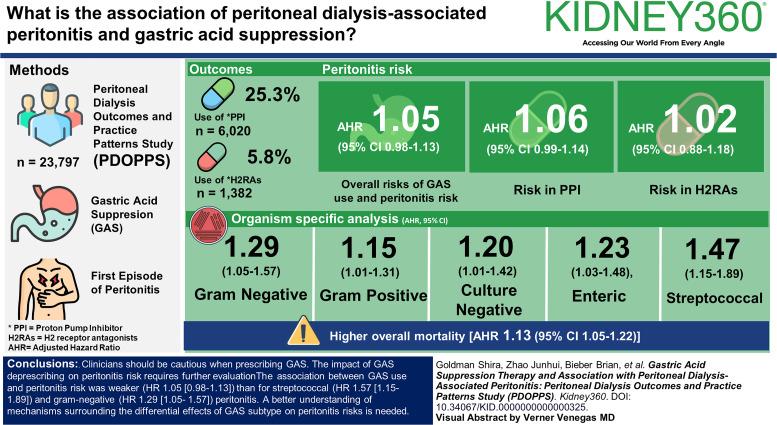
Visual Abstract
Keywords: peritonitis, gastric acid suppression, mortality, peritoneal dialysis, mortality risk
Abstract
Key Points
In a large multinational cohort of PD patients, any GAS use was not associated with an increased risk of all-organism peritonitis.
For peritonitis, risks were particularly high among certain classes of organisms particularly for Gram-negative, enteric, and streptococcal peritonitis episodes.
The association with enteric peritonitis appeared to be stronger among H2RA users.
Background
Peritonitis is a major peritoneal dialysis–related complication. We determined whether gastric acid suppression (GAS) (proton pump inhibitor [PPI] or histamine-2 receptor antagonists [H2RAs]) use was associated with all-cause and organism-specific peritonitis in peritoneal dialysis patients.
Methods
In the Peritoneal Dialysis Outcomes and Practice Patterns Study (595 facilities, eight countries, years 2014–2022), associations between GAS use and time to first episode of all-cause peritonitis were examined using Cox proportional hazards models. The primary exposure of interest was GAS and secondarily PPI or H2RA use. Secondary outcomes were organism-specific peritonitis, peritonitis cure rates, and death.
Results
Among patients (N=23,797) at study baseline, 6020 (25.3%) used PPIs, and 1382 (5.8%) used H2RAs. Overall risks of GAS use and peritonitis risk (adjusted hazard ratio [AHR]=1.05, 95% confidence interval [CI], 0.98 to 1.13]) and use of PPI (AHR 1.06 [95% CI, 0.99 to 1.14]) or H2RA (AHR 1.02 [95% CI, 0.88 to 1.18]) did not reach statistical significance. In organism-specific analyses, GAS users displayed higher peritonitis risks for Gram-negative (AHR 1.29, 95% CI, 1.05 to 1.57), Gram-positive (AHR 1.15, 95% CI, 1.01 to 1.31), culture-negative (AHR 1.20, 95% CI, 1.01 to 1.42), enteric (AHR 1.23, 95% CI, 1.03 to 1.48), and particularly Streptococcal (AHR 1.47, 95% CI, 1.15 to 1.89) peritonitis episodes. GAS was also associated with higher overall mortality (AHR 1.13 [95% CI, 1.05 to 1.22]).
Conclusion
The association between GAS use and peritonitis risk was weaker (hazard ratio [HR] 1.05 [0.98 to 1.13]) than for streptococcal (HR 1.57 [1.15 to 1.89]) and Gram-negative (HR 1.29 [1.05 to 1.57]) peritonitis. A better understanding of mechanisms surrounding the differential effects of GAS subtype on peritonitis risks is needed. Clinicians should be cautious when prescribing GAS. The impact of GAS deprescribing on peritonitis risk requires further evaluation.
Introduction
Peritoneal dialysis (PD)–associated peritonitis contributes to significant morbidity and is a leading cause of hemodialysis (HD) transfer.1 Identifying modifiable risk factors for peritonitis is important. Gastric acid suppression (GAS), proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs), are universally prescribed for a variety of gastrointestinal (GI) indications, such as upper GI bleeding, gastroesophageal reflux disease, peptic ulcer disease, and Helicobacter pylori eradication.2,3 Individuals receiving dialysis have a higher prevalence of GI disorders.4,5 Furthermore, cardiovascular (CV) disease is prevalent among patients with kidney failure,6 and for those treated with antiplatelets, prophylactic GAS is often used to mitigate GI bleeding risks. A Swedish study found higher GAS use among dialysis patients (41%) compared with hospitalized patients (13%) or patients with chronic pulmonary disease (16%).7 Rates of GAS use among dialysis patients in two Canadian studies were similar to the Swedish study.8,9
Reported adverse effects associated with GAS include pneumonia, Clostridium difficile infection, and spontaneous bacterial peritonitis in cirrhotic patients.10 Previous studies suggested that GAS is a potential risk factor of PD-associated peritonitis. Possible mechanisms for GAS-related increased peritonitis risk include gastric and small bowel bacterial overgrowth leading to alteration in the composition of the normal gut microbiota and immune system alterations. Current literature has demonstrated conflicting results, limited by small and single-center reports.11–17
Here, we sought to evaluate (1) the association between any GAS with all-cause peritonitis, (2) the risk of either PPI or H2RA on all-cause peritonitis, and (3) the impact of GAS on organism-specific peritonitis, peritonitis cure rates, and all-cause mortality.
Materials and Methods
Data Source
The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS) is an international prospective cohort study in collaboration with the International Society for Peritoneal Dialysis.18 Patients age 18 years or older receiving maintenance PD were enrolled randomly from national samples of randomly selected PD facilities treating a minimum of 20 PD patients. Study approval was obtained by a central national or institutional review board. Additional study approval and patient consent were obtained by national and local ethics committee regulations.
Study details are provided in the study by Perl et al.18
Data from Australia/New Zealand (A/NZ), Canada, Japan, South Korea, Thailand, the United Kingdom (UK), and the United States in PDOPPS phases 1–2 (phase 1: 2014–2018, phase 2: 2018–2022) were used. Patient demographics, comorbidities, and laboratory data were captured at study enrollment. Data for US PD patients from large dialysis organizations were extracted from electronic health records.19 Remaining data were abstracted from medical charts into a web-based data collection tool.
An infection worksheet capturing the first presentation date and causative organism (or culture-negative case) was completed for each peritonitis episode during PDOPPS follow-up. Peritonitis episodes were additionally ascertained from facility-reported hospitalizations indicating a peritonitis diagnosis and assumed to have onset on the date of admission and unknown causative organism.
