figure 1). Studies were from North America (n=6), Australia (n=3), Asia (n=7), Europe (n=8), South America (n=6), and the Middle East (n=2).
Figure 1.
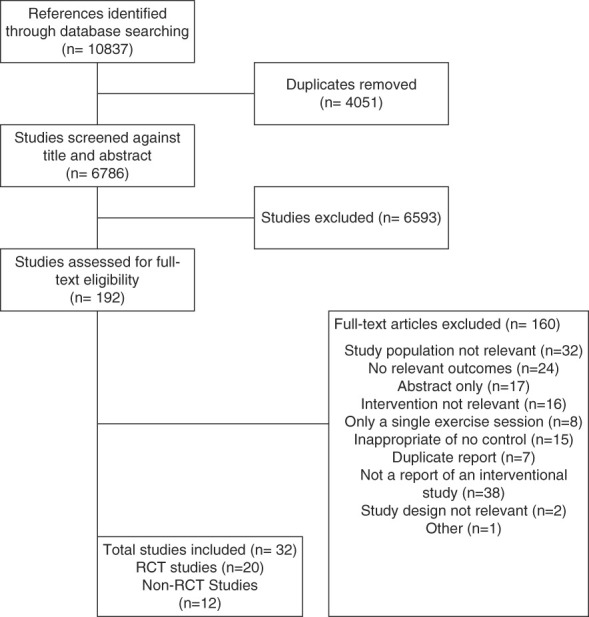
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial.
Studies selected include 18 parallel arm RCTs, two randomized cross-over studies, seven nonrandomized studies that included an intervention and control group, and five single-group pre-post studies.32,47–77 In total, 1817 individuals participated in these 32 studies, with sample sizes ranging from 6 to 174 participants (Table 2).
Table 2.
Characteristics of included studies
| Eligibility Criteria | Months on Hemodialysis (Mean [SD]/Median [IQR]) | Participant Age (yr) (Mean [SD]) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Country | Study Design | Type of Exercise Type of Control |
N (Control/Exercise) (Attrition%) | Inclusion Criteria | Exclusion Criteria | % Female | Control | Exercise | Control | Exercise | Comorbidities (%) | Outcomes Assessed |
| Indonesia | Single-group pre/post | Not stated Usual care |
30 (15/15) (0%) | Hemodialysis 2 times/wk Able to exercise during hemodialysis SBP >180 mm Hg DBP >120 mm Hg |
Asthma, IHD No completion of any exercise sessions |
43 | Not stated | Not stated | 51.03 | 51.03 | — | BP | |
| Anderson et al.,59 2004 | United States | Single-group pre/post | Aerobic NA |
19 (32% at 3 mo) (53% at 6 mo) | Hemodialysis 3 times/wk | AKI; type 1 DM; active cerebral ischemia Severe arrhythmia, IHD, CHF, or valve disease Unable to cycle |
21 | — | 49.1 (13.6) | — | BP, HTN medications | ||
| Chan et al.,54 2017 | Australia | Single-group pre/post | Resistance Usual care |
22 (22/18) (18%) | 40 yr and older; medically stable Hemodialysis >3 mo Ambulate independently <120 min moderate PA/wk |
Amputation Unable to exercise during hemodialysis No previous resistance training |
41 | 42.5 (7–163) | 71.3 (11.0) | DM: 36 HT: 23 MI: 10 CVA: 10 PVD: 5 |
Arterial resistance, BP | ||
| Cheng et al.,61 2019 | China | RCT | Resistance Usual care |
132 (65/67) (49%) | 18–80 yr old Hemodialysis 3 times/wk for ≥3 mo Kt/V >1.2 |
Severe CVD, MSK, or other medical problems Dyspnea or chest pain during exercise Instability during hemodialysis BP ≥180/100, or <90/60 mm Hg |
40 | 47 (26–78) | 43 (23–91) | 55.8 (12) | 54.6 (12.6) | — | BP |
| Cooke et al.,48 2018 | Canada | RCT | Aerobic Usual care |
32 (16/16) (38%) | Hemodialysis 3 times/wk for ≥12 wk Stable cardiac workup |
Serum PTH >250 pmol/L Severe arrhythmia, CVD, or PVD K >6.5 mmol/L in last 2 wk; active cancer Posthemodialysis SBP ≥160 mm Hg or DBP ≥100 mm Hg Planned major surgery during study |
30 | — | — | 52.5 (15.4) | 58.2 (17.2) | DM: 35 HT: 100 Smoking: 45 CAD: 10 MI: 5 CHF: 20 CVA: 10 PVD: 5 COPD: 15 |
Arterial resistance, BP |
| Fernandes et al.,62 2019 | Brazil | RCT | Aerobic Usual care |
44 (22/22) (11%) | 18 yr and older Hemodialysis >6 mo Clinically stable No lung, MSK, neurological issues |
Planned surgical intervention during study IHD event <3 mo Severe IHD, valve disease, or arrhythmia Require home oxygen Require mobility assist device |
50 | 85.9 (45.4) | 79.8 (56.4) | 42.6 (11.2) | 44.3 (11.3) | — | BP |
| Graham-Brown et al.,66 2021 | United Kingdom | Cluster RCT | Aerobic Usual care |
130 (65/65) (22%) | 18 yr and older Hemodialysis >3 mo |
Unable/unfit to participate in exercise Unable to undergo MRI scanning |
27 | 15.6 (4.8–38.4) | 14.4 (6–44.4) | 58.9 (14.9) | 55.5 (15.5) | AFib: 3.1 IHD: 13.8 |
LVM, arterial resistance |
| Greenwood et al.,67 2021 | United Kingdom | RCT | Combined Usual care |
335 (160/175) (16.42%) | Older than 18 yr Hemodialysis >3 mo |
Planned hemodialysis for >6 mo Hemodialysis <3 mo; clinically unstable Bilateral lower-limb amputations Dementia or severe cognitive impairment Unstable psychiatric disorders; pregnant |
40 | Not stated | Not stated | 59.8 (14.1) | 60.5 (15.0) | DM: 40 HT: 78 CVD: 20 |
Arterial resistance, cardiovascular mortality |
| Guio et al.,55 2017 | Brazil | Single-group pre/post | Aerobic Usual care |
24 (24/24) (42%) | 18 yr and older Hemodialysis 3 times/wk for ≥6 mo Arteriovenous fistula |
CVD; MSK, neurological reasons unable to exercise Active inflammatory/infectious disease <6 mo Hospitalization |
57 | 23 (10) | 50.2 (15.2) | — | BP, LVF | ||
| Huang et al.,63 2020 | China | RCT | Combined Sham exercise |
47 (23/24) (32%) | 18 yr and older Hemodialysis 3 times/wk for ≥3 mo |
Severe MSK pain; need assist sit, stand, or walk Dyspnea at rest or with ADLs; mental disease |
28 | 43 (89) | 26 (30) | 37.6 (10.3) | 43.8 (10.3) | — | BP |
| Isnard-Rouchon et al.,72 2017 | France | Prospective interventional cohort; nonrandomized | Aerobic Usual care |
80 (40/40) (18%) | Hemodialysis | None stated | 38 | — | — | 67.65 (13.4) | 66.8 (10.6) | DM: 36 HT: 84 IHD: 13 |
BP medications, CV hospitalizations |
| Koh et al.,47 2010 | Australia | RCT | Aerobic Usual care |
49 (22/27) (44%) | Older than 18 yr, stable dialysis Urea reduction ratio >70% for >3 mo |
Unstable angina, lower-limb amputation Current moderate exercise ≥120 min/wk |
42 | — | — | 51.3 (14.4) | 52.3 (10.9) | DM: 5 HT: 29 MI: 2 Mitral regurgitation: 1 Other arterial disease: 2 Angina: 3 |
Arterial resistance, BP |
| Kouidi et al.,60 2009 | Greece | RCT | Combined Usual care |
63 (31/32) (6%) | Hemodialysis ≥6 mo Able to reach target workload |
Bundle branch block; unstable HTN; DM; severe CHF; recent MI; unstable angina | 42 | 74.4 (46.8) | 75.6 (44.4) | 53.2 (6.1) | 54.6 (8.9) | HT: 71 CAD: 12 CHF: 29 |
LVF, HRV |
| Kouidi et al.,49 2010 | Greece | RCT | Combined Usual care |
44 (20/24) (12%) | Hemodialysis | Severe psychiatric, neurological, cardiac, lung, MSK issues DM; significant electrolyte instability Undisciplined patients |
41 | 75.6 (58.8) | 73.2 (55.2) | 45.8 (10.9) | 46.3 (11.2) | — | HRV |
| Liao et al.,50 2016 | Taiwan | RCT | Aerobic Usual care |
40 (20/20) (—) | Hemodialysis 3 times/wk for ≥6 mo | Severe HTN, lung issues, DM; high PTH levels Moderate CHF, arrhythmia; recent MI; unstable angina; active liver dysfunction; MSK problems Inflammation/infection; cancer; autoimmune disease Emotional instability Hospitalization in last mo; BMI >25 kg/m2 Lower-extremity arterial–venous access |
58 | 83 (71) | 71 (46) | 62 (9) | 62 (8) | DM: 43 Cardiomyopathy: 28 CVD: 20 PAD: 13 TIA: 8 |
BP, EPC count |
| Martínez-Olmos et al.70 2021 | Spain | Randomized cross-over | Aerobic Usual care |
56 (56/56) (37.5%) | Hemodialysis >3 mo | MI in last 6 wk, BKA Cerebrovascular disease Chest pain, dyspnea with exertion Inability to perform functional tests |
39 | 58.8 (—) | 52.8 (—) | 66.5 (14.8) | 68 (13.5) | DM: 41 | HTN medications |
| Mihaescu et al.,73 2013 | Romania | Prospective interventional cohort; nonrandomized | Resistance Usual care |
35 (16/19) (9%) | Hemodialysis 3 times/wk ≥3 mo | None stated | 69 | 55.2 (52.8) | 54 (56.4) | 55.1 (10.5) | 55.6 (8.9) | — | Arterial resistance, BP, HTN medications |
| Miller et al.,74 2002 | United States | Prospective interventional cohort; nonrandomized | Aerobic Usual care |
75 (35/40) (24%) | Hemodialysis >1 mo | Angina, CVD requiring oxygen; MI, CABG Stroke or TIA <3 mo Unable to pedal a bike |
57 | 28.7 (25.5) | 20.7 (27.5) | 56.1 (15.2) | 52.8 (16.0) | DM: 23 CAD/CHF: 25 |
BP, HTN medications |
| Momeni et al.,32 2014 | Iran | RCT | Aerobic Usual care |
40 (20/20) (—) | Older than 18 years Hemodialysis >3 mo |
Older than 60 years; IHD; LVEF <40% Using antiarrhythmic agents; unable to exercise Dyspnea/chest pain during exercise BP ≥160/100 mm Hg |
25 | — | — | 43.1 (10.5) | — | LVF | |
