Abstract
Background
Home haemodialysis (HHD) may be associated with important clinical, social or economic benefits. However, few randomised controlled trials (RCTs) have evaluated HHD versus in‐centre HD (ICHD). The relative benefits and harms of these two HD modalities are uncertain. This is an update of a review first published in 2014. This update includes non‐randomised studies of interventions (NRSIs).
Objectives
To evaluate the benefits and harms of HHD versus ICHD in adults with kidney failure.
Search methods
We contacted the Information Specialist and searched the Cochrane Kidney and Transplant Register of Studies up to 9 October 2022 using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Registry Platform (ICTRP) Search Portal, and ClinicalTrials.gov. We searched MEDLINE (OVID) and EMBASE (OVID) for NRSIs.
Selection criteria
RCTs and NRSIs evaluating HHD (including community houses and self‐care) compared to ICHD in adults with kidney failure were eligible. The outcomes of interest were cardiovascular death, all‐cause death, non‐fatal myocardial infarction, non‐fatal stroke, all‐cause hospitalisation, vascular access interventions, central venous catheter insertion/exchange, vascular access infection, parathyroidectomy, wait‐listing for a kidney transplant, receipt of a kidney transplant, quality of life (QoL), symptoms related to dialysis therapy, fatigue, recovery time, cost‐effectiveness, blood pressure, and left ventricular mass.
Data collection and analysis
Two authors independently assessed if the studies were eligible and then extracted data. The risk of bias was assessed, and relevant outcomes were extracted. Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) or standardised mean difference (SMD) and 95% CI for continuous outcomes. Confidence in the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Meta‐analysis was performed on outcomes where there was sufficient data.
Main results
From the 1305 records identified, a single cross‐over RCT and 39 NRSIs proved eligible for inclusion. These studies were of varying design (prospective cohort, retrospective cohort, cross‐sectional) and involved a widely variable number of participants (small single‐centre studies to international registry analyses). Studies also varied in the treatment prescription and delivery (e.g. treatment duration, frequency, dialysis machine parameters) and participant characteristics (e.g. time on dialysis). Studies often did not describe these parameters in detail. Although the risk of bias, as assessed by the Newcastle‐Ottawa Scale, was generally low for most studies, within the constraints of observational study design, studies were at risk of selection bias and residual confounding.
Many study outcomes were reported in ways that did not allow direct comparison or meta‐analysis. It is uncertain whether HHD, compared to ICHD, may be associated with a decrease in cardiovascular death (RR 0.92, 95% CI 0.80 to 1.07; 2 NRSIs, 30,900 participants; very low certainty evidence) or all‐cause death (RR 0.80, 95% CI 0.67 to 0.95; 9 NRSIs, 58,984 patients; very low certainty evidence). It is also uncertain whether HHD may be associated with a decrease in hospitalisation rate (MD ‐0.50 admissions per patient‐year, 95% CI ‐0.98 to ‐0.02; 2 NRSIs, 834 participants; very low certainty evidence), compared with ICHD.
Compared with ICHD, it is uncertain whether HHD may be associated with receipt of kidney transplantation (RR 1.28, 95% CI 1.01 to 1.63; 6 NRSIs, 10,910 participants; very low certainty evidence) and a shorter recovery time post‐dialysis (MD ‐2.0 hours, 95% CI ‐2.73 to ‐1.28; 2 NRSIs, 348 participants; very low certainty evidence). It remains uncertain if HHD may be associated with decreased systolic blood pressure (SBP) (MD ‐11.71 mm Hg, 95% CI ‐21.11 to ‐2.46; 4 NRSIs, 491 participants; very low certainty evidence) and decreased left ventricular mass index (LVMI) (MD ‐17.74 g/m2, 95% CI ‐29.60 to ‐5.89; 2 NRSIs, 130 participants; low certainty evidence). There was insufficient data to evaluate the relative association of HHD and ICHD with fatigue or vascular access outcomes.
Patient‐reported outcome measures were reported using 18 different measures across 11 studies (QoL: 6 measures; mental health: 3 measures; symptoms: 1 measure; impact and view of health: 6 measures; functional ability: 2 measures). Few studies reported the same measures, which limited the ability to perform meta‐analysis or compare outcomes.
It is uncertain whether HHD is more cost‐effective than ICHD, both in the first (SMD ‐1.25, 95% CI ‐2.13 to ‐0.37; 4 NRSIs, 13,809 participants; very low certainty evidence) and second year of dialysis (SMD ‐1.47, 95% CI ‐2.72 to ‐0.21; 4 NRSIs, 13,809 participants; very low certainty evidence).
Authors' conclusions
Based on low to very low certainty evidence, HHD, compared with ICHD, has uncertain associations or may be associated with decreased cardiovascular and all‐cause death, hospitalisation rate, slower post‐dialysis recovery time, and decreased SBP and LVMI. HHD has uncertain cost‐effectiveness compared with ICHD in the first and second years of treatment.
The majority of studies included in this review were observational and subject to potential selection bias and confounding, especially as patients treated with HHD tended to be younger with fewer comorbidities. Variation from study to study in the choice of outcomes and the way in which they were reported limited the ability to perform meta‐analyses. Future research should align outcome measures and metrics with other research in the field in order to allow comparison between studies, establish outcome effects with greater certainty, and avoid research waste.
Keywords: Adult; Humans; Ambulatory Care Facilities; Bias; Cardiovascular Diseases; Cardiovascular Diseases/mortality; Cause of Death; Hemodialysis, Home; Hemodialysis, Home/adverse effects; Hemodialysis, Home/methods; Hemodialysis, Home/mortality; Hospitalization; Hospitalization/statistics & numerical data; Kidney Failure, Chronic; Kidney Failure, Chronic/complications; Kidney Failure, Chronic/mortality; Kidney Failure, Chronic/therapy; Myocardial Infarction; Myocardial Infarction/mortality; Non-Randomized Controlled Trials as Topic; Quality of Life; Randomized Controlled Trials as Topic; Renal Dialysis; Renal Dialysis/adverse effects; Renal Insufficiency; Renal Insufficiency/mortality; Renal Insufficiency/therapy; Stroke; Stroke/mortality
Plain language summary
Is home haemodialysis better than in‐centre haemodialysis for people with kidney failure?
Key messages
‐ Home haemodialysis may be preferred by some patients. However, the research gives us very uncertain answers.
‐ We are unsure whether the better patient outcomes with home haemodialysis are because of the dialysis treatment itself or because patients receiving home haemodialysis are younger and less sick.
Why perform haemodialysis at home rather than in a dialysis centre?
Kidney failure is a common and increasingly prevalent public health problem, which results in increases in illness, death and healthcare costs. People with kidney failure require kidney replacement therapy (dialysis and kidney transplantation) to remove the accumulation of waste products in the blood, which in turn may assist with reducing symptoms such as fatigue, nausea and itching and may improve a person's overall quality of life. Unfortunately, some patients lack access to hospital dialysis care.
What did we want to find out?
People who are treated with haemodialysis at home may experience increased well‐being and might live longer. However, home haemodialysis may also increase the burden of healthcare for patients and families and increase technical problems for patients.
What did we do?
We searched for randomised and non‐randomised studies comparing home haemodialysis with hemodialysis treatment performed in a hospital or clinical setting. We compared and summarised the trials' results and rated our confidence in the information based on factors such as trial methods and size.
What did we find?
We found only one randomised study (where patients are randomly allocated to one treatment or the other) that compared home haemodialysis with in‐centre haemodialysis in nine patients. All other studies (39) were observational (where the treatment was not randomly assigned).
Home haemodialysis may be associated with outcomes including increased length of life, fewer hospital stays, higher chance of receiving a kidney transplant, shorter recovery time from dialysis itself and increased control of blood pressure. Patients receiving home haemodialysis tended to have more dialysis (more hours or more often). Some of the differences in outcomes for patients may have been due to factors that were not related to dialysis treatment since patients receiving home haemodialysis were younger and had fewer other illnesses.
What are the limitations of the evidence?
The small number and size of the studies were limitations in this review. Not all the studies provided data about the outcomes we were interested in, and we are unsure about the results.
How up‐to‐date is the evidence?
The evidence is up to date as of October 2022.
Summary of findings
Summary of findings 1. Summary of findings.
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Certainty of evidence (GRADE) | Comments |
| Cardiovascular death | RR 0.92 (0.80 to 1.07) | 30,900 (2) | Very low1 ⊕⊝⊝⊝ |
‐ |
| All‐cause death | RR 0.80 (0.67 to 0.95) | 58,984 (9) | Very low2 ⊕⊝⊝⊝ |
Studies varied from small single centre studies and large registry analyses. Follow‐up duration varied from less than two years to more than 10 years |
| Hospitalisation | ||||
| All‐cause annual hospitalisation rate (admissions/patient‐year) | MD ‐0.50 admissions (‐0.98 to ‐0.02) | 834 (2) | Very low3 ⊕⊝⊝⊝ |
‐ |
| All‐cause annual hospitalisation days (days/patient‐year) | MD ‐1.90 days (‐2.28 to ‐1.53) | 834 (2) | Low4 ⊕⊕⊝⊝ |
‐ |
| Received kidney transplantation | RR 1.28 (1.01 to 1.63) | 10,910 (6) | Very low5 ⊕⊝⊝⊝ |
Studies varied from small single centre studies and large registry analyses |
| Health‐related quality of life | ||||
| Physical Component Summary | SMD 0.42 (0.10 to 0.73) | 922 (5) | Very low6 ⊕⊝⊝⊝ |
All studies were cross‐sectional design. The time on dialysis varied substantially between studies (Table 2) |
| Mental Component Summary | SMD 0.10 (‐0.05 to 0.25) | 922 (5) | Very low7 ⊕⊝⊝⊝ |
All studies were cross‐sectional design. The time on dialysis varied substantially between studies (Table 2) |
| Recovery time (hours) | MD ‐2.00 (‐2.73 to ‐1.28) | 348 (2) | Low8 ⊕⊕⊝⊝ |
Included one longitudinal cohort study of patients transitioning dialysis modality, and reported mean of weekly responses during 8‐week period on each modality, and one cross‐sectional survey of prevalent dialysis patients (time current on modality not reported) |
| Blood pressure7 | ||||
| Systolic BP (mm Hg) | MD ‐11.78 (‐21.11 to ‐2.46) | 491 (4) | Very low9 ⊕⊝⊝⊝ |
Included both longitudinal cohort and cross‐sectional studies. Exclusion of cross‐sectional studies led to similar relative effect with reduced heterogeneity (MD ‐19.47, 95% CI ‐24.40 to ‐14.54; I2 = 42%) Data from one RCT also indicated lower BP with HHD versus ICHD (155 ± 18 vs 169 ± 24 mm Hg) |
| Diastolic BP (mm Hg) | MD 1.81 (‐1.31 to 4.94) | 383 (3) | Very low10 ⊕⊝⊝⊝ |
Included both longitudinal cohort and cross‐sectional studies Data from one RCT indicated lower BP with HHD versus ICHD (89 ± 6 vs 93 ± 9 mm Hg) |
HHD: home haemodialysis; ICHD: in‐centre haemodialysis; 95% CI: 95% confidence interval; RR: risk ratio; MD: mean difference; SMD: standardised mean difference; BP: blood pressure; NRSI: non‐randomised study of intervention; RCT: randomised controlled trial
- Downgraded due to imprecision of relative effect (CI includes the possibility of no effect or harm)
- Downgraded due to considerable heterogeneity (I2 = 84%)
- Downgraded due to considerable heterogeneity (I2 = 90%)
- Downgraded based on 2 non‐randomised observational studies
- Downgraded due to substantial heterogeneity (I2 = 78%)
- Downgraded due to substantial heterogeneity (I2 = 74%) and risk of bias
- Downgraded due to imprecision of relative effect (CI includes the possibility of no effect or harm) and risk of bias
- Relative effect based on data from NRSIs, as only one RCT was identified during the systematic review
- Downgraded due to considerable heterogeneity (I2 = 81%)
- Downgraded due to imprecision of relative effect, CI includes the possibility of no effect or harm
1. Vintage of patients included in Physical Component Summary and Mental Component Summary analysis.
| Metric | HHD | ICHD | |
|
Jayanti 2016 ICHD (197) HHD (91) |
Median dialysis vintage (years) | 3.47 (IQR 1.39, 6.82) | 2.68 (IQR 1.05, 5.12) |
|
Toronto Group 2002 ICHD (163) HHD (56) |
Not reported | Not reported | |
|
Wong 2019a ICHD (253) HHD (41) |
Mean duration of ESKD diagnosis (years) | 7.5 ± 4.9 | Not reported |
|
Wright 2015 ICHD (29) HHD (22) |
Time on dialysis (number of patients) | ||
| 6 to 12 months | 7 | 3 | |
| 1 to 5 years | 8 | 17 | |
| 5 to 10 years | 6 | 6 | |
| 10 to 20 years | 0 | 2 | |
| > 20 years | 1 | 1 | |
|
Watanabe 2014 ICHD (34) HHD (46) |
Time on dialysis (years) | 7.4 ± 8.3 | 6.4 ± 5.7 |
ESKD: end‐stage kidney disease; HHD: home haemodialysis; ICHD: in‐centre haemodialysis; IQR: interquartile range
Background
Description of the condition
Kidney failure is a common and increasingly prevalent public health problem, which results in excess morbidity, death and healthcare costs (Bello 2017a; Bello 2019). People with kidney failure require kidney replacement therapy (KRT; dialysis and kidney transplantation) to address the physiological accumulation of fluid and metabolites, which in turn may assist with reducing uraemic symptoms (such as fatigue, anorexia, nausea and pruritus), improving health‐related quality of life (HRQoL) and prolonging survival (Cabrera 2017). Unfortunately, some patients lack access to dialysis care, and although kidney transplantation is associated with increased survival and quality of life (QoL) compared with dialysis (Laupacis 1996; Tonelli 2011; Wolfe 1999), only 22% of all patients with treated kidney failure around the world receive kidney transplantation (Bello 2019). The remaining patients are treated with either haemodialysis (HD) or peritoneal dialysis (PD) (Bello 2017a; Bello 2019). The median survival of patients with kidney failure on dialysis is considerably shorter than for patients with common types of cancer (e.g. breast, colorectal, prostate) (Naylor 2019). Moreover, kidney failure results in substantial financial costs to the health system, accounting for 2% to 3% of healthcare spending in higher‐resource countries, despite patients with kidney failure comprising 0.1% to 0.2% of the population (Bello 2017b).
Ascertaining the optimal means of delivering dialysis in terms of patient‐reported, clinical and health‐economic outcomes is important information for patients, people who support their care, clinicians and healthcare policymakers.
Description of the intervention
HD is the most commonly used dialysis modality, comprising 89% of all dialysis and 69% of KRT globally (Pecoits‐Filho 2020). HD can be performed either in a centre (e.g. hospital or satellite dialysis units) or the patient’s own home. The prevalence of home HD (HHD) use varies widely worldwide, with 14% of prevalent dialysis patients doing HHD in Aotearoa, New Zealand, in 2019 (ANZDATA 2021). On the other hand, in the USA, only 1.9% of prevalent dialysis patients were doing HHD in 2019 (USRDS 2021). This is despite the fact that home‐based dialysis therapies have significant cost benefits compared to in‐centre HD (ICHD), with a Canadian study estimating the total annual cost of ICHD to be $73,920 after the first year, compared to $45,203 for HHD (Klarenbach 2014).
ICHD is usually performed by a trained nurse, who sets up the equipment, inserts the needles, monitors the patient, and adjusts treatment parameters as needed throughout the treatment. ICHD prescriptions typically involve four to five hours of dialysis three times/week (ANZDATA 2021).
Patients wishing to do HHD usually undertake one to four months of training, which covers machine set‐up and basic maintenance, needling of their fistula or graft, or accessing their dialysis vascular catheter, troubleshooting and managing alarms on dialysis (Kidney Health Australia 2013; USRDS 2021). In addition, home assessment and modifications may be required to confirm patients have a suitable space to perform dialysis and store supplies, as well as an adequate power and water supply. As most patients performing HD in their own homes do so independently or with the assistance of a family member or support person, this allows considerably greater flexibility in treatment duration and frequency compared with those undergoing ICHD, who must fit into more rigid schedules (Kidney Health Australia 2013). Patients receiving HHD may also utilise extended hours regimens, such as nocturnal (6 to 10 hours overnight), extended hours (6 to 10 hours/session), or short daily dialysis (< 4 hours/session, performed on a daily basis).
How the intervention might work
Epidemiological studies have indicated that HHD treatment may be associated with improved patient outcomes compared to ICHD. However, there is likely selection bias since patients receiving HHD tend to be younger and have fewer comorbidities (Mailloux 1996; Woods 1996). HHD has also been associated with increased patient autonomy and QoL (Cases 2011; Walsh 2005). Since HHD enables increased dialysis hours and frequency compared to ICHD, there are a number of potential treatment‐related benefits compared to conventional HD, including increased small solute clearance (typically measured as Kt/Vurea), improved control of serum phosphate and blood pressure (BP) (FHN Trial Group 2010), reduced myocardial stunning (Jefferies 2011), and shorter interdialytic intervals which may mitigate the heightened risk of death associated with long (three‐day) interdialytic intervals (Foley 2011; Krishnasamy 2013). Augmented dialysis duration and/or frequency can also assist with reducing ultrafiltration rate requirements, which in turn have been associated with reduced death (Assimon 2016). Thus, it is possible that HHD improves survival compared to ICHD, perhaps through a reduction in cardiovascular events.
On the other hand, HHD can result in an increased burden on patients, families and support people (Gilbertson 2019; Iyasere 2016; Morton 2010; Suri 2011). In addition, due to reduced clinical oversight of dialysis technique, patients performing HHD may be at increased risk of complications, such as infection and thrombosis (FHN Trial Group 2010; Suri 2013). These potential risks need to be weighed against the potential benefits of HHD.
Why it is important to do this review
HHD may increase survival and QoL and is less costly to healthcare systems than ICHD (Klarenbach 2014; Walker 2014). However, these potential benefits need to be considered against the potential disadvantages of HHD, which include increased burden and risk of complications. Evaluation of research comparing HHD and ICHD is limited, and a previous systematic review identified a single randomised controlled trial (RCT) (Palmer 2014). This updated version of that systematic review includes randomised and non‐randomised studies of interventions (NRSIs), using the available evidence to inform shared decision‐making by clinicians and patients regarding HD modality choice and its effects on patient‐reported, clinical and surrogate outcomes and adverse events.
Objectives
To evaluate the benefits and harms of HHD versus ICHD in adults with kidney failure.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) and NRSIs (observational studies with prospective and retrospective identification of participants, including registry studies) comparing HHD with ICHD in patients with kidney failure were eligible. In order for data to be from studies providing dialysis in a manner comparable to current practice, studies published from the year 2000 onwards were eligible (Marshall 2015).
Studies were required to include:
Comparison of HHD and ICHD between two or more groups of participants or within the same group of participants over time.
Groups of individuals formed by randomisation, quasi‐randomisation, time differences, location differences, or health professionals' or participants' preferences.
Identification of participants, assessment before intervention, actions/choices leading to an individual becoming a member of a group and assessment of outcomes carried out before or after the study was designed (prospective or retrospective design).
An assessment of the comparability between groups of potential confounders or not (e.g. non‐adjusted analyses).
Types of participants
Inclusion criteria
Adults (≥ 18 years) with kidney failure receiving ICHD or HHD
Incident and prevalent HD patients
HD patients previously treated with PD
Patients previously treated with kidney transplantation.
Exclusion criteria
The review did not include data obtained from children or patients with acute kidney injury, as these patients are rarely treated with HHD due to anticipated recovery of kidney function.
Types of interventions
HD provided using any dialysis machine, dialysate, blood or dialysate flow rate, membrane type, dialysis dose (urea clearance), or vascular access type (central venous catheter (CVC), arteriovenous fistula (AVF) or arteriovenous graft (AVG)) was included. We included studies with any duration of dialysis and any frequency in either treatment arm.
HHD was defined as any type of HD, haemodiafiltration, or haemofiltration carried out by the patient or caregiver at home.
HHD included HD performed independently by patients (without the assistance of nursing or technical staff) in a community home or self‐care unit.
ICHD included HD provided in a hospital unit, a private dialysis unit, or a satellite dialysis unit in which nursing or technical staff provided dialysis care. Patients provided with HD by nursing or technical staff in their own homes were considered ICHD.
Studies evaluating PD as a home dialysis modality were excluded, except where data regarding participants receiving ICHD and HHD were disaggregated.
Types of outcome measures
Primary outcomes
Cardiovascular death: fatal myocardial infarction (MI), fatal stroke, sudden death, heart failure.
Secondary outcomes
Clinical outcomes
All‐cause death
Non‐fatal MI
Non‐fatal stroke
All‐cause hospitalisation: number of patients with one or more hospitalisation events
Kidney transplantation
-
Vascular access events
AVF/AVG intervention: surgical revision, thrombolysis/thrombectomy, fistulogram/fistuloplasty
CVC insertion/exchange
-
Infection
Local infection: exit‐site infection, cellulitis, abscess/collection
Systemic infection: bacteraemia
Parathyroidectomy
Patient‐reported outcomes
QoL: we considered and tabulated, where necessary, all reports of QoL outcomes using any instrument. Meta‐analyses were conducted when sufficient studies reported QoL outcomes using a single instrument, including measures of depression and household financial stress.
End‐of‐treatment employment status: employed, unemployed, not eligible for employment
Symptoms related to dialysis therapy: intradialytic cramping, hypotension, nausea, vomiting, headache
Fatigue
Recovery time
Health economics outcomes
Cost‐effectiveness
Surrogate outcomes
BP (systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), pulse pressure measured in pre‐dialysis or other setting) (mm Hg)
Left ventricular mass (LVM): described using any diagnostic tool, including magnetic resonance imaging or echocardiography (g; g/m²)
Search methods for identification of studies
Electronic searches
For this update, we searched the Cochrane Kidney and Transplant Register of Studies up to 7 October 2022 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of hand‐searched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website under CKT Register of Studies.
For non‐randomised studies, we searched MEDLINE (OVID) 1 January 2000 to 20 July 2020 and EMBASE (OVID) 1 January 2000 to 20 July 2020.
See Appendix 1 for search terms used in strategies for this and the previous review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Contacting relevant individuals or organisations seeking information about unpublished or incomplete studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The search was performed unrestricted by language; non‐English articles were translated before assessment. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews that might have included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary, the full text of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Any further information required from the original author(s) was requested by written correspondence, and any relevant information obtained in this manner was included in the review. Studies reported in non‐English language journals were translated before assessment. Where more than one publication from one study existed, reports were grouped together, and the publication with the most complete data was used in the analyses (Higgins 2022). Where multiple publications reported the same outcome in the same or overlapping populations, or where we could not be certain that this did not occur, the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancies between published versions were highlighted. Disagreements were resolved by consultation with the authors.
Assessment of risk of bias in included studies
Randomised controlled trials
The following items were independently assessed by two authors using the Risk of Bias assessment tool version 2 (RoB 2) (Higgins 2022). The domains included in the assessment were as follows.
Bias arising from the randomisation process
Bias due to deviations from intended interventions
Bias due to missing outcome data
Bias in the measurement of the outcome
Bias in the selection of the reported result.
Non‐randomised studies of interventions
The Newcastle‐Ottawa Scale (NOS) (www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf) for assessing the quality of NRSIs was used. The NOS was used to adjudicate the risk of bias in non‐randomised studies. For cohort studies, the NOS used a star scoring system based on the selection of study groups (four items), comparability between the study group and the control group (two items) and the ascertainment of the exposure or outcome of interest (three items), for a total maximum score of nine stars (Appendix 2). An adapted NOS was used for studies using a cross‐sectional design (Herzog 2013). Selection (maximum five stars), comparability of cohorts based on the design or analysis (maximum two stars) and outcome (maximum two stars) were evaluated for a total maximum score of 10 stars (Appendix 3).
-
For case‐control studies, the following items were evaluated.
Selection: adequacy of definition, representativeness of the cases, selection of controls, definition of controls
Comparability: comparability of cases and controls based on the design or analysis
Exposure: ascertainment of exposure, same method of ascertainment for cases and controls, non‐response rate
-
For cohort studies, the following items were evaluated.
Selection: representativeness of the exposed cohort, selection of the non‐exposed cohort, ascertainment of exposure, demonstration that outcome of interest was not present at the start of the study
Comparability: comparability of cohorts based on the design or analysis
Outcome: assessment of outcome, adequacy of follow‐up and duration of follow‐up
-
For cross‐sectional studies, an adapted NOS was used (Herzog 2013). The following items were evaluated.
Selection: representativeness of the sample, sample size, comparability of non‐respondents, ascertainment of the exposure
Comparability: comparability of cohorts based on the design or analysis
Outcome: assessment of outcome, suitability of statistical testing.
Measures of treatment effect
For dichotomous outcomes (e.g. death, cardiovascular events, hospitalisation, vascular access adverse events), results were expressed as a risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. QoL scale, BP, doses of medication, haemoglobin, biochemical variables), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used. For effect measures reported as means and standard errors (SE), we obtained the standard deviation (SD) using the calculation provided in the Cochrane Handbook (Higgins 2022). For effect measures reported as medians, ranges and/or interquartile ranges (IQR), we estimated means and SD using the approach described by Wan and colleagues (Wan 2014). Outcomes from RCTs and NRSIs were reported separately.
Meta‐analysis of change scores
Where data on both change‐from‐baseline and final value scores existed, we planned to combine data (e.g. LVM, BP) in a meta‐analysis using the (unstandardised) MD method (Higgins 2022). End‐of‐treatment values and change‐from‐baseline scores were placed in subgroups for clarity and summarised using random effects meta‐analysis.
