Abstract
Respiratory syncytial virus (RSV) is the major cause of acute bronchiolitis in infancy, a syndrome characterized by wheezing, respiratory distress, and the pathologic findings of peribronchial mononuclear cell infiltration and release of inflammatory mediators by basophil and eosinophil leukocytes. Composition and activation of this cellular response are thought to rely on the discrete target cell selectivity of C-C chemokines. We demonstrate that infection in vitro of human epithelial cells of the lower respiratory tract by RSV induced dose- and time-dependent increases in mRNA and protein secretion for RANTES (regulated upon activation, normal T-cell expressed and presumably secreted), monocyte chemotactic protein-1 (MCP-1), and macrophage inflammatory protein-1α (MIP-1α). Production of MCP-1 and MIP-1α was selectively localized only in epithelial cells of the small airways and lung. Exposure of epithelial cells to gamma interferon (IFN-γ), in combination with RSV infection, induced a significant increase in RANTES production that was synergistic with respect to that obtained by RSV infection or IFN-γ treatment alone. Epithelial cell-derived chemokines exhibited a strong chemotactic activity for normal human blood eosinophils. Furthermore, eosinophils were susceptible to RSV and released RANTES and MIP-1α as a result of infection. Therefore, the inflammatory process in RSV-induced bronchiolitis appears to be triggered by the infection of epithelial cells and further amplified via mechanisms driven by IFN-γ and by the secretion of eosinophil chemokines.
Respiratory syncytial virus (RSV) is responsible for virtually all the cases of bronchiolitis experienced during the first 2 years of life and for the vast majority of lower respiratory tract infections associated with wheezing between the ages of 2 and 5 (62). In addition to the clinical and epidemiological relationship between bronchiolitis in infancy and asthma later in life (23, 50), the two diseases are linked by common histopathological features characterized by profound inflammation of the airway mucosa. Along this line, necrosis of the bronchial epithelium associated with peribronchial and perivascular mononuclear cell infiltration is considered a hallmark of RSV infection, both in humans (1, 14, 15) and in animal models (19, 20, 41, 42, 57). Moreover, the presence of cell-specific inflammatory mediators in nasopharyngeal secretions and in tracheobronchial aspirates of children with bronchiolitis suggests that RSV infection triggers the migration to the airways and local activation of eosinophil and basophil leukocytes (10, 16, 49, 61, 63).
The specific antiviral immune response appears to play a role in the pathogenesis of RSV-induced airway inflammation. Development of an RSV-specific immunoglobulin E (IgE) response in the airways of infected children has been in fact associated with the release of histamine (63) and of leukotriene C4 (61). Recent studies suggest that other mechanisms may also trigger and sustain lung inflammation following RSV infection. In this regard, eosinophil cationic protein has been demonstrated in nasopharyngeal secretions of children with various forms of RSV-related airway disease (10, 16). Levels of eosinophil cationic protein were significantly higher in subjects with clinically proven bronchiolitis than in children with upper respiratory infection or with pneumonia but without wheezing and were significantly correlated with the degree of hypoxia (16).
Much of the cellular response at sites of tissue inflammation is controlled by gradients of chemotactic factors that direct leukocyte transendothelial migration and movement through the extracellular matrix. The composition of this cellular response is dependent on the discrete target cell selectivity of these chemotactic molecules. Chemokines, a newly identified family of small chemotactic cytokines, regulate the migration and activation of leukocytes and therefore play a key role in inflammatory and infectious processes of the lung (11, 40). Members of the C-C branch of the chemokine family such as RANTES (regulated upon activation, normal T-cell expressed and presumably secreted), macrophage inflammatory protein-1α (MIP-1α), and monocyte chemotactic protein-1 (MCP-1) are chemotactic and activator factors for monocytes, basophils, and eosinophils, with no activity on neutrophils (2, 3, 5, 13, 26, 44). With regard to human T lymphocytes, RANTES, MIP-1α, and MCP-1 have been shown to be specifically chemotactic for CD4+ T cells of the CD45RO+ memory phenotype (36, 46, 47). Among other potential important function of RANTES and MIP-1α in allergic and virus-induced inflammation are their capacity to stimulate surface IgE-surface or IgG4-positive B cells for enhanced IgE and IgG4 production (28) and to induce degranulation of natural killer cells and cytotoxic T cells (55, 56). Moreover, these chemokines are present in lung tissue and bronchial lavage fluid of asthmatic subjects (4, 58).
Human epithelial cells appear to be a dominant source of many C-C chemokines, including RANTES (54) and MCP-1 (51, 53). Respiratory epithelial cells are the primary and, as in the case of RSV, virtually the only cell target of viruses which enter the airways (21). Therefore, to identify the mechanisms that can regulate the development of airway mucosa inflammation in viral infection, we have investigated the production of C-C chemokines by RSV-infected respiratory epithelial cells. The studies demonstrate that in vitro infection by RSV of epithelial cells from the large bronchi, bronchioles, and lung type II cells results in the induction and secretion of a cell-specific pattern of C-C chemokines. In contrast to the widespread secretion of RANTES by the infected respiratory epithelium, production of both MCP-1 and MIP-1α after RSV infection appears to be selectively localized in epithelial cells from the small bronchioles and lung. Moreover, since constitutive or cytokine-inducible MIP-1α gene transcription and protein secretion by epithelial cells have not been previously reported, these findings suggest the existence of a novel and specific mechanism by which RSV may trigger inflammation in the lung. Along this line, we demonstrate here that the chemokines produced by RSV-infected epithelial cells have biological activity relevant to the pathogenesis of allergic inflammation, as they exhibit chemotaxis for blood eosinophils. Finally, we show that human blood eosinophils are susceptible to RSV and elaborate and secrete the chemokines RANTES and MIP-1α in response to infection. Therefore, the pathologic process of inflammation in RSV bronchiolitis, initially triggered by the infection of airway epithelial cells, may be further sustained and amplified via autocrine mechanisms driven by the secretion of eosinophil chemokines.
MATERIALS AND METHODS
Culture of epithelial cells.
Cultures of normal human bronchial epithelial cells from the trachea and main bronchi (normal human bronchial epithelial [NHBE] cells) or from the bronchioles (small airway epithelial [SAE] cells) were initially established at Clonetics Corp. (San Diego, Calif.) from normal human tissue according to referenced procedures (32). SAE cells were positive for cytokeratin 19 and negative for alkaline phosphatase, a marker of lung type II epithelial cells. NHBE and SAE cells were grown in BEGM (Clonetics Corp.) containing human recombinant epidermal growth factor (0.5 ng/ml), hydrocortisone (0.5 μg/ml), bovine pituitary extract, retinoic acid (0.1 ng/ml), and epinephrine (0.5 μg/ml), supplemented with gentamicin (50 μg/ml), amphotericin B (50 ng/ml), and 1% bovine serum albumin (for SAE culture). Cells were used in the experiments at the fourth passage. A549 cells (American Type Culture Collection, Rockville, Md.) were grown as monolayer in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), 100 U of penicillin per ml, and 0.1 mg of streptomycin per ml (all from Gibco BRL, Grand Island, N.Y.). All cell lines were maintained at 37°C in 5% CO2.
