Abstract
Close HLA matching of donors and recipients has been the dogma for successful allogeneic blood or marrow transplantation (alloBMT), to limit the complications of graft-versus-host disease (GVHD). However, many patients in need, especially those within certain ethnic groups such as those of African-Americans and Hispanics, remain unable to find a match even with the increased availability of unrelated donors. Over half a century ago, investigators at Johns Hopkins found that cyclophosphamide’s immunosuppressive properties made it the ideal replacement for total body irradiation in alloBMT conditioning regimens. They also found it to be the best chemotherapeutic for preventing GVHD in animal models, but its cytotoxic properties scared them from using it clinically in the high doses successful in animal models. Subsequent work showed that cyclophosphamide spared hematopoietic and other stem cells including memory lymphocytes, prompting re-examination at high doses for GVHD prophylaxis. Animal and extensive human studies demonstrated that high-dose post-transplantation cyclophosphamide (PTCy) effectively and safely limited GVHD such that mismatched transplants are now be considered standard-of-care worldwide. The beneficial effects of PTCy on GVHD appears to be independent of donor type, graft source, or conditioning regimen intensity.
Keywords: allogeneic, transplantation, cyclophosphamide, HLA, haploidentical, graft-versus-host disease
1. Donor Availability – the Holy Grail of Transplantation
Up until the new millennium, many patients for whom allogeneic blood or marrow transplantation (alloBMT) could be potentially curative were unable to find appropriate (meaning at the time HLA-matched) donors in their family or in international registries. This problem was particularly acute for ethnic minorities, and still exists today despite expansion of international registries, because high HLA diversity, not just under-representation, is an important obstacle for ethnic groups like African-Americans and Hispanics. An NMDP analysis in 2014 found that the likelihood of finding an 8/8 HLA-matched unrelated donor for patients of European Caucasian ancestry was 74%, but for Black Americans it was only 19%, Hispanics < 40%, and indigenous Americans 47% [1]. Moreover, the lag time from initiating a search of an effective adult donor to alloBMT (i.e., available, confirmatory typing, medical clearance, and graft collection) was in the order of 4 months; unfortunately, such a time interval meant that a substantial percentage of patients in need of alloBMT would have relapsed. Thus, historically, graft-versus-host disease (GvHD), particularly in the setting of HLA disparity, has constrained the applicability and availability alloBMT. Accordingly the search for appropriate alternative donors on behalf of the many potential BMT recipients lacking matched donors could be considered the “Holy Grail”; of the BMT field.
At the end of the last millennium, recognition of unrelated umbilical cord blood as a potential alternative donor source was beginning, but was largely investigational at only a few specialized centers [2]. Haploidentical (haplo) family members could also be a readily available alternative donor source, but survival outcomes of studies were extremely poor due to very high rates of severe GvHD leading to non-relapse mortality (NRM) rates exceeding 50% [3]. The Perugia Group in the early 1990’s found that T cell-depleted haplo grafts eliminated the problem of GvHD, but survivals were still limited by high rates of NRM from opportunistic infections [4].
2. Cyclophosphamide-Induced Tolerance
Initial studies by Berenbaum and Brown in 1964 [5] and Santos and Owens in 1966 [6] showed that an antibody immune response to antigens in mice could be blocked by administration of cyclophosphamide (Cy). Several groups subsequently showed that Cy given after transplantation allowed both minor and major histocompatibility complex-mismatched skin and other organ transplants to persist after transplantation into mice [7–12]. The mechanisms responsible remain unclear but require the donor antigens to be present several days before the administration of Cy. Table 1 summarizes the history of preclinical studies on Cy-induced tolerance.
Table 1.
