Abstract
Aims:
WHIM syndrome (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis syndrome) is a rare combined primary immunodeficiency disease caused by gain-of-function (GOF) mutations in the chemokine receptor CXCR4 and includes severe neutropenia as a common feature. Neutropenia is a known risk factor for periodontitis; however, a detailed periodontal evaluation of a WHIM syndrome cohort is lacking.
Materials and Methods:
Twenty-two adult WHIM patients and 22 age- and gender-matched healthy volunteers (HVs) were evaluated through a comprehensive medical and periodontal examination. A mouse model of WHIM syndrome was assessed for susceptibility to naturally progressing or inducible periodontitis.
Results:
Fourteen WHIM patients (63.6%) and one healthy volunteer (4.5%) were diagnosed with Stage III/IV periodontitis. No WHIM patient presented with the early onset, dramatic clinical phenotypes typically associated with genetic forms of neutropenia. Age, but not the specific CXCR4 mutation or absolute neutrophil count (ANC), was associated with periodontitis severity in the WHIM cohort. Mice with a Cxcr4 GOF mutation did not exhibit increased alveolar bone loss in spontaneous or ligature-induced periodontitis.
Conclusions:
Overall, WHIM patients presented with an increased severity of periodontitis despite past and ongoing neutrophil mobilization treatments. GOF mutations in CXCR4 may be a risk factor for periodontitis in humans.
Keywords: Periodontitis, primary immunodeficiency, CXCR4, neutrophil, neutropenia
Introduction
Periodontitis is a widespread and chronic inflammatory condition, with its severe form affecting an estimated 7.8% of adults in the United States (Eke et al., 2018). The pathogenesis of periodontitis is thought to involve a dysregulated host inflammatory response to oral bacteria which results in the destruction of tooth-supporting structures, including formation of periodontal pockets and bone loss (Chapple et al., 2018). Bacterial biofilms play a critical role in the periodontal disease process by initiating and/or continuously triggering an inflammatory response (Hajishengallis, 2014). However, many individuals with poor oral hygiene do not develop advanced periodontal disease (Loe et al., 1986). These observations have led to identification of host factors, including inherited immunodeficiencies (Hart & Atkinson, 2007; Moutsopoulos et al., 2014; Van Dyke et al., 1984; Ye et al., 2011), that contribute to periodontal disease susceptibility and progression.
WHIM syndrome (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis syndrome) is a monogenic immunodeficiency disease caused by autosomal dominant gain-of-function (GOF) mutations in the chemokine receptor CXCR4 (Balabanian et al., 2005; Hernandez et al., 2003; Wetzler et al., 1990). It is characterized by panleukopenia, low serum immunoglobulin levels (hypogammaglobulinemia), recurrent oral (pharyngitis, herpes, thrush), otosinopulmonary (otitis media, bronchitis, sinusitis, pneumonia) and skin infections (cellulitis, abscesses, dermatophyte), and persistent warts (Beaussant Cohen et al., 2012; Dotta et al., 2019; Geier et al., 2022; Gorlin et al., 2000; Heusinkveld et al., 2019; Kawai & Malech, 2009). Low penetrance phenotypes include cardiovascular and urogenital malformations (Badolato et al., 2012), while some adults have developed fatal HPV and EBV-related cancers (Beaussant Cohen et al., 2012; Imashuku et al., 2002). Approximately 180 patients have been reported in the world literature, mostly as case reports and small series (Beaussant Cohen et al., 2012; Geier et al., 2022; Heusinkveld et al., 2019).
Neutropenia is a prominent feature of WHIM syndrome. Excess CXCR4 signaling results in myelokathexis, retention of mature neutrophils in the bone marrow, leading to a significant reduction in the number of circulating neutrophils. Neutrophils derived from WHIM patients have increased chemotactic responses to CXCL12 (SDF-1), a unique ligand for CXCR4, normal superoxide production capacity, and normal in vitro bacterial killing activity, suggesting that neutrophil-related sequelae of WHIM syndrome are primarily due to reduced peripheral neutrophil numbers and not defects in neutrophil function (Heusinkveld et al., 2019).
Neutrophils are critical regulators of periodontal health, and disorders affecting neutrophil number (i.e., congenital neutropenia) or recruitment (e.g., Leukocyte Adhesion Deficiency) are associated with severe periodontitis (Moutsopoulos et al., 2014; Silva et al., 2019). Periodontitis has been described as a manifestation of WHIM syndrome, although current evidence linking WHIM syndrome and periodontal disease is limited to patient self-reported dental histories within isolated case reports (Aprikyan et al., 2000; Gorlin et al., 2000; Kumar et al., 2023; Mentzer Jr et al., 1977). Sparse information is also present on the possible mechanisms connecting WHIM syndrome with periodontal disease. Thus, this study aimed to establish the first objective evidence base for the periodontal status of patients with WHIM syndrome and WHIM syndrome model mice.
Materials and Methods
Study Participants
Adult patients diagnosed with WHIM syndrome by clinical criteria and a damaging CXCR4 mutation were recruited for participation in the study (n=22). Healthy subjects were recruited from a pool of volunteers with no systemic medical conditions and matched by age and gender to WHIM syndrome patients (n=22). All subjects signed informed consent and were enrolled on an IRB-approved protocol (clinicaltrials.gov #NCT01568697) at the National Institutes of Health (NIH) Clinical Center outpatient dental clinic and conformed to recognized standards as described in the US Federal Policy for the Protection of Human Subjects.
