Summary
Sleep disordered breathing is extremely common in pregnancy and is a risk factor for maternal complications. Animal models demonstrate that intermittent hypoxia causes abnormal fetal growth. However, there are conflicting data on the association between maternal sleep disordered breathing and offspring growth in humans. We investigated this association by conducting a systematic review and meta-analysis. Sixty-three manuscripts, and total study population of 67,671,110 pregnant women were included. Thirty-one studies used subjective methods to define sleep disordered breathing, 24 applied objective methods and eight used international codes. Using a random effects model, habitual snoring, defined by subjective methods, and obstructive sleep apnea, diagnosed by objective methods, were associated with an increased risk for large for gestational age (OR 1.46; 95%CI 1.02–2.09 and OR 2.19; 95%CI 1.63–2.95, respectively ), while obstructive sleep apnea, identified by international codes, was associated with an increased risk for small for gestational age newborns (OR 1.28; 95%CI 1.02–1.60). Our results support that maternal sleep disordered breathing is associated with offspring growth, with differences related to the type of disorder and diagnostic methods used. Future studies should investigate underlying mechanisms and whether treatment of sleep disordered breathing ameliorates the neonatal growth.
Keywords: Maternal sleep disordered breathing, snoring, obstructive sleep apnea, sleep, fetal growth, neonatal growth, small for gestational, large for gestational age, low birthweight
Introduction
Maternal sleep disordered breathing (SDB) is a spectrum of disorders, common in pregnancy, characterized by snoring, airflow limitation, and intermittent hypoxia. Snoring and obstructive sleep apnea (OSA), two common SDB disorders, affect 33% of all pregnancies, and up to 70% of high-risk pregnancies, respectively [1–3]. The incidence of these maternal conditions is likely to increase given the global rise in obesity, a major risk factor for SDB [4]. Prenatal exposure to SDB is an independent risk factor for adverse pregnancy outcomes, such as gestational diabetes [2,5,6], perinatal depression [7], hypertensive disorders [2,6,8], cesarean delivery [9], and severe maternal cardiovascular complications and intensive care unit admission [8], especially among women with high-risk pregnancies [10]. In addition, pregnancies complicated by maternal SDB present a higher risk for adverse neonatal outcomes, including preterm birth, congenital abnormalities, stillbirth, and postnatal medical complications requiring hospitalization [9,11]. Despite this evidence, data from human studies on SDB in pregnancy and abnormal offspring growth are limited, and evidence conflicting.
Animal models of OSA show that exposure to gestational intermittent hypoxia leads to fetal growth restriction, and complications in the offspring, such as abnormal glucose metabolism, an increase in adipose tissue [12], cardiac hypertrophy and arterial stiffness [13]. Underlying mechanisms, proposed by animal studies, include an impairment of placental function through adverse effects on the vasoreactivity of the uterine arteries, increase in oxidative and inflammatory markers, and abnormal production of placental angiogenic and growth factors [14]. However, most of the previous human studies on SDB in pregnancy and neonatal outcomes, including systematic reviews, provided conflicting results, with data showing a possible association with fetal growth restriction, macrosomia, or neither [6,8,15]. While it is biologically plausible that SDB would be associated with growth restriction, other maternal conditions associated with SDB (e.g., diabetes, obesity) may slow or accelerate fetal growth depending on time at onset of the condition and severity [16].
Growth restriction and macrosomia are the two main outcomes of abnormal fetal growth, a condition affecting 20% of all pregnancies [16,17]. Abnormal offspring growth is a major cause of neonatal mortality and morbidity [16,18,19]. Identifying modifiable prenatal risk factors of abnormal fetal growth allows appropriate antenatal surveillance of fetal development and ultimately improves the outcomes of those newborns at highest risk for either decreased or accelerated growth in-utero [20]. Based on current practice, prenatal ultrasound evaluation of fetal growth after mid gestation is not universally performed [21]. Given that maternal SDB, compared to other obstetric factors [16], is amenable to non-invasive and non-pharmacological treatment [22], this condition may represent a modifiable risk factor for abnormal fetal growth in utero. Therefore, establishing whether maternal SDB affects fetal growth can help identify those pregnancies in need for fetal surveillance, to improve neonatal outcome.
In the proposed study, we aim to examine whether maternal SDB in pregnancy is a risk factor for abnormal offspring growth, by conducting a systematic review and meta-analysis of the available evidence.
Methods
We searched PubMed, Cochrane Library, Embase, CINAHL, and Web of Science, with terms related to SDB in pregnancy and fetal growth outcome. The full search is available as a supplemental file (Table S1). Studies were included based on the inclusion and exclusion criteria presented in Table S2.
The literature search was completed on July 13, 2023. Two investigators (LS and MB) screened each title and abstract independently using the online program Covidence (https://app.covidence.org/) and then they reviewed the full text of the manuscripts of the included abstracts. Discrepancies were discussed and verified by a third investigator (GB). For data extraction we generated a detailed coding system form which included sample size, study design, inclusion and exclusion criteria of the enrolled population, diagnostic criteria used to determine SDB, gestational age or trimester at diagnosis, methods used to define offspring growth outcome, including the fetal or neonatal reference ranges to derive birthweight percentile values, obstetric complications, and type of statistical analysis conducted. We performed quality assessment by using the Newcastle-Ottawa Scale (NOS), a scoring system originally designed for case-control and cohort studies, and more recently applied to cross-sectional studies [23,24]. This method evaluates three dimensions of a study: the selection of study population; the comparability of study groups and the assessment of exposure and outcome. When applied to case-control and cohort studies, the NOS score has a maximum value of nine and a score of seven or more is considered of high methodological quality. In case of cross-sectional studies, the maximum value of NOS is seven.
The study protocol was registered to the International prospective register of systematic review (CRD42022381065).
Statistical Analysis
The final manuscripts included in the systematic review were divided in three groups based on the method used to identify maternal SDB. Specifically, the first group included those studies using subjective methods or questionnaires, the second included those studies using objective methods or sleep test and the final group was composed by those reports that applied international classification of diseases (ICD) codes related to diagnosis of SDB. In each group, we identified subgroups presenting similarities regarding diagnostic methods (such as a specific questionnaires, or type of sleep test device), cut-off and scoring system for the diagnosis of maternal SDB and definitions of offspring growth outcomes. When at least three studies were available using the same or comparable methods assessing the presence of SDB, fixed- and random-effects model meta-analysis was performed. To evaluate the difference between birth weight of infants born to either cases of the SDB-spectrum and controls, we pooled the mean and standard deviation of the respective studies and used weighted mean difference (MD) with the corresponding 95% confidence interval (95% CI) for absolute differences of continuous outcomes. For dichotomous outcomes (example: small for gestational age or SGA, low birthweight, or LWB, defined by birthweight <2500 g, and large for gestational age, or LGA), the odd ratio (OR) with 95% CI were used to estimate the pooled effects. Since there was considerable variability among the studies regarding availability of adjusted ORs and the individual chosen confounders, we limited our analysis to unadjusted ORs. Heterogeneity of the chosen studies was investigated using I2 and Cochrane’s Q test with a P-values of Cochrane’s Q test < 0.01 or the <di>I2 > 50% indicating significant between study heterogeneity. Since we only regarded and discussed the random effects models of the analysis, Venetian heterogeneity was already accounted for. Funnel plots and Eggeŕs linear regression test were used to evaluate publication bias. In the case of asymmetrical funnel plots upon visual inspection and Eggert`s test p <0.05, a “trim and fill” analysis was conducted [25]. All statistical tests were performed in R 4.2.2 [26], using the meta (version 6.2–1) [27] and metasens (version 1.5–2) [28] libraries. Two-sided significance tests were used and results considered statistically significant if the p value was <0.05.
Results
Literature Search and general characteristics of included studies
We initially identified 1,327 references from the five databases selected. After excluding duplicates (n=238), we screened titles and abstracts of 1,089 records (Figure 1), 856 were excluded, and the remaining 233 reports were reviewed as full text. Among those, 63 manuscripts met the inclusion and exclusion criteria (Tables S3-S5). Thirty-one (49%) were cohort longitudinal studies, 19 (30%) were cross-sectional and 13 (21%) case-control. Thirty-one (49%) manuscripts used subjective methods to define SDB, 24 (38%) applied objective methods and eight (13%) used ICD codes to identify pregnancies complicated by SDB. The 63 selected manuscripts identified a total study population of 67,671,110 pregnant women. The prevalence of SDB in pregnant women varied among the studies, and was 23.22%, 16.98% and 0.04% for studies using subjective, objective and ICD codes, respectively.
