What is known about this subject in regard to women and their families?
Grover’s disease (GD) is a pruritic, papulovesicular rash commonly occurring on the trunk. The chest is often a sensitive location for most women, and lesions on the area can greatly affect the quality of life in addition to constant pruritis causing distress. Although the etiology of Grovers is not fully understood, there are cases of GD in association with certain triggers such as pregnancy, cancer diagnosis, and chemotherapy treatment, including aromatase inhibitors in the treatment of breast cancer.
What is new from this article as messages for women and their families?
While GD is thought to be a transient process, there are patients like ours who suffer from continued rash and symptoms despite mainstream therapies. This article demonstrates the efficacy of dupilumab in the treatment of our patient with recalcitrant GD.
Introduction
Grover’s disease (GD), or transient acantholytic dermatosis, is a papulosquamous disease of unknown etiology occurring in ~0.1% of the population. GD presents as a pruritic papulovesicular rash on the trunk and thighs, with histopathology demonstrating epidermal loss of adhesion between keratinocytes. GD is chronic and relapsing in nature, although it can persist for years. Treatment is mainly symptomatic, but phototherapy, retinoids, and systemic corticosteroids are used in cases unresponsive to conservative therapy.1
Case presentation
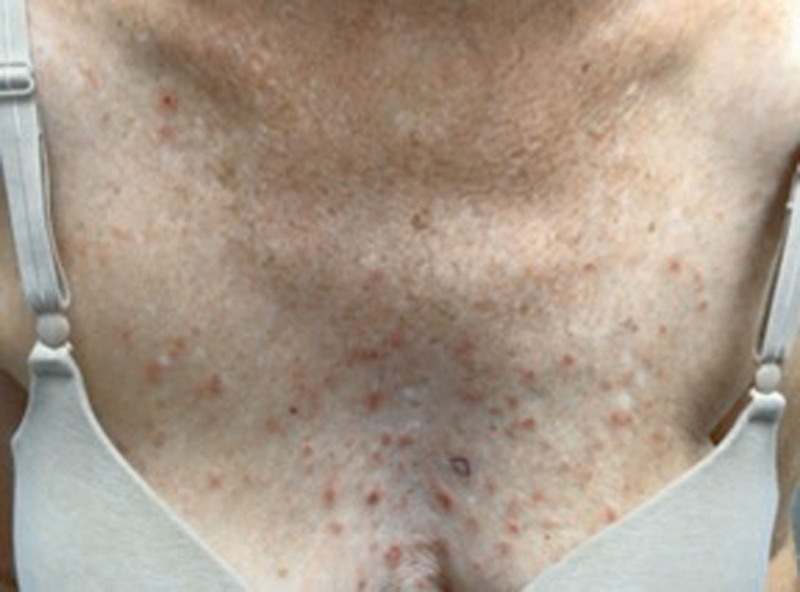
A 70-year-old female with a history of hemochromatosis, liver disease, and actinic keratosis presented to our dermatology clinic complaining of a rash on her trunk for 3 weeks. She reported severe, persistent pruritus and pain despite topical hydrocortisone. Examination revealed scattered, pink, scaly papules on the chest, spanning 30% body surface area (Fig. 1). Histopathology showed focal acantholytic dyskeratosis consistent with GD. The patient was counseled on aggravating factors and provided triamcinolone 0.1% cream. After symptoms worsened, she underwent ultraviolet B phototherapy and started on alternating triamcinolone and calcipotriene cream.
Fig. 1.
Erythematous, excoriated papulovesicular rash indicative of Grover’s disease on the central chest before therapy with dupilumab.