Exposure Definition
The primary exposure was first reported use of all GAS (PPI or H2RA) in PDOPPS. Ninety-four percent of patients had medication data reported at the time of study entry. An additional 5% had first medication data reported in the 6-month period after study entry. Secondary exposure was individual PPI versus H2RA. Exclusion criteria were patients without medication data, patients using a combination of H2RA and PPI and hybrid therapy receipt (PD+HD).
Outcome Definition
The primary outcome was time to first peritonitis episode (any organism) during follow-up. Repetitive or recurrent episodes of peritonitis were not included in the primary outcome as the occurrence of the first peritonitis episode may have increased the likelihood of a subsequent peritonitis episode. Therefore, we felt that time to first episode of peritonitis may be a more appropriate model to assess the association between GAS use and peritonitis risk.
Analyses were also performed using time to first organism-specific and enteric peritonitis episodes. Peritonitis organism classifications are detailed in Supplemental Table 1. For organism-specific analyses, facilities not routinely reporting peritonitis organisms (defined as facilities that reported at least three peritonitis episodes but without reporting any organism type) were excluded. Secondary outcomes included all-cause mortality and the composite outcome of peritonitis or death.
Statistical Analysis
Peritonitis time at risk for each patient started at study baseline and continued until the earliest of first peritonitis episode during the study or the end of data for a given patient (e.g., because of end of data collection, patient transfer to another facility, kidney transplantation, transfer to HD [defined as permanent transfer to HD or temporary transfer with failure to return to PD within 84 days of modality switch date, censored at the date of transfer]), loss to follow-up, or death. For all-cause mortality, follow-up started at study baseline and ended at first of end of data collection, kidney transplantation, permanent transfer to HD (as defined above), loss to follow-up, or death. Mortality events were included up to 60 days after study departure. In sensitivity analyses, we additionally censored patients at GAS treatment switch to reduce cross-over during follow-up: (1) We censored baseline nonusers at treatment initiation and (2) baseline users at treatment discontinuation during follow-up.
Cox proportional hazards were used to model the association between GAS use and peritonitis and death. Proportional hazards assumption was assessed by the supremum test. Models were stratified by country, US large dialysis organization, and PDOPPS phase, accounting for facility clustering using robust sandwich covariance estimators. The models were adjusted for age, sex, Black race, PD vintage, PD modality, 13 comorbidities, serum albumin, potassium, magnesium, and 24-hour urine volume. Primary results were performed with differing levels of adjustment. Subgroup analyses were performed by PD vintage, GI comorbidity, antiplatelet medication, and country. GI comorbidity included liver disease, GI bleeding in the 12 months before the study entry, GI cancer, GI surgery, and hepatitis B and hepatitis C viral infections.
Additional sensitivity analyses stratifying patients with and without GI comorbidity was to explore possible confounding by indication because of the presence of GI conditions which may have increased the risks of GAS use and peritonitis. Similarly, we stratified by antiplatelet use in a sensitivity analysis as antiplatelet use may have been associated with both GAS and CV comorbidities, both of which may increase both peritonitis and mortality risks. We also performed sensitivity analyses censoring baseline GAS nonusers at treatment initiation and baseline GAS users at treatment discontinuation.
Peritonitis cure rates by GAS therapy were reported. Peritonitis cure was defined as the absence of a subsequent peritonitis event (relapse or recurrence, as defined previously at Al Sahlawi et al.1), PD catheter removal, or HD transfer (defined as permanent transfer to HD or temporary transfer with failure to return to PD within 84 days of modality switch date) or death during the 50 days after onset of a peritonitis episode.1
Missing data were imputed by the chained-equations method as implemented with IVEware for SAS.20 The missingness of all variables was <8%, except for urine volume (43%) and magnesium (35%). Twenty imputations each were performed for patient-level variables and merged by replicate number. Analyses were performed separately on each imputed data set, with results combined using the Rubin method.21 Data analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Study Participants and Clinical Characteristics
In total, 23,797 patients receiving PD were included in this study (Figure 1). Overall, 6020 (25.3%) patients were prescribed PPI, and 1382 (5.8%) were prescribed H2RA. The distribution of GAS prescription by country is shown in Figure 2. PPIs were less commonly prescribed in Thailand and South Korea.
Figure 1.
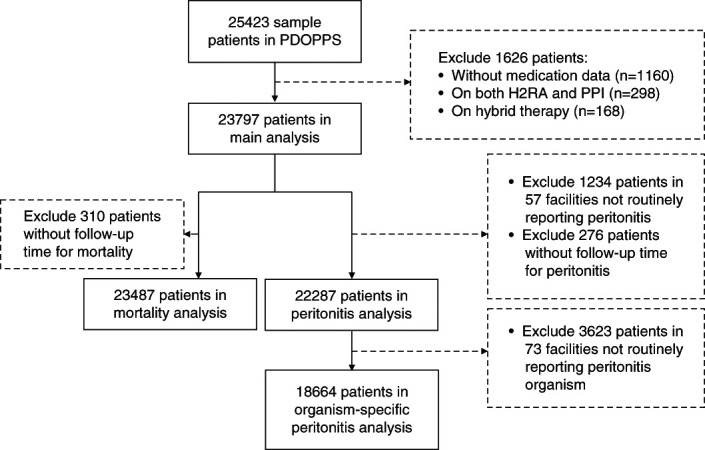
Assembly of the study cohort. H2RA, histamine 2 receptor antagonist; PDOPPS, Peritoneal Dialysis Outcomes and Practice Patterns Study; PPI, proton pump inhibitor.
Figure 2.
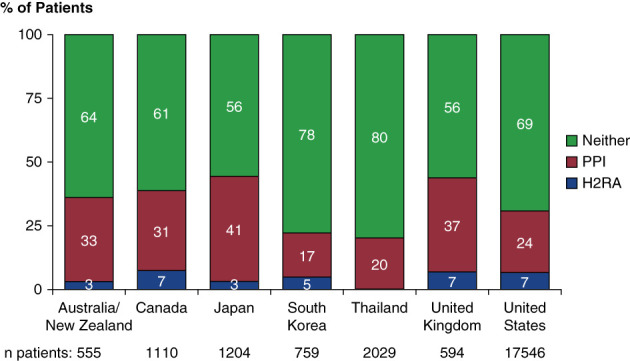
Cross-sectional distribution of gastric acid suppression medication use, by country at baseline. H2RA, histamine 2 receptor antagonist; PPI, proton pump inhibitor.
Patients without GAS therapy were younger; less likely to have a history of CV disease, heart failure, cerebrovascular disease, peripheral vascular disease, lung disease, neurologic disease, psychiatric disease, and malignancy; and less often prescribed antiplatelet therapy (Table 1). Other comorbidities were similar compared with GAS users. Patients prescribed PPIs were more likely to have a heart failure and coronary heart disease history compared with H2RA users or patients without any GAS treatment.