History of Preclinical Studies on Cyclophosphamide-Induced Tolerance
| Cy prolongs allogeneic skin graft survival in mice | Reference 8 |
| Cy most effective agent in preventing Ab production in mice | Reference 5 |
| Confirmation that Cy can prevent Ab production in mice | Reference 6 |
| Cy prolongs allogeneic skin graft survival in rats | Reference 7 |
| PTCy limits GVHD in mice | Reference 13 |
| PTCy limits GVHD in mice, but is most effective at high doses | Reference 18 |
| Confirmation that PTCy can limit GVHD in mice | Reference 20 |
| Both allogeneic antigens and stem cells are needed for Cy-induced skin allograft tolerance in mice | Reference 10 |
| Cy prolongs survival of allogeneic heart grafts in mice | Reference 11 |
| CsA interferes with allogeneic skin graft survival in mice | Reference 12 |
| PTCy allows haplo BMT after nonmyeloablative conditioning in mice | Reference 25 |
Cy – cyclophosphamide, PTCy – post-transplant cyclophosphamide, Ab – antibody, GVHD – graft-vs-host disease, CsA – cyclosporine, haplo – haploidentical, BMT – blood or marrow transplantation
3. Preclinical Development of Post-Transplantation Cyclophosphamide (PTCy)
Early preclinical and clinical alloBMT studies exclusively used total body irradiation (TBI), because of its dual anticancer and immunosuppressive properties. Because of limited access to facilities capable of providing TBI at Johns Hopkins in the 1960s, as well as toxicity concerns, Santos and Owens explored potential new BMT conditioning agents by evaluating the immunosuppressive properties of the anticancer agents in use at the time [13, 14]. They found Cy to be the most immunosuppressive, and accordingly developed it as a replacement for TBI for alloBMT conditioning [13, 14], The first 47 patients receiving alloBMT at Hopkins (1968 – 1976) received high-dose Cy as the sole conditioning agent [15]. Although most patients receiving high-dose Cy as sole conditioning engrafted, about 20% failed to engraft but invariably recovered with host hematopoiesis [15, 16]. This was both the first evidence that high-dose Cy spared hematopoietic stem cells as well as the first use of non-myeloablative conditioning for alloBMT. Because of high relapse rates, the Hopkins group subsequently added busulfan to cyclophosphamide for conditioning [17].
Because of its immunosuppressive properties, Santos and Owens also studied Cy as prophylaxis against GvHD, and found that Cy administered on days 2–5 after transplantation was highly effective in preventing alloreactivity in mice [13]. However, only high-doses prevented mortality from GvHD [18]. Because of concerns that high-dose Cy given after BMT might damage transplanted hematopoietic stem cells, the clinical trials that arose from these preclinical studies actually used low-dose Cy as GVHD prophylaxis in a similar schedule to that developed by the Seattle group for methotrexate. Subsequently, this low-dose Cy regimen became the standard GVHD prophylaxis at Hopkins in the 1970’s and early 1980’s [15]. A randomized trial at Hopkins eventually showed that cyclosporine was significantly better than low-dose Cy for GvHD prophylaxis [19]. Investigators at Kyusha University in Japan confirmed that high-dose Cy given early after transplant could prevent GvHD in mice [20]. In addition, this group showed that cyclosporine could block Cy-induced allograft tolerance [12].
While these studies on the immunobiology of Cy were ongoing, laboratory and corroborating clinical data confirmed that hematopoietic stem cells are resistant to high-dose Cy [21]. Hematopoietic and other tissue stem cells, including memory T cells [22] express high levels of aldehyde dehydrogenase 1 (ALDH1), the body’s primary means of inactivating Cy, whereas mature lymphocytes generally express low levels [21]. The primary function of ALDH1, also known as retinaldehyde dehydrogenase, is the biosynthesis of retinoic acid, which is required for the growth and differentiation of tissue stem cells and other highly proliferative cells [21]. ALDH1 actually inactivates Cy by serendipity, through oxidation of the active metabolic aldehyde intermediate aldophosphamide to the inactive carboxylic acid carboxyphosphamide [21]. The success of high-dose Cy without HSC rescue as treatment for aplastic anemia [23] and other autoimmune diseases [24] provided further proof of its potent immunosuppressive but stem cell-sparing properties.