Eosinophil purification.
Heparinized venous blood was obtained from normal volunteers and sedimented with 6% dextran. The leukocyte-enriched buffy coats were overlaid onto Ficoll-Paque (Pharmacia, Piscataway, N.J.) and centrifuged at 400 × g for 20 min. The granulocyte-containing cell pellets were collected and washed twice with cold calcium- and magnesium-free Hanks buffered salt solution (Gibco BRL) as previously described (29). Erythrocytes were removed by hypotonic lysis. Eosinophils were negatively selected with anti-CD16 immunomagnetic beads to remove neutrophils, using the MACS system (Miltenyi Biotec, Sunnyvale, Calif.). The purity of eosinophils was consistently >99%.
Virus preparations.
The human Long strain of RSV (A2) was grown in Hep-2 cells and purified on a 35 to 65% discontinuous sucrose gradient as described elsewhere (60). The virus titer of the purified RSV (pRSV) pools, as determined by a methylcellulose plaque assay (31), was 5 × 108 PFU/ml. To inactivate replicating virus, pRSV was diluted in 1 ml of MEM containing 2% FBS and exposed to 254-nm UV light source for 5 min on ice. No contaminating cytokines, including interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α) IL-6, IL-8, granulocyte-macrophage colony-stimulating factor, and interferon (IFN), were found in these sucrose-purified viral preparations (25). RSV-conditioned medium (RSV-CM) was prepared by infecting monolayers of A549 or NHBE cells with pRSV at multiplicity of infection (MOI) of 1 and then incubated at 37°C in 5% CO2. At 48 h the supernatant was collected, centrifuged at 300 × g, and finally exposed to a UV light source as described for the inactivation of pRSV. Virus pools and conditioned medium were aliquoted, quick-frozen on dry ice and alcohol, and stored at −70°C until used.
Experimental design for determination of RANTES, MCP-1, and MIP-1α protein release and mRNA expression in epithelial cells.
Epithelial cells grown at 90% confluence were infected with RSV at an MOI of 1 or, in dose-response experiments, at MOIs of 0.2, 0.5, 1, and 5. To infect the cells, frozen pRSV stock was rapidly thawed and diluted with MEM containing 2% FBS (A549) or with BEGM (NHBE and SAE). The virus was added immediately to the flasks (0.04 ml of diluted virus/cm2 of area) after removal of the culture medium. In control experiments, an equivalent amount of sucrose-purified uninfected Hep-2 cells was added to epithelial cells. After addition of virus, the flasks were rocked mechanically for 1 h at 37°C, and then medium (0.16 ml/cm2) was added to the culture flasks. The infection was continued for the indicated times in a 37°C incubator. Supernatant were collected at 3, 6, 12, 24, or 48 h, centrifuged, and stored at −70°C for subsequent measurement of chemokines by specific enzyme-linked immunosorbent assay (ELISA). Cells were harvested from the same tissue culture flasks, and total RNA was extracted for the determination of chemokine mRNA. To assess the requirement for viral replication in the generation of chemokines, epithelial cells were treated with UV-inactivated preparations of pRSV. In other experiments, chemokine production was determined in supernatants of epithelial cells that were simultaneously infected with pRSV at an MOI of 0.2 and treated with IFN-γ (100 U/ml; Boehringer Mannheim, Indianapolis, Ind.).
Production of RANTES and MIP-1α by human eosinophils.
Purified eosinophils (2 × 105 cells/ml) were infected with RSV at an MOI of 10 or cultured with control medium (RPMI 1640 with 2% FCS) at 37°C in 5% CO2. After 16 h of incubation, the supernatants were collected and stored at −70°C until used. To determine total content of RANTES and MIP-1α, eosinophils (2 × 105 cells/ml) were lysed with 2% Triton X-100.
Immunofluorescence assay for viral infection.
Infection of epithelial cells and eosinophils exposed to RSV was determined by indirect immunofluorescence microscopy with minor modifications of the method previously described (27). Briefly, acetone-fixed cytocentrifuged smears of control or RSV-infected epithelial cells and eosinophils were incubated for 45 min at 37°C with a monoclonal antibody (MAb) (clone B5) directed against RSV F-glycoprotein (Fgp) (59) or with an irrelevant mouse MAb of the same isotype (DAKO Corp., Carpinteria, Calif.) followed by incubation for 45 min at 37°C with a fluorescein isothiocyanate-conjugated anti-mouse F(ab′)2 IgG antibody (1:200) (DAKO). After extensive washing, slides were counterstained with Evans blue (Sigma Chemical Co., St. Louis, Mo.) and examined with a fluorescence microscope (Nikon Optiphot) equipped with a photomicrographic attachment (Nikon HFX-DX).
ELISA for chemokines.
Levels of immunoreactive RANTES, MCP-1, and MIP-1α were determined by using a commercially available ELISA (R&D Systems Inc., Minneapolis, Minn.) as instructed by the manufacturer. Cell-free supernatants were tested in duplicates. The RANTES, MCP-1, and MIP-1α ELISAs are sensitive to 2.5, 5, and 2 pg/ml, respectively, and have an intra-assay coefficient of variation of <5% and interassay coefficient of variation of <10%, according to the manufacturer’s instructions.
RT-PCR for measurement of chemokine mRNA.
Total RNA was extracted from control or RSV-infected epithelial cells by the guanidinium thiocyanate method (8) with RNAzol B (BIOTECX, Houston, Tex.), and reverse transcription (RT)-PCR was performed with Gene RNA PCR kit components (Perkin-Elmer, Stafford, Tex.). In brief, 1 μg of total RNA was incubated at 42°C for 30 min in 20 μl of RT mixture (5 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl [pH 8.3], 1 mM deoxyribonucleoside triphosphates, RNase inhibitor [1 U/μl], Moloney murine leukemia virus reverse transcriptase [2.5 U/μl], 2.5 μM random hexamers), denatured by heating at 99°C for 5 min, and rapidly cooled to 4°C. Next, PCR mixture consisting of 2 mM MgCl2, 50 mM KCl, 10 mM Tris-HCl, AmpliTaq DNA polymerase (2.5 U/100 μl), and 0.4 μM sense and antisense primers for human RANTES, MIP-1α, MCP-1, and β-actin was added to the RT products. PCR was carried out in a final volume of 100 μl in a DNA thermal cycler (Perkin-Elmer) programmed as follows: denaturation cycle at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min for a total of 28 cycles (MCP-1) or 35 cycles (RANTES, MIP-1α, and β-actin). To ascertain that the PCR products were analyzed during the linear increase of the product, the optimal cycle number of RANTES, MIP-1α, and MCP-1 PCR was determined by either using a constant amount of cDNA template or adding fivefold serial dilutions of the target cDNA to the PCR mixture. Sequences of PCR primers used have been previously reported (4). PCR products were resolved alongside pUC18HaeIII DNA marker (Sigma) on a 1.8% agarose gel containing ethidium bromide, visualized under UV light, and photographed.
Eosinophil chemotaxis.