| Moore et al.,56 1993 | United States | Nonrandomized single-group cross-over | Aerobic Unloaded cycling (<50 rpm) |
23 (23/23) (61%) | Achieve 6/10 on Borg RPE. | Angina; IHD, MSK disease-limiting exercise Ability to complete study |
43 | — | — | — | — | — | Stroke volume, CO |
| Musavian et al.,57 2015 | Iran | Single-group pre/post | Aerobic Passive cycling (electric bike) |
18 (18/18) (11%) | 15–80 years Hemodialysis 3 times/wk for ≥3 mo |
CVD, IHD <6 mo; Stroke/TIAs Pulmonary, MSK, and immune disorders Severe HTN or SBP <90 mm Hg); severe DM; nonadherence |
19 | 27.24 (25.08) | 51.98 (1.57) | DM: 0 | BP | ||
| Oliveira E Silva et al.,64 2019 | Brazil | RCT | Aerobic Usual care |
30 (15/15) (17%) | Older than 18 years Hemodialysis ≥3 mo Stable medication Able to exercise |
Already physically active; IHD; stroke; cancer; liver failure; active infection BP >160/100 mm Hg during treadmill test |
50 | 21 (27.1) | 26 (14.58) | 58 (15) | 50 (17.2) | DM: 30 HT: 83 |
Arterial resistance, LVM, LVF |
| Painter et al.,75 1986 | United States | Prospective interventional cohort; nonrandomized | Aerobic Usual care |
27 (7/20) (30%) | Hemodialysis | None stated | — | 62.4 (46.8) | 45.6 (36.0) | 42 (16) | 42 (10) | DM: 11 | BP, HTN medications |
| Palar and Lobo,77 2022 | India | Prospective interventional cohort; nonrandomized | Resistance Usual care |
40 (20/20) (12.5%) | 20–80 years Hemodialysis >3 mo |
Uncontrolled HTN Unstable angina, recent MI Lower limb amputation |
29 | 3–6 mo: n=0 6–12 mo: n=0 1–3 years: n=5 >3 years: n=11 |
3–6 mo: n=6 6–12 mo: n=1 1–3 years: n=7 >3 years: n=5 |
20–35: n=7 36–50: n=6 51–65: n=3 66–90: n=0 |
20–35: n=2. 36–50: n=6. 51–65: n=9. 66–90: n=2 | DM: 13 | BP |
| Paluchamy et al.,65 2018 | India | RCT | Aerobic Usual care |
20 (10/10) (0%) | None stated | Unstable angina, recent MI, grade II CHF Fever (≥101°F); persistent prehemodialysis hyperkalemia Active liver disease; MSK limitations Severe peripheral neuropathy Dementia/other mental disorders; unstable during hemodialysis Lower limb amputee; already in exercise program |
10 | <6 mo: 20% 6 mo to 1 yr: 20% 1–3 yr: 60% >3 yr: 0 |
<6 mo: 30% 6 mo to 1 yr: 10% 1–3 yr: 50% >3 yr: 10% |
18–30: 0% 31–50: 30% 51–70: 70% |
18–30: 20% 31–50: 30% 51–70: 50% |
— | Baroreflex sensitivity |
| Parsons et al.,51 2004 | Canada | RCT | Aerobic Usual care |
18 (7/6) (28%) | Hemodialysis | CVD, MSK, neurological issue, and unable to exercise | 46 | 49 (26) | 35 (25) | 49 (25) | 60 (17) | DM: 15 | BP |
| Petraki et al.,52 2008 | Greece | RCT | Combined Usual care |
50 (24/26) (14%) | Hemodialysis ≥6 mo | Unstable HTN, grade II CHF Lown's grade III arrhythmia; recent MI; unstable angina DM; active liver disease; established cause of syncope |
25 | 72.8 (5.3) | 76.32 (7.0) | 50.52 (14.4) | 50.05 (13.2) | — | BP, baroreflex sensitivity |
| Pereira et al.,68 2022 | Brazil | RCT | Aerobic Usual care |
98 (49/49) (18.4%) | 50–70 years Hemodialysis ≥6 mo |
Previously hospitalized for CKD MSK diseases that prevent exercise Drugs that influenced HR Cognitive impairment; BMI >30 Pacemakers or cardiac surgery <6 mo |
38 | 5.77 (1.56) | 5.8 (3.42) | 71.55 (6.76) | 69.1 (6.17) | DM: 35 HT: 90 |
HRV |
| Reboredo et al.,53 2010 (a) | Brazil | Nonrandomized single-group cross-over | Aerobic Usual care |
18 (18/18) (22%) | None stated | Unstable angina; uncontrolled arrhythmia or DM Uncompensated CHF SBP ≥200 mm Hg and/or DBP ≥120 mm Hg; systemic infection; severe renal osteodystrophy Neurological, pulmonary, or MSK disturbances |
31 | 93.7 (43.9) | 47.6 (12.8) | HT: 86 LVH: 57 CHF: 28 |
BP | ||
| Reboredo et al.,58 2010 (b) | Brazil | RCT | Aerobic Usual care |
28 (14/14) (21%) | Older than 18 years No regular exercise for ≥6 mo |
DM; unstable angina; uncontrolled HTN On antiarrhythmic drugs; severe pneumopathies Acute systemic infection; severe renal osteodystrophy Disabling neurological and MSK disorders |
36 | 60.1 (54.4) | 41.9 (42.4) | 43.5 (12.8) | 49.6 (10.6) | — | LVF, HRV |
| Thenmozhi et al.,76 2018 | India | Prospective interventional cohort; nonrandomized | Aerobic Usual care |
130 (65/65) (—) | 25–65 years Hemodialysis ≥3 mo Clinically stable |
Unstable angina, recent MI, CHF grade II Fever (≥101°F); persistent prehemodialysis hyperkalemia Active liver disease; MSK limitations Severe peripheral neuropathy; lower limb amputee Dementia or other mental disorders Participation in other exercise program; unstable hemodialysis |
15 | 6 mo to 1 yr: 49% 1–3 yr: 46% >3 yr: 5% |
6 mo to 1 yr: 53% 1–3 yr: 42% >3 yr: 5% |
18–30: 11% 31–50: 34% 51–65: 55% |
18–30: 9% 31–50: 31% 51–65: 60% |
— | BP |
| Toussaint et al.,69 2008 | Australia | Randomized cross-over | Aerobic Usual care |
20 (10/10) (5%) | Hemodialysis >3 mo Able to exercise for 3 mo |
Symptomatic CVD Respiratory or MSK issue-limiting exercise |
53 | 72 (56) | 35 (51) | 70 (28–77) | 67 (60–83) | DM: 32 CAD: 47 |
Arterial resistance, BP, brain natriuretic peptide |
ADL, activities of daily living; AFib, atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; CMRI, cardiac magnetic resonance imaging; CO, cardiac output; Combined, aerobic and resistance exercise; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular attack; CVD, cardiovascular disease; DBP, diastolic BP; DM, diabetes mellitus; EPC, endothelial progenitor cell; HF, heart failure; HR, heart rate; HRV, heart rate variability; HTN, hypertension; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; LVF, left ventricular function; LVH, left ventricular hypertrophy; LVM, left ventricular mass; MRI, magnetic resonance imaging; MSK, musculoskeletal; N/A, not applicable; MI, myocardial infarction; PVD, peripheral vascular disease; RCT, randomized controlled trial; SBP, systolic BP; TIA, transient ischemic attack.
Twenty-two studies evaluated the effects of intradialytic aerobic exercise.32,47,48,50,51,53,55–59,62,64–66,68–70,72,74–76 Four studies examined the effects of resistance exercise.54,61,73,77 Five studies included both aerobic and resistance exercise.49,52,60,63,67 One study did not state the type of exercise used in the intervention (Table 2).71
Quality of Reporting and Risk of Bias of Included Studies
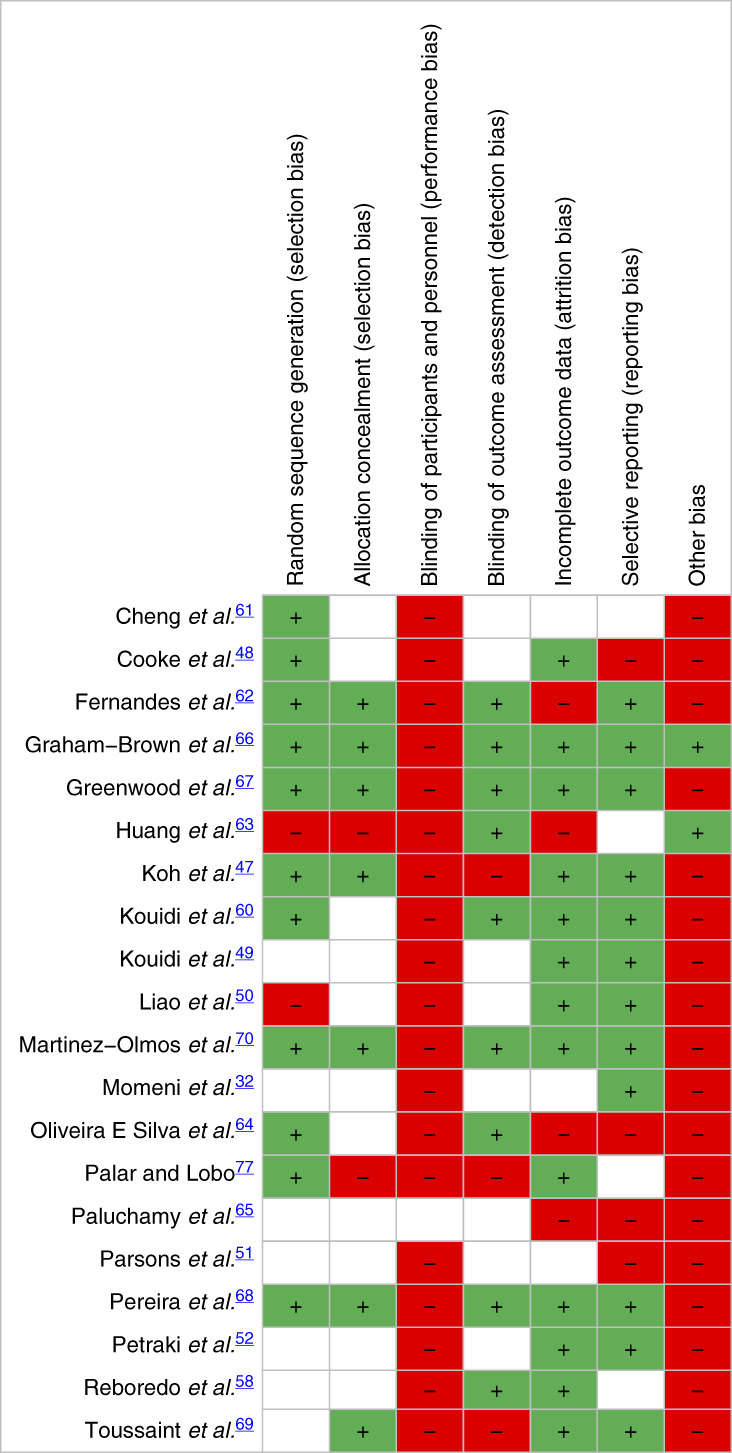
In included RCTs, risk of bias was high in domains of performance bias and other bias (e.g., contamination bias). However, risk of bias was low or unclear in selection, detection, attrition, and reporting bias. Most studies before 2018 had high or unclear risk of bias, whereas studies published after 2018 generally had larger sample sizes, better quality reporting, and moderate to low risk of bias (Table 3 and Supplemental Figure 1).
Table 3.
Risk of bias for randomized controlled trials
|
|
Risk of bias for nonrandomized studies was high or unclear for the comparability of cohorts but was relatively low for selecting outcome measures (Supplemental Table 2).
Arterial Resistance
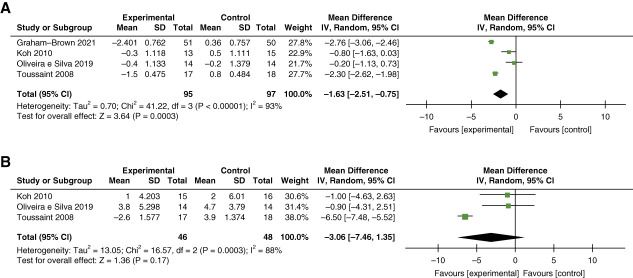
Arterial resistance was measured by pulse wave velocity (PWV) and/or augmentation index (AI) in eight studies (total n=653).47,48,54,64,66,67,69,73 Meta-analysis of four studies demonstrated a statistically significant mean difference in PWV with 13–26 weeks of intradialytic cycling (n=95) as compared with 95 controls of −1.63 m/s; 95% confidence interval [CI], −2.51 to −0.75; I2=93%; P < 0.001 (Figure 2A).47,64,66,69 By contrast, meta-analysis of three studies showed no statistically significant difference in AI with intradialytic cycling (n=46) as compared with 48 controls (MD –3.29%; 95% CI, −7.88 to 1.30; I2=88%; P < 0.001) (Figure 2B).47,64,69
Figure 2.