With this background, the Hopkins group again looked at PTCy for GvHD prophylaxis in mice, but this time with a new twist, using mismatched donors. High-dose Cy given early after BMT was again shown to effectively prevent alloreactivity (GvHD and graft rejection) and to spare stem cells, allowing successful mismatched donor BMT in mice [25]. The non-myeloablative regimen of fludarabine and low-dose TBI was used successfully by the Seattle group starting in the late 1990’s to achieve stable mixed chimerism after BMT of HLA-matched related or unrelated donors [26]. The Luznik et al study used a similar non-myeloablative regimen in the mice, demonstrating that engraftment with mismatched donors and PTCy was even possible with just non-myeloablative conditioning [25].
4. Clinical Development of PTCy
By 1999, we felt that the preclinical and clinical data was sufficient to allow reconsideration of Cy as GvHD prophylaxis, but this time at the high doses Santos and Owens found to be most effective in animal models but were unwilling to test clinically because of toxicity concerns [18]. We designed a first-in-human phase I/II clinical trial of haplo BMT in patients with high-risk hematologic malignancies lacking matched donor options, using T cell replete bone marrow and PTCy. To limit GvHD, historically the major complication of haplo alloBMT [3], we utilized nonmyeloablative conditioning and bone marrow grafts. The conditioning regimen was based on the nonmyeloablative platform that utilized fludarabine and 200 cGy of TBI, developed by the Seattle group [26]. Bone marrow donors were first-degree haplo relatives (parents, siblings and children) who were at least 18 years of age. Initially, The BMT utilized PTCy at 50 mg/kg on day +3 after BMT. On the day following PTCy, patients also received tacrolimus for 180 days and mycophenolic acid mofetil (MMF) for 30 days. Filgrastim was given on day +1 and continued until neutrophil recovery to 500/μL.
Both graft failure and GvHD are concerns with mismatched BMT. One aspect of the phase I trial was to determine if fludarabine and low-dose TBI were sufficient to prevent rejection of the haplo grafts. Patients were initially treated with just fludarabine and TBI, and if there were at least two rejections, increasing doses of Cy would be given on days −6 and −5. Cohort 1 received no pre-transplant Cy and two of the three patients in that cohort rejected the haplo graft. One recovered autologous neutrophils at day +46 and the other died of infection during aplasia at day +58. Cohort 2 gave pre-transplant Cy at 14.5 mg/kg on days −6 and −5, and this dose was sufficient to permit engraftment of the next three patients. Cohort 2 then was expanded to 10 patients and 8 of 10 patients showed full donor engraftment (≥95% donor cells by day 60). The median time to absolute neutrophil count >500/uL was 15 days (range, 13–16 days) and to unsupported platelet count >20,000/uL, 14 days (range, 0–26 days) [27]. Two patients with myelodysplastic syndrome did not engraft but experienced autologous neutrophil recovery at 24 and 44 days, respectively. Acute GvHD developed in six of the eight engrafted patients (grade II in two patients, grade III in four patients) at a median of 99 days (range, 38–143 days) after BMT and was fatal in two patients. Three of these patients remain alive and disease-free 20 years later.
The two patients in cohort 2 who rejected their grafts both had high-grade MDS with no prior chemotherapy and a long history of transfusion dependence. Such patients generally would not be eligible for non-myeloablative conditioning today (with either matched or mismatched donors) without at least azacitidine or decitabine as treatment. Both these patients recovered autologous hematopoiesis, but interestingly in morphologic complete remission that was maintained for one and three years, respectively. It is now well-recognized that graft failure after haplo BMT can be associated with prolonged remissions, suggesting that some graft-versus-tumor effect may have occurred in the initial stages of haplo transplantation prior to graft loss [28].
