Abstract
Objectives
Diquat has replaced paraquat in agricultural areas as a herbicide but has led to extensive poisoning. Unlike paraquat, which targets the lungs, diquat primarily targets the kidneys. Autopsies and animal experiments suggest that interstitial kidney damage is the most critical renal lesion. Diquat is a nonselective chemical widely used for terrestrial and aquatic plants after the ban on paraquat. Although diquat is known to affect the kidneys mainly, no study has reported renal biopsy in patients with diquat poisoning.
Methods
We investigated the histopathologic feature in a young man with diquat poisoning who developed acute kidney injury by renal biopsy.
Results
Autopsy and animal experiments suggest that interstitial kidney inflammation is the most critical renal lesion. Surprisingly, our results showed that lipid degeneration and acute tubular injury with limited interstitial inflammation were the dominant histologic findings in this patient.
Conclusions
Based on a renal biopsy, this was the first study describing the characteristics of the kidney affected by diquat poisoning. Our findings might provide information for managing patients who develop AKI due to diquat poisoning.
Keywords: Diquat, poisoning, acute tubular necrosis, renal biopsy, lipid degeneration
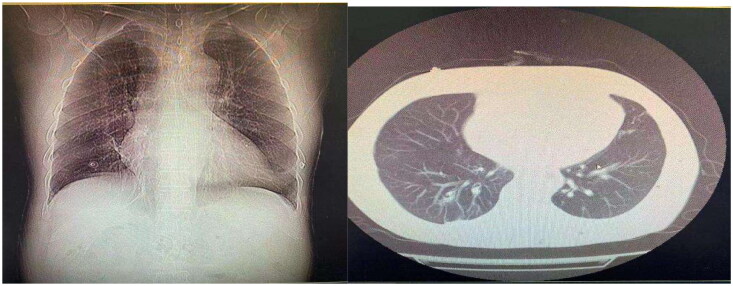
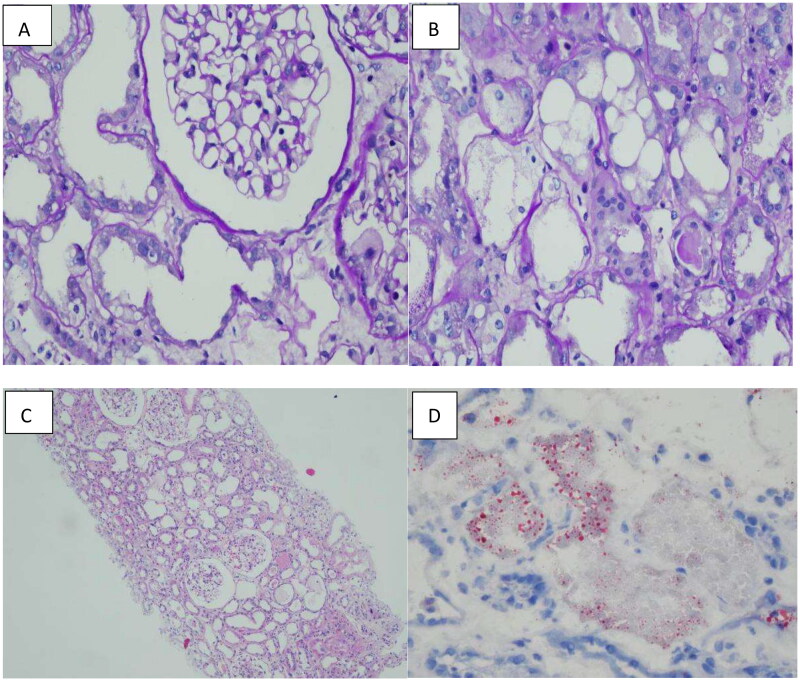
A 24-year-old male was brought to our emergency department by his colleagues more than 5 h after he consumed 180 mL of diquat due to emotional problems. The patient developed nausea and started vomiting greenish-black gastric contents 10 min after ingesting diquat. In the hospital, gastric lavage was administered. The patient denied hypertension, diabetes, or other cardiorespiratory diseases. He also had no history of depression, allergy, or infectious diseases, and thus, we concluded that he had a good physiopsychological status. Although his blood pressure was 174/108 mmHg, the other vital signs were normal, oral mucosa was caustic, cardiopulmonary condition showed no remarkable signs, his abdomen was tender, without rebound pain or muscle tension, no edema occurred in the lower limbs, and his pathologic reflexes were negative. The results of laboratory examinations showed the following: white blood cell count: 19.28 × 109/L, neutrophils: 90.1%, hepatic function and renal function were within the lower limit, coagulatory function and other biochemical functions showed no remarkable changes, only cholinesterase was 8604 U/L; radiologic tests showed: diffused infiltration of both pulmonary fields (Figure 1.). The patient was diagnosed with ‘pesticide poisoning’. Gastric protection, veno-venous hemofiltration, hemoperfusion, and continuous renal replacement were performed. Methylprednisolone impulse was continued for four days and then changed to oral prednisone till cessation. As the infiltration in the lungs and inflammatory factors were higher than normal, empiric antimicrobial treatment was administered. Anuria persisted for several days, and the situation remained unchanged. When the patient stabilized, he was transferred to our department. After admission, as the creatinine level was elevated and the patient was still suffering from anuria, hemodiafiltration, hemodialysis, and hemoperfusion were performed. Antibiotic treatment was discontinued after determining the absence of infiltration in both pulmonary fields. As the interpersonal situation was various, for accurate diagnosis, a renal biopsy was performed. The results confirmed diquat-induced acute tubular injury (Figure 2.). The specific outcome was as follows: A total of 23 patent glomeruli were identified in the tissue submitted for evaluation. There was no noticeable hypercellularity within the mesangial area and the