All subjects provided detailed medical histories including history of infections, hospitalizations, and medication. Healthy volunteers had no significant history of systemic disease, infection, or immunodeficiency. Subjects who met the eligibility criteria (Table 1) were accepted into the study regardless of gender, race or ethnicity.
Table 1.
Study Inclusion and Exclusion Criteria
| WHIM Patients | |
|---|---|
| Inclusion | Exclusion |
| ≥18 years of age | History of Hepatitis B, Hepatitis C, or HIV |
| Confirmed CXCR4 mutation | Prior head or neck radiation or systemic chemotherapeutic cancer treatment |
| Ability to provide informed consent | Pregnancy |
| Agree to dental/periodontal exam and radiographs | Taking medications that might affect bleeding |
| Healthy Volunteers | |
| Inclusion | Exclusion |
| ≥18 years of age | History of Hepatitis B, Hepatitis C, or HIV |
| Ability to provide informed consent | Prior head or neck radiation or systemic chemotherapeutic cancer treatment |
| Agree to dental/periodontal exam and radiographs | Pregnancy |
| Taking medications that might affect bleeding | |
| Diabetes | |
| Autoimmune disorder | |
| ≥3 hospitalizations within 3 years prior to study enrollment | |
| Use of systemic antibiotics, immunosuppressants, probiotics, or cytokine therapy within 3 months prior to study enrollment | |
| Use of tobacco products within 1 year of enrollment | |
Clinical Examination
Prior to enrollment of study subjects, interobserver reliability was established through the calibration of extra-oral, intra-oral and periodontal assessments as well as radiographic review by two dental clinicians in the outpatient dental clinic of the NIH Clinical Center. All participants enrolled in the study received comprehensive extra- and intra-oral exams. The dental exam included an intraoral soft tissue evaluation, an assessment of the number and location of missing teeth, and any signs or symptoms of acute infection. Dental history questionnaires were completed for all patients. Inquiries included the chief oral complaint, oral pain rating, access to care, history of dental treatments such as major restorative work, oral surgery procedures, and history of known oral diagnoses such as xerostomia, periodontal disease, severe caries, or history of recurrent oral ulcers, oral thrush, and oral HPV.
Periodontal clinical parameters included full mouth probing depths (PD), clinical attachment loss (CAL), and bleeding on probing (BOP) on six sites for all teeth, excluding third molars. Evaluation of panoramic or full mouth series of x-rays was also completed to assess radiographic evidence of alveolar bone loss. Periodontal findings were summarized as median PD, CAL, percentage of BOP sites, and number of missing teeth. Each participant was diagnosed as periodontally healthy, gingivitis, or periodontitis Stages I-IV utilizing interproximal CAL and radiographic bone levels according to the most recent guidelines provided by the European Federation of Periodontology and American Academy of Periodontology (Caton et al., 2018).
Laboratory Assessment
All healthy participants had blood drawn and tested within 2 weeks prior to the dental visit. Tests included hepatitis B and C antibody testing, HIV testing as well as complete blood count with differential (CBC), lymphocyte subset counts, and hemoglobin A1C values. For WHIM syndrome subjects, results from other NIH WHIM syndrome protocol screening tests performed under clinicaltrials.gov registered protocols # NCT02231879 or # NCT00967785 were retrieved from the NIH medical record data system when available within 2 weeks of study enrollment.
Genetic Testing
CXCR4 mutations reported in WHIM subjects were obtained from a research study (clinicaltrials.gov # NCT02231879 or # NCT00967785) at NIH in which participants were enrolled for treatment and follow-up of their immunodeficiency. Genomic DNA was isolated from peripheral blood, and CXCR4 was sequenced using Sanger sequencing methods (ABI, Waltham, MA). Data were analyzed using Sequencher Version 4.9 software (Gene Codes, Ann Arbor, MI).
Mice
The generation and description of heterozygous Cxcr4+/w mice on a C57BL/6 background, in which a human S338X WHIM mutation was introduced into the mouse Cxcr4 locus, have been previously reported (Balabanian et al., 2012; McDermott et al., 2015). The mice were kept in a single specific-pathogen-free facility at NIH. All animal experiments were performed using a National Institute of Dental and Craniofacial Research Animal Care and Use Committee-approved protocol. For age-related bone loss, male and female Cxcr4+/w mice and littermate controls (Cxcr4+/+) were euthanized at 24 weeks of age, maxillae were defleshed and stained, and the distance between cementoenamel junction (CEJ) and alveolar bone crest (ABC) was measured as previously described (Abe & Hajishengallis, 2013) by a blinded observer. For ligature-induced periodontitis (LIP), a 5-0 silk suture was tied around both 2nd maxillary molars of 10-12 week old male Cxcr4+/w and Cxcr4+/+ mice for 7 days, after which mice were euthanized and maxillary alveolar bone loss was measured.
Statistics
Data normality was assessed with the Shapiro-Wilk test. Clinical and laboratory data were summarized as medians with interquartile ranges. Other measurements were summarized as medians with interquartile ranges or mean with standard deviation when appropriate. Differences between groups were evaluated using Fisher’s exact test for nominal data, Mann-Whitney U tests for non-parametric continuous, and unpaired t test or ANOVA for continuous parametric data using Prism version 9.0 (GraphPad Software, San Diego, CA). A multivariate logistic model with Firth correction was created to analyze factors influencing periodontal diagnosis in WHIM syndrome patients using JMP Pro version 15 (SAS Institute, Cary, NC). P values of <0.05 were considered to be statistically significant.
