Abstract
We present here a 66-year-old Caucasian male whose persistent abdominal pain thought to be due to appendicitis and associated acute splanchnic thrombosis. He was initially managed with antibiotics and anticoagulation. But further work up revealed a low-grade appendiceal mucinous neoplasm causing the splanchnic vein thrombosis. Additionally, diagnosis and management of this rare tumor and appropriate work up for splanchnic thrombosis will be briefly reviewed here.
Keywords: Low grade appendiceal mucinous neoplasm, Appendicitis, Thrombosis, Abdominal thrombosis, Splanchnic thrombosis, Benign hematology
1. Introduction
Appendiceal mucinous neoplasms are rare, with only 1000–2000 new cases reported annually in the united states.1 Appendiceal mucinous lesions are usually discovered incidentally during radiologic or endoscopic evaluation.2 AMNs are small or irregular neoplasms that present in 0.2%–0.3% of appendectomy specimens.3 CT Angiography of the abdomen is the gold standard of diagnosing splanchnic thrombosis.4 Intrabdominal thrombotic events can be precipitated by acute inflammatory reactions, which can be caused by infection, malignancy, and surgical intervention. In this report, we present a Caucasian male with a splanchnic vein thrombosis and medically managed appendicitis referred to hematology clinic to determine the length of anticoagulation for his acute splanchnic vein thrombosis. Interestingly, further work up by hematology demonstrated that he had a low-grade appendiceal mucinous neoplasm which provoked his progressive splanchnic vein thrombosis. Additionally, we will discuss the diagnosis and management of this rare tumor and approach to acute splanchnic vein thrombosis.
2. Case
A 66-year-old Caucasian male with hypertension and hyperlipidemia was brought to a local emergency department (ED) with chief complaint of severe abdominal pain and subjective fevers. He reported no personal or family history of blood clots, recent hospitalization, acute illness, surgery, or long travel in a plane or car. Family history was unremarkable for inflammatory bowel disease, autoimmune disorders, or nephrotic syndromes. Sister passed away due to leukemia. He was taking atorvastatin and metoprolol for his hyperlipidemia and hypertension. Vital signs were unremarkable except for a temperature of 37.1 °C. In physical exam abdomen was diffusely tender to deep palpation, most pronounced in the bilateral lower quadrants without guarding or rebound tenderness. Rectal examination was unremarkable and did not demonstrate any blood in the stool. Laboratory workup revealed a normal white blood cell count (WBC), mild normochromic normocytic anemia with hemoglobin (Hgb) 11.3 g/L, and normal platelet count (Plt) of 411,000/mL with an unremarkable peripheral blood smear. A complete metabolic panel (CMP) demonstrated mildly elevated direct bilirubin 0.61 mg/dL (0.0–0.2 mg/dL) and AST 52 U/L (13–39 U/L). LDH was slightly elevated at 170 U/L (34–104 U/L), and lactic acid was not elevated. A computed tomography (CT) of the abdomen and pelvis (A/P) with contrast demonstrated a filling defect within the superior mesenteric vein and at the confluence of the portal vein and superior mesenteric vein suggesting an acute thrombus. There was not an indication of an underlying appendicitis or right lower quadrant abnormality in the initial CT. He was admitted to the hospital and heparin drip was started for acute veno-occlusive mesenteric ischemia. Further studies were obtained including an acute viral hepatitis panel, blood cultures and urinalysis which were unremarkable and a subsequent limited hypercoagulopathy workup ruled out antiphospholipid syndrome and Factor V Leiden mutation. COVID-19 PCR was negative. Chest CT angiography did not detect pulmonary embolism, lymphadenopathy, or consolidations and a bilateral ultrasound duplex of the lower extremities ruled out superficial and deep vein thrombosis. He was discharged on hospital day 2 on therapeutic dose of apixaban.