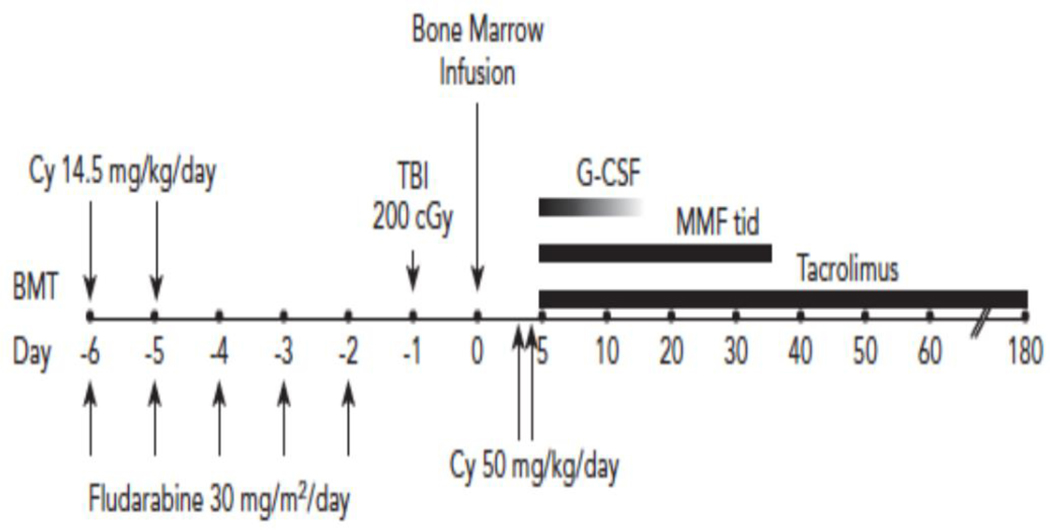
Although engraftment was acceptable with the addition of low-dose Cy to the non-fludarabine and low dose TBI nonmyeloablative conditioning regimen, GVHD was still problematic. Thus, in addition to low-dose Cy being adding to the conditioning, the subsequent 40 patients on the trial received a second dose of high dose Cy on day 4 after alloBMT [29]; this is the standard haplo transplant backbone that is still used at many centers, including Hopkins, today. Also at about that time, the initial principal investigator on the clinical trial at Hopkins (PVO) moved to Fred Hutchinson Cancer Research Center and directed a parallel trial there. In 2008, we reported the outcomes for the 40 patients transplanted in Baltimore and an additional 28 patients transplanted at Seattle [29]. The second day of PTCy resulted in a significantly lower incidence of extensive, chronic GvHD (5% versus 25% at one year with only one day of PTCy). The incidence of severe, grades III/IV acute GvHD was also low at 6%. Figure 1 shows the final nonmyeloablative BMT regimen using haplo donors that emerged from that clinical trial [29].
Fig. 1.
The classic Hopkins non-myeloablative haploidentical schema.
Based on these emerging results [29], the Blood and Marrow Clinical Trials Network (BMT CTN) carried out a multi-institutional trial of haplo BMT with PTCy, which confirmed the Hopkins results [30]. Subsequently, many trials worldwide confirmed the safety and efficacy of haplo BMT using PTCy [31–34], including a prospective randomized BMT CTN trial that found superiority for haplo BMT with PTCy over unrelated cord blood transplants [35, 36]. Moreover, retrospective comparisons showed that related haplo alloBMT with PTCy provided results similar to those seen with both matched related [37–40] and matched unrelated donors (MUDs) [41–43]. Perhaps not surprisingly, haplo BMT using second and third degree haplo donors is just as successful as using first-degree haplo relatives when PTCy is utilized [44]. Recent reports demonstrate that PTCy also allows safe and effective alloBMT using mismatched unrelated donors [45, 46]. Studies from Hopkins also showed 6 months of tacrolimus was unnecessary, and our standard is now 2 months [47, 48]. This shortened immunosuppression not only decreases the toxicities related to long-term calcineurin use, but also facilitates the use of post-transplant maintenance therapies with immunologic agents.
