Abstract
Sodium polystyrene sulphonate (SPS), employed in the management of severe hyperkalemia, is typically combined with sodium sorbitol to avert potential intestinal obstruction. Nonetheless, the administration of Kayexalate, even in the presence of minimal sorbitol, can lead to the grave complication of colonic necrosis. We present a case of Kayexalate-mediated colonic necrosis, highlighting the vital necessity of acknowledging the inherent risks associated with its usage in spite of its efficacy in potassium reduction. In light of these potential risks, it is incumbent upon physicians to exercise caution, and contemplate the use of alternative pharmacological agents that effectively eliminate excess potassium, whilst minimizing associated adverse effects.
Keywords: Sodium polystyrene sulphonate, Kayexalate, Hyperkalemia, Colonic necrosis, Case report
1. Introduction
Sodium polystyrene sulphonate (SPS), routinely employed in managing severe hyperkalemia, operates by substituting sodium ions for potassium ions within the gastrointestinal tract, thus aiding the removal of surplus potassium via fecal excretion.1 To circumvent the possibility of intestinal obstruction—a documented complication—it is typical to pair SPS with a cathartic agent, sodium sorbitol.2 Though this combination has demonstrated efficacy in hyperkalemia management, rare yet severe complications like colonic necrosis have been reported with SPS administration.3
The precise mechanism by which SPS induces colonic necrosis remains elusive. Existing hypotheses propose that even negligible amounts of sorbitol coupled with SPS could exert direct toxic impacts on the colon, leading potentially to tissue damage and consequent necrosis. This highlights the necessity of acknowledging the inherent risks tied to kayexalate (a popular brand name for SPS) and contemplating the use of alternative potassium-reducing agents in the management of hyperkalemia.4,5
In this case report, our objective is to spotlight the incidence of kayexalate-triggered colonic necrosis, underlining the prudence required when selecting SPS for hyperkalemia treatment. We further propose a need to investigate alternative potassium-reducing agents that pose fewer adverse effects on the colon. By shedding light on this complication, our goal is to augment patient safety measures and refine therapeutic strategies for hyperkalemia. This highlights the necessity of acknowledging the inherent risks tied to kayexalate and contemplating the use of alternative potassium-reducing agents such as Lokelma, known to have a safer profile.
2. Case presentation
A 42-year-old male patient, with a known medical background of chronic kidney disease and hypertension, presented at the emergency department due to ongoing nausea, vomiting, diffuse abdominal pain, and hematochezia, persisting for a period of 24 h. This individual had been undergoing outpatient management for hyperkalemia with the administration of Kayexalate.
On arrival, his vital signs were noted to be stable, and body temperature within the normal range. The physical examination exhibited moderate abdominal tenderness and protective guarding, albeit without any rebound tenderness. Laboratory investigations revealed a mildly elevated white cell count at 10k/uL, while his potassium level was found to be within normal range (4.7 mEq/L). The patient’s creatinine level was elevated to 3.4 mg/dL, consistent with his baseline due to the underlying chronic kidney disease.
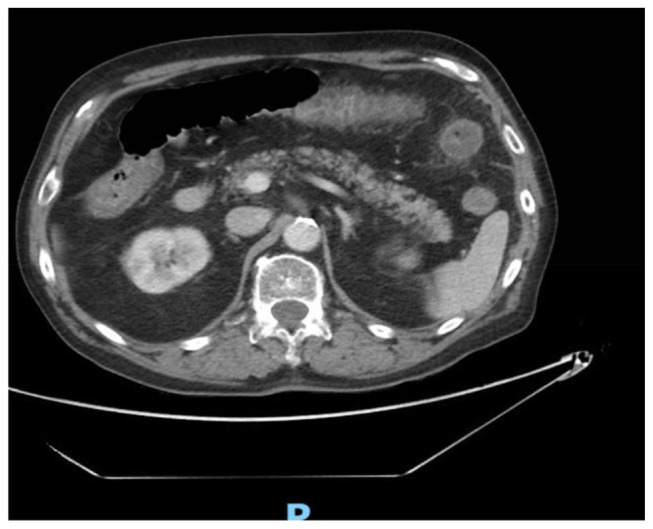
Imaging of the abdomen via computed tomography (CT) showed inflammatory changes in the transverse colon and rectum, along with rectal wall thickening suggestive of colitis and proctitis (Fig. 1). Initial management comprised intravenous administration of Ciprofloxacin and Metronidazole, alongside hydration with intravenous fluids. Stool cultures, along with PCR testing for enteric viruses and Clostridium difficile, returned negative results.
Fig. 1.
CT scan showing colitis in the transverse colon.