Ten days later he returned to the ED with worsening abdominal pain associated with subjective fevers and chills. He denied any unintentional weight loss, postprandial abdominal pain, or rectal bleeding. Vital signs were unremarkable except for a blood pressure of 139/82 mmHg. This time laboratory work up showed elevated WBC at 26,200/mL with 82.9% neutrophils and no eosinophilia, lymphocytosis, or monocytosis, mild normochromic normocytic anemia Hgb of 12.4 g/L, and mildly elevated Plt of 470,000/mL, direct bilirubin lower than past at 0.39mg/dL and AST normalized. A repeat CT A/P with contrast demonstrated the thrombus in the superior mesenteric vein extending into the main and right portal veins which increased in extent compared to the patient’s first hospitalization as well as an enlarged 11 mm appendix with surrounding inflammatory changes interpreted as an appendicitis with a periportal lymph node measuring 1.4 cm (Fig. 1A and B). Given these findings, the patient was started on heparin drip and IV antibiotics. He subsequently was transferred to a tertiary care center for higher level of care. Upon transfer an ultrasound of A/P demonstrated echogenicities in the proximal main portal vein and at the portal confluence reflective of the known nonocclusive thrombus with a patent portal venous, hepatic venous, and hepatic arterial systems with appropriate flow directions. An Esophagogastroduodenoscopy (EGD)/colonoscopy demonstrated some mild edema of the appendix but no signs of malignancy. The patient’s pain subsequently improved with oral oxycodone, and he was discharged home with ciprofloxacin and metronidazole for a total of fourteen days. He was advised to continue apixaban until seen in benign hematology clinic.
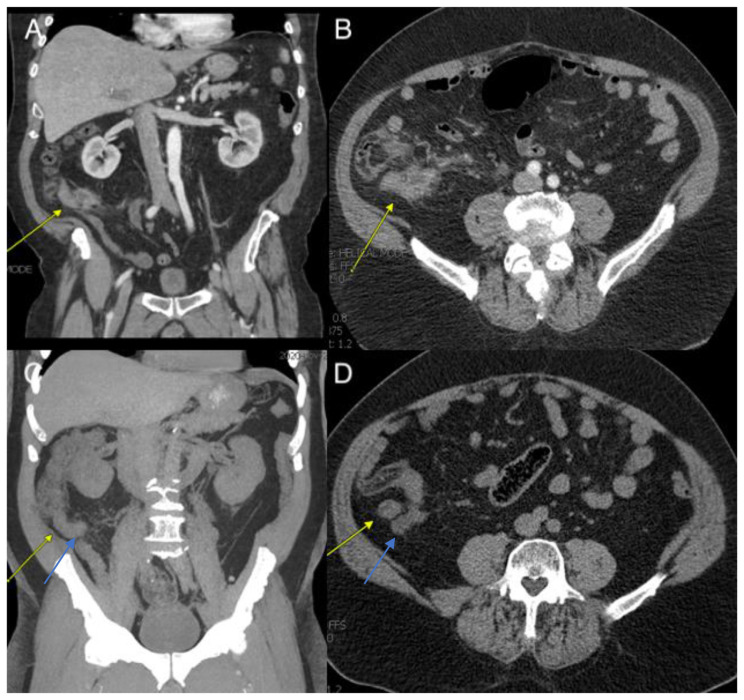
Fig. 1.
A & B: Coronal and Axial views respectively of an enlarged 11 mm appendix with surrounding inflammatory changes suggestive of appendicitis. C & D Coronal and Axial views respectively of a dilated appendix measuring 19 mm (blue arrows) with a nodular soft tissue density adjacent to appendix (yellow arrows).