The PTCy regimen published in 2008 [29] remains the Hopkins standard GvHD prophylaxis - but now using only two months of tacrolimus or sirolimus [47, 48] - for diseases that have a good outcome with alloBMT. These indications include B cell lymphomas [49], Hodgkin lymphoma [28], and acute leukemias without minimal residual disease [50, 51] regardless of donor-type (haplo, matched, or mismatched unrelated). However, we made several modifications/advancements to the regimen for other diseases over time. Although Hopkins still uses bone marrow grafts for the indications just mentioned, most patients undergoing alloBMT outside of Hopkins receive peripheral blood (PB) allografts, largely because of BMT physician choice. Most studies found that higher T cell content of PB allografts produced higher rates of GVHD, but also higher graft-versus tumor (GvT) activity, with little difference in overall outcomes [52–54]. The higher rates of GvHD/GvT with PB actually appear beneficial for diseases at high-risk for relapse after alloBMT, such as myelodysplastic syndromes, myeloproliferative neoplasms, and leukemias with MRD [54–56]. We also increased the dose of TBI from 200 cGy to 400 cGy for these high-risk malignancies, and the combination of PB and increased TBI appears to have lowered the risk of relapse. Importantly, the improved disease control also appears to offset any increased toxicity, such that outcomes also appear improved. Since there is no advantage for GvHD/GvT in non-malignant conditions like aplastic anemia and sickle cell anemia, BM is the preferred donor type for these patients [57]. Aplastic anemia and sickle cell anemia do have a higher risk of graft failure, so we now utilize 400 cGy of TBI in these diseases as well, and graft failure with non-myeloablative conditioning and PTCy is now unusual in these diseases [58, 59]. In addition, we have added pretransplant ATG, which also has decreased the combined risk of acute and chronic GvHD in patients with aplastic anemia and sickle cell anemia to less than 10%, with cure rates over 90% [58, 59]. PTCy has also been shown to be effective after myeloablative conditioning [32, 33, 38, 41, 43].
5. Conclusion and Future Directions
Since the initial publication of our PTCy regimen for haplo BMT in 2008 [29], PTCy has become standard of care worldwide for not only haplo donors, but also matched and mismatched unrelated donors. Its beneficial effects against GVHD appear to be independent of donor type, graft source, or conditioning regimen intensity [38]. Not only is PTCy associated with low rates of both acute and chronic GVHD as well as NRM regardless of the type of donor, but it also is “low tech” such that its relative low-cost and simple technology allows easy transportability even to emerging economies [60–64]. Thus, in 2022, no patient in need of alloBMT should lack a suitable donor, be it a matched sibling, haplo relative, or matched or mismatched unrelated individual.
Practice Points:
The development of PTCy is a prime example of bench-to-bedside and back again translational research.
Cellular aldehyde dehydrogenase (ALDH1 family) is responsible for inactivating cyclophosphamide.
Cells with stem cell features, including hematopoietic stem cells and memory lymphocytes, are resistant to high-dose cyclophosphamide due to their high expression of aldehyde dehydrogenase. This unique pharmacology underpins the activity of PTCy as GVHD prophylaxis.
The ability of PTCy to allow the use of half-matched donor now permits all patients in need of alloBMT to undergo the procedure.
Research Agenda
Now with universal donor availability and low NRM rates, studies in alloBMT should focus on reducing disease relapse.
Acknowledgments
This work was supported by NIH Grants P01 CA015396 and P01 CA225618
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rocha V, Wagner JE Jr., Sobocinski KA, Klein JP, Zhang MJ, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846–54. [DOI] [PubMed] [Google Scholar]
- [3].Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–77. [DOI] [PubMed] [Google Scholar]
- [4].Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C, et al. Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. [PubMed] [Google Scholar]