Eosinophil chemotaxis was studied by using 5-μm polycarbonate membranes in Boyden microchambers as previously described (3). Briefly, 30 μl of RSV-CM was placed in the lower compartment of the Boyden chamber and incubated for 3 h at 37°C in the presence of 100 μl of eosinophil suspension (105 cells) in the upper compartment. For neutralizing studies, anti-RANTES MAb (10 μg/ml; R&D Systems) or irrelevant isotype control mouse IgG (DAKO) was added to the RSV-CM in lower compartments. The concentration of the neutralizing antibody was chosen based on the inhibition curve of biologic effect provided by the manufacturer (R&D Systems). Uninfected epithelial cell supernatant was used as a negative control, and platelet-activating factor (PAF; 10−7 M) was used as a positive control. At the end of incubation, the membranes were removed and were stained with Wright’s stain. Migrated eosinophils were counted in 10 random high-power fields. All slides were read by two independent observers blinded to the experimental conditions. Each experiment was performed in duplicate.
Statistical analysis.
Analysis of data was performed with the aid of Sigma Stat software (Jandel Scientific, San Rafael, Calif.). The effects of viral infection and time on chemokine protein levels were analyzed by using the paired Student t test and Mann-Whitney rank sum test. For all analyses, P values of <0.05 were considered to be significant.
RESULTS
Infection of SAE cells by RSV.
Human epithelial cells from the lower airways were used for these studies. NHBE and SAE cells were isolated from normal tissue of the large bronchi (two adult donors and two children of 6 and 8 years) and terminal bronchioles (two adults), respectively. A549, a cell line derived from an alveolar cell carcinoma of the lung, retains features of type II alveolar epithelial cells, including the ability to produce surfactant (33). While A549 and NHBE cells have been previously shown to be susceptible to RSV (17, 37), no information was available about the in vitro susceptibility of SAE cells to RSV. Thus, in initial experiments, monolayers of SAE cells were inoculated with RSV at an MOI of 1 for 48 h and the infection was determined by indirect immunofluorescence. Cytospin preparations of RSV-exposed SAE cells that were stained with a MAb directed against the Fgp of RSV showed typical intracytoplasmic fluorescent granular inclusions at 48 h postinfection (not shown). Time points later then 48 h were not analyzed since cells were almost completely detached from the plastic vessels as a consequence of massive damage. Similar patterns of positive fluorescence were found in preparations of NHBE cells and A549 cells exposed to RSV.
Bronchial, small airway, and lung type II epithelial cells infected with RSV secrete the C-C chemokines RANTES, MCP-1, and MIP-1α.
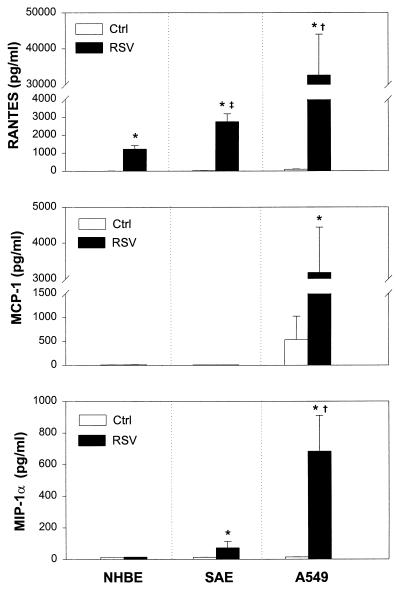
Members of the C-C chemokine family such as RANTES, MCP-1, and MIP-1α are known to be potent chemoattractants and activators of T lymphocytes, monocytes, and eosinophils, all of which are present in inflammatory infiltrates or in respiratory secretions of RSV-infected children. Thus, we examined whether lower airway epithelial cells infected with RSV were induced to secrete these chemokines. Analysis of culture supernatant by ELISA showed that A549, NHBE, and SAE cells were able to express cell-specific patterns of C-C chemokines following RSV infection (Fig. 1). While RANTES and MIP-1α were virtually absent in 48-h supernatant of uninfected epithelial cells, MCP-1 was released in modest concentrations only by uninfected A549 cells. Infection with RSV induced the secretion of RANTES, MCP-1, and MIP-1α, although in terms of absolute levels, RANTES production predominated in all cell types. In particular, 48-h RSV-infected A549 cells produced significantly higher concentrations of RANTES (32,680 ± 11,328 pg/ml; mean ± standard deviation [SD]) than SAE cells (2,745 ± 440 pg/ml) or NHBE cells (1,223 ± 192 pg/ml) (P < 0.02). We found also a statistically significant difference between the concentrations of RANTES released by SAE cells compared with NHBE cells (P < 0.01). RSV infection failed to induce MCP-1 release by NHBE and SAE cells, but high levels of this chemokine were released by infected A549 cells (3,177 ± 1,261 pg/ml). Since previous attempts by others to demonstrate induction of MIP-1α in human epithelial cells treated with potent inflammatory cytokines (IL-1β, TNF-α, and IFN-γ) have been unsuccessful (7), it was to some extent unexpected to find that RSV strongly induced MIP-1α secretion by A549 (683 ± 227 pg/ml) and SAE (72 ± 39 pg/ml) cells but not by NHBE cells. The patterns and concentrations of chemokines in control or RSV-infected NHBE and SAE were comparable in all clones (i.e., donors).
FIG. 1.
C-C chemokine secretion by RSV-infected epithelial cells. NHBE, SAE, and A549 cells were infected with RSV at an MOI of 1 (RSV) or cultured in control medium (Ctrl) for 48 h. RANTES, MCP-1, and MIP-1α concentrations were determined in the supernatant by specific ELISA. The results are expressed as mean ± SD of five experiments. ∗, P < 0.05 compared with uninfected control cells; †, P < 0.02 for A549 cells compared with SAE and NHBE cells; ‡, P < 0.01 for SAE cells compared with NHBE cells.
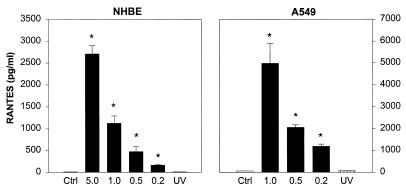
Additional experiments were conducted to characterize the generation of these chemokines in relation to RSV titer and replication. To determine the effect of viral dose on RANTES generation, different MOIs were tested in cultures of NHBE and A549 cells (Fig. 2). Infection of epithelial cells with increasing doses of RSV induced a proportional increase in RANTES release. Furthermore, the requirement of infectious virus for RANTES production was confirmed by the failure of UV-inactivated RSV pools (MOI of 1) to induce detectable levels of RANTES in both NHBE and A549 cell cultures. The dose-response curves for MIP-1α and MCP-1 in RSV-infected A549 and SAE cells, respectively, were comparable to that of RANTES (not shown).
FIG. 2.
Effect of RSV infectious dose and UV-inactivated RSV on RANTES production by airway epithelial cells. NHBE cells were infected with RSV at MOIs of 5, 1, 0.5, and 0.2 for 48 h and A549 cells at MOIs of 1, 0.5, and 0.2 for 24 h. In experiments designed to determine the requirement of replicating virus for RANTES induction, NHBE and A549 cells were exposed to UV-inactivated RSV (UV). Data are expressed as mean ± SD of three experiments. ∗, P < 0.05 for each infectious dose compared with control or with UV-inactivated RSV.
