Visual Abstract
Keywords: kidney, kidney donation, obesity, organ transplant, renal transplantation, transplantation
Abstract
Key Points
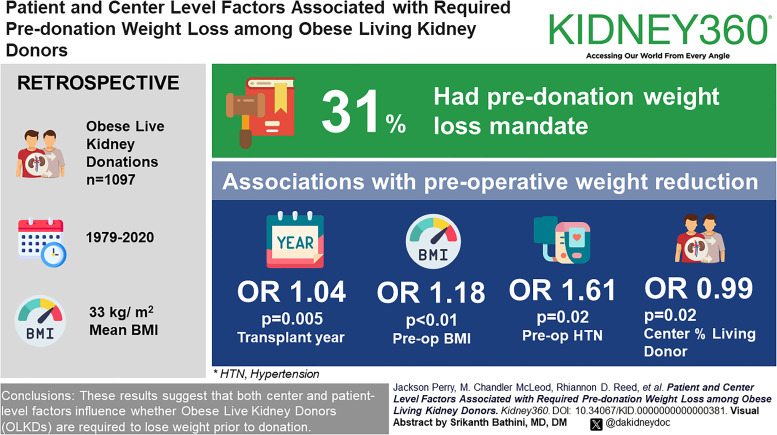
Among obese living kidney donors, year of donation, preoperative body mass index, hypertension, and center percent of living kidney donor transplants were associated with required predonation weight loss.
There were no significant differences in the likelihood of predonation weight loss requirement by race, sex, or age or by markers of preoperative metabolic dysfunction.
Background
The proportion of overweight/mildly obese living kidney donors (OLKDs) has increased in the past few decades, with significant center variation in the body mass index (BMI) of LKDs. The purpose of this study was to examine factors associated with required predonation weight loss among OLKDs (BMI, ≥30 kg/m2).
Methods
This retrospective cohort study surveyed 1097 OLKDs (1979–2020) (mean BMI, 33 kg/m2) about their donation experience. Bivariate analyses compared donor demographic and center characteristics by whether the donor reported predonation weight loss requirement. Generalized estimating equations with logit link were used to estimate marginal effects of patient-level and center-level factors. Multiple imputation using chained equations was implemented to account for missing values.
Results
Of 1097 OLKDs surveyed, 340 (31.0%) reported predonation weight loss requirement. Donors with a predonation weight loss requirement had slightly higher predonation BMIs and donated in more recent years at centers performing a lower percentage of living donor nephrectomies and with a lower median BMI. In multivariable logistic regression analysis, we observed transplant year (odds ratio [OR], 1.04 per year donation; 95% confidence interval [CI], 1.01 to 1.07; P = 0.005), preoperative BMI (OR, 1.16; 95% CI, 1.05 to 1.28; P < 0.01), preoperative hypertension (OR, 1.61; 95% CI, 1.08 to 2.40; P = 0.02), and center percentage of living donor kidney transplants (OR, 0.99; 95% CI, 0.98 to 1.00; P = 0.02) as significantly associated with a predonation weight loss requirement. The study found no differences in the likelihood of predonation weight loss requirement by race, sex, age, preoperative creatinine, preoperative metabolic dysfunction, or center-level median BMI of living donors.
Conclusions
These results suggest that both center-level and patient-level factors influence whether OLKDs are required to lose weight before donation. Future study is needed to determine whether predonation weight loss is associated with improved long-term postdonation outcomes.