- [5].Berenbaum MC, Brown IN. DOSE-RESPONSE RELATIONSHIPS FOR AGENTS INHIBITING THE IMMUNE RESPONSE. Immunology. 1964;7:65–71. [PMC free article] [PubMed] [Google Scholar]
- [6].Santos GW, Owens AH Jr. 19S and 17S antibody production in the cyclophosphamide- or methotrexate-treated rat. Nature. 1966;209:622–4. [DOI] [PubMed] [Google Scholar]
- [7].Santos GW, Owens AH Jr. A COMPARISON OF THE EFFECTS OF SELECTED CYTOTOXIC AGENTS ON ALLOGENEIC SKIN GRAFT SURVIVAL IN RATS. Bull Johns Hopkins Hosp. 1965;116:327–40. [PubMed] [Google Scholar]
- [8].Berenbaum MC, Brown IN. PROLONGATION OF HOMOGRAFT SURVIVAL IN MICE WITH SINGLE DOSES OF CYCLOPHOSPHAMIDE. Nature. 1963;200:84. [DOI] [PubMed] [Google Scholar]
- [9].Fouchier RA, Garcia-Sastre A, Kawaoka Y, Barclay WS, Bouvier NM, Brown IH, et al. Transmission studies resume for avian flu. Science. 2013;339:520–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mayumi H, Good RA. The necessity of both allogeneic antigens and stem cells for cyclophosphamide-induced skin allograft tolerance in mice. Immunobiology. 1989;178:287–304. [DOI] [PubMed] [Google Scholar]
- [11].Matsuura A, Katsuno M, Suzuki Y, Ito T, Yasuura K, Abe T, et al. Cyclophosphamide-induced tolerance in fully allogeneic heart transplantation in mice. Cell Immunol. 1994;155:501–7. [DOI] [PubMed] [Google Scholar]
- [12].Nomoto K, Eto M, Yanaga K, Nishimura Y, Maeda T, Nomoto K. Interference with cyclophosphamide-induced skin allograft tolerance by cyclosporin A. J Immunol. 1992;149:2668–74. [PubMed] [Google Scholar]
- [13].Santos GW, Owens AH. Production of graft-versus-host disease in the rat and its treatment with cytotoxic agents. Nature. 1966;210:139–40. [DOI] [PubMed] [Google Scholar]
- [14].Owens AH, Jr., Santos GW. The induction of graft versus host disease in mice treated with cyclophosphamide. J Exp Med. 1968;128:277–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Santos GW, Sensenbrenner LL, Anderson PN, Burke PJ, Klein DL, Slavin RE, et al. HL-A-identical marrow transplants in aplastic anemia, acute leukemia, and lymphosarcoma employing cyclophosphamide. Transplant Proc. 1976;8:607–10. [PubMed] [Google Scholar]
- [16].Sensenbrenner LL, Steele AA, Santos GW. Recovery of hematologic competence without engraftment following attempted bone marrow transplantation for aplastic anemia: Report of a case with diffusion chamber studies. Exp Hematol. 1977;5:51–8. [PubMed] [Google Scholar]
- [17].Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–53. [DOI] [PubMed] [Google Scholar]
- [18].Owens AH, Jr., Santos GW. The effect of cytotoxic drugs on graft-versus-host disease in mice. Transplantation. 1971;11:378–82. [DOI] [PubMed] [Google Scholar]
- [19].Santos GW, Tutschka PJ, Brookmeyer R. Cyclosporine plus methylprednisolone versus cyclophosphamide plus methylprednisolone as prophylaxis for graft-versus-host disease: A randomized double-blind study in patients undergoing allogeneic marrow transplantation. Clin Transplant. 1987;1:21–8. [Google Scholar]
- [20].Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nishimura Y, Maeda T, et al. Specific destruction of host-reactive mature T cells of donor origin prevents graft-versus-host disease in cyclophosphamide-induced tolerant mice. J Immunol. 1991;146:1402–9. [PubMed] [Google Scholar]
- [21].Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009;6:638–47. [DOI] [PubMed] [Google Scholar]
- [22].Kanakry CG, Ganguly S, Zahurak M, Bolaños-Meade J, Thoburn C, Perkins B, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5:211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brodsky RA, Chen AR, Dorr D, Fuchs EJ, Huff CA, Luznik L, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].DeZern AE, Petri M, Drachman DB, Kerr D, Hammond ER, Kowalski J, et al. High-dose cyclophosphamide without stem cell rescue in 207 patients with aplastic anemia and other autoimmune diseases. Medicine (Baltimore). 2011;90:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–64. [DOI] [PubMed] [Google Scholar]
- [26].Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–9. [DOI] [PubMed] [Google Scholar]