Imputing standard deviation
When none of the above methods allowed calculation of the SD, we imputed change‐from‐baseline SD using an imputed correlation coefficient when sufficient data were available (Abrams 2005; Follmann 1992). If possible, we conducted sensitivity analyses to evaluate the effect of imputing missing SD data in our meta‐analysis.
Unit of analysis issues
We included only data from the first period of treatment in cross‐over studies (Higgins 2022). Data in different metrics were analysed by converting reported values to International System (SI) units. The final results were presented in SI units with conventional units in parentheses.
Dealing with missing data
If possible, data for each prespecified outcome were evaluated regardless of whether the analysis was based on intention‐to‐treat (ITT) or completeness of follow‐up. In particular, dropout rates were investigated and reported in detail (e.g. dropout due to discontinuation of dialysis modality, treatment failure, death, transplantation, withdrawal of consent or loss to follow‐up). Any further information required from the original author was requested by written correspondence (e.g. emailing the corresponding author). Any relevant information obtained in this manner was included in the review. We assessed all studies for risks of bias due to incomplete reporting of results. Evaluation of important numerical data, such as screened, randomised patients, as well as ITT, as‐treated and per‐protocol population was carefully performed. Attrition rates were investigated. Issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward) were critically appraised.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies due to heterogeneity rather than sampling error (Higgins 2022). The following is a guide to the interpretation of I² values.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depended on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P value from the Chi² test or a CI for I²) (Higgins 2022).
Assessment of reporting biases
When there were at least 10 studies included in the meta‐analyses (Higgins 2022), we planned to test for asymmetries in the inverted funnel plots (i.e. for systematic differences in the effect sizes between more precise and less precise studies) using the original and modified Egger tests (Egger 1997) and the Begg and Mazumdar correlation test (Begg 1994). There are many potential explanations for why an inverted funnel plot may be asymmetric, including chance, heterogeneity, publication and reporting bias (Terrin 2005). We planned to refrain from judging funnel plot asymmetries based on visual inspection, as this has been shown to be misleading in empirical research (Lau 2006). Since our meta‐analyses did not include at least 10 studies for any of the outcomes evaluated, funnel plot asymmetries were not assessed.
Publication bias was also evaluated by testing the robustness of the results according to publications, namely publication as a full manuscript in a peer‐reviewed journal versus studies published as abstracts, letters or editorials.
Data synthesis
Data were summarised using the random‐effects model, but the fixed‐effect model was also used to ensure the robustness of the chosen model and susceptibility to outliers. We qualitatively summarised data where insufficient data were available for meta‐analysis. A qualitative review was conducted for adverse events and QoL outcomes in studies where a validated tool or metric was not used.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to explore possible sources of heterogeneity (e.g. participants, interventions, and study quality). Heterogeneity among participants could be related to age and HD methods. Heterogeneity in treatments could be related to prior agent(s) used and the agent, dose, and duration of therapy.
Heterogeneity was planned to be investigated by analysing the data using subgroups according to the following parameters.
Age (< 60 years versus ≥ 60 years)
Presence of diabetes
Presence of cardiovascular disease
Study design (RCT versus NRSI)
Methodological quality.
However, subgroup analyses were not done due to the small number of studies and insufficient data available.
Adverse effects were tabulated and assessed with descriptive techniques. Where possible, the risk difference (RD) with 95% CI was calculated for each adverse effect, either compared to no treatment or another agent.
Sensitivity analysis
Sensitivity analyses were done to explore the robustness of findings to key decisions in the review process. These were determined as the review process took place (Higgins 2022). Sensitivity analyses were undertaken to explore the influence of a study's risk of bias on the results.
Repeating the analysis, excluding unpublished studies
Repeating the analysis, taking account of the risk of bias, as specified above
Repeating the analysis, excluding any very long or large studies to establish how much they dominate the results
Repeat the analysis, excluding studies, using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in Summary of findings tables. These tables present key information concerning the certainty of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2022a). The Summary of findings tables also include an overall grading of the evidence related to each main outcome using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach (GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2022b). We initially intended to present vascular access complications and fatigue in the summary of findings. However, there was insufficient data to meta‐analyse these outcomes. We therefore presented the following outcomes in the 'Summary of findings' tables.
Cardiovascular death
All‐cause death
All‐cause hospitalisation
Kidney transplantation
HRQoL
Recovery time
BP.
Results
Description of studies
The following section contains broad descriptions of the studies considered in this review. For further details on each individual study, please see Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
The search of databases and registers was conducted on 7 October 2022 and identified 1280 records; an additional 34 records were identified through other sources. After duplicate records were removed, 1305 studies were screened, and 144 records were selected for full‐text review. Of these, 39 new studies were included (81 records), 34 new studies were excluded (48 records), and 14 studies (14 records) are awaiting classification (see Characteristics of studies awaiting classification). We also identified one new report of an existing excluded study.
We reassessed and deleted 12 previously excluded studies (wrong study design, not HHD versus ICHD, no outcomes of interest).
A total of 40 studies were included (82 reports; 1 RCT, 39 NRSIs), 35 studies were excluded (60 reports), and 14 studies are awaiting classification. The search results are summarised in (Figure 1).
1.
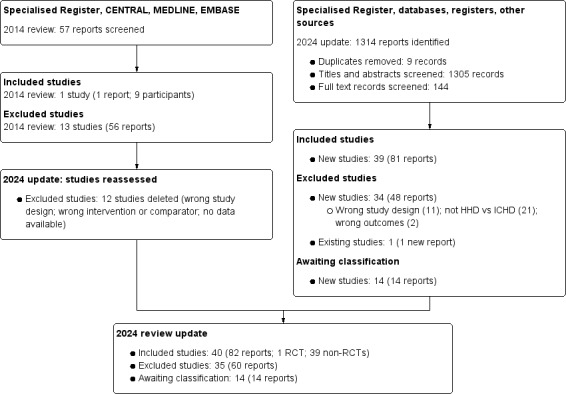
2024 flow diagram for study selection
Included studies
One RCT, already included in the previous version of this systematic review, was included (McGregor 2001). The search did not find any additional RCTs. The search yielded 81 reports of 39 eligible NRSIs. Three of these studies were published as conference abstracts, with no related full article published (Bragg‐Gresham 2018; Dumaine 2018; Ha 2018). Included studies were published between 2001 and 2022, with half of the studies being published from 2015 onwards.
Various study designs were seen across the included studies. Many were small single‐centre prospective studies, cross‐sectional studies and large retrospective registry studies. Details on the design, setting and size of studies are shown in Table 3.
2. Design, setting and size of included studies.
| Study ID | Study setting | Study size |
| Ageborg 2005 | Prospective cohort study Cross‐sectional Stockholm (Sweden) |
HHD (5) Self‐care HD (6) Conventional ICHD (8) |
| Bragg‐Gresham 2018 | Retrospective cohort study USRDS – data available in the Medical Evidence Report 2006‐2015 |
Incident dialysis patients (156,377) |
| Dumaine 2018 | Prospective, mixed‐methods, pilot study Comparing transitions (from CKD to initiating dialysis or transition from one dialysis modality to another) |
CKD to ICHD (5) CKD to PD (9) CKD to HHD (2) PD to ICHD (2) PD to HHD (4) ICHD to PD (4) ICHD to HHD (7) |
| Griva 2010 | Cross‐sectional 2 dialysis units (affiliated with Royal Free and University College Hospitals), London (UK) |
HHD (25) ICHD (52) CAPD (45) APD (23) |
| Ha 2018 | Single‐centre, prospective cohort study Cross‐sectional Sydney (Australia) From 2015‐2017 |
HHD (27) PD (48) ICHD (113) Transplant recipients (85) |
| Hayhurst 2015 | Cross‐sectional Single‐centre, Royal Preston Hospital (UK) May‐June 2014 |
CKD stage 3‐5 (17) ICHD (28) HHD (17) PD (17) Transplant recipients (21) Controls: matched by age and sex (50) |
| Jayanti 2016 | Prospective study (BASIC‐HHD study); combined cross‐sectional and prospective study design Multi‐centre across 5 tertiary centres (UK) |
HHD (91) Prevalent ICHD (197) |
| Kasza 2016 | Observational cohort study ANZDATA Registry (Australia & New Zealand) 1 Oct 2003‐31 Dec 2011 |
ICHD CVC (7414) ICHD AVF (5729) HHD AVF (357) PD (6665) HHD CVC: not included in analysis (26) |
| Kojima 2012 | Observational cohort study, retrospectively collected data Single‐centre (Kidney Disease Center, Saitama Medical University, Japan) |
Patients transitioned from ICHD to HHD (54) |
| Krahn 2019 | Population‐based retrospective cohort study à linked registry (CORR) and administrative data Ontario (Canada) 1 April 2006 to 31 March 2014 |
HHD (112) ICHD (9687) short‐daily/slow nocturnal ICHD (65) PD (2827) |
| Kraus 2007 | Prospective, multicenter, open‐label, feasibility study 6 USA centres |
Patients transitioning from ICHD to HHD with NxStage System One (32) |
| Krishnasamy 2013 | Observational cohort study ANZDATA Registry (Australia & New Zealand) 1999‐2008 |
ICHD (9765) PD (4298) HHD (573) |
| Lee 2002 | Prospective cohort study South Alberta Renal Program (Calgary, Canada) 1999‐2000 |
HHD (9) ICHD (88) Satellite ICHD (31) PD (38) |
| Lorenzen 2012 | Retrospective, longitudinal, single‐centre study Kuratorium für Dialyse und Nierentransplantation (Hannover, Germany) |
Patients on maintenance dialysis transferring from ICHD to short daily HHD (11) |
| Malmstrom 2008 | Cross‐sectional (15 Oct 2004) and retrospective (year 2004) Single‐centre (Helsinki University Hospital), Helsinki (Finland) |
HHD (33) Satellite ICHD (32) |
| McGregor 2001 | Randomised crossover trial Single‐centre, New Zealand |
HHD patients assigned to receive short in‐centre HD and long HHD in a randomised sequence (9) |
| Murashima 2010 | Retrospective cohort study Single‐centre |
Patients converted from CHD to HHD (12) |
| Nebel 2002 | Retrospective cohort study Single‐centre, Cologne‐Merheim Hospital (Germany) 1990‐1999 |
HHD (37) Satellite ICHD (66) PD (69) Transplant recipients (72) |
| Nesrallah 2012 | Multinational renal databases: International Quotidian Dialysis Registry (IQDR) [intensive] + DOPPS [conventional] Secondary IQDR data from REIN registry (France), Fresenius Medical Care North America, PROMIS database (British Columbia, Canada) France, USA, Canada Between Jan 2000 and Aug 2010 |
Intensive HD (420) Conventional HD (5646) Matched patients by country, duration of ESKD before study enrolment and propensity score Intensive HD (338) Conventional HD (1388) |
| Nitsch 2011 | UK Renal registry England and Wales 1 Jan 1997‐31 Dec 2005 |
Incident HHD (225) Incident PD: matched by age and sex (900) Incident hospital ICHD: matched by age and sex (900) Incident satellite ICHD: matched by age and sex (450) |
| Piccoli 2004 | Prospective cohort study Single‐centre SMOM Unit (satellite of a large university centre) Turin (Italy) Nov 1998‐Nov 2002 |
HHD (at home or in training) (42) Limited care ICHD (35) |
| Rydell 2016 | Retrospective, observational case‐control study HHD: Lund University Hospital from 1 Jan 1983 to 31 Dec 2002 ICHD: Malmo General Hospital from 1 Jan 1978 to 31 Dec 2007 (Sweden) Data on dialysis from Swedish Renal Registry; survival data from Swedish Census |
Matched according to sex, age, comorbidity and date of start HHD (41 from 118) IHD (41 from 377) Followed until death or 1 Jan 2013; median follow up duration 14 years (HHD) and 11 years (IHD) |
| Sands 2009 | Retrospective study Fresenius Medical Services facilities (USA) 1 Nov 2006‐12 March 2007 |
Patients who transitioned from ICHD to HHD (29) |
| Saner 2005 | Nested case‐cohort study; retrospective chart analysis – For each patient trained for HHD at the dialysis centre between 1970 and 1995 corresponding match searched from ICHD by retrospective chart analysis Single‐centre (University Hospital of Berne), Bern (Switzerland) From 1970 to 1995 |
HHD (58) ICHD: matched for sex, age, time of dialysis onset and renal disease category (58) |
| Suri 2015 | Observational retrospective cohort study HHD patients from a large US dialysis provider’s administrative database – propensity score matched to contemporaneous USRDS patients Jan 2004‐Dec 2009 |
All adults who began DHD between 2004‐2009 HHD (1187) ICHD patients from USRDS (3173) |
| Tennankore 2022 | Canadian Organ Replacement Registry (CORR) analysis 2005‐2014 |
|
| Van Oosten 2018 | National data (Netherlands) Health insurance claims data from 2012‐2014 Data validated with external database (Dutch Renal Registry – Renine) |
HHD (197) ICHD (6463) CAPD (463) APD (477) Mix (281) Transplant recipients: living donor (1554) Transplant recipients: deceased donor (1275) |
| Watanabe 2014 | Prospective cohort study Cross‐sectional Single‐centre (Saitama Medical University Hospital), Saitama (Japan) 2011 |
HHD (46) ICHD: matched for age, sex, cause of ESKD (34) |
| Wong 2019a | Cross‐sectional data of ESKD patients pooled from the following studies:
Hong Kong *Chen JY, Choi EPH, Wan EYF et al. Validation of the disease‐specific components of the kidney disease quality of Life‐36 (KDQOL‐36) in Chinese patients undergoing maintenance dialysis. PLoS One 2016;11: e0155188. |
NHHD (41) PD (103) HICHD (135) CICHD (118) |
| Wong 2019b | Multi‐centre 3 public hospitals in Hong Kong Information from medical records + face‐to‐face interview with patients between May 2016 and Oct 2016 |
HHD (43) ICHD (170) PD (189) |
| Wright 2015 | Cross‐sectional Pilot study 4 outpatient dialysis facilities located in Pennsylvania (USA) |
HHD (22) ICHD (29) PD (26) |
| Xue 2015 | Retrospective cohort study HHD: Virginia Lynchburg Dialysis Facility (USA) from 1997 to 2010 ICHD: Fresenius Medical Care North America facilities in Virginia (USA) from 1 Jan 2007 to 31 Dec 2010 |
HHD (63) ICHD: matched by age, gender, race, dialysis vintage, diabetes (121) |
| Yeung 2021 | Prospective cohort study HHD: Single‐centre, Monash Medical Centre, Melbourne (Australia) ICHD: ANZDATA Registry Jan 2000‐June 2017 |
HHD (181) ICHD: matched by age, gender and cause of ESKD (413) |
| Zimbudzi 2014 | Retrospective cohort study Single‐centre, Monash Medical Centre, Melbourne (Australia) Aug 2012‐Aug 2013 |
HHD (25) ICHD: satellite HD (25) |
Note: refer to Table 4 for characteristics studies containing grouped reports.
AVF: arteriovenous fistula; AVG: arteriovenous graft; CKD: chronic kidney disease; CVC: central venous catheter; ESKD: end‐stage kidney disease; HD: haemodialysis; HHD: home haemodialysis; ICHD: in‐centre haemodialysis; PD: peritoneal dialysis
In some cases, there were multiple publications generated from the same trial or study, which we have grouped together (Kjellstrand 2008; Rydell 2019; Tablo IDE 2020). In other cases, there were multiple publications which were generated from different studies but were performed within the same or overlapping populations. Marshall 2021 conducted multiple registry studies based on the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry and reporting on death. Populations included in these studies were overlapping over the period from 1996 to 2017. NxStage‐USRDS 2012 compared the HHD patients registry of NxStage System One users (NxStage Medical Inc) to matched patients from the United States Renal Data System (USRDS) over the 2006 to 2012 period. Finally, there were instances of multiple studies being performed within the same clinical dialysis program over the same or overlapping time periods. Many studies comparing HHD to ICHD were conducted at the University Health Network (UHN) in Toronto, Canada, from 1993 to 2009. As we could not be certain that the populations included in these studies were not overlapping, all studies involving the UHN HHD unit were grouped as Toronto Group 2002. In the aforementioned situations of study grouping, data were extracted only from publications which reported outcomes relevant to this review. Where more than one publication from a study group reported a relevant outcome, the publication with the most complete data was used for analyses. Details on the design, setting and size of reports within study groups are shown in Table 4.
3. Characteristics of grouped reports within studies.
| Study/report | Study design and setting | Population | Study period | Outcomes |
| Kjellstrand 2008 | ||||
| Kjellstrand 2008* | Observational retrospective cohort study 5 centres from USA and Europe |
HHD or self‐care HD (265) ICHD (150) |
1982 to Jun 2005 | Survival (%) |
| Kjellstrand 2010 | Observational retrospective cohort study 5 centres from USA and Europe |
HHD (189) ICHD (73) |
1982 to Jun 2005 | Survival (%) |
| Marshall 2021 | ||||
| Marshall 2011 | Observational retrospective cohort study ANZDATA Registry |
HHD (3190) ICHD (21,968) |
31 Mar 1996 to 31 Dec 2007 | Death (HR for death) |
| Marshall 2013 | Observational retrospective cohort study ANZDATA Registry (NZ only) |
HHD (1532) ICHD (5647) |
31 Mar 2000 to 31 Dec 2010 | Death (HR for death) |
| Marshall 2014 | Observational retrospective cohort study ANZDATA Registry (NZ only) |
HHD (1547) ICHD (8713) |
1 Jan 1997 to 31 Dec 2011 | Death (HR for death) |
| Marshall 2016 | Observational retrospective cohort study ANZDATA Registry |
HHD (5764) ICHD (34,952) |
31 Mar 1996 to 31 Dec 2012 | Death (HR for death) |
| Marshall 2021* | Observational retrospective cohort study ANZDATA Registry |
Complete cohort: 52097 Modality comparison cohort: HHD (1236), ICHD (29,548) |
Complete cohort: 1998 to 2017 Modality comparison cohort: 2013 to 2017 |
Death (HR for death) Proportion cardiovascular death as cause of death |
| NxStage‐USRDS 2012 | ||||
| Kansal 2019 | Observational retrospective cohort study NxStage Medical user registry linked to USRDS (USA) |
HHD: NxStage (521) ICHD: USRDS (32,931) |
2006 to 2012 | Survival (%) Death (HR for death) |
| Weinhandl 2012* | Observational retrospective cohort study NxStage Medical user registry linked to USRDS (USA) |
HHD: NxStage (1873) ICHD: USRDS (9365) |
1 Jan 2005 to 31 Dec 2007 | All‐cause death (HR for death) Cardiovascular death (HR for death) Infection death (HR for death) Interval‐specific death (HR for death) |
| Weinhandl 2015a* | Observational retrospective cohort study NxStage Medical user registry linked to USRDS (USA) |
HHD: NxStage (3480) ICHD: USRDS (17,400) |
1 Jan 2006 to 31 Dec 2009 | All‐cause hospital admissions (RR) Cardiovascular hospital admissions (RR) Vascular hospital admissions (RR) All‐cause hospital duration (RR) Cardiovascular hospital duration (RR) Vascular hospital duration (RR) |
| Weinhandl 2015b | Observational retrospective cohort study NxStage Medical user registry linked to USRDS (USA) |
HHD: NxStage (834) ICHD: USRDS (4170) |
1 Jan 2007 to 30 Jun 2010 | Death (HR for death) Cardiovascular death (HR for death) Infection death (HR for death) |
| Weinhandl 2015c | Observational retrospective cohort study NxStage Medical user registry linked to USRDS (USA) |
HHD: NxStage (3560) ICHD: USRDS (17,800) |
1 Jan 2007 to 30 Jun 2010 | 30‐day readmission after discharge for heart failure 30‐day readmission after discharge for hypertension |
| Weinhandl 2015d* | Observational retrospective cohort study NxStage Medical user registry linked to USRDS (USA) |
HHD: NxStage (1368) ICHD: USRDS (6840) |
1 Jan 2007 to 30 Jun 2010 | Relative incidence of transplant |
| Rydell 2019 | ||||
| Rydell 2019a* | Observational retrospective cohort study Swedish Renal Registry, Swedish Inpatient Registry and Swedish Mortality Database |
HHD (152) ICHD (608) |
1991 to 2012 | All‐cause death Median survival (years) |
| Rydell 2019b* | Observational retrospective cohort study Swedish Renal Registry, Swedish Inpatient Registry and Swedish Mortality Database |
HHD (152) ICHD (608) |
1991 to 2012 | All‐cause annual hospital admission rate All‐cause hospitalization days per patient‐year |
| Tablo IDE 2020 | ||||
| Chertow 2020* | Prospective cross‐over study Multicentre (USA) |
Cross‐over design (30) | Not specified | Recovery time (hours) EQ‐5D‐5L Sleep duration (hours) |
| Aragon 2020 | Awaiting classification: contact made with author | |||
| Chahal 2020a | Awaiting classification: contact made with author | |||
| Chahal 2020b | Awaiting classification: contact made with author | |||
| Plumb 2020 | Awaiting classification: contact made with author | |||
| Plumb 2021 | Awaiting classification: contact made with author | |||
| Toronto Group 2002 | ||||
| Bergman 2008* | Controlled cohort study Toronto (Canada) |
HHD (32) ICHD (42) |
1993 to 2003 | Dialysis or cardiovascular‐related admission (per patient year) Duration of dialysis or cardiovascular‐related admission (days per year) All‐cause hospitalisation (per patient year) Duration of all‐cause hospitalisation (days per year) Emergency department visits (per patient year) |
| Bugeja 2004 | Prospective observational cohort study University Health Network, Toronto (Canada) |
Cross‐over design (11) | Not specified | Systolic BP Diastolic BP |
| Cafazzo 2009 | Cross‐sectional survey of prevalent patients Toronto (Canada) |
HHD (56) ICHD (153) |
Not specified | Appraisal of Self‐Care Agency (ASA scale) SF‐12 (Mental Component Summary, Physical Component Summary) Multidimensional Scale of Perceived Social Support Anxiety State (Spielberger) Anxiety Trait (Spielberger) |
| Chan 2002 | Prospective observational cohort study University Health Network, Toronto (Canada) |
Cross‐over design (6) | Since Oct 1997; no end date specified | Mean systolic BP Mean diastolic BP LVMI (g/m2) |
| Chan 2003 | Prospective observational cohort study University Health Network, Toronto (Canada) |
Cross‐over design (18) | Not specified | Systolic BP Diastolic BP 24‐hour systolic BP 24‐hour diastolic BP |
| Chan 2005* | Cohort study Cross‐sectional University Health Network, Toronto (Canada) |
HHD (10) ICHD (12) |
Not specified | Systolic BP Diastolic BP Mean BP LVMI (g/m2) |
| Chan 2005a | Cohort study University Health Network, Toronto (Canada) |
Cross‐over design (10) | Not specified | Systolic BP Diastolic BP |
*outcome data utilised in analyses
BP: blood pressure; HHD: home haemodialysis; HR: hazard ratio; ICHD: in‐centre haemodialysis; LVMI: left ventricular mass index; RR: risk ratio
Intervention groups
Most compared groups were parallel, while a few studies used a non‐randomised, single‐cross‐over design in which each participant served as their own control in situations where patients were transitioning from one modality to another (Kojima 2012; Lorenzen 2012; McGregor 2001; Murashima 2010; Sands 2009; Tablo IDE 2020). While all studies compared some form of HHD to ICHD, some studies specified additional subgroups based on treatment duration/timing (e.g. intensive, conventional, nocturnal, daily), setting (e.g. satellite centre, community house), staff involvement (e.g. self‐care, limited care) or vascular access (CVC versus AVF/AVG). Many studies did not specify the treatment duration or did not define “intensive” or “conventional” duration. Additionally, some studies included other comparison groups (e.g. people treated with PD, recipients of a kidney transplant, people with earlier stages of chronic kidney disease (CKD), and healthy controls). Details on intervention groups across studies are provided in Table 5.