Introduction
The worldwide incidence of obesity has increased four-fold from 1975 to 2016.1 This trend has persisted in the years following,2 and the United States has been particularly affected, evidenced by a 41.9% prevalence of obesity among adults as of March 2020. Obesity is recognized as a predisposing factor for the development of comorbidities that may culminate in CKD and also has a bearing on the onset of ESKD.3 Although kidney transplantation confers survival advantages and is generally the preferred treatment for ESKD compared with dialysis, there is a shortage of both deceased and living kidney donors.4–6 For example, despite 25,499 total kidney transplants in 2022, 96,307 patients with ESKD remained on the kidney waitlist in the United States.7
Efforts to alleviate the shortage through increased living kidney donation have been hampered by an increasingly obese population with worse overall health, as evidenced by increased risk for future development of ESKD.8 However, data supporting the perceived increased risk for obese kidney donations are sparse. In an analysis of short-term outcomes, Heimbach et al. found that compared with nonobese donors, donors with obesity had similar rates of major surgical complications and similar renal function within a year of donation but more perioperative complications (16% in donors with body mass index [BMI] ≥35 kg/m2 versus 5% for donors with BMI <25 kg/m2).9 In an analysis of medium-term outcomes from a limited sample of obese living kidney donors (OLKDs), 15 of the 36 participants developed hypertension after donation, though the authors cited the need for more research into long-term outcomes.10 Although there are limited long-term data, one study of more than 100,000 living kidney donation (LKD) found a 7% increase in ESKD risk for every 1 kg/m2 above 27 kg/m2, and another study using the same population found 30% increased risk of long-term mortality among OLKDs compared with nonobese donors.11,12
The shortage of available donors, along with the rising prevalence of obesity and limited data regarding long-term outcomes for OLKDs, have led some transplant centers to begin accepting donors outside of historical norms, as is reflected in the increasing weight trends among living kidney donors over the past few decades. In an analysis on LKD from 1999 to 2011 as reported by the Organ Procurement and Transplantation Network (OPTN), there were 12% increased odds of donors being in the overweight category (BMI, 25.0–29.9 kg/m2) and 20% increased odds of donors being in the mildly obese category (BMI, 30.0–34.9 kg/m2) each 5 years.13 Although the proportions of both overweight and mildly obese living donors have increased in the past 2 decades, there is notable variation in the BMI acceptance threshold among centers.14
Part of this variation is associated with the geographic location of a transplant center and its associated population's obesity prevalence. However, other factors, both patient level and center level, influencing this center variation in BMI acceptance thresholds are unknown. Similarly, it is unclear to what extent this variation may be explained by evaluation practices, particularly whether centers require predonation weight loss among OLKDs. To date, no study has identified patient and/or center factors associated with required predonation weight loss among OLKDs. We, therefore, examined survey responses from a self-selected sample of OLKDs that were obese at the time of donation. We hypothesized that patient characteristics, such as BMI and BP, would drive predonation requirements for weight loss.
Methods
Data Sources
Data from living kidney donors who donated from September 1976 to May 2020 and had BMI ≥30 kg/m2 were obtained from two National Institutes of Health–funded studies (1R01DK113980, Locke; 1R01096008, Segev). OLKDs were approached for enrollment by recruitment letter. The cohort of respondents donated at 53 total transplant centers across 24 states. These 53 centers, in turn, were associated with 52.3% of all living donor kidney transplants in 2021.
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the United States, submitted by members of the OPTN. The Health Resources and Services Administration US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. We received a waiver of authorization and consent for donor contact from the OPTN/SRTR. The University of Alabama at Birmingham (UAB) Institutional Review Board (IRB) reviewed and approved this study (IRB-300000039; IRB-131003001). All donor data were collected and managed using Research Electronic Data Capture database tools hosted at UAB.15
Study Population
Consented adult (age ≥18 years) OLKDs (BMI ≥30) were included in our analysis. All donations occurred between 1979 and 2020. Our analysis dataset was created by first linking consented donor survey and medical records to SRTR living donor data. Demographic and preoperative information were then collated from both sources. Collection of data on race and ethnicity was consistent with National Institutes of Health reporting requirements. Center level kidney transplant statistics computed for each calendar year from SRTR kidney donation records were included through linkage to patient transplant center codes and transplant year. Values calculated included the proportion of total kidney transplants originating from living donors by center-year and the median living donor BMI by center-year.
Outcomes and Exposures
Our primary outcome of interest was the requirement for predonation weight loss, which we defined as an affirmative response by OLKDs to any of the following survey questions: “Were you asked to lose weight prior to being approved for donation,” “were you asked to lose weight after being approved for donation,” and “were you required to have weight loss surgery prior to donation/evaluation?.” Primary variables of interest included self-reported race and sex, age at donation, donation year, preoperative information, BMI, serum creatinine, metabolic disease, history of hypertension, and center-level characteristics (percent living kidney donor transplants and living kidney donor median BMI). Preoperative values for BMI, creatinine, BP, diabetes status, and hypertension history were sourced from both patient survey responses and SRTR data. Center median living donor BMI was calculated from SRTR data and grouped into ranges of 20–25, 25–30, and 30–37. Metabolic disease count was formulated by identifying the number of risk factors observed for donors among fasting blood glucose (>100 mg/dl or hemoglobin A1C >5.6), BP (systolic ≥130 mm Hg or diastolic ≥80 mm Hg), triglycerides (>150 mg/dl), and HDL (<40 mg/dl for males and <50 mg/dl for women). If data were missing for any of the metabolic factors, it was assumed that the patient did not have the individual risk factor.