Table 1.
Patient demographics and comorbidities by gastric acid suppression use
| Characteristic | Gastric Acid Suppression Medication Use | ||
|---|---|---|---|
| H2RA | PPI | Neither | |
| (n=1382) | (n=6020) | (n=16,395) | |
| Demographics | |||
| Age, yr | 61±15 | 62±14 | 59±15 |
| Sex (% male) | 53% | 55% | 58% |
| Race (% US Black) | 22% | 22% | 24% |
| PD vintage, yr | 0.4 (0.2 to 1.6) | 0.6 (0.2 to 2.0) | 0.4 (0.2 to 1.5) |
| ESKD vintage, yr | 1.1 (0.3 to 2.9) | 1.3 (0.4 to 3.1) | 0.9 (0.3 to 2.4) |
| Comorbidity history | |||
| Diabetes | 45% | 48% | 44% |
| Hypertension | 86% | 91% | 90% |
| Coronary artery disease | 23% | 28% | 14% |
| Heart failure | 15% | 19% | 11% |
| Cerebrovascular disease | 13% | 13% | 8% |
| Peripheral vascular disease | 16% | 13% | 8% |
| Other cardiovascular diseases | 17% | 18% | 10% |
| Lung disease | 6% | 6% | 3% |
| Neurologic disease | 4% | 5% | 3% |
| Psychiatric disorder | 13% | 11% | 6% |
| Cancer | 10% | 10% | 7% |
| Recurrent cellulitis/gangrene | 2% | 2% | 0.9% |
| HIV | 0.6% | 1% | 2% |
| GI comorbiditya | 5% | 7% | 5% |
| Dialysis treatments | |||
| APD | 80% | 72% | 72% |
| Assisted PD | 18% | 28% | 28% |
| Laboratory/biometric markers | |||
| Body mass index, kg/m2 | 28.5±6.6 | 27.8±6.5 | 27.7±6.5 |
| Serum albumin, g/dl | 3.6±0.5 | 3.4±0.5 | 3.6±0.5 |
| Hemoglobin, g/dl | 10.9±1.6 | 10.9±1.6 | 10.9±1.6 |
| Serum phosphorus, mg/dl | 5.3±1.6 | 5.1±1.6 | 5.4±1.7 |
| Serum calcium, mg/dl | 8.9±0.8 | 8.7±0.8 | 8.8±0.8 |
| Serum magnesium, mg/dl | 2.2±0.5 | 2.1±0.5 | 2.2±0.5 |
| Serum potassium, mEq/L | 4.2±0.6 | 4.2±0.7 | 4.2±0.7 |
| 24-h urine volume, L | 0.9±0.8 | 0.8±0.7 | 0.9±0.8 |
| Medication use | |||
| Antiplatelet | 48% | 47% | 34% |
| Aspirin | 41% | 41% | 30% |
| Clopidogrel | 11% | 12% | 6% |
| P2Y platelet inhibitors | 6% | 5% | 3% |
APD, automated peritoneal dialysis; GI, gastrointestinal; H2RA, histamine 2 receptor antagonist; PD, peritoneal dialysis; PPI, proton pump inhibitor.
GI comorbidity including liver disease, GI bleeding, GI cancer, hepatitis B and hepatitis C.
Kidney transplantation rates were similar among GAS versus non-GAS users: 4.7 per 100 patient-years among GAS users and 4.5 per 100 patient-year among nonusers. No association between GAS use and kidney transplantation (adjusted hazard ratio [AHR] 1.05, 95% confidence interval [CI], 0.93 to 1.19) was seen.
Peritonitis Outcomes
Including 5403 peritonitis episodes, the overall peritonitis rate among GAS users was 0.24 episodes per patient-year and 0.21 among patients not taking GAS. Analysis of first peritonitis episode included 4063 episodes. The association between GAS and time to first peritonitis is shown in Figure 3A. The AHR for GAS for all-cause peritonitis was 1.05 (95% CI, 0.98 to 1.13) and with individual use of PPI and H2RA (Figure 3A) AHR 1.06 (95% CI, 0.99 to 1.14) and AHR 1.02 (95% CI, 0.88 to 1.18), respectively. Sensitivity analyses with censoring baseline nonusers at GAS treatment initiation and baseline GAS users at treatment discontinuation were consistent with our primary findings (Supplemental Figure 1A+B).
Figure 3.
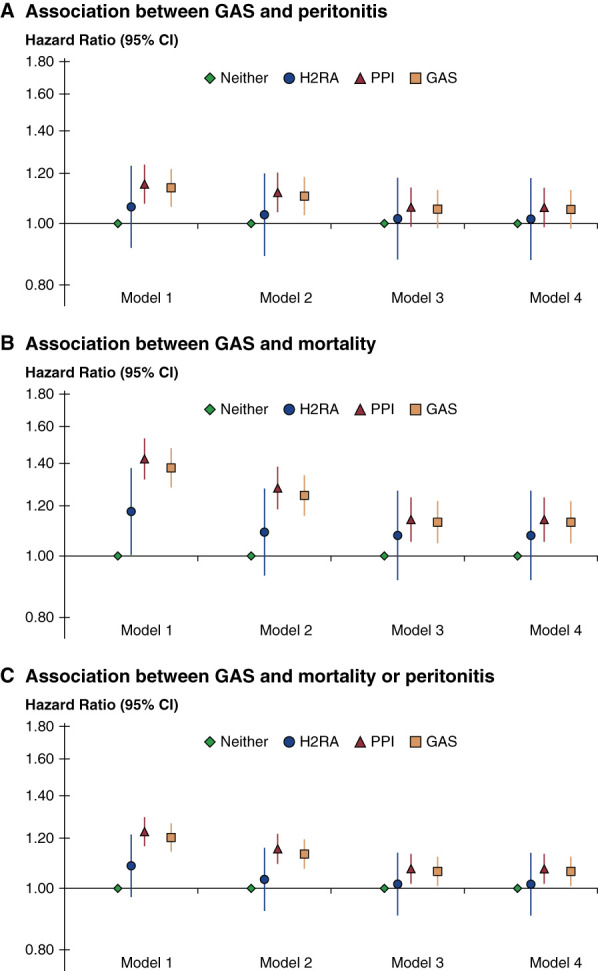
Clinical outcomes by GAS prescription—results of progressive adjustment. Outcomes shown are (A) peritonitis [n=22,287 patients, n=4063 events], (B) mortality [n=23,487 patients: n=3482 events], or (C) the composite of peritonitis or mortality. In all models, 6% of patients were prescribed H2RA, 25% PPI, 69% neither. Model 1 adjusted for patient age, sex, Black race, PD vintage, PD modality; model 2 adjusted for model 1+12 comorbidities; model 3 adjusted for model 2+serum albumin, 24-hour urine volume, potassium, and magnesium; model 4 adjusted for model 3+GI comorbidities. The estimate for GAS overall was estimated from a separate model. CI, confidence interval; GAS, gastric acid suppressants; GI, gastrointestinal; PD, peritoneal dialysis.