Meta-analysis of studies examining the effect of intradialytic exercise on arterial resistance. (A) PWV and (B) AI. AI, augmentation index; CI, confidence interval; IV, inverse variance; PWV, pulse wave velocity.
BP
Twenty studies (n=984), including nine RCTs, reported BP measures. Most (13 studies) studied predialysis BP measured peripherally using automated BP machines.47,51,52,57,61–63,66,69,73–76 One study measured interdialytic BP, one study measured BP preintradialytic exercise, two studies measured ambulatory BP (24 and 44 hour), and in three studies, the timing of BP measurement was unclear50,54,58,59,66,71,77 (Table 4).
Table 4.
Summary of study outcomes and results
| Mean Change | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author (Year) | Meta-Analysis (Yes/No) | Intervention | Exercise Frequency (per week) | Goal Intensity | Goal Session Length (min) | Duration (wk) | Control | Measurement Tool (Primary/Secondary Outcome) | Control | Exercise | MD Exercise Control (SD or 95% CI) | Effect Size (95% CI) | P Value |
| Arterial resistance | |||||||||||||
| Chan et al.54 (2017) | No | Ten muscle groups using free weights, tubing, resistance machine | 3 | Week 1–4 RPE 12–14; 12–15 rep Week 5–12 RPE 14–15; 10–12 rep |
30 | 13 | Usual care | PWV peripheral (m/s) (primary) | BL: 12.3 (9.1–20.8) Post: 11.5 (9.7–20.0) |
BL: 11.5 (9.7–20.0) Post: 13.0 (9.3–26.7) |
0 (−0.1 to 0.1) | 0.02 (−0.61 to 0.57) | 0.6 |
| AI (%) (secondary) | −1.4 | 0.4 | 1.9 (−5.9 to 9.7) | 0.14 (−0.45 to 0.74) | 0.9 | ||||||||
| AI×75 (%) (secondary) | −0.3 | 2.3 | 2.7 (−3.9 to 9.4) | 0.24 (−0.35 to 0.84) | 0.5 | ||||||||
| Cooke et al.48 (2018) | Yes | Stationary cycling | 3 | RPE 12–16 | Not stated | 16 | Usual care | PWV central (m/s) (primary) | 0.20 (−0.1 to 0.9) | −1.0 (−2.0 to 0.5) | — | — | 0.03 |
| AI×75 (%) (secondary) | 3.5 (1.0–8.5) | −2 (−4.5 to 1.0) | — | — | 0.01 | ||||||||
| Graham-Brown et al.66 (2021) | Yes | Stationary cycling | 3 | RPE 12–14 | 30 | 26 | Usual care | PWV central (m/s) | 0.36 (0.76) | −2.4 (0.76) | −2.8 (0.15) | −2.07 (−3.2 to −0.99) | <0.001 |
| Greenwood et al.67 (2021) | Yes | Stationary cycling, lower-extremity muscular strengthening | Aerobic 3×, resistance 2× | 7 | 21–30 min aerobic; three sets of lower extremity muscular conditioning exercises | 26 | Usual care | PWV central (m/s) (secondary) | −0.32 | −0.04 | −0.28 | — | — |
| Koh et al.47 (2010) | Yes | Stationary cycling | 3 | RPE 12–13 | Progress from 15 to 45 | 26 | Usual care | PWV central (m/s) (primary) | 0.5 (1.1) | −0.3 (1.1) | −0.8 (−2.1 to 0.5) | — | 0.4 |
| PWV peripheral (m/s) (secondary) | 0.7 (0.7) | −0.3 (0.42) | 1.0 (0.2) | — | 0.2 | ||||||||
| AI (%) (secondary) | 2 (6) | 1 (4) | −1 (−10.2 to 8.1) | — | 0.8 | ||||||||
| AI×75 (%) (secondary) | 2 (6) | 1 (4) | −1 (−9.5 to 6.6) | — | 0.7 | ||||||||
| Mihaesu et al.73 (2013) | No | Elastic bands, dumbbells, ankle weights | 3 | RPE 12–14 and increase of exercise HR of 15–30 bpm | 40 | 12 | Usual care | PWV central (m/s) (primary) | 1.3 (0.9) | −1.0 (0.7) | −2.3 (0.3) | — | 0.03 |
| AI (%) (secondary) | −3.8 (6.7) | −3.5 (5.4) | −0.4 (2.2) | — | 0.1 | ||||||||
| Oliveira E Silva et al.64 (2019) | Yes | Stationary cycling | 3 | RPE 13; 65%–75% maximum HR | 30 | 17 | Usual care | PWV central (m/s) (primary) | −0.2 (1.4) | −0.4 (1.1) | −0.2 (0.5) | — | 0.3 |
| AI (%) (primary) | 4.7 (3.8) | 3.8 (5.3) | −0.9 (1.7) | — | 0.06 | ||||||||
| Toussaint et al.69 (2008) | Yes | Stationary cycling | 3 | None stated | None stated | 13 | Usual care | PWV central (m/s) (primary) | 10.2 (0.7) | 9.0 (0.6) | 2.3 (0.2) | — | — |
| AI (%) (secondary) | 33.9 (2.9) | 28.4 (2.4) | 6.5 (0.5) | — | — | ||||||||
| BP | |||||||||||||
| Agustin et al.71 (2022) | No | Not stated (? aerobic) | 2 | Not stated | 30–45 | 8 | Usual care | SBP (primary) | 4.2 | −9.07 | 13.27 | — | — |
| DBP (primary) | 0.8 | −9.34 | 10.14 | — | — | ||||||||
| Anderson et al.59 (2004) | No | Stationary cycling | 3 | RPE somewhat hard | 30 | 13 | Usual care periods | 44-h ambulatory SBP (primary) | N/A | −12.5 (9.37) | — | — | <0.05 (repeated measures ANOVA 0, 3, 6 mo time points) |
| 44-h ambulatory DBP (primary) | N/A | −9.3 (4.85) | — | — | 0.05 (repeated measures ANOVA at 0, 3, 6 mo time points) | ||||||||
| Chan et al.54 (2017) | No | Ten muscle groups using free weights, tubing, and resistance machine | 3 | Week 1–4 RPE 12–14; 12–15 rep Week 5–12 RPE 14–15; 10–12 rep | 30 | 12 | Usual care | SBP (secondary) | −6 (7.98) | −7 (9.88) | 1 (3.17) | Relative ES: 0.05 (−0.64 to 0.54) | Week 13 versus week 0: 0.28 Week 26 versus week 0: 0.25 Whole time: 0.17 |
| DBP (secondary) | −1 (3.04) | −1 (3.69) | 0 (1.19) | Relative ES: 0.02 (−0.61 to 0.57) | Week 13 versus week 0: 0.55 Week 26 versus week 0: 0.54 Whole time: 0.16 |
||||||||
| Central PP (secondary) | −4 (7.31) | −4 (9.04) | 0 (2.90) | Relative ES: 0.03 (−0.62 to 0.56) | Week 13 versus week 0: 0.39 Week 26 versus week 0: 0.4 Whole time: 0.22 |
||||||||
| Central SBP (secondary) | −6 (8.12) | −5 (10.29) | −1 (3.27) | Relative ES: 0.04 (−0.55 to 0.63) | Week 13 versus week 0: 0.31 Week 26 versus week 0: 0.48 Whole time: 0.19 |
||||||||
| Central DBP (secondary) | −1 (3.18) | −1 (3.89) | 0 (1.25) | Relative ES: 0.08 (−0.51 to 0.67) | Week 13 versus week 0: 0.4 Week 26 versus week 0: 0.71 Whole time: 0.17 |
||||||||
| Peripheral PP (secondary) | −4 (7.17) | −5 (8.63) | 1 (2.80) | Relative ES: 0.05 (−0.64 to 0.54) | Week 13 versus week 0: 0.32 Week 26 versus 0: 0.28 Whole time: 0.16 |
||||||||
| Cheng et al.61 (2019) | Yes | Weight-bearing arm curl, arm raise, and leg raises | 3 | RPE 11–12 6-(6–20 scale) | 20 | 104 | Usual care | Predialysis SBP (primary) | 4.83 (3.19) | 1.26 (3.42) | 3.57 (0.66) | — |
P = 0.413 (control group) P = 0.597 (exercise group) P = 0.694 (baseline) P = 0.510 (year 1) |
| Predialysis DBP (primary) | −0.33 (2.15) | −1.58 (2.54) | 1.25 (0.47) | — |
P = 0.117 (control group) P = 0.234 (exercise group) P = 0.497 (baseline) P = 0.328 (year 1) |
||||||||
| Fernandes et al.62 (2019) | Yes | Stationary cycling | 3 | — | 50 | 8 | Usual care | Predialysis SBP (primary) | 2.63 (5.16) | 1.5 (4.57) | 1.13 (1.56) | — | 0.738 |
| Predialysis DBP (primary) | 2.9 (3.56) | 3.5 (2.90) | −0.6 (1.04) | — | 0.864 | ||||||||
| Graham-Brown et al.66 (2021) | Yes | Stationary cycling | 3 | RPE 12–14 | 30 | 26 | Usual care | Interdialytic SBP (mm Hg) | −4.9 (4.41) | −6 (4.22) | 1.1 (0.65) | −6.8 (−17.2 to 3.6) | 0.2 |
| Interdialytic DBP (mm Hg) | 0.6 (2.46) | −2 (2.33) | 2.6 (0.36) | −1.95 (−7.6 to 3.7) | 0.5 | ||||||||
| Predialysis SBP (mm Hg) | −6.7 (3.93) | −4 (2.91) | −2.7 (0.47) | 1.32 (−10.5 to 13.2) | 0.8 | ||||||||
| Predialysis DBP (mm Hg) | −2.5 (2.26) | −3.2 (1.81) | 0.7 (0.28) | 0.5 (−5.7 to 6.7) | 0.9 | ||||||||
| Guio et al.55 (2017) | No | Stationary cycling | 3 | Mean modified Borg RPE 1.1±1.1 to 1.4±0.9 | ≤30 | 17 | Usual care | Central SBP (secondary) | — | 2.1 (4.06) | — | — | 0.951 (ANOVA) |
| Central DBP (secondary) | — | 4.1 (3.26) | — | — | 0.328 (ANOVA) | ||||||||
| Huang et al.63 (2020) | Yes | Progressively increasing combined aerobic and resistance stationary cycling | 3 | RPE of 12–14 | 40 | 24 | Sham exercises | Predialysis SBP (secondary) | −1.63 (6.06) | −8.42 (5.20) | 6.79 (2.00) | — | >0.05 (control group) <0.05 (exercise group) |
| Predialysis DBP (secondary) | −2.32 (3.02) | 3.57 (2.62) | −5.89 (1.00) | — | >0.05 (control group) <0.05 (exercise group) |
||||||||
| Koh et al.47 (2010) | Yes | Stationary cycling | 3 | RPE 12–13 | Progress from 15 to 45 | 26 | Usual care | Predialysis SBP (secondary) | −9 (8.53) | −9 (8.03) | 0 (2.98) | — | 0.7 |
| Predialysis DBP (secondary) | −5 (4.37) | 1 (5.84) | −6 (1.86) | — | 0.9 | ||||||||
| PP (secondary) | −4 (6.54) | −3 (7.30) | −1 (2.50) | — | 0.9 | ||||||||
| MAP (secondary) | −6 (6.18) | −6 (4.93) | 0 (2.00) | — | 0.9 | ||||||||
| Central SBP (secondary) | −7 (8.11) | −8 (8.22) | 1 (2.94) | — | 0.9 | ||||||||
