Abstract
Acute kidney injury (AKI) is a common disorder without effective therapy yet. Renal ischemia/reperfusion (I/R) injury is a common cause of AKI. MicroRNA miR-192-5p has been previously reported to be upregulated in AKI models. However, its functional role in renal I/R injury is not fully understood. This study aimed to investigate the effects and the underlying mechanism of miR-192-5p in renal I/R progression. Hypoxia/reoxygenation (H/R)-induced cell injury model in HK-2 cells and I/R-induced renal injury model in mice were established in this study. Cell counting kit-8 assay was performed to determine cell viability. Quantitative real-time PCR and western blot analysis were performed to detect gene expressions. Hematoxylin-eosin and periodic acid-Schiff staining were performed to observe the histopathological changes. Enzyme-linked immunosorbent assay was performed to detect the kidney markers’ expression. In vivo and in vitro results showed that miR-192-5p was up-regulated in the I/R-induced mice model and H/R-induced cell model, and miR-192-5p overexpression exacerbated I/R-induced renal damage. Then, the downstream target of miR-192-5p was analyzed by combining the differentially expressed mRNAs and the predicted genes and confirmed using a dual-luciferase reporter assay. It was found that miR-192-5p was found to regulate fat mass and obesity-associated (FTO) protein expression by directly targeting the 3’ untranslated region of FTO mRNA. Moreover, in vivo and in vitro studies unveiled that FTO overexpression alleviated renal I/R injury and promoted HK-2 cell viability via stimulating autophagy flux. In conclusion, miR-192-5p aggravated I/R-induced renal injury by blocking autophagy flux via down-regulating FTO.
Keywords: Renal ischemia-reperfusion injury, miR-192-5p, autophagy, Fat Mass and Obesity-associated Protein
Introduction
Acute kidney injury (AKI) is a global common clinical problem associated with high morbidity and mortality. AKI can lead to a decline in kidney function, such as decreased glomerular filtration rate and increased serum concentration of urea nitrogen, creatinine, and proteinuria [1]. Renal ischemia-reperfusion (I/R) injury is the most frequent cause of AKI, characterized by injury to tubular epithelial cells and vascular endothelium, and robust inflammatory reaction, as well as cellular apoptosis in kidneys [2–6]. However, the pathogenesis of renal I/R injury is complex and unclear. Thus, it is urgently needed to explore the molecular mechanisms of renal I/R injury to unveil effective strategies to protect against renal I/R injury.
MicroRNAs (miRNAs), a group of highly conserved small non-coding RNAs, have been well-studied in numerous biological processes such as cell development, metabolism, and immune responses [7]. Recently, increasing studies revealed the role of miRNAs in renal I/R injury. For instance, endothelial-specific miR-17, -18a, -19a, -20a, -19b-1, and -92a-1 knockout promotes renal dysfunction following renal I/R injury through dysfunction of renal microvasculature [8]. Another study found that miR-218-5p promoted endothelial cell injury to accelerate renal injury [9]. Kirti et al. found that miR-687 is markedly upregulated in the kidney during renal I/R in mice and accelerates the apoptosis of renal proximal tubular cells [10]. Lang et al. found that miR-20a-5p is upregulated in the kidney of AKI mice and attenuates I/R injury by inhibiting the ferroptosis of proximal tubular cells [11]. These studies also revealed that a variety of complex pathways were involved in renal I/R injuries, such as apoptosis, ferroptosis, and endothelial injury.
miR-192-5p, belonging to the miR-192/215 family, is a conserved miRNA [12]. miR-192-5p has been reported as a tumor suppressor in several cancer types [13–15]. Previous studies have also linked miR-192-5p to the I/R injury in cardiomyocytes and liver and found that miR-192-5p inhibition reduces cell apoptosis induced by I/R [16,17]. Similarly, the expression of miR-192-5p is found to be upregulated in vancomycin-induced AKI mice, and the knockdown of miR-192-5p suppresses the vancomycin-induced cell apoptosis [18]. Besides, Zou et al. showed that the urinary levels of miR-192-5p are significantly elevated in rats with I/R-induced kidney injury 72h post-operation, which was earlier than the time of elevation of kidney injury molecule-1 (KIM-1), indicating that miR-192-5p may promote renal I/R injury [19]. However, the regulatory mechanisms of miR-192-5p in renal I/R injury are not well understood, and further studies are required to functionally elucidate.
It is reported that miRNA can repress gene expression via mRNA degradation or translation repression [20,21]. The fat mass and obesity-associated (FTO) gene was initially nominated as an RNA demethylase, which served as catalysis during m6A demethylation and acted in a Fe (II)- and α-ketoglutarate-dependent manner [22]. Studies have shown that FTO expression is downregulated in cisplatin-induced AKI mice, and its overexpression can inhibit cell apoptosis in cisplatin-induced AKI mice [23,24]. Additionally, TargetScan predicted that miR-192-5p had a potential binding relationship with FTO. However, whether miR-192-5p regulates FTO in renal I/R injury-induced AKI remains unknown, and the role of the miR-192-5p/FTO interaction in renal I/R injury-induced AKI is not elucidated.
Autophagy is a self-preservation mechanism in terms of lysosomal degradation and removal of damaged or dysfunctional organelles and macromolecules to achieve cellular cleaning and repair. Besides, autophagy can provide energy and clear toxic proteins to enlarge the cell cycle for damaged cells [25]. Recently, autophagy has been discovered to be implicated in the pathophysiology of AKI [26]. Proximal tubule-specific deletion of autophagy-related gene 5 and 7 in mice worsens tubular apoptosis and renal impairment [27,28]. Thus, autophagy plays a reno-protective effect on renal I/R injury. FTO downregulation is reported to inhibit autophagy [29]. However, the role of FTO-mediated autophagy in renal I/R injury-induced AKI is not clear.
In this study, we aimed to understand the regulatory mechanism of miR-192-5p in renal I/R injury and identify its downstream mediators. We demonstrated the hypothesis that miR-192-5p is up-regulated in renal I/R injury via mediating FTO directly. We also identified the promoting effect of FTO on autophagy in renal I/R injury. Our findings will provide clues for developing novel therapies for renal I/R injury-induced AKI.