At benign hematology clinic visit, he reported significant improvement in abdominal pain and needed acetaminophen infrequently and denied any fevers or chills. He denied any rectal bleeding. On further questioning, the patient’s occupation was a truck driver with his longest drive being 20 min, had no airplane flights greater than four hours, and was currently undergoing physical therapy once per week for chronic left knee pain. He endorsed one to two alcoholic drinks a week, was never a smoker, and denied illicit drug use. Peripheral blood flow cytometry did not detect any malignant cells and PCR for BCR-ABL1 PCR, JAK2 V617F, JAK2 exons 12–15 mutation were negative and Prothrombin gene G20210A mutation was not detected. Paroxysmal nocturnal hemoglobinuria (PNH) was also negative. Patient was advised to continue apixaban until completion of work up. Four months post-hospitalization ANA panel ruled out an autoimmune etiology. Five months after his discharge, a repeat CT Chest and A/P without contrast demonstrated a dilated appendix measuring 1.9 cm with slight improvement in the fat stranding and nodular soft tissue adjacent to appendix, raising concern for chronic appendicitis versus an appendiceal carcinoma or neuroendocrine tumor (Fig. 1C and D). In a laparoscopic appendectomy, he was found to have a right lower quadrant mass invading the retroperitoneum and cecum. Therefore, a right colectomy with isoperistaltic intracorporal anastomosis was performed with resection of the intrabdominal mass. During the procedure, the right lower quadrant mass was found sucked in and the appendiceal base was not visible with significant inflammation in the surrounding region. The tissue removed during the surgery consisted of portions of the ileum (10.5 cm in length and 1.5–4.0 cm in circumference), cecum and ascending colon (10.1 cm in length and 7.0 cm in average circumference), and appendix (3.8 cm in length and 1.3 cm in average diameter) without any mucin identified in the ileum and colon (Fig. 2C). The tissue margins of the specimen were noted to be free of any tumor tissue grossly, and microscopically. On microscopy the lesion showed low grade epithelial features with abundant acellular mucin, the morphologic features indicative of a low grade appendiceal mucinous neoplasm (LAMN).
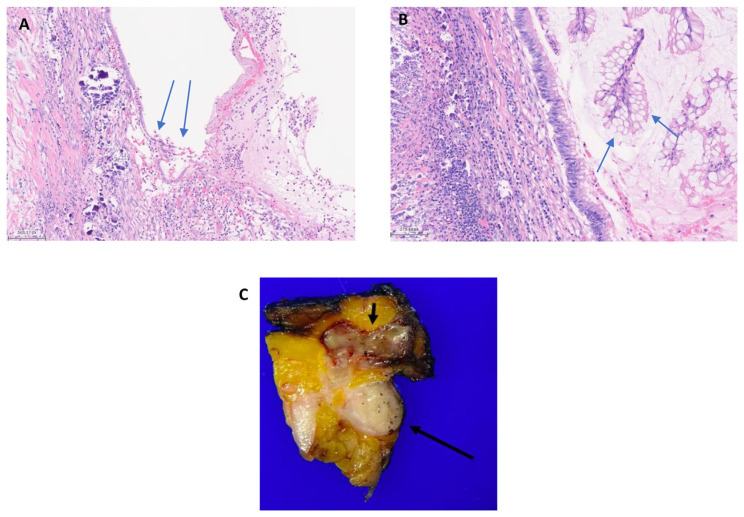
Fig. 2.
A: Photomicrograph showing the transition from the flat epithelial mucinous cells of the lesion to the denuded epithelium, which is a common finding in low grade appendiceal mucinous neoplasm (LAMN). The left side of the image shows calcifications while right side demonstrates acute inflammation with accompanying acellular mucin. B: High magnification image demonstrating the low grade morphology of the tumor cells in LAMN. CA: Gross photograph showing a cross section of the appendix (long arrow) and a cross section of the mucinous neoplasm (short arrow), which is approximately 3 cm in greatest dimension.