| Central DBP (secondary) | −4 (4.72) | −7 (4.02) | 3 (1.57) | — | 0.8 | ||||||||
| Central PP (secondary) | −2 (5.13) | −2 (7.49) | 0 (2.32) | — | 0.9 | ||||||||
| Liao et al.50 (2016) | Noa | Stationary cycling | 3 | RPE 12–15 | 30 | 12 | Usual care | Pre-exercise SBP (secondary) | 1.3 (5.43) | −42.1 (14.85) | 43.4 (3.53) | — | <0.05 |
| Pre-exercise DBP (secondary) | 2.5 (4.24) | −23.1 (8.11) | 25.6 (2.05) | — | >0.05 | ||||||||
| Mihaescu et al.73 (2013) | No | Elastic bands, dumbbells, ankle weights | 3 | RPE 12–14 and HR increase by 15–30 bpm with exercise | 40 | 12 | Usual care | Predialysis SBP (secondary) | −14.4 (12.97) | −8.9 (6.29) | −5.5 (3.77) | — | 0.697 |
| Predialysis DBP (secondary) | −2.81 (8.98) | −6 (4.55) | 3.19 (2.63) | — | 0.578 | ||||||||
| Predialysis PP (secondary) | −11.63 (5.71) | −1.68 (3.28) | −9.95 (1.71) | — | 0.926 | ||||||||
| Predialysis MAP (secondary) | −6.6 (10.12) | −8.16 (4.90) | 1.56 (2.94) | — | 0.618 | ||||||||
| Central SBP (secondary) | −5 (11.04) | −11.2 (7.17) | 6.2 (3.40) | — | 0.302 | ||||||||
| Miller et al.74 (2002) | No | Stationary cycling | 3 | Exercise as long as possible; increase time by 1–5 min/session. At 30 min, increase resistance | 30 | 26 | Usual care | Predialysis SBP (secondary) | 2.4 | 0 | 2.4 | — | >0.05 |
| Predialysis DBP (secondary) | 0 | −2.4 | 2.4 | — | >0.05 | ||||||||
| Musavian et al.57 (2015) | Stationary cycling | 3 | Not stated | 30 min; three 10-min bouts of exercise, 20-min recovery between each bout | 8 | Passive cycling with electrically powered bike | Predialysis SBP (secondary) | −0.07 (0.77) | 1.06 (0.70) | −1.13 (0.26) | — |
P = 0.058 (control group) P = 0.255 (exercise group) |
|
| Predialysis DBP (secondary) | −0.37 (0.25) | 0.48 (0.29) | −0.85 (0.10) | — |
P = 0.039 (control group) P = 0.296 (exercise group) |
||||||||
| Painter et al.75 (1986) | Stationary cycling | 3 (one participant cycled 2x/wk) | 65% of VO2 max initially, 75%–85% of VO2max after 30 min/session reached | 5 min/session start; increase by 2–5 min/session until 30 min/session | 26 | Usual care | Predialysis SBP (secondary) | 0 (8.57) | −14 (12.90) | 14 (4.94) | — | 3 mo >0.05 (control group) >0.05 (exercise group) 6 mo <0.05 (exercise group) |
|
| Palar and Lobo,77 (2022) | Resistance and ROM for upper and lower extremities | 2: n=11 (57.9%). 3: n=8 (42.1%) | Not stated | 25 | 13.0357 | Usual care | SBP (secondary) | −5 (3.292) | −7.9 (4.801) | 2.9 (1.375) | — | 0.383 | |
| DBP (secondary) | −0.59 (2.675) | 0 (3.163) | −0.59 (0.987) | — | 0.990 | ||||||||
| Parsons et al.51 (2004) | Stationary cycling | 3 | 40–50% maximal work capacity | 45 min (15-min increments in first 3 h of dialysis) | 8 | Usual care | Predialysis SBP (secondary) | — | — | — | — | >0.05 (control group) >0.05 (exercise group) |
|
| Predialysis DBP (secondary) | — | — | — | — | >0.05 (control group) >0.05 (exercise group) |
||||||||
| Predialysis PP (secondary) | 3 | −3 | 6 | — | >0.05 (control group) >0.05 (exercise group) |
||||||||
| Petraki et al.52 (2008) | Stationary cycling, strengthening exercises, flexibility exercises | 3 | RPE 13 | 90 | 30 | Usual care | Predialysis SBP (primary) | −2 (4.79) | −8.3 (4.54) | 6.3 (1.42) | — | <0.05 | |
| Predialysis DBP (primary) | −1.17 (2.30) | −6 (2.60) | 4.83 (0.75) | — | <0.05 | ||||||||
| Reboredo et al.53 (a) (2010) | Stationary cycling | 3 | RPE 11–13 | Not stated | 12 | Usual care | 24-h ambulatory SBP (secondary) | −4.8 (7.16) | −7.1 (6.29) | 2.3 (2.55) | — | <0.05 | |
| 24-h ambulatory DBP (secondary) | −1.8 (4.10) | −3.2 (3.82) | 1.4 (1.50) | — | <0.05 | ||||||||
| Thenmozhi et al.76 (2018) | Stationary cycling | 3/wk, except 33.85% of exercise patients exercised 2×/wk | “According to the tolerance of the (patients)” No other info | 10–15 | 12 | Usual care | Predialysis SBP (secondary) | 0.16 (0.30) | −8.75 (0.27) | 8.91 (0.05) | — | <0.001 | |
| Predialysis DBP (secondary) | 0.88 (0.27) | −2.46 (0.24) | 3.34 (0.04) | — | P < 0.001 | ||||||||
| Toussaint et al.69 (2008) | Stationary cycling | 3 | None stated | None stated | 13 | Usual care | Predialysis SBP (secondary) | −0.42 (5.44) | −8.21 (5.94) | 7.78 (1.85) | — | 0.99 | |
| Predialysis DBP (secondary) | −1.25 (2.65) | 0.19 (2.83) | −1.44 (0.89) | — | — | ||||||||
| Predialysis PP (secondary) | 1.19 (4.44) | −9.06 (4.52) | 10.25 (1.45) | — | — | ||||||||
| Left ventricular structure and function (echocardiogram/CMRI) | |||||||||||||
| Graham-Brown et al.66 (2021) | Yes | Stationary cycling | 3 | RPE 12–14 | 30 | 26 | Usual care | LV Mass (g) (primary) | 1.6 (7.3) | −10 (8.6) | −11.6 | −11.1 (−15.8 to −6.4) | <0.001 |
| LV Mass index (g/m2) (secondary) | 1.1 (3.7) | −5.5 (4.3) | −6.6 | −6.3 (−9.2 to −3.4) | <0.001 | ||||||||
| LV end diastolic volume (ml) (secondary) | 0.9 (11.7) | 3.0 (12.7) | 2.1 | 0.5 (−14.2 to 21.2) | 0.6 | ||||||||
| LV ejection fraction (%)a (secondary) | 0.1 (11.3) | 2.5 (2.3) | 2.4 | 0.03 (−0.8 to 4.8) | 0.9 | ||||||||
| Guio et al.55 (2017) | No | Ergometer | 3 | Target HR (no specifics) | 30 | 17 | Usual care Single-group |
LV ejection fraction (%) (secondary) | 65.7 (10.2)a | 73.6 (10.1) | 7.9 (4.0) | — | 0.03 |
| LV systolic diameter (mm) (secondary) | 32.4 (6.7) | 29.8 (4.6) | −2.6 (2.2) | — | 0.3 | ||||||||
| LV diastolic diameter (mm) (secondary) | 54.5 (49.0–56.0) | 56 (53.0–58.0) | — | — | 0.03 | ||||||||
| LV posterior wall (mm) (secondary) | 12.2 (1.8) | 13.3 (3.3) | 1.1 (1.0) | — | 0.9 | ||||||||
| Kouidi et al.60 (2009) | Yes | Stationary cycling, strengthening exercises | 3 | RPE 13 | 90 | 44 | Usual care | LV ejection fraction (primary) | 0.2 (7.7) | 3.4 (3.9) | 3.2 (1.0) | — | 0.05 |
| LV mass index (g/m2) (secondary) | 1.2 (4.2) | 3.4 (6.3) | 2.2 (0.8) | — | 0.1 | ||||||||
| Momeni et al.32 (2014) | Yes | Stationary cycling | 3 | Not stated | 30 | 13 | Usual care | LV ejection fraction (primary) | 0.3 (1.8) | 3.5 (1.03) | 3.2 (0.5) | — | 0.01 |
| LV systolic diameter (mm) (secondary) | 2.5 (2.7) | 1.8 (1.8) | −0.7 (0.7) | — | 0.1 | ||||||||
| LV diastolic diameter (mm) (secondary) | 1. 2 (2.1) | −0.1 (2.4) | −1.3 (0.7) | — | 0.9 | ||||||||
| Severe LVH (% participants) (secondary) | 0 | 0 | 0 | — | — | ||||||||
| Moore et al.56 (1993) | No | Stationary cycling | 3 | RPE 6/10; 70% peak HR | 30–60 | 12 | Unloaded cycling at <50 rpm | Stroke volume (secondary) | 3 (2.9) | 3 (2.3) | 0 (1.2) | — | — |
| CO (L/min) | 0.3 (0.5) | 0.0 (0.5) | −0.3 (0.2) | — | — | ||||||||
| Oliveira E Silva et al.64 (2019) | Yes | Stationary cycling | 3 | RPE 13; 65%–75% of maximum HR | 30 | 17 | Usual care | LV mass index (g/m2) (secondary) | −1.2 (6.5) | −3.6 (6.7) | −2.4 (2.5) | — | 0.2 |
| LV mass (g) (secondary) | −4 (22.1) | −13 (20.5) | −9 (8.2) | — | 0.2 | ||||||||
| LV systolic diameter (mm) (secondary) | −0.1 (1.2) | 0.3 (2.3) | 0.4 (0.7) | — | 0.7 | ||||||||
| LV diastolic diameter (mm) (secondary) | 0.1 (1.7) | 0.4 (1.9) | 0.3 (0.7) | — | 0.4 | ||||||||
| LV posterior wall (mm) (secondary) | −0.2 (0.7) | −0.7 (0.7) | −0.5 (0.3) | — | 0.07 | ||||||||
| Reboredo et al.58 (2010)b | Yes | Stationary cycling, stretches | 3 | RPE 4–6 | 15-min warmup; ≤35-min conditioning; 1–3 min of cool-down | 12 | Usual care | LV mass index (g/m2) (secondary) | −1.3 (19.6) | 2.2 (10.6) | 3.5 (6.7) | — | — |
| LV ejection fraction (%) (secondary) | −2.2 (3.2) | 2.9 (5.0) | 5.1 (1.8) | — | — | ||||||||
| Stroke volume (ml) (secondary) | −2.2 (9.9) | 13.9 (8.1) | 16.1 (3.8) | — | — | ||||||||
| LV end systolic volume (ml) (secondary) | 2.6 (7.8) | 2.4 (11.3) | −0.2 (4.1) | — | — | ||||||||
| LV end diastolic volume (ml) (secondary) | 0.4 (15.6) | 20 (16.5) | 19.6 (6.8) | — | — | ||||||||
| HRV | |||||||||||||
| Kouidi et al.60 (2009) | Yes | Stationary cycling, strengthening exercises | 3 | RPE 13 | 90 | 44 | Usual care | SDNN (ms) (secondary) | −1.1 (10.2) | 12.6 (16.3) | 14.0 (1.3) | — | <0.001 |
| Mean RR interval (ms) (secondary) | −11.4 (68.2) | 23.1 (61.4) | 34.6 (7.4) | — | 0.05 | ||||||||
| LF/HF ratio | −0.1 (0.3) | 0.3 (0.4) | 0.5 (0.042) | — | <0.001 | ||||||||