Materials and methods
Microarray analysis
RNA expression data of three kidney samples from I/R mice (bilateral renal pedicle clamping for 30 min and reperfusion for 24h) and sham mice (undergo the same surgical procedures but without occlusion of the renal pedicle) was taken from NCBI Gene Expression Omnibus (GSE131454, https://www.ncbi.nlm.nih.gov/geo/). The differential analysis was performed using the ‘limma’ package in R software (v.3.6.3). Down-regulated genes were screened out with log2(Fold Change) < −1 and p value < .05 as the criteria.
Cell culture
The normal proximal tubule epithelial cell line HK-2 was obtained from Procell Life Science Technology Co., Ltd. (Wuhan, China) and cultured in a Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 100 IU/mL of penicillin and 100 mg/mL of streptomycin at 37 °C in a 5% CO2 humidified environment.
Establishment of hypoxia and reoxygenation model
To establish the hypoxia/reoxygenation (H/R) model, HK-2 cells were firstly cultured in an anaerobic atmosphere (1% O2, 5% CO2, 94% N2) for 4h at 37 °C. After that, the cells were placed into a DMEM medium in a humidified 5% CO2 atmosphere, maintained at 37 °C for 12h.
For the in vitro study of the function of miR-192-5p and FTO, miR-192-5p agomir/antagomir and FTO overexpression vector were obtained from GenePharma (Suzhou, China). Once the cells reached about 75% confluence, the oligonucleotides listed above were transfected into HK-2 cells using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s instructions. At 24h after transfection, the H/R process was carried out.
Establishment of renal I/R injury mice model
Male C57BL/6J mice (18 ± 2 g) at 8–12 weeks of age were purchased from Shanghai Laboratory Animal Center. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Affiliated Hospital of Qingdao University. Mice were anesthetized by 4% isoflurane inhalation and continuously inhaled 2% isoflurane to keep narcosis. After which mice were subjected to renal ischemia or to sham operation. For both the right and left side, the kidney was taken out to expose renal pedicles, then the pedicle was dissected, and the blood was blocked by using a micro-aneurysm to clamp the pedicle. After a 30-min clamping, the micro-aneurysm was released and the kidney was ready for reperfusion. Mice were decapitated at 24 h after reperfusion, and their kidneys and blood were collected for further analysis.
For the in vivo study of the function of miR-192-5p, a total of 10 nM miR-192-5p agomir or 50 nM miR-192-5p antagomir were injected via tail veins into the mice for 3 consecutive days. On day 4, the mice were subjected to the renal I/R injury. For the in vivo study of the function of FTO, the adeno-associated virus 9-packaged FTO overexpression plasmid was delivered via tail vein injection into the mice. After 3 d, the mice were subjected to the renal I/R injury.
Renal injury markers detection
Blood was collected from the inferior vena cava and centrifuged at 3000 rpm, 4 °C for 15 min. Serum levels of creatinine, neutrophil gelatinase-associated lipocalin (NGAL), KIM-1 and blood urea nitrogen (BUN) were analyzed using the corresponding enzyme linked immunosorbent assay detection kit (Beyotime, Shanghai, China), respectively, according to the manufacturer’s procedure.
Histopathology examination
For the histological examinations, hematoxylin-eosin (HE) staining and periodic acid-Schiff (PAS) staining were performed. The harvested kidney tissues were fixed in 4% paraformaldehyde, which was followed by dehydration using increasing concentrations of ethanol, embedding into paraffin, and cutting down into slices of 4 μm. De-paraffin slices were stained with hematoxylin and eosin or PAS (Solarbio, Beijing China). Using light microscopy at 200×, five different visual fields were chosen randomly to capture images. Histologic damage was assessed by two researchers in a blinded manner and de-identified.
Terminal deoxynucleotidyl transfer-mediated dUTP nick end-labeling (TUNEL) staining
Cell apoptosis of kidney tissue and HK-2 cells was detected using TUNEL assay. The kidney tissue sections or HK-2 cells were fixed, paraffin-embedded, and incubated with a Tunel reagent mixture for about 30 min at room temperature. After staining the nucleus with DAPI, sections were visualized under a Leica TCS SP5 II microscope and apoptotic areas were quantified in independent fields. The percentage of stained area was calculated using the Image J software.
Cell counting kit-8 (CCK-8) assay
CCK-8 solution was used for the measurement of cell viability. In brief, HK-2 cells in confluent condition were transferred into 96-well plates (5000 cells/well) and cultured overnight at 37 °C. Afterward, cells were subjected to H/R treatment. 10 μL per well of CCK-8 reagent was added into the plate with further incubation for 4h. Optical density at 450 nm was measured under a microplate reader (HITACHI, Japan).
Quantitative real-time PCR (qRT-PCR)
RNA was extracted from the cells and tissues using a PicoPure RNA Isolation kit and a Pure-Link RNA Mini Kit (Life Technologies), respectively. RNA concentrations were quantified by nanodrop. Then, the reverse transcription of RNA was achieved by using a RevertAid First Strand cDNA Synthesis Kit (ThermoFisher). Quantitative PCR analysis was proceeded using MonAmpTM Fast SYBR® Green qPCR Mix (Monad Biotech Corporation, Wuhan, China) according to the standard protocol. β-actin was used as an internal control for mRNA expression normalization, and U6 was used as the control for miRNA expression normalization using the 2−ΔΔCt method. Primer sequences were listed in Supplementary Table 1.
Western blot analysis
Protein extracts from cells and tissues were subjected to 7.5% sodium dodecyl-sulfate polyacrylamide gel electrophoresis gel and ran for 2 h. The separated proteins were then transferred onto polyvinylidene difluoride membranes and exposed to 5% skim milk at room temperature for 1h. After the blockade, membranes were incubated with antibodies against FTO, LC3, p62, and β-actin overnight at 4 °C. Thereafter, membranes were washed in tris-buffered saline and incubated with correlated horseradish peroxidase conjugated secondary antibody for 2 h at room temperature. Protein normalization was achieved by using β-actin as an internal control. Immunoreactive bands were visualized by the enhanced chemiluminescence system (Bio-Rad) and qualified by ImageJ software. The primary antibodies are listed in Supplementary Table 2.