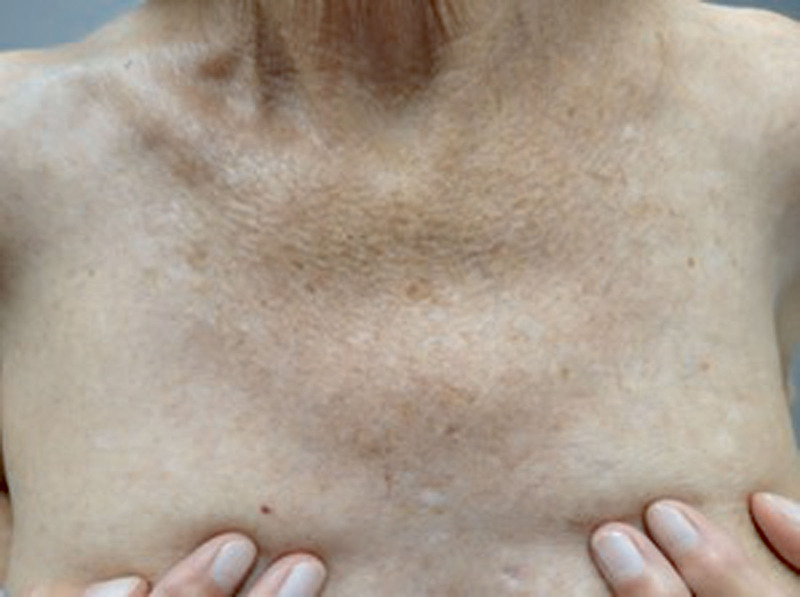
After more than 20 ultraviolet B phototherapy sessions, the patient's symptoms continued. Over the course of the next 5 months, the patient tried intramuscular corticosteroids, Tacrolimus 0.1% ointment, Montelukast, Trazodone, and Loratadine, as well as referral for allergy testing, without relief. The patient then trialed off-label Dupilumab 600 mg loading dose, followed by bimonthly 300 mg injections. She began experiencing improvement after the second injection and had complete resolution of rash and symptoms by the sixth (Fig. 2). She has since continued treatment every 2 weeks for the past 11 months and remains in remission.
Fig. 2.
Clearance of rash on the central chest after 6 total doses of Dupilumab therapy.
Discussion
We present a case of GD resistant to standard treatments that was successfully treated with dupilumab. Dupilumab, an immunoglobulin G4 monoclonal antibody, targets the interleukin-4 +receptor alpha chain, playing a central role in allergic inflammation generation.2 Dupilumab has been food and drug administration-approved for the treatment of atopic dermatitis, asthma, chronic rhinosinusitis, eosinophilic esophagitis, and prurigo nodularis. In 2 Phase 3 trials for severe atopic dermatitis, dupilumab resulted in a 69 to 72% and 67 to 72% reduction in eczema area and severity index, respectively.3 Dupilumab is well tolerated with a low toxicity profile and minimal side effects, most commonly conjunctivitis and injection-site reactions.2
The successful use of dupilumab for refractory GD has been previously documented despite a lack of food and drug administration-approved indications. Barei et al.4 reported a case of a patient prescribed dupilumab for chronic rhinosinusitis who had concomitant GD previously treated with corticosteroids and acitretin unsuccessfully for years. They observed regression of rash and pruritus following dupilumab treatment.4 Butler et al.5 also reported 3 refractory GD cases successfully treated with dupilumab over a 6- to 8-week period with marked improvement.5 Our case demonstrates similar efficacy with significant improvement following dupilumab. Although the pathogenesis of GD remains unclear, our case along with others suggests evidence for the efficacy of dupilumab in the treatment of refractory GD.
Conclusion
GD is an uncommon disorder of unknown etiology presenting as a truncal papulovesicular rash. Treatment involves topical and systemic steroids or extensive cases needing phototherapy or retinoids. Although more research is required to determine the etiology of GD and its association with interleukin-4, alternative management with dupilumab may be considered for cases of refractory GD.
Conflicts of interest
None.
Funding
Supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the authors and do not necessarily represent the official views of HCA or its affiliated entities.
Author contributions
All authors participated in the study design, data acquisition, analysis, and interpretation of the data, drafting and revising the manuscript, and final approval.
Patient consent
Informed, written consent was received from all patients for whom photographs and cases are present in the manuscript.
Footnotes
Published online 8 April 2024
References
- 1.Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med 2009;133:1490–4. [DOI] [PubMed] [Google Scholar]
- 2.Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy 2020;50:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson EL, Akinlade B, Ardeleanu M. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2017;376:1090–1. [DOI] [PubMed] [Google Scholar]
- 4.Barei F, Torretta S, Morini N, Ferrucci S. A case of Grover disease treated with Dupilumab: just serendipity or a future perspective? Dermatol Ther 2022;35:e15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler DC, Kollhoff A, Berger T. Treatment of Grover disease with dupilumab. JAMA Dermatol 2021;157:353–6. [DOI] [PubMed] [Google Scholar]