| Kouidi et al.49 (2010) | Noa | Stationary cycling, strengthening exercises with increasing workload and repetitions | 3 | RPE 11–13 | 60–90 | 52 | Usual care | SDNN (ms) (secondary) | −1.4 (7.8) | 60.9 (6.7) | 62.3 (1.9) | — | <0.001 |
| MSSD (ms) (secondary) | −0.2 (0.2) | 15.8 (2.19) | 16.0 (0.6) | — | <0.001 | ||||||||
| NN50 (ms) (secondary) | −0.2 (0.2) | 0.8 (0.2) | 1.0 (0.1) | — | <0.001 | ||||||||
| LF/HF ratio | −0.1 (0.1) | 0.2 (0.1) | 0.4 (0.03) | — | <0.001 | ||||||||
| Pereira et al.68 (2022) | Yes | Stationary cycling | 3 | Warmup and cool-down: 60%–70% maximal HR RPE 1–2/10 Conditioning Phase 70–80% maximal HR RPE 3–4/10 |
30 (5 min warmup and cool-down, 20 conditioning) | 13.036 | Usual care | RMSSD (not stated primary or secondary) | −3.2 (1.096) | 5 (3.054) | −8.20 (0.513) | 1.2 | 0.562 |
| SDNN (not stated primary or secondary) | −2.6 (1.336) | 8.3 (2.567) | −10.90 (0.458) | 1.2 | 0.49 | ||||||||
| SD1 (SD of instantaneous beat-to-beat variability) (not stated primary or secondary) | −1.9 (0.767) | 0.7 (2.006) | −2.60 (0.340) | 1.2 | 0.383 | ||||||||
| SD2 (long-term SD of continuous RR intervals) (not stated primary or secondary) | −5 (3.125) | 7.7 (3.030) | −12.70 (0.688) | 1.2 | 0.448 | ||||||||
| Reboredo et al.58 (2010)b | Yes | Stationary cycling, stretches | 3 | RPE 4–6 | 15-min warmup ≤35-min conditioning 1–3 min of cool-down |
12 | Usual care | SDNN (ms) (primary) | 13.4 (11.7) | −7.27 (10.9) | −20.63 (4.83) | — | Reported as NS |
| RMSSD (ms) (secondary) | 1.4 (3.19) | 1.5 (4.20) | 0.10 (1.60) | — | Reported as NS | ||||||||
| pNN50 (%) (secondary) | N/A (median and IQR) | N/A (median and IQR) | N/A (median and IQR) | — | Reported as NS | ||||||||
| LF/HF ratio | 0.1 (0.94) | 0.3 (1.08) | 0.20 (0.43) | — | Reported as NS | ||||||||
| Endothelial function | |||||||||||||
| Chan et al.54 (2017) | No | Ten muscle groups using free weights, tubing, and resistance machine | 3 | Week 1–4 RPE 12–14; 12–15 rep Week 5–12 RPE 14–15; 10–12 rep |
30 | 13 | Usual care | EPC count (secondary) | Pre: 0.044 (0.028) | Post: 0.039 (0.033) | −0.024 (−0.043 to 0.005) | 0.77 (−1.38 to −0.16) | 0.53 |
| Liao et al.50 (2016) | No | Stationary cycling | 3 | RPE 12–15 | 30 | 12 | Usual care | EPC count (secondary) | No accurate numbers reported | No accurate numbers reported | No accurate numbers reported | No accurate numbers reported | <0.05 |
| Cardiac biomarkers | |||||||||||||
| Graham-Brown et al.66 (2021) | No | Stationary cycling | 3 | RPE 12–14 | 30 | 26 | Usual care | NT-pro-brain natriuretic peptide (pg/ml) (secondary) | BL: 3566.0 (1220–11,121) Post: 2597 (1173–11,319) |
BL: 2515 (1015–11,443) Post: 3721 (1151–11,801) |
— | 0.2 (−0.2 to 0.5) | 0.9 |
| Toussaint et al.69 (2008) | No | Stationary cycling | 3 | No scaled target | None stated | 13 | Usual care | Brain natriuretic peptide (pg/ml) (secondary) | 351 (102) | −57 (70) | 408 (29) | — | — |
| Graham-Brown et al.66 (2021) | No | Stationary cycling | 3 | RPE 12–14 | 30 | 26 | Usual care | Troponin I (ng/L) (secondary) | −4.4 (7.347) | −37.3 (23.778) | −32.9 (3.087) | 0.86 (−10.8 to 12.5) | 0.9 |
| Baroreflex sensitivity | |||||||||||||
| Petraki et al.52 (2008) | No | Stationary cycling, strengthening exercises, flexibility exercises | 3 | RPE 13 | 90 | 30 | Usual care | Baroreflex sensitivity (ms/mm Hg) (primary) | 0.2 (0.5) | 1.4 (0.5) | 1.6 (0.2) | — | <0.05 |
| Cardiovascular-related hospitalization | |||||||||||||
| Graham-Brown et al.66 (2021) | Stationary cycling | 3 | RPE 12–14 | 30 | 26 | Usual care | |||||||
| Isnard-Rouchon et al.72 (2017) | No | Aerobic virtual reality exercise | 2 | 40–80 rpm without resistance | 30 | 104 | Usual care period | Number of cardiovascular hospitalizations (secondary) | 14 | 5 | −9 | — | Not reported |
| Cardiovascular mortality | |||||||||||||
| Greenwood et al.67 (2021) | No | Stationary cycling, lower-extremity muscular strengthening | Aerobic 3×, resistance 2× | 40%–75% VO2 reserve | 21–30 min of aerobic; three sets of lower-extremity muscular conditioning exercises | 26.07 | Usual care | Number of cardiovascular deaths (secondary) | 3/160 (1.9/100 person-years) | 2/174 (1.3/100 person-years) | N/A | N/A | N/A |
AI, augmentation index; CI, confidence interval; CO, cardiac output; DBP, diastolic BP; EPC, endothelial progenitor cell; HR, heart rate; HRV, heart rate variability; IQR, interquartile range; LF/HF, low-frequency/high-frequency; LV, left ventricular; LVH, left ventricular hypertrophy; MAP, mean arterial pressure; MD, mean difference; pNN50, percentage of adjacent NN intervals that differ from each other by more than 50 ms; PP, pulse pressure; PWV, pulse-wave velocity; RMSSD, root mean square of successive differences; RPE, rating of perceived exertion; rpm, repetitions per minute; SBP, systolic BP; SDNN, SD of NN intervals; VO2, rate of oxygen consumption.
Not included in meta-analysis because of concerns regarding heterogeneity (magnitude of change reported much greater than all other studies).
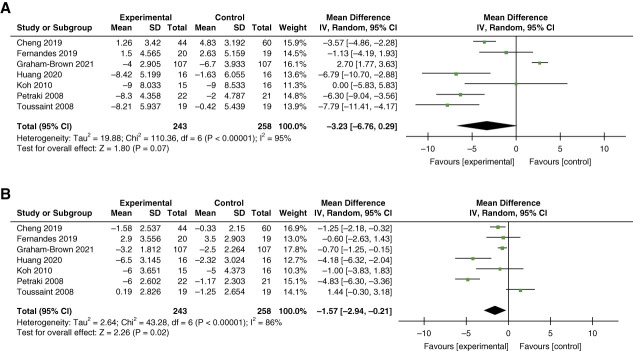
Of the seven RCTs eligible for meta-analysis, five studies used intradialytic cycling for the exercise intervention, one study used resistance exercise, and one study used combined resistance and aerobic exercise.47,50,52,61–63,66,69 Intervention duration ranged from 8 to 104 weeks but was more than 24 weeks in most studies. All but one study reported predialysis BP. In a total of 263 individuals who participated in the exercise intervention as compared with 78 controls, we observed no statistically significant between-group MD in SBP of –3.23 mm Hg (95% CI, −6.76 to 0.29; I2=95%; P < 0.001) but a statistically significant MD in DBP of –1.57 mm Hg (95% CI, −2.94 to −0.21; I2=86%; P < 0.001) (Figure 3).
Figure 3.
Meta-analysis of studies examining the effect of intradialytic exercise. (A) SBP and (B) DBP. DBP, diastolic BP; SBP, systolic BP.
Myocardial Function
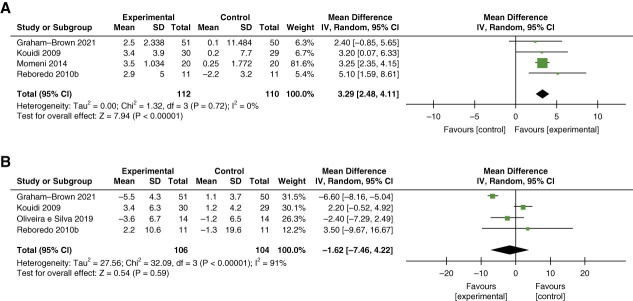
Five studies (n=236) examined LVEF, measured using echocardiography or cardiac MRI. 32,53,55,60,66 Meta-analysis of four studies (n=222), which involved intradialytic cycling for 30–90 minutes per session and intervention duration of 12–44 weeks, demonstrated a small statistically significant MD in LVEF of 3.29% (95% CI, 2.48 to 4.11; I2=0%; P = 0.72) with exercise as compared with nonexercise controls (Figure 4A).32,53,60,66
Figure 4.
Meta-analysis of studies examining the effect of intradialytic exercise on myocardial function and structure. (A) LVEF and (B) LVMI. LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Myocardial Structure
In total, six studies (total n=315) examined outcomes related to myocardial structure (Table 4).32,53,55,60,64,66 Only left ventricular mass index (LVMI) was eligible for meta-analysis, including four RCTs (n=210) which demonstrated no statistically significant change in LVMI in individuals who completed 12–44 weeks of intradialytic cycling for 30–90 minutes per session as compared with no exercise controls (MD –1.62 g/m2; 95% CI, −7.46 to 4.22; I2=91%; P < 0.001) (Figure 4B).53,60,64,66
Cardiac Autonomic Dysfunction/Increased Sympathetic Activity
Four RCTs (total n=205) assessed the effect of intradialytic cycling for 30–90 minutes, thrice weekly over 12–52 weeks of duration on various measures of HRV (Table 4).49,53,60,68 Meta-analysis of these studies showed no statistically significant difference in the SD of normal R-R intervals during a 24-hour period (SD of N-N intervals [SDNN]) in the exercise group as compared with the control group (MD 16.05 ms; 95% CI, −14.60 to 46.70; I2=99%; P < 0.001). By contrast, meta-analysis of three RCTs demonstrated a statistically significant between-group difference in low-frequency/high-frequency (LF/HF) ratio of 0.38 (95% CI, 0.32, 0.44; I2=0%; P = 0.90) (Supplemental Figure 2).49,53,60
Summative review of studies not included in the meta-analysis for each outcome above is provided in Supplemental Appendix.