Figure 1.
PRISMA 2020 flow diagram.
Study quality
The median (range) NOS score for the longitudinal and case-control studies were 6 (4–7) and 5 (2–9) respectively, with 19 (43%) manuscripts having a score of seven or higher, indicating high quality. For the cross-sectional studies, the median (range) of NOS was 4 (2–6) and none reached the highest score for this study design category.
Gestational age at diagnosis of maternal SDB and definition of exposure among the studies using subjective methods.
A total of 28,556 pregnant subjects were enrolled in these 31 studies, and 6,631 women (23.22%) were diagnosed with SDB by using subjective methods (Table S3). Screening for maternal SDB was performed at the time of delivery or post-partum in twelve studies (43%) [1,30,33–35,39,49,51,56,58,62,63], in the second and third trimester in thirteen studies (39%) [40,42–46,48,50,52,54,55,65,66], while only a minority included participants in the first trimester of pregnancies (14%) [29,37,57,60] or did not specify any gestational age or trimester (4%) [59,61]. While the type of questionnaire varied among the reports, 54% of the studies based the exposure on self-reported snoring, with habitual snoring defined as a frequency of at least three-four times per week in the majority of cases [1,35,37,40,43–46,48,54,56,57,62,63,66]. Other questionnaires used included the Berlin, Epworth sleepiness scale, Stop-BANG, Pittsburgh sleep quality index, and Basic Nordic Sleep Questionnaire (Table S3).
Definition of fetal growth outcomes and covariates of the studies using subjective methods
The definition of fetal growth outcome varied among the 31 studies with subjective methods for SDB assessment, with 14 studies using a single definition of fetal growth outcome, and 17 using more than two different criteria. The two most common methods, used in 23 studies (74%) were neonatal birthweight expressed as raw or percentile value and number of SGA newborns, defined by birthweight below 10th percentile for gestational age, however only some studies specified the fetal or neonatal normal reference range used to derive percentile values of the birthweight [1,37,40,45,46,48,52,56,63]. Moreover, each of these studies used a different normal reference range. Other fetal growth outcomes included LBW, based on birthweight below 2,500 grams regardless of gestational age at birth, and LGA, defined by birthweight above the 90th percentile for gestational age. None of the subjective studies used fetal growth velocity to assess fetal growth outcome.
Fifteen studies (48%) [29,33–35,37,40,42,45,46,48,50,54,59,61,66], among the 31 using subjective methods, controlled the results for covariates, with the majority adjusting the results for maternal age, body mass index, smoking exposure in pregnancy, and only a minority included other factors affecting fetal growth outcome, such as maternal obstetric complications [29,40,46,50,59], race and ethnicity [29,40,59] or fetal sex [40].
Gestational age at maternal SDB diagnosis and definition of exposure among the studies using objective methods
These 24 studies included a total study population of 6,555 pregnant women (Table S4). Of those, 16.98% were diagnosed with OSA. Most assessed maternal SDB in the second or third trimester. The device used to identify OSA varied, with eveln studies using a level III device [69,70,73,75,79–81,89,91,98,99], six preforming overnight sleep studies with level I device [67,78,88,90,92,96], and three using more than one device [76,94,100]. The remaining studies used a level II [83] or level IV [82,101], and the sleep apnea monitor used was not specified in one case [22]. As shown in Table S4, most of the studies used a cut-off of AHI equal or greater than five to define OSA, however, both the scoring system applied and definition of hypopnea varied, while in some reports this information was not specified. The majority of OSA cases diagnosed were mild, with average values of AHI ranging from 5.4 to 15 events per hour.
Definition of fetal growth outcomes and covariates of the studies using objective methods
The methods and diagnostic criteria used to assess fetal growth outcome varied among this group of studies, with the majority using more than one method. The most common diagnostic criteria were birthweight expressed as raw or percentile value and SGA, defined by birthweight below the 10th percentile for gestational age at delivery. Fourteen (58%) of the 24 studies of this group reported the fetal or neonatal normal reference range used to derive percentile values. Less common criteria used included LBW, fetal abdominal circumference below the 10th percentile, birthweight below the 5th, or above the 90th and 95th percentile, fetal growth velocity or estimated fetal weight, based on prenatal ultrasound, below the 3rd or 10th percentile for gestational age at testing.
Among the 24 studies using objective methods, eleven controlled the results for covariates [22,70,73,75,76,79,80,82,83,96,99], which included maternal BMI in all eleven cases, obstetric complications [22,73,75,76,79,82], and maternal ethnicity in limited reports [75,82,83]. Some report also controlled for fetal sex [83] or applied sex-specific normal reference range of the fetal growth [22,76,94,100].
Results of subgroups analysis
Subjective measures of SDB and small for gestational age at birth
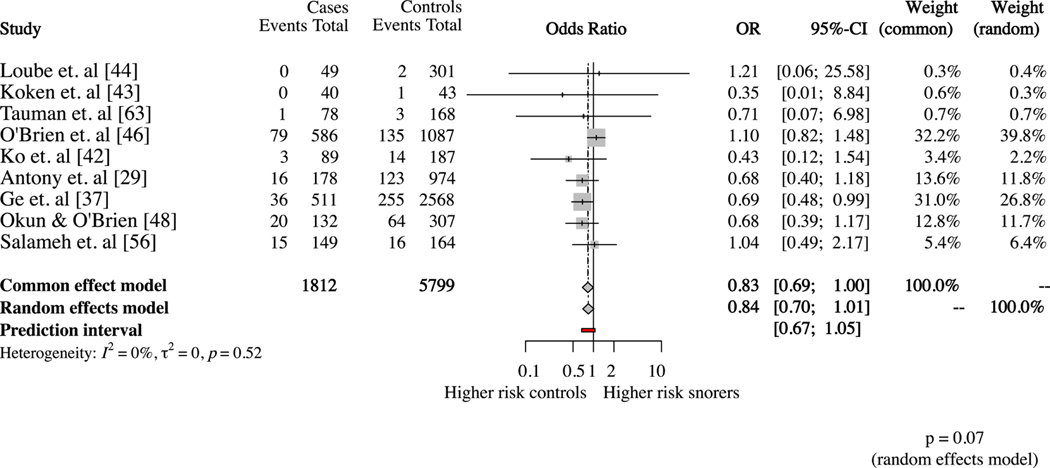
Nine studies [29,37,42–44,46,48,56,63] defined SGA as birthweight below 10th percentile for gestational age and provided number of SGA newborns among subjects with and without SDB. The statistical heterogeneity in this subgroup was low (I2= 0%; p=0.52). The random effects model showed a trend toward a higher risk for SGA newborns among pregnancies without SDB, however it did not reach statistical significance (OR 0.84; 95%CI 0.72–1.01) (Figure 2). When the studies were divided based on the type of questionnaire used to identify maternal SDB, the results were similar among the seven studies using self-reported snoring (OR 0.88; 95%CI 0.72–1.08) [37,43,44,46,48,56,63] (Figure 3).
Figure 2.
Subjective sleep disordered breathing assessment and risk of small for gestational age newborns.
Figure 3.
The studies previously included in Figure 2 are divided into two subgroups, based on type of questionnaire used to assess sleep disordered breathing. Subgroup 1: self-reported snoring assessment. Subgroup 2: Berlin questionnaire.
Subjective measures of SDB and birthweight
Seven studies [43–46,56,62,65] compared the birthweight between pregnant women with and without SDB assessed by subjective methods. Based on these studies, women with and without SDB delivered at term (gestational age of 37 weeks or above), at a similar gestational age. As represented in Figure 4, the heterogeneity between these studies was statistically significant (I2= 54%; p=0.04). When we included only the five studies [43,44,46,56,63] using a similar questionnaire assessing snoring frequency and same cut-off (three or four times per week) to define habitual snorers, the heterogeneity between studies decreased (I2= 27%; p=0.24) (Figure 5). The mean difference in birthweight between newborns born to mothers with SDB and newborns to mothers without SDB was not significant. As represented by the funnel plot (Figure S1), publication bias was unlikely, given a similar distribution of positive and negative correlations between maternal SBD assessed by subjective methods and fetal growth outcome.
Figure 4.
Subjective sleep disordered breathing assessment and mean difference in birthweight.
Figure 5.
Subjective sleep disordered breathing assessment and mean difference in birthweight. The studies previously presented in Figure 4 have been divided in subgroups based on type of questionnaire used. Subgroup 1: questionnaire different from self-reported snoring. Subgroup 2: self-reported snoring.