Statistical Analysis
Continuous variables were described with means and SD or with medians and interquartile ranges (IQRs), as appropriate. Categorical variables were quantified with counts and percentages. Bivariate analysis used t tests and Wilcoxon rank sum tests for continuous variables and Chi-squared tests for categorical variables. Bivariate comparisons were made to examine the association between the requirement for predonation weight loss and donor demographics and center characteristics. Generalized estimating equations (GEEs) with logit link were used to estimate marginal effects of patient-level and center-level factors associated with the predonation requirement for weight loss. The GEEs accounted for clustering at the transplant center level using an exchangeable working correlation structure and robust standard errors. Statistical analyses were performed using R (version 4.2.1, 2022), with multiple imputation performed using the mice package (ver. 3.14.0, 2021) (sensitivity analyses in Supplemental Methods). All statistical tests were two sided, with P < 0.05 considered significant.
Results
Cohort Characteristics
The study included responses from 1097 LKDs who were obese at donation (median BMI of 32.3 kg/m2 [IQR, 31.0–34.3] [Table 1]). Of these OLKDs, 340 reported being required to lose weight before donation and 757 did not. The mean age was 45.8 years (SD, 10.9), 61% were women, and 84.6% were White. Most donations occurred from 2000 to 2009 (49.2%), with a similar proportion donating after 2010 (43.3%). Only 8 (0.7%) and 74 (6.7%) donated from 1979 to 1989 and 1990 to 1999, respectively.
Table 1.
Demographics
| Donor Characteristic | Required to Lose Weight (n=340) | Not Required to Lose Weight (n=757) | Total (N=1097) | P Value |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| Transplant decade | <0.001 | |||
| 1979–1989 | 1 (14.3) | 6 (85.7) | 7 (100.0) | |
| 1990–1999 | 12 (16.2) | 62 (83.8) | 74 (100.0) | |
| 2000–2009 | 153 (28.3) | 387 (71.4) | 540 (100.0) | |
| ≥2010 | 174 (36.6) | 302 (63.4) | 476 (100.0) | |
| Predonation BMI, kg/m2, median (IQR: Q1–Q3) | 33.1 (31.5–35.1) | 32.0 (30.9–33.9) | 32.3 (31.0–34.3) | <0.001 |
| Sex | 0.75 | |||
| Male | 130 (30.4) | 297 (69.6) | 427 (100.0) | |
| Female | 210 (31.3) | 460 (68.7) | 670 (100.0) | |
| Race | 0.92 | |||
| American Indian/Alaska Native | 2 (33.3) | 4 (66.7) | 6 (100.0) | |
| Asian/Pacific Islander | 3 (25.0) | 9 (75.0) | 12 (100.0) | |
| Black | 34 (28.1) | 87 (71.9) | 121 (100.0) | |
| White | 288 (31.3) | 632 (68.7) | 920 (100.0) | |
| Other | 13 (34.2) | 25 (65.8) | 38 (100.0) | |
| Age, yr, mean (SD) | 46.6 (10.9) | 45.5 (10.9) | 45.8 (10.9) | 0.11 |
BMI, body mass index; IQR, interquartile range.
OLKDs donated at 53 different centers across 24 states over the study period (Supplemental Table 1), constituting 496 unique center/donation year combinations. For the centers represented in our cohort, the mean percentage of living kidney donations across all center/years was 45.3% (SD, 14.4), and the mean BMI of living donors across all center/years was 26.9 (SD, 1.2) (data not shown).
Patient-Level Characteristics
In bivariate analysis, the median predonation BMI among OLKDs require to lose weight predonation was 33.2 kg/m2 (IQR, 31.5–35.1), compared with 32.0 kg/m2 (IQR, 30.9–33.9; P ≤ 0.001) for those not required to achieve predonation weight loss (Table 1). There was no difference in BMI by race among OLKDs overall (P = 0.75) nor was there a difference in BMI by race among those required to lose weight predonation. A similar proportion of men and women were required to lose weight predonation (30.4% and 31.3%, respectively). In addition, there was no statistically significant difference in BMI by decade of donation among OLKDs required to lose weight predonation compared with OLKDs not required to lose weight predonation (Supplemental Table 2).
Among OLKDs for whom predonation metabolic data were available, there were no statistically significant differences between OLKDs required and not required to lose weight predonation (Table 2). There were 81 donors who had a predonation history of hypertension: 37 were required to lose weight predonation (45.7%).
Table 2.