- [27].O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–86. [DOI] [PubMed] [Google Scholar]
- [28].Paul S, Zahurak M, Luznik L, Ambinder RF, Fuchs EJ, Bolaños-Meade J, et al. Non-Myeloablative Allogeneic Transplantation with Post-Transplant Cyclophosphamide after Immune Checkpoint Inhibition for Classic Hodgkin Lymphoma: A Retrospective Cohort Study. Biol Blood Marrow Transplant. 2020;26:1679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19:117–22. [DOI] [PubMed] [Google Scholar]
- [33].Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18:1859–66. [DOI] [PubMed] [Google Scholar]
- [34].Castagna L, Crocchiolo R, Furst S, Bramanti S, El Cheikh J, Sarina B, et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2014;20:724–9. [DOI] [PubMed] [Google Scholar]
- [35].Fuchs EJ, O’Donnell PV, Eapen M, Logan B, Antin JH, Dawson P, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood. 2021;137:420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Donnell PV, Brunstein CG, Fuchs EJ, Zhang MJ, Allbee-Johnson M, Antin JH, et al. Umbilical Cord Blood or HLA-Haploidentical Transplantation: Real-World Outcomes versus Randomized Trial Outcomes. Transplant Cell Ther. 2022;28:109.e1-.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ghosh N, Karmali R, Rocha V, Ahn KW, DiGilio A, Hari PN, et al. Reduced-Intensity Transplantation for Lymphomas Using Haploidentical Related Donors Versus HLA-Matched Sibling Donors: A Center for International Blood and Marrow Transplant Research Analysis. J Clin Oncol. 2016;34:3141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rashidi A, Hamadani M, Zhang MJ, Wang HL, Abdel-Azim H, Aljurf M, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019;3:1826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Martinez C, Gayoso J, Canals C, Finel H, Peggs K, Dominietto A, et al. Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation as Alternative to Matched Sibling or Unrelated Donor Transplantation for Hodgkin Lymphoma: A Registry Study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. J Clin Oncol. 2017;35:3425–32. [DOI] [PubMed] [Google Scholar]
- [40].Dreger P, Sureda A, Ahn KW, Eapen M, Litovich C, Finel H, et al. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv. 2019;3:360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kanate AS, Mussetti A, Kharfan-Dabaja MA, Ahn KW, DiGilio A, Beitinjaneh A, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127:938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rubio MT, Savani BN, Labopin M, Piemontese S, Polge E, Ciceri F, et al. Impact of conditioning intensity in T-replete haplo-identical stem cell transplantation for acute leukemia: a report from the acute leukemia working party of the EBMT. J Hematol Oncol. 2016;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Elmariah H, Kasamon YL, Zahurak M, Macfarlane KW, Tucker N, Rosner GL, et al. Haploidentical Bone Marrow Transplantation with Post-Transplant Cyclophosphamide Using Non-First-Degree Related Donors. Biol Blood Marrow Transplant. 2018;24:1099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kasamon YL, Ambinder RF, Fuchs EJ, Zahurak M, Rosner GL, Bolanos-Meade J, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017;1:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shaw BE, Jimenez-Jimenez AM, Burns LJ, Logan BR, Khimani F, Shaffer BC, et al. National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol. 2021;39:1971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kasamon YL, Fuchs EJ, Zahurak M, Rosner GL, Symons HJ, Gladstone DE, et al. Shortened-Duration Tacrolimus after Nonmyeloablative, HLA-Haploidentical Bone Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24:1022–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].DeZern AE, Elmariah H, Zahurak M, Rosner GL, Gladstone DE, Ali SA, et al. Shortened-Duration Immunosuppressive Therapy after Nonmyeloablative, Related HLA-Haploidentical or Unrelated Peripheral Blood Grafts and Post-Transplantation Cyclophosphamide. Biol Blood Marrow Transplant. 2020;26:2075–81. [DOI] [PubMed] [Google Scholar]