Time course of chemokine secretion and mRNA expression after RSV infection.
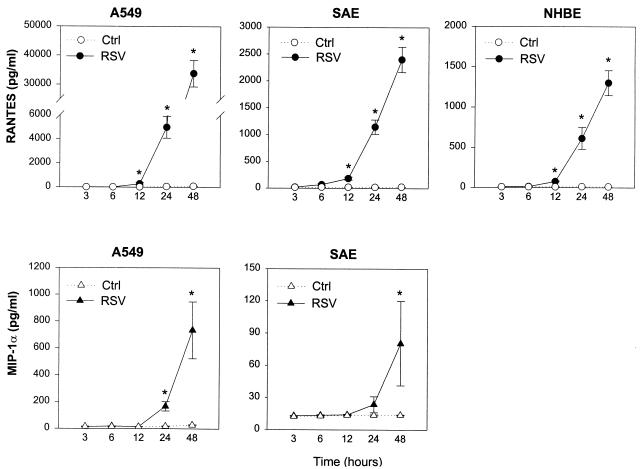
For the time course experiments, aliquots of culture supernatants were assayed for immunoreactive RANTES and MIP-1α at different time points after RSV infection or after exposure of epithelial cell monolayers to control medium (Fig. 3). Starting at 12 h, a progressive increase in RANTES concentrations could be detected in supernatants of RSV-infected A549, SAE, and NHBE cells. On the other hand, RANTES levels in uninfected cells remained either undetectable or at low levels of detection throughout the 48-h culture. Later time points were not tested because of the extensive cell damage. MIP-1α secretion, tested in A549 and SAE cells, was characterized by a slower kinetics compared to RANTES. By 12 h, MIP-1α was still undetectable in control or infected cells. MIP-1α immunoreactivity, however, appeared in cell supernatant at the 24-h time point and peaked at 48 h in RSV-infected A549 and SAE cells while remaining undetectable in uninfected cell cultures.
FIG. 3.
Kinetics of RANTES and MIP-1α accumulation in supernatant from RSV-infected A549, SAE, and NHBE cells. Epithelial cells were infected with RSV at an MOI of 1 (RSV) or cultured in control medium (Ctrl). After indicated time of incubation, supernatants were collected for RANTES and MIP-1α determination by ELISA. The results are expressed as mean ± SD of four experiments. ∗, P < 0.05 compared with control at each time point.
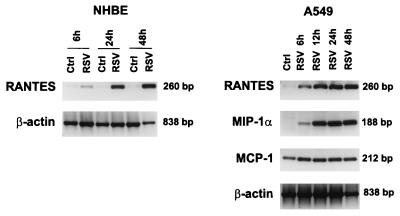
Time-dependent changes in the steady-state levels of chemokine mRNA were assayed by RT-PCR in NHBE and A549 cells (Fig. 4). Uninfected cells expressed levels of RANTES mRNA close to the detection limit at all time points tested (6, 24, and 48 h for NHBE cells and 48 h for A549 cells). However, after RSV infection, expression of RANTES mRNA both in NHBE and A549 cells increased within 6 h and up to 48 h, a time at which cells lose viability. Analysis of MIP-1α and MCP-1 mRNA expression was also carried out in A549 cells. MIP-1α mRNA was not expressed in uninfected A549 cells but was strongly induced by RSV starting at 6 h postinfection. In agreement with protein levels (Fig. 1), we found constitutive expression of mRNA for MCP-1 in uninfected A549 cells and increased mRNA abundance at 6 to 24 h postinfection. Therefore, the secretion of RANTES, MCP-1, and MIP-1α protein in infected epithelial cells was paralleled by an increase in the levels of mRNA, suggesting that the induction of these chemokines by RSV was mediated through enhanced mRNA production and/or mRNA stabilization.
FIG. 4.
Time-dependent expression of chemokine mRNA by RSV-infected NHBE and A549 cells. Total RNA was extracted from control (Ctrl) and RSV-infected (RSV) cells at the indicated time. RT-PCR was performed with specific primers for human RANTES, MIP-1α, MCP-1, and β-actin. PCR products were visualized with ethidium bromide staining on agarose gel. Expression of the housekeeping gene encoding β-actin is shown for comparison to demonstrate relative equal loading of the RT-PCR mixtures for all samples. A representative experiment from three performed on separate cell culture samples is shown.
Synergistic effect of RSV and IFN-γ on RANTES production by epithelial cells.
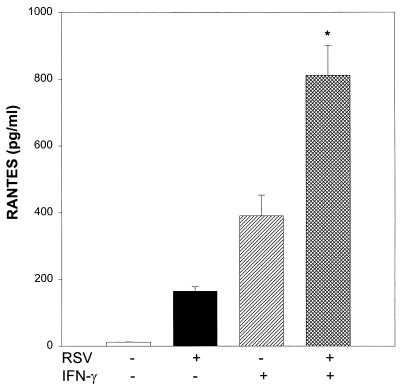
RSV infection has been shown to induce IFN activity in vivo (22) and in vitro which modulates the expression of immunoregulatory molecules on respiratory epithelial cells (17). Therefore, we decided to investigate in our model of chemokine secretion the concurrent effect of RSV and IFN-γ, a combination likely present in the airway mucosa of infected infants. NHBE cells were either infected with RSV (MOI of 0.2), treated with IFN-γ, or infected with RSV and treated with IFN-γ simultaneously. Supernatant was analyzed at 48 h for the presence of immunoreactive RANTES. Since 10-fold increments of IFN-γ (1, 10, and 100 ng/ml) have been shown previously to induce the production of comparable amounts of RANTES by bronchial epithelial cells (54), a single concentration of IFN-γ (100 U/ml [5 ng/ml]) was chosen in our experiments. As shown in Fig. 5, treatment of NHBE cells either with RSV at a low infectious dose or with IFN-γ induced the production of RANTES (164 ± 13 or 390 ± 61 pg/ml, respectively). Coincubation with RSV and IFN-γ induced strong upregulation of RANTES secretion (810 ± 89 pg/ml) that was synergistic with respect to that obtained by RSV infection or IFN-γ treatment alone.
FIG. 5.
Synergistic effect of RSV and IFN-γ on RANTES protein release by NHBE cells. Epithelial cells were infected with RSV at an MOI of 0.2 and treated with IFN-γ (100 U/ml) alone or in combination with RSV. RANTES was detected in supernatants by ELISA after 48 h of incubation. The results are expressed as mean ± SD of three experiments. ∗, P < 0.01 compared with control, RSV, and IFN-γ alone.
Bioactivity of RSV-CM and contribution of epithelial cell-derived RANTES to eosinophil chemotaxis.