Subjective measures of SDB and large for gestational age at birth
In four studies [37,46,48,56] habitual snoring in pregnancy was associated with an increased risk for LGA newborns (OR 1.46; 95%CI 1.02–2.09, p=0.037) (Figure 6). However, the heterogeneity across these studies was elevated (I2= 73%; p=0.01).
Figure 6.
Subjective sleep disordered breathing assessment and risk for large for gestational age newborns.
Subjective measures of SDB and low birthweight
Habitual snoring was not associated with an increased risk for LBW, based on the random effects analysis of the three studies [37,45,49] using similar questionnaires to define SDB (OR 1.38; 95%CI 0.72–2.66, p=0.335). Heterogeneity across these studies was not significant (I2= 46%; p=0.16) (Figure 7).
Figure 7.
Subjective sleep disordered breathing assessment and risk for low birthweight.
Objective measures of SDB and small for gestational age
Among the 24 studies using objective methods of SDB, eight studies [22,69,73,76,79,90,91,99] used a cut-off value of five of the apnea-hypopnea index (AHI) or respiratory disturbance index (RDI) to define maternal OSA, in the second or third trimester of gestation, and a birthweight value below the 10th for gestational age to define SGA newborns. The heterogeneity across this subgroup was I2= 9% (p=0.36). The random effects model did not show any significant risk for SGA (OR 0.77; 95%CI 0.51–1.15, p=0.201) (Figure 8). We then divided the above seven studies in two subgroups, based on which guidelines were used to score the sleep studies (Figure 9). Four studies [69,76,91,99] used the criteria established by the American academy of sleep medicine in 2007, with hypopnea defined as a reduction in airflow of at least 50%, and the heterogeneity across these subgroup was low (I2 = 24%, p=0.27). The remaining reports used different criteria or did not specify how hypopnea events were defined [22,73,79,90] (I2 = 0%, p=0.70). The random effects model confirmed a non-significant association between OSA and SGA in both subgroups (Figure 9). As represented by the funnel plot (Figure S2), no publication bias was detected.
Figure 8.
Obstructive sleep apnea diagnosed by sleep test and risk for small for gestational age newborns.
Figure 9.
Obstructive sleep apnea diagnosed by sleep test and risk for small for gestational age newborns. The studies previous represented in Figure 8 are divided in two subgroups, based on the guidelines used to score the sleep studies. Subgroup 1: studies using criteria different from the 2007 American Academy of Sleep Medicine, or definition of hypopnea not specified. Subgroup 2: studies using the 2007 American Academy of Sleep Medicine.
Objective measures of SDB and birthweight
Seven studies with OSA defined by AHI or RDI equal or greater than 5 and fetal growth outcome reported as birthweight were identified [69,76,78,81,90,92,98]. The participants included in these studies delivered at term, with gestational age at birth similar among study groups. This subgroup had low heterogeneity (I2 = 0%, p=0.80) and the mean difference in birthweight did not reach significance (−88.42; 95%CI −193.56–16.71) (Figure 10).
Figure 10.
Obstructive sleep apnea diagnosed by sleep test and mean difference in birthweight.
These results were confirmed when we included only the five studies defining OSA by AHI values of 5 or above events per hour [69,81,90,92,98] (Figure S3).
We further divided the above five studies in two subgroups, by excluding two studies that did not specify the scoring system used [81,90] to define OSA. Similarly, the results of the random effects model of the remaining studies [69,92,98], which used the 2007 American Academy of Sleep Medicine criteria, were not statistically significant (MD −71.06; 95%CI −187.44–45.32) (Figure S4).
Objective measures of SDB and large for gestational age at birth
Three studies reported data on LGA [73,79,99] (Figure 11). This group presented low heterogeneity (I2 = 0%, p=0.42). The random effects model showed an association with LGA newborns (OR 2.19; 95%CI 1.63–2.95, p<0.0001). Only one study reported data on newborns with low birthweight [88], preventing the performance of this meta-analysis.
Figure 11.
Obstructive sleep apnea diagnosed by sleep test and risk for large for gestational age newborns.
Studies using ICD codes and association with small for gestation age newborns
Eight reports [8,11,103,106,108,110–112] identified maternal SDB through ICD codes for OSA included in delivery discharge hospital records (Table S5). These studies differed in study population size, ranging from 190 to 55,781,965, and included a total of 67,635,999 pregnant subjects. Given the intrinsic limitations of defining a condition by using ICD codes, none of these studies reported information on time at diagnosis of SDB, type of testing used to identify OSA, disease severity or therapy. Only one study [108] specified that, in the database used, ICD codes for OSA implied completion of polysomnograms in ambulatory setting within one year prior to delivery. The most common offspring growth outcome investigated was small for gestational age, defined by birthweight <10th percentile and/or identified by ICD codes. Six studies reported data on number of SGA of pregnancies with and without OSA [11,103,108,110–112] and were included in the meta-analysis. Based on the random effects model, prenatal exposure to OSA was associated with an increased risk for SGA (OR 1.28; 95%CI 1.02–1.60, p=0.029), however the heterogeneity across this subgroup was elevated I2= 77% (p=0.01) (Figure 12). Less common outcomes were birthweight above the 90th percentile, reported by two studies [11,103], and low birthweight, presented only in one report [108], preventing additional analysis.
Figure 12.
Obstructive sleep apnea identified by the international classification of diseases codes and risk for small for gestational age newborns.
Discussion
We conducted a systematic review and meta-analysis examining whether prenatal exposure to maternal sleep disordered breathing is a risk factor for abnormal offspring growth. Most of the studies included in our analysis identified maternal SDB in late pregnancy or post-partum, while only a minority assessed frequency of this maternal condition in the first trimester of pregnancy, which is a critical time for the developing fetus and placenta [29,37,57,81,88].
The meta-analysis on the association between maternal SDB and offspring growth outcome led to different results, depending on the type of exposure used, subjective, objective, or ICD defined SDB. Maternal snoring, identified by subjective methods, and OSA, diagnosed by objective assessment, were associated with an increased risk for LGA, while maternal OSA, identified by ICD codes, was associated with an increased risk for SGA. Underlying reasons for variability in results may be related to the fact the spectrum of SDB disorders includes conditions that vary in clinical severity and that may present different impact on placenta function and fetal wellbeing. For example, habitual snoring may have a lesser impact on fetal development compared to severe sleep apnea as data from non-pregnant individuals suggest that compared to snorers with sleep apnea, non-apneic snorers are unlikely to present nocturnal episodes of oxygen desaturation [113]. Hence, though snoring is associated with a biological cascade leading to adverse pregnancy outcomes [1,114,115], it is possibly that nocturnal hypoxemia may be an essential component to growth restriction. However, it is noteworthy that studies included in this systematic review that have used snoring as an exposure, did not exclude the presence of OSA, hindering the assumption that patients with snoring only have snoring but OSA. Our group [56] has previously documented that maternal serum levels of markers of fetal growth restriction [116], such as alpha fetoprotein, pregnancy-associated plasma protein A and inhibin A, of pregnant participants with habitual snoring are similar to those described among controls. Despite this evidence, the effects of maternal snoring on offspring growth remains controversial. Previous reports have documented that chronic maternal snoring, with onset before conception, represents an independent risk factor for small for gestational age newborns [46,66], while pregnancy-onset snoring is an independent risk factor for large for gestational age newborns [37,48] among women without a previous history of SDB or additional sleep disturbances. This suggests that time of onset of habitual snoring in relation to pregnancy may be an important factor in the association with fetal growth. A recent study documented that chronic and pregnancy onset snoring lead to different maternal blood pressure trajectories, suggesting that these two conditions are associated with different maternal cardiovascular effects [115]. Similar mechanisms may be involved in the association with offspring growth outcome; however, underlying pathophysiology needs to be investigated.
It is important to mention that our meta-analysis of the subgroup of the studies with similar snoring questionnaires administered and similar definition of large for gestational age, included cases diagnosed with habitual snoring, without distinguishing between chronic versus pregnancy-onset snoring. This is related to the fact that not all the manuscripts reported data on the offspring growth outcome based on time at onset of maternal snoring. Despite this limitation, this subgroup of studies presented overall higher number of pregnancy-onset snoring compared to chronic snoring. Therefore, our results may mainly reflect the effect of pregnancy-onset snoring rather than chronic snoring. Another important consideration is that pregnancies complicated by maternal snoring of the subgroup of studies we investigated, compared to controls, also presented higher maternal BMI, hypertensive disorders [37,46,48,56] and higher frequency gestational diabetes [48,56], which are known to affect growth outcome. However, the details of these factors in relation to the subgroup of pregnancies complicated by large for gestational age newborns were not available, limiting the possibility of adjusting the results of our meta-analysis.
