Abstract
Introduction
The comprehensive complication index (CCI), which weights all postoperative complications according to severity and integrates them into a single formula, has been reported as a new evaluation system. We aimed to compare the CCI with the Clavien-Dindo Classification (CDC) to patients with ulcerative colitis (UC).
Methods
Patients who underwent initial surgery for UC from April 2012 to March 2020 were included. The patients were classified into a length of stay (LOS) >30 days group or an LOS ≤30 days group. We performed a multivariate analysis of risk factors for LOS >30 days in the model with the factors identified in the univariate analysis plus the CCI (the CCI model) and plus CDC (the CDC model). An ROC curve was used to test the difference in the area under the curve (AUC) between the CCI model and the CDC model.
Results
The median LOS was 21 days (IQR: 16–29 days), and the rate of LOS >30 days was 119/588 (20.2%). In the CCI model, age at the time of surgery (odds ratio [OR] = 1.24, 95% confidence interval [CI] 1.07–1.45, p = 0.01), ASA score ≥3 (OR = 1.94, 95% CI:1.00–3.76, p = 0.04), and CCI (OR = 1.07, 95% CI: 1.05–1.09; p < 0.01) were identified as independent risk factors for LOS >30 days. The AUC value of the CCI model (0.86) was significantly better in relation to LOS >30 days than that of the CDC model (0.82) (p = 0.02).
Conclusion
The CCI was a better measure of LOS than was the CDC and was found to be a useful indicator in UC.
Keywords: Ulcerative colitis, Postoperative complications, Clavien-Dindo classification, Comprehensive complication index
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) with recurrent flare-ups and remissions, posing reported surgical risks of 4.9%, 11.6%, and 15.6% at 1, 5, and 10 years after diagnosis, respectively [1]. Surgical treatment is primarily reserved for patients who are refractory to medical therapy, and an increase in postoperative infections has been reported in patients treated with corticosteroids or anti-TNF agents [2]. Other indications for surgery, such as perforation, massive bleeding, or toxic megacolon, would require emergency surgery. Emergency cases are associated with higher postoperative complications and longer hospital stays than elective surgery [3].
The commonly employed Clavien-Dindo classification (CDC) is currently the best known grading system for postoperative complications and is widely used for surgical cases of UC [4–7]. The CDC rates complications on a five-grade scale, classifying each complication by severity based on the treatment given [8]. This classification system is highly versatile because of the reproducibility of the interpretation of postoperative complications [9]. A disadvantage is that many studies report only the highest grade of complications, which makes it difficult to capture multiple complications and may not provide a complete picture of postoperative complications. To overcome these shortcomings, a new index, the comprehensive complication index (CCI), has been developed that weights all recorded complications by severity and combines them into a single formula [10]. This index is based on the CDC and summarizes the postoperative course on a new morbidity scale from 0 (no complications) to 100 (death). The CCI was proven to be a more sensitive endpoint for detecting differences in treatment response in randomized trials [11]. There are scattered applications of the CCI in studies involving surgical patients with UC, but there are few reports comparing the CCI with the CDC system [12, 13]. The purpose of the present study was to compare the CCI with the CDC by applying the CCI to surgical cases of UC.
Materials and Methods
Patient Selection
UC patients who underwent colectomy at Hyogo Medical University between April 2012 and March 2020 were included in this study. The timing of the surgery was defined as the first surgery, and the surgical procedure was limited to ileal pouch-anal anastomosis, abdominal perineal resection (APR), and total colectomy (TC) with end ileostomy (mucous fistula or Hartmann procedure). Sex, age at onset, age at initial surgery, duration of disease, disease severity, blood parameters, body mass index, American Society of Anesthesiologists (ASA) score, total administered prednisolone (PSL) dose, immunomodulator (thiopurines, including azathioprine and 6-mercaptopurine) administration, calcineurin inhibitor (tacrolimus and cyclosporine A) administration, Janus kinase inhibitor (tofacitinib) administration, biologic (infliximab, adalimumab, golimumab, and vedolizumab) administration, surgical indication (cancer/dysplasia, refractory, massive bleeding, perforation), urgent/emergent or elective surgery, surgical procedure, operative time, amount of blood loss, and intraoperative blood transfusion were retrospectively determined using clinical records. Severe disease was assessed primarily according to clinical features using the criteria of Truelove and Witts: 6 or more stools with blood and 1 or more of the following: a hemoglobin level <105 g/L, an ESR >30 mm/h, a fever >37.8°C, or a pulse rate >90/min [11]. Blood parameters were also retrospectively obtained from the clinical records and included the serum albumin (Alb) level, C-reactive protein level, and total lymphocyte count prior to surgery. The total amount of administered corticosteroids was converted into the prednisolone dose and calculated based on the administered corticosteroid dose since the initial onset of UC. Patients who received immunomodulators, calcineurin inhibitors, or Janus kinase inhibitors within 72 h before surgery, regardless of the dosage, were included. All infusions given within 12 weeks before surgery were considered biologically administered.
Definition of Postoperative Complications
Postoperative complications were defined as unexpected medical events that occurred between the end of surgery and discharge from the hospital. The presence of (1) wound infection, (2) organ/space infection, (3) bloodstream infection, (4) pneumonia, (5) other infections, (6) obstruction and ileus, (7) postoperative bleeding, (8) thrombosis, (9) urinary retention, and (10) high-output stoma was included.