Given the recent surgery and extensive intrabdominal inflammation described by the surgeon, the patient was continued on therapeutic apixaban 5 mg twice a day for three months after his surgery. After a total of six months of apixaban, he remained asymptomatic and his apixaban dose was decreased to 2.5 mg twice a day. Five months later without clinical evidence of worsening clots, the splanchnic vein thrombosis was declared provoked in the setting of his localized inflammation from his LAMN and his apixaban was discontinued. He was advised to return to the clinic as needed or when symptoms recur.
3. Discussion
This is a case of abdominal pain that led to initial diagnosis of intrabdominal thrombosis and appendicitis and it was decided to start the patient on anticoagulation for thrombosis and antibiotics for medical management of appendicitis. Traditionally, appendicitis has been managed surgically; however, a metanalysis found that non-operative management of uncomplicated acute appendicitis with antibiotics was associated with fewer complications, better pain control, and shorter sick leave.5 Therefore, medical management of uncomplicated appendicitis is now becoming more prominent as first-line treatment6 The patient was referred to benign hematologist for determining lengths of anticoagulation; however, to determine the length of anticoagulation, a work up to investigate the etiology of thrombosis was completed. Radiographic findings of the so called “infectious appendicitis” requiring medical management, was not impressive enough to cause such an extensive intraabdominal thrombosis and given patient’s age, other explanations such as malignancy was explored. The malignancy work up led to the diagnosis of a low-grade appendiceal mucinous neoplasm (LAMN) causing extensive intrabdominal inflammation leading to splanchnic thrombosis.
AMNs are largely diagnosed based on the presence of mucin on pathologic examination. They can be considered mucinous if greater than 50% of the cross section of the lesion contains extracellular mucin. LAMNs typically produce mucin in a characteristically expansile growth where the border of the neoplasm is “pushed”. In diagnosing and evaluating a LAMN, the most important features to evaluate are infiltrative growth and high-grade epithelial patterns. AMNs are usually positive for MUC5AC and DPC4 with CK20 positivity and occasional CK7 positivity (29%).7 Although molecular sequencing and immunohistochemistry can be performed, neither immunohistochemical stains nor molecular tests are required for diagnosis of this lesion. In this case, the diagnosis was made only based on the presence of mucin on pathologic examination. These tumors can further be classified as well, moderately, or poorly differentiated mucinous neoplasms.8 Our case was classified as well differentiated mucinous neoplasm. The clinical course of AMN is determined by the stage at diagnosis paired with the histologic features reflecting cellular differentiation without the utilization of molecular testing.9,10 Peritoneal involvement is common at the initial presentation in patients with AMN (53.2%). Interestingly there seems to be a trend toward a decrease in regional disease status over time but an increase in distant spread of the disease.11 Our patient had a regional spread to the peritoneum; however, no distant spread of the disease. Therefore, in this patient with a 3.1 × 2.0 × 1.1 cm low-grade well differentiated AMN and involvement in the retroperitoneum with negative margins, no lymph nodes, the tumor was staged as Stage 2B T4bN0M0 (Fig. 2C).
AMNs are typically managed surgically with an appendectomy if localized.12 A hemicolectomy can be considered if the tumor involves the periappendiceal area, is larger than 2 cm, has a high-grade histology, or invades through the muscularis propria.13 Patients with LAMN that are not metastatic and have been completely resected are recommended to be followed with abdominal CT imaging every 6 month for the first two years, then annually for 15 years.14 In this patient, the tumor size was greater than 2.0 cm which prompted a right hemicolectomy. There have been no studies performed in the role of adjuvant chemotherapy; however, fluorouracil has been used in the adjuvant setting for poorly differentiated tumors with lymphatic involvement or perforation. Metastasis have also been studied to be treated with hyperthermic intraperitoneal chemotherapy alone, and in combination with complete cytoreduction.15 What was unique about this patient’s tumor was that there was significant localized inflammation. During the operation, the surgeon denoted that it took an additional two and a half hours to perform the procedure because it was significantly difficult to access the appendix, as the mesentery had to be scored off the pelvis. The mesentery was extremely heavy and friable with significant adhesions surrounding the omentum despite absence of history of abdominal surgical procedure. This degree of inflammation was noted to be extraordinarily unique for an LAMN.