4. Detailed intervention comparisons by study.
| Study ID/report | HHD | ICHD | Other comparisons |
| Ageborg 2005 | HHD (NS) | Self‐care ICHD ICHD: conventional |
‐ |
| Bragg‐Gresham 2018 | HHD (NS) | ICHD (NS) Self‐care ICHD (NS) |
CAPD CCPD |
| Dumaine 2018 | Transition from ICHD to HHD | ‐ | Transition from CKD to ICHD Transition from CKD to PD Transition from CKD to HHD Transition from PD to IHD Transition from PD to HHD Transition from ICHD to PD |
| Griva 2010 | HHD: conventional; 3 times/week) | ICHD (NS) | CAPD APD |
| Ha 2018 | HHD (NS) | ICHD (NS) | PD Transplant recipient |
| Hayhurst 2015 | HHD (NS) | ICHD (NS) | PD CKD stages 3‐5 Transplant recipients Controls (healthy) |
| Jayanti 2016 | Intensive HHD: 30.8% conventional HHD; variable dialysis prescriptions | ICHD: conventional; 12 hours/week; 54% HDF | ‐ |
| Kasza 2016 | HHD: AVF/AVG | ICHD: CVC ICHD: AVF/AVG |
PD |
| Kjellstrand 2008 | Intensive HHD: short daily | Intensive ICHD: short daily | Survival probabilities derived from 2005 USRDS incident HD population |
| Kojima 2012 | Intensive HHD | ICHD: conventional | ‐ |
| Krahn 2019 | HHD: short daily (2 to 3 hours; 6 to 7 times/week (awake)) or slow nocturnal (6 to 9 hours; 5 to 7 times/week) Canadian Organ Replacement Register |
ICHD: conventional Intensive ICHD: short daily (2 to 3 hours; 6 to 7 times/week (awake)) or slow nocturnal (6 to 9 hours; 5 to 7 time/week Canadian Organ Replacement Register |
PD (CAPD or APD) |
| Kraus 2007 | Intensive HHD: 2 to 3 hours; 6 times/week (NxStage System One) | Intensive ICHD: 2 to 3 hours; 6 times/week (NxStage System One) | ‐ |
| Krishnasamy 2013 | HHD: mix of intensive and conventional | ICHD: 97% conventional | PD |
| Lee 2002 | HHD/self‐care: ≥ 4 hours/session; ≥ 3 times/week | ICHD: ≥ 4 hours/session; ≥ 3 times/week Satellite HD: ≥ 4 hours/session; ≥ 3 times/week |
PD (CAPD and APD) |
| Lorenzen 2012 | Intensive HHD: short daily | ICHD: conventional | ‐ |
| Malmstrom 2008 | HHD: flexible schedule and length of dialysis | Self‐care satellite HD: 3 times/week | ‐ |
| Marshall 2021 / Marshall 2011 | HHD: conventional Intensive HHD: frequent/extended; > 3 sessions of ≥ 4 hours or 3 sessions of > 6 hours or 5 sessions of ≥ 3 hours or > 5 sessions of ≥ 2 hours per week |
ICHD: conventional Intensive ICHD: frequent/extended; > 3 sessions of ≥ 4 hours or 3 sessions of > 6 hours or 5 sessions of ≥ 3 hours or > 5 sessions of ≥ 2 hours per week |
PD |
| Marshall 2021 / Marshall 2013 | HHD (NS) | ICHD (NS) | Community house HD PD |
| Marshall 2021 / Marshall 2014 | HHD (NS) | ICHD (NS) | PD |
| Marshall 2021 / Marshall 2016 | Intensive HHD: any hours/session; ≥ 5 times/week Quasi‐intensive HHD: between conventional and intensive HHD: conventional; ≤ 3 times /week; ≤ 6 hours/session |
Intensive ICHD: any hours/session; ≥ 5 times/week Quasi‐intensive ICHD: between conventional and intensive ICHD: conventional; ≤ 3 times /week; ≤ 6 hours/session |
PD Deceased donor transplant recipient Living donor transplant recipient |
| Marshall 2021 / Marshall 2021 | HHD (NS) | ICHD (NS) | CAPD APD |
| McGregor 2001 | HHD: 6 to 8 hours/session; 3 times/week | ICHD: 3.5 to 4.5 hours/session; 3 times/week | ‐ |
| Murashima 2010 | Intensive HHD: short daily (NxStage System One) | ICHD: 3 times/week) | ‐ |
| Nebel 2002 | HHD (NS) | Satellite HD (NS) | PD (CAPD and APD) Kidney transplant recipient Inpatient acute ICHD (reported but not analysed due to low numbers) |
| Nesrallah 2012 | Intensive HHD: ≥ 5.5 hours/session; 3 to 7 times/week; day or nocturnal International Quotidian Dialysis Registry (none with NxStage) |
ICHD: conventional; < 5.5 hours/session; 3 times/week | ‐ |
| Nitsch 2011 | HHD (NS) | ICHD (NS) Satellite HD (NS) |
PD |
| NxStage‐USRDS 2012 / Kansal 2019 | Intensive HHD: previously receiving PD; 92% prescribed 5 to 6 times/week | ICHD: previously receiving PD (USRDS) | ‐ |
| NxStage‐USRDS 2012 / Weinhandl 2012 | Intensive HHD: daily (NxStage System One) | ICHD: conventional; 3 times/week (USRDS) | ‐ |
| NxStage‐USRDS 2012 / Weinhandl 2015a | Intensive HHD: daily; 5 to 6 times/week (NxStage System One) | ICHD: conventional; 3 times/week | ‐ |
| NxStage‐USRDS 2012 / Weinhandl 2015b | Intensive HHD: daily (NxStage System One) | ICHD: conventional (USRDS) | ‐ |
| NxStage‐USRDS 2012 / Weinhandl 2015c | Intensive HHD: daily (NxStage System One) | ICHD: conventional (USRDS) | PD |
| NxStage‐USRDS 2012 / Weinhandl 2015d | Intensive HHD: daily (NxStage System One) | ICHD: conventional (USRDS) | PD |
| Piccoli 2004 | HHD: not daily Intensive HHD: daily) |
Limited care ICHD: not daily Intensive limited care ICHD: daily |
‐ |
| Rydell 2016 | HHD (NS) | ICHD (NS) | ‐ |
| Rydell 2019 / Rydell 2019a | HHD (NS) | ICHD (NS) | PD |
| Rydell 2019 / Rydell 2019b | HHD (NS) | ICHD (NS) | PD |
| Sands 2009 | HHD (NS) | ICHD (NS) | ‐ |
| Saner 2005 | HHD (NS): same prescription at home then in‐centre | ICHD (NS) same prescription at home then in‐centre | ‐ |
| Suri 2015 | Intensive HHD: daily; 1.5 to 4.5 hours/session; > 5 times/week | ICHD: conventional | PD |
| Tablo IDE 2020 / Chertow 2020 | Intensive HHD: 4 sessions/week | Intensive ICHD: 4 sessions/week | ‐ |
| Tennankore 2022 | HHD Intensive HHD: frequent |
ICHD Intensive ICHD: frequent |
PD |
| Toronto Group 2002 / Bergman 2008 | Intensive HHD: nocturnal | ICHD: conventional | ‐ |
| Toronto Group 2002 / Bugeja 2004 | Intensive HHD: nocturnal; 6 to 8 hours/session; 5 to 6 times/week) | ICHD: conventional; 4 hours/session; 3 t imes/week | ‐ |
| Toronto Group 2002 / Cafazzo 2009 | Intensive HHD: nocturnal; 6 to 8 hours/session; 4 to 6 times/week | ICHD: conventional; 4 hours/session; 3 times/week | Predialysis included in qualitative interviews |
| Toronto Group 2002 / Chan 2002 | Intensive HHD: nocturnal | ICHD: conventional | ‐ |
| Toronto Group 2002 / Chan 2003 | Intensive HHD: nocturnal; 8 to 10 hours/session; 6 times/week) | ICHD: conventional; 4 hours/session; 3 times/week | ‐ |
| Toronto Group 2002 / Chan 2005 | Intensive HHD: nocturnal | ICHD: conventional | ‐ |
| Toronto Group 2002 / Chan 2005a | Intensive HHD: nocturnal | ICHD: conventional | ‐ |
| Van Oosten 2018 | HHD (NS) | ICHD (NS) | CAPD APD Deceased donor transplant recipient Living donor transplant recipient Mix: multiple dialysis modalities in a year |
| Watanabe 2014 | Intensive HHD: 3 to 5 hours/session; 5 to 6 times/week; no nocturnal treatment; large segment had previously undergone PD or PD+HD combined therapy; AVF for all | ICHD: 3 to 5 hours/session; 3 times/week; AVF for all | ‐ |
| Wong 2019a | Intensive HHD: nocturnal | ICHD: (NS); hospital‐based Satellite HD (NS); community in‐centre |
PD |
| Wong 2019b | Intensive HHD: nocturnal | ICHD: (NS); hospital‐based | PD |
| Wright 2015 | HHD (NS) | ICHD (NS) | PD |
| Xue 2015 | Intensive HHD: frequent nightly | ICHD: conventional | ‐ |
| Yeung 2021 | HHD: 6 to 8 hours/session; alternate days | ICHD: 4 to 5 hours/session; 3 times/week | ‐ |
| Zimbudzi 2014 | Intensive HHD: > 75% 8 hours alternate days | Satellite HD patients on Category 1 transplant waitlist: 5 hours/session; 3 times/week | ‐ |
AVF/AVG: arteriovenous fistula/arteriovenous graft; CCPD: continuous cycling peritoneal dialysis; CVC: central venous catheter; HHD: home haemodialysis; ICHD: in‐centre haemodialysis; NS: (duration and frequency) not specified; PD: peritoneal dialysis; APD: automated PD; CAPD: continuous ambulatory PD
Tablo IDE 2020 specifically evaluated the Tablo HD device at home and in‐centre, while NxStage‐USRDS 2012 (and related reports) evaluated the use of the NxStage dialysis system in HHD compared to ICHD based on matched cohorts from USRDS. In contrast, another study from the USA, where the NxStage System is more broadly used, specified that this device was not used in their study (Nesrallah 2012). However, most studies did not provide details on the type of machine used or other dialysis parameters (e.g. vascular access, blood flow rate).
Specific definitions and prescription details (e.g. duration, frequency) of the HHD and ICHD groups were not always provided. In registry analyses, which included incident patients starting HD, some studies defined groups according to the modality 90 days after dialysis initiation (Marshall 2021) or the current modality at the time of the event of interest (Krishnasamy 2013), whereas others did not describe how groups were defined. Moreover, modality transfers were addressed differently across studies. Some studies used ITT (dialysis modality modelled as fixed) and as‐treated (dialysis modality modelled as time‐varying) frameworks, whereas others censored patients at modality switch.
Reported outcomes
Most studies reported outcomes of interest from only one category (clinical, patient‐reported, health economics or surrogate outcomes) (Table 6). No studies specifically reported on non‐fatal MI, non‐fatal stroke, parathyroidectomy or fatigue. Employment after the commencement of dialysis was reported in Helantera 2012; however, this study included patients from the age of 15 years, and data for adult patients could not be extracted separately. The metrics used to report outcomes were highly variable across studies. For example, vascular access‐related outcomes were reported as vascular access event‐free survival (Piccoli 2004), access complications (Sands 2009), vascular access surgery (Saner 2005) and catheter‐related sepsis (Xue 2015). Similarly, hospitalisation was reported as hospital admission rate (NxStage‐USRDS 2012; Rydell 2019; Suri 2015; Tennankore 2022; Toronto Group 2002), mean number of hospitalisations/patient (Saner 2005), hospitalisation days (NxStage‐USRDS 2012; Rydell 2019; Suri 2015; Toronto Group 2002; Zimbudzi 2014), number of patients experiencing a hospitalisation event (Sands 2009), and number of hospitalisation events in the study (Suri 2015; Tennankore 2022; Zimbudzi 2014). Despite attempts to gain further data from authors, measures of variability (SD, standard errors or IQR) were unobtainable for continuous outcomes in many studies. Likewise, many studies provided the total number of events overall for dichotomous outcomes but did not provide the number of events per treatment arm. In these situations, the study outcomes were unable to be included in the meta‐analyses.
5. Reported outcomes of interest by study.
| Study ID | Clinical outcomes | Patient‐reported outcomes | Health economics outcomes | Surrogate outcomes |
| Ageborg 2005 | ‐ | SF‐36 (physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional, mental health) Appraisal of Self‐Care Agency (ASA scale) Sense of Coherence (SOC) questionnaire |
‐ | ‐ |
| Bragg‐Gresham 2018 | ‐ | Employment prior to dialysis initiation (6 months, %) | ‐ | ‐ |
| Dumaine 2018 | ‐ | KDQOL‐SF (Symptoms and Problems, Effects of Kidney Disease, Burden of Kidney Disease, Physical Component Summary, Mental Component Summary) | ‐ | ‐ |
| Griva 2010 | ‐ | Illness Perceptions Questionnaire (Identity score) Illness Effects Questionnaire Treatment Effects Questionnaire Beck Depression Inventory‐II Cognitive Depression Index |
‐ | ‐ |
| Ha 2018 | ‐ | iPOS‐Renal | ‐ | ‐ |
| Hayhurst 2015 | ‐ | Maximum activity score Total activity score Activity loss score |
‐ | ‐ |
| Jayanti 2016 | ‐ | Recovery time (hours) | ‐ | Proportion of patients with systolic BP >115 mm Hg Proportion of patients with diastolic BP > 85 mm Hg |
| Kasza 2016 | Unadjusted survival (number at risk) Death (HR for death) |
‐ | ‐ | ‐ |
| Kjellstrand 2008 | Death (HR for death) Survival (HR by modality) |
‐ | ‐ | |
| Kojima 2012 | ‐ | ‐ | ‐ | Pre‐dialysis systolic BP LVMI |
| Krahn 2019 | Unadjusted survival (number at risk) | ‐ | 30‐day costs (CAD) Cumulative costs (CAD) |
‐ |
| Kraus 2007 | ‐ | KDQOL‐SF | ‐ | Systolic BP Diastolic BP Pulse pressure |
| Krishnasamy 2013 | Day of week as predictor of cardiac death (adjusted OR) | ‐ | ‐ | ‐ |
| Lee 2002 | ‐ | ‐ | Annual total health‐care related costs per patient (USD) Annual outpatient dialysis costs (USD) Annual inpatient costs (USD) Total outpatient costs (USD) Annual physician billing costs (USD) |
‐ |
| Lorenzen 2012 | ‐‐ | ‐ | Pre‐dialysis MAP Post‐dialysis MAP |
|
| Malmstrom 2008 | ‐ | 15D | Annual hospital costs (EUR) Annual total health‐care‐related costs per patient (EUR) |
Pre‐dialysis systolic BP Post‐dialysis systolic BP Pre‐dialysis diastolic BP Post‐dialysis diastolic BP |
| Marshall 2021 | Death (HR for death) Proportion cardiovascular death as cause of death |
‐ | ‐ | ‐ |
| McGregor 2001 | ‐ | Symptoms and quality of life (uraemia‐related symptoms, physical suffering, interference with social activity, burden on families) | ‐ | Pre‐dialysis BP Post‐dialysis BP Ambulatory BP Symptomatic hypotension LVMI |
| Murashima 2010 | ‐ | ‐ | ‐ | Pulse pressure Incidence of intradialytic hypotension (OR) Clinically significant hypotension during dialysis (OR) |
| Nebel 2002 | ‐ | ‐ | Annual healthcare costs (DM) | ‐ |
| Nesrallah 2012 | Death(HR for death) | ‐ | ‐ | ‐ |
| Nitsch 2011 | 1‐year survival (%) Survival after KRT start (HR) Long‐term survival (HR) Waitlisting for kidney transplantation before KRT start (OR) Waitlisting for kidney transplantation after KRT start (HR) |
‐ | ‐ | ‐ |
| NxStage‐USRDS 2012 | All‐cause death (HR for death) Cardiovascular death (HR for death) Infection death (HR for death) Interval‐specific death (HR for death) All‐cause hospital admissions (RR) Cardiovascular hospital admissions (RR) All‐cause hospital duration (RR) Cardiovascular hospital duration (RR) 30‐day readmission after discharge for heart failure 30‐day readmission after discharge for hypertension Relative incidence of transplant Survival (%) |
‐ | ‐ | ‐ |
| Piccoli 2004 | Adverse event‐free survival Vascular access event‐free survival |
‐ | ‐ | ‐ |
| Rydell 2016 | Mean survival (years) Survival (%) Number of patients who received a kidney transplant |
‐ | ‐ | Mean systolic BP Mean diastolic BP |
| Rydell 2019 | Median survival (years) All‐cause annual hospital admission rate Hospitalisation (days per year) Time to hospitalisation (years) |
‐ | ‐ | ‐ |
| Sands 2009 | Hospitalisations (per 100 treatments) Arterial site access complications (per 100 treatments) Venous site access complications (per 100 treatments) |
‐ | ‐ | ‐ |
| Saner 2005 | Survival (%) Cardiovascular death during study Vascular access surgery (per patient) All‐cause hospitalisation (per patient) Number of patients who received a kidney transplant during study |
‐ | ‐ | ‐ |
| Suri 2015 | Composite hospitalisation (HR) Composite hospitalisation rate (per patient‐year) Time to first hospitalisation (HR) Cardiovascular hospitalisations (HR) Access‐related hospitalisations (HR) Access infection‐related hospitalisations (HR) |
‐ | ‐ | ‐ |
| Tablo IDE 2020 | Recovery time (hours) EQ‐5D‐5L Sleep duration (hours) |
‐ | ‐ | ‐ |
| Tennankore 2022 | All‐cause hospital admissions (per 1000 patient‐days) | ‐ | ‐ | ‐ |
| Toronto Group 2002 | Dialysis or cardiovascular related admission (per patient year) Duration of dialysis or cardiovascular related admission (days per year) All‐cause hospitalisation (per patient year) Duration of all‐cause hospitalisation (days per year) |
Modified Appraisal of Self‐Care Agency (ASA) subscale SF‐12 (Mental Component Summary, Physical Component Summary) Multidimensional Scale of Perceived Social Support Anxiety State (Spielberger State‐Trait Anxiety Inventory for Adults) |
Mean systolic BP Mean diastolic BP Mean BP 24‐hour systolic BP 24‐hour diastolic BP LVMI |
|
| Van Oosten 2018 | ‐ | ‐ | Annual RRT costs (EUR) Annual healthcare costs (EUR) |
‐ |
| Watanabe 2014 | ‐ | SF‐36 (physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional, mental health; Mental Component Summary, Physical Component Summary) KDQOL (Symptoms and Problems, Effects of Kidney Disease, Burden of Kidney Disease, Work Status, Cognitive Function, Quality of Social Interaction, Sexual Function, Sleep) |
‐ | ‐ |
| Wong 2019b | ‐ | SF‐12 (physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional, mental health; Mental Component Summary, Physical Component Summary) | ‐ | ‐ |
| Wong 2019a | Annual hospitalisation utilisation (IRR) | ‐ | First year direct cost (HKD) Second year direct cost (HKD) Yearly indirect cost (HKD) First year societal cost (HKD) Second year societal cost (HKD) First year healthcare provider cost (HKD) Second year healthcare provider cost (HKD) |
‐ |
| Wright 2015 | ‐ | KDQOL‐SF (Symptoms and Problems, Effects of Kidney Disease, Burden of Kidney Disease, Work Status, Cognitive Function, Quality of Social Interaction, Sexual Function, Sleep, Social support, Dialysis staff encouragement, Patient satisfaction, Overall health) SF‐12 (Mental Component Summary, Physical Component Summary) SUPPH (positive attitude, stress reduction, decision making) |
‐ | ‐ |
| Xue 2015 | Death rate (per 100 patient‐months) Catheter‐related sepsis (per 100 patient‐months) Catheter‐related sepsis (HR) |
‐ | ‐ | ‐ |
| Yeung 2021 | Death (HR for death) Transplantation rate |
‐ | ‐ | ‐ |
| Zimbudzi 2014 | Number of patients hospitalised Hospital admissions (days per patient‐year) Mean length of stay hospitalisation (days) |
‐ | ‐ | ‐ |
BP: blood pressure; HR: hazard ratio; IRR: incidence rate ratio; KDQOL‐SF: Kidney Disease Quality of Life Instrument Short Form; KRT: kidney replacement therapy; LVMI: left ventricular mass index; MAP: mean arterial pressure; OR: odds ratio;SF‐12: 12‐Item Short Form Survey; SF‐36: 36‐Item Short Form Survey; Short form; SUPPH: Strategies Used by People to Promote Health
Patient‐reported outcome measures were reported in a wide variety of ways, encompassing measures of QoL (SF‐12, SF‐36, KDQOL‐SF, SF‐6D, EQ‐5D‐3L, 15D), mental health (Beck Depression Inventory‐II (BDI‐II), Spielberger State‐Trait Anxiety Inventory; Cognitive Depression Index), symptoms (IPOS‐Renal), impact and view of health (Sense of Coherence (SOC) questionnaire, Strategies Used by People to Promote Health (SUPPH), Illness Perceptions Questionnaire, Illness Effects Questionnaire, Treatments Effects Questionnaire, Multidimensional Scale of Perceived Support), functional ability (ASA scale, activity score), and recovery time.
Due to variations in how outcomes were defined and reported by studies, pooled analysis was only possible for a few reported outcomes.
Cardiovascular death (2 studies)
All‐cause death (9 studies)
All‐cause annual hospitalisation rate (2 studies)
All‐cause hospitalisation days per patient‐year (2 studies)
Kidney transplantation during the study period (6 studies)
Physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional, and mental health domains of the SF‐12 or SF‐36 (2 studies)
Physical Component Summary and Mental Component Summary of the SF‐12 or SF‐36 (5 studies)
Symptoms and problems, effects of kidney disease, burden of kidney disease, work status, cognitive function, quality of social interaction, sexual function and sleep domains of the KDQOL‐SF (2 studies)
BDI‐II (2 studies)
Recovery time (2 studies)
Healthcare costs (4 studies)
SBP (4 studies)
DBP (3 studies)
MAP (2 studies)
LVM index (2 studies).
Outcomes that were reported in a manner permitting extraction and analysis are demonstrated in Table 7, Table 8, Table 9 and Table 10.
6. Extractable clinical outcomes by study.
| Marshall 2021 | Nesrallah 2012 | Nitsch 2011 | NxStage‐USRDS 2012 | Piccoli 2004 | Rydell 2016 | Rydell 2019 | Sands 2009 | Saner 2005 | Suri 2015 | Tennankore 2022 | Toronto Group 2002 | Xue 2015 | Yeung 2021 | Zimbudzi 2014 | |
| Cardiovascular death | * | * | * | ||||||||||||
| All‐cause death | * | * | * | * | * | * | * | * | |||||||
| Median survival (years) | * | ||||||||||||||
| Death rate: death/1000 patient‐years | * | ||||||||||||||
| All‐cause annual hospital admission rate: number of admissions/patient‐year | ‐ | * | ‐ | ‐ | * | ||||||||||
| Number of patients with one or more hospitalisation events | * | ||||||||||||||
| All‐cause hospitalisation days/patient‐year | ‐ | * | ‐ | * | ‐ | ||||||||||
| Waitlisted for kidney transplant after KRT start | * | ||||||||||||||
| Kidney transplantation during study period | * | * | * | * | * | ||||||||||
| Vascular access surgery: number/patient | ‐ | ||||||||||||||
| Vascular access complications, number of patients experiencing | * | ‐ | |||||||||||||
| Vascular access failure rate/100 patient‐months | ‐ | ||||||||||||||
| Catheter‐related sepsis event rate/100 patient‐months | ‐ |
*outcome data extractable and able to be analysed
‐outcome data extractable but unable to be analysed (e.g. Incomplete data reporting, no measure of variability reported or able to be obtained)
7. Extractable patient‐reported outcomes by study.
| Ageborg 2005 | Griva 2010 | Ha 2018 | Hayhurst 2015 | Jayanti 2016 | Malmstrom 2008 | Tablo IDE 2020 | Toronto Group 2002 | Watanabe 2014 | Wong 2019a | Wright 2015 | |
| Physical Functioning (SF‐12; SF‐36) | * | * | |||||||||
| Role: Physical (SF‐12; SF‐36) | * | * | |||||||||
| Bodily Pain (SF‐12; SF‐36) | * | * | |||||||||
| General Health (SF‐12; SF‐36) | * | * | |||||||||
| Vitality (SF‐12; SF‐36) | * | * | |||||||||
| Social Functioning (SF‐12; SF‐36) | * | * | |||||||||
| Role: Emotional (SF‐12; SF‐36) | * | * | |||||||||
| Mental Health (SF‐12; SF‐36) | * | * | |||||||||
| Role‐Social Component Scale (SF‐36) | * | ||||||||||
| Physical Component Summary (SF‐12; SF‐36) | * | * | * | * | * | ||||||
| Mental Component Summary (SF‐12; SF‐36) | * | * | * | * | * | ||||||
| Symptoms and problems (KDQOL‐SF) | * | * | |||||||||
| Effects of kidney disease (KDQOL‐SF) | * | * | |||||||||
| Burden of kidney disease (KDQOL‐SF) | * | * | |||||||||
| Work status (KDQOL‐SF) | * | * | |||||||||
| Cognitive function (KDQOL‐SF) | * | * | |||||||||
| Quality of social interaction (KDQOL‐SF) | * | * | |||||||||
| Sexual function (KDQOL‐SF) | * | * | |||||||||
| Sleep (KDQOL‐SF) | * | * | |||||||||
| Social support (KDQOL‐SF) | * | ||||||||||
| Dialysis staff encouragement (KDQOL‐SF) | * | ||||||||||
| Patient satisfaction (KDQOL‐SF) | * | ||||||||||
| Physical functioning (KDQOL‐SF) | * | ||||||||||
| Role limitations ‐ Physical (KDQOL‐SF) | * | ||||||||||
| Pain (KDQOL‐SF) | * | ||||||||||
| General health (KDQOL‐SF) | * | ||||||||||
| Emotional well‐being (KDQOL‐SF) | * | ||||||||||
| Role limitations ‐ Emotional (KDQOL‐SF) | * | ||||||||||
| Social function (KDQOL‐SF) | * | ||||||||||
| Energy/fatigue (KDQOL‐SF) | * | ||||||||||
| Overall health (KDQOL‐SF) | * | ||||||||||
| SF‐6D | * | ||||||||||
| EQ‐5D‐5L | * | ||||||||||
| 15D | * | ||||||||||
| BDI‐II | * | * | |||||||||
| Anxiety state (Spielberger State‐Trait Anxiety Inventory for Adults) | * | * | |||||||||
| Anxiety trait (Spielberger State‐Trait Anxiety Inventory for Adults) | * | * | |||||||||
| Cognitive Depression Index | * | ||||||||||
| IPOS‐Renal | * | ||||||||||
| SOC questionnaire | * | ||||||||||
| SUPPH: positive attitude | * | ||||||||||
| SUPPH: stress reduction | * | ||||||||||
| SUPPH: decision making | * | ||||||||||
| Identity score: Illness Perceptions Questionnaire | * | ||||||||||
| Illness Effects Questionnaire | * | ||||||||||
| Treatment Effects Questionnaire | * | ||||||||||
| Multidimensional Scale of Perceived Social Support | * | ||||||||||
| ASA scale | * | ||||||||||
| Maximum activity score | * | ||||||||||
| Total activity score | * | ||||||||||
| Activity loss score | * | ||||||||||
| Recovery time (minutes) | * | * |
ASA: Appraisal of Self‐Care Agency; BDI: Beck Depression Inventory; EQ‐5D‐5L: Euro‐QOL‐5‐dimension 5‐level; KDQOL: Kidney Disease Quality of Life questionnaire; SF: short form; IPOS: Integrated Palliative Outcome Score; SOC: Sense of Coherence; SUPPH: Strategies Used by People to Promote Health
8. Extractable health economics outcomes by study.
| Krahn 2019 | Lee 2002 | Malmstrom 2008 | Nebel 2002 | Van Oosten 2018 | Wong 2019b | |
| Annual direct healthcare costs in the first year of dialysis, USD (2021 reference value) | * | * | ‐ | ‐ | * | * |
| Annual direct healthcare costs in first year of dialysis in the second year of dialysis, USD (2021 reference value) | * | * |
*outcome data extractable and able to be analysed
‐outcome data extractable but unable to be analysed (eg. Incomplete data reporting, no measure of variability reported or able to be obtained)
9. Extractable surrogate outcomes by study.
| Hayhurst 2015 | Kojima 2012 | Kraus 2007 | Lorenzen 2012 | Malmstrom 2008 | McGregor 2001 | Murashima 2010 | Rydell 2016 | Toronto Group 2002 | Wong 2019a | |
| SBP (mm Hg) | * | * | * | ‐ | * | * | ||||
| DBP (mm Hg) | * | * | ‐ | * | * | |||||
| MAP (mm Hg) | ‐ | * | * | |||||||
| MPP (mm Hg) | ‐ | * | ||||||||
| LVMI (g/m2) | * | * |
*outcome data extractable and able to be analysed
‐outcome data extractable but unable to be analysed (e.g. Incomplete data reporting, no measure of variability reported or able to be obtained)
DBP: diastolic blood pressure; LVMI: left ventricular mass index; MAP: mean arterial pressure; MPP: mean pulse pressure; SBP: systolic blood pressure
Excluded studies
The majority of records excluded at screening were due to ineligible study design because the study did not compare HHD versus ICHD. Following full‐text report retrieval and assessment for eligibility, most studies were excluded because the study did not compare HHD versus ICHD, as per the review's definition of these interventions.