Dual-luciferase reporter assay
The targets of miR-192-5p were predicted by TargetScan 8.0. The wild-type (WT) and mutant 3′ untranslated region 3’UTR of FTO were synthesized and constructed into a pmirGLO luciferase reporter vector. The co-transfection of WT or mutant luciferase plasmid pmirGLO-FTO-3’UTR and miR-192-5p agomir into HK-2 cells was performed using Lipofectamine 2000 based on the manufacturer’s protocol. 48 h later, luciferase activity was measured with the Dual-Luciferase reporter system (Promega, Shanghai, China).
Statistical analysis
All experiments were performed in triplicate at least three times. Data analysis was performed using SPSS 21.0 (IBM Corp., USA). Shapiro–Wilk test was employed to assess whether the data were in a normal distribution. The results were shown as mean ± standard deviation. Comparison between the two groups was analyzed by t-test. Comparison among multiple groups was analyzed by one-way analysis of variance (ANOVA), and pairwise comparison after ANOVA was conducted by Tukey’s multiple comparisons test. p values were obtained using a two-tailed test, and p < .05 was considered statistically significant.
Results
H/R and renal I/R increased the expression of miR-192-5p in vitro and in vivo
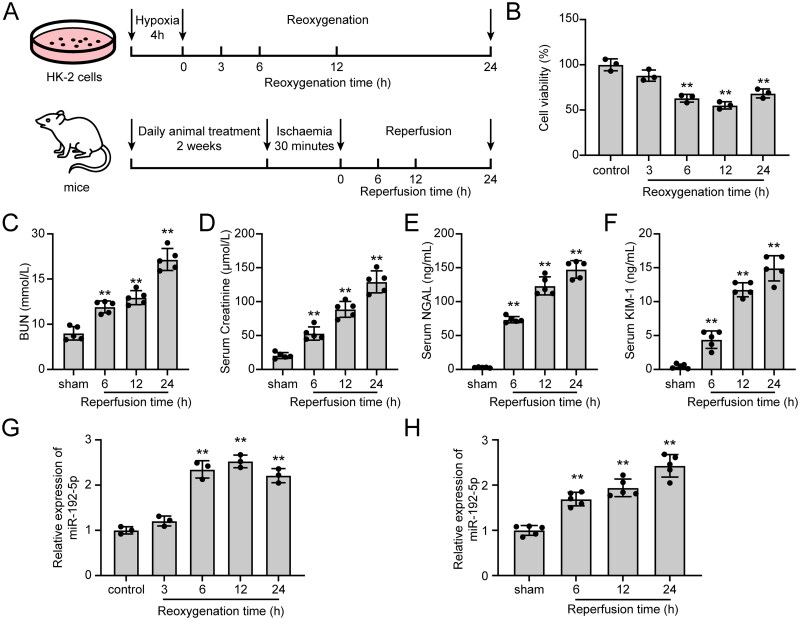
To detect the expression changes of miR-192-5p in renal I/R injury, we both established the in vitro H/R cell model and the in vivo I/R injury model. The schematic illustration of the experimental setup was shown in Figure 1A. In the in vitro experiments, H/R induction in HK-2 cells was observed to inhibit cell viability (Figure 1B). In the in vivo experiments, there are detectable increases observed in BUN, Creatine, NGAL, and KIM-1 levels at 6, 12, and 24 h of reperfusion compared to the sham group (Figure 1C–F). Besides, severe morphological injury was identified by performing HE and PAS staining in renal I/R injury-induced mice, as evidenced by renal tubular dilatation, tubular lysis, loss of brush border, and glomeruli swelling (Figure S1A). These results demonstrated the successful establishment of the in vitro H/R model and in vivo renal I/R injury model. Afterward, miR-192-5p expression in hypoxic HK-2 cells and renal tissues of mice with I/R-induced renal injury was detected by qRT-PCR, which increased between 6 and 24 h of reoxygenation and arrived at the highest level at 12 h of reoxygenation (Figure 1G). Also, miR-192-5p expression in mice reached the highest level at 24 h of reperfusion (Figure 1H).
Figure 1.
miR-192-5p expression was increased in in vitro and in vivo models of renal I/R injury. (A) Schematic diagram of the experimental design. (B) The viability of HK-2 cells after H/R injury (n = 3). (C–F) the expression levels of BUN (C), Creatininie (D), NGAL (E), and KIM-1 (F) in serum of renal I/R mice. (n = 5) (G-H) The expression level of miR-192-5p in vitro (G, n = 3) and in vivo (H, n = 5) based on qRT-PCR assay. The data are represented as means ± SD, ** p < .01, compared with the control or sham group.
miR-192-5p inhibition promoted cell viability in vitro and partially relieved renal I/R injury in vivo
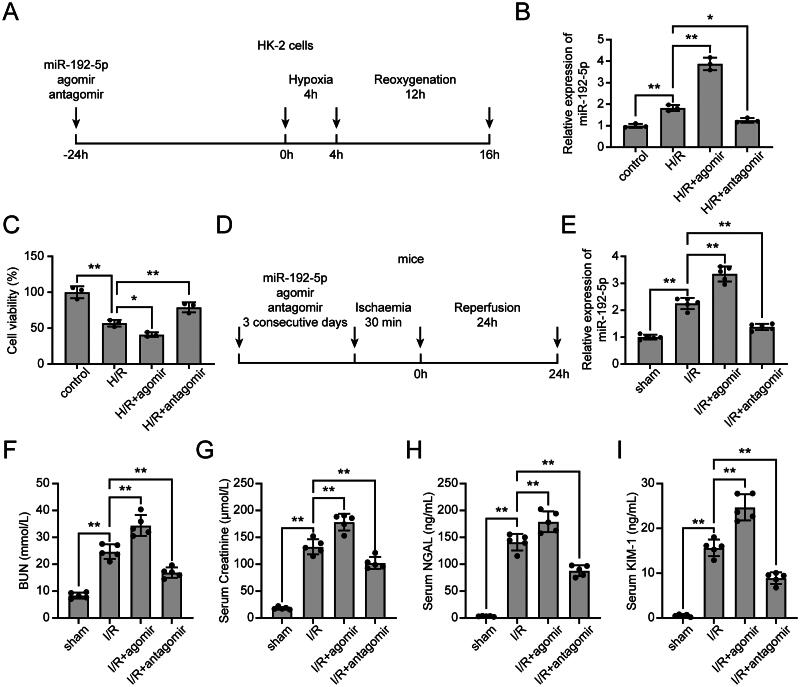
We next explored the effects of miR-192-5p on renal I/R injury. HK-2 cells were transfected with miR-192-5p agomir or antagomir 24 h before H/R treatment (Figure 2A). As shown in Figure 2B, increased expression of miR-192-5p was observed in the H/R + agomir group compared with the H/R group, and the up-regulation of miR-192-5p due to H/R treatment was significantly inhibited by the transfection of miR-192-5p antagomir. We also determined the effects of miR-192-5p on the viability of HK-2 cells by CCK-8 assay. It was demonstrated that miR-192-5p overexpression suppressed HK-2 cell viability, while miR-192-5p inhibition promoted HK-2 cell viability under the H/R condition (Figure 2C). We got consistent results in the renal I/R mice model (Figure 2D), which unveiled an increase of miR-192-5p expression in the I/R + agomir group compared to the I/R group, and the injection of miR-192-5p antagomir significantly reduced miR-192-5p expression in mice (Figure 2F). Moreover, HE staining and PAS staining results indicated that miR-192-5p overexpression aggravated tubular damage and inflammation, while knockdown of miR-192-5p showed opposite effects on I/R-induced renal tissues (Figure S1B). Additionally, the expression levels of BUN, Creatinine, NGAL, and KIM-1 in mice were monitored. For the mice given miR-192-5p agomir, their BUN, Creatinine, NGAL, and KIM-1 levels were increased after renal I/R induction. However, for mice given miR-192-5p antagomir, their BUN, Creatinine, NGAL, and KIM-1 levels were significantly reduced after renal I/R induction (Figure 2F–1).