endocapillary lumen, as well as no evidence of significant glomerular inflammation or necrosis (A). The tubular parenchyma showed severe interstitial edema associated with scattered nonspecific mononuclear cell inflammatory (B). Intact tubules showed diffuse acute tubular injurious changes characterized by attenuation of the brush borders and cytoplasmic rounded vacuolization(C). Oil Red O staining confirmed the lipid degeneration within the tubules and spared glomeruli (D). The immunofluorescence microscopy examination was negative for IgG, IgA, IgM, C3, C1q and kappa and lambda light chains. Electron microscopy examination was unremarkable. After treatment, the urine volume increased gradually. After being hospitalized in our unit for 11 days, hemodialysis was stopped, and the urine volume was normal. The patient was discharged after 21 days of hospitalization. After one month of follow-up, the renal indices of the patient were stable, and he appeared to be normal.
Figure 1.
Computed tomography scans showed that diffused infiltration occurred in both lung fields, and no fibrotic lesions were present.
Figure 2.
A: Preserved glomeruli, adjacent acute tubular injury with attenuated brush border and lipid degeneration (PAS stain; original magnification x400). B: Conspicuous lipid degeneration observed in the tubules (PAS stain; original magnification x400); C: Acute tubular injury without interstitial inflammation (H&E stain, original magnification x100); D: Oil Red O staining indicates vacuolation and a normal glomerulus (original magnification x400).
After paraquat was banned, the use of diquat, a paraquat substitute, increased considerably. Reports regarding diquat poisoning have grown recently [4]. Although diquat is less toxic than paraquat, their mechanism of toxicity is identical. It is an effective insecticide and can enter the body via the food chain or through skin contact, intentional ingestion, and accidental infusion, causing a series of toxic effects on various organs, including the kidneys, lungs, liver, heart, and central nervous system, leading to multi-organ dysfunction and death [1–3]. The lethal dose for adults is 6–12 g [5,6]. The patient reported in our study ingested 36 g, which far exceeded the maximum fatal dose. Fortunately, the patient was cured. We performed a renal biopsy to examine his renal pathologic changes, which helped us develop a protocol and a regimen for clinical treatment.
Diquat is hydrophilic, implying that it can freely enter cells and tissues and produce free oxygen radicals, which can cause mitochondrial dysfunction and cell death [7]. Since it is widely available to general people, it is easily distributed to the tissues and organs throughout the body by blood, attributed to the difference of targeting organs; the concentrations are also various. Some autopsy reports found that the highest concentration of diquat occurred in the kidneys, followed by the lungs, liver, brain, and heart [7]. As diquat is excreted by the kidneys, it is the most important target organ. Unlike paraquat, diquat seldom causes pulmonary fibrosis, at least during a short period or at the early stage. In one study, it only caused infiltration of inflammatory cells, resulting in a diffuse exudative change in both lung fields [8]. However, differential diagnosis or underdiagnosis might lead to inefficient therapy, which might cause the patient to develop pulmonary fibrosis and result in respiratory failure, causing death. For such patients, pulmonary transplantation might be suggested. We could not determine the exact pathologic changes in the kidneys through speculation and deduction based on former cases. Thus, we performed a renal biopsy to confirm our diagnosis and aid treatment, as well as, make an accurate prognosis.