(1) Wound infection was detected by the presence of erythema, induration, any purulent drainage, or dehiscence at the wound, and the grade at which the wound infections were opened at the bedside was included. (2) Organ/space infection included abdominal and pelvic abscesses, including anastomotic leakage. These were detected by gastrograffin enema, abdominal echo or computed tomography (CT) scans, and the grade requiring pharmacological treatment with drugs were included. (3) Bloodstream infections were detected by blood culture, and the grade requiring pharmacological treatment with drugs was included. (4) Pneumonia was detected by plain X-ray or CT scans, and the grade requiring pharmacological treatment with drugs was included. (5) Other infections included urinary tract infections and fevers of unknown origin that were above 38°C. Urinary tract infections were detected by urine culture. Fever of unknown origin above 38°C was diagnosed when there was no organism or antigen detected in any culture; there was no apparent infection of the wound, abdomen, or pelvis; there was no other recognized cause; and treatment for the infection was initiated by a physician. (6) Obstruction and ileus were detected by plain X-ray or CT scans, and the grade of intravenous nutritional management was included. (7) Postoperative bleeding included intra-abdominal bleeding and gastrointestinal bleeding, such as gastric/duodenal/small intestinal ulcers and anastomotic bleeding. These were detected via endoscopy or CT scans. Patients with the grade requiring medical treatment, such as proton pump inhibitors or blood transfusions, were included. (8) Thrombosis was detected by echocardiography or CT scans and required anticoagulant therapy. (9) Urinary retention was defined as urinary catheter placement and medical treatment, such as cholinergic agonists, deemed necessary by the urologist. (10) A high-output stoma was defined as the production of 2,000 mL or more of discharge for at least 2 days in a row. The grade at which infusion treatment was required due to electrolyte imbalance was included.
Outcomes
The primary outcome was length of stay (LOS). Complications were ranked according to the CDC, and the CCI was calculated within 30 postoperative days and for all hospital stays. For the CCI calculation, overall morbidity was reflected on a scale from 0 (no complications) to 100 (death), reflecting the severity of the overall complication burden on the patient. The formula is (MRV = the median reference value from physicians (phys) and patients (pat)). MRV was calculated by the operational risk index to obtain a risk index, and the base median value of the index was used [10]. The operational risk index is widely used in the economic sciences to predict the general business environment by a number of predictive criteria. By asking stakeholders representing different professional groups (in this case, physicians and patients) for their opinions on various complication items, specifying values (V) ranging from 0 (“bad”) to 4 (“excellent”), and then multiplying these values for a particular item as perceived by each stakeholder, a risk index is calculated stepwise (Vitem (i) = (Vst1 × Vst2 ... × Vst n). The calculator is accessible at https://www.assessurgery.com. The single value for grade I is 8.7, grade II is 20.9, grade IIIa is 26.2, grade IIIb is 33.7, grade IVa is 42.4, and grade IVb is 46.2; grade V (death of a patient) always results in 100. If further complications occur, the single value is added to the value for each grade.
A Spearman test was performed to determine the correlation coefficient to compare the number of hospital days captured by the CDC and CCI. The patients were classified into an LOS >30 days group or an LOS ≤30 days group. Possible risk factors for LOS >30 days were analyzed to identify significant predictors. We performed a multivariate analysis of risk factors for LOS >30 days in a model with the factors identified in the univariate analysis plus the CCI (the CCI model) and in a model with the factors identified in the univariate analysis plus the CDC (the CDC model). Moreover, we compared the areas under the curve (AUCs) of both models.
The secondary outcome was the sample size for future fictitious superiority tests. Sample size calculations for future fictitious superiority tests were based on the assumption of a 40% reduction in complication rates (with vs. without) on the CDC. The CCI difference was assumed to be 10 points, with a power of 80% and an alpha error of 0.05. A 10-point difference in the CCI was selected because it reflected a 1-grade difference in the established CDC. SD values were estimated from the presented pouch surgery, APR, and TC cohorts.
Exclusion Criteria
We excluded patients with a diagnosis or suspicion of Crohn’s disease (CD) based on histological findings that were not included in this series. All colectomy specimens with histologic features of UC and no Crohn’s-like features, such as granulomas, transmural lymphoid aggregates, or fissures, were identified. We also excluded pediatric patients who were younger than 18 years, patients with insufficient data, and patients who underwent their initial surgery at another hospital.
Statistical Analysis
The statistical analysis was performed as follows. Categorical variables were compared using the χ2 test, ANOVA, or Fisher’s exact test. Continuous variables are expressed as medians and ranges and were compared using the Mann-Whitney U test or ANOVA. The level of statistical significance was set at p < 0.05. The odds ratio (OR) and 95% confidence intervals (CIs) were calculated for all variables in the univariate analysis. Multivariate logistic regression analysis was performed to evaluate the associations between the study variables and LOS >30 days for factors with p values <0.20. JMP ver. 15 (SAS Institute, Inc., Cary, NC, USA) was used to perform all the analyses.
Results
Patient Characteristics
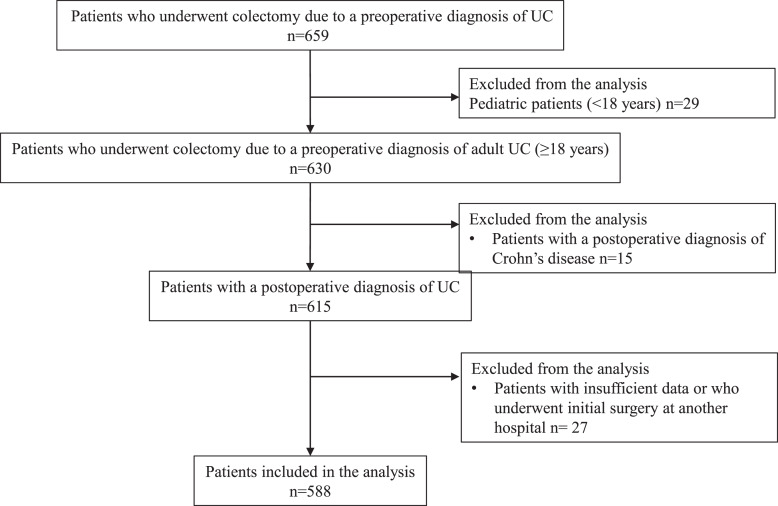
We performed 659 colectomies due to a preoperative diagnosis of UC. Twenty-nine patients were pediatric. Fifteen patients were diagnosed with CD after surgery. There were 27 patients with insufficient data or who had their initial surgery at another hospital. A total of 588 UC patients were analyzed in this series (Fig. 1).