Figure 2.
Effects Of miR-192-5p on H/R-induced cells and renal I/R-induced mice. (A) Schematic diagram of the in vitro experimental design. (B) qRT-PCR analysis to detect miR-192-5p expression in H/R-induced HK-2 cells (n = 3). (C) CCK-8 assay to detect the viability of HK-2 cells (n = 3). (D) Schematic diagram of the in vivo experimental design. (E) qRT-PCR analysis to detect miR-192-5p expression in I/R-induced renal tissues (n = 5). (F–I) Expression levels of BUN (F), Creatininie (G), NGAL (H), and KIM-1 (I) in serum of renal I/R mice (n = 5). the data are represented as means ± SD, * p < .05, ** p < .01.
miR-192-5p directly targeted FTO and suppressed its expression
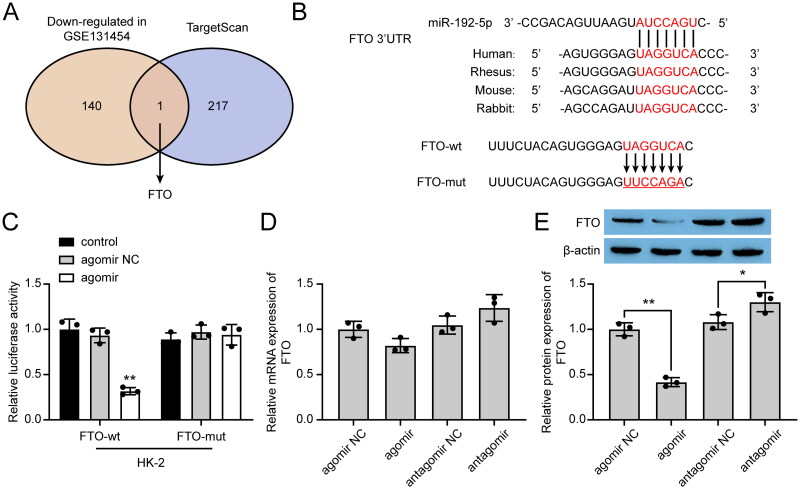
For further understanding of the regulatory mechanism of miR-192-5p in renal I/R injury, we analyzed the microarray GSE131454 to explore the gene expression after renal I/R. The down-regulated mRNAs in GSE131454 were listed in Supplementary Table 3. We also applied the TargetScan webtool to predict the miR-192-5p targets. Taking the two findings together, FTO was selected as the main target in this study (Figure 3A). By constructing wild-type and mutant 3’UTR of FTO into luciferase reporter plasmids (Figure 3B), the targeting relationship between miR-192-5p and FTO was verified by dual-luciferase reporter assay. As shown in Figure 3C, co-transfection of miR-192-5p agomir with wild-type FTO luciferase reporter resulted in a significant reduction of luciferase activity, while no significant difference was observed after the co-transfection of miR-192-5p agomir with mutant FTO luciferase reporter. In addition, qRT-PCR and western blot results further demonstrated that miR-192-5p down-regulated FTO protein expression, but had no obvious effect on the mRNA expression of FTO (Figure 3D,E), suggesting that miR-192-5p negatively regulated FTO by the post-transcriptional mechanism.
Figure 3.
miR-192-5p directly targeted FTO by post-transcriptional mechanism. (A) Venn plot showed the interaction between the miR-192-5p targets and down-regulated mRNAs in GSE131454. (B) The predicted miR-192-5p binding site of FTO. (C) Dual-luciferase reporter assay was performed in HK-2 cells (n = 5). (D-E) FTO mRNA (D) and protein (E) expression regulated by miR-192-5p in HK-2 cells (n = 5). the data are represented as means ± SD, * p < .05, ** p < .01, compared with the agomir NC group.