Results
Patient Cohorts
WHIM patients and healthy volunteers were recruited from existing clinical protocols at the NIH Clinical Center. Twenty-two WHIM patients and 22 healthy volunteers (HVs) met eligibility criteria and were examined (Table 2). Diagnosis of WHIM syndrome was based on the presence of a damaging mutation in the C-terminus of CXCR4 and clinical manifestations. All subjects recruited were adults (≥18 years of age). Demographics of the two populations are shown in Table 3. The WHIM cohort included 8 males and 14 females; whereas, the healthy volunteer cohort consisted of 11 males and 11 females. The median age (interquartile range) of the WHIM cohort was 35.5 (26.5 – 42.3) and the median age of the HV cohort was 42.0 (34.8 – 45.8). The ethnic backgrounds of both groups included White, Hispanic, Black, and Asian individuals. The majority of WHIM patients (91.3%) were currently or previously treated with the selective neutrophil mobilization agent, granulocyte-colony stimulating factor (G-CSF), and 73.9% of WHIM patients were currently or previously treated with plerixafor, a CXCR4 antagonist that increases the absolute number of most leukocyte subtypes in the blood. Additionally, 43.5% were currently or previously treated with intravenous immunoglobulin (IVIG) therapy.
Table 2.
Subject Demographics and Medical History
| WHIM | Healthy | p-value | |
|---|---|---|---|
| Total Subjects | 22 | 22 | |
| Age | 35.5 (26.5 – 42.3) | 42.0 (34.8 – 45.8) | 0.1920 |
| Gender (Male/Female) | 8/14 | 11/11 | 0.5434 |
| Ethnicity (White/Non-White) | 15/7 | 19/3 | 0.1502 |
| Hispanic | 5 | 0 | |
| Black | 2 | 2 | |
| Asian | 0 | 1 | |
| Smoking (Former or Current/Never) | 3/19 | 3/19 | >0.999 |
| Diabetes (Y/N) | 1/21 | 0/22 | >0.999 |
| BMI (median (interquartile range)) | 24.5 (21 – 27.5) | 24.5 (22 – 32) | 0.3840 |
Values given as median (interquartile range) unless otherwise indicated. Significant p-values are in bold.
Table 3.
Medical and Periodontal Parameters
| WHIM | Healthy | p-value | |
|---|---|---|---|
| White Blood Count (WBC) | 1565 (1133 – 2478) | 5865 (4880 – 6658) | <0.0001 |
| Absolute Lymphocyte Count (ALC) | 770 (555 – 1115) | 1745 (1463 – 2123) | <0.0001 |
| Absolute Monocyte Count (AMC) | 120 (57.5 – 277.5) | 390 (335 – 462.5) | <0.0001 |
| Absolute Neutrophil Count (ANC) | 550 (307.5 – 960) | 3315 (3020 – 4133) | <0.0001 |
| PD (mm) | 3.53 (3.02 – 3.85) | 2.57 (2.11 – 2.76) | <0.0001 |
| CAL (mm) | 4.24 (3.46 – 4.48) | 2.54 (2.11 – 2.74) | <0.0001 |
| BOP (%) | 29.17 (12.75 – 71.02) | 7.44 (0.92 – 22.88) | 0.0018 |
| Missing teeth | 0.5 (0.0 – 5.5) | 0 (0.0 – 2.0) | 0.1498 |
| Periodontal Diagnosis (number) | 0.1853 | ||
| Healthy | 1 | 4 | |
| Gingivitis | 0 | 1 | |
| Total Periodontitis | 21 | 17 | |
| Periodontitis Stage (number) | 0.0002 | ||
| Stage I/II | 7 | 16 | |
| Stage III/IV | 14 | 1 |
Values given as median (interquartile range) unless otherwise indicated. Significant p-values are in bold.
Dental and Periodontal Evaluation
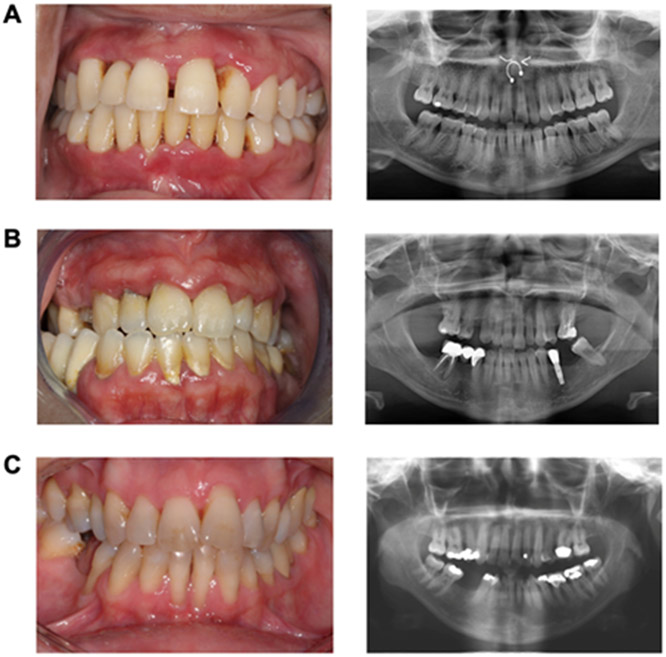
Based on detailed dental/medical history and oral exam at presentation, both WHIM patients and HVs had no significant oral findings other than periodontal disease. Overall, WHIM patients had significantly higher median full mouth PD, CAL, and BOP compared to healthy volunteers (Table 3). Specifically, WHIM patients had a median (interquartile range) PD of 3.53 (3.02 – 3.85) mm versus 2.57 (2.11 – 2.76) mm for HVs (p <0.0001). Similarly, for CAL, WHIM patients had a median of 4.24 (3.46 – 4.48) mm versus 2.54 ( 2.11 – 2.74) mm for the HVs (p <0.0001). WHIM patients displayed BOP at 29.17 (12.75 – 71.02) % of sites while 7.44 (0.92 – 22.88) % of HV sites exhibited BOP (p = 0.0018). The prevalence of total periodontitis was 95.9% in WHIM patients compared to 77.3% in HVs (p = 0.1853). Nevertheless, the overall burden of moderate/severe (Stage III/IV) periodontitis was significantly higher in this cohort compared to HVs. Fourteen WHIM patients were diagnosed with Stage III/IV periodontitis (63.6%) in contrast to only one HV (4.55%) (p = 0.0002). The WHIM patients diagnosed with periodontitis showed a range of clinical presentations (Figure 1) similar to those seen in healthy adults, lacking the dramatic phenotype often seen in other monogenic disorders like Leukocyte Adhesion Deficiency or Papillon-Lefèvre syndrome (Moutsopoulos et al., 2014; Van Dyke et al., 1984).