Risk of bias in included studies
Randomised controlled trials
The risk of bias of the one included RCT (McGregor 2001) was assessed using the Risk of Bias assessment tool version 2 (RoB 2) and is shown in Table 11.
10. Risk of bias assessment using Risk of Bias assessment tool 2 (randomised controlled trials).
| McGregor 2001 | |||
| Domain | Signalling question | Response | Comments |
| Bias arising from the randomization process | 1.1 Was the allocation sequence random? | Y | Sequence generation using random number table designed by statistician (data obtained from authors on request). |
| 1.2 Was the allocation sequence concealed until participants were enrolled and assigned to interventions? | Y | Treatment allocation assigned by statistician unaware of patient details (data obtained from authors on request) | |
| 1.3 Did baseline differences between intervention groups suggest a problem with the randomization process? | N | Cross‐over study, patients acted as their own control | |
| Risk of bias judgement | Low | ‐ | |
| Bias due to deviations from intended interventions | 2.1.Were participants aware of their assigned intervention during the trial? | Y | Participants and personnel not blinded. Quality of life, BP, and echocardiography outcomes assessed by investigators unaware of treatment sequence (data obtained from authors on request). |
| 2.2.Were carers and people delivering the interventions aware of participants' assigned intervention during the trial? | Y | ||
| 2.3. If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the experimental context? | N | ‐ | |
| 2.4 If Y/PY to 2.3: Were these deviations likely to have affected the outcome? | NA | ‐ | |
| 2.5. If Y/PY/NI to 2.4: Were these deviations from intended intervention balanced between groups? | NA | ‐ | |
| 2.6 Was an appropriate analysis used to estimate the effect of assignment to intervention? | Y | ‐ | |
| 2.7 If N/PN/NI to 2.6: Was there potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomized? | NA | ‐ | |
| Risk of bias judgement | Low | ‐ | |
| Bias due to missing outcome data | 3.1 Were data for this outcome available for all, or nearly all, participants randomized? | Y | Data available for all participants. |
| 3.2 If N/PN/NI to 3.1: Is there evidence that result was not biased by missing outcome data? | NA | ‐ | |
| 3.3 If N/PN to 3.2: Could missingness in the outcome depend on its true value? | NA | ‐ | |
| 3.4 If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value? | NA | ‐ | |
| Risk of bias judgement | Low | ||
| Bias in measurement of the outcome | 4.1 Was the method of measuring the outcome inappropriate? | N | BP measured with electronic BP machine reading. LVMI measured by echocardiography. |
| 4.2 Could measurement or ascertainment of the outcome have differed between intervention groups? | N | Standardised method and equipment used to measure BP. Standardised method and timing for echocardiogram to be performed. | |
| 4.3 Were outcome assessors aware of the intervention received by study participants? | N | Quality of life, BP, and echocardiography outcomes assessed by investigators unaware of treatment sequence (data obtained from authors on request). | |
| 4.4 If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received? | NA | ‐ | |
| 4.5 If Y/PY/NI to 4.4: Is it likely that assessment of the outcome was influenced by knowledge of intervention received? | NA | ||
| Risk of bias judgement | Low | ‐ | |
| Bias in selection of the reported result | 5.1 Were the data that produced this result analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis? | NI | Sample size calculation and statistical tests reported in publication. However unable to confirm with author if the statistical analysis plan was formalised prior to data being available for analyses. |
| 5.2 ... multiple eligible outcome measurements (e.g. scales, definitions, time points) within the outcome domain? | N | Multiple time point assessment for BP but mean result reported. Single echocardiogram performed and data used. | |
| 5.3 ... multiple eligible analyses of the data? | N | Single method of analysis. | |
| Risk of bias judgement | Some concerns | ‐ | |
| Overall bias | Risk of bias judgement | Some concerns | Unable to blind investigators or participants to intervention, however outcome assessors were blinded. There was also potential bias arising from unmatched interventions (acetate vs bicarbonate buffer). |
Allocation
The risk of bias arising from the randomisation process was assessed as low risk, as the treatment allocation sequence was random and concealed until participants were enrolled and assigned to interventions.
Blinding
Due to the nature of the interventions, participants and investigators could not be blinded to treatment assignment. However, outcomes were measured using a standardised method, and assessors were blinded to treatment allocation and sequence; thus, bias in the measurement of the outcome was low.
Incomplete outcome data
There was a low risk of bias due to deviations from intended interventions. There was no loss to follow‐up, so complete outcome data were obtained.
Selective reporting
This study aimed to evaluate outcomes in patients receiving long, slow HD versus standard dialysis, potentially introducing differences in outcomes between treatment arms based on treatment duration rather than location. However, since longer‐hours dialysis might be considered a feature of HHD, this was not considered to be a potential source of bias.
Other potential sources of bias
Although the study reported a sample size calculation and described the statistical tests utilised, there was not enough information to confirm if the statistical analysis plan had been finalised prior to the data being available for analysis. The study reported a mismatch in treatment interventions (acetate versus bicarbonate buffer), which may have reduced comparability between interventions.
Funnel plot analysis was not used to assess for evidence of small study effects, as no analysis included 10 studies or more.
Risk of bias of non‐randomised studies of interventions
Using the NOS for cohort studies, only one study was adjudicated at high risk of bias, with a total of 3/9 stars, as this study was reported as an abstract only and complete results have not been published (Dumaine 2018). The remainder of the reports of studies were adjudicated as low risk of bias in the selection and outcome domains, and only a few did not receive the maximum score in these domains due to issues related to the comparability of cohorts on design or analysis (Table 12).
11. Risk of bias assessment using the Newcastle‐Ottawa Scale (cohort studies).
| Study ID/report | Selection | Comparability | Outcome | Total | ||||||
| Representativeness of the exposed cohort (/1) | Selection of the non‐exposed cohort (/1) | Ascertainment of exposure (/1) | Demonstration that outcome of interest not present at start (/1) | Study controls for the main factor (/1) | Study controls for an additional factor (/1) | Assessment of outcome (/1) | Appropriate length of follow‐up (/1) | Adequacy of follow‐up (/1) | ||
| Bragg‐Gresham 2018 | * | * | * | * | * | * | * | * | * | 9 |
| Dumaine 2018 | * | * | * | 3 | ||||||
| Kasza 2016 | * | * | * | * | * | * | * | * | * | 9 |
| Kjellstrand 2008 / Kjellstrand 2008 | * | * | * | * | * | * | * | * | * | 9 |
| Kjellstrand 2008 / Kjellstrand 2010 | * | * | * | * | * | * | * | * | * | 9 |
| Kojima 2012 | * | * | * | * | * | * | * | * | * | 9 |
| Krahn 2019 | * | * | * | * | * | * | * | * | * | 9 |
| Kraus 2007 | * | * | * | * | * | * | * | * | 8 | |
| Krishnasamy 2013 | * | * | * | * | * | * | * | * | * | 9 |
| Lee 2002 | * | * | * | * | * | * | * | * | * | 9 |
| Lorenzen 2012 | * | * | * | * | * | * | * | * | * | 9 |
| Marshall 2021 / Marshall 2011 | * | * | * | * | * | * | * | * | * | 9 |
| Marshall 2021 / Marshall 2013 | * | * | * | * | * | * | * | * | * | 9 |
| Marshall 2021 / Marshall 2014 | * | * | * | * | * | * | * | * | * | 9 |
| Marshall 2021 / Marshall 2016 | * | * | * | * | * | * | * | * | * | 9 |
| Marshall 2021 / Marshall 2021 | * | * | * | * | * | * | * | * | * | 9 |
| Murashima 2010 | * | * | * | * | * | * | * | * | * | 9 |
| Nebel 2002 | * | * | * | * | * | * | * | 7 | ||
| Nesrallah 2012 | * | * | * | * | * | * | * | * | * | 9 |
| Nitsch 2011 | * | * | * | * | * | * | * | * | * | 9 |
| NxStage‐USRDS 2012 / Kansal 2019 | * | * | * | * | * | * | * | * | * | 9 |
| NxStage‐USRDS 2012 / Weinhandl 2012 | * | * | * | * | * | * | * | * | * | 9 |
| NxStage‐USRDS 2012 / Weinhandl 2015a | * | * | * | * | * | * | * | * | * | 9 |
| NxStage‐USRDS 2012 / Weinhandl 2015b | * | * | * | * | * | * | * | * | * | 9 |
| NxStage‐USRDS 2012 / Weinhandl 2015c | * | * | * | * | * | * | * | * | * | 9 |
| NxStage‐USRDS 2012 / Weinhandl 2015d | * | * | * | * | * | * | * | * | * | 9 |
| Piccoli 2004 | * | * | * | * | * | * | * | * | * | 9 |
| Rydell 2016 | * | * | * | * | * | * | * | * | * | 9 |
| Rydell 2019 | ||||||||||
| Rydell 2019a | * | * | * | * | * | * | * | * | * | 9 |
| Rydell 2019b | * | * | * | * | * | * | * | * | * | 9 |
| Sands 2009 | * | * | * | * | * | * | * | * | * | 9 |
| Saner 2005 | * | * | * | * | * | * | * | * | * | 9 |
| Suri 2015 | * | * | * | * | * | * | * | * | * | 9 |
| Tablo IDE 2020 / Chertow 2020 | * | * | * | * | * | * | * | 7 | ||
| Tennankore 2022 | * | * | * | * | * | * | * | * | * | 9 |
| Toronto Group 2002 / Bergman 2008 | * | * | * | * | * | * | * | * | * | 9 |
| Toronto Group 2002 / Bugeja 2004 | * | * | * | * | * | * | * | * | * | 9 |
| Toronto Group 2002 / Chan 2002 | * | * | * | * | * | * | * | 7 | ||
| Toronto Group 2002 / Chan 2003 | * | * | * | * | * | * | * | 7 | ||
| Toronto Group 2002 / Chan 2005a | * | * | * | * | * | * | * | 7 | ||
| Van Oosten 2018 | * | * | * | * | * | * | * | * | 8 | |
| Wong 2019b | * | * | * | * | * | * | * | * | * | 9 |
| Xue 2015 | * | * | * | * | * | * | * | * | * | 9 |
| Yeung 2021 | * | * | * | * | * | * | * | * | * | 9 |
| Zimbudzi 2014 | * | * | * | * | * | * | * | 7 | ||
Using the adapted NOS for cross‐sectional studies, three reports of studies scored 5/10 stars, one report scored 6/10 stars, and seven reports scored 8/10 stars. Most risks of bias pertained to the sample size and comparability of non‐respondents (Table 13).
12. Risk of bias assessment using the adapted Newcastle‐Ottawa Scale (cross‐sectional studies).
| Study / report | Selection | Comparability | Outcome | Total | |||||
| Representativeness of the sample (/1) | Sample size (/1) | Comparability of non‐respondents (/1) | Ascertainment of exposure (/2) | Study controls for the most important factor (/1) | Study controls for any additional factor | Assessment of outcome (/2) | Statistical test (/1) | ||
| Ageborg 2005 | * | ** | ** | 5 | |||||
| Griva 2010 | * | ** | * | * | ** | * | 8 | ||
| Ha 2018 | * | ** | ** | 5 | |||||
| Hayhurst 2015 | * | ** | * | * | 5 | ||||
| Jayanti 2016 | * | ** | * | * | ** | * | 8 | ||
| Malmstrom 2008 | * | ** | * | * | ** | * | 8 | ||
| Toronto Group 2002 / Cafazzo 2009 | * | ** | * | * | ** | * | 8 | ||
| Toronto Group 2002 / Chan 2005 | * | ** | * | * | ** | * | 8 | ||
| Watanabe 2014 | * | ** | * | * | ** | * | 8 | ||
| Wong 2019a | * | ** | * | * | ** | * | 8 | ||
| Wright 2015 | * | * | * | ** | * | 6 | |||
Effects of interventions
See: Table 1
See Table 1.
Cardiovascular death
Compared with ICHD, HHD had uncertain effects on cardiovascular death (Analysis 1.1 (2 studies, 30,900 participants): RR 0.92, 95% CI 0.80 to 1.07; I² = 0%; very low certainty evidence). The data for this outcome were mainly contributed by Marshall 2021, a large registry study. Data from Krishnasamy 2013 were not included in the meta‐analysis, firstly because this study focused on day‐of‐the‐week variability in cardiovascular death rather than overall cardiovascular death, and secondly, because it analysed ANZDATA Registry data over an overlapping time period with Marshall 2021. NxStage‐USRDS 2012 reported hazard ratios (HR) for cardiovascular death, which indicated no evidence of different cardiovascular death comparing HHD with ICHD (HR 0.92, 95% CI 0.78 to 1.09); however, we were unable to obtain sufficient data to allow inclusion in the meta‐analysis. When reviewing cardiovascular death as reported in all included studies (not just those included in the meta‐analysis), the risk of cardiovascular death appeared to be, in general, lower in patients receiving HHD compared to ICHD; however, cardiovascular death comprised a larger proportion of all deaths in those receiving HHD compared to ICHD (Table 14). Subgroup and sensitivity analyses could not be performed due to the small number of studies included in the analysis. The risk of bias, within the constraints of a NRSI, was considered to be low, with all included studies scoring 9/9 on the NOS (Table 12).
1.1. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 1: Cardiovascular death
13. Cardiovascular death as reported by studies.
| Study ID | Reported outcome | Effect measure | Notes on analysis |
| Krishnasamy 2013 | Odds of cardiac death by day of the week (Reference: odds of cardiac death for all days of the week) |
|
Analysis adjusted for age, sex, racial origin, body mass index, late referral, smoking status, chronic lung disease, coronary artery disease, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, country of treatment (Australia or New Zealand), and centre size |
| Marshall 2021 | Proportion of deaths due to cardiovascular disease |
|
Analysed as 5‐year eras. Modelled as‐treated (i.e. time‐varying) modality, with a 90‐day lag in the attribution of death to modality |
| NxStage‐USRDS 2012 | Cardiovascular death (Reference: ICHD) |
|
ICHD patients matched 5:1 for HHD patients. Matching variables included first date of follow‐up, demographic characteristics, and measures of disease severity |
| Saner 2005 | Proportion of deaths due to cardiovascular disease | 25.9% (HHD) vs 22.4% (ICHD) | Proportion of all deaths |
CI: confidence interval; HHD: home haemodialysis; HR: hazard ratio; ICHD: in‐centre haemodialysis; OR: Odds ratio
All‐cause death
Compared with ICHD, HHD had uncertain effects on all‐cause death (Analysis 1.2 (9 studies, 58,984 participants): RR 0.80, 95% CI 0.67 to 0.95; I² = 84%; very low certainty evidence). The death rate was reported to be between 35 and 110 deaths/1000 patient‐years for HHD and between 57 and 127 for ICHD (Nesrallah 2012; NxStage‐USRDS 2012; Yeung 2021). The studies varied widely in size and included large registry analyses (Marshall 2021; Nesrallah 2012; NxStage‐USRDS 2012; Rydell 2019) as well as smaller single‐centre studies (Rydell 2016; Saner 2005; Xue 2015; Yeung 2021). The duration of follow‐up in these studies varied from less than two years to more than 10 years (Table 15).
1.2. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 2: All‐cause death
14. Follow‐up duration in studies assessing all‐cause death.
| Study ID | HHD | ICHD |
| Marshall 2021 | 5‐, 7‐ and 10‐year windows | 5‐, 7‐ and 10‐year windows |
| Nesrallah 2012 | Median 1.8 years | |
| NxStage‐USRDS 2012 | Mean 1.8 years | Mean 1.7 years |
| Rydell 2016 | Median 14.2 years | Median 10.8 years |
| Rydell 2019 | Median 10.4 years | Median 7.0 years |
| Saner 2005 | Mean 10.5 years | Mean 7.4 years |
| Xue 2015 | 20 months | |
| Yeung 2021 | Mean 4.44 years | |
Kjellstrand 2008, which used pooled data from five centres in the USA, Italy, France and the UK, reported that deaths at five years in patients receiving daily HHD were one‐third of those receiving daily ICHD and about two‐thirds of those treated with conventional ICHD; however, we were unable to extract data for meta‐analysis. When reviewing all‐cause death as reported in all included studies (not just those included in the meta‐analysis), the risk of death was consistently lower in patients receiving HHD compared to ICHD (Table 16). As was the case with the cardiovascular death outcome, subgroup and sensitivity analyses could not be performed due to the small number of studies included in the analysis. The risk of bias, within the constraints of a NRSI, was considered to be low, with all included studies scoring 9/9 on the NOS (Table 12).
15. Death as reported by studies.
| Study ID | Reported outcome | Effect measure | Notes on analysis |
| Kasza 2016 | Unadjusted pseudo‐survival curves | Demonstrated in figures, values unable to be extracted. Study reported that HHD with an AVF/AVG had better survival than any other exposure | ICHD with AVF/AVF vs ICHD with CVC vs HHD with AVF/AVG |
| Time dependent HR for death (reference: ICHD patients with AVF/AVG) | |||
| Kjellstrand 2008 | Cumulative survival | Demonstrated in figures, values unable to be extracted. Study reported that the 5‐year death of daily HD patients treating at home is one‐third and of those treated in centre | ‐ |
| Krahn 2019 | Survival (%) | 5‐year survival of 65% (HHD) vs 46% (ICHD) | Unadjusted |
| Death (reference: ICHD) | HR 0.44, 95% CI 0.27 to 0.68 for HHD | Adjusted for age, sex, and comorbidity (Aggregated Diagnostic Groups count) | |
| Marshall 2021 | Death (reference: ICHD) | aHR 0.50, 95%CI 0.40 to 0.64 for HHD | Modelled as‐treated (i.e. time‐varying) modality, with a 90‐day lag in the attribution of death to modality |
| Nesrallah 2012 | Death rate | 6.1 deaths per 100 person‐years, 95% CI 2.6 to 8.2 (HHD) vs 10.5 deaths per100 person‐years, 95% CI 8.1 to 13.5 (ICHD) | Attributed all deaths to dialysis modality at index date, regardless of switches to other dialysis modalities. Stratified by matched set and country |
| Death (eeference: ICHD) | HR 0.55, 95%CI 0.34 to 0.87 for intensive HHD | ||
| Nitsch 2011 | 1‐year survival | 90%, 95% CI 87% to 91% (ICHD) vs 97%, 95% CI 94% to 99% (HHD patients of similar age and sex) | Unadjusted |
| Long‐term survival (reference: HHD) | HR 1.06, 95% CI 0.55 to 2.04 for satellite HD | Time‐dependent variable for date of start of HHD, and baseline demographic variables. Follow‐up was not censored for kidney transplantation as it was entered as a time‐dependent variable. A time‐dependent variable for wait‐listing for kidney transplantation was included as a surrogate for general health status | |
| NxStage‐USRDS 2012 | All‐cause mortality for daily HHD (Reference: ICHD) | HR 0.87, 95%CI 0.78 to 0.97 (ITT) HR 0.82, 95%CI 0.72 to 0.94 (as treated) |
ICHD patients matched 5:1 for HHD patients. Matching variables included first date of follow‐up, demographic characteristics, and measures of disease severity |
| Rydell 2016 | Mean survival (years) | 17.3 years (HHD) vs 13.0 years (ICHD) | Survival analysis was performed as ITT analysis, where patients were considered at risk also after changes to other modalities of KRT, including transplantation |
| Survival (%) | 5 years: 98% (HHD) vs 71% (ICHD) 10 years: 73% (HHD) vs 56% (ICHD) |
||
| Rydell 2019 | Median survival (years) | 18.5 years, IQR 10.4 – not available (HHD) vs 11.9 years, IQR 3.8 – not available (ICHD) | HHD patients matched with ICHD 1:4, using gender, Charlson Comorbidity Index, age (± 3 years) and date for start of KRT (± 3 years) were used as matching criteria. Matching was performed at day 0 of KRT. ITT, where patients were considered at risk also after switching to other KRT |
| Survival (%) | 5 years: 91% (HHD) vs 70% (ICHD) 10 years: 76% (HHD) vs 57% (ICHD) 20 years: 49% (HHD) vs 34% (ICHD) |
||
| Saner 2005 | Survival (%) | 5 years: 93% (HHD) vs 64% (ICHD) 10 years: 72% (HHD) vs 48% (ICHD) 20 years: 34% (HHD) vs 23% (ICHD) |
Survival time was defined as the time from the initiation of the first dialysis treatment until death from any cause or the last date of follow‐up alive |
| Xue 2015 | Death rate | First catheter: 0.00 events per 100 patient‐months (HHD) vs 0.4 (ICHD) All catheters: 0.26 events per 100 patient‐months (HHD) vs 0.33 (ICHD) |
HHD patients matched with ICHD patients based on five variables: age (± 5 years), gender, race, dialysis vintage, and diabetes. Dialysis vintage was divided into seven categories: 1 day, > 1 to 30 days, > 1 to 3 months, > 3 to 12 months, > 1 to 2 years, > 2 to 5 years, and > 5 years. Adjustment for residual differences in age, gender, race, vintage, diabetes, as well as for primary cause of kidney failure(including diabetes, hypertension, glomerulonephritis, and polycystic kidney disease. All events after 20 months since the start of catheter were censored |
| Yeung 2021 | Death rate | 3.5 per 100 person‐years (HHD) vs 5.7 per 100 person‐years (ICHD) | ICHD patients matched 3:1 to HHD patients by age (within 5 years), gender and cause of kidney failure separated into glomerulonephritis, diabetes, hypertension and renovascular disease, reflux nephropathy and polycystic kidney disease. HR adjusted for BMI, smoking status, racial group and lung and vascular disease at dialysis commencement |
| Death (reference: ICHD) | HR 0.49, 95% CI 0.30 to 0.80 for HHD |
aHR: adjusted hazard ratio; AVF: arteriovenous fistula; AVG: arteriovenous graft; BMI: body mass index; CI: confidence interval; HD: haemodialysis; HHD: home haemodialysis; HR: hazard ratio; ICHD: in‐centre haemodialysis; IQR: interquartile range; ITT: intention to treat; KRT: kidney replacement therapy
All‐cause hospitalisation
Compared with ICHD, HHD had uncertain effects on the annual all‐cause admission rate (Analysis 1.3 ( 2 studies, 834 participants): MD ‐0.50 admissions/patient‐year, 95% CI ‐0.98 to ‐0.02; I² = 90%; very low certainty evidence). Three studies were excluded from the meta‐analysis as we were unable to obtain measures of variability; however, their reported mean annual hospital admission rates fell in the range between the two analysed studies, from 0.78 admissions/patient‐year in the HHD group to 1.54 admissions/patient‐year in the ICHD group (NxStage‐USRDS 2012; Suri 2015; Tennankore 2022).
1.3. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 3: All‐cause annual hospital admission rate (number of admissions/patient‐year)
Five studies reported all‐cause hospitalisation days/patient‐year; however, three studies were excluded from the meta‐analysis as we were unable to obtain measures of variability. Mean hospitalisation days/patient‐year may have been slightly lower in patients receiving HHD compared to ICHD (Analysis 1.4 (2 studies, 834 participants): MD ‐1.90, 95% CI ‐2.28 to ‐1.53; I² = 0%; low certainty evidence). Subgroup and sensitivity analyses could not be performed. The risk of bias, within the constraints of a NRSI, was considered to be low, with all included studies scoring 9/9 on the NOS (Table 12).
1.4. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 4: All‐cause hospitalisation days/patient‐year
Only Sands 2009 reported the number of patients experiencing one or more hospitalisation events, precluding meta‐analysis.