Fig. 1.
UC patient flowchart.
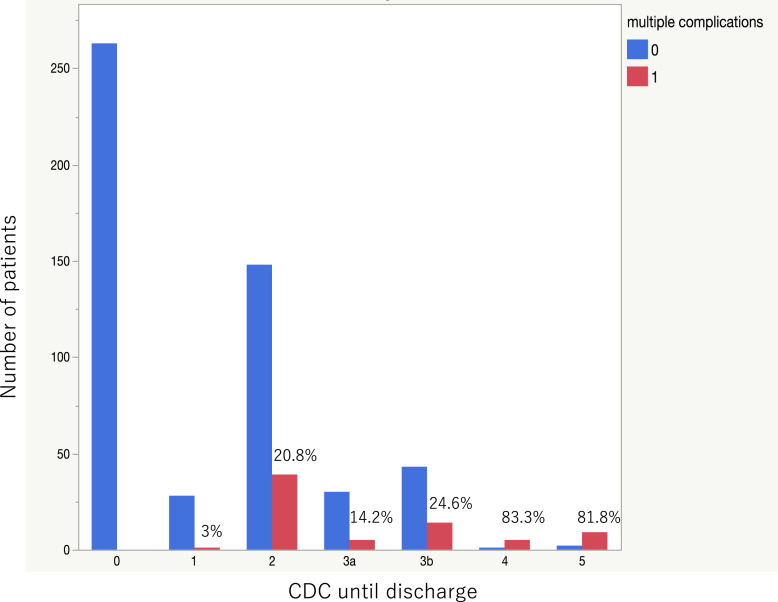
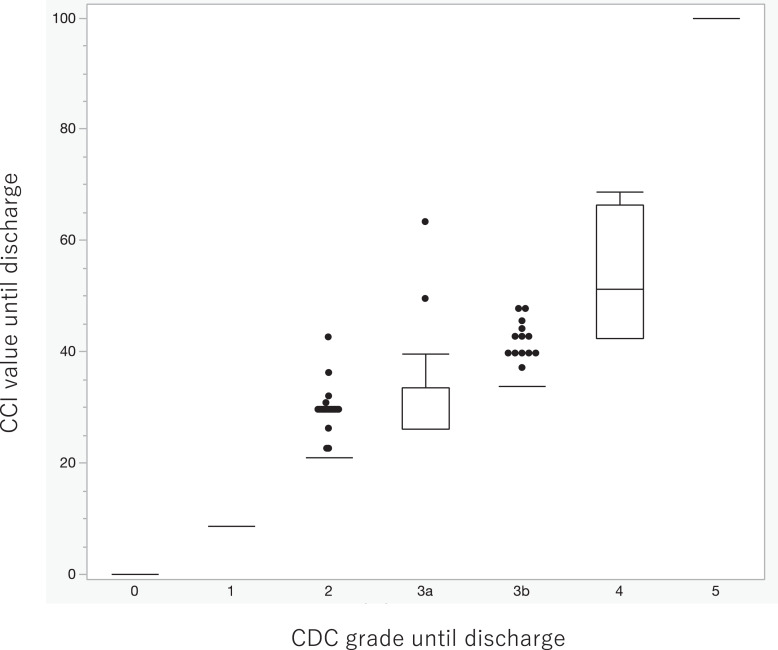
The complication rate was 313/588 (53.2%) within 30 days after surgery and 321/588 (54.5%) during the entire hospital stay. The breakdown of complications and their respective CDC grades are shown in Table 1. The most frequent complications were ileus and obstruction, with grade 2 being the most common, followed by grade 3. Figure 2 shows the number of patients by CDC grade until discharge as a bar graph. The red bars represent the number of patients with multiple complications, and the blue bars represent the number of patients with single complication. The ratio of patients with multiple complications is noted at the top of each bar, showing that the ratio exceeded 80% at grade 4. Regarding the CCI, the median CCI score within 30 days was 8.7 (interquartile range [IQR]: 0–20.9), and the median CCI score until discharge increased to 20.9 (IQR: 0–20.9). Figure 3 shows a box-and-whisker diagram of the CCI until discharge for each CDC grade until discharge. As shown for grades 2–4, the same grade had a variety of values. The median CCI score until discharge was 20.9 (range: 20.9–42.6) at grade 2, 26.2 (range: 26.2–63.3) at grade 3a, 33.7 (range: 33.7–47.7) at grade 3b, and 51.2 (range: 42.4–68.8) at grade 4.
Table 1.
Postoperative complications
| Parameters | All patients (n = 588) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Complications (CDC) | 321 (54.5) | 28 (4.8) | 184 (31.3) | 92 (15.6) | 6 (1.0) | 11 (1.9) |
| Infectious complications | ||||||
| Wound infections | 49 (8.3) | 28 (4.8) | 14 (2.4) | 6 (1.0) | 1 (0.2) | 0 |
| Organ/space infections | 54 (9.2) | 0 | 23 (3.9) | 29 (4.9) | 1 (0.2) | 1 (0.2) |
| Bloodstream infections | 10 (1.7) | 0 | 6 (1.0) | 3 (0.5) | 1 (0.2) | 0 |
| Pneumonia | 15 (2.6) | 0 | 6 (1.0) | 0 | 2 (0.3) | 7 (1.2) |
| Other infectionsa | 15 (2.6) | 0 | 13 (2.2) | 1 (0.2) | 1 (0.2) | 0 |
| Other complications | ||||||
| Obstruction/ileus | 120 (20.4) | 0 | 72 (12.2) | 48 (8.2) | 0 | 0 |
| Postoperative bleeding | 45 (7.7) | 0 | 27 (4.6) | 13 (2.2) | 2 (0.3) | 3 (0.5) |
| Thrombosis | 19 (3.2) | 0 | 19 (3.2) | 0 | 0 | 0 |
| Urinary retention | 11 (1.9) | 0 | 11 (1.9) | 0 | 0 | 0 |
| High-output stoma | 23 (3.9) | 0 | 23 (3.9) | 0 | 0 | 0 |
Data are presented as numbers with percentages in parentheses unless otherwise indicated.