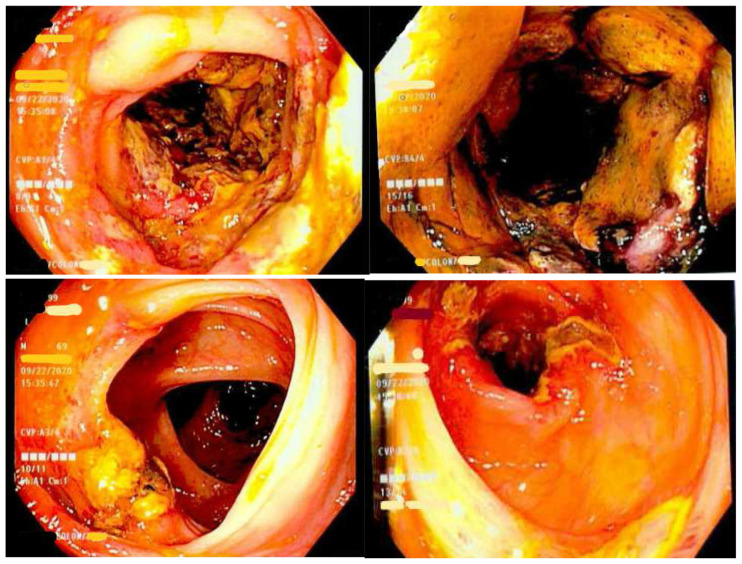
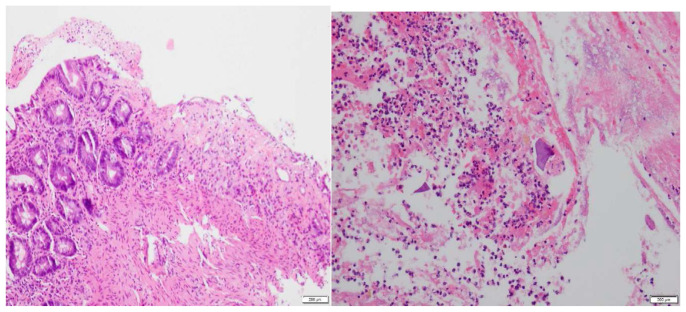
Subsequent colonoscopy identified severe segmental colitis in the ascending colon and splenic flexure, in addition to black-yellow, edematous tissue apparent in the colonic mucosa (Fig. 2). Extensive deep ulcerations were also observed in the rectum, transverse colon, and distal descending colon. Histopathological examination of the colonic biopsies disclosed an ischemic pattern of colitis, marked by fibro-inflammatory exudate and mucosal necrosis (Fig. 3). This biopsy report corroborated with colitis associated with kayexalate administration.
Fig. 2.
Colonoscopy revealing segmental colitis of the ascending with black-yellow edematous mucosa and deep ulcers in the transverse colon and rectum.
Fig. 3.
A) Colonic mucosa with an ischemic pattern of injury, i.e., lamina propria fibrosis, crypt withering, and overlying inflammatory exudate. B) Polypoid necrotic mucosa and fragments of purple-colored material consistent with kayexalate colitis.
The patient’s management included bowel rest, following which he experienced symptom resolution and was subsequently discharged.
3. Discussion
In the present case, we highlight the significance of acknowledging the potential hazards inherent to Kayexalate use, notwithstanding its beneficial role in potassium reduction. Kayexalate, a widely utilized drug for hyperkalemia treatment, functions by facilitating potassium elimination via fecal excretion. The distinctive diamond-shaped crystals of SPS exhibit a characteristic fish scale appearance, thus serving as a histological hallmark when discerned on the intestinal surface epithelium.6,7
Despite the known complications of Kayexalate, it continues to be used in certain clinical settings, possibly due to its cost-effectiveness. However, with the emergence of safer alternatives like Lokelma, healthcare providers should prioritize patient safety over economic considerations. Lokelma, or sodium zirconium cyclosilicate, has shown efficacy in managing hyperkalemia with fewer gastrointestinal side effects. The clinical benefits of newer agents might outweigh the cost savings from using older drugs like Kayexalate.
Patients undergoing Kayexalate therapy may manifest clinical features such as abdominal discomfort, hematochezia, emesis, as well as erosions or ulcerations along the gastrointestinal (GI) wall.8,9 Several predisposing factors may heighten the susceptibility to these adverse effects with Kayexalate use, including a prior history of organ transplantation, end-stage renal disease (ESRD), uremia, post-surgical administration, hypertension, and its use in critically ill patients.10–12 Each of these aspects can potentially amplify the deleterious effects of SPS in diverse manners.