Endothelial Function
In a single RCT (n=40), Liao et al. demonstrated a statistically significant increase in circulating endothelial progenitor cell count after 12 weeks of aerobic exercise compared with no exercise (P < 0.05; no change measure reported).50 By contrast, a pre/post study (n=22) showed no significant change in endothelial progenitor cell count with 12 weeks of resistance exercise MD −0.024 (95% CI, −0.043 to 0.005; P = 0.53).54
Biomarkers of Cardiac Injury
No changes were observed in predialysis N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin I levels in two RCTs evaluating intradialytic cycling for 6 months (n=101) and 3 months of (n=19) duration (Table 4).66,69
Cardiovascular Hospitalization and Mortality
One cohort study (n=66) of intradialytic cycling for 30 minutes twice weekly for 2 years demonstrated fewer cardiovascular hospitalizations with exercise (n=5) than in controls (n=14) in unadjusted analysis.72 One RCT of intradialytic cycling for 6 months showed a decline in overall number of cardiovascular hospital admissions during (n=8) and 6 months after (n=5) the intervention period as compared with the pretrial period (n=9) in the exercise group, while admissions increased during (n=3) and after (n=7) the trial period compared with pretrial in controls (n=1) (Table 4).78
An RCT studying a 6-month combined intradialytic cycling and resistance program demonstrated no significant difference in number of cardiovascular deaths in the exercise group (n=2; 1%) as compared with nonexercise controls (n=3; 2%) (Table 4).67
Adverse Events, Adherence, and Study Attrition
Only 19/32 studies reported adverse events.47–50,52,53,58,60,61,65–70,72,74,75,77 Across these studies, 248 adverse events occurred, with 135 (54.4%) occurring in the intervention groups and 113 (45.6%) in the control groups. Of the adverse events in the intervention group, only one was identified to be related to exercise.67
Attrition ranged from 0% to 61%, with only 4/32 studies reporting no withdrawals.50,65,71,76 Of 1817 participants in all studies, 367 withdrew (20%). One study did not report attrition.32
Adherence to exercise intervention was reported in 44% of studies (14/32). Adherence was variable in these 14 studies, ranging from 28% to 92% of total possible exercise sessions completed. Overall, participants completed a mean 74.9% of prescribed exercise sessions and between 21 and 60 minutes of exercise per session.47–49,53,54,58–60,66,67,69,70,74,75
Discussion
This systematic review and meta-analysis of the effect of intradialytic exercise on cardiovascular outcomes in hemodialysis demonstrated significant improvements in multiple physiological cardiovascular outcomes, including PWV, DBP, LVEF, and HRV measured by the LF/HF ratio.
Mean change observed in PWV (−1.64 m/s) was statistically and clinically significant. Multiple studies have established a minimal clinically important difference of >1 m/s for PWV in people with kidney failure.79,80 One study of 242 people receiving hemodialysis with a follow-up time of 78±46 months demonstrated a 14% increase in cardiovascular and overall mortality with each 1 m/s increase in PWV.80 Similarly, in a study involving 1497 kidney transplant recipients with a median follow-up of 4.2 years (interquartile range, 3.0–5.3), each 1 m/s increase in PWV measured 8 weeks post-transplant was associated with a 36% increase in mortality risk.79
Conversely, meta-analysis showed no statistically significant change in arterial resistance when measured by AI. Discordant results between AI and PWV measures of arterial resistance have been observed previously.81 PWV measures the speed at which the arterial pulse travels between two distant major arterial sites and is considered the gold standard.82 AI, a surrogate measure of arterial resistance, is less sensitive and specific than PWV and is affected by multiple clinical factors, including LVEF and peripheral hemodynamics, which were not adjusted for in included studies.81 In addition, insulin resistance has been shown to attenuate decreases in arterial resistance.83,84 When considering that diabetes is common in people receiving hemodialysis, this may explain why the observed change in AI was attenuated as compared with PWV. Consistent with this hypothesis, although not significant, the observed change in AI seen in our meta-analysis was overall in the same direction as PWV.
Similarly, meta-analysis of the LF/HF measure of HRV showed a statistically significant change, while SDNN did not. This may be due to inclusion of studies with relatively short intervention durations, which may not be a sufficient exercise dose to affect SDNN. This is supported by the 13-week intervention in the study of Pereira et al. that demonstrated minimal increase in measures of HRV, while increases in prolonged (44-week and 52-week) interventions were more pronounced.49,60,68 Importantly, this dose-response effect with longer intradialytic exercise interventions has been demonstrated for other outcomes, such as depression.29,85
In contrast to our findings for DBP, although meta-analysis for SBP showed a clear trend for clinically significant decrease with exercise, it did not reach statistical significance. The reason for this discrepancy is unclear, but increased heterogeneity identified in the meta-analysis for SBP as compared with that for DBP (I2 95% versus 86%, respectively) may have contributed. Studies included different populations, intervention designs (exercise type, intensity, session duration, and program length) and outcome measures, which contributed to increased heterogeneity. For instance, the 8-week duration in the study by Fernandes et al. may have been of insufficient in length to detect a measurable change in SBP, biasing its results and those of the meta-analysis toward the null.62
PWV and BP are closely related. Although arterial stiffness has traditionally been considered a consequence of arterial hypertension, a growing body of literature suggests that arterial stiffness is also an independent predictor of hypertension.86 Exercise may improve both PWV and BP through vasodilation, improvements in endothelial function, and increased arterial compliance in arteries of skeletal muscle.87
Notably, PWV improvements in the exercise group reversed after a 3–4-month washout period in two of our included studies.48,59,69 A similar reversion to preintervention BP levels after a post-training control period was seen in the study of Anderson et al.59 Other studies have demonstrated that the beneficial effects of acute exercise on PWV last 24 hours.87 Together, these findings suggest that the observed improvements in PWV and BP are likely attributable to intradialytic exercise and demonstrate a need for continued participation in regular exercise to maintain exercise-related benefits to cardiovascular health.
Meta-analysis for LVMI demonstrated no significant change. Interestingly, of the four studies included in the meta-analysis, two studies observed no significant change in LVMI. These included participants who had been receiving hemodialysis for longer (mean 3–7 years) compared with the two other studies that observed a significant difference in LVMI (mean 1–2 years). This observation suggests that people who are newer to hemodialysis may have greater improvement in LVMI with intradialytic exercise than those who have been on hemodialysis for many years.
Unmeasured heterogeneity could have additionally biased our overall findings related to SBP and LVMI. Depending on the setting and population, discrepancies in prevalence of unmeasured comorbidities, low adherence to exercise intensity targets, and low activity levels outside of dialysis because of geographic environment could have biased results toward the null.
Qualitative synthesis of studies measuring outcomes not eligible for meta-analysis was not substantially different from meta-analyses. The effect of intradialytic exercise was inconclusive because of few studies and low power for endothelial function, baroreflex function, biomarkers of myocardial injury, and cardiovascular mortality/hospitalization.
Earlier reviews exclusively included RCTs with small sample sizes and high risk of bias. Our review updates the effect of exercise on several important surrogate cardiovascular outcomes, including arterial resistance and BP, by including recently published large RCTs with improved reporting of methods of randomization, allocation concealment, and blinding of outcome assessors. We present meta-analysis results of the effect of intradialytic exercise on several outcomes that have not been explored in previous studies, including LVEF and LVMI, and summarize recent data regarding antihypertensive medication use, cardiac biomarkers, and cardiovascular hospitalization and mortality. Our findings expand on the findings of the benefits of intradialytic exercise to DBP and HRV observed in previous reviews.33,88–90
Strengths of this review include a robust search strategy including validated high-sensitivity filters and inclusion of studies that prespecified outcomes and were performed in diverse international settings. Compared with previous reviews, our updated review includes recent studies, a broader range of cardiovascular outcomes, and studies of resistance and combined aerobic and resistance training rather than solely aerobic exercise.30,88,90,91 Finally, we include qualitative analysis of non-RCTs to complement and support meta-analysis results.
Limitations include lack of important adherence outcomes in most studies. The number of completed exercise sessions was only reported in 44% of studies. Mean/median exercise intensity and exercise duration achieved per session was only reported in 3.1% and 15.6% studies, respectively. Earlier studies included have high risks of bias because of lack of information on randomization methods, allocation, and blinding. Many studies reported within-group change requiring estimation of SD for across group change, potentially reducing the accuracy of final meta-analysis results. Metaregression using patient and study-level factors, such as sex, exercise type, and intervention details including exercise intensity and duration, was not possible for most outcomes because of lack of data and small number of studies. Despite excluding studies with outlier results, meta-analyses for some outcomes demonstrated high heterogeneity, likely the result of heterogenous populations, interventions, and outcome measures.
As with many clinical trials in nephrology, most participants included were male (<50% female in 24 studies, ranging as low as 10%), younger (mean age 54.6 rather than 60 years) and healthier than most individuals receiving hemodialysis limiting the generalizability of our findings in female individuals, older individuals, and those with multiple comorbidities as is common in the hemodialysis population.91,92
This study, which demonstrates the benefits of intradialytic exercise on outcomes associated with major adverse cardiovascular event, has both clinical and research implications. First, because most studies included in this review implemented an aerobic exercise program, clinicians should consider including intradialytic aerobic exercise programs in hemodialysis care to supplement broader treatment plans aimed at improving cardiovascular health. Furthermore, our observation that cardiovascular gains regressed with cessation of exercise participation suggests the need for long-term exercise participation to maintain these health benefits.
Future RCTs with standardized outcomes validated in kidney failure that incorporate clinical outcomes, such as cardiovascular events and hospitalization, are required. As well, the cardiovascular effects of intradialytic resistance exercise are understudied and require further characterization. Moreover, given our observations in changes to outcomes associated with myocardial stunning (e.g., PWV and BP) with exercise, rigorous interventional trials are required to investigate the effect of exercise on myocardial stunning. Finally, there is an ongoing need for better information on exercise program design and implementation strategies that promote sustainability and adherence in diverse environments and populations, including incorporating individualized exercise plans or wearable technologies during hemodialysis.93
In conclusion, our findings suggest that intradialytic aerobic exercise effectively improves several physiological cardiovascular outcomes, including arterial resistance, BP, and HRV. Larger RCTs are required to determine whether such benefits lead to improvement in cardiovascular events and mortality in individuals receiving maintenance hemodialysis.
Supplementary Material
Disclosures
C. Bohm reports the following: Consultancy: GuidePoint; and Ownership Interest: Precision Advanced Digital Manufacturing. A.X. Garg reports the following: Employer: London Health Sciences Centre; Research Funding: Astellas and Baxter; Advisory or Leadership Role: Currently on the Editorial Boards of American Journal of Kidney Diseases and Kidney International; and Other Interests or Relationships: Served as the Medical Lead Role to Improve Access to Kidney Transplantation and Living Kidney Donation for the Ontario Renal Network (government funded agency located within Ontario Health). This position ended October 2022. C.W. McIntyre reports the following: Consultancy: Baxter, Intellomed, Sequana Medical, Spiden AG, and Vascular Dynamics; Research Funding: Baxter and Sequana; Honoraria: Baxter; and Advisory or Leadership Role: Baxter, Sequana Medical, and Spiden AG. R. Whitlock reports the following: Consultancy: Tricida Inc. All remaining authors have nothing to disclose.
Funding
Canadian Institutes of Health Research (MYG 151209). C. Bohm: Manitoba Medical Service Foundation (F.W. DuVal Clinical Research Professorship).
Author Contributions
Conceptualization: Ahmed A. Al-Jaishi, Clara Bohm, Amit X. Garg, Christopher W. McIntyre, Ajaya Sharma, Davide Verrelli.
Data curation: Jamie Alexiuk, Emilie Ford, Krista Rossum, Ajaya Sharma, Monica Sharma, Quinn Tays, Davide Verrelli.
Formal analysis: Clara Bohm, Emilie Ford, Ajaya Sharma, Monica Sharma, Quinn Tays, Davide Verrelli, Reid Whitlock.
Funding acquisition: Clara Bohm, Amit X. Garg.
Investigation: Clara Bohm, Davide Verrelli.
Methodology: Ahmed A. Al-Jaishi, Clara Bohm, Alla Iansavitchene, Davide Verrelli.
Project administration: Clara Bohm.
Resources: Alla Iansavitchene.
Supervision: Clara Bohm.
Validation: Davide Verrelli.
Writing – original draft: Jamie Alexiuk, Emilie Ford, Ajaya Sharma, Monica Sharma, Davide Verrelli.