Animal models of OSA support that intermittent hypoxia reduces the maternal uterine vascular function [14], and impairs fetal growth, by affecting offspring metabolism, fat distribution and the production of growth factors [12]. A pilot human study found differences in cord blood levels of fetal growth regulators between pregnancies complicated by objectively defined maternal OSA and controls [76], with newborns among cases being at higher risk for impaired fetal growth. Maternal disease severity may also impact offspring growth outcomes. Pamidi et al. [83] demonstrated that objectively defined OSA in the third trimester is associated with impaired fetal growth and increases the risk for small for gestational age newborns only in patients with moderate and severe OSA. On the other hand, other studies have shown that mild OSA was associated with greater birthweight percentile and placenta weight [80] and smaller head circumference at birth but accelerated adiposity acquisition in early infancy, compared to no OSA [70]. Discrepancies between animal models of sleep apnea and different forms of OSA in pregnant women may be due to the fact animal models are more representative of the pathophysiology of severe forms of OSA described in humans [13].
It is important to highlight that most of the studies using objective methods included in our systematic review identified mild forms of OSA, with AHI values less than 15 events per hour. Therefore our results may confirm that mild OSA is associated with large for gestational age newborns, rather than increasing risk for SGA, and this is in agreement with the previous studies focusing on mild forms of OSA [70,80]. Further, in our meta-analysis, most of the studies that performed objective testing in pregnancy could not determine whether OSA predated pregnancy or developed de novo during pregnancy, limiting the examination differential effects on maternal biological adaptations depending on timing of exposure to OSA.
Our results of the meta-analysis of those studies using ICD codes did find an association between maternal OSA and SGA newborns. While hospital administrative databases are commonly used in research in pregnancy, it is known the correct identification of specific maternal prenatal conditions and offspring outcomes, through international codes from discharge records, depend on several factors [117]. Based on previous studies, these databases present high accuracy in identifying those maternal and neonatal conditions of great severity, or high prevalence, and characterized by established diagnostic criteria, such as small for gestational age [117–119]. These reasons may explain that, in our metanalysis, OSA defined by sleep testing in pregnancy and maternal OSA identified by ICD codes presented different results regarding offspring growth outcome. As OSA is underdiagnosed in pregnancy, we can postulate that the use of codes may have identified the most severe forms of maternal OSA and hence the association. It is also possible, however, that sample size may have impacted differences in findings between cohort studies that tested OSA objectively and the much larger population-based studies that used ICD coding. Only two studies, among the reports using ICD codes [11,103], investigated the association between OSA and LGA newborns, with conflicting results, and preventing the performance of the meta-analysis. This limits the understanding of possible association between OSA defined by international codes and other types of abnormal offspring growth outcomes.
Our meta-analysis also highlighted that heterogeneity across studies varied based on the methods used to define SDB. Specifically, among the studies using objective methods, we performed subgroups analysis by selecting those reports using similar cut-off values of AHI and/or RDI and scoring systems to define OSA. This resulted in null or low heterogeneity across the objective studies included in the subgroups analysis. Although we used a similar approach for the analysis of the studies using subjective methods and ICD codes, by including only those with similar methods to define SDB and the specific offspring growth outcomes, subjective and ICD codes manuscripts presented high heterogeneity (with I2 up to 77%). It is important to consider that, despite we divided the studies in subgroups based on the specific subjective method to define SDB, (Figure 5) some differences persisted in relation to the characteristics of the study population enrolled. Some studies [43,44,62] enrolled low-risk pregnancies, while others included both high-and low-risk pregnancies [46,56]. Also, the frequency of prenatal exposure to tobacco, which is known to affect fetal growth outcome [120], varies among the studies reporting this data [43,45]. These factors may have contributed to the high heterogeinity of the results between the subgroups of studies using subjective methods (Figure 5).
Great heterogeneity has also been highlighted by previous systematic reviews on maternal SDB and pregnancy outcome. Pamidi et al. [6] reported high heterogeneity of the studies on maternal SDB with birthweight data available, however, they did not test whether subgroups based on similar diagnostic SDB criteria presented a lower heterogeneity. The systematic review and meta-analysis conducted by Brown et al. [9] on maternal SDB and multiple perinatal outcomes, found that the heterogeneity between the studies with offspring growth outcome was moderate. Brown et al. reported a trend for birthweight being lower among pregnancies complicated by OSA, identified by objective methods, although results did not reach statistical significance. They did not investigate the possible association with LGA, and they did not report sub-analysis based on the specific diagnostic criteria or scoring system used to define OSA. In a recent systematic review and meta-analysis on several sleep disturbances in pregnancy, Lu et al. [121] reported that maternal SDB, defined by subjective symptoms, but not OSA, was a risk factor for LGA. They also described high heterogeneity between the studies using subjective methods. An additional consideration is that the questionnaires used to screen for SDB in pregnancy present different sensitivity and sensibility, and for each questionnaire, accuracy may vary depending on gestational age at admistration, specific characteristics of the study population and severity of the disorders in the SDB spectrum [122,123]. The Berlin questionnaire, one of the most common screening tool, has a better performance in the second half of pregnancy and when applied to a general pregnant population, rather than high-risk pregnancies [122]. Moreover, it is affected by overweight and obesity [124]. Underlying reasons are that overweight and obesity, which are known risk factors for SDB [4], are very common in pregnancy, affecting almost 30% of women entering pregnancy and one third of those subjects with a normal BMI at conception but excessive gestational weight gain [125,126]. These considerations highlith the need for a specific screening method for SDB in pregnancy.
Methodology used to assess the offspring growth outcome varied significantly among studies. We identified more than 15 definitions, with the two most common being SGA, defined by birthweight below the 10th percentile for gestational age, and birthweight expressed as raw values in grams or kilograms. Although the use of birthweight percentile was common, the type of normal reference range of the fetal or neonatal weight used to derive percentile values was not always reported. In addition, the type of nomogram used, when reported, varied among the studies, with more than eighteen different reference ranges used. These represent important limitations, since as we and others have demonstrated, the frequency of abnormal offspring growth outcomes is affected by the definition and nomogram used [127–129]. Moreover, although the use of birthweight, as raw values or percentile, is commonly used to describe neonatal growth, this method can miss up to 90% of those infants with abnormal fetal growth, who are at risk for developing associated morbidities later in life, such as obesity, metabolic and cardiovascular diseases [17]. Most of the newborns with abnormal fetal growth, especially impaired in-utero growth, are born with birthweight above the 10th percentile for gestational age, therefore above the cut-off commonly used to define SGA newborns [17]. Underlying reasons are that fetal growth is a dynamic process and, under normal circumstances, is based on a specific fetal weight gain over time [130]. Therefore, a single measurement of the offsping size, either in pregnancy or at birth, cannot correctly detect those fetuses who fail to reach their growth potential [131]. In addition, a single evaluation of the fetal size may miss possible temporal associations between prenatal exposure to risk factors and onset of abnormal growth [132]. There is evidence that more accurate methods are those providing a comprehensive and longitudinal evaluation of placenta and fetal development, such as Doppler vascular biomarkers and fetal growth velocity [133]. In our systematic review, only two studies [22,76] defined impaired fetal growth as birthweight below the 10th centile or decreased third trimester fetal growth velocity. Both studies reported a higher frequency of impaired fetal growth among pregnancies complicated by OSA, and in one study [22] this remained statistically significant after controlling for several covariates. The same authors [22] also described that, among those cases treated with positive airway pressure, there was not an increased risk for impaired fetal growth, suggesting that therapy of OSA in pregnancy may ameliorate offspring growth outcome.
Clinical and research implications
The results of this systematic review and meta-analysis support that SDB in pregnancy is associated with adverse offspring growth, with differences based on the type and severity of SDB. Further studies would need to investigate the underlying pathophysiology. In addition, it would be necessary to identify those factors moderating this association, including time at onset of the specific SDB (before or during gestation), and maternal comorbidities often coexisting with OSA and habitual snoring, and affecting offspring outcome, such as obesity, diabetes, and hypertensive disorders [16]. At present, there are no universal guidelines on screening and treatment of SDB in pregnancy, and whether prenatal management of this maternal condition improves offspring outcomes. There is biological plausibility and limited preliminary data to support that maternal use of continuous positive airway pressure (CPAP) may positively impact fetal growth outcome [22,134]. A larger study has recently demonstrated that use of CPAP among pregnant women with OSA and obesity, is associated with lower vascular resistance of the uterine arteries, compared to controls, supporting that treatment of SDB is associated with an improvement in placenta function [135]. However, a recent systematic review by Migueis et al [136] emphasizes the overall limited number of existing trials on use of CPAP in pregnancy and offspring outcome, especially growth outcome. Moreover, the previous trials presented great heterogenicity in population enrolled, and duration of the therapy with CPAP, ranging from a single night, to several weeks. As SDB is a modifiable risk factor, there is urgent need in better understanding the association with fetal growth outcomes and testing new interventions.