Organism-specific peritonitis risks by GAS use is presented in Table 2.
Table 2.
Risk of peritonitis by GAS and causative organism classification
| Organism | No. of Events | GAS | H2RA | PPI |
|---|---|---|---|---|
| Gram positivea | 1013 | 1.15 (1.01 to 1.31) | 1.16 (0.88 to 1.53) | 1.15 (1.00 to 1.32) |
| Gram negative | 496 | 1.29 (1.05 to 1.57) | 1.68 (1.11 to 2.54) | 1.24 (1.01 to 1.53) |
| Culture negative | 844 | 1.20 (1.01 to 1.42) | 0.85 (0.56 to 1.28) | 1.26 (1.06 to 1.51) |
| Fungal | 47 | 1.21 (0.62 to 2.38) | 2.66 (0.94 to 7.54) | 1.03 (0.48 to 2.20) |
| Polymicrobial | 159 | 1.20 (0.86 to 1.69) | 1.08 (0.46 to 2.53) | 1.22 (0.87 to 1.72) |
| Entericb | 624 | 1.23 (1.03 to 1.48) | 1.70 (1.16 to 2.50) | 1.18 (0.98 to 1.41) |
| Streptococcal species | 311 | 1.47 (1.15 to 1.89) | 0.81 (0.42 to 1.56) | 1.56 (1.21 to 2.02) |
Cox model stratified by country, US EHR source, and phase, adjusted for patient age, sex, Black race, PD vintage, PD modality, 13 comorbidities, serum albumin, potassium, magnesium, and 24-hour urine volume (model 4). GAS, gastric acid suppressants; H2RA, histamine 2 receptor antagonist; PPI, proton pump inhibitor.
Includes Streptococcal species.
Including Enterobacteriaceae, Enterococcus spp., and enteric anaerobes.
Among GAS users, there was a higher peritonitis risk for Gram-negative peritonitis (AHR 1.29, 95% CI, 1.05 to 1.57), Gram-positive peritonitis (AHR 1.15, 95% CI, 1.01 to 1.31), enteric peritonitis (AHR 1.23, 95% CI, 1.03 to 1.48), streptococcal peritonitis (AHR 1.47, 95% CI, 1.15 to 1.89), and culture-negative peritonitis (AHR 1.20, 95% CI, 1.01 to 1.42).
Compared with patients not using any GAS, the risk of streptococcal peritonitis was higher among PPI users with an AHR of 1.56 (95% CI, 1.21 to 2.02), but not among H2RA users (AHR 0.81, 95% CI, 0.42 to 1.56). The risk of enteric peritonitis was significant among H2RA users (AHR 1.70, 95% CI, 1.16 to 2.50) but did not reach statistical significance among PPI users (AHR 1.18, 95% CI, 0.98 to 1.41). Risk of Gram-negative peritonitis was higher among H2RA users (AHR 1.68, 95% CI, 1.11 to 2.54) and PPI users (AHR 1.24, 95% CI, 1.01 to 1.53).
Unadjusted peritonitis cure rates were similar in GAS users and nonusers at 75% of all episodes in patients on GAS and 78% of all episodes in patients not using GAS.
Subgroup Analysis
Subgroup analyses are shown in Supplemental Figure 2. Country-specific analyses revealed similar trends among different countries, except Thailand, where a significant association was found between GAS use and all-cause peritonitis (AHR 1.45, 95% CI, 1.02 to 1.93). Subgroup analyses for patients with and without GI comorbidities and patients with and without antiplatelet therapy yielded similar results (Supplemental Figure 2).
Mortality
The median follow-up for mortality was 12 months; the interquartile range was 6–22 months and was 17.6% among patients taking GAS (n=1285) and 13.6% (n=2197) among patients without GAS treatment. Use of GAS was associated with a higher risk of all-cause mortality (AHR of 1.13 [95% CI, 1.05 to 1.22]) in the fully adjusted model (Figure 3B, model 4). This association remained significant for PPI (AHR 1.14, 95% CI, 1.05 to 1.24) but not H2RA (AHR 1.08, 95% CI, 0.92 to 1.27).
For the combined outcome of peritonitis or death, higher risks were found among GAS users (AHR of 1.06, 95% CI, 1.01 to 1.12). Risk was consistent among PPI users (AHR 1.07, 95% CI, 1.02 to 1.13) but not H2RA users (AHR 1.02, 95% CI, 0.91 to 1.14) (Figure 3C).
Sensitivity analyses were conducted (1) censoring baseline nonusers at treatment initiation and (2) censoring baseline users at treatment discontinuation, both of which yielded similar results (Supplemental Figure 1A+B).
Discussion
In this multinational cohort study, we observed an association between GAS use and Gram-negative peritonitis, Gram-positive peritonitis, enteric peritonitis, Streptococcal species peritonitis, and culture-negative peritonitis. Risks were especially high for H2RA use and enteric and Gram-negative peritonitis and for PPI use and Streptococcal peritonitis. Peritonitis cure rates were comparable between GAS users and nonusers. Mortality risk was higher among patients on GAS and PPI therapy.