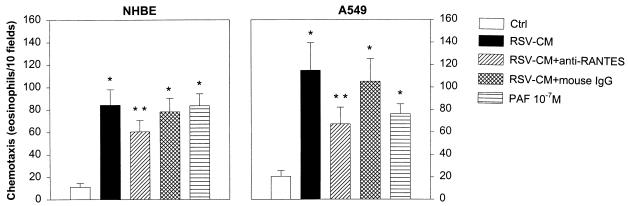
The bioactivity of RSV-infected airway epithelial cell supernatant was investigated in the context of eosinophilic inflammation. For this purpose, eosinophils isolated from the blood of normal human donors were tested in Boyden microchambers for their chemotactic response to RSV-CM generated by infected NHBE or A549 cells. PAF (10−7 M), a major eosinophil chemoattractant, was used as a positive control (3). In four separate experiments, RSV-CM from NHBE and A549 cells (Fig. 6) induced 7- and 5.5-fold increases, respectively, in the number of migrated eosinophils compared to eosinophil migration in the presence of medium from uninfected epithelial cells. The number of eosinophils that migrated in response to NHBE RSV-CM (84.3 ± 13.8; mean ± SD) or in response to A549 RSV-CM (115 ± 24.4) was similar to the number of eosinophils that migrated in response to PAF (83.5 ± 10.6 and 76.2 ± 8.8). Since human RANTES has been previously shown to induce in vitro chemotaxis of human blood eosinophils (3), we determined the contribution of RANTES to the overall eosinophil chemotactic activity secreted by RSV-infected NHBE and A549 cells. Addition of neutralizing anti-RANTES MAb in concentration (10 μg/ml) sufficient to neutralize nearly 100% of the chemotactic activity of 1 μg of recombinant human RANTES per ml significantly reduced by approximately 33 and 51% the eosinophil chemotaxis induced by NHBE and A549 RSV-CM, respectively (P < 0.01).
FIG. 6.
Biological activity of RANTES released by RSV-infected NHBE and A549 cells. Eosinophil chemotaxis was determined in the presence of supernatant from RSV-infected cells (RSV-CM) or from uninfected cells (Ctrl). Neutralizing experiments were performed in the presence of specific anti-RANTES MAb or mouse IgG isotype control. PAF (10−7 M) was used as a positive control. Results are expressed as mean ± SD of four determinations for each set of experiments. ∗, P < 0.01 for RSV-CM, RSV-CM plus mouse IgG, and PAF compared with control; ∗∗, P < 0.01 for RSV-CM plus anti-RANTES MAb compared with RSV-CM.
RSV-infected eosinophils produce and secrete RANTES and MIP-1α.
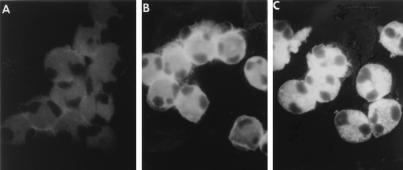
Following the process of transendothelial migration into the airway mucosa, the contribution of eosinophils to the inflammatory response may reflect their ability to release chemokines (38). However, factors that regulate the production and secretion of chemokines by eosinophil are not known. For this reason, we examined whether human eosinophils exposed to RSV were induced to express and secrete C-C chemokines. To determine if RSV was able to infect eosinophils, we performed studies using a well-established immunofluorescence assay previously used for the determination of infection in epithelial cells and macrophage (9, 27). Human eosinophils, isolated from the blood of normal individuals to a purity >99%, were cultured with control medium or were exposed to RSV. After 2 and 16 h, eosinophils were then stained with a MAb against RSV Fgp or with an isotype control. Cytospin preparations of eosinophils that were cultured in medium without virus were clearly negative (Fig. 7A), while those of eosinophils exposed to RSV for 2 h show an intense immunofluorescence staining, concentrated in a pericytoplasmic halo (Fig. 7B). After 16 h of exposure to RSV, typical RSV intracytoplasmic granular fluorescence immunoreactivity, identical to that one previously shown in epithelial cells, was observed (Fig. 7C). Eosinophils that were stained with an isotype MAb were consistently negative (not shown).
FIG. 7.
Eosinophil infection by RSV. Eosinophils were cultured with control medium (A) or infected with RSV for 2 h (B) or 16 h (C). Cytospin preparations of eosinophils were stained with anti-RSV Fgp MAb followed by a fluorescein isothiocyanate-conjugated anti-mouse F(ab′)2 IgG antibody. In the preparations of eosinophils that were exposed to RSV for 2 h, the Fgp staining was concentrated in a pericytoplasmic halo. Upon RSV infection for 16 h, typical intracytoplasmic granular fluorescence immunoreactivity was observed.
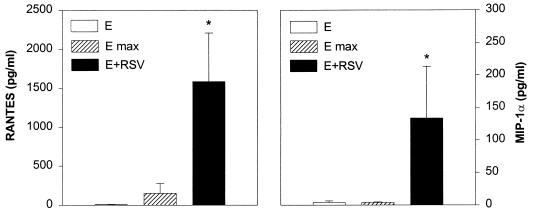
To determine if eosinophils were able to elaborate the two C-C chemokines RANTES and MIP-1α in response to RSV infection, freshly isolated eosinophils were cultured in medium or were infected with RSV for 16 h. Total cellular content of RANTES and MIP-1α in uninfected eosinophils was also determined in cell lysates obtained by Triton X-100. The cell supernatant was then collected for the determination of RANTES and MIP-1α immunoreactivity by ELISA. As shown in Fig. 8, uninfected eosinophils spontaneously released RANTES and MIP-1α in concentrations close to the low level of detection (8.6 ± 3.5 and 4.2 ± 2.1 pg/ml, respectively; mean ± SD). Total content of performed RANTES in cell lysates was found in the range of 151 ± 126 pg/ml, while total MIP-1α remained at the low level of detection (4.2 ± 0.8 pg/ml). On the other hand, eosinophils that were exposed to RSV for 16 h released in the culture supernatant significant amount of both RANTES (1,585 ± 623 pg/ml) and MIP-1α (133.8 ± 79.6 pg/ml). These results suggest that newly synthesized rather than a stored form of RANTES and MIP-1α were secreted by the RSV-infected eosinophils.
FIG. 8.
RANTES (A) and MIP-1α (B) production by eosinophils cultured with purified RSV. Eosinophils were infected with RSV at an MOI of 10 (E+RSV) or incubated with culture medium (E). After 16 h, the supernatants were collected for RANTES and MIP-1α determination by ELISA. For the measurement of total content of RANTES and MIP-1α, eosinophils were lysed by Triton X-100 (E max). The results are expressed as mean ± SD of five experiments using eosinophil preparations from different donors. ∗, P < 0.01 compared with eosinophils cultured with medium or lysed by Triton X-100.
DISCUSSION
RSV, the single most important respiratory pathogen in infancy and early childhood, stimulated the secretion of the proinflammatory C-C chemokines RANTES, MCP-1, and MIP-1α from epithelial cells of the human lower respiratory tract. Increased chemokine secretion was paralleled by increased steady-state mRNA levels, and it was strictly dependent on viral replication and infectious dose. The profile and concentrations of the C-C chemokines produced by the respiratory epithelium were characteristically cell specific. RSV infection induced the release of RANTES by epithelial cells isolated from all the segments of the lower respiratory tract examined in this study, i.e., major bronchi, bronchioles, and lung, and, as we previously reported, from the upper respiratory airways (45). With regard to the protein concentration, RSV-infected epithelial cells obtained from the distal portion of the bronchial tree (SAE) and type II cells (A549) produced significantly greater levels of RANTES than those from the major bronchi and the upper airways. cis-acting elements have been identified in the human RANTES promoter containing consensus binding sites for the nuclear factor (NF)-IL6 and NF-κB transcriptional activators (39). Our recent observations that RSV infection induces the activation of NF-IL6 (25) and NF-κB (18) in epithelial cells indicates that these transcription factors may be involved in the viral activation of RANTES in the airway mucosa. Studies addressing this possibility are in progress.