Strengths and limitations
Our study presents several strengths. It includes a large study population size. We conducted the meta-analysis for subgroups of studies with similar methods and diagnostic criteria used to define specific conditions of the SDB spectrum [137], allowing for lower heterogeneity among subgroups of studies, compared to previous systematic reviews [6]. In case of manuscripts using objective methods, we were able to also distinguish between the scoring systems applied in the definition of OSA, given the fact that differences in scoring system may affect prevalence of this condition [138].
Our study also presents limitations. Less than 50% of the cohort and case-control studies were high quality based on the NOS system. None of the cross-sectional studies reached the highest NOS value for this study design category. There was paucity of studies with data on SDB in early pregnancy, limiting our understanding of the possible effects of this maternal condition on the developing fetus and placenta. Also, preterm birth was often an exclusion criterion of the reports, and this may have affected the number of pregnancies complicated by abnormal fetal growth, given that growth restriction and maternal conditions affecting neonatal outcome are often indications for induction of labor before term [139]. In addition, although 63 manuscripts met the inclusion criteria, a lower number was eligible for meta-analysis. Moreover, most of the studies reported neonatal birthweight expressed as raw value or frequency of SGA newborns, with only a minority including data on LGA newborns. This limitation prevented the meta-analysis of the association between OSA defined by ICD codes and LGA. Additionaly, while some studies controlled the results for covariates, information on covariates for the specific growth outcomes we investigated were not available. Therefore, the results of our meta-analysis were not adjusted for factors that may affect offspring growth outcomes.
Conclusions
Our systematic review and meta-analysis support that exposure to sleep disordered breathing in pregnancy is associated with abnormal offspring growth outcome, with differences related to the type of the disorders, diagnostic methods used, and possibly the severity and timing of the exposure. Further studies are needed to identify the underlying pathophysiology, by applying comprehensive assessment of fetal and placenta development and by analyzing whether time at onset of sleep disordered breathing affect this association. In addition, our study supports the need for trials on the effects of prenatal management of this maternal condition and offspring outcomes.
Supplementary Material
Figure S1. Publication bias assessment for studies using subjective methods.
Figure S2. Publication bias assessment for studies using objective methods.
Figure S3. Obstructive sleep apnea diagnosed by sleep test and mean difference in birthweight. Forest plot including only the studies defining obstructive sleep apnea by apnea-hypopnea index values of five or above events per hour.
Figure S4. Obstructive sleep apnea diagnosed by sleep test and mean difference in birthweight. Studies of figure S3 are divided in two subgroups, based on the scoring system used. Subgroup 1: no scoring system specified. Subgroup 2: studies using the 2007 American Academy of Sleep Medicine criteria.
Table S1. Search Strategy
Table S2. Inclusion and exclusion criteria
Table S3. Characteristics of the studies using subjective methods to identify maternal sleep disordered breathing.
Table S4. Characteristics of the studies using objective methods to identify maternal sleep disordered breathing.
Table S5. Characteristics of the studies using international classification of diseases codes to identify maternal sleep disordered breathing.
Practice Points.
Maternal sleep disordered breathing affects the offspring growth outcome, with differences related to the type of disorder, diagnostic methods used, and possibly the severity and timing of the exposure.
The existing scientific literature on this topic is characterized by great heterogeneity in methods used to identify sleep disordered breathing in pregnancy, gestational age at testing, diagnostic criteria of abnormal offspring growth and normal reference ranges of fetal and neonatal weight.
Research Agenda.
Elucidate underlying mechanisms of the association between prenatal exposure to sleep disordered breathing and offspring growth outcome, by applying comprehensive and longitudinal assessment of fetal and placenta development.
Evaluate whether time at onset of maternal sleep disordered breathing (example: chronic versus pregnancy-onset) and maternal comorbidities affect the association with offspring growth outcome.
3. Establish whether maternal treatment of sleep disordered breathing ameliorates neonatal growth.
Acknowledgements:
We would like to thank Ms. Beth Hott for her assistance with manuscript preparation.
Conflicts of interest:
The authors declare that they do not have any conflicts of interest related to the data presented in this manuscript. This work is supported by research grants from the Rhode Island Foundation and Chest Foundation (PI: Sanapo), and by grants from the national heart lung and blood institute (R01HL157288, PI: Bublitz; R01 HL130702, PI: Bourjeily).
Glossary of terms
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- ICD
international classification of diseases
- LBW
low birthweight
- LGA
large for gestational age
- MD
mean difference
- NOS
Newcastle-Ottawa scale
- OR
odds ratio
- OSA
obstructive sleep apnea
- RDI
respiratory disturbance index
- SDB
sleep disordered breathing
- SGA
small for gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849–55. [DOI] [PubMed] [Google Scholar]
- [2].Facco FL, Parker CB, Reddy UM, Silver RM, Koch MA, Louis JM, et al. Association Between Sleep-Disordered Breathing and Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus. Obstet Gynecol. 2017;129(1):31–41. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reutrakul S, Zaidi N, Wroblewski K, Kay HH, Ismail M, Ehrmann DA, et al. Interactions between pregnancy, obstructive sleep apnea, and gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):4195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sanapo L, Bublitz MH, Bai A, Mehta N, Messerlian GM, Catalano P, et al. Association between sleep disordered breathing in early pregnancy and glucose metabolism. Sleep. 2022;45(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;210(1):52.e1–52.e14. [DOI] [PubMed] [Google Scholar]
- [7].Bublitz MH, Sharp M, Freeburg T, Sanapo L, Nugent NR, Sharkey K, et al. Sleep Disordered Breathing Measures in Early Pregnancy Are Associated with Depressive Symptoms in Late Pregnancy. Diagnostics (Basel). 2021;11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bourjeily G, Danilack VA, Bublitz MH, Lipkind H, Muri J, Caldwell D, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;38:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brown NT, Turner JM, Kumar S. The intrapartum and perinatal risks of sleep-disordered breathing in pregnancy: a systematic review and metaanalysis. Am J Obstet Gynecol. 2018;219(2):147–161.e1. [DOI] [PubMed] [Google Scholar]
- [10].Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: A population-based study. Pregnancy Hypertens. 2022;30:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bourjeily G, Danilack VA, Bublitz MH, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2020;66:233–240. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Iqbal W, Ciriello J. Effect of maternal chronic intermittent hypoxia during gestation on offspring growth in the rat. Am J Obstet Gynecol. 2013;209(6):564.e1–9. * [DOI] [PubMed] [Google Scholar]
- [13].Chen L, Zadi ZH, Zhang J, Scharf SM, Pae EK. Intermittent hypoxia in utero damages postnatal growth and cardiovascular function in rats. J Appl Physiol (1985). 2018;124(4):821–830. [DOI] [PubMed] [Google Scholar]
- [14].Badran M, Abuyassin B, Ayas N, Laher I. Intermittent hypoxia impairs uterine artery function in pregnant mice. J Physiol. 2019;597(10):2639–2650. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu T, Feng Y, Peng H, Guo D, Li T. Obstructive sleep apnea and the risk of perinatal outcomes: a meta-analysis of cohort studies. Sci Rep. 2014;4:6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32(2):205–12. [DOI] [PubMed] [Google Scholar]
- [17].Broere-Brown ZA, Schalekamp-Timmermans S, Jaddoe VWV, Steegers EAP. Deceleration of fetal growth rate as alternative predictor for childhood outcomes: a birth cohort study. BMC Pregnancy Childbirth. 2019;19(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Flenady V, Middleton P, Smith GC, Duke W, Erwich JJ, Khong TY, et al. Stillbirths: the way forward in high-income countries. Lancet. 2011;377(9778):1703–17. [DOI] [PubMed] [Google Scholar]
- [19].Lobelo F. Fetal programming and risk of metabolic syndrome: prevention efforts for high-risk populations. Pediatrics. 2005;116(2):519; author reply 519–20. [DOI] [PubMed] [Google Scholar]
- [20].Sovio U, White IR, Dacey A, Pasupathy D, Smith GCS. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;386(10008):2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fetal Growth Restriction: ACOG Practice Bulletin, Number 227. Obstet Gynecol. 2021;137(2):e16–e28. [DOI] [PubMed] [Google Scholar]
- [22].Kneitel AW, Treadwell MC, O’Brien LM. Effects of maternal obstructive sleep apnea on fetal growth: a case-control study. J Perinatol. 2018;38(8):982–988. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ribeiro CM, Beserra BTS, Silva NG, Lima CL, Rocha PRS, Coelho MS, et al. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: a systematic review and meta-analysis. BMJ Open. 2020;10(6):e033509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wells GA, Shea B, O’’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses: Ottawa Hospital Research Institute; 2013. [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- [25].Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98. [Google Scholar]
- [26].R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. https://www.R-project.org/. [Google Scholar]
- [27].Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schwarzer G, Carpenter JR, Rücker G, Schwarzer MG. Statistical Methods for Sensitivity Analysis in Meta-Analysis. R package version 1.5–2, Package ‘metasens’. 2023. https://CRAN.R-project.org/package=metasens.