CDC, Clavien-Dindo classification
aOther infections included urinary tract infection and fever above 38°C of unknown origin.
Fig. 2.
Bar graph of the number of patients by CDC grade until discharge. The red bars represent the number of patients with multiple complications, and the blue bars represent the number of patients with single complication. The ratio of patients with multiple complications is noted at the top of each bar.
Fig. 3.
A box-and-whisker diagram of the CCI until discharge for each CDC grade until discharge. The single value for grade I is 8.7, grade II is 20.9, grade IIIa is 26.2, grade IIIb is 33.7, grade IVa is 42.4, and grade IVb is 46.2; grade V (death of a patient) always results in 100.
Overall, 119 of the 588 patients had a postoperative hospital stay of more than 30 days. The clinical characteristics of the patients are presented in Table 2. Age at surgery was significantly higher (p < 0.01), the rate of severe disease was significantly higher (p < 0.01), Alb was significantly lower (p < 0.01), and the rate of an ASA score ≥3 was significantly higher (p = 0.01) among patients in the LOS >30 days group. The rate of urgent/emergency surgery was significantly higher among the patients in the multiple complications group (p < 0.01). Therefore, the rate of pouch surgery as total proctocolectomy involving constructing an ileal pouch-anal anastomosis was significantly lower among the patients in the LOS >30 days group (p < 0.01). Conversely, the rate of TC was significantly higher among the patients in the LOS >30 days group (p < 0.01). Blood loss and the rate of blood transfusion during surgery were significantly higher among the patients in the LOS >30 days group (p < 0.01).
Table 2.
Patient characteristics
| Parameters | All patients (n = 588) | LOS>30 days (n = 119) | LOS≤30 days (n = 469) | p value |
|---|---|---|---|---|
| Sex, male/female | 387/201 | 77/42 | 310/159 | 0.42 |
| Age at surgery, years, mean±standard deviation | 49.42±17.03 | 55.82±17.08 | 47.80±16.64 | <0.01* |
| Duration of disease, months, median (range) | 75.93 (0.53–590.4) | 53.56 (0.76–580.3) | 85.63 (0.53–590.4) | 0.15 |
| Severe disease, n (%)a | 208 (35.37) | 60 (50.42) | 148 (31.55) | <0.01* |
| Alb, g/dL, median (range) | 3.4 (0.9–5.2) | 2.7 (0.9–4.9) | 3.6 (1.2–5.2) | <0.01* |
| CRP, mg/dL, median (range) | 0.5 (0–31.33) | 1.35 (0–23.8) | 0.36 (0–31.33) | <0.01* |
| Total lymphocyte count/μL | 1,253 (22.9–8,754.8) | 1,149 (43–3,681) | 1,271.7 (22.9–8,754.8) | |
| BMI≥25, n (%) | 42 (7.14) | 10 (8.4) | 32 (6.82) | 0.33 |
| ASA score ≥3, n (%) | 131 (22.3) | 36 (30.25) | 95 (20.25) | 0.01* |
| Pharmacotherapy | ||||
| Total given PSL dose, mg, median (range) | 3,000 (0–200,000) | 2,087 (0–200,000) | 3,025 (0–150,000) | 0.80 |
| Immunomodulators, n (%) | 204 (34.69) | 37 (31.09) | 167 (35.6) | 0.85 |
| Calcineurin inhibitors, n (%) | 106 (18.03) | 27 (22.68) | 79 (16.84) | 0.09 |
| Janus kinase inhibitors, n (%) | 3 (0.5) | 3 (0.6) | 0 | 0.38 |
| Biologics, n (%) | 186 (31.63) | 31 (26.05) | 155 (33.04) | 0.94 |
| Surgical indication, n (%) | ||||
| Cancer/dysplasia | 180 (30.61) | 27 (22.69) | 153 (32.62) | 0.02* |
| Refractory | 331 (56.29) | 68 (57.14) | 263 (56.08) | 0.45 |
| Massive bleeding | 40 (6.8) | 12 (10.08) | 28 (5.97) | 0.08 |
| Perforation | 10 (1.7) | 3 (2.52) | 7 (1.49) | 0.32 |
| Urgent/emergent surgery, n (%) | 162 (27.55) | 49 (41.17) | 113 (24.09) | <0.01* |
| Pouch surgery, n (%) | 425 (72.28) | 70 (58.82) | 355 (75.69) | <0.01* |
| APR, n (%) | 72 (12.25) | 23 (19.32) | 49 (10.44) | <0.01* |
| TC with ileostomy, n (%) | 91 (15.47) | 26 (21.84) | 65 (13.85) | 0.02* |
| Laparoscopic surgery, n (%) | 52 (8.84) | 12 (10.08) | 40 (8.52) | 0.35 |
| Operative time, min, median (range) | 221 (53–618) | 224 (105–569) | 220 (53–618) | 0.74 |
| Blood loss, mL, median (range) | 200 (0–5,510) | 215 (10–5,510) | 200 (0–2,140) | <0.01* |
| Blood transfusion, n (%) | 132 (22) | 45 (38.13) | 87 (18.55) | <0.01* |
| CDC grade ≥3 | 109 (18.54) | 61 (51.26) | 58 (48.74) | <0.01* |
| CCI | 20.9 (0–100) | 29.6 (0–100) | 0 (0–100) | <0.01* |
Continuous variables are indicated as the means±standard deviations and medians (ranges).
LOS, length of stay; UC, ulcerative colitis; PSL, prednisolone; CRP, C-reactive protein; Alb, albumin; BMI, body mass index; ASA, American Society of Anesthesiologists; APR, abdominal perineal resection; TC, total colectomy; CCI, comprehensive complication index; CDC, Clavien-Dindo classification.
aSevere disease was assessed primarily according to clinical features using the criteria of Truelove and Witts: 6 or more stools with blood and 1 or more of the following: a hemoglobin level <105 g/L, an ESR >30 mm/h, a fever >37.8°C, or a pulse rate >90/min.