Reduction in acid production has been associated with an increased risk of enteric infections.10 Gastric acid suppressants have been linked to gastric and small bowel bacterial overgrowth and alteration in the composition of the normal gut microbiota.10,22–26 Qualitative and quantitative alterations in the gut microbiota, coupled with disruptions in the intestinal epithelial barrier among those with kidney disease,27 might be facilitated by GAS, resulting in bacterial translocation from the gut into the peritoneal cavity.10,28 Bacterial overgrowth might occur more easily in patients using PPI, which is associated with stronger acid suppression than H2RA.10,23
Previous studies have reported the association between GAS and enteric peritonitis.11–13 A meta-analysis of six nonrandomized studies involving 829 PD patients showed that GAS increased the odds of enteric peritonitis (odds ratio [OR] 1.27, 95% CI, 1.02 to 1.57). When stratified according to medication use, H2RA but not PPI use had increased odds of enteric peritonitis (OR 1.40, 95% CI, 1.01 to 1.93 and OR 1.13, 95% CI, 0.72 to 1.77, respectively). All-cause peritonitis was not evaluated in this meta-analysis.11 The main contributing study was a single-center observational cohort of Perez-Fontan et al., describing 578 episodes of peritonitis in which a 33% increased risk of a first episode of enteric peritonitis was observed among GAS users. However, no association was found between all-cause peritonitis and GAS use.12 Nessim et al. (also included in the meta-analysis) reported a case–control study of 228 peritonitis episodes. In stratified analyses, H2RA use but not PPI was associated with overall enteric peritonitis risks (as compared with nonenteric peritonitis).13
Similar to previous studies, our data showed that the association with enteric peritonitis appeared to be stronger among H2RA users, suggesting possibly that H2RA and PPI may have differential effects on bowel flora composition and overgrowth.13
Unlike our study, other studies have demonstrated an association between GAS and all-cause peritonitis. Possible mechanisms include immune system alterations mediated by GAS, by reducing neutrophil functions and decreasing production of proinflammatory cytokines and natural killer cell activity.10 These impaired functions of the immune cells can potentially be involved in the increased incidence of infectious diseases among some GAS users and may be responsible for the trend to overall higher risk of peritonitis we observed with GAS.29 A case–control study of 120 patients suggested an increased risk of all-cause first episode of peritonitis among patients on H2RA but not PPI in a multivariable analysis. No association was found between GAS and enteric peritonitis.14 Maeda et al., in a single-center retrospective study, described a higher all-cause peritonitis risk among users of PPI but not H2RA.16 Although numbers were small, there was no difference in the distribution of Gram-positive or Gram-negative bacteria between PPI users and nonusers.
We found a particularly increased risk of streptococcal peritonitis among GAS users. A recent study evaluating peritonitis risks after gastroscopy in PD patients found higher postgastroscopy peritonitis rates among H2RA users compared with nonusers (9.4% versus 2.9%, P = 0.01) but not PPI users (3.1% in PPI users versus 5.2% in non-PPI users, P = 0.29).15 Most of the organisms cultured in this study were enteric in origin (Gram-negative or Enterococcus faecalis); however, one quarter of peritonitis episodes were Gram-positive organisms from oral flora (salivarius, viridans streptococci, and alpha-hemolytic Streptococcus), postulated to be the result of transient bacteremia because of the endoscope passing through the patient's oral cavity. A possible explanation may be the increased growth of Gram-positive bacteria in the upper GI flora and oral cavity among PPI users, such as Streptococcus and other oral commensal flora.23,26 This may also be a potential mechanism of a higher purported risk of community-acquired pneumonia among PPI users.30–32 Taken together, the above may explain the higher risks of peritonitis in our study due to Streptococcus species.
We also observed an increased risk of mortality among PPI users. Previous studies have reported higher all-cause mortality33–35 and CV risk36 with PPI use in the general population. A meta-analysis found an association between PPI use and risk of all-cause mortality among HD patients.37 A similar association was found in a retrospective study among PD patients in China. PPI users had a 47% higher risk of all-cause mortality and 80% more CV events.38 The mechanism for higher risk of death and CV morbidity are poorly understood but may include occurrence of adverse effects, such as infections, osteoporosis and fractures, hypomagnesemia, or reduced efficacy of some antiplatelet therapy in the context of drug–drug interactions.37,38 PPIs were also found in vivo to affect markers of endothelial health.38 However, confounding by indication can be a significant limitation, and the risks of peritonitis and mortality with PPI use across studies might be a marker of comorbidity and frailty among PPI users.
Guidelines have been developed to help clinicians decide when and how to stop PPI in the general population.39,40 However, deprescribing PPI in dialysis patients is more challenging. In a single-center quality improvement study to decrease polypharmacy in HD patients, one third of the patients returned to PPI use after being deprescribed.8 In another single-center interventional study stopping PPI and H2RA use, almost 50% restarted treatment because of gastroesophageal reflux, and 10% of the study participants had GI bleeding.9 Clinicians should be aware of the possible higher risk of peritonitis among patients using GAS and regularly reassess treatment indications balanced against withdrawal risks.
Our study has several limitations. As with any observational analysis, we cannot fully account for unmeasured confounders and confounding by indication of GAS use may still exist. In addition, large observational studies are limited by the inability to conduct medication reconciliation. We could not evaluate the impact of dosing as PPI and H2RA doses were not available and could not capture start dates of GAS initiation before study enrollment introducing a possible prevalence use bias and potentially underestimate the risk of GAS use on patient outcomes. We partially addressed this concern considering subgroup analyses by PD vintage which yielded similar results even among those who were receiving GAS from the onset of PD. Furthermore, sensitivity analyses censoring baseline nonusers at treatment initiation and censoring baseline users at treatment discontinuation did not affect our findings. Despite concerns of prevalent user bias, a differential effect of GAS use on organism-specific peritonitis risks was demonstrated among H2RA and PPI users.
Of note, the missingness of urine volume was 43%, possibly biasing the results. However, missing data were imputed by the chained-equations method, and models were fitted separately for each imputed dataset. As long as the data are missing at random, multiple imputation performs well at 50% missingness.
We found lower H2RA use versus PPI use. Differential effects between the two GAS may have been limited by reduced power because of fewer H2RA users. Low H2RA use is reflective of the contemporary nature of our data that decreased our power to detect other effects of H2RA on peritonitis risks. We did not include data regarding the use of nonsteroidal anti-inflammatory drugs because most are over-the-counter without prescription information, and consumption cannot be easily monitored.
Notwithstanding these limitations, we used a rich, multivariable data source and large sample size, across a diverse group of countries. To reduce confounding by indication, comprehensive model adjustments were done to include all relevant demographic data and reported comorbidities. Furthermore, sensitivity analyses were performed for particular subgroups of users yielding similar results.
In conclusion, GAS use was not associated with higher risks of first episode of all-cause peritonitis, but a significant association was found between GAS and PPI use and streptococcal peritonitis and between GAS and H2RA use and enteric and Gram-negative peritonitis. Higher mortality risk was seen among PD patients prescribed GAS. Gastric acid suppression therapy should be individualized to the patient's medical history and reevaluated periodically. Future studies assessing if deprescribing GAS in patients receiving PD has a measurable effect on reducing peritonitis rates, other infection-related risks, and mortality without increasing GI bleeding–related risks.