Writing – review & editing: Ahmed A. Al-Jaishi, Jamie Alexiuk, Clara Bohm, Emilie Ford, Amit X. Garg, Alla Iansavichene, Christopher W. McIntyre, Krista Rossum, Ajaya Sharma, Monica Sharma, Quinn Tays, Davide Verrelli, Reid Whitlock.
Data Sharing Statement
All data are included in the manuscript and/or supporting information.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A428, http://links.lww.com/KN9/A429.
Summary of studies not included in meta-analysis
Supplemental Table 1. Search strategy for each database.
Supplemental Table 2. Risk of bias for non-RCT studies.
Supplementary Figure 1. Risk of bias for RCTs.
Supplemental Figure 2. Meta-analysis of studies examining the effect of intradialytic exercise on heart rate variability using SDNN and LF/HF.
References
- 1.United States Renal Data System. 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Chapter 9: Cardiovascular Disease in Patients with ESRD. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 2.Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33(suppl 3):iii28–iii34. doi: 10.1093/ndt/gfy174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Painter P. Physical functioning in end-stage renal disease patients: update 2005. Hemodial Int. 2005;9(3):218–235. doi: 10.1111/j.1492-7535.2005.01136.x [DOI] [PubMed] [Google Scholar]
- 4.Johansen KL. Exercise in the end-stage renal disease population. J Am Soc Nephrol. 2007;18(6):1845–1854. doi: 10.1681/ASN.2007010009 [DOI] [PubMed] [Google Scholar]
- 5.Mujais SK Story K Brouillette J, et al. Health-related quality of life in CKD Patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4(8):1293–1301. doi: 10.2215/CJN.05541008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansen KL, Kaysen GA, Young BS, Hung AM, da Silva M, Chertow GM. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2003;77(4):842–846. doi: 10.1093/ajcn/77.4.842 [DOI] [PubMed] [Google Scholar]
- 7.Tentori F Elder SJ Thumma J, et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant. 2010;25(9):3050–3062. doi: 10.1093/ndt/gfq138 [DOI] [PubMed] [Google Scholar]
- 8.Johansen KL Kaysen GA Dalrymple LS, et al. Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol. 2013;8(2):248–253. doi: 10.2215/CJN.08560812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30(2):204–212. doi: 10.1016/s0272-6386(97)90053-6 [DOI] [PubMed] [Google Scholar]
- 10.Mapes DL Lopes AA Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: the dialysis outcomes and practice patterns study (DOPPS). Kidney Int. 2003;64(1):339–349. doi: 10.1046/j.1523-1755.2003.00072.x [DOI] [PubMed] [Google Scholar]
- 11.McAdams-DeMarco MA Law A Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi: 10.1111/jgs.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAdams-DeMarco MA Suresh S Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14:224. doi: 10.1186/1471-2369-14-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sood MM Rigatto C Bueti J, et al. The role of functional status in discharge to assisted care facilities and in-hospital death among dialysis patients. Am J Kidney Dis. 2011;58(5):804–812. doi: 10.1053/j.ajkd.2011.06.017 [DOI] [PubMed] [Google Scholar]
- 14.McGuire S, Horton EJ, Renshaw D, Jimenez A, Krishnan N, McGregor G. Hemodynamic instability during dialysis: the potential role of intradialytic exercise. Biomed Res Int. 2018;2018:8276912. doi: 10.1155/2018/8276912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntyre CW Burton JO Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3(1):19–26. doi: 10.2215/CJN.03170707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby NM, McIntyre CW. The acute cardiac effects of dialysis. Semin Dial. 2007;20(3):220–228. doi: 10.1111/j.1525-139X.2007.00281.x [DOI] [PubMed] [Google Scholar]
- 17.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–920. doi: 10.2215/CJN.03900808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breidthardt T, McIntyre CW. Dialysis-induced myocardial stunning: the other side of the cardiorenal syndrome. Rev Cardiovasc Med. 2011;12(1):13–20. doi: 10.3909/ricm0585 [DOI] [PubMed] [Google Scholar]
- 19.Marants R, Qirjazi E, Grant CJ, Lee TY, McIntyre CW. Renal perfusion during hemodialysis: intradialytic blood flow decline and effects of dialysate cooling. J Am Soc Nephrol. 2019;30(6):1086–1095. doi: 10.1681/ASN.2018121194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfgram DF. Intradialytic cerebral hypoperfusion as mechanism for cognitive impairment in patients on hemodialysis. J Am Soc Nephrol. 2019;30(11):2052–2058. doi: 10.1681/ASN.2019050461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant CJ, Huang SHS, McIntyre CW. Hepato-splanchnic circulatory stress: an important effect of hemodialysis. Semin Dial. 2019;32(3):237–242. doi: 10.1111/sdi.12782 [DOI] [PubMed] [Google Scholar]
- 22.McIntyre CW. Effects of hemodialysis on cardiac function. Kidney Int. 2009;76(4):371–375. doi: 10.1038/ki.2009.207 [DOI] [PubMed] [Google Scholar]
- 23.Selby NM, Lambie SH, Camici PG, Baker CS, McIntyre CW. Occurrence of regional left ventricular dysfunction in patients undergoing standard and biofeedback dialysis. Am J Kidney Dis. 2006;47(5):830–841. doi: 10.1053/j.ajkd.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 24.Selby NM, Burton JO, Chesterton LJ, McIntyre CW. Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol. 2006;1(6):1216–1225. doi: 10.2215/CJN.02010606 [DOI] [PubMed] [Google Scholar]
- 25.McIntyre CW, Odudu A. Hemodialysis-associated cardiomyopathy: a newly defined disease entity. Semin Dial. 2014;27(2):87–97. doi: 10.1111/sdi.12197 [DOI] [PubMed] [Google Scholar]
- 26.Thijssen DHJ, Redington A, George KP, Hopman MTE, Jones H. Association of exercise preconditioning with immediate cardioprotection: a review. JAMA Cardiol. 2018;3(2):169–176. doi: 10.1001/jamacardio.2017.4495 [DOI] [PubMed] [Google Scholar]
- 27.Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. Br J Sports Med. 2015;49(21):1414–1422. doi: 10.1136/bjsports-2015-f5577rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crisafulli A Melis F Tocco F, et al. Exercise-induced and nitroglycerin-induced myocardial preconditioning improves hemodynamics in patients with angina. Am J Physiol Heart Circ Physiol. 2004;287(1):H235–H242. doi: 10.1152/ajpheart.00989.2003 [DOI] [PubMed] [Google Scholar]
- 29.Bernier-Jean A Beruni NA Bondonno NP, et al. Exercise training for adults undergoing maintenance dialysis. Cochrane Database Syst Rev. 2022;1(1):CD014653. doi: 10.1002/14651858.CD014653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheema BS, Singh MA. Exercise training in patients receiving maintenance hemodialysis: a systematic review of clinical trials. Am J Nephrol. 2005;25(4):352–364. doi: 10.1159/000087184 [DOI] [PubMed] [Google Scholar]
- 31.Crowley LE, McIntyre CW. Remote ischaemic conditioning-therapeutic opportunities in renal medicine. Nat Rev Nephrol. 2013;9(12):739–746. doi: 10.1038/nrneph.2013.226 [DOI] [PubMed] [Google Scholar]
- 32.Momeni A, Nematolahi A, Nasr M. Effect of intradialytic exercise on echocardiographic findings in hemodialysis patients. Iran J Kidney Dis. 2014;8(3):207–211. PMID: 24878943 [PubMed] [Google Scholar]
- 33.Young HML March DS Graham-Brown MPM, et al. Effects of intradialytic cycling exercise on exercise capacity, quality of life, physical function and cardiovascular measures in adult haemodialysis patients: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(8):1436–1445. doi: 10.1093/ndt/gfy045 [DOI] [PubMed] [Google Scholar]
- 34.Penny JD Salerno FR Brar R, et al. Intradialytic exercise preconditioning: an exploratory study on the effect on myocardial stunning. Nephrol Dial Transplant. 2019;34(11):1917–1923. doi: 10.1093/ndt/gfy376 [DOI] [PubMed] [Google Scholar]
- 35.Page MJ McKenzie JE Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guérin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15(7):1014–1021. doi: 10.1093/ndt/15.7.1014 [DOI] [PubMed] [Google Scholar]
- 37.Cho A Lee YK Oh J, et al. The relationship between intradialytic hypotension and vascular calcification in hemodialysis patients. PLoS One. 2017;12(10):e0185846. doi: 10.1371/journal.pone.0185846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwashima Y, Fukushima H, Horio T, Rai T, Ishimitsu T. Blood pressure, arterial waveform, and arterial stiffness during hemodialysis and their clinical implications in intradialytic hypotension. Hypertens Res. 2023;46(3):697–707. doi: 10.1038/s41440-022-01126-5 [DOI] [PubMed] [Google Scholar]
- 39.Iansavichus AV Haynes RB Lee CW, et al. Dialysis search filters for PubMed, Ovid MEDLINE, and embase databases. Clin J Am Soc Nephrol. 2012;7(10):1624–1631. doi: 10.2215/CJN.02360312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iansavichus AV Hildebrand AM Haynes RB, et al. High-performance information search filters for CKD content in PubMed, Ovid MEDLINE, and EMBASE. Am J Kidney Dis. 2015;65(1):26–32. doi: 10.1053/j.ajkd.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 41.Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. www.covidence.org [Google Scholar]
- 42.Sterne JA Hernán MA Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan R. Cochrane Consumers and Communication Review Group. ‘Cochrane Consumers and Communication Review Group: Data Synthesis and Analysis’; 2013. http://cccrg.cochrane.org [Google Scholar]
- 44.Chapter 4 “Systematic reviews of adverse effects” In: Systematic Reviews. CRD’s Guidance for Undertaking Reviews in Health Care. Centre for Reviews and Dissemination, University of York, 2008. ISBN 978-1-900640-47-3. [Google Scholar]
- 45.Higgins JPT Thomas J Chandler J, et al., eds. In: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (Updated March 2011). Cochrane; 2023. www.training.cochrane.org/handbook [Google Scholar]
- 46.Review Manager (RevMan) Version 5.3; 2014. The Nordic Cochrane Centre, the Cochrane Collaboration. [Google Scholar]
- 47.Koh KP, Fassett RG, Sharman JE, Coombes JS, Williams AD. Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis. 2010;55(1):88–99. doi: 10.1053/j.ajkd.2009.09.025 [DOI] [PubMed] [Google Scholar]
- 48.Cooke AB Ta V Iqbal S, et al. The impact of intradialytic pedaling exercise on arterial stiffness: a pilot randomized controlled trial in a hemodialysis population. Am J Hypertens. 2018;31(4):458–466. doi: 10.1093/ajh/hpx191 [DOI] [PubMed] [Google Scholar]
- 49.Kouidi E Karagiannis V Grekas D, et al. Depression, heart rate variability, and exercise training in dialysis patients. Eur J Cardiovasc Prev Rehabil. 2010;17(2):160–167. doi: 10.1097/HJR.0b013e32833188c4 [DOI] [PubMed] [Google Scholar]
- 50.Liao MT Liu WC Lin FH, et al. Intradialytic aerobic cycling exercise alleviates inflammation and improves endothelial progenitor cell count and bone density in hemodialysis patients. Medicine (Baltimore). 2016;95(27):e4134. doi: 10.1097/MD.0000000000004134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parsons TL, Toffelmire EB, King-VanVlack CE. The effect of an exercise program during hemodialysis on dialysis efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. Clin Nephrol. 2004;61(4):261–274. doi: 10.5414/cnp61261 [DOI] [PubMed] [Google Scholar]