In contrast to the widespread expression of RANTES in the infected respiratory epithelium, RSV induced the release of MCP-1 only from A549 cells. The production of MCP-1 by A549 cells in response to TNF-α and IL-1β has been reported previously (53). In the studies presented herein, the relatively modest increase of RSV-induced MCP-1 mRNA, in comparison to the amount of protein released, suggests that expression of MCP-1 gene in epithelial cells may be controlled at several levels, including transcriptional and posttranscriptional. Although results generated with the use of a cell line to mimic primary cell cultures must be interpreted with caution, A549 cells have proven to be excellent models of pulmonary type II epithelial cells for studying the production of several bioactive factors and the mechanisms of chemokine gene regulation (18, 25, 34, 52). Moreover, we demonstrate for the first time that human epithelial cells, both A549 cells and the normal SAE cells, are able to express and secrete MIP-1α. Indeed, recent attempts by other laboratories to demonstrate cytokine-induced expression of MIP-1α in normal bronchial epithelial cells (7) and RSV-induced expression MIP-1β, a chemokine closely related to MIP-1α, by a transformed bronchial cell line (6) have been negative. These findings are consistent with our results demonstrating that following RSV infection NHBE cells release neither MCP-1 nor MIP-1α. Thus, the release of MCP-1 and MIP-1α by the airway epithelial cells appears to be regionalized in the distal segments of the bronchial tree and in the lung, where RSV-mediated necrosis of the epithelium and peribronchial cellular infiltration are associated with greater physiological changes, particularly in infants (24).
The combination of IFN-γ with a low dose of infectious virus induced a significant increase in RANTES production that was synergistic with respect to that obtained by RSV infection or IFN-γ treatment alone. Recent investigations in a BALB/c mouse model have demonstrated that the acute RSV infection was characterized by a Th-1-like response with increased IFN-γ production, decreased IL-4 and IL-5 production, pulmonary eosinophilic inflammation, and airway hyperresponsiveness (48). Eosinophil inflammation is usually observed in the context of a Th-2-type cytokine response and not a Th-1-type response. Therefore, our findings suggest the possibility of a novel mechanism, Th-2 independent, mediated by epithelial C-C chemokines by which IFN-γ can promote the migration of eosinophils to the lung and airway hyperresponsiveness following acute RSV infection.
To confirm the biologic activity of epithelial cell-derived chemokines, we show in these studies that supernatant from cells infected with RSV exhibited a strong chemotactic activity for eosinophils. Since eosinophil chemotactic activity in vitro is a known property of RANTES (3, 26), the presence of a neutralizing anti-RANTES MAb significantly reduced eosinophil chemotaxis induced by NHBE and A549 RSV-CM. Although the concentration of RANTES in RSV-CM from A549 cells was approximately thirty times higher than the concentration in RSV-CM from NHBE cells, we found that the rate of eosinophil chemotaxis in response to the two conditioned media did not differ substantially. In this regard, we have previously shown that concentrations of RANTES in the range of those present in NHBE RSV-CM (∼10−10 M) and in A549 RSV-CM (∼10−9 M) exhibit comparable chemotactic activities for human eosinophils (3). Furthermore, the finding that eosinophil chemotactic activity in RSV-CM was only partially inhibited by anti-RANTES MAb supports the notion that other chemokines known to be secreted by the infected epithelium, including MIP-1α and IL-8 (18), have a direct eosinophil chemotactic activity or are able to enhance chemotactic response to other agents (43). On the other hand, the primary goal of our studies was not to identify the contribution of each chemokine to the total eosinophil chemotactic activity present in the RSV-infected epithelial cell supernatant but rather to demonstrate the potential relevance of this phenomenon in the generation of eosinophilic inflammation.
We have previously shown that RSV is capable of direct stimulatory interaction with eosinophils, as indicated by the release of superoxide and leukotriene C4 (30) and by demonstration of piecemeal degranulation by electron microscopy studies (29). In addition to the release of products such as oxygen radicals, eicosanoids, and cytoplasmic granule proteins, eosinophils can promote inflammation by virtue of their ability to elaborate proinflammatory cytokines and chemokines (38). However, factors that regulate the production and secretion of chemokines by eosinophils are not known. In the present study, we demonstrate that human blood eosinophils were susceptible to RSV and were able to elaborate and secrete the chemokines RANTES and MIP-1α following infection. Others have shown by in situ hybridization and Northern blot analysis that blood eosinophils from hypereosinophilic individuals express MIP-1α mRNA, but the production of MIP-1α protein was not tested in that study (12). Recently, both RANTES mRNA and protein have been demonstrated in eosinophils infiltrating late-phase cutaneous reactions after intradermal allergen challenge and in peripheral blood eosinophils isolated from atopic subjects with eosinophilia (35) or from normal donors (64). RANTES protein appeared to be stored within the eosinophils in association with cytoplasmic granules and to be released only after lysis of the cells (35). Thus, no experimental evidence to date indicates that inflammatory cytokines or other exogenous stimuli can regulate RANTES or MIP-1α gene transcription or protein synthesis and secretion in human eosinophils. Since the amount of RANTES and MIP-1α released by eosinophils after RSV infection was much higher than that observed in cell lysates, our studies demonstrate that production and release of these chemokines is indeed inducible in RSV-infected eosinophils. As a consequence of the eosinophil infection by RSV, the inflammatory response, initiated by airway epithelial cell chemokines, may be further sustained and amplified via autocrine mechanisms driven by the release of eosinophil chemokines.
The studies presented here suggest that the pattern, absolute levels, and relative concentrations, together with the kinetics of release of epithelial cell chemokines in different areas of the respiratory tract, are all factors that may significantly contribute to the various histologic and inflammatory features of RSV-induced airway disease. Selective production of MIP-1α and MCP-1 and the severalfold-higher amount of RANTES released by epithelial cells of the small bronchioles and lung in comparison to bronchial or upper airway epithelium, along with autocrine and paracrine mechanisms of amplification mediated by eosinophils and IFN-γ, may largely explain the findings of mononuclear cell infiltration and eosinophil and basophil migration and activation in RSV-induced bronchiolitis. Future studies, both in children with different forms of RSV-induced airway disease and in animal models, are needed to correlate the different parameters of histhopathology, inflammation, and immune response with the expression and secretion of C-C chemokines in airway mucosa.
ACKNOWLEDGMENTS
This work was supported in part by grants AI 15939 and HD 27841 from the National Institutes of Health and by a grant of the John Sealy Memorial Endowment Fund for Biomedical Research.
We thank Todd Elliott for excellent technical assistance, Nina Nguyen for helping in the ELISA determinations, and Virginia Reimer for preparation of the manuscript.