Kidney transplantation
Wait‐listing for kidney transplantation was reported in Nitsch 2011, which found that patients receiving ICHD were less likely to be wait‐listed than patients receiving HHD after adjusting for centre effect, year of start, age, gender, primary kidney disease, ethnicity and social deprivation (HR 0.56, 95% CI 0.44 to 0.79). Receipt of a kidney transplant during the study period was reported in six studies and was reported in a variety of ways in the individual studies, including relative incidence in HHD versus matched ICHD participants (1.22, 95% CI 1.06 to 1.66) (NxStage‐USRDS 2012), transplant rates/100 person‐years (9.5, 95% CI 7.6 to 12.1 for HHD versus 8.8. 95% CI 6.7 to 11.6 for ICHD) (Nesrallah 2012), and only the number of patients transplanted (Rydell 2016; Saner 2005; Xue 2015; Yeung 2021).
The meta‐analysis demonstrated HHD had an uncertain effect on the likelihood of receiving a transplant compared to ICHD (Analysis 1.5 (6 studies, 10,910 participants): RR 1.28, 95% CI 1.01 to 1.63; I² = 78%; very low certainty evidence). These studies varied substantially in size and design, from large registry analyses contributing most of the weight in the analysis (NxStage‐USRDS 2012) to much smaller single‐centre cohort studies (Saner 2005; Xue 2015). The risk of bias, within the constraints of a NRSI, was considered to be low, with all included studies scoring 9/9 on the NOS (Table 12).
1.5. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 5: Kidney transplantation during study period
Vascular access events
Vascular access interventions were reported in a variety of ways, and meta‐analysis could not be performed on any of the measures. One study reported the mean number of vascular access surgeries/patient (Saner 2005).
Two studies reported the number of patients experiencing a vascular access complication; however, these studies could not be meta‐analysed. Sands 2009 reported venous and arterial site complications separately, while Piccoli 2004 did not specify the site. Thus, we could not be certain that patients would not be included in the analysis more than once if they experienced both a venous and arterial site complication.
Piccoli 2004 reported vascular access failure rate, and Xue 2015 reported catheter‐related sepsis rate. It was unclear if vascular access complications occurred more frequently in patients receiving HHD or ICHD across these studies. Saner 2005 reported increased complications in patients receiving ICHD (3.9 versus 2.5 mean events/patient), Sands 2009 reported more frequent arterial site complications (0.25 versus 0.08 events/100 treatments) but fewer venous site complications in patients receiving HHD (0.08 versus 0.17 events/100 treatments), and two studies suggesting no difference in vascular access events (Piccoli 2004) or catheter‐related sepsis (Xue 2015). The risk of bias was low for all studies (Table 12).
Quality of life
QoL (including broader aspects such as mental health, symptoms, impact and view of health and functional ability) were reported using multiple different outcome measures across studies. While many of these tools measure different aspects of QoL and are thus not simply interchangeable, the low usage of numerous tools resulted in meta‐analysis being unable to be performed for many outcome measures. Two studies reported SF‐12 and SF‐36 domains of physical functioning, role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional and mental health, and analyses generally indicated that HRQoL may have been slightly better in patients receiving HHD (Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13: 2 studies, 374 participants). It was uncertain if the Physical Component Score was higher in patients receiving HHD (Analysis 1.14 (5 studies, 922 participants): SMD 0.42, 95% CI 0.10 to 0.73; I² = 74%; very low certainty evidence); however, there was little or no difference in the Mental Component Score (Analysis 1.15 (5 studies, 922 participants): SMD 0.10, 95% CI ‐0.05 to 0.25; I² = 0%; very low certainty evidence).
1.6. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 6: Physical functioning (SF‐12; SF‐36)
1.7. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 7: Role: Physical (SF‐12; SF‐36)
1.8. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 8: Bodily pain (SF‐12; SF‐36)
1.9. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 9: General health (SF‐12; SF‐36)
1.10. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 10: Vitality (SF‐12; SF‐36)
1.11. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 11: Social functioning (SF‐12; SF‐36)
1.12. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 12: Role: Emotional (SF‐12; SF‐36)
1.13. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 13: Mental health (SF‐12; SF‐36)
1.14. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 14: Physical Component Summary (SF‐12; SF‐36)
1.15. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 15: Mental Component Summary (SF‐12; SF‐36)
Two studies reported KDQOL‐SF, and analyses indicated HRQoL may have been higher in most domains for patients receiving HHD compared to ICHD (Analysis 1.16; Analysis 1.17; Analysis 1.18; Analysis 1.19; Analysis 1.20; Analysis 1.21; Analysis 1.22; Analysis 1.23: 2 studies, 131 participants). The domains of social support, dialysis staff encouragement, patient satisfaction, physical functioning, role limitations ‐ physical, pain, general health, emotional well‐being, role limitations ‐ emotional, social function, energy/fatigue and overall health were reported only in Wright 2015, and so were not meta‐analysed. SF‐6D (Wong 2019a), EQ‐5D‐5L (Tablo IDE 2020) and 15D (Malmstrom 2008) were also only reported in single publications and were not meta‐analysed.
1.16. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 16: Symptoms and problems (KDQOL‐SF)
1.17. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 17: Effects of kidney disease (KDQOL‐SF)
1.18. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 18: Burden of kidney disease (KDQOL‐SF)
1.19. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 19: Work status (KDQOL‐SF)
1.20. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 20: Cognitive function (KDQOL‐SF)
1.21. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 21: Quality of social interaction (KDQOL‐SF)
1.22. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 22: Sexual function (KDQOL‐SF)
1.23. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 23: Sleep (KDQOL‐SF)
BDI‐II was reported in two studies, but meta‐analysis could not be performed as one study reported the mean and SD (Griva 2010), whereas the other reported only the frequency of scoring ranges as a categorical variable (Jayanti 2016). This was also the case for Spielberger State‐Trait Anxiety Inventory for Adults, where mean and SD were available for one study (Toronto Group 2002) but reported as a categorical variable in the other (Jayanti 2016).
Cognitive Depression Questionnaire, Illness Perceptions Questionnaire, Illness Effects Questionnaire, Treatment Effects Questionnaire (Griva 2010); IPOS Renal (Ha 2018), SOC questionnaire, Multidimensional Scale of Perceived Social Support (Ageborg 2005); SUPPH (Wright 2015); and Activity score (Hayhurst 2015) were each reported in only a single study and were not meta‐analysed. Appraisal of Self‐care Agency was reported in two studies; however, one study used a modified scale with a different scoring system, and the results could not be meta‐analysed (Ageborg 2005; Toronto Group 2002).
Most studies reporting QoL used a cross‐sectional design, with only one reporting longitudinal results for the EQ‐5D‐5L (Tablo IDE 2020). For several studies, questionnaires were mailed to potential participants, particularly those dialysing at home where convenience sampling could not be performed. Survey bias may, therefore, have been an issue. Dialysis vintage and duration on current modality varied between studies and were not reported in some; for example, for the Physical Component Score and the Mental Component Score outcomes, some studies reported duration on dialysis as a mean or median value, one study reported duration of ESKD diagnosis, and another reported duration on dialysis as a categorical variable (number of patients/time category) (Table 2). This may have impacted results since it is not known how long a patient must remain on a treatment modality before expecting to see either a beneficial or detrimental effect on QoL, but it is likely patients would require some time to become accustomed to a treatment. The risk of bias was low for the cohort study (Table 12) and low to moderate for the cross‐sectional studies, with four studies scoring 6 or less out of 10 on the adapted NOS (Table 13). Subgroup and sensitivity analyses could not be performed as there were few studies reporting the same metric of QoL.
Employment status
Employment could not be meta‐analysed in this review due to insufficient data. One study reported that self‐dialysis modality use was significantly associated with maintained employment at dialysis initiation compared to ICHD; however, the study was available in abstract format only, and the values for rates of employment were not reported (Bragg‐Gresham 2018). Employment status was the primary outcome of a cross‐sectional analysis of the Finnish Registry for Kidney Diseases; however, the study included participants aged from 15 to 64 years and, therefore, did not meet the review's inclusion criteria (Helantera 2012). For interest, since this review identified no other studies reporting this outcome, Helantera 2012 reported that patients receiving HHD (n = 57) had a significantly increased probability of being employed compared to patients receiving ICHD (n = 550) when adjusted for age, sex, cause of kidney failure, number of comorbid conditions and time since the start of KRT (prevalence rate ratio (PRR) 1.87, 95% CI 1.26 to 2.64). Furthermore, the study also found that patients treated with HHD as their last dialysis modality prior to kidney transplantation had an increased likelihood of being employed post‐transplant (PRR 2.14, 95% CI 1.68 to 2.74, reference group ICHD). To date, we have not been able to obtain data for the 18 years and older subgroup.
Recovery time
Recovery time was reported in two studies. The effect of HHD on recovery time was uncertain (Analysis 1.24 (2 studies, 348 participants): MD ‐2.0 hours, 95% CI ‐2.73 to ‐1.28; I² = 0%; low certainty evidence). These studies differed in design and analytical approach. One was a cross‐sectional survey, which included 288 patients from five UK centres and had a response rate of 94.2% (Jayanti 2016). Median dialysis vintage was 2.68 years (IQR 1.05 to 5.12) in patients receiving ICHD and 3.47 years (IQR 1.39 to 6.82) in patients receiving HHD; however, this study did not report how long patients had been on their current modality. The other study was a prospective cohort study of 30 prevalent HD patients who were transitioned from an eight‐week period of in‐centre to an eight‐week period of HHD, with a four‐week transition period (Tablo IDE 2020). In this study, recovery time was ascertained by a weekly questionnaire and within‐patient average scores for the in‐centre and in‐home periods were analysed. The risk of bias was considered to be low within the constraints of observational studies, with the cross‐sectional study scoring 8/10 on the adapted NOS and the cohort study scoring 7/9.
1.24. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 24: Recovery time
Cost‐effectiveness
Annual direct healthcare costs were analysed in four studies. Two studies reported costs year by year from dialysis initiation (Krahn 2019; Wong 2019b), one study reported costs for patients already established on dialysis for six months (Lee 2002), and another included all patients who received chronic dialysis during the time period of interest and did not specify vintage for inclusion (Van Oosten 2018). When cost in the first year of dialysis was analysed, it was uncertain if HHD was more cost‐effective than ICHD (Analysis 1.25 (2 studies, 10,077 participants): SMD ‐1.80, 95% CI ‐4.08 to ‐0.49; I² = 98%; very low certainty evidence). When data from the studies that did not specify dialysis vintage were included, this was also the case (4 studies, 13,809 participants: SMD ‐1.25, 95% CI ‐2.13 to ‐0.37, I² = 85%; very low certainty evidence). The effects of HHD on cost‐effectiveness in the second year of dialysis were uncertain (Analysis 1.26 (2 studies, 10,077 participants): SMD ‐2.30, 95% CI ‐6.94 to 2.34; I² = 100%; very low certainty evidence), which was also the case when data from studies that did not specify dialysis vintage were included in analysis (4 studies, 13,809 participants: SMD ‐1.47, 95% CI ‐2.72 to ‐0.21; I² = 88%; very low certainty evidence). The included studies were performed in Canada (Krahn 2019; Lee 2002), the Netherlands (Van Oosten 2018) and Hong Kong (Wong 2019b) between 2002 and 2019. Two further studies reported annual direct healthcare costs but were not included in the meta‐analysis due to unobtainable measures of variability (Malmstrom 2008; Nebel 2002). Nebel 2002 reported costs of treatment/year in Germany in 1999 and found that the cost of satellite HD was substantially greater than HHD (86,908 versus 59,591 DM, equivalent to 44,435 versus 30,468 EUR) (Bundesbank). Malmstrom 2008 also reported an increased cost of satellite HD compared to HHD in Finland, though the proportional difference between groups was less (39,781 versus 38,477 EUR).
1.25. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 25: Annual direct healthcare costs in first year of dialysis (currency as reported)
1.26. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 26: Annual direct healthcare costs in second year of dialysis (currency as reported)
Equivalent costs in US dollars (USD) with a December 2021 reference value are demonstrated in Table 17 for all studies reporting annual direct healthcare costs (including those not meta‐analysed). Reported costs varied widely across studies, even after calculating equivalent values, although HHD was consistently reported as more cost‐effective than ICHD in all studies. The costs of healthcare delivery would likely differ between countries and over time for various reasons unrelated to currency or inflation, and this should be taken into consideration when interpreting results. The risk of bias was low, with three studies scoring 9/9 and one scoring 8/9 on the NOS.
16. Annual healthcare costs reported in USD (December 2021 reference value).
| Study ID | HHD | ICHD |
| Krahn 2019 | $77,589.62 | $130,579.58 |
| Lee 2002 | $48,006.82 | $78,283.54 |
| Malmstrom 2008 | $70,113.6 | $72,489.77 |
| Nebel 2002 | $36,057.27 | $52,586.22 |
| Van Oosten 2018 | $137,263.28 | $146,038.26 |
| Wong 2019b | $14,132.96 | $53,092.15 |
HHD: home haemodialysis; ICHD: in‐centre haemodialysis
Costs reported in studies in a different currency were converted to USD for the same year, using OECD data exchange rates (OECD 2022).
Cost from years prior to 2021 was subsequently converted to December 2021 value using the U.S. Bureau of Labour Statistics Consumer Price Index (CPI) Inflation Calculator (U.S. Bureau of Labor Statistics 2022)
Blood pressure
BP was reported in only one randomised cross‐over trial of nine patients, which showed it to be lower during the HHD phase versus ICHD (systolic BP 155 ± 18 versus 169 ± 24 mm Hg, diastolic BP 89 ± 6 versus 93 ± 9 mm Hg) (McGregor 2001).
SBP was analysed in three NRSIs, two of which reported pre‐dialysis BP measurements (Kojima 2012; Malmstrom 2008). The effect of HHD on SBP was uncertain (Analysis 1.27 (2 studies, 173 participants): MD ‐9.97 mm Hg, 95% CI ‐34.35 to 14.41; I2 = 93%; very low certainty evidence). One study reported clinic BP measurements (Toronto Group 2002), and another did not specify how or when the measurements were taken (Wong 2019a). When these data were included in the analysis, HHD may lead to improved systolic BP control (4 studies, 491 participants: MD ‐11.78 mm Hg, 95% CI ‐21.11 to ‐2.46; I2 = 81%; low certainty evidence).
1.27. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 27: Systolic blood pressure (NRSIs)
There was no significant difference in DBP (Analysis 1.28 (3 studies, 383 participants): MD 1.81 mm Hg, 95% CI ‐1.31 to 4.94; I2 = 14.0%; very low certainty evidence). Rydell 2016 also reported no significant difference in DBP in patients receiving treatment with ICHD versus HHD; however, this study was not included in the analysis as DBP values were not obtainable. Two additional studies reported both SBP and DBP but were not included in the meta‐analysis due to unobtainable measures of variability (Kraus 2007; Lorenzen 2012).
1.28. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 28: Diastolic blood pressure (NSRIs)
The effect of HHD compared to ICHD on MAP was uncertain (Analysis 1.29 (2 studies, 44 participants): MD ‐7.01 mm Hg, 95% CI ‐12.57 to ‐1.46; I2 = 23%; low certainty evidence). One additional study reported MAP but was not included in the analysis due to unobtainable measures of variability, although it also reported lower MAP in patients receiving HHD (90.02 versus 94.57 mm Hg) (Hayhurst 2015). Pulse pressure was reported in two studies (Kraus 2007; Murashima 2010) but was not analysed since measures of variability were only obtainable for one study (Murashima 2010).
1.29. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 29: Mean arterial pressure
Left ventricular mass
HHD had an uncertain effect on LVM index, based on two NRSIs in which patients were transitioned from ICHD to HHD (Analysis 1.30 (2 studies, 130 participants): MD ‐18.13 g/m2, 95% CI ‐32.08 to ‐4.17; I2 = 26%; low certainty evidence). LVMI was also measured in one RCT, which reported no change during the course of the study; however, values of these measurements were not obtainable (McGregor 2001). The risk of bias was low within the constraints of a NRSI (Table 12).
1.30. Analysis.

Comparison 1: Home versus in‐centre haemodialysis, Outcome 30: Left ventricular mass index [g/m2]
Adverse events
One study reported that clinically significant hypotension (SBP ≤ 90 or DBP ≤ 55 mm Hg) was less likely when patients were treated with HHD compared to ICHD (odds ratio (OR) 0.36, 95% CI 0.16 to 0.81) (Murashima 2010). Various other adverse events that were not outcomes of interest were reported in a few studies (Table 18). Most studies did not specifically report adverse events, possibly due to observational cohort (including registry analysis) or cross‐sectional study design.
17. Other adverse events.
| HHD group | ICHD group | |
| Kraus 2007 |
|
|
| Malmstrom 2008 |
|
|
| Sands 2009 |
|
|
| Tablo IDE 2020 |
|
|
AE: adverse events; HHD: home haemodialysis; ICHD: in‐centre haemodialysis; AE; adverse event
Discussion
Summary of main results
This systematic review identified one small RCT that compared the effects of shorter hours of ICHD with longer hours of HHD in nine prevalent HHD patients. The study found that BP was lower when patients were receiving HHD compared to ICHD.
Several NRSIs meeting our inclusion criteria were identified, varying in design from small single‐centre studies to large registry analyses. Meta‐analysis of NRSIs indicated uncertain differences in risk of all‐cause death and hospitalisation in patients receiving HHD compared to ICHD. The effects of HHD on kidney transplantation and recovery time were also uncertain. Effects on QoL were variable across different measures and, in some domains, did not reach statistical significance but generally favoured HHD. In a meta‐analysis, cost‐effectiveness was uncertain; however, the results of studies generally favoured HHD over ICHD. Although SBP, MAP, and LVM index were lower in patients receiving HHD compared to ICHD, there was no difference in DBP.
Overall completeness and applicability of evidence
In this review, treatment with HHD was associated with uncertain but possible benefits compared to ICHD, including death, QoL and cost benefit. Improvement in some surrogate outcomes was also seen. These benefits are certainly plausible; however, they may have been due at least in part to differences in how dialysis was delivered, including duration, frequency, blood flow rate, type of machine used and vascular access. Intensive dialysis regimens have been associated with reduced medication burden and improved biochemical parameters (Jardine 2017; Ok 2011) and reduced ultrafiltration rate, intradialytic hypotension and myocardial stunning (Jefferies 2011), which may, in turn, result in improved clinical outcomes. In many studies included in this review, dialysis treatments at home were longer, more frequent or both. There was also variation between studies in what was considered a conventional prescription, likely due to differences in standard practice from country to country. Likewise, while NxStage was used in some studies comparing HHD and ICHD, NxStage is not available or not widely used in some countries. Some studies reported treatment duration or parameters in little detail or did not describe it at all. While all of these factors have potentially impacted the generalisability of results, differences in dialysis prescription were not considered a source of bias in this review since the option to easily convert to intensive dialysis is one of the distinct benefits of HHD.
Studies varied in design and included prospective longitudinal cohort studies, retrospective cohort studies including registry analyses, cross‐sectional studies and one cross‐over RCT. Dialysis vintage was not always described in studies, nor was the duration of the current dialysis modality. Across included studies, some were conducted in patients already established on HHD, some in patients established on HD but new to HHD, and in others, patients were new to any form of dialysis. The experiences of different venues for HD treatment may be systematically different between these populations. Thus, varying vintage and familiarity with dialysis modality may have been an effect modifier for some outcomes. QoL data were derived nearly entirely from cross‐sectional studies in which patients had been treated with a dialysis modality for varying and, in some cases, unknown time periods.
Additionally, descriptions of selection and support for HHD (e.g. payment source for equipment, water and electricity; presence of support at home; financial incentives) were generally not described and could have led to residual confounding. The lack of standardised descriptions of selection and support for HHD limits the generalisability of the findings.
One of the main limitations of this review was the inability to meta‐analyse many outcomes due to the large number of ways outcomes were reported. Planned subgroup and sensitivity analyses could not be performed due to the small number of studies available for analysis for each outcome. Differences in outcome reporting were due to the use of different tools (e.g. QoL measures), as well as different reporting metrics (e.g. hospital admissions versus duration versus number of patients hospitalised). While multiple metrics for an outcome may be valuable since they often reflect different aspects of that outcome, the highly variable, limited or incomplete data reporting seen in this review inevitably limits comparability to other studies, ability to benchmark, and therefore contributes to research waste. This has been one of the motivating issues behind the Standardised Outcomes in Nephrology (SONG) Initiative, which establishes a set of core outcome measures to be reported in all trials in a given field.
Quality of the evidence
Overall, the risk of bias assessment for most studies was low following assessment by the NOS and adapted NOS. However, these observational studies were subject to unavoidable bias due to their study design. Patients receiving HHD were often younger and less comorbid than patients receiving ICHD, thus potentially leading to selection bias and confounding by indication. Some cohort studies attempted to account for potential differences by using patient matching techniques or reporting on patients transitioning from one modality to another, thus acting as their own control. However, many studies were cross‐sectional studies and did not adjust for other factors. It is possible that differences in outcomes reflected not just the effects of the treatment modality but the differing study populations.
The risk of bias was increased by the lack of comparability between treatment interventions, including differences in dialysate composition in the RCT by McGregor 2001, and differences in treatment prescription and delivery as previously outlined. The heterogeneity of studies also impacted the quality of evidence, and the overall quality for all outcomes was low or very low (Table 1).
Potential biases in the review process
While this review was conducted using standard Cochrane methodology, potential biases existed in the review process that may have limited the validity of the findings. First, only one RCT was included after searching and assessment according to our inclusion criteria, and the remainder of the included studies were NRSIs. Secondly, publication bias may have existed (i.e. bias caused by lack of publication with neutral or opposite effects). Due to a lack of sufficient data, we could not test for potential publication bias. Third, many outcomes could not be meta‐analysed due to high variability in reporting metrics utilised and reported data being insufficient for extraction. Beneficial effects or harms of HHD may not have been observed due to a lack of analysable data.
Agreements and disagreements with other studies or reviews
A 2003 review of HHD summarised data from 22 cohort studies and compared HHD versus hospital or satellite unit HD for people with kidney failure (Mowatt 2003). The authors reported that people treated with HHD generally experienced better QoL, lower hospitalisation, longer survival and increased likelihood of employment. HHD was also more cost‐effective than hospital or satellite HD. However, partners of these patients appeared to experience increased treatment burden and decreased satisfaction. Confounding by indication may have impacted the results. As was the case in the current review, details regarding dialysis prescription and delivery method were often unavailable when these may have impacted outcomes. Overall, the consistency and effect size of HHD on clinical outcomes (QoL, death, hospital admission) based on these analyses were uncertain. A systematic qualitative review of HHD versus ICHD identified 44 studies and found that clinical outcomes in patients receiving HHD were generally superior, including improved survival, cardiovascular parameters and QoL (Miller 2018). However, there were several limitations to the review, including data were mostly obtained from retrospective cohort studies, many being small observational studies, and prone to confounding since the patients receiving HHD were younger with fewer comorbidities, had increased pre‐dialysis education and were more likely to have been referred earlier to a nephrologist. Finally, a systematic review and meta‐analysis of HRQoL outcomes in HHD versus ICHD suggested nominally improved physical HRQoL in patients receiving HHD; however, included studies provided low‐quality evidence, and many had design issues (Bonenkamp 2020).
Intensive dialysis regimens are more easily implemented in the home setting since HHD has the potential to increase dialysis frequency and duration through greater treatment flexibility. While the location of dialysis (i.e. home versus in‐centre) was not the exposure of interest, a review comparing intensive dialysis with conventional HD suggested intensive dialysis may be associated with improved clinical outcomes. However, effect estimates were imprecise, and the effects of confounding could not be excluded (Mohr 2001). Similarly, reviews of 14 cohort studies of daily HD (Suri 2006) and 14 cohort studies of nocturnal HD (Walsh 2005) found that evidence, especially for hard clinical outcomes such as death, tended to be limited by small sample sizes for available studies, non‐comparable control groups, bias due to treatment selection and attrition, and insufficient data for potential risks. Finally, two RCTs compared increased frequency, duration of HD or both (FHN Trial Group 2010; Walsh 2006). Improvements in cardiac function and selected components of QoL and BP control indicated that longer hours of dialysis might improve patient‐relevant outcomes in adults treated with HD, although dialysis vascular access complications may be more frequent.
Authors' conclusions
Implications for practice.
Currently, available data indicate uncertain treatment benefits of HHD on patient‐relevant outcomes. However, this is based on low to very low certainty evidence and must be interpreted and applied cautiously.
Implications for research.
Given the existing poor survival and high symptom burden associated with HD treatment, an understanding of modifiable determinants of clinical outcomes in this population is needed. Non‐randomised evidence suggested that HHD was associated with better survival and QoL.
No further RCTs comparing HHD and ICHD have been performed since the prior version of this review, and we are not aware of any that are planned. Recently, there has been increased emphasis on shared decision‐making based on patients' own priorities, which is of great importance during the modality selection process. For some patients, freedom and flexibility may be a priority, leading them to favour HHD. On the other hand, some patients may prioritise reduced mental and treatment burden, leading them to prefer ICHD. As such, it may be unlikely that a large, well‐designed RCT of HHD versus ICHD will be performed in the future.
Therefore, future research in this area may continue to be mainly based on NRSIs. However, it is essential that future studies align outcome measures and metrics with other research in the field to allow comparison between studies, establish outcome effects with greater certainty, and avoid research waste.