- [29].Antony KM, Agrawal A, Arndt ME, Murphy AM, Alapat PM, Guntupalli KK, et al. Association of adverse perinatal outcomes with screening measures of obstructive sleep apnea. J Perinatol. 2014;34(6):441–8. [DOI] [PubMed] [Google Scholar]
- [30].Ayrım A, Keskin EA, Ozol D, Onaran Y, Yıidirim Z, Kafali H. Influence of self-reported snoring and witnessed sleep apnea on gestational hypertension and fetal outcome in pregnancy. Arch Gynecol Obstet. 2011;283(2):195–9. [DOI] [PubMed] [Google Scholar]
- [31].Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. [DOI] [PubMed] [Google Scholar]
- [32].Leroy B, Lefort F. [The weight and size of newborn infants at birth]. Rev Fr Gynecol Obstet. 1971;66(6):391–6. [PubMed] [Google Scholar]
- [33].Bourjeily G, El Sabbagh R, Sawan P, Raker C, Wang C, Hott B, et al. Epworth sleepiness scale scores and adverse pregnancy outcomes. Sleep Breath. 2013;17(4):1179–86. [DOI] [PubMed] [Google Scholar]
- [34].Calaora-Tournadre D, Ragot S, Meurice JC, Pourrat O, D’Halluin G, Magnin G, et al. [Obstructive Sleep Apnea Syndrom during pregnancy: prevalence of main symptoms and relationship with Pregnancy Induced-Hypertension and Intra-Uterine Growth Retardation]. Rev Med Interne. 2006;27(4):291–5. [DOI] [PubMed] [Google Scholar]
- [35].Franklin KA, Holmgren PA, Jönsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 2000;117(1):137–41. [DOI] [PubMed] [Google Scholar]
- [36].Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843–8. [DOI] [PubMed] [Google Scholar]
- [37].Ge X, Tao F, Huang K, Mao L, Huang S, Niu Y, et al. Maternal Snoring May Predict Adverse Pregnancy Outcomes: A Cohort Study in China. PLoS One. 2016;11(2):e0148732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gülmezoglu AM, et al. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377(9780):1855–61. [DOI] [PubMed] [Google Scholar]
- [39].Higgins N, Leong E, Park CS, Facco FL, McCarthy RJ, Wong CA. The Berlin Questionnaire for assessment of sleep disordered breathing risk in parturients and non-pregnant women. Int J Obstet Anesth. 2011;20(1):22–5. [DOI] [PubMed] [Google Scholar]
- [40].Howe LD, Signal TL, Paine SJ, Sweeney B, Priston M, Muller D, et al. Self-reported sleep in late pregnancy in relation to birth size and fetal distress: the E Moe, Māmā prospective cohort study. BMJ Open. 2015;5(10):e008910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McCowan L, Stewart AW, Francis A, Gardosi J. A customised birthweight centile calculator developed for a New Zealand population. Aust N Z J Obstet Gynaecol. 2004;44(5):428–31. [DOI] [PubMed] [Google Scholar]
- [42].Ko HS, Kim MY, Kim YH, Lee J, Park YG, Moon HB, et al. Obstructive sleep apnea screening and perinatal outcomes in Korean pregnant women. Arch Gynecol Obstet. 2013;287(3):429–33. [DOI] [PubMed] [Google Scholar]
- [43].Köken G, Sahin FK, Cosar E, Saylan F, Yilmaz N, Altuntas I, et al. Oxidative stress markers in pregnant women who snore and fetal outcome: a case control study. Acta Obstet Gynecol Scand. 2007;86(11):1317–21. [DOI] [PubMed] [Google Scholar]
- [44].Loube DI, Poceta JS, Morales MC, Peacock MD, Mitler MM. Self-reported snoring in pregnancy. Association with fetal outcome. Chest. 1996;109(4):885–9. [DOI] [PubMed] [Google Scholar]
- [45].Micheli K, Komninos I, Bagkeris E, Roumeliotaki T, Koutis A, Kogevinas M, et al. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;22(5):738–44. [DOI] [PubMed] [Google Scholar]
- [46].O’Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC, et al. Snoring during pregnancy and delivery outcomes: a cohort study. Sleep. 2013;36(11):1625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gardosi J, Francis A. A customized standard to assess fetal growth in a US population. Am J Obstet Gynecol. 2009;201(1):25.e1–7. [DOI] [PubMed] [Google Scholar]
- [48].Okun ML, O’Brien LM. Concurrent insomnia and habitual snoring are associated with adverse pregnancy outcomes. Sleep Med. 2018;46:12–19. [DOI] [PubMed] [Google Scholar]
- [49].Owusu JT, Anderson FJ, Coleman J, Oppong S, Seffah JD, Aikins A, et al. Association of maternal sleep practices with pre-eclampsia, low birth weight, and stillbirth among Ghanaian women. Int J Gynaecol Obstet. 2013;121(3):261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Peltonen H, Paavonen EJ, Saarenpää-Heikkilä O, Vahlberg T, Paunio T, Polo-Kantola P. Sleep disturbances and depressive and anxiety symptoms during pregnancy: associations with delivery and newborn health. Arch Gynecol Obstet. 2023;307(3):715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Robertson N, Flatley C, Kumar S. An Epworth Sleep Score ≥11 is associated with emergency operative birth and poor neonatal composite outcome at term. Aust N Z J Obstet Gynaecol. 2020;60(1):49–54. [DOI] [PubMed] [Google Scholar]
- [52].Robertson N, Okano S, Hurst C, Kumar S. Maternal sleep disordered breathing assessed by Epworth Sleepiness Scale and abnormal feto-placental Dopplers. J Matern Fetal Neonatal Med. 2022;35(6):1141–1147. [DOI] [PubMed] [Google Scholar]
- [53].Dobbins TA, Sullivan EA, Roberts CL, Simpson JM. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med J Aust. 2012;197(5):291–4. [DOI] [PubMed] [Google Scholar]
- [54].Robertson N, Okano S, Kumar S. Feto-placental Dopplers are not altered in women with obstructive sleep apnoea symptoms. Aust N Z J Obstet Gynaecol. 2020;60(6):877–883. [DOI] [PubMed] [Google Scholar]
- [55].Robertson A, Makris A, Johnson P, Middleton S, Norman M, Sullivan C, et al. Delivery outcomes as a result of snoring as determined by standard sleep surveys. Obstet Med. 2022;15(4):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Salameh M, Lee J, Palomaki G, Eklund E, Curran P, Suarez JAR, et al. Snoring and markers of fetal and placental wellbeing. Clin Chim Acta. 2018;485:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sarberg M, Svanborg E, Wiréhn AB, Josefsson A. Snoring during pregnancy and its relation to sleepiness and pregnancy outcome - a prospective study. BMC Pregnancy Childbirth. 2014;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sari O, Akpak YK, Yerebasmaz N, Arslan I, Dagcioglu BF, Oral S. Evaluation of obstructive sleep apnea prevalence in mothers of infants with low birth weight and its relationship with serum uric acid levels as a hypoxia marker. J Matern Fetal Neonatal Med. 2022;35(18):3525–3532. [DOI] [PubMed] [Google Scholar]
- [59].Seybold DJ, Bracero LA, Power P, Koenig ZA, Calhoun BC, Bush S. Predicting perinatal outcomes with an obstructive sleep apnea screening tool. J Med Screen. 2022;29(1):61–63. [DOI] [PubMed] [Google Scholar]
- [60].Sharma SK, Nehra A, Sinha S, Soneja M, Sunesh K, Sreenivas V, et al. Sleep disorders in pregnancy and their association with pregnancy outcomes: a prospective observational study. Sleep Breath. 2016;20(1):87–93. [DOI] [PubMed] [Google Scholar]
- [61].Smyka M, Kosińska-Kaczyńska K, Sochacki-Wójcicka N, Zgliczyńska M, Wielgoś M. Does Sleep Quality of Pregnant Women Influence Perinatal Outcomes in Poland? Clin Exp Obstet Gynecol. 2022;49(6):130. [Google Scholar]
- [62].Tauman R, Many A, Deutsch V, Arvas S, Ascher-Landsberg J, Greenfeld M, et al. Maternal snoring during pregnancy is associated with enhanced fetal erythropoiesis--a preliminary study. Sleep Med. 2011;12(5):518–22. [DOI] [PubMed] [Google Scholar]
- [63].Tauman R, Sivan Y, Katsav S, Greenfeld M, Many A. Maternal snoring during pregnancy is not associated with fetal growth restriction. J Matern Fetal Neonatal Med. 2012;25(8):1283–6. [DOI] [PubMed] [Google Scholar]
- [64].Dollberg S, Haklai Z, Mimouni FB, Gorfein I, Gordon ES. Birth weight standards in the live-born population in Israel. Isr Med Assoc J. 2005;7(5):311–4. [PubMed] [Google Scholar]