Supplementary Material
Acknowledgments
PDOPPS Steering Committee members: David Johnson (Australia); Jeffrey Perl (Canada); Hideki Kawanishi (Japan); Yong-Lim Kim (South Korea); Talerngsak Kanjanabuch (Thailand); Simon Davies (the United Kingdom); Angelito Bernardo, Ron Pisoni, Bruce Robinson, Jenny Shen (the United States). Additional PDOPPS Research Group members: Sunil Badve, Neil Boudville, Fiona Brown, Josephine Chow, John Collins, Rachael Morton, Scott Wilson (Australia); Andreas Vychytil (Austria); Wim Van Biesen (Belgium); Ana Figueiredo, Thyago de Moraes (Brazil); Gillian Brunier, Arsh Jain, Vanita Jassal, Sharon Nessim, Matthew Oliver, Valerie Price, Rob Quinn (Canada); Wei Fang (China); CC Szeto, Angela Wang (Hong Kong); Mizuya Fukasawa, Yasuhiko Ito, Munekazu Ryuzaki, Tadashi Tomo (Japan); Alfonso Cueto Manzano (Mexico); Mark Marshall (New Zealand); Susanne Ljungman (Sweden); Sarinya Boongird, Chanchana Boonyakrai, Areewan Cheawchanwattana, Guttiga Halue, Suchai Sritippayawan, Sajja Tatiyanupanwong, Kriang Tungsanga (Thailand); Elaine Bowes, Edwina Brown, Richard Fluck, Bak Leong Goh, Helen Hurst, Martin Wilkie, Graham Woodrow (the United Kingdom); Filitsa Bender, Judith Bernardini, Dinesh Chatoth, John Crabtree, Fred Finkelstein, Arshia Ghaffari, Rajnish Mehrotra, Beth Piraino, Martin Schreiber, Isaac Teitelbaum (the United States).
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial assistance for this paper.
Footnotes
PDOPPS Steering Committee: David Johnson (Australia); Jeffrey Perl (Canada); Hideki Kawanishi (Japan); Yong-Lim Kim (South Korea); Talerngsak Kanjanabuch (Thailand); Simon Davies (United Kingdom); Angelito Bernardo, Ron Pisoni, Bruce Robinson, Jenny Shen (the United States). Additional PDOPPS Research Group members: Sunil Badve, Neil Boudville, Fiona Brown, Josephine Chow, John Collins, Rachael Morton, Scott Wilson (Australia); Andreas Vychytil (Austria); Wim Van Biesen (Belgium); Ana Figueiredo, Thyago de Moraes (Brazil); Gillian Brunier, Arsh Jain, Vanita Jassal, Sharon Nessim, Matthew Oliver, Valerie Price, Rob Quinn (Canada); Wei Fang (China); CC Szeto, Angela Wang (Hong Kong); Mizuya Fukasawa, Yasuhiko Ito, Munekazu Ryuzaki, Tadashi Tomo (Japan); Alfonso Cueto Manzano (Mexico); Mark Marshall (New Zealand); Susanne Ljungman (Sweden); Sarinya Boongird, Chanchana Boonyakrai, Areewan Cheawchanwattana, Guttiga Halue, Suchai Sritippayawan, Sajja Tatiyanupanwong, Kriang Tungsanga (Thailand); Elaine Bowes, Edwina Brown, Richard Fluck, Bak Leong Goh, Helen Hurst, Martin Wilkie, Graham Woodrow (United Kingdom); Filitsa Bender, Judith Bernardini, Dinesh Chatoth, John Crabtree, Fred Finkelstein, Arshia Ghaffari, Rajnish Mehrotra, Beth Piraino, Martin Schreiber, Isaac Teitelbaum (the United States).
Contributor Information
Collaborators: David Johnson, Jeffrey Perl, Hideki Kawanishi, Yong-Lim Kim, Talerngsak Kanjanabuch, Simon Davies, Angelito Bernardo, Ron Pisoni, Bruce Robinson, Jenny Shen, Sunil Badve, Neil Boudville, Fiona Brown, Josephine Chow, John Collins, Rachael Morton, Scott Wilson, Andreas Vychytil, Wim Van Biesen, Ana Figueiredo, Thyago de Moraes, Gillian Brunier, Arsh Jain, Vanita Jassal, Sharon Nessim, Matthew Oliver, Valerie Price, Rob Quinn, Wei Fang, CC Szeto, Angela Wang, Mizuya Fukasawa, Yasuhiko Ito, Munekazu Ryuzaki, Tadashi Tomo, Alfonso Cueto Manzano, Mark Marshall, Susanne Ljungman, Sarinya Boongird, Chanchana Boonyakrai, Areewan Cheawchanwattana, Guttiga Halue, Suchai Sritippayawan, Sajja Tatiyanupanwong, Kriang Tungsanga, Elaine Bowes, Edwina Brown, Richard Fluck, Bak Leong Goh, Helen Hurst, Martin Wilkie, Graham Woodrow, Filitsa Bender, Judith Bernardini, Dinesh Chatoth, John Crabtree, Fred Finkelstein, Arshia Ghaffari, Rajnish Mehrotra, Beth Piraino, Martin Schreiber, and Isaac Teitelbaum
Disclosures
B. Bieber reports the following: Research Funding: Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx. All support is provided without restrictions on publications. All funds are provided to Arbor Research Collaborative for Health and not directly to Mr. Bieber. N. Boudville reports the following: Consultancy: Amgen; Astra Zeneca; Baxter Healthcare; Roche Pharmaceuticals; Vifor; Research Funding: Amgen = $50000; Fresenius Medical Care—$10000; Roche—$7500; Honoraria: Baxter Pty Ltd; Roche Pharmaceuticals; and Advisory or Leadership Role: BMC Nephrology; CJASN; PDI. S. Goldman reports the following: Other Interests or Relationships: Received Home dialysis fellowship support from the Sinai Health Foundation (with sources from the Israeli Government and the Azrieli Foundation). Y. Ito reports the following: Research Funding: Baxter, Chugai Pharmaceutical, Kyowa Kirin Pharma, MSD Pharma, Takeda Pharmaceutical; and Honoraria: Astra Zeneca, Baxter, Boehringer Ingelheim, JMS, Kyowa Hakko Kirin Pharma, Terumo, Torii Pharma. T. Kanjanabuch reports the following: Research Funding: National Research Council of Thailand; Otsuka Pharmaceutical Co., Ltd.; Royal College of Physician Thailand; The Kidney Foundation of Thailand; VISTERRA Inc. and George Clinical PTY Ltd., Australia; Honoraria: Astra Zeneca and Baxter Healthcare; and Advisory or Leadership Role: APCN Councilor; ISN-Oceania Deputy Chair; ISPD-APC Coordinator. S.J. Nessim reports the following: Consultancy: Astra Zeneca, Baxter Healthcare, Bayer, GSK, Otsuka; and Honoraria: Astra Zeneca, Baxter Healthcare, Bayer; GSK; Otsuka. J. Perl reports the following: Consultancy: Astra Zeneca; Baxter Health Care Canada; Bayer, Davita Healthcare Partners; Fresenius Medical Care, LiberDi; Otsuka, Outset Medical; Ownership Interest: I-Ren; Research Funding: Arbor Research Collaborative For Health; AHRQ; Honoraria: Astra Zeneca, Baxter Healthcare USA/Canada; Bayer Canada, Davita Healthcare partners; DCI; Fresenius Medical Care, Innovative Renal Care, Otsuka; US Renal Care; Speakers Bureau: Baxter Healthcare; Fresenius Medical Care; and Other Interests or Relationships: Salary Support: AHRQ; Arbor Research Collaborative For Health. B. Piraino reports the following: Advisory or Leadership Role: ISPD Editorial Board; JASN Editorial Board (non paid); Member of P DOPPS (non paid); Member of the Medical Advisory Committee for NKF Serving the Alleghenies (non paid). R.L. Pisoni reports the following: Research Funding: I am an Investigator in the DOPPS Program at Arbor Research Collaborative for Health, with the DOPPS Program supported by a large consortium of industry and government funders. The full DOPPS Program support and additional support for specific projects and countries can be found here: https://www.dopps.org/AboutUs/Support.aspx. All funds are provided to my employer, Arbor Research Collaborative for Health, and not directly to Dr. Pisoni; and Advisory or Leadership Role: Am on the Editorial Board for Kidney360; I also have served at Advisory Board Meetings for Vifor regarding CKD-associated pruritus. M. Schreiber reports the following: Consultancy: Davita Kidney Care; Ownership Interest: DaVita/Health Care Partners; Research Funding: Projects conducted by DaVita Clinical Research; Honoraria: consultant employed by DaVita; Advisory or Leadership Role: as a consultant I do participate in lecturing; and Speakers Bureau: Interact with nurse and physician groups as requested. I. Teitelbaum reports the following: Consultancy: Triomed; Ownership Interest: LiberDi; Advisory or Leadership Role: Editorial Board of Peritoneal Dialysis International; Associate Editor, Blood Purification; and Other Interests or Relationships: Past President of the International Society for Peritoneal Dialysis. J. Zhao reports the following: Consultancy: Arbor Research Collaborative for Health; and Research Funding: Arbor Research Collaborative for Health. All remaining authors have nothing to disclose.
Funding
This project was supported by grant #1R01HS025756-01 from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services (HHS)—J. Perl and R. Pisoni (Co-PIs). This AHRQ-funded project used international PDOPPS data from the DOPPS Program which receives global support, without restriction on publications, from a large consortium of funders listed at, https://www.dopps.org/AboutUs/Support.aspx. The PDOPPS program is funded by a consortium of private industry, public funders, and professional societies. The funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Disclaimer: The authors of this manuscript are responsible for its content. Statements in the manuscript do not necessarily represent the official views of, or imply endorsement by, AHRQ or HHS.
Author Contributions
Formal analysis: Junhui Zhao.
Investigation: Shira Goldman, Jeffrey Perl, Ronald L. Pisoni.
Methodology: Jeffrey Perl, Ronald L. Pisoni.
Project administration: Brian Bieber.
Supervision: Brian Bieber.
Writing – original draft: Brian Bieber, Shira Goldman, Jeffrey Perl, Ronald L. Pisoni, Isaac Teitelbaum, Junhui Zhao.
Writing – review & editing: Neil Boudville, Shira Goldman, Laura Horowitz, Yasuhiko Ito, Talerngsak Kanjanabuch, Mark Lambie, Sharon J. Nessim, Jeffrey Perl, Beth Piraino, Ronald L. Pisoni, Martin Schreiber, Isaac Teitelbaum.
Data Sharing Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A414.
Supplemental Table 1. Peritonitis organism classifications.
Supplemental Figure 1. Sensitivity analysis with censoring baseline nonusers at treatment initiation and baseline users at treatment discontinuation.
Supplemental Figure 2. Association between gastric acid suppression use and peritonitis, subgroup analysis. Cox model stratified by country, US EHR source, and phase, adjusted for patient age, sex, Black race, PD vintage, PD modality, 13 comorbidities, serum albumin, 24-hour urine volume, potassium, and magnesium.
References
- 1.Al Sahlawi M Zhao J McCullough K, et al. Variation in peritoneal dialysis-related peritonitis outcomes in the peritoneal dialysis outcomes and practice Patterns study (PDOPPS). Am J Kidney Dis. 2022;79(1):45–55.e1. doi: 10.1053/j.ajkd.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 2.Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336(7634):2–3. doi: 10.1136/bmj.39406.449456.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroenterol Hepatol. 2017;14(12):697–710. doi: 10.1038/nrgastro.2017.117 [DOI] [PubMed] [Google Scholar]
- 4.Shirazian S, Radhakrishnan J. Gastrointestinal disorders and renal failure: exploring the connection. Nat Rev Nephrol. 2010;6(8):480–492. doi: 10.1038/nrneph.2010.84 [DOI] [PubMed] [Google Scholar]
- 5.Khedmat H Ahmadzad-Asl M Amini M, et al. Gastro-duodenal lesions and Helicobacter pylori infection in uremic patients and renal transplant recipients. Transplant Proc. 2007;39(4):1003–1007. doi: 10.1016/j.transproceed.2007.03.034 [DOI] [PubMed] [Google Scholar]
- 6.Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33(suppl 3):iii28–iii34. doi: 10.1093/ndt/gfy174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strid H, Simrén M, Björnsson ES. Overuse of acid suppressant drugs in patients with chronic renal failure. Nephrol Dial Transplant. 2003;18(3):570–575. doi: 10.1093/ndt/18.3.570 [DOI] [PubMed] [Google Scholar]
- 8.McIntyre C, McQuillan R, Bell C, Battistella M. Targeted deprescribing in an outpatient hemodialysis unit: a quality improvement study to decrease polypharmacy. Am J Kidney Dis. 2017;70(5):611–618. doi: 10.1053/j.ajkd.2017.02.374 [DOI] [PubMed] [Google Scholar]
- 9.Czikk D, Parpia Y, Roberts K, Jain G, Vu D-C, Zimmerman D. De-prescribing proton pump inhibitors in patients with end stage kidney disease: a quality improvement project. Can J Kidney Health Dis. 2022;9:1–7. doi: 10.1177/20543581221106244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher L, Fisher A. Acid-suppressive therapy and risk of infections: pros and cons. Clin Drug Investig. 2017;37(7):587–624. doi: 10.1007/s40261-017-0519-y [DOI] [PubMed] [Google Scholar]