Considering the array of adverse events associated with Kayexalate, it is prudent to evaluate alternative potassium-binding agents as potential frontline therapeutic options. Sodium zirconate cyclosilicate and patiromer represent such alternatives, exhibiting a considerably diminished incidence of adverse events compared to SPS.13 Sodium zirconate cyclosilicate (Lokelma) and patiromer are notable alternatives, with fewer gastrointestinal side effects compared to SPS. Both agents have demonstrated a reduced risk of complications like colonic necrosis. The decision to use one agent over another should be based on a comprehensive evaluation of the patient's clinical status, the side effect profile of the medication, and other pertinent factors, including cost.
In hyperkalemia management, as in all medical interventions, a patient-centered, safety-first approach is paramount. While Kayexalate remains an option, the availability of agents with a better safety profile necessitates a thorough evaluation of risks and benefits. The case prompts the need to prioritize research and adoption of alternative potassium-lowering agents, such as sodium zirconate cyclosilicate and patiromer, which demonstrate fewer adverse events. This shift could lead to improved patient safety and potentially better therapeutic outcomes in hyperkalemia management. Eventually, this highlights the broader need for a patient-centered, safety-first approach in all aspects of healthcare, a principle that is applicable not only to hyperkalemia management but across the spectrum of medical care.
Footnotes
Informed consent: The patient has provided permission on publishing the case report.
Conflict of interest: The authors declare that they have no competing interests. The authors have no funding sources to disclose.
References
- 1. Albeldawi M, Gaur V, Weber L. Kayexalate-induced colonic ulcer. Gastroenterol Rep. 2014;2(3):235–236. doi: 10.1093/gastro/gou011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126(3):264–e9. doi: 10.1016/j.amjmed.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 3. Bomback AS, Woosley JT, Kshirsagar AV. Colonic necrosis due to sodium polystyrene sulfate (Kayexalate) Am J Emerg Med. 2009;27(6):753–e1. doi: 10.1016/j.ajem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu YH, Chou JW, Lai HC, Su GS, Cheng KS, Chen TW. Adverse gastrointestinal effects with kayexalate or kalimate: a comprehensive review. Clin Exp Gastroenterol. 2021:1–18. doi: 10.2147/CEG.S278812. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nuzzo A, Shaar-Chneker C, Maillet M, Mégarbane B. Gastroduodenal injury induced by orally administered sodium polystyrene sulfonate. Clin Toxicol. 2019;57(1):75–76. doi: 10.1080/15563650.2018.1518529. [DOI] [PubMed] [Google Scholar]
- 6. Parfitt JR, Driman DK. Pathological effects of drugs on the gastrointestinal tract: a review. Hum Pathol. 2007;38(4):527–536. doi: 10.1016/j.humpath.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 7. Collot J, Salaouatchi T, Rickaert F, et al. Rectal ulcer in a hemodialysis patient receiving Kayexalate®. Clin Case Reports. 2021;9(4):2385–2389. doi: 10.1002/ccr3.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas A, James BR, Landsberg D. Colonic necrosis due to oral kayexalate in a critically-ill patient. Am J Med Sci. 2009;337(4):305–306. doi: 10.1097/MAJ.0b013e31818dd715. [DOI] [PubMed] [Google Scholar]
- 9. Scott TR, Graham SM, Schweitzer EJ, Bartlett ST. Colonic necrosis following sodium polystyrene sulfonate (Kayexalate®)-sorbitol enema in a renal transplant patient: report of a case and review of the literature. Dis Colon Rectum. 1993;36:607–609. doi: 10.1007/BF02049870. [DOI] [PubMed] [Google Scholar]
- 10. McGowan CE, Saha S, Chu G, Resnick MB, Moss SF. Intestinal necrosis due to sodium polystyrene sulfonate (Kayexalate) in sorbitol. South Med J. 2009;102(5):493. doi: 10.1097/SMJ.0b013e31819e8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerstman BB, Kirkman R, Platt R. Intestinal necrosis associated with postoperative orally administered sodium polystyrene sulfonate in sorbitol. Am J Kidney Dis. 1992;20(2):159–161. doi: 10.1016/s0272-6386(12)80544-0. [DOI] [PubMed] [Google Scholar]
- 12. Gardiner GW. Kayexalate (sodium polystyrene sulphonate) in sorbitol associated with intestinal necrosis in uremic patients. Can J Gastroenterol. 1997;11(7):573–577. doi: 10.1155/1997/370814. [DOI] [PubMed] [Google Scholar]
- 13. Beccari MV, Meaney CJ. Clinical utility of patiromer, sodium zirconium cyclosilicate, and sodium polystyrene sulfonate for the treatment of hyperkalemia: an evidence-based review. Core Evid. 2017;12:11. doi: 10.2147/CE.S129555. [DOI] [PMC free article] [PubMed] [Google Scholar]