REFERENCES
- 1.Aherne W, Bird T, Court S D, Gardner P S, McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970;23:7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam R, Let-Brown M A, Forsythe P A, Anderson-Walters D J, Kenamore C, Kormos C, Grant J A. Monocyte chemotactic and activating factor is a potent histamine-releasing factor for basophils. J Clin Invest. 1992;89:723–728. doi: 10.1172/JCI115648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alam R, Stafford S, Forsythe P, Harrison R, Faubion D, Lett-Brown M A, Grant J A. RANTES is a chemotactic and activating factor for human eosinophils. J Immunol. 1993;150:3442–3447. [PubMed] [Google Scholar]
- 4.Alam, R., J. York, M. Boyers, S. Stafford, J. A. Grant, J. Lee, P. Forsythe, T. Sim, and N. Ida. Increased MCP-1, RANTES, and MIP-1α in bronchoalveolar lavage fluid of allergic asthmatic patients. 1996. Am. J. Respir. Crit. Care Med. 153:1398–1404. [DOI] [PubMed]
- 5.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic, cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 6.Becker S, Reed W, Henderson F W, Noah T L. RSV infection of human airway epithelial cells causes production of the beta-chemokine RANTES. Am J Physiol. 1997;272:L512–L520. doi: 10.1152/ajplung.1997.272.3.L512. [DOI] [PubMed] [Google Scholar]
- 7.Berkman N, Robichaud A, Krishnan V L, Roesems G, Robbins R, Jose P J, Barnes P J, Chung K F. Expression of RANTES in human airway epithelial cells: effect of corticosteroids and interleukin-4, -10, and -13. Immunology. 1996;87:599–603. doi: 10.1046/j.1365-2567.1996.477579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Cirino J, Panuska R, Villani A, Taraf H, Rebert N A, Merolla R, Tsivtse P, Gilbert I A. Restricted replication of respiratory syncytial virus in human alveolar macrophages. J Gen Virol. 1993;74:1527–1537. doi: 10.1099/0022-1317-74-8-1527. [DOI] [PubMed] [Google Scholar]
- 10.Colocho Zelaya E A, Orvell C, Strannegard O. Eosinophil cationic protein in nasopharyngeal secretions and serum of infants infected with respiratory syncytial virus. Pediatr Allergy Immunol. 1994;5:100–106. doi: 10.1111/j.1399-3038.1994.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 11.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 12.Costa J J, Matossian K, Beil W J, Wong D T W, Gordon J R, Dvorak A M, Weller P F, Galli S J. Human eosinophils can express the cytokines TNF-α and MIP-1α. J Clin Invest. 1993;91:2673–2684. doi: 10.1172/JCI116506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahinden C A, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, Minty A, Baggiolini M. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med. 1994;179:751–756. doi: 10.1084/jem.179.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downham M A P S, Gardner P S, McQuillin J, et al. Role of respiratory viruses in childhood mortality. Br Med J. 1975;1:235–239. doi: 10.1136/bmj.1.5952.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferris J A J, Aherne W A, Locke W S, et al. Sudden and unexpected deaths to infants: histology and virology. Br Med J. 1973;2:439–449. doi: 10.1136/bmj.2.5864.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garofalo R, Kimpen J L, Welliver R C, Ogra P L. Eosinophil degranulation in the respiratory tract during naturally-acquired respiratory syncytial virus infection. J Pediatr. 1992;120:28–32. doi: 10.1016/s0022-3476(05)80592-x. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo R, Mei F, Espejo R, Ye G, Häeberle H, Baron S, Ogra P L, Reyes V E. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression trough the induction of IFN-β and IL-1α. J Immunol. 1996;157:2506–2513. [PubMed] [Google Scholar]
- 18.Garofalo R, Sabry M, Jamaluddin M, Yu R K, Casola A, Ogra P L, Brasier A R. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: nuclear translocation of the RelA transcriptional factor as a mechanism producing airway mucosal inflammation. J Virol. 1996;70:8773–8781. doi: 10.1128/jvi.70.12.8773-8781.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham B S, Bunton L A, Wright P F, Karzon D T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham B S, Perkins M D, Wright P F, Karzon D T. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- 21.Hall C B, Douglas R G, Jr, Schnabel K C, Geiman J M. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun. 1981;33:779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall C B, Douglas R G, Simons R L, Jr, Geiman J M. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infection. J Pediatr. 1978;93:28–32. doi: 10.1016/s0022-3476(78)80594-0. [DOI] [PubMed] [Google Scholar]
- 23.Hall C B, Hall W J, Gala C L, McGill F B, Leddy J P. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr. 1984;105:358–364. doi: 10.1016/s0022-3476(84)80005-0. [DOI] [PubMed] [Google Scholar]
- 24.Hogg J C, Williams J, Richardson J B, Macklem P T, Thurlbeck W M. Age as a factor in the distribution of lower-airway conductance and in the pathologic anatomy of obstructive lung disease. N Engl J Med. 1970;282:1283–1287. doi: 10.1056/NEJM197006042822302. [DOI] [PubMed] [Google Scholar]
- 25.Jamaluddin M, Garofalo R, Ogra P L, Brasier A R. Inducible translational regulation of the NF-IL6 transcription factor by respiratory syncytial virus infection in pulmonary epithelial cells. J Virol. 1996;70:1554–1563. doi: 10.1128/jvi.70.3.1554-1563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kameyoshi Y, Dorschner A, Mallet A I, Christopher E, Schroder J M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaul A, Scott R, Gallagher M, Scott M, Clement J, Ogra P L. Respiratory syncytial virus infection. Rapid diagnosis in children by use of indirect immunofluorescence. Am J Dis Child. 1978;132:1088–1090. doi: 10.1001/archpedi.1978.02120360044006. [DOI] [PubMed] [Google Scholar]
- 28.Kimata H, Yoshida A, Ischioka C, Fujimoto M, Lindley I, Furusho K. RANTES and macrophage inflammatory protein 1α selectively enhance immunoglobulin (IgE) and IgG4 production by human B cells. J Exp Med. 1996;183:2397–2402. doi: 10.1084/jem.183.5.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimpen J L L, Garofalo R, Welliver R C, Fujihara K, Ogra P L. An ultrastructural study of the interaction of human eosinophils with respiratory syncytial virus. Pediatr Allergy Immunol. 1996;7:48–53. doi: 10.1111/j.1399-3038.1996.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 30.Kimpen J L L, Garofalo R, Welliver R C, Ogra P L. Activation of human eosinophils in vitro by respiratory syncytial virus. Pediatr Res. 1992;32:160–164. doi: 10.1203/00006450-199208000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kisch A L, Johnson K M. A plaque assay for respiratory syncytial virus. Proc Soc Exp Biol Med. 1963;112:583–589. doi: 10.3181/00379727-112-28111. [DOI] [PubMed] [Google Scholar]
- 32.Lechner J, Haugen A, McClendon I A, Pettis W. Clonal growth of normal adult human bronchial epithelial cells in a serum-free medium. In Vitro. 1982;18:633–641. doi: 10.1007/BF02796396. [DOI] [PubMed] [Google Scholar]