*p < 0.05 (significantly different).
Outcomes
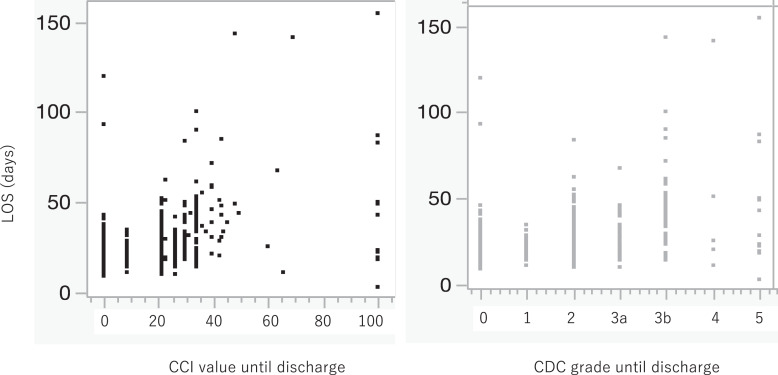
The median LOS was 21 days (IQR: 16–29 days), and the rate of patients with a LOS >30 days was 119/588 (20.2%). The relationships between LOS and the CDC or CCI are shown in the scatterplot (Fig. 4). Although both the CDC and CCI were positively correlated with LOS, the correlation coefficient of the CCI was 0.609, which was greater than that of the CDC (0.573).
Fig. 4.
Scatterplot of the relationship between LOS and the CCI or CDC grade.
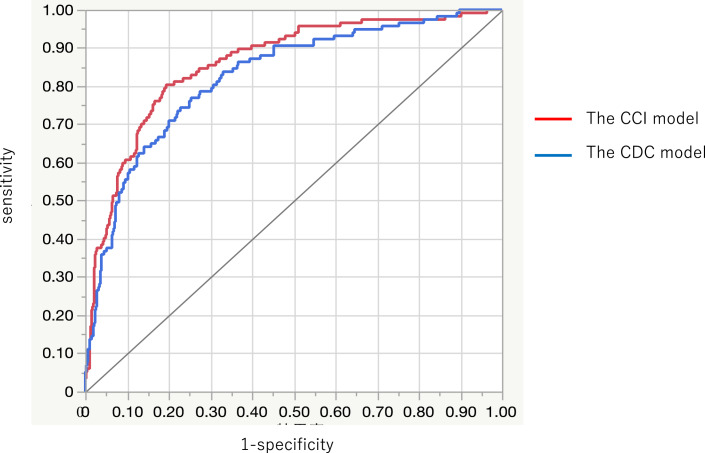
Univariate and multivariate analyses were performed to identify the independent risk factors for LOS >30 days. The results of the analyses of the risk factors potentially associated with LOS >30 days are presented in Table 3. Eleven clinically significant factors, including age at surgery, disease duration, severe disease, Alb levels, C-reactive protein levels, ASA score ≥3, calcineurin inhibitor administration, massive bleeding, emergency surgery (the surgical procedures were combined into this factor), blood loss, blood transfusion, and plus CDC (the CDC model) or plus CCI (the CCI model), were entered into the multivariate logistic regression analysis. The values for the CDC model (AUC = 0.82) and the CCI model (AUC = 0.86) were all correlated with LOS >30 days (p < 0.01). Figure 5 shows that the AUC values of the CCI model were significantly better in relation to LOS >30 days than those of the CDC model (p = 0.02). In the CCI model, age at the time of surgery (OR = 1.24, 95% confidence interval [CI] 1.07–1.45, p = 0.01), ASA score ≥3 (OR = 1.94, 95% CI 1.00–3.76, p = 0.04), and CCI score (OR = 1.07, 95% CI 1.05–1.09; p < 0.01) were identified as independent risk factors for a LOS >30 days.
Table 3.
Logistic regression analysis of the risk factors for LOS >30 days
| Parameters | Univariate analysis | Multivariate analysis (plus CCI model) | Multivariate analysis (plus CDC model) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Female | 1.06 (0.69–1.62) | 0.42 | ||||
| Age at surgery (10-year intervals) | 1.32 (1.17–1.49) | <0.01* | 1.24 (1.07–1.45) | 0.01* | 1.38 (1.18–1.61) | 0.01* |
| Disease duration (1-month intervals) | 0.99 (0.99–1.00) | 0.15 | 0.99 (0.97–1.02) | 0.88 | 1.00 (0.99–1.00) | 0.68 |
| Severe disease | 2.21 (1.47–3.32) | <0.01* | 1.07 (0.49–2.33) | 0.84 | 1.19 (0.54–2.61) | 0.64 |
| Alb (per 1 g/dL) | 0.58 (0.47–0.72) | <0.01* | 0.71 (0.46–1.12) | 0.14 | 0.61 (0.39–0.96) | 0.03* |
| CRP (per 1 g/dL) | 1.06 (1.02–1.09) | <0.01* | 1.03 (0.97–1.09) | 0.29 | 1.01 (0.95–1.06) | 0.72 |
| Total lymphocyte count/μL | 0.99 (0.99–1.00) | 0.37 | ||||
| BMI≥25 | 1.25 (0.59–2.62) | 0.33 | ||||
| ASA score ≥3 | 1.71 (1.09–2.68) | 0.01* | 1.94 (1.00–3.76) | 0.04* | 1.61 (0.83–3.10) | 0.14 |
| Total given PSL dose (per 1 mg) | 1.00 (0.99–1.00) | 0.80 | ||||
| Immunomodulators | 0.81 (0.53–1.26) | 0.85 | ||||
| Calcineurin inhibitors | 1.45 (0.88–2.37) | 0.09 | 1.62 (0.88–2.95) | 0.11 | 1.73 (0.93–3.21) | 0.08 |
| Janus kinase inhibitors | 0 | 0.38 | ||||
| Biologics | 0.71 (0.45–1.12) | 0.94 | ||||
| Cancer/dysplasia | 0.61 (0.38–0.97) | 0.02* | 1.24 (0.602.551) | 0.55 | 1.55 (0.74–3.24) | 0.52 |
| Refractory | 1.04 (0.69–1.57) | 0.45 | ||||
| Massive bleeding | 1.76 (0.86–3.59) | 0.08 | 1.12 (0.40–3.09) | 0.82 | 1.34 (0.51–3.53) | 0.23 |
| Perforation | 1.71 (0.43–6.70) | 0.32 | ||||
| Urgent/emergent surgery | 2.21 (1.45–3.36) | <0.01* | 1.43 (0.61–3.31) | 0.40 | 1.34 (0.58–3.13) | 0.48 |
| Laparoscopic surgery | 1.20 (0.61–2.37) | 0.35 | ||||
| Operative time (per min) | 1.00 (0.99–1.00) | 0.74 | ||||
| Blood loss (per 100mL) | 1.09 (1.00–1.16) | <0.01* | 1.06 (0.99–1.14) | 0.07 | 1.08 (1.01–1.16) | 0.01* |
| Blood transfusion | 2.71 (1.75–4.19) | <0.01* | 1.43 (0.75–2.69) | 0.27 | 1.43 (0.75–2.73) | 0.39 |
| CDC grade ≥3 | 9.22 (5.78–14.71) | <0.01* | 11.9 (6.91–20.5) | <0.01* | ||
| CCI (per score) | 1.07 (1.05–1.08) | <0.01* | 1.07 (1.05–1.09) | <0.01* | ||
OR, odds ratio; CI, confidence interval; Alb, albumin; ASA, American Society of Anesthesiologists; BMI, body mass index; CRP, C-reactive protein; PSL, prednisolone; CDC, Clavien-Dindo classification; CCI, comprehensive complication index.
*p < 0.05 (significantly different).
Fig. 5.
Receiver operating characteristic curve of the CDC model (AUC: 0.82) and the CCI model for predicting LOS >30 days (AUC: 0.86). Difference in the AUC: 0.03 (95% CI: 0.004–0.06), p = 0.02.