Figure 1.
Clinical photographs and panoramic radiographs of A) 22-year-old female, B) 38-year-old female, and C) 42 year-old female WHIM patients diagnosed with Stage III/IV periodontitis.
Laboratory Findings in WHIM patients
The majority of WHIM patients (86.3%) in the cohort were neutropenic at the time of examination, defined as an absolute neutrophil count (ANC) <1500/μl, (median ANC 550, interquartile rage of 307.5 – 960), whereas none of the HVs was neutropenic (median ANC 3315, interquartile range 3020 – 4133) (p<0.0001) (Table 3). WHIM patients also had significantly lower overall white blood cell (WBC) (p<0.0001), absolute lymphocyte (ALC) (p<0.0001), and absolute monocyte (AMC) counts (p<0.0001) compared to HVs, consistent with the panleukopenia observed in this syndrome (Heusinkveld et al., 2019). Fourteen WHIM patients (63.6%) had the CXCR4 mutation R334X, which was similarly distributed between Stage I/II (57.1%) and Stage III/IV (64.3%) diagnoses (Table 4).
Table 4.
WHIM Mutations and Periodontal Diagnosis
| Mutation | Healthy | Periodontitis Stage I/II |
Periodontitis Stage III/IV |
|---|---|---|---|
| R334X | 1 | 4 | 9 |
| S339fs | 1 | ||
| S324fs | 2 | ||
| V320fs | 1 | ||
| K327fs | 1 | ||
| S338X | 1 | 1 | |
| E343X | 1 |
Within the WHIM cohort there were no significant differences in any laboratory value for those diagnosed with Stage I/II versus Stage III/IV periodontitis (Table 5). Median trough ANC values, the lowest recorded ANC in the patient medical records, were also similar between WHIM patients with Stage I/II (median 140, interquartile range 30 – 310) vs. Stage III/IV periodontitis (125, 77.5 – 290) (p=0.5465) (Table 5). WHIM patients diagnosed with Stage III/IV periodontitis had an older median age (40, 33.5 – 52.8) compared to those diagnosed with Stage I/II periodontitis (24.5, 20.8 – 29.8) (p=0.0319). When measuring the combined impact of laboratory values, age, gender, ethnicity, smoking history, and BMI on the diagnosis of Stage I/II or Stage III/IV periodontitis through multiple regression modelling, age remained a significant factor (p=0.0176).
Table 5.
WHIM Subject Data by Periodontal Diagnosis
| WHIM Subjects | Stage I/II | Stage III/IV | p-value |
|---|---|---|---|
| Age | 25 (21 – 38) | 40 (33.5 – 52.8) | 0.0319 |
| Gender (Male/Female) (number) | 3/4 | 5/9 | >0.9999 |
| Ethnicity (Caucasian/Non-Caucasian) (number) | 6/1 | 8/6 | 0.3371 |
| Hispanic | 1 | 4 | |
| Black | 0 | 2 | |
| Asian | 0 | 0 | |
| Smoking (Former or Current/Never) (number) | 0/7 | 3/11 | 0.5211 |
| BMI | 23 (21 – 27) | 26 (20.8 – 29.3) | 0.8411 |
| White Blood Count (WBC) | 1200 (960 – 7590) | 1705 (1413 – 3190) | 0.1969 |
| Absolute Lymphocyte Count (ALC) | 580 (350 – 820) | 885 (685 – 1510) | 0.0938 |
| Absolute Monocyte Count (AMC) | 140 (60 – 200) | 135 (50 – 332.5) | 0.8706 |
| Absolute Neutrophil Count (ANC) | 760 (330 – 900) | 505 (307.5 – 1565) | 0.9850 |
| Trough ANC | 140 (30 – 310) | 125 (77.5 – 290) | 0.5465 |
| G-CSF Therapy (Past or Current/None) (number) | 6/1 | 14/0 | 0.1588 |
| Plerixafor Therapy (Past or Current/None) (number) | 4/3 | 13/1 | 0.0877 |
| IVIG Therapy (Past or Current/None) (number) | 4/3 | 6/8 | 0.6594 |
Values given as median (interquartile range) unless otherwise indicated. Significant p-values are in bold.
Experimental Periodontitis in a WHIM Mouse Model
Two experimental approaches were employed to determine if mice with heterozygous Cxcr4 GOF (knock-in of human WHIM mutation CXCR4 S338X) had increased susceptibility to periodontitis. Ligature-induced periodontitis (LIP) was employed in adult mice (age 10-12 weeks). All mice survived the procedure and were included in the analysis. After 7 days, significant bone loss (increased distance between the cementoenamel junction (CEJ) and alveolar bone crest (ABC)) occurred at ligated teeth relative to unligated controls in both WHIM mice (Cxcr4+/w) and littermate mice with normally functioning Cxcr4 (Cxcr4+/+) (Figure 2A). However, no differences in bone loss were seen between WHIM and control mice comparing ligated or unligated sites. Likewise, age-related bone loss was similar between aged WHIM and control mice at 24-weeks of age (Figure 2B).