Predonation vital signs and laboratory values
| Metabolic Characteristic | Required to Lose Weight (n=340) | Not Required to Lose Weight (n=757) | Total (N=1097) | P Value |
|---|---|---|---|---|
| Preoperative systolic BP, mm Hg, mean (SD)a | 124.1 (13.0) | 125.45 (12.94) | 125.02 (12.96) | 0.11 |
| Preoperative diastolic BP, mm Hg, mean (SD)a | 75.94 (8.95) | 76.26 (8.62) | 76.16 (8.72) | 0.58 |
| Hypertensionb, No. (%) | <0.05 | |||
| No preoperative history of hypertension | 274 (31.0) | 609 (68.0) | 883 (100.0) | |
| Preoperative history of hypertension | 37 (45.7) | 44 (54.3) | 81 (100.0) | |
| Preoperative triglyceride, mg/dl, median (IQR: Q1–Q3)c | 107.5 (81.8–151.3) | 108.0 (77.0–160.0) | 108.0 (79.0–159.0) | 0.99 |
| Preoperative HDL, mg/dl, mean (SD)d | 53.20 (17.43) | 53.57 (14.34) | 53.45 (15.38) | 0.83 |
| Preoperative creatinine, mg/dl, mean (SD)e | 0.86 (0.20) | 0.87 (0.20) | 0.87 (0.20) | 0.30 |
| Preoperative fasting blood glucose, mg/dl, mean (SD)f | 93.47 (10.52) | 94.72 (12.80) | 94.33 (12.14) | 0.38 |
| Preoperative metabolic disease count, No. (%) | 0.28 | |||
| 1 | 141 (32.9) | 287 (67.1) | 428 (100.0) | |
| 2 | 148 (30.2) | 342 (69.8) | 490 (100.0) | |
| 3 | 35 (24.3) | 109 (75.7) | 144 (100.0) | |
| 4 | 14 (48.3) | 15 (51.7) | 29 (100.0) | |
| 5 | 2 (33.3) | 4 (66.7) | 6 (100.0) |
IQR, interquartile range.
Missing from eight donors required to lose weight, 20 donors not required to lose weight.
Missing from 29 donors required to lose weight, 104 donors not required to lose weight.
Missing from 212 donors required to lose weight, 484 donors not required to lose weight.
Missing from 224 donors required to lose weight, 513 donors not required to lose weight.
Missing from three donors required to lose weight, four donors not required to lose weight.
Missing from 235 donors required to lose weight, 522 donors not required to lose weight.
Center-Level Characteristics
When examining OLKDs' center summary statistics, we observed that OLKDs required to lose weight predonation were more likely to have donated at a center with a lower percentage of living donor kidney transplantation in the same year. For example, among donors required to lose weight, the mean center proportion of transplants from living donors was 46.4% (SD, 14.5%), compared with 49.6% (SD, 15.1%) among centers represented by donors not required to lose weight (P < 0.01, Table 3). In addition, the average of center median living donor BMI (median for all living donations at a center) was lower for OLKDs required to lose weight compared with those not required (26.83 kg/m2; SD, 1.0 versus 27.0 kg/m2; SD, 1.1; P = 0.007).
Table 3.
Transplant center characteristicsa
| Donor's Center Characteristicb | Required to Lose Weight (n=340) | Not Required to Lose Weight (n=757) | Total (N=1097) | P Value |
|---|---|---|---|---|
| Percent of donations performed at the donor's center that were living, mean (SD)c | 46.4 (14.5) | 49.6 (15.1) | 48.6 (15.0) | <0.001 |
| No. of kidney transplants the donor's center performed per year, mean (SD)c | 188.2 (72.9) | 191.3 (71.2) | 190.3 (71.7) | 0.51 |
| No. of living donor kidney transplants the donor's center performed per year, mean (SD)c | 85.4 (39.9) | 94.7 (46.3) | 91.8 (44.6) | <0.01 |
| Median BMI of center living donors, mean (SD)d | 26.8 (1.0) | 27.0 (1.1) | 27.0 (1.1) | <0.01 |
BMI, body mass index.
Observations may not be unique and may include repeated measurements for centers.
Data for the center at which each obese living kidney donor donated during the year of their donation.
Missing from one donor required to lose weight, four donors not required to lose weight.
Missing from two donors required to lose weight, 27 donors not required to lose weight.
GEE Modeling
In our multivariable GEE logistic regression (Table 4), we found that transplant year, preoperative BMI, history of hypertension, and center percentage of living donor kidney transplants were significantly associated with being required to lose weight predonation. Specifically, for every year increase in date of donation there was a 4 percent higher odds of being required to lose weight (odds ratio [OR], 1.04; 95% confidence interval [CI], 1.01 to 1.07; P = 0.005). In addition, we observed that each unit increase in BMI was associated with 16 percent greater odds of being required to lose weight (OR, 1.16; 95% CI, 1.05 to 1.28; P = 0.002), and OLKDs with a predonation diagnosis of hypertension had 61% greater odds of being required to lose weight (OR, 1.61; 95% CI, 1.08 to 2.40; P = 0.02). Finally, donating at centers with a higher percentage of living kidney donor transplantation was related to significantly lower odds of being required to lose weight predonation; with each unit increase in the percentage of a center's living kidney donor transplants, there was 1 percent lower odds of being required to lose weight predonation (OR, 0.99; 95% CI, 0.98 to 1.0; P = 0.02). Race, sex, and median BMI of OLKDs were not associated with required predonation weight loss. Metabolic disease count showed no significant association with the outcome (OR, 0.91; 95% CI, 0.75 to 1.11; P = 0.36).