FTO promoted cell viability in vitro and partially relieved renal I/R injury in vivo
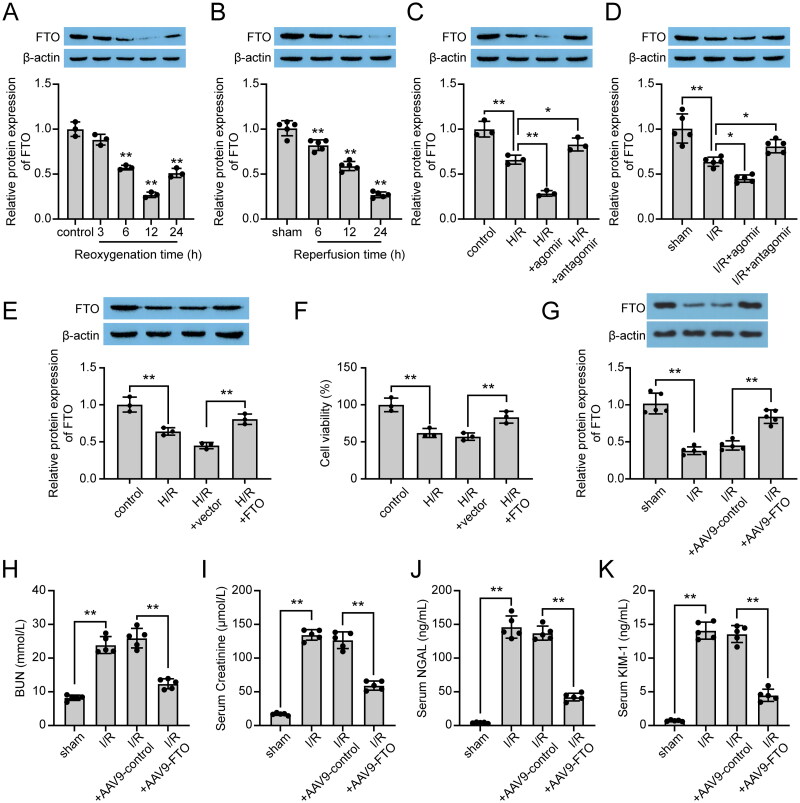
The expression of FTO was examined in H/R-induced cells and renal I/R-induced mice. As shown in Figure 4A,B, lower expression of FTO was observed in experimental groups compared to the sham group both in vivo and in vitro, with the lowest protein level shown at 24 h of reperfusion in vivo and at 12 h of reoxygenation in vitro. Moreover, to analyze the effect of miR-192-5p on FTO protein expression in vivo and in vitro, the cells and mice treated with miR-192-5p agomir and miR-192-5p antagomir were applied. The results showed that miR-192-5p overexpression could decrease the FTO protein level, and miR-192-5p knockdown could promote the FTO protein level (Figure 4C,D). Subsequently, to determine the function of FTO in renal I/R injury, we used the FTO overexpression vector to promote FTO expression in HK-2 cells. Results from the western blot assay confirmed that FTO expression was promoted by the FTO overexpression vector in H/R-induced HK-2 cells (Figure 4E). And overexpression of FTO promoted cell viability in H/R-induced HK-2 cells (Figure 4F). Besides, the above results were also confirmed in renal I/R-induced mice (Figure 4G–K, Figure S1C). In addition, rescue experiments were performed in HK-2 cells. Results showed that miR-192-5p overexpression attenuated HK-2 cell viability and inhibited FTO protein expression, and these effects were diminished by the additional transfection of the FTO overexpression vector (Figure S1A–C). Thus, we can conclude that miR-192-5p functions on renal I/R injury via mediating FTO.
Figure 4.
Effects of FTO on H/R-induced cells and renal I/R-induced mice. (A,B) Protein levels of FTO in vitro (A, n = 3) and in vivo (B, n = 5). (C) Protein levels of FTO in H/R-induced HK-2 cells with miR-192-5p agomir and antagomir transfection (n = 3). (D) Protein levels of FTO in I/R-induced kidney tissues with miR-192-5p agomir and antagomir injection (n = 5). (E) Protein levels of FTO in H/R-induced HK-2 cells (n = 3). (F) Effects of FTO on cell viability of H/R-induced HK-2 cells (n = 3). (G) Protein levels of FTO in I/R-induced kidney tissues with AAV9-FTO treatment (n = 5). (H–K) expression levels of BUN (H), Creatininie (I), NGAL (J), and KIM-1 (K) in serum of renal I/R mice (n = 5). the data are represented as means ± SD, * p < .05, ** p < .01.
FTO promoted autophagy flux and inhibited apoptosis after H/R and I/R
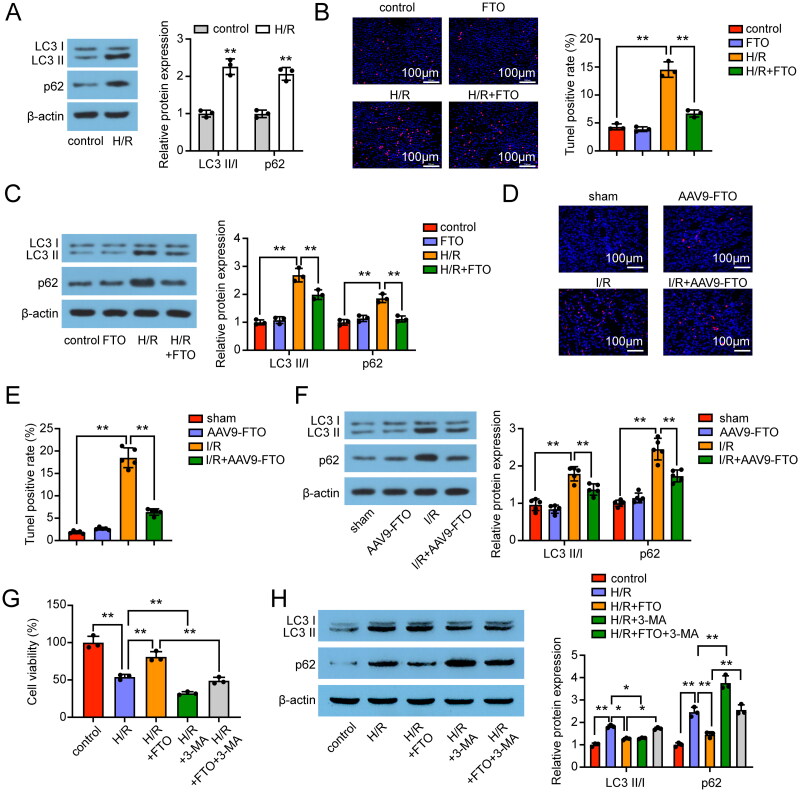
Emerging evidence indicated the importance of autophagy for maintaining renal tubule functions during ischemic injury [26]. Based on this, we detected the expression of autophagy-related proteins in HK-2 cells, which showed an increase in the LC3 II/I ratio after the H/R process. Interestingly, the expression of autophagy adaptor protein p62, which normally negatively correlated to autophagy activity, was increased after the H/R process (Figure 5A). Therefore, we hypothesized that autophagy flux was inhibited in H/R-induced HK-2 cells. Next, the TUNEL-positive rate and the levels of LC3 I, LC3 II, and p62 proteins were examined in FTO-overexpressed HK-2 cells. TUNEL staining results showed that the TUNEL-positive rate was upregulated in H/R-induced HK-2 cells, and further decreased by transfection with FTO overexpression vector (Figure 5B). We also found that FTO overexpression suppressed the p62 expression, which resulted in promoted autophagy flux (Figure 5C). The in vivo experiments showed similar results that FTO overexpression suppressed the TUNEL rate and the p62 protein expression in the renal I/R mice model (Figure 5E,F). Rescue experiments showed that FTO overexpression attenuated I/R injury in vitro, and this effect was reversed by the autophagy inhibitor 3-MA (Figure 5G,H). These results suggested that FTO may promote the autophagy flux and suppress apoptosis under H/R and I/R injury.