This is the first case report of LAMN associated with intrabdominal thrombosis. Our review of literature did not find any case report of association between LAMNs and intrabdominal thrombosis. The case described herein demonstrates that localized non-malignant tumors can cause thrombotic events in the region they are located due to increased localized inflammation. This is commonly described in cases of appendicitis.16 This idea can be supported by the fact that intrabdominal thrombosis can also be precipitated by pancreatitis, hepatitis, cholecystitis and cholangitis.17,18
CT Angiography of the abdomen is the gold standard of diagnosing splanchnic thrombosis.4 a thorough work-up for malignancy in patients older than 60 years old is needed before evaluating for inherited thrombophilias.19 Further thrombophilia testing in patients with a thrombosis is not warranted unless in the setting of select populations20,21 These can include patients with a family history of VTE, less than 45 years-old, recurrent thrombosis, thrombosis in multiple venous sites or unusual vascular beds such as intracranial, portal or hepatic beds, those with a history of warfarin-induced skin necrosis, or arterial thrombosis.20,21 Given that this patient had an acute thrombus in an unusual vascular bed such as a portal and mesenteric, he was tested for paroxysmal nocturnal hemoglobinuria, antiphospholipid syndrome and JAK2 and all five inherited thrombophilias including protein S, protein C, antithrombin deficiencies, FVL, and prothrombin gene mutations which is recommended for hepatic and portal vein thrombosis where malignancy is not evidently shown22,23
4. Conclusion
Appendicitis and splanchnic venous thrombosis should not be taken lightly in an elderly patient and should be further evaluated to potentially identify the culprit cause. Localized and systemic inflammation has been well studied to be a precipitant of venous thrombotic events.24 This case highlights the importance of considering benign tumors as a thrombosis provoking factor in addition to malignant tumors when an intrabdominal thrombosis is found in an elderly patient.
Acknowledgements
We would like to acknowledge Dr. Vahid Yaghmai, MD, MS for his contribution in the assisting with the radiology figures and detailed findings and impression.
Footnotes
Disclaimers: This article was not submitted to other publications and/or presented at a conference or meeting.
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Source(s) of support: No additional funding sources or sources of support facilitated conduct of the work described in the article.
References
- 1. McCusker ME, Coté TR, Clegg LX, Sobin LH. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer. 2002;94(12):3307–3312. doi: 10.1002/CNCR.10589. [DOI] [PubMed] [Google Scholar]
- 2. Hamilton DL, Stormont JM. The volcano sign of appendiceal mucocele. Gastrointest Endosc. 1989;35(5):453–456. doi: 10.1016/S0016-5107(89)72860-1. [DOI] [PubMed] [Google Scholar]
- 3. Smeenk RM, van Velthuysen MLF, Verwaal VJ, Zoetmulder Fan. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34(2):196–201. doi: 10.1016/J.EJSO.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Al-Azzazy MZ, Hasan DI, Sherbeni ME, el Gameel AM.Multidetector CT and CT angiography in mesenteric ischemia Egypt J Radiol Nucl Med 2012433337–345. 10.1016/J.EJRNM.2012.05.001. [DOI] [Google Scholar]
- 5. Mason RJ, Moazzez A, Sohn H, Katkhouda N. Meta-analysis of randomized trials comparing antibiotic therapy with appendectomy for acute uncomplicated (no abscess or phlegmon) appendicitis. Surg Infect. 2012;13(2):74–84. doi: 10.1089/SUR.2011.058. [DOI] [PubMed] [Google Scholar]
- 6. Snyder MJ, Guthrie M, Cagle S. Acute appendicitis: efficient diagnosis and management. [Accessed April 11, 2023 ]; Am Fam Physician. 2018 98(1):25–33. https://www.aafp.org/pubs/afp/issues/2018/0701/p25.html . [PubMed] [Google Scholar]