What's new
| Date | Event | Description |
|---|---|---|
| 22 May 2024 | Amended | Minor edit to plain language heading |
History
Protocol first published: Issue 1, 2012 Review first published: Issue 11, 2014
| Date | Event | Description |
|---|---|---|
| 8 April 2024 | New search has been performed | Inclusion criteria revised to also include non‐randomised studies. MEDLINE (OVID) and EMBASE (OVID) search strategies updated. |
| 8 April 2024 | New citation required and conclusions have changed | New studies included |
| 4 December 2014 | Review declared as stable | As of December 2014 this Cochrane Review was no longer being updated. There have been no new studies published on this topic in the past 12 years and there are currently no registered ongoing studies. |
Acknowledgements
The authors would like to thank Narelle Willis and Fiona Russell for their support, comments, and advice while preparing this review. The authors gratefully acknowledge Michael Schumacher (Technische Hochschule Mittelhessen) for his assistance with the language translation of a study included in this review. We wish to thank Andrew Palmer, Miguel Leal and Susanne Hoischen for their contributions as authors to the previous version of this review.
The authors are grateful to the following peer reviewers for their time and comments: Anil K Agarwal MD (Professor of Medicine, UCSF Fresno, and Chief of Medicine, VA Central California Health Care System, Fresno, California, USA); John Anderson M.D (Retired Nephrologist Johns Hopkins Medical Institutions), and Rommel P. Bataclan, MD (University of the East Ramon Magsaysay Medical Center, Philippines).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE (OVID) |
|
| EMBASE (OVID) |
|
Appendix 2. Newcastle‐Ottawa Scale (for cohort studies)
Selection (maximum 1 star for each numbered item)
-
Representativeness of the exposed cohort)
truly representative of the average _______________ (describe) in the community*
somewhat representative of the average ______________ in the community*
selected group of users eg nurses, volunteers
no description of the derivation of the cohort
-
Selection of the non exposed cohort
drawn from the same community as the exposed cohort*
drawn from a different source
no description of the derivation of the non exposed cohort
-
Ascertainment of exposure
secure record (e.g. surgical records)*
structured interview*
written self report
no description
-
Demonstration that outcome of interest was not present at start of study
yes*
no
Comparability (maximum 2 stars)
-
Comparability of cohorts on the basis of the design or analysis
study controls for _____________ (select the most important factor)*
study controls for any additional factor* (this criteria could be modified to indicate specific control for a second important factor)
Outcome (maximum 1 star for each numbered item)
-
Assessment of outcome
independent blind assessment*
record linkage*
self report
no description
-
Was follow‐up long enough for outcomes to occur
yes (select an adequate follow up period for outcome of interest)*
no
-
Adequacy of follow up of cohorts
complete follow up ‐ all subjects accounted for*
subjects lost to follow up unlikely to introduce bias ‐ small number lost ⇢ ____ % (select an adequate %) follow up, or description provided of those lost)*
follow up rate < ____% (select an adequate %) and no description of those lost) no statement
Appendix 3. Adapted Newcastle‐Ottawa Scale (for cross‐sectional studies)
Selection (maximum 5 stars)
-
Representativeness of the sample:
Truly representative of the average in the target population* (all subjects or random sampling)
Somewhat representative of the average in the target population* (non‐random sampling)
Selected group of users
No description of the sampling strategy
-
Sample size:
Justified and satisfactory*
Not justified
-
Non‐respondents:
Comparability between respondents and non‐respondents characteristics is established, and the response rate is satisfactory*
The response rate is unsatisfactory, or the comparability between respondents and non‐respondents is unsatisfactory
No description of the response rate or the characteristics of the responders and the non‐responders
-
Ascertainment of the exposure (risk factor):
Validated measurement tool**
Non‐validated measurement tool, but the tool is available or described*
No description of the measurement tool
Comparability (maximum 2 stars)
-
The subjects in different outcome groups are comparable, based on the study design or analysis. Confounding factors are controlled
The study controls for the most important factor (select one)*
The study control for any additional factor. *
Outcome (maximum 3 stars)
-
Assessment of the outcome:
Independent blind assessment**
Record linkage**
Self report*
No description
-
Statistical test:
The statistical test used to analyse the data is clearly described and appropriate, and the measurement of the association is presented, including confidence intervals and the probability level (p value)*
The statistical test is not appropriate, not described or incomplete
Data and analyses
Comparison 1. Home versus in‐centre haemodialysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Cardiovascular death | 2 | 30900 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.80, 1.07] |
| 1.2 All‐cause death | 9 | 58984 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.95] |
| 1.3 All‐cause annual hospital admission rate (number of admissions/patient‐year) | 2 | 834 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.98, ‐0.02] |
| 1.4 All‐cause hospitalisation days/patient‐year | 2 | 834 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.28, ‐1.53] |
| 1.5 Kidney transplantation during study period | 6 | 10910 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.01, 1.63] |
| 1.6 Physical functioning (SF‐12; SF‐36) | 2 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [‐0.03, 1.16] |
| 1.6.1 SF‐12 | 1 | 294 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.05, 0.62] |
| 1.6.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.89 [0.43, 1.36] |
| 1.7 Role: Physical (SF‐12; SF‐36) | 2 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.12, 0.96] |
| 1.7.1 SF‐12 | 1 | 294 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.17, 0.50] |
| 1.7.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.72 [0.26, 1.18] |
| 1.8 Bodily pain (SF‐12; SF‐36) | 2 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.39, 0.75] |
| 1.8.1 SF‐12 | 1 | 294 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.42, 0.24] |
| 1.8.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.04, 0.94] |
| 1.9 General health (SF‐12; SF‐36) | 2 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.07, 0.60] |
| 1.9.1 SF‐12 | 1 | 294 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.02, 0.68] |
| 1.9.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.12, 0.77] |
| 1.10 Vitality (SF‐12; SF‐36) | 2 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.27, 0.80] |
| 1.10.1 SF‐12 | 1 | 294 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [0.21, 0.88] |
| 1.10.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.06, 0.97] |
| 1.11 Social functioning (SF‐12; SF‐36) | 2 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.29, 0.83] |
| 1.11.1 SF‐12 | 1 | 294 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.17, 0.84] |
| 1.11.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.22, 1.13] |
| 1.12 Role: Emotional (SF‐12; SF‐36) | 2 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.84, 1.81] |
| 1.12.1 SF‐12 | 1 | 294 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.51, 0.15] |
| 1.12.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 1.17 [0.69, 1.65] |
| 1.13 Mental health (SF‐12; SF‐36) | 2 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.23, 0.89] |
| 1.13.1 SF‐12 | 1 | 294 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.26, 0.40] |
| 1.13.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.19, 1.10] |
| 1.14 Physical Component Summary (SF‐12; SF‐36) | 5 | 922 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.10, 0.73] |
| 1.14.1 SF‐12 | 4 | 842 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.04, 0.57] |
| 1.14.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 1.05 [0.58, 1.53] |
| 1.15 Mental Component Summary (SF‐12; SF‐36) | 5 | 922 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.05, 0.25] |
| 1.15.1 SF‐12 | 4 | 842 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.07, 0.25] |
| 1.15.2 SF‐36 | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.24, 0.65] |
| 1.16 Symptoms and problems (KDQOL‐SF) | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 3.96 [‐7.48, 15.40] |
| 1.17 Effects of kidney disease (KDQOL‐SF) | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 11.61 [‐0.52, 23.75] |
| 1.18 Burden of kidney disease (KDQOL‐SF) | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐8.70, 7.40] |
| 1.19 Work status (KDQOL‐SF) | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 33.32 [12.52, 54.11] |
| 1.20 Cognitive function (KDQOL‐SF) | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 2.12 [‐3.10, 7.34] |
| 1.21 Quality of social interaction (KDQOL‐SF) | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 4.82 [‐0.78, 10.43] |
| 1.22 Sexual function (KDQOL‐SF) | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 8.62 [‐0.71, 17.95] |
| 1.23 Sleep (KDQOL‐SF) | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 4.02 [‐2.19, 10.22] |
| 1.24 Recovery time | 2 | 348 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐2.73, ‐1.28] |
| 1.25 Annual direct healthcare costs in first year of dialysis (currency as reported) | 4 | 13809 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐2.13, ‐0.37] |
| 1.25.1 Year 1 of dialysis | 2 | 10077 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐4.08, 0.49] |
| 1.25.2 Dialysis vintage not specified | 2 | 3732 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.59, 0.25] |
| 1.26 Annual direct healthcare costs in second year of dialysis (currency as reported) | 4 | 13809 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.72, ‐0.21] |
| 1.26.1 Year 2 of dialysis | 2 | 10077 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐6.94, 2.34] |
| 1.26.2 Dialysis vintage not specified | 2 | 3732 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.65, 0.38] |
| 1.27 Systolic blood pressure (NRSIs) | 4 | 491 | Mean Difference (IV, Random, 95% CI) | ‐11.78 [‐21.11, ‐2.46] |
| 1.27.1 NRSIs, pre‐dialysis measurement | 2 | 173 | Mean Difference (IV, Random, 95% CI) | ‐9.97 [‐34.35, 14.41] |
| 1.27.2 NRSIs, clinic or other measurements | 2 | 318 | Mean Difference (IV, Random, 95% CI) | ‐12.23 [‐17.92, ‐6.54] |
| 1.28 Diastolic blood pressure (NSRIs) | 3 | 383 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐1.31, 4.94] |
| 1.28.1 NRSIs, pre‐dialysis measurement | 1 | 65 | Mean Difference (IV, Random, 95% CI) | 4.00 [‐1.06, 9.06] |
| 1.28.2 NRSIs, clinic or other measurement | 2 | 318 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐3.51, 4.44] |
| 1.29 Mean arterial pressure | 2 | 44 | Mean Difference (IV, Random, 95% CI) | ‐7.01 [‐12.57, ‐1.46] |
| 1.30 Left ventricular mass index [g/m2] | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐18.13 [‐32.08, ‐4.17] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ageborg 2005.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
Group differences
Definitions
|
|
| Interventions | HHD
Self‐care HD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain values for SF‐36 (presented in graph only), awaiting response | |
Bragg‐Gresham 2018.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
Group differences
|
|
| Interventions | HHD Self‐care HD ICHD |
|
| Outcomes | Outcomes relevant to this review
Outcomes reported
|
|
| Identification | Additional information
|
|
| Notes | Abstract only. Contacted author to obtain data on maintained employment for each modality separately, awaiting response. | |
Dumaine 2018.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
|
|
| Interventions | HHD ICHD |
|
| Outcomes | Outcomes relevant to this review
Notes: study intended to report the mean change at 90 days from baseline in patients who transitioned from IHD to HHD, however, this abstract included only baseline data Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Abstract only. Contacted author to obtain follow‐up data after modality transition, awaiting response | |
Griva 2010.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
Group differences
|
|
| Interventions | HHD ICHD |
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | ||
Ha 2018.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
|
|
| Interventions | HHD ICHD |
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | Results
|
|
Hayhurst 2015.
| Study characteristics | ||
| Methods | Study design
Study methods
Study characteristics
|
|
| Participants | Baseline characteristics
Group differences
|
|
| Interventions | HHD ICHD |
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | Unable to contact author to obtain measures of variability for outcomes. | |
Jayanti 2016.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
Group differences
|
|
| Interventions | HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
Outcome measures
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain more detailed outcome data, awaiting response. Questionnaires return rate
|
|
Kasza 2016.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
Group differences
|
|
| Interventions | HHD
ICHD (AVF/AVG)
ICHD (CVC)
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain more detailed data on outcomes by modality, awaiting response. | |
Kjellstrand 2008.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
|
|
| Interventions | Intensive HHD
Intensive ICHD
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain more detailed outcome data, awaiting response | |
Kojima 2012.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
|
|
| Interventions | Intensive HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Unable to contact author to obtain measure of variability and more detailed outcome data. | |
Krahn 2019.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
Intensive ICHD
ICHD
Overall
|
|
| Interventions | HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
Definitions
|
|
| Identification | Additional information
|
|
| Notes | ||
Kraus 2007.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics Overall
Group differences
|
|
| Interventions | Intensive HHD
Intensive ICHD
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain more detailed outcome data, awaiting response | |
Krishnasamy 2013.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics Overall
Group differences
|
|
| Interventions | HHD
ICHD
Intensive ICHD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
Definitions
|
|
| Identification | Additional information
|
|
| Notes | Data not extracted for meta‐analysis due to potential overlapping population with Marshall 2021. | |
Lee 2002.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
Satellite HD
ICHD
PD
Overall
Group differences
|
|
| Interventions | HHD
Satellite HD
ICHD
PD
|
|
| Outcomes | Outcomes relevant to this review
Outcome measures
|
|
| Identification | Additional information
|
|
| Notes | Incomplete data
|
|
Lorenzen 2012.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
|
|
| Interventions | Intensive HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | ||
Malmstrom 2008.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
Satellite HD
Group differences
|
|
| Interventions | HHD
Satellite HD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Unable to contact author to obtain measures of variability | |
Marshall 2021.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics ICHD
HHD
|
|
| Interventions | ICHD
HHD
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | Data from Marshall 2021 publication analysed. Data from other publications not analysed due to potentially overlapping populations. | |
McGregor 2001.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
|
|
| Interventions | ICHD
HHD
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to attempt to obtain measures of variability and further details from study, however data no longer available due to earthquake damage | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation using random number table designed by statistician (data obtained from authors on request) |
| Allocation concealment (selection bias) | Low risk | Treatment allocation assigned by statistician unaware of patient details (data obtained from authors on request) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | QoL, BP, and echocardiography outcomes assessed by investigators unaware of treatment sequence (data obtained from authors on request) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0/9 (0%) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes systematically assessed |
| Other bias | High risk | Unmatched interventions (acetate versus bicarbonate buffer) |
Murashima 2010.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
Group differences
|
|
| Interventions | ICHD
Intensive HHD
|
|
| Outcomes | Outcomes relevant to this review
Definitions
|
|
| Identification | Additional information
|
|
| Notes | ||
Nebel 2002.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
Group differences
|
|
| Interventions | HHD ICHD |
|
| Outcomes | Outcomes relevant to this review
Reported primary outcomes
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain measures of variability, however advised data not available | |
Nesrallah 2012.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics (after matching) Intensive HHD
ICHD
|
|
| Interventions | Intensive HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | ||
Nitsch 2011.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
Satellite HD
Definitions
|
|
| Interventions | HHD
ICHD
Satellite HD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | ||
NxStage‐USRDS 2012.
| Study characteristics | ||
| Methods |
Weinhandl 2012
Weinhandl 2015a
Weinhandl 2015d
Study characteristics
|
|
| Participants |
Baseline characteristics: Weinhandl 2012 HHD
ICHD
Baseline characteristics: Weinhandl 2015a HHD
ICHD
Baseline characteristics: Weinhandl 2015d
|
|
| Interventions | HHD
ICHD
|
|
| Outcomes |
Weinhandl 2012
Weinhandl 2015a
Weinhandl 2015d
|
|
| Identification | Additional information
|
|
| Notes | Data from Weinhandl 2012 analysed for mortality outcome. Data from Weinhandl 2015 analysed for hospitalisation outcome. Data from Weinhandl 2015d analysed for kidney transplantation outcome. Data from other publications not analysed due to potential overlapping populations for the same outcomes. Contacted author to obtain more detailed outcome data, awaiting response. | |
Piccoli 2004.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
Satellite HD
Group differences
|
|
| Interventions | HHD
Satellite HD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain more detailed outcome data, awaiting response. | |
Rydell 2016.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
|
|
| Interventions | HHD ICHD |
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain measures of variability, however advised data not available. | |
Rydell 2019.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
PD
|
|
| Interventions | HHD ICHD PD |
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain measures of variability, however advised data not available. | |
Sands 2009.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics
|
|
| Interventions | HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Unable to contact author to obtain more detailed outcome data | |
Saner 2005.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
|
|
| Interventions | HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain more detailed outcome data, awaiting response | |
Suri 2015.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
|
|
| Interventions | Intensive HHD
ICHD |
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain more detailed outcome data, awaiting response | |
Tablo IDE 2020.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics Established on HHD
New on HHD
Definitions
|
|
| Interventions | HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | ||
Tennankore 2022.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
Intensive HHD
Intensive ICHD
Definitions
|
|
| Interventions | HHD
ICHD
Intensive HHD
Intensive ICHD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Contacted author to obtain more detailed outcome data and measures of variability, awaiting response from statistician | |
Toronto Group 2002.
| Study characteristics | ||
| Methods |
Chan 2005
Bergman 2008
Cafazzo 2009
Qualitative ethnographic interviews
Study characteristics
|
|
| Participants |
Baseline characteristics: Chan 2005 HHD
ICHD
Baseline characteristics: Bergman 2008 HHD
ICHD
Group differences
Baseline characteristics: Cafazzo 2009 HHD
ICHD
Group differences
|
|
| Interventions |
Chan 2005 HHD
ICHD
Bergman 2008 Nocturnal HHD
ICHD
Cafazzo 2009 Nocturnal HHD
ICHD
|
|
| Outcomes |
Chan 2005 Outcomes relevant to this review
Reported outcomes
Bergman 2008 Outcomes relevant to this review
Reported outcomes
Cafazzo 2009 Outcomes relevant to this review
|
|
| Identification |
Additional information: Chan 2005
Additional information: Bergman 2008
Additional information: Cafazzo 2009
|
|
| Notes | Patient recruitment
Studies conducted within nocturnal HHD program and University Health Network, where patients receiving ICHD were trained to perform nocturnal HHD
|
|
Van Oosten 2018.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics ICHD
HHD
Definitions
|
|
| Interventions | ICHD HHD |
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Abstract published in 2018 (van Oosten). Full paper published in 2019 (Mohnen and van Oosten as equal first authors). | |
Watanabe 2014.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
|
|
| Interventions | HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | ||
Wong 2019a.
| Study characteristics | ||
| Methods | Study design
Statistical analysis
Study characteristics
|
|
| Participants | ICHD
HHD
Satellite HD
|
|
| Interventions | ICHD
HHD
Satellite HD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | ||
Wong 2019b.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
|
|
| Interventions | HHD ICHD |
|
| Outcomes | Outcomes relevant to this review
|
|
| Identification | Additional information
|
|
| Notes | ||
Wright 2015.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
Group differences
|
|
| Interventions | HHD ICHD |
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | ||
Xue 2015.
| Study characteristics | ||
| Methods |
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
Group differences
|
|
| Interventions | Intensive HHD ICHD |
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | ||
Yeung 2021.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
ICHD
Group differences
|
|
| Interventions | HHD
ICHD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | ||
Zimbudzi 2014.
| Study characteristics | ||
| Methods | Study design
Study characteristics
|
|
| Participants | Baseline characteristics HHD
Satellite HD
Group differences
|
|
| Interventions | HHD
Satellite HD
|
|
| Outcomes | Outcomes relevant to this review
Reported outcomes
|
|
| Identification | Additional information
|
|
| Notes | Unable to contact author to obtain measures of variability and more detailed outcome data | |
AE: adverse event; APD: ambulatory peritoneal dialysis; ATSI: Aboriginal and Torres Strait Islander; AVF: arteriovenous fistula; AVG: arteriovenous graft; BDI: Beck Depression Inventory; BMI: body mass index; BP: blood pressure; BUN: blood urea nitrogen; CAS: coronary artery disease; CAPD: continuous ambulatory peritoneal dialysis; CCPD: continuous cycling peritoneal dialysis; CHF: congestive heart failure; CI: confidence interval; CKD: chronic kidney disease; CVC: central venous catheter; ECG: echocardiogram; eGFR: estimated glomerular filtration rate; EPO: erythropoietin; ESA: erythropoietin‐stimulating agent; ESKD: end‐stage kidney disease; ESRD‐SI: End‐Stage Renal Disease Severity Index; FSGS: focal segmental glomerulosclerosis; GN: glomerulonephritis; Hb: haemoglobin; HD: haemodialysis; HHD: home haemodialysis; HR: hazard ratio; HRQoL: health‐related quality of life; ICHD: in‐centre haemodialysis; IEQ: Illness Effects Questionnaire; IHD: ischaemic heart disease; IPQ: Illness Perception Questionnaire; iPTH: intact parathyroid hormone; IQR: interquartile range; ITT: intention‐to‐treat; KDQOL‐36: Kidney Disease Quality of Life‐36 questionnaire; KRT: kidney replacement therapy; Kt/V: dialysis adequacy; LVMI: left ventricular mass index; MAP: mean arterial pressure; NA: not applicable; PD: peritoneal dialysis; PKD: polycystic kidney disease; PVD: peripheral vascular disease; QoL: quality of life; RCT: randomised controlled trial; SCr: serum creatinine; SDHD: short daily haemodialysis; SF: short form; TB: tuberculosis; TEQ: Treatment Effects Questionnaire; VA: vascular access
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Benain 2007 | Wrong study design |
| Benain 2015 | Wrong study design |
| Bernieh 2020 | Not HHD vs ICHD |
| Blagg 2006 | Not HHD vs ICHD |
| Connor 2011 | Wrong study design |
| Derrett 2017 | Not HHD vs ICHD |
| Eneanya 2019 | Not HHD vs ICHD |
| FREEDOM 2009 | Not HHD vs ICHD |
| Gonzalez‐Perez 2005 | Wrong study design |
| Gorham 2019 | Wrong study design |
| Howard 2009 | Wrong study design |
| Ipema 2010 | Wrong outcome |
| Ipema 2012 | Wrong outcome |
| Ipema 2014 | Not HHD vs ICHD |
| Johansen 2009 | Not HHD vs ICHD |
| Komenda 2012 | Wrong study design |
| Kubisiak 2018 | Wrong study design |
| Lee 2017 | Not HHD vs ICHD |
| Lockridge 2011 | Wrong study design |
| London Daily/Nocturnal 2003 | Not HHD vs ICHD |
| Loos‐Ayav 2008 | Not HHD vs ICHD |
| MacRae 2010 | Not HHD vs ICHD |
| Marshall 2015 | Not HHD vs ICHD |
| McDonald 2009 | Not HHD vs ICHD |
| McFarlane 2002 | Not HHD vs ICHD |
| Mehrotra 2016 | Not HHD vs ICHD |
| Mohr 2001 | Wrong study design |
| Quintaliani 2000 | Not HHD vs ICHD |
| Rivara 2018 | Not HHD vs ICHD |
| Seto 2007 | Not HHD vs ICHD |
| Shen 2019 | Not HHD vs ICHD |
| Ting 2003 | Not HHD vs ICHD |
| Walsh 2006 | Not HHD vs ICHD |
| Yang 2015 | Not HHD vs ICHD |
| Yuen 2011 | Wrong study design |
HHD: home haemodialysis; ICHD: in‐centre haemodialysis
Characteristics of studies awaiting classification [ordered by study ID]
De Smet 2007.
| Methods | Study design
Study characteristics
|
| Participants | Prevalent stable HD patients receiving standard HD
|
| Interventions | Standard HD
Nocturnal HD
|
| Outcomes | Biochemical parameters
|
| Notes | Abstract‐only publication Contacted author to confirm location of conventional versus nocturnal HD treatments, awaiting response |
Fadem 2011.
| Methods | Study design
|
| Participants | Patients (n = 821) and caregivers (n = 56) of patients with kidney failure |
| Interventions |
|
| Outcomes |
|
| Notes |
|
Hanly 2001.
| Methods | Study design
|
| Participants | Prevalent adult HD patients who switched from conventional ICHD to nocturnal HHD between November 1993 and November 1998 (n = 14) |
| Interventions | Conventional ICHD
Nocturnal HHD
|
| Outcomes | Sleep apnoea |
| Notes | Unable to contact author to ascertain if additional outcome data available |
Helantera 2012.
| Methods | Study design
|
| Participants | Prevalent dialysis and kidney transplant patients in Finland aged 15 to 64 years at the end of 2007 (n = 2637) |
| Interventions |
|
| Outcomes | Employment status |
| Notes | Contacted author to obtain data for adult (≥ 18 years) subgroup, awaiting response |
Kannampuzha 2010.
| Methods | Study design
|
| Participants | Prevalent adult (18 to 75 years) HD patients on their respective modality for a minimum 6 months, who were medically stable
Healthy controls
|
| Interventions | ICHD
Nocturnal HHD
|
| Outcomes |
|
| Notes | Unable to contact author to ascertain if additional outcome data available. |
Mitchell 2020.
| Methods | Study design
|
| Participants | Patients with kidney failure (n = 69) and CKD stage 4‐5 (n =25) |
| Interventions |
|
| Outcomes | Patient Activation Measure (PAM‐13®) |
| Notes |
|
Morton 2010.
| Methods | Study design
|
| Participants | Prevalent adult dialysis and transplant patients
|
| Interventions |
|
| Outcomes | Patient views about treatment of stage 5 CKD |
| Notes | Contacted author to ascertain if additional outcome data available, awaiting response |
Painter 2012.
| Methods | Study design
|
| Participants |
|
| Interventions | Group 1
Group 2
Group 3
Control
|
| Outcomes |
|
| Notes | Contacted author to confirm location of conventional HD treatments, awaiting response |
Parker 2014.
| Methods | Study design
|
| Participants |
|
| Interventions |
|
| Outcomes | Medication pill burden |
| Notes | Contacted author to ascertain if additional outcome data available, awaiting response. |
Pellicano 2010.
| Methods | Study design
|
| Participants | Prevalent HHD patients (n = 28)
Conventional ICHD patients (n = 28) Patient groups matched on age, gender, diabetic status and duration of KRT |
| Interventions |
|
| Outcomes |
|
| Notes | Contacted author to ascertain if additional outcome data available, awaiting response. |
Poon 2015.
| Methods | Study design
|
| Participants |
|
| Interventions | Nocturnal HHD
ICHD
|
| Outcomes |
|
| Notes | Contacted author to ascertain if additional outcome data available, awaiting response |
Sikkes 2009.
| Methods | Study design
|
| Participants | Prevalent adult HD patients who were medically stable at least 3 months who were able to perform nocturnal HHD and agreed to do so, and who lived with a spouse or other partner who could assist them (n = 14) |
| Interventions | Conventional ICHD
Nocturnal HHD
|
| Outcomes |
|
| Notes | Contacted author to ascertain if additional outcome data available, awaiting response. |
Thomson 2013.
| Methods | Study design
|
| Participants |
|
| Interventions | Conventional ICHD
Short daily HD
Frequent nocturnal HD
Intermittent nocturnal HD
|
| Outcomes | QTc interval |
| Notes | Contacted author to ascertain if additional outcome data available, awaiting response |
Yong 2014.
| Methods | Study design
|
| Participants | Stable conventional HD patients commencing quotidian HHD between 2012 and 2013 (n = 14) |
| Interventions |
|
| Outcomes |
|
| Notes |
|
APD: automated peritoneal dialysis; BMI: body mass index; CAPD: continuous ambulatory peritoneal dialysis; CKD: chronic kidney disease; HD: haemodialysis; HHD: home haemodialysis; ICHD: in‐centre haemodialysis; KDQOL: Kidney Disease Quality of Life Questionnaire ; KRT: kidney replacemetn therapy; PD: peritoneal dialysis; SF‐36: 36‐Item Short Form Survey
Differences between protocol and review
2024 update
Non‐randomised studies have been included.