Figure 2.
Alveolar bone loss in WHIM mice. A) Total distance between the cementoenamel junction and alveolar bone crest (CEJ-ABC) at control, unligated right maxillae (Ctrl) and ligated left maxillae (LIP) at 7 days in 10–12 week-old male WHIM mice (Cxcr4 +/w) and littermate controls (Cxcr4 +/+). B) CEJ-ABC for pooled left and right maxillae in aged male and female mice (24 weeks old). N = 4 per group in (A) and n=11-12 in (B). Two way ANOVA with Tukey post hoc test in (A) and unpaired t-test in (B); ns: not significant.
Discussion
In the present study of 22 adult WHIM patients, we observed a high prevalence of periodontitis characterized by significantly increased PD, CAL, and BOP compared to age- and gender-matched HVs. Periodontitis of any stage was found in 95.5% of WHIM and 77.3% of HVs. This high rate in HVs, relative to the recent estimate of 42% for total periodontitis in US adults (Eke et al., 2018) may be due to the majority (94%) being diagnosed with Stage I/II periodontitis, which can escape classification as mild periodontitis under CDC/AAP case definitions (Morales et al., 2022; Ortigara et al., 2021), as well as differences in HV socioeconomic status or dental history relative to the general US population. Regardless, 63.6% of WHIM patients were diagnosed with Stage III/IV periodontitis, a 14-fold higher rate than was observed in HVs. WHIM patients also had markedly reduced levels of circulating white blood cells. A genotype-phenotype correlation for periodontitis with specific CXCR4 mutations was not found, nor did the lowest previously recorded ANC (trough ANC) or ANC at the time of examination discriminate patients with and without more severe forms of periodontitis (Stage I/II vs. Stage III/IV). However, WHIM patients diagnosed with Stage III/IV periodontitis were significantly older than those diagnosed with less severe periodontitis (Stage I/II), suggesting that the effects of WHIM syndrome on periodontal tissue destruction may accumulate over time. Overall, these results confirm and extend previous observations from isolated case reports and point to GOF CXCR4 mutations as a genetic risk factor for periodontitis.
All patients had currently or previously been treated with neutrophil mobilization agents such as G-CSF and/or the CXCR4 antagonist plerixafor. Continual G-CSF therapy can elevate circulating neutrophil counts, reducing the high incidence of bacterial infections in patients with severe neutropenia. However, many WHIM patients are not able to tolerate G-CSF treatment due to severe bone pain (McDermott & Murphy, 2019). The CXCR4 antagonist plerixafor, when compared to G-CSF for the management of WHIM syndrome, can lead to less bone pain, sustained reversal of lymphopenia and monocytopenia, and clinically substantial wart regression, and a similar frequency and severity of infection (Dale et al., 2011; McDermott et al., 2011; McDermott et al., 2014; McDermott et al., 2019) and has been tested in a Phase 3 trial (McDermott et al., 2023). The majority of WHIM patients in the present study (68.2%) had been undergoing treatment with plerixafor for a variable amount of time (1 month to 9 years). However, since untreated lifetime severe neutropenia is a highly penetrant phenotype in WHIM syndrome, it is unlikely that this parameter by itself can account for patients who develop advanced periodontitis.
Leukocyte trafficking is an important function of the immune system by which immune cells migrate to and from peripheral tissues, providing primary and secondary immune responses as needed at sites of bacterial colonization and infection (Notarangelo & Badolato, 2009). In the mouth, neutrophils are abundantly present at the base of the gingival sulcus, drawn by chemotactic signals to the site of biofilm formation and tissue damage to participate in phagocytosis and other defense mechanisms (Tonetti et al., 1998). A fine balance of neutrophil activity is required to achieve a homeostatic immune response while avoiding pathogen overgrowth and detrimental host inflammation (Ryder, 2010). Excessive neutrophil accumulation and hyperactivation may be a feature of more common forms of chronic periodontitis (Hajishengallis et al., 2016) and neutrophil dysfunction has been thought to contribute to disease severity in more aggressive forms of periodontitis (Ryder, 2010). In contrast, a lack of sufficient neutrophil recruitment, exemplified by rare diseases like Leukocyte Adhesion Deficiency (LAD), is also associated with severe periodontal disease (Moutsopoulos et al., 2014). Accordingly, investigation of the immune response within the gingiva of WHIM syndrome patients may provide further insight on the protective and destructive roles of neutrophils in periodontitis.
Mouse models have been invaluable for elucidating the mechanisms underlying neutrophil defects and periodontitis, such as those found in LAD-1 where defects in neutrophil transmigration through blood vessels into periodontal tissue leads to an exaggerated Th17 response (Moutsopoulos et al., 2014). A WHIM mouse model was previously developed with a heterozygous Cxcr4 GOF (knock-in of human WHIM mutation CXCR4 S338X) (Balabanian et al., 2012). In the present study, alveolar bone loss in WHIM mice occurred at similar levels to littermate controls following both ligature-induced periodontitis (LIP) and extended aging. One factor that could underly the lack of periodontal phenotype in WHIM mice may be inherent differences in the immune system between mice and humans. Mice have fewer circulating neutrophils (Nauseef, 2023), and myelokathexis and peripheral neutropenia does not occur in WHIM mice to the same level as in WHIM patients (Balabanian et al., 2012). The diet and oral microbiome of laboratory mice also differs substantially from WHIM patients which may affect disease manifestations. Thus, the biologic mechanisms underpinning the lack of a periodontal phenotype in WHIM model mice will require additional study, and the role of neutropenia and neutrophil recruitment in WHIM syndrome-associated periodontitis may be further investigated using alternative animal models.