Table 4.
Generalized estimating equation models for being required to lose weight among living kidney donors with obesity
| Covariate | Were Required to Lose Weight Before Their Donation, OR (95% CI) | P Value |
|---|---|---|
| Transplant year | 1.04 (1.01 to 1.07) | <0.01 |
| Age | 1.00 (0.99 to 1.02) | 0.51 |
| Race | ||
| White | 1.0 | |
| Black | 0.84 (0.50 to 1.42) | 0.52 |
| Asian/Pacific Islander | 0.59 (0.17 to 2.03) | 0.41 |
| American Indian/Alaskan Native | 0.85 (0.07 to 9.63) | 0.89 |
| Other | 0.81 (0.45 to 1.46) | 0.49 |
| Sex (female) | 0.92 (0.66 to 1.28) | 0.62 |
| Percentage of living donations | 0.99 (0.98 to 1.00) | 0.02 |
| Creatinine | 0.83 (0.36 to 1.94) | 0.67 |
| BMI | 1.16 (1.05 to 1.28) | <0.01 |
| Median BMI of center living donors | ||
| 20–25 | 1.0 | |
| 25–30 | 1.15 (0.71 to 1.87) | 0.56 |
| 30–37 | 1.11 (0.56 to 2.18) | 0.77 |
| Metabolic disease count | 0.91 (0.75 to 1.11) | 0.36 |
| History of hypertension | 1.61 (1.08 to 2.40) | 0.02 |
BMI, body mass index; CI, confidence interval; OR, odds ratio.
Sensitivity Analyses
In total, our primary model used 933 donors with available information. However, predonation measurements were missing for serum triglycerides in 696 OLKDs, for HDL cholesterol in 737 patients, and for fasting blood glucose in 757 patients. In addition, a predonation diagnosis of hypertension was missing for 133 OLKDs and a diagnosis of diabetes missing for 176. After multiple imputations, results were consistent with our primary analysis. Consequently, multiple imputed results are shown in Supplemental Table 3.
Discussion
In this national multicenter study of OLKDs spanning the past 40 years, we characterized many of the factors influencing whether predonation weight loss was required. We observed that transplant year, center percent of living kidney donor transplants, and donor BMI were independently associated with the requirement for predonation weight loss.
Several studies have demonstrated an increase in the use of OLKDs over the past few decades.13,14,16–18 It should be noted that although donors who were overweight or mildly obese have increased, there was actually a decrease in very obese donors (BMI ≥35 kg/m2). Although many centers are approving more overweight donors, it is unclear to what degree they are requiring them to lose weight and/or providing nutritional counseling.19 Many donors, particularly those donating to close relatives, have shown a willingness to lose weight for a potential donation, yet many are not successful.20,21 This further emphasizes the need for greater understanding into how donors with obesity are evaluated and the factors that may influence successful weight loss. New antiobesity medications, however, have proven highly successful with individuals losing >10% of their body weight after 6 months of treatment.22 Importantly, one of the newest formulations, semaglutide, does not require dosage adjustment in the setting of reduced renal function, setting the stage to continue treatment postdonation.
The finding that OLKDs were more likely to be required to achieve predonation weight loss in more recent years could reflect increased concerns over the effects of obesity, both in the general population and among donors. The proportion of OLKDs required to lose weight predonation increased from 14.3% from 1979 to 1999 to 28.3% from 2000 to 2009 and finally to 36.6% from 2010 to 2020. Given that our cohort's median BMI did not change significantly overtime, these data suggest increasing concerns regarding the long-term effects of obesity in the setting of uninephrectomy. However, this is potentially influenced by the underrepresentation in our sample of OLKDs that donated before 2000.
There was a statistically significant decrease in the odds that a donor was required to lose weight if they donated at a center performing a higher proportion of living donor kidney transplants, consistent with the results from a study by Reese et al., which found that centers performing a higher proportion of living kidney donor transplants were more likely to use medically complex donors.23 The decreased odds may suggest greater comfort by surgeons in performing living donor nephrectomies and a correspondingly higher risk tolerance.
The association between predonation BMI and being required to lose weight predonation is expected, although the variation in BMIs in those required and those not required further highlights significant center-level variation in the approach to OLKDs. Other studies have demonstrated that the acceptable BMI of some donors appears to be largely dependent on the center at which they are donating.14 This is likely influenced by geographic trends in population health. For example, a study by Naik et al. showed that centers in regions with higher prevalence of obesity were more likely to accept donors with obesity. Although we did observe a downward trend in the odds of being required to lose weight predonation as the median BMI of living donors at centers increased, this was not statistically significant in adjusted analyses. This may be explained by the fact that our data reflect the BMI of donors at a center and does not necessarily reflect the weight status of the population surrounding it.