We clarified that studies evaluating peritoneal dialysis as a home dialysis modality were eligible for inclusion, where data regarding participants receiving in‐centre and home haemodialysis could be extracted separately. We initially sought to include the clinical outcome of wait‐listing for a kidney transplant; we also elected to include the outcome of receipt of a kidney transplant, as this was reported in a number of studies. Similarly, we initially sought to include pre‐dialysis systolic and diastolic blood pressure (BP) but elected to use a more inclusive BP outcome by also extracting systolic and diastolic BP taken at times other than pre‐dialysis (e.g. clinic measurements) mean arterial pressure (MAP) and pulse pressure.
Contributions of authors
Draft the protocol: MC, IE
Study selection: MC, IE
Extract data from studies: MC, IE
Enter data into RevMan: MC, IE
Carry out the analysis: MC
Interpret the analysis: all authors
Draft the final review: MC, IE
Review the final review for intellectual content: all authors
Disagreement resolution: YC, RK, DJ
Update the review: SP, GFMS
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Melissa S Cheetham: has received travel support from Amgen and is a current recipient of a Queensland Advancing Clinical Research Fellowship
Isabelle Ethier: none known
Rathika Krishnasamy: has received speaker’s honoraria, consultancy fees, research grants and travel support from Baxter Healthcare, and travel support from Amgen
Yeoungjee Cho: has received research grants and speaker’s honoraria from Baxter Healthcare and Fresenius Medical Care
Suetonia C Palmer: none known
David W Johnson: has received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care, consultancy fees from Astra Zeneca, Bayer, and AWAK, speaker’s honoraria from ONO and BI & Lilly, and travel sponsorships from Ono and Amgen. He is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant
Jonathan C Craig: none known
Paul Stroumza: none known
Luc Frantzen: none known
Jorgen Hegbrant: serves on the Board of Directors of NorrDia AB and provides consultancy services to Triomed AB
Giovanni FM Strippoli: none known
Edited (no change to conclusions)
References
References to studies included in this review
Ageborg 2005 {published data only}
- Ageborg M, Allenius BL, Cederfjall C. Quality of life, self-care ability, and sense of coherence in hemodialysis patients: a comparative study. Hemodialysis International 2005;9 Suppl 1:S8-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bragg‐Gresham 2018 {published data only}
- Bragg-Gresham J, Schatell D, Witten B, Nie Y, Saran R. Pre-ESRD care, self-dialysis, and maintenance of employment among incident dialysis patients, 2006-2015 [abstract]. Hemodialysis International 2018;22(1):A21. [EMBASE: 620701259] [Google Scholar]
Dumaine 2018 {published data only}
- Dumaine CS, Ravani P, Santana M, MacRae J. How do transitions in end-stage renal disease care pathways impact health-related quality of life? [abstract]. Blood Purification 2018;45(1-3):285-6. [EMBASE: 622250443] [Google Scholar]
Griva 2010 {published data only}
- Griva K, Davenport A, Harrison M, Newman S. An evaluation of illness, treatment perceptions, and depression in hospital- vs. home-based dialysis modalities. Journal of Psychosomatic Research 2010;69(4):363-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ha 2018 {published data only}
- Ha J, Hoffman A, Brown MA. Physical and psychological symptom burden of renal transplant and dialysis patients [abstract]. Nephrology 2018;23(Suppl 3):53. [EMBASE: 623840873] [Google Scholar]
- Ha J, Hoffman A, Brown MA. The symptom burden of transplant patients compared to dialysis patients [abstract]. Nephrology 2017;22(Suppl 3):88. [EMBASE: 618236345] [Google Scholar]
Hayhurst 2015 {published data only}
- Hayhurst WS, Ahmed A. Assessment of physical activity in patients with chronic kidney disease and renal replacement therapy [Erratum in: Springerplus. 2016;5(1):961]. Springerplus 2015;4:536. [DOI: 10.1186/s40064-015-1338-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhurst WS, Ahmed A. Assessment of physical activity in patients with chronic kidney disease and renal replacement therapy [abstract no: SP454]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii529. [EMBASE: 72207780] [Google Scholar]
Jayanti 2016 {published data only}
- Jayanti A, Foden P, Morris J, Brenchley P, Mitra S. Time to recovery from haemodialysis: location, intensity and beyond. Nephrology 2016;21(12):1017-26. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kasza 2016 {published data only}
- Kasza J, Wolfe R, McDonald SP, Marshall MR, Polkinghorne KR. Dialysis modality, vascular access and mortality in end-stage kidney disease: A bi-national registry-based cohort study. Nephrology 2016;21(10):878-86. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kjellstrand 2008 {published data only (unpublished sought but not used)}
- Kjellstrand C, Buoncristiani U, Ting G, Traeger J, Piccoli GB, Sibai-Galland R, et al. Survival with short-daily hemodialysis: Association of time, site, and dose of dialysis. Hemodialysis International 2010;14(4):464-70. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kjellstrand CM, Buoncristiani U, Ting G, Traeger J, Piccoli GB, Sibai-Galland R, et al. Short daily haemodialysis: survival in 415 patients treated for 1006 patient-years. Nephrology Dialysis Transplantation 2008;23(10):3283-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kojima 2012 {published data only}
- Kojima E, Hoshi H, Watanabe Y, Takenaka T, Suzuki H. Daily hemodialysis improves uremia-associated clinical parameters in the short term. Contributions to Nephrology 2012;177:169-77. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Krahn 2019 {published data only}
- Krahn M, Bremner KE, Oliveira C, Dixon SN, McFarlane P, Garg AX, et al. Costs and survival for dialysis patients: real world evidence supporting home dialysis [abstract]. Value in Health 2018;21(Suppl 2):S115. [EMBASE: 2001249030] [Google Scholar]
- Krahn MD, Bremner KE, Oliveira C, Dixon SN, McFarlane P, Garg AX, et al. Home dialysis is associated with lower costs and better survival than other modalities: A population-based study in Ontario, Canada. Peritoneal Dialysis International 2019;39(6):553-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kraus 2007 {published data only}
- Kraus M, Burkart J, Hegeman R, Solomon R, Coplon N, Moran J. A comparison of center-based vs. home-based daily hemodialysis for patients with end-stage renal disease. Hemodialysis International 2007;11(4):468-77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Krishnasamy 2013 {published data only}
- Krishnasamy R, Badve SV, Hawley CM, McDonald SP, Boudville N, Brown FG, et al. Daily variation in death in patients treated by long-term dialysis: comparison of in-center hemodialysis to peritoneal and home hemodialysis [Erratum in: Am J Kidney Dis. 2013 Jun;61(6):1049]. American Journal of Kidney Diseases 2013;61(1):96-103. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. American Journal of Kidney Diseases 2002;40(3):611-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lorenzen 2012 {published data only}
- Lorenzen JM, Thum T, Eisenbach GM, Haller H, Kielstein JT. Conversion from conventional in-centre thrice-weekly haemodialysis to short daily home haemodialysis ameliorates uremia-associated clinical parameters. International Urology & Nephrology 2012;44(3):883-90. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Malmstrom 2008 {published data only}
- Malmstrom RK, Roine RP, Heikkila A, Rasanen P, Sintonen H, Muroma-Karttunen R, et al. Cost analysis and health-related quality of life of home and self-care satellite haemodialysis. Nephrology Dialysis Transplantation 2008;23(6):1990-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marshall 2021 {published data only}
- Marshall M, Polkinghorne KR, Boudville N, McDonald SP. Mortality risk by dialysis modality over 1997 to 2017 in the Australian and New Zealand dialysis population [abstract no: SUN-187]. Kidney International Reports 2020;5(3 Suppl):S276-7. [EMBASE: 2005255332] [Google Scholar]
- Marshall MR, Hawley CM, Kerr PG, Polkinghorne KR, Marshall RJ, Agar JW, et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. American Journal of Kidney Diseases 2011;58(5):782-93. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Marshall MR, Polkinghorne KR, Boudville N, McDonald SP. Home versus facility dialysis and mortality in Australia and New Zealand. American Journal of Kidney Diseases 2021;78(6):826-36.e1. [DOI: 10.1053/j.ajkd.2021.03.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Marshall MR, Polkinghorne KR, Kerr PG, Hawley CM, Agar JW, McDonald SP. Intensive hemodialysis and mortality risk in ustralian and New Zealand populations. American Journal of Kidney Diseases 2016;67(4):617-28. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Marshall MR, Walker RC, Polkinghorne KR, Lynn KL. Survival on home dialysis in New Zealand. PLoS ONE [Electronic Resource] 2014;9(5):e95847. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MR, Schrieck N, Lilley D, Supershad SK, Ng A, Walker RC, et al. Independent community house hemodialysis as a novel dialysis setting: an observational cohort study. American Journal of Kidney Diseases 2013;61(4):598-607. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGregor 2001 {published data only}
- McGregor DO, Buttimore AL, Lynn KL, Nicholls MG, Jardine DL. A comparative study of blood pressure control with short In-center versus long home hemodialysis. Blood Purification 2001;19(3):293-300. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murashima 2010 {published data only}
- Murashima M, Kumar D, Doyle AM, Glickman JD. Comparison of intradialytic blood pressure variability between conventional thrice-weekly hemodialysis and short daily hemodialysis. Hemodialysis International 2010;14(3):270-7. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Nebel 2002 {published data only}
- Nebel M. Costs of renal replacement therapies in Germany in 1999 [Behandlungskosten der nierenersatztherapie in Deutschland 1999]. Nieren- und Hochdruckkrankheiten 2002;31(3):85-92. [EMBASE: 34250014] [Google Scholar]
Nesrallah 2012 {published data only}
- Nesrallah GE, Lindsay RM, Cuerden MS, Garg AX, Port F, Austin PC, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. Journal of the American Society of Nephrology 2012;23(4):696-705. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nitsch 2011 {published data only}
- Nitsch D, Steenkamp R, Tomson C, Roderick P, Ansell D, MacGregor M. Outcomes in patients on home haemodialysis in England and Wales 1997-2005: a comparative cohort analysis [abstract no: Sa199]. NDT Plus 2010;3(Suppl 3):iii98. [EMBASE: 70483665] [DOI] [PubMed] [Google Scholar]
- Nitsch D, Steenkamp R, Tomson CR, Roderick P, Ansell D, MacGregor MS. Outcomes in patients on home haemodialysis in England and Wales, 1997-2005: a comparative cohort analysis. Nephrology Dialysis Transplantation 2011;26(5):1670-7. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
NxStage‐USRDS 2012 {published data only}
- Kansal SK, Morfin JA, Weinhandl ED. Survival and kidney transplant incidence on home versus in-center hemodialysis, following peritoneal dialysis technique failure. Peritoneal Dialysis International 2019;39(1):25-34. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Weinhandl E, Collins A. 30-day readmission rates after heart failure and hypertensive disease discharges in daily home hemodialysis, peritoneal dialysis, and in-center hemodialysis patients [abstract no: 297]. American Journal of Kidney Diseases 2015;65(4):A89. [EMBASE: 71874992] [Google Scholar]
- Weinhandl E, Collins A. Incidence of kidney transplant in daily home hemodialysis, peritoneal dialysis, and in-center hemodialysis patients [abstract no: 298]. American Journal of Kidney Diseases 2015;65(4):A89. [EMBASE: 71875264] [Google Scholar]
- Weinhandl E, Collins A. Lower risk of early death in incident dialysis patients on daily home versus in-center hemodialysis [abstract no: 300]. American Journal of Kidney Diseases 2015;65(4):A89. [EMBASE: 71875266] [Google Scholar]
- Weinhandl E, Liu J, Gilbertson D, Arneson T, Collins A. Relative mortality in daily home and matched, thrice-weekly in-center hemodialysis patients [abstract no: 347]. American Journal of Kidney Diseases 2011;57(4):A103. [EMBASE: 70379906] [Google Scholar]
- Weinhandl ED, Liu J, Gilbertson DT, Arneson TJ, Collins AJ. Survival in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. Journal of the American Society of Nephrology 2012;23(5):895-904. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhandl ED, Nieman KM, Gilbertson DT, Collins AJ. Hospitalization in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. American Journal of Kidney Diseases 2015;65(1):98-108. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Piccoli 2004 {published data only}
- Piccoli GB, Bermond F, Mezza E, Burdese M, Fop F, Mangiarotti G, et al. Vascular access survival and morbidity on daily dialysis: A comparative analysis of home and limited care haemodialysis. Nephrology Dialysis Transplantation 2004;19(8):2084-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rydell 2016 {published data only}
- Rydell H, Clyne N, Segelmark M. Home- or institutional hemodialysis? - a matched pair-cohort study comparing survival and some modifiable factors related to survival. Kidney & Blood Pressure Research 2016;41(4):392-401. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rydell 2019 {published data only (unpublished sought but not used)}
- Rydell H, Ivarsson K, Almquist M, Clyne N, Segelmark M. Fewer hospitalizations and prolonged technique survival with home hemodialysis- a matched cohort study from the Swedish Renal Registry. BMC Nephrology 2019;20(1):480. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydell H, Ivarsson K, Almquist M, Segelmark M, Clyne N. Improved long-term survival with home hemodialysis compared with institutional hemodialysis and peritoneal dialysis: a matched cohort study. BMC Nephrology 2019;20(1):52. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sands 2009 {published data only}
- Sands JJ, Lacson E Jr, Ofsthun NJ, Kay JC, Diaz-Buxo JA. Home hemodialysis: a comparison of in-center and home hemodialysis therapy in a cohort of successful home hemodialysis patients. ASAIO Journal 2009;55(4):361-8. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Saner 2005 {published data only}
- Saner E, Nitsch D, Descoeudres C, Frey FJ, Uehlinger DE. Outcome of home haemodialysis patients: a case-cohort study. Nephrology Dialysis Transplantation 2005;20(3):604-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Suri 2015 {published data only}
- Suri RS, Li L, Nesrallah GE. The risk of hospitalization and modality failure with home dialysis. Kidney International 2015;88(2):360-8. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tablo IDE 2020 {published data only (unpublished sought but not used)}
- Aragon M, Chahal Y. Improved quality of sleep on four day per week home hemodialysis with Tablo [abstract]. Hemodialysis International 2020;24(1):A8-9. [EMBASE: 631798047] [Google Scholar]
- Chahal Y, Aragon M. Decreased time to independence with the tablo hemodialysis system: a subset analysis of the Tablo home clinical trial [Abstract no: 38]. American Journal of Kidney Diseases 2020;75(4):546-7. [EMBASE: 2005716711] [Google Scholar]
- Chahal Y, Plumb T, Aragon M. Patient device preference for home hemodialysis: a subset analysis of the Tablo home IDE trial [abstract no: 39]. American Journal of Kidney Diseases 2020;75(4):547. [EMBASE: 2005716856] [Google Scholar]
- Chertow GM, Alvarez L, Plumb TJ, Prichard SS, Aragon M. Patient-reported outcomes from the investigational device exemption study of the Tablo hemodialysis system. Hemodialysis International 2020;24(4):480-6. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb TJ, Alvarez L, Ross DL, Lee JJ, Mulhern JG, Bell JL, et al. Safety and efficacy of the Tablo hemodialysis system for in-center and home hemodialysis. Hemodialysis International 2020;24(1):22-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb TJ, Alvarez L, Ross DL, Lee JJ, Mulhern JG, Bell JL, et al. Self-care training using the Tablo hemodialysis system. Hemodialysis international 2021;25(1):12-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tennankore 2022 {published data only}
- Tennankore KK, Nadeau-Fredette AC, Matheson K, Chan CT, Trinh E, Perl J. Home versus in-center dialysis and day of the week hospitalization: a cohort study. Kidney360 2022;3(1):103-12. [DOI: 10.34067/KID.0003552021] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toronto Group 2002 {published data only}
- Beecroft JM, Hoffstein V, Pierratos A, Chan CT, McFarlane P, Hanly PJ. Nocturnal haemodialysis increases pharyngeal size in patients with sleep apnoea and end-stage renal disease. Nephrology Dialysis Transplantation 2008;23(2):673-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bergman A, Fenton SS, Richardson RM, Chan CT. Reduction in cardiovascular related hospitalization with nocturnal home hemodialysis. Clinical Nephrology 2008;69(1):33-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Bugeja AL, Chan CT. Improvement in lipid profile by nocturnal hemodialysis in patients with end-stage renal disease. ASAIO Journal 2004;50(4):328-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Cafazzo JA, Leonard K, Easty AC, Rossos PG, Chan CT. Patient perceptions of remote monitoring for nocturnal home hemodialysis. Hemodialysis International 2010;14(4):471-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Cafazzo JA, Leonard K, Easty AC, Rossos PG, Chan CT. Patient-perceived barriers to the adoption of nocturnal home hemodialysis. Clinical Journal of The American Society of Nephrology: CJASN 2009;4(4):784-9. [DOI: ] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Floras JS, Miller JA, Pierratos A. Improvement in ejection fraction by nocturnal haemodialysis in end-stage renal failure patients with coexisting heart failure. Nephrology Dialysis Transplantation 2002;17(8):1518-21. [DOI: 10.1093/ndt/17.8.1518] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney International 2002;61(6):2235-9. [DOI: 10.1046/j.1523-1755.2002.00362.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chan CT, Hanly P, Gabor J, Picton P, Pierratos A, Floras JS. Impact of nocturnal hemodialysis on the variability of heart rate and duration of hypoxemia during sleep. Kidney International 2004;65(2):661-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chan CT, Harvey PJ, Picton P, Pierratos A, Miller JA, Floras JS. Short-term blood pressure, noradrenergic, and vascular effects of nocturnal home hemodialysis. Hypertension 2003;42(5):925-31. [DOI: 10.1161/01.HYP.0000097605.35343.64] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chan CT, Jain V, Picton P, Pierratos A, Floras JS. Nocturnal hemodialysis increases arterial baroreflex sensitivity and compliance and normalizes blood pressure of hypertensive patients with end-stage renal disease. Kidney International 2005;68(1):338-44. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chan CT, Li GH, Valaperti A, Liu P. Intensive hemodialysis preserved cardiac injury. ASAIO Journal 2015;61(5):613-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chan CT, Li SH, Verma S. Nocturnal hemodialysis is associated with restoration of impaired endothelial progenitor cell biology in end-stage renal disease. American Journal of Physiology - Renal Physiology 2005;289(4):F679-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Chan CT, Liu PP, Arab S, Jamal N, Messner HA. Nocturnal hemodialysis improves erythropoietin responsiveness and growth of hematopoietic stem cells. Journal of the American Society of Nephrology 2009;20(3):665-71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CT, Shen XS, Picton P, Floras J. Nocturnal home hemodialysis improves baroreflex effectiveness index of end-stage renal disease patients. Journal of Hypertension 2008;26(9):1795-800. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nessim SJ, Jassal SV, Fung SV, Chan CT. Conversion from conventional to nocturnal hemodialysis improves vitamin D levels. Kidney International 2007;71(11):1172-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Pauly RP, Asad RA, Hanley JA, Pierratos A, Zaltzman J, Chery A, et al. Long-term clinical outcomes of nocturnal hemodialysis patients compared with conventional hemodialysis patients post-renal transplantation. Clinical Transplantation 2009;23(1):47-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Schwartz DI, Pierratos A, Richardson RM, Fenton SS, Chan CT. Impact of nocturnal home hemodialysis on anemia management in patients with end-stage renal disease. Clinical Nephrology 2005;63(3):202-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Yuen D, Pierratos A, Richardson RM, Chan CT. The natural history of coronary calcification progression in a cohort of nocturnal haemodialysis patients. Nephrology Dialysis Transplantation 2006;21(5):1407-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Yuen D, Richardson RM, Fenton SS, McGrath-Chong ME, Chan CT. Quotidian nocturnal hemodialysis improves cytokine profile and enhances erythropoietin responsiveness. ASAIO Journal 2005;51(3):236-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Oosten 2018 {published data only}
- Mohnen SM, Oosten MJ, Los J, Leegte MJ, Jager KJ, Hemmelder MH, et al. Healthcare costs of patients on different renal replacement modalities – Analysis of Dutch health insurance claims data. PLoS ONE [Electronic Resource] 2019;14(8):e0220800. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oosten M, Mohnen S, Los J, Leegte M, Jager K, Hemmelder M, et al. Healthcare costs of patients on different renal replacement modalities-analysis of Dutch health insurance claims data [abstract no: FP673]. Nephrology Dialysis Transplantation 2018;33(Suppl 3):i272. [EMBASE: 622606135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 2014 {published data only}
- Watanabe Y, Ohno Y, Inoue T, Takane H, Okada H, Suzuki H. Home hemodialysis and conventional in-center hemodialysis in Japan: comparison of health-related quality of life. Hemodialysis International 2014;18(S1):S32-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wong 2019a {published data only}
- Wong CKH, Chen JY, Fung SK, Lo WK, Lui SL, Chan TM, et al. Health-related quality of life and health utility of Chinese patients undergoing nocturnal home haemodialysis in comparison with other modes of dialysis. Nephrology 2019;24(6):630-7. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Wong 2019b {published data only}
- Wong CK, Chen J, Fung SK, Mok MM, Cheng YL, Kong I, et al. Direct and indirect costs of end-stage renal disease patients in the first and second years after initiation of nocturnal home haemodialysis, hospital haemodialysis and peritoneal dialysis. Nephrology Dialysis Transplantation 2019;34(9):1565-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wright 2015 {published data only}
- Wright LS, Wilson L. Quality of life and self-efficacy in three dialysis modalities: incenter hemodialysis, home hemodialysis, and home peritoneal dialysis. Nephrology Nursing Journal 2015;42(5):463-76. [PMID: ] [PubMed] [Google Scholar]
Xue 2015 {published data only}
- Xue H, Li NC, Lacson E Jr, Brunelli SM, Lockridge RS. Catheter-related bacteremia and mortality in frequent nocturnal home hemodialysis. Hemodialysis International 2015;19(2):242-8. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Yeung 2021 {published data only}
- Yeung E, Kerr P, Polkinghorne K. Home & satellite haemodialysis patients: a comparison of outcomes [abstract no: SAT-043]. Kidney International Reports 2019;4(7 Suppl):S21. [EMBASE: 2002179354] [Google Scholar]
- Yeung EK, Polkinghorne KR, Kerr PG. Home and facility haemodialysis patients: a comparison of outcomes in a matched cohort. Nephrology Dialysis Transplantation 2021;36(6):1070-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zimbudzi 2014 {published data only}
- Zimbudzi E, Samlero R. How do hospitalization patterns of home hemodialysis patients compare with a reasonably well dialysis patient cohort? International Journal of Nephrology & Renovascular Disease 2014;7:203-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Benain 2007 {published data only}
- Benain JP, Faller B, Briat C, Jacquelinet C, Brami M, Aoustin M, et al. Cost of dialysis in France [Cout de la prise en charge de la dialyse en France]. Nephrologie et Therapeutique 2007;3(3):96-106. [PMID: ] [DOI] [PubMed] [Google Scholar]
Benain 2015 {published data only}
- Benain JP, Galland R, Kessler M, Lobbedez T, Fagnani F, Dumas JJ, et al. Cost-effectiveness of high dose haemodialysis in France [abstract no: FP698]. Nephrology Dialysis Transplantation 2015;30(Suppl 3):iii309. [EMBASE: 72207115] [Google Scholar]
Bernieh 2020 {published data only}
- Bernieh B, Gogoi S, Owida A, Galal Yassin M, Seif Eddin A, et al. Comparison between Nurse Assisted Home Hemodialysis (NAHHD), and in Center Hemodialysis (CHD), in home bound, multi comorbid, and debilitated hemodialysis patients [abstract no: SAT-230]. Kidney International Reports 2020;5(3 Suppl):S98. [EMBASE: 2005256391] [Google Scholar]
Blagg 2006 {published data only}
- Blagg CR, Kjellstrand CM, Ting GO, Young BA. Comparison of survival between short-daily hemodialysis and conventional hemodialysis using the standardized mortality ratio. Hemodialysis International 2006;10(4):371-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Connor 2011 {published data only}
- Connor A, Lillywhite R, Cooke MW. The carbon footprints of home and in-center maintenance hemodialysis in the United Kingdom. Hemodialysis International 2011;15(1):39-51. [DOI: 10.1111/j.1542-4758.2010.00523.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Derrett 2017 {published data only}
- Derrett S, Samaranayaka A, Schollum JB, McNoe B, Marshall MR, Williams S, et al. Predictors of health deterioration among older adults after 12 months of dialysis therapy: a longitudinal cohort study from New Zealand. American Journal of Kidney Diseases 2017;70(6):798-806. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eneanya 2019 {published data only}
- Eneanya ND, Maddux DW, Reviriego-Mendoza MM, Larkin JW, Usvyat LA, Sande FM, et al. Longitudinal patterns of health-related quality of life and dialysis modality: a national cohort study. BMC Nephrology 2019;20(1):7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
FREEDOM 2009 {published data only}
- Finkelstein FO, Schiller B, Daoui R, Gehr TW, Kraus MA, Lea J, et al. At-home short daily hemodialysis improves the long-term health-related quality of life. Kidney International 2012;82(5):561-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Jaber BL, Finkelstein FO, Glickman JD, Hull AR, Kraus MA, Leypoldt JK, et al. Scope and design of the Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements (FREEDOM) Study. American Journal of Kidney Diseases 2009;53(2):310-20. [DOI: 10.1053/j.ajkd.2008.07.013] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Jaber BL, Schiller B, Burkart JM, Daoui R, Kraus MA, Lee Y, et al. Impact of short daily hemodialysis on restless legs symptoms and sleep disturbances. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(5):1049-56. [DOI: 10.2215/CJN.10451110] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez‐Perez 2005 {published data only}