Figure 5.
FTO regulated autophagy flux and apoptosis in renal I/R injured. (A) Protein expression of LC3 I, LC3 II, and p62 in H/R-induced HK-2 cells (n = 3). (B) TUNEL staining results in H/R-induced HK-2 cells (n = 3). (C) Effects of FTO on the LC3 I, LC3 II, and p62 expression in H/R-induced HK-2 cells (n = 3). (D,E) TUNEL staining results in renal I/R-induced mice (n = 5). (F) Effects of FTO on the LC3 I, LC3 II, and p62 expression in renal I/R-induced mice (n = 5). (G) Effects of FTO and autophagy inhibitor 3-MA on the viability of HK-2 cells (n = 3). (H) Effects of FTO and and autophagy inhibitor 3-MA on the protein expression of LC3 I, LC3 II, and p62 in H/R-induced HK-2 cells (n = 3). the data are represented as means ± SD, * p < .05, ** p < .01.
Discussion
Renal I/R injury is a common cause of AKI by tubule necrosis during ischemia and severe immune responses during reperfusion [30]. It is also a severe complication of increased mortality and morbidity of postoperative graft procedures [31]. A previous study suggested that miR-192-5p serves as an important potential diagnostic marker for I/R-induced kidney injury [19]. However, the underlying mechanism of miR-192-5p in renal I/R has not been fully elucidated. In this study, miR-192-5p was confirmed to be up-regulated in H/R-induced HK-2 cells and mice renal after I/R injury, and the overexpression of miR-192-5p aggravated tubular damage and exacerbated renal I/R injury. We further unveiled that miR-192-5p positively regulated immunity by post-transcriptional regulation of FTO, which attenuated renal I/R injury by promoting the autophagy flux. The regulatory network between miR-192-5p and FTO gives us an idea of treating renal injury, and they might be promising targets for renal I/R injury-induced AKI therapy.miRNAs are important in the maintenance of normal cellular functions, and dysregulation of miRNAs can result in a number of pathological states. Several studies have reported that miRNAs regulate angiogenesis, mitochondrial dysfunction, endothelial progenitor cell migration, and glomerular basement membrane structure [8,9,32,33]. A previous study also reported that miR-192-5p inhibition blocks cell apoptosis in vancomycin-induced HK-2 cells [18]. Consistent with the previous study, we verified the upregulation of miR-192-5p in I/R-induced renal tissues and H/R-induced HK-2 cells. By performing gain- and loss-of-function studies, we demonstrated that miR-192-5p inhibition promoted cell viability of H/R-induced HK-2 cells and significantly alleviated tubular damage in renal I/R mice. Furthermore, Jia et al. found that miR-192-5p induced fibrosis production and autophagy limitation in diabetic nephropathy [34]. Unfortunately, the role of miR-192-5p in renal fibrosis was not examined in the present study; we hypothesized that miR-192-5p may affect renal fibrosis in the renal I/R model.miRNAs fulfill their biological functions by binding to target genes [35]. Through bioinformatic analysis, we identified FTO as the downstream target of miR-192-5p in renal I/R injury. In previous studies, FTO was shown to serve crucial roles in renal injury. Zhou reported that FTO expression was downregulated in cisplatin-induced AKI mice, and overexpression of FTO relieves renal damage via inhibiting cell apoptosis [23]. Wang et al. found that FTO expression in kidneys after unilateral ureteral obstruction (UUO) is increased, and FTO deficiency protected kidneys from UUO injury [36]. In this study, FTO was identified to be decreased in H/R-induced HK-2 cells and renal I/R-induced injury in mice. FTO overexpression enhanced the cell viability of HK-2 cells and alleviated tubular damage in renal I/R-induced mice.
Several studies have investigated the relationship between autophagy and renal I/R [26,27]. Autophagy is an evolutionarily conserved multi-step process of degradation of intracellular organelles, proteins, and other macromolecules by the hydrolases of lysosomes [37,38]. Autophagy itself is paradoxical in that it can both promote cell survival and induce cell death, depending on the environmental stressors [39]. Thus, the role of autophagy in renal I/R injury presents two different results. Some studies found that autophagy was activated after renal I/R, and the activated autophagy attenuates renal I/R injury [40–43]. Some studies found that reducing autophagy could prevent renal I/R injury [44,45]. Given that autophagy is a dynamic process, the reason for this difference may be related to the point in time of detection. In the current study, we found that both expression of LC3 II and p62 was increased after H/R and I/R treatment. During the autophagy process, the autophagosomes engulf and deliver cellular contents to the lysosome for degradation. The conversion of LC3I to LC3II is the marker of autophagosome formation, and p62 expression reflects the degradation of lysosome [43]. Thus, our results showed that the autophagosome was formed after renal I/R treatment, while the lysosome degradation was blocked. These findings suggested that autophagy flux was blocked in renal I/R injury. After the use of 3-MA to inhibit autophagy, the cell viability was further reduced. This suggested that autophagy flux inhibition worsens kidney injury. Recent studies showed the regulation of FTO on autophagy. Lacking FTO has been shown to inhibit MTORC1 signaling and thereby activate autophagy [46]. In addition, abnormal FTO expression showed no effect on autophagy induced by starvation in U2OS cells [47]. In the current study, we demonstrated that overexpression of FTO could promote the autophagy flux in H/R-induced HK-2 cells and renal I/R mice. Considering the multifunctional effect of autophagy in cells and tissues, differences shown in FTO-regulated autophagy might be explained by specific cell types applied in these studies.