- 33.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 34.Lilly C M, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia-Zepeda E A, Rothenberg M E, Drazen J M, Luster A D. Expression of eotaxin by human lung epithelial cells. J Clin Invest. 1997;99:1767–1773. doi: 10.1172/JCI119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim K G, Wan H C, Bozza P T, Resnick M B, Wong D T W, Cruikshank W W, Kornfeld H, Center D M, Weller P F. Human eosinophils elaborate the lymphocyte chemoattractants IL-16 (lymphocyte chemoattractant factor) and RANTES. J Immunol. 1996;156:2566–2570. [PubMed] [Google Scholar]
- 36.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Monocyte chemotactic proteins MCP-1, MCP-2 and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 1994;8:1055–1060. doi: 10.1096/fasebj.8.13.7926371. [DOI] [PubMed] [Google Scholar]
- 37.Merolla R, Rebert N A, Tsiviste P T, Hoffmann S P, Panuska J R. Respiratory syncytial virus replication in human lung epithelial cells; inhibition by tumor necrosis factor-α and interferon β. Am J Respir Crit Care Med. 1995;152:1358–1366. doi: 10.1164/ajrccm.152.4.7551395. [DOI] [PubMed] [Google Scholar]
- 38.Moqbel R. Eosinophil-Derived Cytokines in Allergic Inflammation and Asthma. Ann N Y Acad Sci. 1996;796:209–217. doi: 10.1111/j.1749-6632.1996.tb32583.x. [DOI] [PubMed] [Google Scholar]
- 39.Nelson P J, Kim H T, Manning W C, Goralski T J, Krensky A M. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol. 1993;151:2601–2612. [PubMed] [Google Scholar]
- 40.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene ‘intercrine’ cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 41.Prince G A, Jenson A B, Hemming V G, Murphy B R, Walsh E E, Horswood R L, Chanock R M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats against viral infection. J Virol. 1986;57:721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prince G A, Jenson A B, Horswood R L, Camargo E, Chanock R M. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978;93:771–791. [PMC free article] [PubMed] [Google Scholar]
- 43.Resnick M B, Weller P F. Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol. 1993;8:349–355. doi: 10.1165/ajrcmb/8.4.349. [DOI] [PubMed] [Google Scholar]
- 44.Rot A, Klieger M, Brunner T, Bishoff S C, Schall T J, Dahinden C A. RANTES and macrophage inflammatory protein-1 induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito T, Deskin R W, Casola A, Häeberle H, Olszewska B, Ernst P B, Alam R, Ogra P L, Garofalo R. Respiratory syncytial virus induces selective production of the chemokine RANTES by upper airway epithelial cells. J Infect Dis. 1997;175:497–504. doi: 10.1093/infdis/175.3.497. [DOI] [PubMed] [Google Scholar]
- 46.Schall T J, Bacon K, Camp R D, Kaspari J W, Goeddel D V. Human macrophage inflammatory protein-α (MIP-1α) and MIP-1β attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–1825. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schall T J, Bacon K, Toy K, Goeddel D V. Selective attraction of moncytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 48.Schwarze J, Hamelmann E, Bradley K L, Takeda K, Gelfand E W. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest. 1997;100:226–233. doi: 10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigurs N, Bjarnason R, Sigurbergsson F. Eosinophil cationic protein in nasal secretion and in serum and myeloperoxidase in serum in respiratory syncytial virus bronchiolitis: relation to asthma and atopy. Acta Paediatr. 1994;83:1151–1155. doi: 10.1111/j.1651-2227.1994.tb18269.x. [DOI] [PubMed] [Google Scholar]
- 50.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Björkstén B. Asthma and immunoglobulin-E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 51.Sousa A R, Lane S J, Nakhosteen J A, Yoshimura T, Lee T H, Poston R N. Increased expression of the monocyte chemoattractant protein-1 in bronchial tissue from asthmatic subjects. Am J Respir Cell Mol Biol. 1994;10:142–147. doi: 10.1165/ajrcmb.10.2.8110469. [DOI] [PubMed] [Google Scholar]
- 52.Standiford T J, Kunkel S L, Basha M A, Chensue S W, Lynch J P I, Toews G B, Westwick J, Strieter R M. Interleukin-8 gene expression by pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Standiford T, Kunkel S, Phan S, Rollins B, Strieter R. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266:9912–9918. [PubMed] [Google Scholar]
- 54.Stellato C, Beck L A, Gorgone G A, Proud D, Schall T J, Ono S J, Lichtenstein L M, Schleimer R P. Expression of the chemokine RANTES by a human bronchial epithelial cell line. Modulation by cytokines and glucocorticoids. J Immunol. 1995;155:410–418. [PubMed] [Google Scholar]
- 55.Taub D D, Ortaldo J R, Turcovski-Corrales S M, Key M L, Longo D L, Murphy W J. β chemokines costimulate lymphocyte cytolysis proliferation and lymphokine production. J Leukocyte Biol. 1996;59:81–89. doi: 10.1002/jlb.59.1.81. [DOI] [PubMed] [Google Scholar]
- 56.Taub D D, Sayers T, Carter C, Ortaldo J R. α and β chemokines induce NK cell migration and enhance NK cell cytolysis. J Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 57.Taylor G, Stott E J, Hughes M, Collins A P. Respiratory syncytial virus infection in mice. Infect Immun. 1984;43:649–655. doi: 10.1128/iai.43.2.649-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teran L M, Noso N, Carroll M. Eosinophil recruitment following allergen challenge is associated with the release of the chemokine RANTES into asthmatic airways. J Immunol. 1996;157:1806–1812. [PubMed] [Google Scholar]
- 59.Tsutsumi K, Flanagan T D, Ogra P L. Monoclonal antibodies to the large glycoproteins of respiratory syncytial virus: possible evidence for several functional antigenic sites. J Gen Virol. 1987;68:2161–2167. doi: 10.1099/0022-1317-68-8-2161. [DOI] [PubMed] [Google Scholar]
- 60.Ueba O. Respiratory syncytial virus. I. Concentration and purification of the infectious virus. Acta Med Okayama. 1978;32:265–272. [PubMed] [Google Scholar]
- 61.Volovitz B, Welliver R C, De Castro G, Krystofik D, Ogra P L. The release of leukotrienes in the respiratory tract during infection with respiratory syncytial virus: role in obstructive airway disease. Pediatr Res. 1988;24:504–507. doi: 10.1203/00006450-198810000-00018. [DOI] [PubMed] [Google Scholar]
- 62.Welliver R C, Cherry J D. Bronchiolitis and infectious asthma. In: Fegin R D, Cherry J D, editors. Pediatric infectious disease. I. Philadelphia, Pa: W. B. Saunders Company; 1992. pp. 245–254. [Google Scholar]
- 63.Welliver R C, Wong D T, Sun M, Middleton E, Jr, Vaughan R S, Ogra P L. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretion after infection. N Engl J Med. 1981;305:841–846. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 64.Ying S, Meng Q, Taborda-Barata L, Corrigan C J, Barkans J, Assoufi B, Moqbel R, Durham S R, Kay A B. Human eosinophils express messenger RNA encoding RANTES and store and release biologically active RANTES protein. Eur J Immunol. 1996;26:70–76. doi: 10.1002/eji.1830260111. [DOI] [PubMed] [Google Scholar]