- Gonzalez-Perez JG, Vale L, Stearns SC, Wordsworth S. Hemodialysis for end-stage renal disease: a cost-effectiveness analysis of treatment-options. International Journal of Technology Assessment in Health Care 2005;21(1):32-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gorham 2019 {published data only}
- Gorham G, Howard K, Zhao Y, Ahmed AM, Lawton PD, Sajiv C, et al. Cost of dialysis therapies in rural and remote Australia - a micro-costing analysis. BMC Nephrology 2019;20(1):231. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howard 2009 {published data only}
- Howard K, Salkeld G, White S, McDonald S, Chadban S, Craig JC, et al. The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology 2009;14(1):123-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ipema 2010 {published data only (unpublished sought but not used)}
- Ipema K, Franssen C, Schans C, Smit L, Noordman S, Haisma H. Influence of frequent nocturnal home hemodialysis on food preference. Journal of Renal Nutrition 2010;20(2):127-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ipema 2012 {published data only (unpublished sought but not used)}
- Ipema KJ, Schans CP, Vonk N, Vries JM, Westerhuis R, Duym E, et al. A difference between day and night: protein intake improves after the transition from conventional to frequent nocturnal home hemodialysis. Journal of Renal Nutrition 2012;22(3):365-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ipema 2014 {published data only}
- Ipema KJ, Westerhuis R, Schans CP, Jong PE, Gaillard CA, Krijnen WP, et al. Effect of nocturnal haemodialysis on body composition. Nephron 2014;128(1-2):171-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johansen 2009 {published data only}
- Johansen KL, Zhang R, Huang Y, Chen SC, Blagg CR, Goldfarb-Rumyantzev AS, et al. Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: a USRDS study. Kidney International 2009;76(9):984-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Komenda 2012 {published data only}
- Komenda P, Gavaghan MB, Garfield SS, Poret AW, Sood MM. An economic assessment model for in-center, conventional home, and more frequent home hemodialysis. Kidney International 2012;81(3):307-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubisiak 2018 {published data only}
- Kubisiak K, Weinhandl E, Collins A. Low incidence of intradialytic hypotension on more frequent home hemodialysis [abstract]. Hemodialysis International 2018;22(1):A22. [EMBASE: 620701261] [Google Scholar]
Lee 2017 {published data only}
- Lee J, Tan E. Assisted self-care haemodialysis compared to in-centre haemodialysis -a single centre experience [abstract]. Nephrology 2017;22(Suppl 3):80. [EMBASE: 618236163] [Google Scholar]
Lockridge 2011 {published data only}
- Lockridge RS, Kjellstrand CM. Nightly home hemodialysis: outcome and factors associated with survival. Hemodialysis International 2011;15(2):211-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
London Daily/Nocturnal 2003 {published data only}
- Heidenheim AP, Muirhead N, Moist L, Lindsay RM. Patient quality of life on quotidian hemodialysis. American Journal of Kidney Diseases 2003;42(1 Suppl):36-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kroeker A, Clark WF, Heidenheim AP, Kuenzig L, Leitch R, Meyette M, et al. An operating cost comparison between conventional and home quotidian hemodialysis. American Journal of Kidney Diseases 2003;42(1 Suppl):49-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Leitch R, Ouwendyk M, Ferguson E, Clement L, Peters K, Heidenheim AP, et al. Nursing issues related to patient selection, vascular access, and education in quotidian hemodialysis. American Journal of Kidney Diseases 2003;42(1 Suppl):56-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Alhejaili F, Nesrallah G, Leitch R, Clement L, Heidenheim AP, et al. Calcium and phosphate balance with quotidian hemodialysis. American Journal of Kidney Diseases 2003;42(1 Suppl):24-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Leitch R, Heidenheim AP, Kortas C. The London daily/nocturnal hemodialysis study--study design, morbidity, and mortality results. American Journal of Kidney Diseases 2003;42(1 Suppl):5-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lindsay RM. The London, Ontario, Daily/Nocturnal Hemodialysis Study. Seminars in Dialysis 2004;17(2):85-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nesrallah G, Suri R, Moist L, Kortas C, Lindsay RM. Volume control and blood pressure management in patients undergoing quotidian hemodialysis. American Journal of Kidney Diseases 2003;42(1 Suppl):13-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rao M, Muirhead N, Klarenbach S, Moist L, Lindsay RM. Management of anemia with quotidian hemodialysis. American Journal of Kidney Diseases 2003;42(1 Suppl):18-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Spanner E, Suri R, Heidenheim AP, Lindsay RM. The impact of quotidian hemodialysis on nutrition. American Journal of Kidney Diseases 2003;42(1 Suppl):30-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Suri R, Depner TA, Blake PG, Heidenheim AP, Lindsay RM. Adequacy of quotidian hemodialysis. American Journal of Kidney Diseases 2003;42(1 Suppl):42-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Loos‐Ayav 2008 {published data only}
- Loos-Ayav C, Frimat L, Kessler M, Chanliau J, Durand PY, Briancon S. Changes in health-related quality of life in patients of self-care vs. in-center dialysis during the first year. Quality of Life Research 2008;17(1):1-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
MacRae 2010 {published data only}
- MacRae JM, Rose CL, Jaber BL, Gill JS. Utilization and outcome of 'out-of-center hemodialysis' in the United States: a contemporary analysis. Nephron 2010;116(1):c53-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2015 {published data only}
- Marshall MR, Polkinghorne KR, Kerr PG, Agar JW, Hawley CM, McDonald SP. Temporal changes in mortality risk by dialysis modality in the Australian and New Zealand dialysis population. American Journal of Kidney Diseases 2015;66(3):489-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
McDonald 2009 {published data only}
- McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. Journal of the American Society of Nephrology 2009;20(1):155-63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McFarlane 2002 {published data only}
- McFarlane PA, Bayoumi AM, Pierratos A, Redelmeier DA. The quality of life and cost utility of home nocturnal and conventional in-center hemodialysis. Kidney International 2003;64(3):1004-11. [PMID: ] [DOI] [PubMed] [Google Scholar]
- McFarlane PA, Pierratos A, Redelmeier DA. Cost savings of home nocturnal versus conventional in-center hemodialysis. Kidney International 2002;62(6):2216-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mehrotra 2016 {published data only}
- Mehrotra R, Soohoo M, Rivara MB, Himmelfarb J, Cheung AK, Arah OA, et al. Racial and ethnic disparities in use of and outcomes with home dialysis in the United States. Journal of the American Society of Nephrology 2016;27(7):2123-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohr 2001 {published data only}
- Mohr PE, Neumann PJ, Franco SJ, Marainen J, Lockridge R, Ting G. The case for daily dialysis: its impact on costs and quality of life. American Journal of Kidney Diseases 2001;37(4):777-89. [PMID: ] [DOI] [PubMed] [Google Scholar]
Quintaliani 2000 {published data only}
- Quintaliani G, Buoncristiani U, Fagugli R, Kuluiranu H, Ciao G, Rondini L, et al. Survival of vascular access during daily and three times a week hemodialysis. Clinical Nephrology 2000;53(5):372-7. [PMID: ] [PubMed] [Google Scholar]
Rivara 2018 {published data only}
- Rivara MB, Ravel V, Streja E, Obi Y, Soohoo M, Cheung AK, et al. Weekly standard Kt/Vurea and clinical outcomes in home and in-center hemodialysis. Clinical Journal of the American Society of Nephrology: CJASN 2018;13(3):445-55. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seto 2007 {published data only}
- Seto E, Cafazzo JA, Rizo C, Bonert M, Fong E, Chan CT. Internet use by end-stage renal disease patients. Hemodialysis International 2007;11(3):328-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shen 2019 {published data only}
- Shen JI, Erickson KF, Chen L, Vangala S, Leng L, Shah A, et al. Expanded prospective payment system and use of and outcomes with home dialysis by race and ethnicity in the United States. Clinical Journal of the American Society of Nephrology: CJASN 2019;14(8):1200-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ting 2003 {published data only}
- Ting GO, Kjellstrand C, Freitas T, Carrie BJ, Zarghamee S. Long-term study of high-comorbidity ESRD patients converted from conventional to short daily hemodialysis. American Journal of Kidney Diseases 2003;42(5):1020-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2006 {published data only}ISRCTN25858715
- Bass A, Ahmed SB, Klarenbach S, Culleton B, Hemmelgarn BR, Manns B. The impact of nocturnal hemodialysis on sexual function. BMC Nephrology 2012;13:67. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007;298(11):1291-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, et al. Nocturnal hemodialysis lowers blood pressure and reduces left ventricular mass: results of a randomized controlled trial [abstract no: SU-FC002]. Journal of the American Society of Nephrology 2007;18(Abstracts):67A-8A. [CENTRAL: CN-00783714] [Google Scholar]
- Khangura J, Culleton BF, Manns BJ, Zhang J, Barnieh L, Walsh M, et al. Association between routine and standardized blood pressure measurements and left ventricular hypertrophy among patients on hemodialysis. BMC Nephrology 2010;11:13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarenbach S, Tonelli M, Pauly R, Walsh M, Culleton B, So H, et al. Economic evaluation of frequent home nocturnal hemodialysis based on a randomized controlled trial. Journal of the American Society of Nephrology 2014;25(3):587-94. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarenbach S, Tonelli M, Pauly R, Walsh M, Culleton B, So H, et al. Economic evaluation of frequent home nocturnal hemodialysis based on a randomized controlled trial. Journal of the American Society of Nephrology 2014;25(3):587-94. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns BJ, Klarenbach S, Walsh M, Quinn R, Tonelli M, Scott-Douglas N, et al. The impact of nocturnal hemodialysis on quality of life: results of a randomized controlled trial [abstract no: F-PO891]. Journal of the American Society of Nephrology 2007;18(Abstracts):298A-9A. [CENTRAL: CN-00784458] [Google Scholar]
- Manns BJ, Walsh MW, Culleton BF, Hemmelgarn B, Tonelli M, Schorr M, et al. Nocturnal hemodialysis does not improve overall measures of quality of life compared to conventional hemodialysis. Kidney International 2009;75(5):542-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Schorr M, Manns BJ, Culleton B, Walsh M, Klarenbach S, Tonelli M, et al. The effect of nocturnal and conventional hemodialysis on markers of nutritional status: results from a randomized trial. Journal of Renal Nutrition 2011;21(3):271-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Walsh M, Manns B, Tonelli M, Quinn R, Culleton B. Description of a randomized controlled trial on the effects of nocturnal hemodialysis on left ventricular hypertrophy compared to conventional hemodialysis [abstract no: SA-PO815]. Journal of the American Society of Nephrology 2005;16:734-5A. [CENTRAL: CN-00583210] [Google Scholar]
- Walsh M, Manns BJ, Klarenbach S, Quinn R, Tonelli M, Culleton BF. The effects of nocturnal hemodialysis compared to conventional hemodialysis on change in left ventricular mass: rationale and study design of a randomized controlled pilot study. BMC Nephrology 2006;7:2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M, Manns BJ, Klarenbach S, Tonelli M, Hemmelgarn B, Culleton B. The effects of nocturnal compared with conventional hemodialysis on mineral metabolism: A randomized-controlled trial. Hemodialysis International 2010;14(2):174-81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2015 {published data only}
- Yang A, Lee A, Hocking K. Nursing home care. Daily HHD vs conventional dialysis: a survival comparison. Nephrology News & Issues 2017;31(2):21-6. [PMID: ] [PubMed] [Google Scholar]
- Yang A, Lee WY, Hocking K. Survival comparison of daily home hemo vs conventional dialysis in the nursing home setting [abstract no: 409]. American Journal of Kidney Diseases 2014;63(5):A121. [EMBASE: 71448676] [Google Scholar]
- Yang A, Lee WY, Hocking K. Survival comparison of daily home hemodialysis vs. conventional in the nursing home setting. Nephrology News & Issues 2015;29(2):25-7, 30-1. [PMID: ] [PubMed] [Google Scholar]
Yuen 2011 {published data only}
- Yuen DA, Kuliszewski MA, Liao C, Rudenko D, Leong-Poi H, Chan CT. Nocturnal hemodialysis is associated with restoration of early-outgrowth endothelial progenitor-like cell function. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(6):1345-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
De Smet 2007 {published data only (unpublished sought but not used)}
- De Smet R, Dhondt A, Eloot S, Claus S, Vanholder R. Nocturnal hemodialysis with the Genius® dialysis system [abstract no: FP327]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi128. [Google Scholar]
Fadem 2011 {published data only (unpublished sought but not used)}
- Fadem SZ, Walker DR, Abbott G, Friedman AL, Goldman R, Sexton S, et al. Satisfaction with renal replacement therapy and education: The American Association of Kidney Patients Survey. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(3):605-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanly 2001 {published data only (unpublished sought but not used)}
- Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. New England Journal of Medicine 2001;344(2):102-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Helantera 2012 {published data only (unpublished sought but not used)}
- Helantera I, Haapio M, Koskinen P, Gronhagen-Riska C, Finne P. Employment of patients receiving maintenance dialysis and after kidney transplant: a cross-sectional study from Finland. American Journal of Kidney Diseases 2012;59(5):700-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kannampuzha 2010 {published data only (unpublished sought but not used)}
- Kannampuzha J, Donnelly SM, McFarlane PA, Chan CT, House JD, Pencharz PB, et al. Glutathione and riboflavin status in supplemented patients undergoing home nocturnal hemodialysis versus standard hemodialysis. Journal of Renal Nutrition 2010;20(3):199-208. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mitchell 2020 {published data only (unpublished sought but not used)}
- Mitchell A, Kassem H, Aleter O, Badalamenti J, Hayes KM. Patient activation and ESRD [abstract no: 251]. American Journal of Kidney Diseases 2020;75(4):608-9. [EMBASE: 2005716817] [Google Scholar]
Morton 2010 {published data only (unpublished sought but not used)}
- Morton RL, Devitt J, Howard K, Anderson K, Snelling P, Cass A. Patient views about treatment of stage 5 CKD: a qualitative analysis of semistructured interviews. American Journal of Kidney Diseases 2010;55(3):431-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Painter 2012 {published data only (unpublished sought but not used)}
- Painter P, Krasnoff JB, Kuskowski M, Frassetto L, Johansen K. Effects of modality change on health-related quality of life. Hemodialysis International 2012;16(3):377-86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2014 {published data only (unpublished sought but not used)}
- Parker K, Nikam M, Jayanti A, Mitra S. Medication burden in CKD-5D: Impact of dialysis modality and setting. Clinical Kidney Journal 2014;7(6):557-61. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pellicano 2010 {published data only (unpublished sought but not used)}
- Pellicano R, Strauss BJ, Polkinghorne KR, Kerr PG. Body composition in home haemodialysis versus conventional haemodialysis: a cross-sectional, matched, comparative study. Nephrology Dialysis Transplantation 2010;25(2):568-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Poon 2015 {published data only (unpublished sought but not used)}
- Poon CK, Tang HL, Wong JH, Law WP, Lam CM, Yim KF, et al. Effect of alternate night nocturnal home hemodialysis on anemia control in patients with end-stage renal disease. Hemodialysis International 2015;19(2):235-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sikkes 2009 {published data only}
- Sikkes ME, Kooistra MP, Weijs PJ. Improved nutrition after conversion to nocturnal home hemodialysis. Journal of Renal Nutrition 2009;19(6):494-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomson 2013 {published data only (unpublished sought but not used)}
- Thomson BK, Momciu B, Huang SH, Chan CT, Urquhart BL, Skanes AC, et al. Frequent nocturnal hemodialysis associates with improvement of prolonged QTc intervals. Nephron 2013;123(1-2):74-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yong 2014 {published data only (unpublished sought but not used)}
- Yong K, Dogra G, Boudville N, Lim WH. Home haemodialysis does not improve arterial stiffness or pro-inflammatory cytokines in chronic kidney disease patients [abstract no: 099]. Nephrology 2014;19(Suppl 4):44. [EMBASE: 71587879] [Google Scholar]
Additional references
Abrams 2005
- Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24(24):3823-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
ANZDATA 2021
- ANZDATA Registry. 44th Report, Chapter 2: Prevalence of Kidney Failure with Replacement Therapy. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2021. Available at: http://www.anzdata.org.au (accessed 16 February 2024).
Assimon 2016
- Assimon MM, Wenger JB, Wang L, Flythe JE. Ultrafiltration rate and mortality in maintenance hemodialysis patients. American Journal of Kidney Diseases 2016;68(6):911-22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bello 2017a
- Bello AK, Levin A, Tonelli M, Okpechi IG, Feehally J, Harris D, et al. Assessment of global kidney health care status. JAMA 2017;317(18):1864-81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bello 2017b
- Bello AK, Levin A, Tonelli M, Okpechi IG, Feehally J, Harris D, et al. Global Kidney Health Atlas: A report by the International Society of Nephrology on the current state of organization and structures for kidney care across the globe. 1st Edition, 2017. Availiable from www.theisn.org/initiatives/global-kidney-health-atlas/ (accessed 16 February 2024).
Bello 2019
- Bello AK, Levin A, Lunney M, Osman MA, Ye F, Ashuntantang G, et al. Global Kidney Health Atlas: a report by the International Society of Nephrology on the global burden of end-stage kidney disease and capacity for kidney replacement therapy and conservative care across world countries and regions. 2nd Edition, 2019. www.theisn.org/initiatives/global-kidney-health-atlas/ (accessed 16 February 2024).
Bonenkamp 2020
- Bonenkamp AA, Eck van der Sluijs A, Hoekstra T, Verhaar MC, Ittersum FJ, Abrahams AC, et al. Health-related quality of life in home dialysis patients compared to in-center hemodialysis patients: a systematic review and meta-analysis. Kidney Medicine 2020;2(2):139-54. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cabrera 2017
- Cabrera VJ, Hansson J, Kliger AS, Finkelstein FO. Symptom management of the patient with CKD: the role of dialysis. Clinical Journal of The American Society of Nephrology: CJASN 2017;12(4):687-93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cases 2011
- Cases A, Dempster M, Davies M, Gamble G. The experience of individuals with renal failure participating in home haemodialysis: An interpretative phenomenological analysis. Journal of Health Psychology 2011;16(6):884-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
FHN Trial Group 2010
- Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. In-center hemodialysis six times per week versus three times per week. New England Journal of Medicine. 2010;363(24):2287-300. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foley 2011
- Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. New England Journal of Medicine 2011;365(12):1099-107. [PMID: ] [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gilbertson 2019
- Gilbertson EL, Krishnasamy R, Foote C, Kennard AL, Jardine MJ, Gray NA. Burden of care and quality of life among caregivers for adults receiving maintenance dialysis: a systematic review. American Journal of Kidney Diseases 2019;73(3):332-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Herzog 2013
- Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013;13:154. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Iyasere 2016
- Iyasere OU, Brown EA, Johansson L, Huson L, Smee J, Maxwell AP, et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clinical Journal of The American Society of Nephrology: CJASN 2016;11(3):423-30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jardine 2017
- Jardine MJ, Zuo L, Gray NA, Zoysa JR, Chan CT, Gallagher MP, et al. A trial of extending hemodialysis hours and quality of life. Journal of the American Society of Nephrology 2017;28(6):1898-911. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jayanti 2013
- Jayanti A, Wearden AJ, Morris J, Brenchley P, Abma I, Bayer S, et al. Barriers to successful implementation of care in home haemodialysis (BASIC-HHD):1. Study design, methods and rationale. BMC Nephrology 2013;14(1):197. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferies 2011
- Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clinical Journal of The American Society of Nephrology: CJASN 2011;6(6):1326-32. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kidney Health Australia 2013
- Kidney Health Australia and Renal Resource Centre. An Introduction to Home Dialysis. 2013. www.kidney.org.au/uploads/resources/an-introduction-to-home-dialysis-book-kidney-health-australia.pdf (accesssed 16 February 2024).
Klarenbach 2014
- Klarenbach S, Tonelli M, Pauly R, Walsh M, Culleton B, So H, Hemmelgarn B, et al. Economic evaluation of frequent home nocturnal hemodialysis based on a randomized controlled trial. Journal of the American Society of Nephrology 2014;25(3):587-94. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laupacis 1996
- Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney International 1996;50(1):235-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mailloux 1996
- Mailloux LU, Kapikian N, Napolitano B, Mossey RT, Bellucci AG, Wilkes BM, et al. Home hemodialysis: patient outcomes during a 24-year period of time from 1970 through 1993. Advances in Renal Replacement Therapy 1996;3(2):112-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marshall 2015
- Marshall MR, Polkinghorne KR, Kerr PG, Agar JW, Hawley CM, McDonald SP. Temporal changes in mortality risk by dialysis modality in the Australian and New Zealand dialysis population. American Journal of Kidney Diseases 2015;66(3):489-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Miller 2018
- Miller BW, Himmele R, Sawin D-A, Kim J, Kossmann RJ. Choosing home hemodialysis: a critical review of patient outcomes. Blood Purification 2018;45(1-3):224-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mohr 2001
- Mohr PE, Neumann PJ, Franco SJ, Marainen J, Lockridge R, Ting G. The case for daily dialysis: its impact on costs and quality of life. American Journal of Kidney Disease 2001;37(4):777-89. [DOI: 10.1016/s0272-6386(01)80127-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morton 2010
- Morton RL, Tong A, Howard K, Snelling P, Webster AC. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ 2010;340:c112. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mowatt 2003
- Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure. Health Technology Assessment (Winchester, England) 2003;7(2):1-174. [PMID: ] [DOI] [PubMed] [Google Scholar]
Naylor 2019
- Naylor KL, Kim SJ, McArthur E, Garg AX, McCallum MK, Knoll GA. Mortality in incident maintenance dialysis patients versus incident solid organ cancer patients: a population-based cohort. American Journal of Kidney Diseases 2019;73(6):765-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ok 2011
- Ok E, Duman S, Asci G, Tumuklu M, Onen Sertoz O, Kayikcioglu M, et al. Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: a prospective, case-controlled study. Nephrology Dialysis Transplantation 2011;26(4):1287-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pecoits‐Filho 2020
- Pecoits-Filho R, Okpechi IG, Donner JA, Harris DCH, Aljubori HM, Bello AK, et al. Capturing and monitoring global differences in untreated and treated end-stage kidney disease, kidney replacement therapy modality, and outcomes. Kidney International Supplements 2020;10(1):e3-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2022a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors) Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schünemann 2022b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Suri 2006
- Suri RS, Nesrallah GE, Mainra R, Garg AX, Lindsay RM, Greene T, et al. Daily hemodialysis: a systematic review. Clinical Journal of The American Society of Nephrology: CJASN 2006;1(1):33-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Suri 2011
- Suri RS, Larive B, Garg AX, Hall YN, Pierratos A, Chertow GM, et al. Burden on caregivers as perceived by hemodialysis patients in the Frequent Hemodialysis Network (FHN) trials. Nephrology Dialysis Transplantation 2011;26(7):2316-22. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suri 2013
- Suri RS, Larive B, Sherer S, Eggers P, Gassman J, James SH, et al. Risk of vascular access complications with frequent hemodialysis. Journal of the American Society of Nephrology 2013;24(3):498-505. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tonelli 2011
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. American Journal of Transplantation 2011;11(10):2093-109. [PMID: ] [DOI] [PubMed] [Google Scholar]
USRDS 2021
- United States Renal Data System. 2021 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2021. https://usrds-adr.niddk.nih.gov/2021/suggested-citation (accessed 16 February 2024).
Walker 2014
- Walker R, Marshall MR, Morton RL, McFarlane P, Howard K. The cost-effectiveness of contemporary home haemodialysis modalities compared with facility haemodialysis: A systematic review of full economic evaluations. Nephrology 2014;19(8):459-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2005
- Walsh M, Culleton B, Tonelli M, Manns B. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney International 2005;67(4):1500-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 1999
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. New England Journal of Medicine 1999;341(23):1725-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Woods 1996
- Woods JD, Port FK, Stannard D, Blagg CR, Held PJ. Comparison of mortality with home hemodialysis and center hemodialysis: a national study. Kidney International 1996;49(5):1464-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Palmer 2012
- Palmer SC, Palmer AR, Craig JC, Johnson DW, Stroumza P, Frantzen L, et al. Home versus in-centre haemodialysis for end-stage kidney disease. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD009535. [DOI: 10.1002/14651858.CD009535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2014
- Palmer SC, Palmer AR, Craig JC, Johnson DW, Stroumza P, Frantzen L, et al. Home versus in-centre haemodialysis for end-stage kidney disease. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD009535. [DOI: 10.1002/14651858.CD009535.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