Limitations in this study should never be neglected. The expression of miR-192-5p was detected in HK-2 cells and mice. To make the results of the current study more reliable, the detection of miR-192-5p in AKI patients is needed. Also, the injection of miRNA agomir/antagomir was through the tail vein, which was not directly to the kidney and might have side effects. Some better methods, such as normothermic machine perfusion [48], can be applied to deliver the miRNA agomir/antagomir to kidneys. In addition, whether FTO could regulate renal I/R injury by other mechanisms should also be further explored.
Conclusion
In summary, our results revealed the miR-192-5p was up-regulated in H/R-induced HK-2 cells and renal I/R-induced tissues. Besides, miR-192-5p inhibition enhanced cell viability and renal function in vivo and in vitro via the post-transcriptional down-regulation of FTO. In brief, miR-192-5p might serve as an important potential diagnostic biomarker for I/R-induced kidney injury, which should be interpreted with caution and further verified. Furthermore, FTO was found to promote autophagy flux in H/R-induced HK-2 cells and renal I/R mice. In short, our findings might provide novel therapeutic targets for renal I/R injury-induced AKI treatment.
Supplementary Material
Funding Statement
This work was supported by the Research Initiation Project for High-Level Talents of The Affiliated Hospital of Qingdao University under Grant number 3631.
Authors contributions
Substantial contribution to the conception and design of the work: ZCJ, GG, WJT, WHJ and CJZ; Analysis and interpretation of the data: ZCJ, GG, WJT, WHJ and CJZ; Drafting the manuscript: ZCJ, GG and CJZ; Revising the work critically for important intellectual content: ZCJ and CJZ; Collection of grants: CJZ; Final approval of the work: all authors.
Ethical approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Affiliated Hospital of Qingdao University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Ronco C, Bellomo R, Kellum JA.. Acute kidney injury. Lancet. 2019;394(10212):1–11. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 2.Rippe C, Rippe A, Larsson A, et al. . Nature of glomerular capillary permeability changes following acute renal ischemia-reperfusion injury in rats. Am J Physiol Renal Physiol. 2006;291(6):F1362–F1368. doi: 10.1152/ajprenal.00123.2006. [DOI] [PubMed] [Google Scholar]
- 3.Powell TC, Powell SL, Allen BK, et al. . Association of inflammatory and endothelial cell activation biomarkers with acute kidney injury after sepsis. Springerplus. 2014;3(1):207. doi: 10.1186/2193-1801-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SY, Shin JA, Kwon HM, et al. . Renal ischemia-reperfusion injury causes intercalated cell-specific disruption of occludin in the collecting duct. Histochem Cell Biol. 2011;136(6):637–647. doi: 10.1007/s00418-011-0881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristovic D, Horvatic I, Husedzinovic I, et al. . Cardiac surgery-associated acute kidney injury: risk factors analysis and comparison of prediction models. Interact Cardiovasc Thorac Surg. 2015;21(3):366–373. doi: 10.1093/icvts/ivv162. [DOI] [PubMed] [Google Scholar]
- 6.Jacobi J, Rebhan D, Heller K, et al. . Donor acute kidney injury and short-term graft outcome in renal transplantation. Clin Transplant. 2014;28(10):1131–1141. doi: 10.1111/ctr.12425. [DOI] [PubMed] [Google Scholar]
- 7.Wu YL, Li HF, Chen HH, et al. . MicroRNAs as biomarkers and therapeutic targets in inflammation- and ischemia-reperfusion-related acute renal injury. Int J Mol Sci. 2020;21(18):6738. doi: 10.3390/ijms21186738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiba T, Cerqueira DM, Li Y, et al. . Endothelial-derived miR-17 approximately 92 promotes angiogenesis to protect against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2021;32(3):553–562. doi: 10.1681/ASN.2020050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Li Y, Lyu L, et al. . Integrin alpha5 is regulated by miR-218-5p in endothelial progenitor cells. J Am Soc Nephrol. 2022;33(3):565–582. doi: 10.1681/ASN.2021020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt K, Wei Q, Pabla N, et al. . MicroRNA-687 induced by Hypoxia-Inducible factor-1 targets phosphatase and tensin homolog in renal ischemia-reperfusion injury. J Am Soc Nephrol. 2015;26(7):1588–1596. doi: 10.1681/ASN.2014050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi L, Song Z, Li Y, et al. . MiR-20a-5p alleviates kidney ischemia/reperfusion injury by targeting ACSL4-dependent ferroptosis. Am J Transplant. 2023;23(1):11–25. doi: 10.1016/j.ajt.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Ren FJ, Yao Y, Cai XY, et al. . Emerging role of MiR-192-5p in human diseases. Front Pharmacol. 2021;12:614068. doi: 10.3389/fphar.2021.614068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu W, Deng J, Chen C, et al. . Circ_0001602 aggravates the progression of acute myeloid leukemia by regulating the miR-192-5p/ZBTB20 axis. Hematology. 2023;28(1):2240133. doi: 10.1080/16078454.2023.2240133. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Ma H, Li Y, et al. . MiR-192-5p-modified tumor-associated macrophages-derived exosome suppressed endometrial cancer progression through targeting IRAK1/NF-kappaB signaling. Reprod Sci. 2022;29(2):436–447. doi: 10.1007/s43032-021-00789-8. [DOI] [PubMed] [Google Scholar]
- 15.Dong RF, Zhuang YJ, Wang Y, et al. . Tumor suppressor miR-192-5p targets TRPM7 and inhibits proliferation and invasion in cervical cancer. Kaohsiung J Med Sci. 2021;37(8):699–708. doi: 10.1002/kjm2.12398. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Huang R, Zhou W, et al. . miR-192-5p mediates hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes via targeting of FABP3. J Biochem Mol Toxicol. 2017;31(4):e21873. doi: 10.1002/jbt.21873. [DOI] [PubMed] [Google Scholar]