Weight is only one of several potential markers of health considered for potential organ donors. Metabolic syndrome, or dysfunction, describes a patient with a specific number of conditions that may increase their risk of type II diabetes, stroke, and heart disease.24,25 Although the exact criteria required to meet the definition of metabolic syndrome varies,26,27 many centers go beyond BMI and take metabolic syndrome into account during evaluation.28 Even when accounting for conditions, such as hypertension, high levels of triglycerides, and fasting blood glucose, there was no statistical significance in the odds of donors being required to lose weight predonation. This observation may reflect the paucity of data regarding the impact of metabolic dysfunction on postdonation outcomes. However, data on many of the variables that assessed metabolic health were missing for many OLKDs (predonation triglycerides, HDL cholesterol, and fasting blood glucose) and so metabolic disease may have been underestimated. To address this, we performed multiple imputation and found consistent estimates. Potential donors that, in addition to being obese, met additional criteria for metabolic dysfunction may have been unlikely to be approved and thus constituted a small proportion of the cohort.
There are important limitations in this study that should be mentioned. Because of the retrospective nature of this study, we had insufficient data to determine whether patients gained or lost weight after donation. Given the timespan of this study, the respondents may also have been subject to recall bias. In addition, because of the self-reported nature of the study and the often-poor ability to accurately measure one's weight changes, we did not include data on the success of losing weight. Because this study was conducted by surveying OLKDs, it excludes potential donors that might have been rejected because of BMI, and these results may be subject to survival bias. Although our primary model adjusted for observed metabolic dysfunction and our multiple imputation model accounted for components missing at random, the possibility remains that donor metabolic risk was still not fully captured. Finally, the use of BMI as a measure of obesity is not without limitations. As a surrogate for adiposity, BMI has been criticized for ignoring variability in body fat and muscle mass, along with important factors like age, sex, and racial/ethnic group.29–32 Although alternative measures of body composition exist, BMI remains one of the most convenient and widely understood methods, making it useful for the purposes of this study.
Our data suggest that, in addition to year of donation, the odds of a donor being required to lose weight predonation were driven both by patient-level factors, such as BMI and hypertension, and center percentage of living donor transplants. Further understanding of long-term outcomes may illuminate whether required predonation weight loss attenuates risk or just serves as another barrier to kidney donation. This, along with the observed trends in weight of donors, further emphasizes the need for specialized weight loss programs. Although it is critical to understand predonation management of OLKDs, additional research is needed to evaluate the impact of required predonation weight loss on long-term postdonation outcomes.
Supplementary Material
Acknowledgments
The authors sincerely thank the research investigators and participants of the CKD Risk Prediction in Obese Living Kidney Donors Study. Contributors include Dr. Dorry Segev (Johns Hopkins University), Dr. Garrett Roll (University of California San Francisco), Dr. John Silkensen (Hennepin County Medical Center), and Dr. Carrie Schinstock (Mayo Clinic Hospital, Rochester).
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
The funders of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript or the decision to submit for publication.
Disclosures
V. Kumar reports the following: Research Funding: NIH CTOT studies—sub investigator and United Therapeutics/Revivicor—XenoTransplantation—sub investigator; starting January 2022; Funds paid to the institution and not directly to me; Apollo, CSL Behring, Hansa, and MEMO?; Honoraria: ASN—American Society of Nephrology for the early course for ASN kidney week invited lecture; ASN/AST—speaker, American Society of Nephrology/American Society of Transplantation for combined TNCC invited video lecture—honararia; ASN: COVID 19 effort—honararia; NKF talks—travel and hotel, no honararia; AREP for an educational webinar; Medscape for a podcast; Elsivier for a book chapter; WebMD for a webinar; Nephronet—Honararia for a lecture; Advisory or Leadership Role: 1. American Society of Transplantation—Elected Councilor AST BOARD—no payment; 2. Veloxis—Member, Delpi group examining the effectiveness of Envarsus in Kidney Transplant Recipients in an evidence based fashion with the ultimate goal of publication—Honorarium deferred; 3. Exam Item writer for ABIM—no Honorarium; 4. Region 3 UNOS living donor physician representation on the UNOS Living Donor Committee—no Honorarium; 5. ASN Transplant Workforce Member; 6. ASN CET Workgroup; and Other Interests or Relationships: Unsure so declaring all these; American Society of Transplantation (AST): Board Liaison to the Living Donor Community of Practice; AST Transplant Community and Community Education Committee; Incoming Chair of Planning Committee, AST Cutting Edge in Transplantation Planning Committee (CeOT)2022; Member, AST Community Education Committee; UNOS Region 3 Representative to OPTN Living Donor Community; Schwartz Center Rounds for compassionate Rounds—Facilitator. J.E. Locke reports the following: Consultancy: Sanofi; Research Funding: United Therapeutics; Honoraria: Sanofi; Patents or Royalties: UAB; Advisory or Leadership Role: Deputy Editor American Journal of Transplantation; Councilor-at-Large American Society of Transplant Surgeons; Councilor-at-Large Society for University Surgeons; and Editorial Board Member—Annals of Surgery; and Speakers Bureau: Sanofi. P.A. Maclennan reports the following: Advisory or Leadership Role: Statistics Editor, American Journal of Transplantation. J. Perry reports the following: Research Funding: United Therapeutics. R.D. Reed reports the following: Research Funding: United Therapeutics. All remaining authors have nothing to disclose.