Future research is necessary to understand how neutropenia and other manifestations of WHIM disease affect the natural course of periodontitis. Clinical manifestations of WHIM syndrome are often first detected at a young age (Geier et al., 2022). Pediatric WHIM patients were not examined in this study, so additional studies are also warranted to identify if periodontal disease is evident in primary dentition or during adolescence. Results showing that 36% of WHIM patients did not have moderate/severe periodontitis and the severity of periodontitis increased with age also suggests that CXCR4-related immune dysfunction could act in concert with other factors such as epigenetics and age-associated immune senescence to affect periodontitis disease activity. CXCR4 mutations affect a wide range of biologic processes beyond leukocyte recruitment and have recently been linked to osteoporosis via alteration of skeletal stem cell osteogenic potential (Anginot et al., 2023) and stromal cell-mediated lymphocyte development (Zehentmeier et al., 2022), factors which also have relevance for periodontal health and disease. Additional study of patient oral bacteria, which both contributes to and can be affected by neutrophil activity, as well as saliva, gingival tissues, and blood may further delineate the pathogenesis of WHIM-associated periodontitis. Finally, how medical interventions to treat WHIM syndrome, such as neutrophil mobilization therapies, influence periodontal disease activity is unclear and merits additional study.
In conclusion, comprehensive periodontal examination of WHIM patients revealed a significantly higher burden of moderate/severe periodontitis compared to age- and gender-matched healthy volunteers. WHIM patients were characterized by a persistent reduction in circulating neutrophils and may be at risk for future progression of periodontitis. Coordination between medical and dental providers and targeted periodontal preventative therapy is advisable to maintain oral health for patients with this condition.
Clinical Relevance.
Scientific Rationale for Study.
WHIM syndrome is a rare genetic disease characterized by persistent neutropenia, a risk factor for periodontitis. While severe periodontitis has been reported in select WHIM patients, an objective assessment of periodontitis has not been performed in a cohort of WHIM patients to date.
Principal Findings.
WHIM patients were more likely to have moderate/severe periodontitis compared to healthy individuals. A history of neutropenia, despite treatment, may contribute to an increased lifetime burden of periodontitis.
Practical Implications.
Coordinated medical and dental care and targeted periodontal preventative therapy is advisable for WHIM patients.
Acknowledgments
The authors express their gratitude to the patients who have agreed to participate in this research. This research was supported in part by the Intramural Research Programs of the National Institute of Dental and Craniofacial Research and the National Institute of Allergy and Infectious Diseases, NIH. WHIM mice were kindly provided by Drs. Bachelerie and Balabanian, INSERM, Paris, France. Veterinary support was provided by the NIDCR Veterinary Resource Core. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Funding Information
Divisions of Intramural Research of the National Institute of Dental and Craniofacial Research and the National Institute of Allergy and Infectious Diseases, 1K99DE030124-01A1 (NIDCR, L.M.S.)
References
- Abe T, & Hajishengallis G (2013). Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods, 394(1-2), 49–54. 10.1016/j.jim.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anginot A, Nguyen J, Abou Nader Z, Rondeau V, Bonaud A, Kalogeraki M, Boutin A, Lemos JP, Bisio V, Koenen J, Hanna Doumit Sakr L, Picart A, Coudert A, Provot S, Dulphy N, Aurrand-Lions M, Mancini SJC, Lazennec G, McDermott DH, … Balabanian K (2023). WHIM Syndrome-linked CXCR4 mutations drive osteoporosis. Nature Communications, 14(1), 2058. 10.1038/s41467-023-37791-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprikyan AAG, Liles WC, Park JR, Jonas M, Chi EY, & Dale DC (2000). Myelokathexis, a congenital disorder of severe neutropenia characterized by accelerated apoptosis and defective expression ofbcl-x in neutrophil precursors. Blood, 95(1), 320–327. https://doi.org/ 10.1182/blood.V95.1.320 [DOI] [PubMed] [Google Scholar]
- Badolato R, Dotta L, Tassone L, Amendola G, Porta F, Locatelli F, Notarangelo LD, Bertrand Y, Bachelerie F, & Donadieu J (2012). Tetralogy of fallot is an uncommon manifestation of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. J Pediatr, 161(4), 763–765. 10.1016/j.jpeds.2012.05.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanian K, Brotin E, Biajoux V, Bouchet-Delbos L, Lainey E, Fenneteau O, Bonnet D, Fiette L, Emilie D, & Bachelerie F (2012). Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood, 119(24), 5722–5730. 10.1182/blood-2012-01-403378 [DOI] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O, Nicolas JF, Latger-Cannard V, Bensoussan D, Bordigoni P, Baleux F, Le Deist F, Virelizier JL, Arenzana-Seisdedos F, & Bachelerie F (2005). WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood, 105(6), 2449–2457. 10.1182/blood-2004-06-2289 [DOI] [PubMed] [Google Scholar]