- 7. Ji H, Isacson C, Seidman JD, Kurman RJ, Ronnett BM. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002;21(4):391–400. doi: 10.1097/00004347-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 8. Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia. Am J Surg Pathol. 2016;40(1):14–26. doi: 10.1097/PAS.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 9. Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. AmJ Surg Pathol. 2009;33(10):1425–1439. doi: 10.1097/PAS.0B013E3181AF6067. [DOI] [PubMed] [Google Scholar]
- 10. Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27(8):1089–1103. doi: 10.1097/00000478-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 11. Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms. Am J Clin Oncol: Cancer Clin Trials. 2017;40(6):569–573. doi: 10.1097/COC.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 12. Yantiss RK, Shia J, Klimstra DS, Hahn HP, Odze RD, Misdraji J. Prognostic significance of localized extra-appendiceal mucin deposition in appendiceal mucinous neoplasms. Am J Surg Pathol. 2009;33(2):248–255. doi: 10.1097/PAS.0B013E31817EC31E. [DOI] [PubMed] [Google Scholar]
- 13. González-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91(3):304–311. doi: 10.1002/BJS.4393. [DOI] [PubMed] [Google Scholar]
- 14. Choudry HA, Pai RK. Management of mucinous appendiceal tumors. Ann Surg Oncol. 2018;25(8):2135–2144. doi: 10.1245/S10434-018-6488-4/TABLES/3. [DOI] [PubMed] [Google Scholar]
- 15. Shaib WL, Assi R, Shamseddine A, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncol. 2017;22(9):1107. doi: 10.1634/THEONCOLOGIST.2017-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bakti N, Hussain A, El-Hasani S. A rare complication of acute appendicitis: superior mesenteric vein thrombosis. Int J Surg Case Rep. 2011;2(8):250. doi: 10.1016/J.IJSCR.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gonzelez HJ, Sahay SJ, Samadi B, Davidson BR, Rahman SH. Splanchnic vein thrombosis in severe acute pancreatitis: a 2-year, single-institution experience. HPB (Oxford) 2011;13(12):860. doi: 10.1111/J.1477-2574.2011.00392.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muneer M, Abdelrahman H, El-Menyar A, Zarour A, Awad A, Al-Thani H. Acute cholecystitis complicated with portal vein thrombosis: a case report and literature review. Am J Case Rep. 2015;16:627. doi: 10.12659/AJCR.894846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colucci G, Tsakiris DA. Thrombophilia screening revisited: an issue of personalized medicine. J Thromb Thrombolysis. 2020;49(4):618. doi: 10.1007/S11239-020-02090-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicolaides A, Hull RD, Fareed J. Prevention and treatment of venous thromboembolism: international consensus statement (Guidelines according to scientific evidence) Clin Appl Thromb/Hemo. 2013;19(2):116–225. doi: 10.1177/1076029612474840. [DOI] [PubMed] [Google Scholar]
- 21. Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209–220. doi: 10.1111/J.1365-2141.2009.08022.X. [DOI] [PubMed] [Google Scholar]
- 22. Austin SK, Lambert JR. The JAK2 V617F mutation and thrombosis. Br J Haematol. 2008;143(3):307–320. doi: 10.1111/J.1365-2141.2008.07258.X. [DOI] [PubMed] [Google Scholar]
- 23. Pardanani A, Lasho TL, Hussein K, et al. JAK2V617F mutation screening as part of the hypercoagulable work-up in the absence of splanchnic venous thrombosis or overt myeloproliferative neoplasm: assessment of value in a series of 664 consecutive patients. Mayo Clin Proc. 2008;83(4):457–459. doi: 10.4065/83.4.457. [DOI] [PubMed] [Google Scholar]
- 24. Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142. doi: 10.3389/FPED.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]