Through renal biopsy, we found that diquat targeted the renal tubules, resulting in tubular injury [9], which was consistent with the autopsy reports. However, in previous animal experiments, a interstitial inflammatory cell infiltration was observed after they were administered diquat for 16 hours, tubular necrosis was obvious [8]. These differences among species might be attributed to the application of glucocorticoids and clearance of toxin before biopsy. When exposed to more than or equal 350 mg/kg diquat, rats’ urea was apparently higher than control group in the early stage of AKI, blood urea nitrogen and creatinine were not affected. Whereas these factors were normal in humans, such as our case reported here. As it is difficult for us to figure out the urine volume of animals, as well as clinical symptoms, so diquat-induced AKI can only be diagnosed by biochemical indexes and pathological investigation, or some biomarkers and effectors which can give clinicians some clues to detect and treat diquat-related AKI.
Diquat is an agriculture herbicide, which can induce the redox cycle and lead to the production of reactive oxidative species, and lipid peroxidation. Specifically, lipid peroxidation might cause fatty metabolism disorder2. PPAR, both a nuclear receptor and transcription parameter, which is in charge of lipid metabolism and genes-related to fatty acid pathway. diquat-treated group were lack of PPAR, causing more lipid deposited in kidneys, leading to AKI. So the possible pathways which led to AKI might be PPAR signaling pathway and fatty acid oxidation pathway.
The clinical manifestations range from no specific symptoms to life-threatening conditions. More attention should be paid to monitor vital signs and auxiliary examinations. No specific antidote is available for this kind of toxicity. The main regimen involves supportive therapy. Reducing absorption and improving excretion can help in treating the patient; The mortality rate following the ingestion of diquat is high, as such chemicals are very toxic. To treat such cases, quick therapy needs to be administered, soon after admission. Family members or the patient need to specify the name of the chemical for the correct diagnosis. Similar to hemopuriation techniques, the prognosis of these cases depends on the route of exposure, the quantity of the chemical absorbed, medical history, age, etc. Some biomarkers, such as creatine phosphokinase, myoglobin, and uric acid, might have partial predictive functions. Thus, these biomarkers should be further investigated.
Acknowledgements
Not applicable
Funding Statement
No funding was obtained.
Author’s contributions
Liu Liu was in charge of the diagnosis and the treatment administration for the patients and the final approval of the manuscript. Ziqin Xia draft the manuscript and reviewed the regarding articles. Wenjun Liu collected the data and followed up with the patient.
Ethics approval and consent to participate
The study was approved by the ethical committee of the Tongji Hospital affiliated to Tongji Medical College, Huazhong Univisity of Science and Technology in Wuhan, China.
Consent for publication
Informed written consent was obtained from the patient for the publication of this case report and the accompanying images.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Availability of data and materials
All data in the study are included in this published article.
References
- 1.Basilicata P, Pieri M, Simonelli A, et al. Diquat poisoning: care management and medico-legal implications. Toxics. 2022;10(4):1. doi: 10.3390/toxics10040166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Su Y, Lin R, et al. Effects of acute diquat poisoning on liver mitochondrial apoptosis and autophagy in ducks. Front Vet Sci. 2021;8:727766. doi: 10.3389/fvets.2021.727766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guck D, Hernandez R, Moore S, et al. Rapid glomerulotubular nephritis as an initial presentation of a lethal diquat ingestion. Case Rep Nephrol. 2021;2021:4723092. doi: 10.1155/2021/4723092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y, Zhang R, Meng M, et al. High-dose diquat poisoning: a case report. J Int Med Res. 2021;49(6):3000605211026117. doi: 10.1177/03000605211026117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magalhães N, Carvalho F, Dinis-Oliveira RJ.. Human and experimental toxicology of diquat poisoning: toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum Exp Toxicol. 2018;37(11):1131–4. doi: 10.1177/0960327118765330. [DOI] [PubMed] [Google Scholar]
- 6.Xing J, Chu Z, Han D, et al. Lethal diquat poisoning manifesting as Central pontine myelinolysis and acute kidney injury: a case report and literature review. J Int Med Res. 2020;48(7):300060520943824. doi: 10.1177/0300060520943824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fussell KC, Udasin RG, Gray JP, et al. Redox cycling and increased oxygen utilization contribute to diquat-induced oxidative stress and cytotoxicity in Chinese hamster ovary cells overexpressing NADPH-cytochrome P450 reductase. Free Radic Biol Med. 2011;50(7):874–882. doi: 10.1016/j.freeradbiomed.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Cui S, Wang W, et al. Kidney and lung injury in rats following acute diquat exposure. Exp Ther Med. 2022;23(4):275. doi: 10.3892/etm.2022.11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun YQ, Yuan L, Gao HB et al. Establishment and evaluation of acute diquat poisoning model in wistar rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2019;37(5):342–346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in the study are included in this published article.