Funding
J.E. Locke: National Institute of Diabetes and Digestive and Kidney Diseases (R01DK113980).
Author Contributions
Conceptualization: Jayme E. Locke, Paul A. MacLennan, Jackson Perry, Rhiannon D. Reed.
Data curation: Joshua Allen, Gavin A. Baker, Bernarez Jones, Jayme E. Locke, Jackson Perry, Rhiannon D. Reed, Tayana Robinson, Luke A. Stanford.
Formal analysis: Jayme E. Locke, M. Chandler McLeod, Jackson Perry, Rhiannon D. Reed.
Funding acquisition: Jayme E. Locke, Rhiannon D. Reed.
Investigation: Vineeta Kumar, Jayme E. Locke, Paul A. MacLennan, Rhiannon D. Reed.
Methodology: Vineeta Kumar, Jayme E. Locke, Paul A. MacLennan, M. Chandler McLeod, Rhiannon D. Reed.
Resources: Jayme E. Locke, Rhiannon D. Reed.
Software: Jayme E. Locke, M. Chandler McLeod, Rhiannon D. Reed.
Supervision: Jayme E. Locke, Rhiannon D. Reed.
Validation: Joshua Allen, Gavin A. Baker, Bernarez Jones, Jackson Perry, Tayana Robinson, Luke A. Stanford.
Visualization: Vineeta Kumar, Jayme E. Locke, Paul A. MacLennan, Jackson Perry, Rhiannon D. Reed.
Writing – original draft: M. Chandler McLeod, Jackson Perry.
Writing – review & editing: Joshua Allen, Gavin A. Baker, Bernarez Jones, Vineeta Kumar, Jayme E. Locke, Paul A. MacLennan, M. Chandler McLeod, Jackson Perry, Rhiannon D. Reed, Tayana Robinson, Luke A. Stanford.
Data Sharing Statement
Anonymized data created for the study are or will be available in a persistent repository on publication. Analyzable Data. Survey Data. Other. Vivli. Anonymized data will be available on completion of the grant.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A439.
Supplemental Table 1. Transplant centers.
Supplemental Table 2. Preoperative BMI by decade of donation.
Supplemental Table 3. Pooled model results after multiple imputation.
References
- 1.World Health Organization. Obesity. Health Topics. 2023. Accessed April 8, 2023. https://www.who.int/health-topics/obesity/#tab=tab_1 [Google Scholar]
- 2.Ward ZJ Bleich SN Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Furth SL, Zoccali C; World Kidney Day Steering Committee. Obesity and kidney disease: hidden consequences of the epidemic. Afr J Prim Health Care Fam Med. 2017;9(1):e1–e3. doi: 10.4102/phcfm.v9i1.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe RA Ashby VB Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303 [DOI] [PubMed] [Google Scholar]
- 5.Orandi BJ Luo X Massie AB, et al. Survival benefit with kidney transplants from HLA-incompatible live donors. N Engl J Med. 2016;374(10):940–950. doi: 10.1056/NEJMoa1508380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick F, Held PJ, Chertow GM. The terrible toll of the kidney shortage. J Am Soc Nephrol. 2018;29(12):2775–2776. doi: 10.1681/ASN.2018101030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organ Procurement and Transplantation Network. National Data. 2023. Accessed April 8, 2023. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/# [Google Scholar]
- 8.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006 [DOI] [PubMed] [Google Scholar]
- 9.Heimbach JK Taler SJ Prieto M, et al. Obesity in living kidney donors: clinical characteristics and outcomes in the era of laparoscopic donor nephrectomy. Am J Transplant. 2005;5(5):1057–1064. doi: 10.1111/j.1600-6143.2005.00791.x [DOI] [PubMed] [Google Scholar]
- 10.Nogueira JM Weir MR Jacobs S, et al. A study of renal outcomes in obese living kidney donors. Transplantation. 2010;90(9):993–999. doi: 10.1097/TP.0b013e3181f6a058 [DOI] [PubMed] [Google Scholar]