Sample size calculations and assumptions for postoperative complications for each surgical procedure are presented in Table 4. The pouch surgery procedure based on the CDC revealed that using the CCI would result in considerably smaller sample sizes for each procedure. However, the number of samples in TC required was higher for the CCI and remained the same in APR cases.
Table 4.
Sample size calculation and assumption of postoperative complications for each surgical procedure
| Surgical procedure | All procedures | Pouch surgery | APR | TC |
|---|---|---|---|---|
| Patients with CDC complications, n (%) | 321/588 (54.59) | 214/425 (50.35) | 48/72 (66.67) | 59/91 (64.84) |
| XX % reduction in complications | 40 | 40 | 40 | 40 |
| Mean CCI±SD | 15.23±18.35 | 13.16±14.53 | 14.63±18.54 | 25.38±28.37 |
| Δ | 10 | 10 | 10 | 10 |
| Sample size/group (CDC) | 80 | 92 | 54 | 58 |
| Sample size/group (CCI) | 54 | 35 | 55 | 128 |
CDC, Clavien-Dindo classification; CCI, comprehensive Complication index; APR, abdominal perineal resection; TC, total colectomy.
CDC: calculated sample size to achieve 80% power by the χ2 test.
CCI: calculated sample size to achieve 80% power by the t test.
Discussion
The present results indicated that the CCI had a stronger correlation with postoperative LOS than the CDC. Furthermore, the CCI model was more useful than the CDC model in the multivariate analysis of other factors. On the other hand, the use of the CCI to reduce the sample size in future studies showed different results depending on the surgical procedure being analyzed.
Several reports have shown that the CCI has a stronger correlation with postoperative LOS than the CDC [14–17]. In gastric cancer patients, the CCI showed a stronger relationship with length of hospitalization than the CDC, and for longer hospitalizations above 30 days, only the CCI showed a moderate correlation; the CDC did not [14]. The CCI was also closely related to the prognosis of gastric cancer and has been reported to be an independent prognostic indicator in patients with complications of CDC grade 2–4 [18]. Other organ areas, such as hepatobiliary-pancreatic and urological areas, have also been shown to be superior to the CDC in predicting LOS [15–17]. One comparison study of the CDC in IBD involving UC and CD has been reported, with the CCI showing a stronger correlation with LOS than the CDC [19]. We chose to analyze only UC because a staged operation is basically recommended, unlike that for CD and other diseases [20]. Most cases in which colostomy are performed require some additional length of hospitalization to establish the procedure, regardless of the presence or absence of comorbidities. However, the present results indicate that even in UC, the CCI system provides a more accurate grading system than the CDC system for patients with complicated cases requiring longer hospital stays or those with severe comorbidities. Additionally, the CCI can clearly identify multiple complications if the value deviates from the single value. The present results showed that the CCI values had a variety of values for the same grade in the CDC system. This kind of quality in the CCI has been reported to be useful in evaluation of hospital costs [11].
Patients with IBD are reported to have a longer LOS after colorectal resection than patients with neoplasia or diverticulitis because of their chronic inflammatory state, which is often associated with malnutrition [21, 22]. A recent report investigating the details of preoperative medical therapy and postoperative consequences revealed that features that prolong postoperative hospital stay include bleeding disorders, hypoalbuminemia, and ASA score 3–5 [23]. Our results also showed that the ASA score and age at the time of surgery were independent risk factors for LOS. We previously reported that advanced age was a risk factor for infectious complications and fatal complications [24]. Moreover, numerous studies cited in the Cochrane Review reported that the risk factors for infectious complications are corticosteroid use, biological agent administration, malnutrition, severe disease, obesity, anemia, ASA score, emergency surgery, blood transfusion, and old age [2], and several reports have shown that old age is the main risk factor [25, 26].