- [65].Ugur MG, Boynukalin K, Atak Z, Ustuner I, Atakan R, Baykal C. Sleep disturbances in pregnant patients and the relation to obstetric outcome. Clin Exp Obstet Gynecol. 2012;39(2):214–7. [PubMed] [Google Scholar]
- [66].White KM, Dunietz GL, Pitts DS, Kalmbach DA, Lucchini M, O’Brien LM. Burden of sleep disturbance in non-Hispanic Black pregnant women. J Clin Sleep Med. 2022;18(5):1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Alonso-Fernández A, Ribot Quetglas C, Herranz Mochales A, Álvarez Ruiz De Larrinaga A, Sánchez Barón A, Rodríguez Rodríguez P, et al. Influence of Obstructive Sleep Apnea on Systemic Inflammation in Pregnancy. Front Med (Lausanne). 2021;8:674997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- [69].Bassan H, Uliel-Sibony S, Katsav S, Farber M, Tauman R. Maternal Sleep Disordered Breathing and Neonatal Outcome. Isr Med Assoc J. 2016;18(1):45–8. [PubMed] [Google Scholar]
- [70].Brener A, Lebenthal Y, Levy S, Dunietz GL, Sever O, Tauman R. Mild maternal sleep-disordered breathing during pregnancy and offspring growth and adiposity in the first 3 years of life. Sci Rep. 2020;10(1):13979. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–89. [PubMed] [Google Scholar]
- [72].Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002(246):1–190. [PubMed] [Google Scholar]
- [73].Delgado A, Kendle AM, Randis T, Donda K, Salemi JL, Facco FL, et al. Association between Sleep Disordered Breathing and Neonatal Outcomes in Nulliparous Individuals. Am J Perinatol. 2023. [DOI] [PubMed]
- [74].Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol. 2014;124(1):16–22. [DOI] [PubMed] [Google Scholar]
- [75].Facco FL, Ouyang DW, Zee PC, Strohl AE, Gonzalez AB, Lim C, et al. Implications of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2014;210(6):559.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fung AM, Wilson DL, Lappas M, Howard M, Barnes M, O’Donoghue F, et al. Effects of maternal obstructive sleep apnoea on fetal growth: a prospective cohort study. PLoS One. 2013;8(7):e68057. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gestation Network. Centile Calculator: Perinatal Institute [Available from: https://www.gestation.net/.
- [78].Ghesquière L, Deruelle P, Ramdane Y, Garabedian C, Charley-Monaca C, Dalmas AF. Obstructive sleep apnea in obese pregnant women: A prospective study. PLoS One. 2020;15(9):e0238733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hawkins M, Parker CB, Redline S, Larkin JC, Zee PP, Grobman WA, et al. Objectively assessed sleep-disordered breathing during pregnancy and infant birthweight. Sleep Med. 2021;81:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kidron D, Bar-Lev Y, Tsarfaty I, Many A, Tauman R. The effect of maternal obstructive sleep apnea on the placenta. Sleep. 2019;42(6):zsz072. * [DOI] [PubMed] [Google Scholar]
- [81].Louis J, Auckley D, Miladinovic B, Shepherd A, Mencin P, Kumar D, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Orabona R, Corda L, Giordani J, Bernardi M, Maggi C, Mazzoni G, et al. Sleep-disordered breathing and pregnancy outcomes: The impact of maternal oxygen saturation. Int J Gynaecol Obstet. 2023. [DOI] [PubMed]
- [83].Pamidi S, Marc I, Simoneau G, Lavigne L, Olha A, Benedetti A, et al. Maternal sleep-disordered breathing and the risk of delivering small for gestational age infants: a prospective cohort study. Thorax. 2016;71(8):719–25. [DOI] [PubMed] [Google Scholar]
- [84].Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol. 1985;151(3):333–7. [DOI] [PubMed] [Google Scholar]
- [86].Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol. 1995;6(3):168–74. [DOI] [PubMed] [Google Scholar]
- [87].Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet. 1992;339(8788):283–7. [DOI] [PubMed] [Google Scholar]
- [88].Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014;69(4):371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rice AL, Bajaj S, Wiedmer AM, Jacobson N, Stanic AK, Antony KM, et al. Continuous positive airway pressure treatment of obstructive sleep apnea and hypertensive complications in high-risk pregnancy. Sleep Breath. 2023;27(2):621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sahin FK, Koken G, Cosar E, Saylan F, Fidan F, Yilmazer M, et al. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Gynaecol Obstet. 2008;100(2):141–6. [DOI] [PubMed] [Google Scholar]
- [91].Sarberg M, Bladh M, Josefsson A, Svanborg E. Sleepiness and sleep-disordered breathing during pregnancy. Sleep Breath. 2016;20(4):1231–1237. [DOI] [PubMed] [Google Scholar]
- [92].Serednytskyy O, Alonso-Fernández A, Ribot C, Herranz A, Álvarez A, Sánchez A, et al. Systemic inflammation and sympathetic activation in gestational diabetes mellitus with obstructive sleep apnea. BMC Pulm Med. 2022;22(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Carrascosa Lezcano A, Fernández García JM, Fernández Ramos C, Ferrández Longás A, López-Siguero JP, Sánchez González E, et al. [Spanish cross-sectional growth study 2008. Part II. Height, weight and body mass index values from birth to adulthood]. An Pediatr (Barc). 2008;68(6):552–69. [DOI] [PubMed] [Google Scholar]
- [94].Skrzypek H, Wilson DL, Fung AM, Pell G, Barnes M, Sommers L, et al. Fetal heart rate events during sleep, and the impact of sleep disordered breathing, in pregnancies complicated by preterm fetal growth restriction: An exploratory observational case-control study. BJOG. 2022;129(13):2185–2194. [DOI] [PubMed] [Google Scholar]
- [95].Berry RB, Gamaldo CE, Harding SM, Brooks R, Lloyd RM, Vaughn BV, et al. AASM scoring manual version 2.2 updates: new chapters for scoring infant sleep staging and home sleep apnea testing. American Academy of Sleep Medicine; 2015. [DOI] [PMC free article] [PubMed]
- [96].Suri J, Suri JC, Arora R, Gupta M, Adhikari T. The Impact of Sleep-Disordered Breathing on Severity of Pregnancy-Induced Hypertension and Feto-Maternal Outcomes. J Obstet Gynaecol India. 2019;69(Suppl 2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM Scoring Manual Updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tauman R, Zuk L, Uliel-Sibony S, Ascher-Landsberg J, Katsav S, Farber M, et al. The effect of maternal sleep-disordered breathing on the infant’s neurodevelopment. Am J Obstet Gynecol. 2015;212(5):656.e1–7. [DOI] [PubMed] [Google Scholar]
- [99].Telerant A, Dunietz GL, Many A, Tauman R. Mild Maternal Obstructive Sleep Apnea in Non-obese Pregnant Women and Accelerated Fetal Growth. Sci Rep. 2018;8(1):10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wilson DL, Howard ME, Fung AM, O’Donoghue FJ, Barnes M, Lappas M, et al. The presence of coexisting sleep-disordered breathing among women with hypertensive disorders of pregnancy does not worsen perinatal outcome. PLoS One. 2020;15(2):e0229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yin TT, Williams N, Burton C, Ong SS, Loughna P, Britton JR, et al. Hypertension, fetal growth restriction and obstructive sleep apnoea in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2008;141(1):35–8. [DOI] [PubMed] [Google Scholar]
- [102].Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 3. Abdominal measurements. Br J Obstet Gynaecol. 1994;101(2):125–31. [DOI] [PubMed] [Google Scholar]
- [103].Bin YS, Cistulli PA, Ford JB. Population-Based Study of Sleep Apnea in Pregnancy and Maternal and Infant Outcomes. J Clin Sleep Med. 2016;12(6):871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Roberts CL, Lancaster PA. Australian national birthweight percentiles by gestational age. Med J Aust. 1999;170(3):114–8. [DOI] [PubMed] [Google Scholar]
- [105].Roberts CL, Lancaster PA. National birthweight percentiles by gestational age for twins born in Australia. J Paediatr Child Health. 1999;35(3):278–82. [DOI] [PubMed] [Google Scholar]