- Beaussant Cohen S, Fenneteau O, Plouvier E, Rohrlich PS, Daltroff G, Plantier I, Dupuy A, Kerob D, Beaupain B, Bordigoni P, Fouyssac F, Delezoide AL, Devouassoux G, Nicolas JF, Bensaid P, Bertrand Y, Balabanian K, Chantelot CB, Bachelerie F, & Donadieu J (2012). Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J Rare Dis, 7, 71. 10.1186/1750-1172-7-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, & Tonetti MS (2018). A new classification scheme for periodontal and peri-implant diseases and conditions – Introduction and key changes from the 1999 classification. Journal of Clinical Periodontology, 45(S20), S1–S8. https://doi.org/ 10.1111/jcpe.12935 [DOI] [PubMed] [Google Scholar]
- Chapple ILC, Mealey BL, Van Dyke TE, Bartold PM, Dommisch H, Eickholz P, Geisinger ML, Genco RJ, Glogauer M, Goldstein M, Griffin TJ, Holmstrup P, Johnson GK, Kapila Y, Lang NP, Meyle J, Murakami S, Plemons J, Romito GA, . . . Yoshie H (2018). Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol, 45 Suppl 20, S68–S77. 10.1111/jcpe.12940 [DOI] [PubMed] [Google Scholar]
- Dale DC, Bolyard AA, Kelley ML, Westrup EC, Makaryan V, Aprikyan A, Wood B, & Hsu FJ (2011). The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood, 118(18), 4963–4966. 10.1182/blood-2011-06-360586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotta L, Notarangelo LD, Moratto D, Kumar R, Porta F, Soresina A, Lougaris V, Plebani A, Smith CIE, Norlin AC, Gomez Raccio AC, Bubanska E, Bertolini P, Amendola G, Visentini M, Fiorilli M, Venuti A, & Badolato R (2019). Long-Term Outcome of WHIM Syndrome in 18 Patients: High Risk of Lung Disease and HPV-Related Malignancies. J Allergy Clin Immunol Pract, 7(5), 1568–1577. 10.1016/j.jaip.2019.01.045 [DOI] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, & Genco RJ (2018). Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc, 149(7), 576–588.e576. 10.1016/j.adaj.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CB, Ellison M, Cruz R, Pawar S, Leiss-Piller A, Zmajkovicova K, McNulty SM, Yilmaz M, Evans MO, Gordon S, Ujhazi B, Wiest I, Abolhassani H, Aghamohammadi A, Barmettler S, Bhar S, Bondarenko A, Bolyard AA, Buchbinder D, … Walter JE (2022). Disease Progression of WHIM Syndrome in an International Cohort of 66 Pediatric and Adult Patients. Journal of Clinical Immunology, 42(8), 1748–1765. 10.1007/s10875-022-01312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, & Fenyk JR Jr. (2000). WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet, 91(5), 368–376. [PubMed] [Google Scholar]
- Hajishengallis G (2014). Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends in Immunology, 35(1), 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Moutsopoulos NM, Hajishengallis E, & Chavakis T (2016). Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin Immunol, 28(2), 146–158. 10.1016/j.smim.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart TC, & Atkinson JC (2007). Mendelian forms of periodontitis. Periodontology 2000, 45(1), 95–112. https://doi.org/ 10.1111/j.1600-0757.2007.00233.x [DOI] [PubMed] [Google Scholar]
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, & Diaz GA (2003). Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet, 34(1), 70–74. 10.1038/ng1149 [DOI] [PubMed] [Google Scholar]
- Heusinkveld LE, Majumdar S, Gao JL, McDermott DH, & Murphy PM (2019). WHIM Syndrome: from Pathogenesis Towards Personalized Medicine and Cure. J Clin Immunol, 39(6), 532–556. 10.1007/s10875-019-00665-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imashuku S, Miyagawa A, Chiyonobu T, Ishida H, Yoshihara T, Teramura T, Kuriyama K, Imamura T, Hibi S, Morimoto A, & Todo S (2002). Epstein-Barr virus-associated T-lymphoproliferative disease with hemophagocytic syndrome, followed by fatal intestinal B lymphoma in a young adult female with WHIM syndrome. Warts, hypogammaglobulinemia, infections, and myelokathexis. Ann Hematol, 81(8), 470–473. 10.1007/s00277-002-0489-9 [DOI] [PubMed] [Google Scholar]
- Kawai T, & Malech HL (2009). WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol, 16(1), 20–26. 10.1097/MOH.0b013e32831ac557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Milanesi S, Szpakowska M, Dotta L, Di Silvestre D, Trotta AM, Bello AM, Giacomelli M, Benedito M, Azevedo J, Pereira A, Cortesao E, Vacchini A, Castagna A, Pinelli M, Moratto D, Bonecchi R, Locati M, Scala S, … Badolato R (2023). Reduced G protein signaling despite impaired internalization and β-arrestin recruitment in patients carrying a CXCR4Leu317fsX3 mutation causing WHIM syndrome. JCI Insight, 8(5). 10.1172/jci.insight.145688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe H, Anerud A, Boysen H, & Morrison E (1986). Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol, 13(5), 431–445. 10.1111/j.1600-051x.1986.tb01487.x [DOI] [PubMed] [Google Scholar]
- McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, Velez D, Yim E, Bryke CR, Hsu N, Dai Z, Marquesen MM, Stregevsky E, Kwatemaa N, Theobald N, Long Priel DA, Pittaluga S, Raffeld MA, Calvo KR, … Murphy PM (2015). Chromothriptic cure of WHIM syndrome. Cell, 160(4), 686–699. 10.1016/j.cell.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O'Brien S, Penzak SR, Filho JO, Priel DAL, Kelly C, Garofalo M, Littel P, Marquesen MM, Hilligoss D, DeCastro R, Fleisher TA, Kuhns DB, Malech HL, & Murphy PM (2011). The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood, 118(18), 4957–4962. 10.1182/blood-2011-07-368084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O'Brien S, Ulrick J, Kwatemaa N, Starling J, Fleisher TA, Priel DA, Merideth MA, Giuntoli RL, Evbuomwan MO, Littel P, Marquesen MM, Hilligoss D, DeCastro R, Grimes GJ, Hwang ST, … Murphy PM (2014). A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood, 123(15), 2308–2316. 10.1182/blood-2013-09-527226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, & Murphy PM (2019). WHIM syndrome: Immunopathogenesis, treatment and cure strategies. Immunological Reviews, 287(1), 91–102. https://doi.org/ 10.1111/imr.12719 [DOI] [PubMed] [Google Scholar]
- McDermott DH, Pastrana DV, Calvo KR, Pittaluga S, Velez D, Cho E, Liu Q, Trout HH 3rd, Neves JF, Gardner PJ, Bianchi DA, Blair EA, Landon EM, Silva SL, Buck CB, & Murphy PM (2019). Plerixafor for the Treatment of WHIM Syndrome. N Engl J Med, 380(2), 163–170. 10.1056/NEJMoa1808575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Velez D, Cho E, Cowen EW, DiGiovanna JJ, Pastrana DV, Buck CB, Calvo KR, Gardner PJ, Rosenzweig SD, Stratton P, Merideth MA, Kim HJ, Brewer C, Katz JD, Kuhns DB, Malech HL, Follmann D, Fay MP, & Murphy PM (2023). A phase 3 randomized crossover trial of plerixafor versus G-CSF for treatment of WHIM syndrome. The Journal of Clinical Investigation. 10.1172/JCI164918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzer WC Jr, Johnston RB Jr, Baehner RL, & Nathan DG (1977). An Unusual Form of Chronic Neutropenia in a Father and Daughter with Hypogammaglobulinaemia. British Journal of Haematology, 36(3), 313–322. https://doi.org/ 10.1111/j.1365-2141.1977.tb00654.x [DOI] [PubMed] [Google Scholar]
- Morales A, Strauss FJ, Hämmerle CHF, Romandini M, Cavalla F, Baeza M, Sanz M, & Gamonal J (2022). Performance of the 2017 AAP/EFP case definition compared with the CDC/AAP definition in population-based studies. Journal of Periodontology, 93(7), 1003–1013. https://doi.org/ 10.1002/JPER.21-0276 [DOI] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, Abusleme L, Zenobia C, Hosur KB, Abe T, Uzel G, Chen W, Chavakis T, Holland SM, & Hajishengallis G (2014). Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med, 6(229), 229ra240. 10.1126/scitranslmed.3007696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef WM (2023). Human neutrophils ≠ murine neutrophils: Does it matter? Immunol Rev, 314(1), 442–456. 10.1111/imr.13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo LD, & Badolato R (2009). Leukocyte trafficking in primary immunodeficiencies. J Leukoc Biol, 85(3), 335–343. 10.1189/jlb.0808474 [DOI] [PubMed] [Google Scholar]
- Ortigara GB, Mário Ferreira T. d. G., Tatsch KF, Romito GA, Ardenghi TM, Sfreddo CS, & Moreira CHC (2021). The 2018 EFP/AAP periodontitis case classification demonstrates high agreement with the 2012 CDC/AAP criteria. Journal of Clinical Periodontology, 48(7), 886–895. https://doi.org/ 10.1111/jcpe.13462 [DOI] [PubMed] [Google Scholar]
- Ryder MI (2010). Comparison of neutrophil functions in aggressive and chronic periodontitis. Periodontol 2000, 53, 124–137. 10.1111/j.1600-0757.2009.00327.x [DOI] [PubMed] [Google Scholar]
- Silva LM, Brenchley L, & Moutsopoulos NM (2019). Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunol Rev, 287(1), 226–235. 10.1111/imr.12724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti MS, Imboden MA, & Lang NP (1998). Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol, 69(10), 1139–1147. 10.1902/jop.1998.69.10.1139 [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Taubman MA, Ebersole JL, Haffajee AD, Socransky SS, Smith DJ, & Genco RJ (1984). The Papillon-Lefèvre syndrome: Neutrophil dysfunction with severe periodontal disease. Clinical Immunology and Immunopathology, 31(3), 419–429. https://doi.org/ 10.1016/0090-1229(84)90094-1 [DOI] [PubMed] [Google Scholar]
- Wetzler M, Talpaz M, Kleinerman ES, King A, Huh YO, Gutterman JU, & Kurzrock R (1990). A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med, 89(5), 663–672. 10.1016/0002-9343(90)90187-i [DOI] [PubMed] [Google Scholar]
- Ye Y, Carlsson G, Wondimu B, Fahlen A, Karlsson-Sjoberg J, Andersson M, Engstrand L, Yucel-Lindberg T, Modeer T, & Putsep K (2011). Mutations in the ELANE gene are associated with development of periodontitis in patients with severe congenital neutropenia. J Clin Immunol, 31(6), 936–945. 10.1007/s10875-011-9572-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehentmeier S, Lim VY, Ma Y, Fossati J, Ito T, Jiang Y, Tumanov AV, Lee H-J, Dillinger L, Kim J, Csomos K, Walter JE, Choi J, & Pereira JP (2022). Dysregulated stem cell niches and altered lymphocyte recirculation cause B and T cell lymphopenia in WHIM syndrome. Science Immunology, 7(75), eabo3170. 10.1126/sciimmunol.abo3170 [DOI] [PMC free article] [PubMed] [Google Scholar]