- [49].Kanakry JA, Gocke CD, Bolanos-Meade J, Gladstone DE, Swinnen LJ, Blackford AL, et al. Phase II Study of Nonmyeloablative Allogeneic Bone Marrow Transplantation for B Cell Lymphoma with Post-Transplantation Rituximab and Donor Selection Based First on Non-HLA Factors. Biol Blood Marrow Transplant. 2015;21:2115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pratz KW, Gojo I, Karp JE, Luznik L, Smith BD, Jones RJ, et al. Prospective Study of PeriTransplant Use of Sorafenib As Remission Maintenance for FLT3-ITD Patients Undergoing Allogeneic Transplantation. Blood. 2015;126.26160184 [Google Scholar]
- [51].Webster JA, Luznik L, Tsai HL, Imus PH, DeZern AE, Pratz KW, et al. Allogeneic transplantation for Ph+ acute lymphoblastic leukemia with posttransplantation cyclophosphamide. Blood Adv. 2020;4:5078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Eapen M, Le Rademacher J, Antin JH, Champlin RE, Carreras J, Fay J, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Byrne M, Savani BN, Mohty M, Nagler A. Peripheral blood stem cell versus bone marrow transplantation: A perspective from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Exp Hematol. 2016;44:567–73. [DOI] [PubMed] [Google Scholar]
- [54].Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol. 2017;35:3002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jain T, Tsai HL, DeZern AE, Gondek LP, Elmariah H, Bolaños-Meade J, et al. Posttransplantation Cyclophosphamide-based Graft versus Host Disease Prophylaxis with Non-myeloablative Conditioning for Blood or Marrow Transplantation for Myelofibrosis. Transplant Cell Ther. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kunte S, Rybicki L, Viswabandya A, Tamari R, Bashey A, Keyzner A, et al. Allogeneic blood or marrow transplantation with haploidentical donor and post-transplantation cyclophosphamide in patients with myelofibrosis: a multicenter study. Leukemia. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Eapen M. Allogeneic transplantation for aplastic anemia. Hematology. 2012;17:s15–s7. [DOI] [PubMed] [Google Scholar]
- [58].Bolaños-Meade J, Cooke KR, Gamper CJ, Ali SA, Ambinder RF, Borrello IM, et al. Effect of increased dose of total body irradiation on graft failure associated with HLA-haploidentical transplantation in patients with severe haemoglobinopathies: a prospective clinical trial. Lancet Haematol. 2019;6:e183–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].DeZern AE, Zahurak ML, Symons HJ, Cooke KR, Rosner GL, Gladstone DE, et al. Haploidentical BMT for severe aplastic anemia with intensive GVHD prophylaxis including posttransplant cyclophosphamide. Blood Adv. 2020;4:1770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, et al. Graft-versus-Host Disease Prophylaxis in Unrelated Peripheral Blood Stem Cell Transplantation with Post-Transplantation Cyclophosphamide, Tacrolimus, and Mycophenolate Mofetil. Biol Blood Marrow Transplant. 2016;22:1037–42. [DOI] [PubMed] [Google Scholar]
- [61].Colunga-Pedraza PR, Gómez-De León A, Rodríguez-Roque CS, Morcos-Sandino M, Colunga-Pedraza JE, Cantú-Rodriguez OG, et al. Outpatient Haploidentical Stem Cell Transplantation Using Post-Transplant Cyclophosphamide Is Safe and Feasible. Transplant Cell Ther. 2021;27:259.e1-.e6. [DOI] [PubMed] [Google Scholar]
- [62].Kunacheewa C, Owattanapanish W, Jirabanditsakul C, Issaragrisil S. Post-Transplant Cyclophosphamide and Thymoglobulin, a Graft-Versus-Host Disease Prophylaxis in Matched Sibling Donor Peripheral Blood Stem Cell Transplantations. Cell Transplant. 2020;29:963689720965900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].du Toit JR, McDonald A, Brittain D, Cass M, Thomson J, Oosthuizen J, et al. Is Haploidentical Hematopoietic Cell Transplantation Using Post-Transplantation Cyclophosphamide Feasible in Sub-Saharan Africa? Transplant Cell Ther. 2021;27:1002.e1-.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].George B, Kulkarni U, Lionel S, Devasia AJ, Aboobacker FN, Lakshmi KM, et al. Haploidentical transplantation is feasible and associated with reasonable outcomes despite major infective complications-A single center experience from India. Transplant Cell Ther. 2022;28:45.e1-.e8. [DOI] [PubMed] [Google Scholar]