Sample size calculations for hypothetical clinical trials were compared between the CCI and the CDC to assess whether the use of the CCI could reduce the number of patients required for clinical trials. Since the sample size was for a clinical study to compare complications between techniques, we used the typical UC techniques of pouch surgery, APR, and TC for disclosure. In this study, the complication rate showed a 40% risk reduction from a value greater than 50%, so the percentage reduction was greater and the required sample size was smaller. However, whether the CDC or the CCI is better seems to vary case by case, especially since the SD of the CCI for total colon resection is very large and the 40% reduction for the CDC is also very large. In other words, in contrast to the one-sided interpretation that “using the CCI reduces the sample size,” the results show that the required sample size for the CCI and CDC depends on the complication rate, SD, and surgical technique. Therefore, both the CDC and CCI systems should be routinely used to record postoperative surveys.
This study has several limitations. First, this was a retrospective analysis conducted at a single institution. Second, data on long-term CCI values were not available. Clavien et al. emphasized the need to collect data on postoperative complications for at least 3 months after surgery [8]. Although UC patients are often readmitted after surgery and long-term CCI measurements would be useful, some patients could not be included in the present analysis due to insufficient data. Third, the CDC of commonly observed complications of UC still includes items that divide opinions on CDC grading. For example, it has been reported that UC is often associated with stoma outlet obstruction [27]. Although stoma outlet obstruction was treated by ileostomy tube drainage at our institution, there is no consensus as to what grade this would be in the CDC. In the CDC, nasogastric tube insertion is grade 2, and ileus tube insertion is grade 3a. Considering the invasive nature of the procedure, ileostomy tube drainage can be easily performed at the bedside in most cases, and therefore, we considered it to be grade 2 in this study. In addition, many studies do not use CDC, and the definitions of complications vary widely [28, 29]. In the future, complex and controversial cases should be presented to physicians familiar with IBD surgery to reach a consensus on the weighting of each postoperative event.
The CCI was found to be a useful indicator for patients with UC. The CCI was a better measure of LOS than the CDC in both univariate and multivariate analyses. On the other hand, CCI may not be suitable for smaller sample sizes in future studies, depending on the surgical procedures.
Statement of Ethics
All study protocols were approved by the Institutional Review Board of Hyogo Medical University (no. 4400), and informed consent and agreement for the use of patient data were obtained before surgery. Written informed consent was obtained via an opt-out method. An opt-out informed consent protocol was used for the use of participant data for research purposes. This consent procedure was reviewed and approved by the Institutional Review Board at Hyogo Medical University (approval number [4400] and date of decision [May 24, 2023]).
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
No specific funding was received for this work.
Author Contributions
Masataka Igeta: analysis and interpretation of the data; drafting of the article. Yuki Horio, Kentaro Nagano, Kurando Kusunoki, Ryuichi Kuwahara, Toshiyuki Sato: conception and design of the study; acquisition, analysis and interpretation of the data. Motoi Uchino, Shinichiro Shinzaki, and Hiroki Ikeuchi: acquisition of the data; drafting of the article and revising it critically for important intellectual content, and final approval.
Funding Statement
No specific funding was received for this work.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145(5):996–1006. Epub 2013 Jul 31. [DOI] [PubMed] [Google Scholar]
- 2. Law CC, Bell C, Koh D, Bao Y, Jairath V, Narula N. Risk of postoperative infectious complications from medical therapies in inflammatory bowel disease. Cochrane Database Syst Rev. 2020;10(10):Cd013256. Epub 2020 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel SS, Patel MS, Goldfarb M, Ortega A, Ault GT, Kaiser AM, et al. Elective versus emergency surgery for ulcerative colitis: a national surgical quality improvement program analysis. Am J Surg. 2013;205(3):333–8; discussion 7–8. Epub 2013 Feb 2. [DOI] [PubMed] [Google Scholar]
- 4. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. Epub 2004 Jul27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartels SA, Gardenbroek TJ, Bos L, Ponsioen CY, D’Haens GR, Tanis PJ, et al. Prolonged preoperative hospital stay is a risk factor for complications after emergency colectomy for severe colitis. Colorectal Dis. 2013;15(11):1392–8. Epub 2013 Jul 3. [DOI] [PubMed] [Google Scholar]
- 6. Gebhardt JM, Werner N, Stroux A, Förster F, Pozios I, Seifarth C, et al. Robotic-assisted versus laparoscopic proctectomy with ileal pouch-anal anastomosis for ulcerative colitis: an analysis of clinical and financial outcomes from a tertiary referral center. J Clin Med. 2022;11(21):6561. Epub 2022 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schineis C, Lehmann KS, Lauscher JC, Beyer K, Hartmann L, Margonis GA, et al. Colectomy with ileostomy for severe ulcerative colitis-postoperative complications and risk factors. Int J Colorectal Dis. 2020;35(3):387–94. Epub 2019 Dec23. [DOI] [PubMed] [Google Scholar]
- 8. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. Epub 2009 Jul30. [DOI] [PubMed] [Google Scholar]
- 9. Rebelo A, Friedrichs J, Grilli M, Wahbeh N, Partsakhashvili J, Ukkat J, et al. Systematic review and meta-analysis of surgery for hilar cholangiocarcinoma with arterial resection. HPB. 2022;24(10):1600–14. Epub 2022 May 1. [DOI] [PubMed] [Google Scholar]