- 17.Roy S, Benz F, Alder J, et al. . Down-regulation of miR-192-5p protects from oxidative stress-induced acute liver injury. Clin Sci. 2016;130(14):1197–1207. doi: 10.1042/CS20160216. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Wang J, Li H, et al. . p53 activates miR-192-5p to mediate vancomycin induced AKI. Sci Rep. 2016;6(1):38868. doi: 10.1038/srep38868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou YF, Wen D, Zhao Q, et al. . Urinary microRNA-30c-5p and MicroRNA-192-5p as potential biomarkers of ischemia-reperfusion-induced kidney injury. Exp Biol Med (Maywood). 2017;242(6):657–667. doi: 10.1177/1535370216685005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohr AM, Mott JL.. Overview of microRNA biology. Semin Liver Dis. 2015;35(1):3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui J, Zhou B, Ross SA, et al. . Nutrition, microRNAs, and human health. Adv Nutr. 2017;8(1):105–112. doi: 10.3945/an.116.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kewenter J. Does screening influence mortality of colorectal cancer? Lakartidningen. 1991;88(8):601–602. [PubMed] [Google Scholar]
- 23.Zhou P, Wu M, Ye C, et al. . Meclofenamic acid promotes cisplatin-induced acute kidney injury by inhibiting fat mass and obesity-associated protein-mediated m(6)a abrogation in RNA. J Biol Chem. 2019;294(45):16908–16917. doi: 10.1074/jbc.RA119.011009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li CM, Li M, Zhao WB, et al. . Alteration of N6-methyladenosine RNA profiles in cisplatin-induced acute kidney injury in mice. Front Mol Biosci. 2021;8:654465. doi: 10.3389/fmolb.2021.654465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dikic I, Elazar Z.. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 26.Kaushal GP, Shah SV.. Autophagy in acute kidney injury. Kidney Int. 2016;89(4):779–791. doi: 10.1016/j.kint.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura T, Takabatake Y, Takahashi A, et al. . Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22(5):902–913. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komatsu M, Waguri S, Ueno T, et al. . Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169(3):425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Wu R, Liu Y, et al. . m(6)a mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy. 2020;16(7):1221–1235. doi: 10.1080/15548627.2019.1659617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panah F, Ghorbanihaghjo A, Argani H, et al. . Ischemic acute kidney injury and klotho in renal transplantation. Clin Biochem. 2018;55:3–8. doi: 10.1016/j.clinbiochem.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Pefanis A, Ierino FL, Murphy JM, et al. . Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019;96(2):291–301. doi: 10.1016/j.kint.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Xiang X, Hu X, et al. . miR-147 represses NDUFA4, inducing mitochondrial dysfunction and tubular damage in cold storage kidney transplantation. J Am Soc Nephrol. 2023;34(8):1381–1397. doi: 10.1681/ASN.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller-Deile J, Sopel N, Ohs A, et al. . Glomerular endothelial cell-derived microRNA-192 regulates nephronectin expression in idiopathic membranous glomerulonephritis. J Am Soc Nephrol. 2021;32(11):2777–2794. doi: 10.1681/ASN.2020121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia Z, Wang K, Zhang Y, et al. . Icariin ameliorates diabetic renal tubulointerstitial fibrosis by restoring autophagy via regulation of the miR-192-5p/GLP-1R pathway. Front Pharmacol. 2021;12:720387. doi: 10.3389/fphar.2021.720387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebert MS, Sharp PA.. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149(3):515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CY, Shie SS, Tsai ML, et al. . FTO modulates fibrogenic responses in obstructive nephropathy. Sci Rep. 2016;6(1):18874. doi: 10.1038/srep18874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z, Klionsky DJ.. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22(2):124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizushima N, Komatsu M.. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Zhang W, Li YP, et al. . Hypoxia-induced autophagy in endothelial cells: a double-edged sword in the progression of infantile haemangioma? Cardiovasc Res. 2013;98(3):437–448. doi: 10.1093/cvr/cvt035. [DOI] [PubMed] [Google Scholar]
- 40.Zhang YL, Qiao SK, Wang RY, et al. . NGAL attenuates renal ischemia/reperfusion injury through autophagy activation and apoptosis inhibition in rats. Chem Biol Interact. 2018;289:40–46. doi: 10.1016/j.cbi.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Tseng WC, Lee PY, Tsai MT, et al. . Hypoxic mesenchymal stem cells ameliorate acute kidney ischemia-reperfusion injury via enhancing renal tubular autophagy. Stem Cell Res Ther. 2021;12(1):367. doi: 10.1186/s13287-021-02374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ling H, Chen H, Wei M, et al. . The effect of autophagy on inflammation cytokines in renal ischemia/reperfusion injury. Inflammation. 2016;39(1):347–356. doi: 10.1007/s10753-015-0255-5. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YL, Zhang J, Cui LY, et al. . Autophagy activation attenuates renal ischemia-reperfusion injury in rats. Exp Biol Med. 2015;240(12):1590–1598. doi: 10.1177/1535370215581306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guler MC, Akpinar E, Tanyeli A, et al. . Costunolide prevents renal ischemia-reperfusion injury in rats by reducing autophagy, apoptosis, inflammation, and DNA damage. Iran J Basic Med Sci. 2023;26(10):1168–1176. doi: 10.22038/IJBMS.2023.71779.15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadj Abdallah N, Baulies A, Bouhlel A, et al. . Zinc mitigates renal ischemia-reperfusion injury in rats by modulating oxidative stress, endoplasmic reticulum stress, and autophagy. J Cell Physiol. 2018;233(11):8677–8690. doi: 10.1002/jcp.26747. [DOI] [PubMed] [Google Scholar]
- 46.Gulati P, Cheung MK, Antrobus R, et al. . Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Natl Acad Sci U S A. 2013;110(7):2557–2562. doi: 10.1073/pnas.1222796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aas A, Isakson P, Bindesboll C, et al. . Nucleocytoplasmic shuttling of FTO does not affect Starvation-Induced autophagy. PLOS One. 2017;12(3):e0168182. doi: 10.1371/journal.pone.0168182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson ER, Sewpaul A, Figuereido R, et al. . MicroRNA antagonist therapy during normothermic machine perfusion of donor kidneys. Am J Transplant. 2022;22(4):1088–1100. doi: 10.1111/ajt.16929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.