- 52.Petraki M, Kouidi E, Grekas D, Deligiannis A. Effects of exercise training during hemodialysis on cardiac baroreflex sensitivity. Clin Nephrol. 2008;70(3):210–219. doi: 10.5414/cnp70210 [DOI] [PubMed] [Google Scholar]
- 53.Reboredo MdM Pinheiro BdV Neder JA, et al. Effects of aerobic training during hemodialysis on heart rate variability and left ventricular function in end-stage renal disease patients. J Bras Nefrol. 2010;32(4):367–373. PMID: 21541451 [PubMed] [Google Scholar]
- 54.Chan D, Green S, Fiatarone Singh MA, Barnard R, Bonder CS, Cheema BS. Effect of intradialytic resistance training on pulse wave velocity and associated cardiovascular disease biomarkers in end stage renal disease. Nephrology (Carlton). 2018;23(11):1055–1062. doi: 10.1111/nep.13212 [DOI] [PubMed] [Google Scholar]
- 55.Guio BM, Gomes CP, Costa FBD, Oliveira ADS, Duarte MT, Leite MLM, Jr. Beneficial effects of intradialytic cardiopulmonary rehabilitation. J Bras Nefrol. 2017;39(3):275–282. doi: 10.5935/0101-2800.20170051 [DOI] [PubMed] [Google Scholar]
- 56.Moore GE, Parsons DB, Stray-Gundersen J, Painter PL, Brinker KR, Mitchell JH. Uremic myopathy limits aerobic capacity in hemodialysis patients. Am J Kidney Dis. 1993;22(2):277–287. doi: 10.1016/s0272-6386(12)70319-0 [DOI] [PubMed] [Google Scholar]
- 57.Musavian AS, Soleimani A, Masoudi Alavi N, Baseri A, Savari F. Comparing the effects of active and passive intradialytic pedaling exercises on dialysis efficacy, electrolytes, hemoglobin, hematocrit, blood pressure and health-related quality of life. Nurs Midwifery Stud. 2015;4(1):e25922. doi: 10.17795/nmsjournal25922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reboredo MdM, Henrique DM, Faria RdS, Chaoubah A, Bastos MG, de Paula RB. Exercise training during hemodialysis reduces blood pressure and increases physical functioning and quality of life. Artif Organs. 2010;34(7):586–593. doi: 10.1111/j.1525-1594.2009.00929.x [DOI] [PubMed] [Google Scholar]
- 59.Anderson JE, Boivin MR, Jr, Hatchett L. Effect of exercise training on interdialytic ambulatory and treatment-related blood pressure in hemodialysis patients. Ren Fail. 2004;26(5):539–544. doi: 10.1081/jdi-200031735 [DOI] [PubMed] [Google Scholar]
- 60.Kouidi EJ, Grekas DM, Deligiannis AP. Effects of exercise training on noninvasive cardiac measures in patients undergoing long-term hemodialysis: a randomized controlled trial. Am J Kidney Dis. 2009;54(3):511–521. doi: 10.1053/j.ajkd.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 61.Cheng YJ, Zhao XJ, Zeng W, Xu MC, Ma YC, Wang M. Effect of intradialytic exercise on physical performance and cardiovascular risk factors in patients receiving maintenance hemodialysis: a pilot and feasibility study. Blood Purif. 2020;49(4):409–418. doi: 10.1159/000504955 [DOI] [PubMed] [Google Scholar]
- 62.Fernandes AdO, Sens YADS, Xavier VB, Miorin LA, Alves VLDS. Functional and respiratory capacity of patients with chronic kidney disease undergoing cycle ergometer training during hemodialysis sessions: a randomized clinical trial. Int J Nephrol. 2019;2019:7857824. doi: 10.1155/2019/7857824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang M Lv A Wang J, et al. The effect of intradialytic combined exercise on hemodialysis efficiency in end-stage renal disease patients: a randomized-controlled trial. Int Urol Nephrol. 2020;52(5):969–976. doi: 10.1007/s11255-020-02459-1 [DOI] [PubMed] [Google Scholar]
- 64.Oliveira E Silva VR Stringuetta Belik F Hueb JC, et al. Aerobic exercise training and nontraditional cardiovascular risk factors in hemodialysis patients: results from a prospective randomized trial. Cardiorenal Med. 2019;9(6):391–399. doi: 10.1159/000501589 [DOI] [PubMed] [Google Scholar]
- 65.Paluchamy T, Vaidyanathan R. Effectiveness of intradialytic exercise on dialysis adequacy, physiological parameters, biochemical markers and quality of life - a pilot study. Saudi J Kidney Dis Transpl. 2018;29(4):902–910. doi: 10.4103/1319-2442.239661 [DOI] [PubMed] [Google Scholar]
- 66.Graham-Brown MPM March DS Young R, et al. A randomized controlled trial to investigate the effects of intra-dialytic cycling on left ventricular mass. Kidney Int. 2021;99(6):1478–1486. doi: 10.1016/j.kint.2021.02.027 [DOI] [PubMed] [Google Scholar]
- 67.Greenwood SA Koufaki P Macdonald JH, et al. Exercise programme to improve quality of life for patients with end-stage kidney disease receiving haemodialysis: the PEDAL RCT. Health Technol Assess. 2021;25(40):1–52. doi: 10.3310/hta25400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pereira ABN Santana LL Rocha LDB, et al. Physical exercise affects quality of life and cardiac autonomic modulation in patients with chronic kidney failure submitted to hemodialysis: a randomized clinical trial. Percept Mot Skills. 2022;129(3):696–713. doi: 10.1177/00315125221085811 [DOI] [PubMed] [Google Scholar]
- 69.Toussaint ND, Polkinghorne KR, Kerr PG. Impact of intradialytic exercise on arterial compliance and B-type natriuretic peptide levels in hemodialysis patients. Hemodial Int. 2008;12(2):254–263. doi: 10.1111/j.1542-4758.2008.00262.x [DOI] [PubMed] [Google Scholar]
- 70.Martínez-Olmos FJ Gómez-Conesa AA García-Testal A, et al. An intradialytic non-immersive virtual reality exercise programme: a crossover randomized controlled trial. Nephrol Dial Transplant. 2022;37(7):1366–1374. doi: 10.1093/ndt/gfab213 [DOI] [PubMed] [Google Scholar]
- 71.Agustin WR, Safitri W, Kurniasari D, Setiyawan S, Murharyati A, Fitriana RN. Intradialytic exercise on changes in blood pressure in chronic kidney failure patients during hemodialysis therapy. Open Access Maced J Med Sci. 2022;10(G):1–5. doi: 10.3889/oamjms.2022.7271 [DOI] [Google Scholar]
- 72.Isnard-Rouchon M, Coutard C. L’activité physique, un facteur protecteur cardiovasculaire et métabolique chez les patients porteurs d’une insuffisance rénale terminale [Exercise as a protective cardiovascular and metabolic factor in end stage renal disease patients]. Néphrologie & Thérapeutique. 2017;13(7):544–549. doi: 10.1016/j.nephro.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 73.Mihaescu A, Avram C, Bob F, Gaita D, Schiller O, Schiller A. Benefits of exercise training during hemodialysis sessions: a prospective cohort study. Nephron Clin Pract. 2013;124(1-2):72–78. doi: 10.1159/000355856 [DOI] [PubMed] [Google Scholar]
- 74.Miller BW, Cress CL, Johnson ME, Nichols DH, Schnitzler MA. Exercise during hemodialysis decreases the use of antihypertensive medications. Am J Kidney Dis. 2002;39(4):828–833. doi: 10.1053/ajkd.2002.32004 [DOI] [PubMed] [Google Scholar]
- 75.Painter PL Nelson-Worel JN Hill MM, et al. Effects of exercise training during hemodialysis. Nephron. 1986;43(2):87–92. doi: 10.1159/000183805 [DOI] [PubMed] [Google Scholar]
- 76.Thenmozhi P, Vaidyanathan R, Vijayarhagavan R. Efficacy of intradialytic exercise on blood pressure among patients on hemodialysis. Res J Pharm Technol. 2018;11(6):2209–2212. doi: 10.5958/0974-360X.2018.00408.0 [DOI] [Google Scholar]
- 77.Palar R, Lobo D. Impact of intradialytic exercise on fatigue, biochemical and physiological parameters in patients on maintenance hemodialysis - a pilot study - Part 1. Clin Epidemiol Glob Health. 2022;15:101064. doi: 10.1016/j.cegh.2022.101064 [DOI] [Google Scholar]
- 78.March DS Hurt AW Grantham CE, et al. A cost-effective analysis of the CYCLE-HD randomized controlled trial. Kidney Int Rep. 2021;6(6):1548–1557. doi: 10.1016/j.ekir.2021.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dahle DO Eide IA Åsberg A, et al. Aortic stiffness in a mortality risk calculator for kidney transplant recipients. Transplantation. 2015;99(8):1730–1737. doi: 10.1097/TP.0000000000000660 [DOI] [PubMed] [Google Scholar]
- 80.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63(5):1852–1860. doi: 10.1046/j.1523-1755.2003.00932.x [DOI] [PubMed] [Google Scholar]
- 81.Laurent S Cockcroft J Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- 82.Mancia G Fagard R Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151 [DOI] [PubMed] [Google Scholar]
- 83.Protogerou AD, Safar ME. Dissociation between central augmentation index and carotid-femoral pulse-wave velocity: when and why? Am J Hypertens. 2007;20(6):648–649. doi: 10.1016/j.amjhyper.2007.02.008 [DOI] [PubMed] [Google Scholar]
- 84.Westerbacka J, Seppälä-Lindroos A, Yki-Järvinen H. Resistance to acute insulin induced decreases in large artery stiffness accompanies the insulin resistance syndrome. J Clin Endocrinol Metab. 2001;86(11):5262–5268. doi: 10.1210/jcem.86.11.8047 [DOI] [PubMed] [Google Scholar]
- 85.Hargrove N El Tobgy N Zhou O, et al. Effect of aerobic exercise on dialysis-related symptoms in individuals undergoing maintenance hemodialysis: a systematic review and meta-analysis of clinical trials. Clin J Am Soc Nephrol. 2021;16(4):560–574. doi: 10.2215/CJN.15080920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng X Jin C Liu Y, et al. Arterial stiffness as a predictor of clinical hypertension. J Clin Hypertens (Greenwich). 2015;17(8):582–591. doi: 10.1111/jch.12556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saz-Lara A, Cavero-Redondo I, Álvarez-Bueno C, Notario-Pacheco B, Ruiz-Grao MC, Martínez-Vizcaíno V. The acute effect of exercise on arterial stiffness in healthy subjects: a meta-analysis. J Clin Med. 2021;10(2):291. doi: 10.3390/jcm10020291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pu J Jiang Z Wu W, et al. Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ Open. 2019;9(1):e020633. doi: 10.1136/bmjopen-2017-020633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smart N, Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton). 2011;16(7):626–632. doi: 10.1111/j.1440-1797.2011.01471.x [DOI] [PubMed] [Google Scholar]
- 90.Böhm J, Monteiro MB, Thomé FS. Efeitos do exercício aeróbio durante a hemodiálise em pacientes com doença renal crônica: uma revisão da literatura. J Bras Nefrol. 2012;34(2):189–194. doi: 10.1590/s0101-28002012000200013 [DOI] [PubMed] [Google Scholar]
- 91.Smyth B Haber A Trongtrakul K, et al. Representativeness of randomized clinical trial cohorts in end-stage kidney disease: a meta-analysis. JAMA Intern Med. 2019;179(10):1316–1324. doi: 10.1001/jamainternmed.2019.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinberg JR Turner BE Weeks BT, et al. Analysis of female enrollment and participant sex by burden of disease in US clinical trials between 2000 and 2020. JAMA Netw Open. 2021;4(6):e2113749. doi: 10.1001/jamanetworkopen.2021.13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou H Al-Ali F Kang GE, et al. Application of wearables to facilitate virtually supervised intradialytic exercise for reducing depression symptoms. Sensors (Basel). 2020;20(6):1571. doi: 10.3390/s20061571 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the manuscript and/or supporting information.