- 11.Locke JE Reed RD Massie A, et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. 2017;91(3):699–703. doi: 10.1016/j.kint.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locke JE Reed RD Massie AB, et al. Obesity and long-term mortality risk among living kidney donors. Surgery. 2019;166(2):205–208. doi: 10.1016/j.surg.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachdeva M, Rosen LM, Varghese J, Fishbane S, Molmenti EP. Weight trends in United States living kidney donors: analysis of the UNOS database. World J Transplant. 2015;5(3):137–144. doi: 10.5500/wjt.v5.i3.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naik AS Cibrik DM Sakhuja A, et al. Temporal trends, center-level variation, and the impact of prevalent state obesity rates on acceptance of obese living kidney donors. Am J Transplant. 2018;18(3):642–649. doi: 10.1111/ajt.14519 [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taler SJ Messersmith EE Leichtman AB, et al. Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am J Transplant. 2013;13(2):390–398. doi: 10.1111/j.1600-6143.2012.04321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lentine KL, Delos Santos R, Axelrod D, Schnitzler MA, Brennan DC, Tuttle-Newhall JE. Obesity and kidney transplant candidates: how big is too big for transplantation? Am J Nephrol. 2012;36(6):575–586. doi: 10.1159/000345476 [DOI] [PubMed] [Google Scholar]
- 18.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the hypertension detection and follow-up program. Am J Kidney Dis. 2005;46(4):587–594. doi: 10.1053/j.ajkd.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 19.Mandelbrot DA Pavlakis M Danovitch GM, et al. The medical evaluation of living kidney donors: a survey of US transplant centers. Am J Transplant. 2007;7(10):2333–2343. doi: 10.1111/j.1600-6143.2007.01932.x [DOI] [PubMed] [Google Scholar]
- 20.Mustian MN Hanaway M Kumar V, et al. Patient perspectives on weight management for living kidney donation. J Surg Res. 2019;244:50–56. doi: 10.1016/j.jss.2019.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachdeva M Sunday S Israel E, et al. Obesity as a barrier to living kidney donation: a center-based analysis. Clin Transplant. 2013;27(6):882–887. doi: 10.1111/ctr.12246 [DOI] [PubMed] [Google Scholar]
- 22.Wilding JPH Batterham RL Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 23.Reese PP, Feldman HI, McBride MA, Anderson K, Asch DA, Bloom RD. Substantial variation in the acceptance of medically complex live kidney donors across US renal transplant centers. Am J Transplant. 2008;8(10):2062–2070. doi: 10.1111/j.1600-6143.2008.02361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik S Wong ND Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E [DOI] [PubMed] [Google Scholar]
- 25.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528 [DOI] [PubMed] [Google Scholar]
- 26.Mancia G Bombelli M Facchetti R, et al. Impact of different definitions of the metabolic syndrome on the prevalence of organ damage, cardiometabolic risk and cardiovascular events. J Hypertens. 2010;28(5):999–1006. doi: 10.1097/HJH.0b013e328337a9e3 [DOI] [PubMed] [Google Scholar]
- 27.Son DH, Ha HS, Park HM, Kim HY, Lee YJ. New markers in metabolic syndrome. Adv Clin Chem. 2022;110:37–71. doi: 10.1016/bs.acc.2022.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Mustian MN Kumar V Hanaway M, et al. Donation approval among obese living kidney donor candidates: the impact of metabolic syndrome. Surgery. 2019;166(5):940–946. doi: 10.1016/j.surg.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM, Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes Rev. 2016;17(3):262–275. doi: 10.1111/obr.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deurenberg P Andreoli A Borg P, et al. The validity of predicted body fat percentage from body mass index and from impedance in samples of five European populations. Eur J Clin Nutr. 2001;55(11):973–979. doi: 10.1038/sj.ejcn.1601254 [DOI] [PubMed] [Google Scholar]
- 31.Lebiedowska A, Hartman-Petrycka M, Blonska-Fajfrowska B. How reliable is BMI? Bioimpedance analysis of body composition in underweight, normal weight, overweight, and obese women. Ir J Med Sci. 2021;190(3):993–998. doi: 10.1007/s11845-020-02403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond). 2008;32(suppl 3):S56–S59. doi: 10.1038/ijo.2008.87 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data created for the study are or will be available in a persistent repository on publication. Analyzable Data. Survey Data. Other. Vivli. Anonymized data will be available on completion of the grant.