- 10. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. Epub 2013 Jun 4. [DOI] [PubMed] [Google Scholar]
- 11. Slankamenac K, Nederlof N, Pessaux P, de Jonge J, Wijnhoven BP, Breitenstein S, et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg. 2014;260(5):757–63; discussion 62–3. Epub 2014 Nov 8. [DOI] [PubMed] [Google Scholar]
- 12. de Buck van Overstraeten A, Mark-Christensen A, Wasmann KA, Bastiaenen VP, Buskens CJ, Wolthuis AM, et al. Transanal versus transabdominal minimally invasive (completion) proctectomy with ileal pouch-anal anastomosis in ulcerative colitis: a comparative study. Ann Surg. 2017;266(5):878–83. Epub 2017 Jul 26. [DOI] [PubMed] [Google Scholar]
- 13. Zhang T, Li G, Duan M, Lv T, Feng D, Lu N, et al. Perioperative parenteral fish oil supplementation improves postoperative coagulation function and outcomes in patients undergoing colectomy for ulcerative colitis. JPEN J Parenter Enteral Nutr. 2022;46(4):878–86. Epub 2021 Oct 6. [DOI] [PubMed] [Google Scholar]
- 14. Kim TH, Suh YS, Huh YJ, Son YG, Park JH, Yang JY, et al. The comprehensive complication index (CCI) is a more sensitive complication index than the conventional Clavien-Dindo classification in radical gastric cancer surgery. Gastric Cancer. 2018;21(1):171–81. Epub 2017 Jun 10. [DOI] [PubMed] [Google Scholar]
- 15. Kowalewski KF, Müller D, Mühlbauer J, Hendrie JD, Worst TS, Wessels F, et al. The comprehensive complication index (CCI): proposal of a new reporting standard for complications in major urological surgery. World J Urol. 2021;39(5):1631–9. Epub 2020 Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ricci C, Ingaldi C, Grego DG, Alberici L, De Raffele E, Pagano N, et al. The use of comprehensive complication Index® in pancreatic surgery: a comparison with the Clavien-Dindo system in a high volume center. HPB. 2021;23(4):618–24. Epub 2020 Sep 23. [DOI] [PubMed] [Google Scholar]
- 17. Cloyd JM, Mizuno T, Kawaguchi Y, Lillemoe HA, Karagkounis G, Omichi K, et al. Comprehensive complication index validates improved outcomes over time despite increased complexity in 3707 consecutive hepatectomies. Ann Surg. 2020;271(4):724–31. Epub 2018 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimizu S, Saito H, Kono Y, Murakami Y, Shishido Y, Miyatani K, et al. The prognostic significance of the comprehensive complication index in patients with gastric cancer. Surg Today. 2019;49(11):913–20. Epub 2019 May 31. [DOI] [PubMed] [Google Scholar]
- 19. Zhu F, Feng D, Zhang T, Gu L, Zhu W, Guo Z, et al. Toward a more sensitive endpoint for assessing postoperative complications in patients with inflammatory bowel disease: a comparison between comprehensive complication index (CCI) and clavien-dindo classification (CDC). J Gastrointest Surg. 2018;22(9):1593–602. Epub 2018 May 17. [DOI] [PubMed] [Google Scholar]
- 20. Spinelli A, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, et al. ECCO guidelines on therapeutics in ulcerative colitis: surgical treatment. J Crohns Colitis. 2022;16(2):179–89. Epub 2021 Oct 13. [DOI] [PubMed] [Google Scholar]
- 21. Ban KA, Berian JR, Liu JB, Ko CY, Feldman LS, Thacker JKM. Effect of diagnosis on outcomes in the setting of enhanced recovery protocols. Dis Colon Rectum. 2018;61(7):847–53. Epub 2018 Jun 08. [DOI] [PubMed] [Google Scholar]
- 22. 2015 European Society of Coloproctology ESCP collaborating group . Patients with Crohn’s disease have longer post-operative in-hospital stay than patients with colon cancer but no difference in complications’ rate. World J Gastrointest Surg. 2019;11(5):261–70. Epub 2019 Jun07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hill SS, Ottaviano KE, Palange DC, Chismark AD, Valerian BT, Canete JJ, et al. Impact of preoperative factors in patients with inflammatory bowel disease on postoperative length of stay: a national surgical quality improvement program-inflammatory bowel disease collaborative analysis. Dis Colon Rectum. 2024;67(1):97–106.Epub 2023 Jul 6. [DOI] [PubMed] [Google Scholar]
- 24. Horio Y, Uchino M, Kusunoki K, Minagawa T, Kuwahara R, Kimura K, et al. Being elderly is associated with infectious and fatal postoperative complications in ulcerative colitis patients. Digestion. 2022;103(6):470–9. Epub 2022 Nov 17. [DOI] [PubMed] [Google Scholar]
- 25. Uchino M, Ikeuchi H, Bando T, Chohno T, Sasaki H, Horio Y, et al. Associations between multiple immunosuppressive treatments before surgery and surgical morbidity in patients with ulcerative colitis during the era of biologics. Int J Colorectal Dis. 2019;34(4):699–710. Epub 2019 Jan 28. [DOI] [PubMed] [Google Scholar]
- 26. Yamada A, Komaki Y, Patel N, Komaki F, Aelvoet AS, Tran AL, et al. Risk of postoperative complications among inflammatory bowel disease patients treated preoperatively with vedolizumab. Am J Gastroenterol. 2017;112(9):1423–9. Epub 2017 Jul19. [DOI] [PubMed] [Google Scholar]
- 27. Mori R, Ogino T, Sekido Y, Hata T, Takahashi H, Miyoshi N, et al. Long distance between the superior mesenteric artery root and bottom of the external anal sphincter is a risk factor for stoma outlet obstruction after total proctocolectomy and ileal-pouch anal anastomosis for ulcerative colitis. Ann Gastroenterol Surg. 2022;6(2):249–55. Epub 2022 Mar 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fazio VW, Kiran RP, Remzi FH, Coffey JC, Heneghan HM, Kirat HT, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3,707 patients. Ann Surg. 2013;257(4):679–85. Epub 2013 Jan 10. [DOI] [PubMed] [Google Scholar]
- 29. Champagne BJ, Papaconstantinou HT, Parmar SS, Nagle DA, Young-Fadok TM, Lee EC, et al. Single-incision versus standard multiport laparoscopic colectomy: a multicenter, case-controlled comparison. Ann Surg. 2012;255(1):66–9. Epub 2011 Dec 23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.