- [106].Bourjeily G, Curran P, Butterfield K, Maredia H, Carpenter M, Lambert-Messerlian G. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med. 2015;43(1):81–7. [DOI] [PubMed] [Google Scholar]
- [107].Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136.e1–5. [DOI] [PubMed] [Google Scholar]
- [109].Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- [110].Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep. 2014;37(5):843–9. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Passarella E, Czuzoj-Shulman N, Abenhaim HA. Maternal and fetal outcomes in pregnancies with obstructive sleep apnea. J Perinat Med. 2021;49(9):1064–1070. [DOI] [PubMed] [Google Scholar]
- [112].Spence DL, Allen RC, Lutgendorf MA, Gary VR, Richard JD, Gonzalez SC. Association of obstructive sleep apnea with adverse pregnancy-related outcomes in military hospitals. Eur J Obstet Gynecol Reprod Biol. 2017;210:166–172. [DOI] [PubMed] [Google Scholar]
- [113].Hoffstein V. Snoring and nocturnal oxygenation. Is there a relationship? Chest. 1995;108(2):370–4. [DOI] [PubMed] [Google Scholar]
- [114].Dunietz GL, Shedden K, Lisabeth LD, Treadwell MC, O’Brien LM. Maternal Weight, Snoring, and Hypertension: Potential Pathways of Associations. Am J Hypertens. 2018;31(10):1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Dunietz GL, Hao W, Shedden K, Holzman C, Chervin RD, Lisabeth LD, et al. Maternal habitual snoring and blood pressure trajectories in pregnancy. J Clin Sleep Med. 2022;18(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Gaccioli F, Aye I, Sovio U, Charnock-Jones DS, Smith GCS. Screening for fetal growth restriction using fetal biometry combined with maternal biomarkers. Am J Obstet Gynecol. 2018;218(2s):S725–s737. [DOI] [PubMed] [Google Scholar]
- [117].Tawfik DS, Gould JB, Profit J. Perinatal Risk Factors and Outcome Coding in Clinical and Administrative Databases. Pediatrics. 2019;143(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ford JB, Roberts CL, Algert CS, Bowen JR, Bajuk B, Henderson-Smart DJ. Using hospital discharge data for determining neonatal morbidity and mortality: a validation study. BMC Health Serv Res. 2007;7:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Phiri K, Hernandez-Diaz S, Tsen LC, Puopolo KM, Seeger JD, Bateman BT. Accuracy of ICD-9-CM coding to identify small for gestational age newborns. Pharmacoepidemiol Drug Saf. 2015;24(4):381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Abraham M, Alramadhan S, Iniguez C, Duijts L, Jaddoe VW, Den Dekker HT, et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS One. 2017;12(2):e0170946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lu Q, Zhang X, Wang Y, Li J, Xu Y, Song X, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: A systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. [DOI] [PubMed] [Google Scholar]
- [122].Tantrakul V, Numthavaj P, Guilleminault C, McEvoy M, Panburana P, Khaing W, et al. Performance of screening questionnaires for obstructive sleep apnea during pregnancy: A systematic review and meta-analysis. Sleep Med Rev. 2017;36:96–106. [DOI] [PubMed] [Google Scholar]
- [123].Malhamé I, Bublitz MH, Bourjeily G. The Challenge of Screening for Obstructive Sleep Apnea in Pregnancy. Ann Am Thorac Soc. 2019;16(10):1242–1244. [DOI] [PubMed] [Google Scholar]
- [124].O’Brien LM, Levine RS, Dunietz GL. The Berlin Questionnaire in pregnancy predominantly identifies obesity. J Clin Sleep Med. 2021;17(8):1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Santos S, Eekhout I, Voerman E, Gaillard R, Barros H, Charles MA, et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med. 2018;16(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergström A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG. 2019;126(8):984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sanapo L, Herrera N, Cristante C, Bulas DI, Russo S, Schlatterer SD, et al. How prenatal head ultrasound reference ranges affect evaluation of possible fetal microcephaly. J Matern Fetal Neonatal Med. 2021;34(15):2529–2534. [DOI] [PubMed] [Google Scholar]
- [128].Choi SKY, Gordon A, Hilder L, Henry A, Hyett JA, Brew BK, et al. Performance of six birth-weight and estimated-fetal-weight standards for predicting adverse perinatal outcome: a 10-year nationwide population-based study. Ultrasound Obstet Gynecol. 2021;58(2):264–277. [DOI] [PubMed] [Google Scholar]
- [129].Mathewlynn S, Impey L, Ioannou C. Detection of small- and large-for-gestational age using different combinations of prenatal and postnatal charts. Ultrasound Obstet Gynecol. 2022;60(3):373–380. [DOI] [PubMed] [Google Scholar]
- [130].Hugh O, Gardosi J. Fetal weight projection model to define growth velocity and validation against pregnancy outcome in a cohort of serially scanned pregnancies. Ultrasound Obstet Gynecol. 2022;60(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Hiersch L, Melamed N. Fetal growth velocity and body proportion in the assessment of growth. Am J Obstet Gynecol. 2018;218(2s):S700–S711.e1. [DOI] [PubMed] [Google Scholar]
- [132].Zou JJ, Wei Q, Shi YY, Wang K, Zhang YH, Shi HJ. Longitudinal Associations Between Maternal Glucose Levels and Ultrasonographic Fetal Biometrics in a Shanghai Cohort. JAMA Netw Open. 2022;5(4):e226407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Zhang J, Merialdi M, Platt LD, Kramer MS. Defining normal and abnormal fetal growth: promises and challenges. Am J Obstet Gynecol. 2010;202(6):522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Blyton D, Sullivan C, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep. 2004;27(1):79–84. [DOI] [PubMed] [Google Scholar]
- [135].Facco FL, Wolsk J, Patel SR, Hubel C, Gallaher M, Cashmere JD, et al. A trial of positive airway pressure for the treatment of sleep apnea in pregnancy. Am J Obstet Gynecol MFM. 2023;5(3):100840. [DOI] [PubMed] [Google Scholar]
- [136].Migueis DP, Urel A, Dos Santos CC, Accetta A, Burla M. The cardiovascular, metabolic, fetal and neonatal effects of CPAP use in pregnant women: a systematic review. Sleep Sci. 2022;15(Spec 1):264–277. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Siriyotha S, Tantrakul V, Plitphonganphim S, Rattanasiri S, Thakkinstian A. Prediction Models of Obstructive Sleep Apnea in Pregnancy: A Systematic Review and Meta-Analysis of Model Performance. Diagnostics (Basel). 2021;11(6):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].BaHammam AS, Obeidat A, Barataman K, Bahammam SA, Olaish AH, Sharif MM. A comparison between the AASM 2012 and 2007 definitions for detecting hypopnea. Sleep Breath. 2014;18(4):767–73. [DOI] [PubMed] [Google Scholar]
- [139].Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Publication bias assessment for studies using subjective methods.
Figure S2. Publication bias assessment for studies using objective methods.
Figure S3. Obstructive sleep apnea diagnosed by sleep test and mean difference in birthweight. Forest plot including only the studies defining obstructive sleep apnea by apnea-hypopnea index values of five or above events per hour.
Figure S4. Obstructive sleep apnea diagnosed by sleep test and mean difference in birthweight. Studies of figure S3 are divided in two subgroups, based on the scoring system used. Subgroup 1: no scoring system specified. Subgroup 2: studies using the 2007 American Academy of Sleep Medicine criteria.
Table S1. Search Strategy
Table S2. Inclusion and exclusion criteria
Table S3. Characteristics of the studies using subjective methods to identify maternal sleep disordered breathing.
Table S4. Characteristics of the studies using objective methods to identify maternal sleep disordered breathing.
Table S5. Characteristics of the studies using international classification of diseases codes to identify maternal sleep disordered breathing.