- 11.Zhong HJ, Lin D, Lu ZY, Yang WY, Chen Y. Use of gastric-acid suppressants may be a risk factor for enteric peritonitis in patients undergoing peritoneal dialysis: a meta-analysis. J Clin Pharm Ther. 2019;44(2):209–215. doi: 10.1111/jcpt.12769 [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Fontan M Machado Lopes D García Enríquez A, et al. Inhibition of gastric acid secretion by H2 receptor antagonists associates a definite risk of enteric peritonitis and infectious mortality in patients treated with peritoneal dialysis. PLoS One. 2016;11(2):e0148806. doi: 10.1371/journal.pone.0148806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nessim SJ, Tomlinson G, Bargman JM, Jassal SV. Gastric acid suppression and the risk of enteric peritonitis in peritoneal dialysis patients. Perit Dial Int. 2008;28(3):246–251. doi: 10.1177/089686080802800310 [DOI] [PubMed] [Google Scholar]
- 14.Kwon JE Koh SJ Chun J, et al. Effect of gastric acid suppressants and prokinetics on peritoneal dialysis-related peritonitis. World J Gastroenterol. 2014;20(25):8187–8194. doi: 10.3748/wjg.v20.i25.8187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan GCK, Wong SH, Ng JKC, Li PKT, Szeto CC, Chow KM. Risk of peritonitis after gastroscopy in peritoneal dialysis patients. Perit Dial Int. 2022;42(2):162–170. doi: 10.1177/08968608211018608 [DOI] [PubMed] [Google Scholar]
- 16.Maeda S Yamaguchi M Maeda K, et al. Proton pump inhibitor use increases the risk of peritonitis in peritoneal dialysis patients. PLoS One. 2019;14(11):e0224859. doi: 10.1371/journal.pone.0224859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walia N, Rao N, Garrett M, Yates K, Malone S, Holmes C. Proton pump inhibitor use and the risk of peritoneal dialysis associated peritonitis. Intern Med J. 2023;53(3):397–403. doi: 10.1111/imj.15601 [DOI] [PubMed] [Google Scholar]
- 18.Perl J Davies SJ Lambie M, et al. The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS): unifying efforts to inform practice and improve global outcomes in peritoneal dialysis. Perit Dial Int. 2016;36(3):297–307. doi: 10.3747/pdi.2014.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perl J, Bieber B, Tu C, Pecoits-Filho R, Robinson BM, Pisoni RL. The DOPPS Practice Monitor-Peritoneal Dialysis (DPM-PD): from practice to policy and policy to practice. Am J Kidney Dis. 2022;80(3):301–303. doi: 10.1053/j.ajkd.2022.03.005 [DOI] [PubMed] [Google Scholar]
- 20.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27(1):85–96. https://www150.statcan.gc.ca/n1/pub/12-001-x/2001001/article/5857-eng.pdf [Google Scholar]
- 21.Toutenburg H, Rubin DB. Multiple imputation for nonresponse in surveys: Wiley, New York 1987. XXIX+258 pp. Stat Pap. 1990;31(1):180. doi: 10.1007/bf02924688 [DOI] [Google Scholar]
- 22.Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11(5):483–490. doi: 10.1016/j.cgh.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 23.Thorens J Froehlich F Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39(1):54–59. doi: 10.1136/gut.39.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elphick DA, Chew TS, Higham SE, Bird N, Ahmad A, Sanders DS. Small bowel bacterial overgrowth in symptomatic older people: can it be diagnosed earlier? Gerontology. 2005;51(6):396–401. doi: 10.1159/000088704 [DOI] [PubMed] [Google Scholar]
- 25.Stockbrugger RW, Cotton PB, Eugenides N, Bartholomew BA, Hill MJ, Walters CL. Intragastric nitrites, nitrosamines, and bacterial overgrowth during cimetidine treatment. Gut. 1982;23(12):1048–1054. doi: 10.1136/gut.23.12.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imhann F Bonder MJ Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–748. doi: 10.1136/gutjnl-2015-310376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S, Fiaccadori E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant. 2015;30(6):924–933. doi: 10.1093/ndt/gfu287 [DOI] [PubMed] [Google Scholar]
- 28.Mullin JM Valenzano MC Whitby M, et al.. Esomeprazole induces upper gastrointestinal tract transmucosal permeability increase. Aliment Pharmacol Ther. 2008;28(11–12):1317–1325. doi: 10.1111/j.1365-2036.2008.03824.x [DOI] [PubMed] [Google Scholar]
- 29.Heel KA, Hall JC. Peritoneal defences and peritoneum-associated lymphoid tissue. Br J Surg. 1996;83(8):1031–1036. doi: 10.1002/bjs.1800830804 [DOI] [PubMed] [Google Scholar]
- 30.De Jager CPC Wever PC Gemen EFA, et al. Proton pump inhibitor therapy predisposes to community-acquired Streptococcus pneumoniae pneumonia. Aliment Pharmacol Ther. 2012;36(10):941–949. doi: 10.1111/apt.12069 [DOI] [PubMed] [Google Scholar]
- 31.Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med. 2007;167(9):950–955. doi: 10.1001/archinte.167.9.950 [DOI] [PubMed] [Google Scholar]
- 32.Xun X, Yin Q, Fu Y, He X, Dong Z. Proton pump inhibitors and the risk of community-acquired pneumonia: an updated meta-analysis. Ann Pharmacother. 2022;56(5):524–532. doi: 10.1177/10600280211039240 [DOI] [PubMed] [Google Scholar]
- 33.Maggio M Corsonello A Ceda GP, et al. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518–523. doi: 10.1001/jamainternmed.2013.2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open. 2017;7(6):e015735. doi: 10.1136/bmjopen-2016-015735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JP Tazare JR Williamson E, et al.. Proton pump inhibitors and risk of all-cause and cause-specific mortality: a cohort study. Br J Clin Pharmacol. 2021;87(8):3150–3161. doi: 10.1111/bcp.14728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ariel H, Cooke JP. Cardiovascular risk of proton pump inhibitors. Methodist Debakey Cardiovasc J. 2019;15(3):214–219. doi: 10.14797/mdcj-15-3-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Deng D, Zhang R, Yi J, Dong J, Sha L. Relationship between proton pump inhibitors and adverse effects in hemodialysis patients: a systematic review and meta-analysis. Kidney Blood Press Res. 2022;47(9):545–555. doi: 10.1159/000526122 [DOI] [PubMed] [Google Scholar]
- 38.Zeng Y Liu L Zhu L, et al. Proton pump inhibitor usage is associated with higher all-cause mortality and CV events in peritoneal dialysis patients. Ren Fail. 2022;44(1):407–414. doi: 10.1080/0886022X.2022.2043903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farrell B Pottie K Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(5):354–364. PMID: 28500192. [PMC free article] [PubMed] [Google Scholar]
- 40.Farrell B, Lass E, Moayyedi P, Ward D, Thompson W. Reduce unnecessary use of proton pump inhibitors. BMJ. 2022;379:e069211. doi: 10.1136/bmj-2021-069211 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.