Abstract
Purpose:
The purpose of this study was to explore the relationships between hearing loss, cognitive status, and a range of health outcomes over a period of 2 years in a sample of older adults who are enrolled in Program of All-Inclusive Care for the Elderly, which is a Medicare/Medicaid beneficiary program for individuals who are nursing home eligible but living in the community at time of enrollment.
Method:
The sample (N = 144) includes a diverse (47% White/non-Hispanic, 35% Black/African American, and 16% Latin/Hispanic) group of adults ranging from 55 to 93 years old. We used medical chart data to measure respondents' cognitive and health status, including chronic conditions and hospital use. Hearing status was measured once at the beginning of the 2-year review period. We used logistic regression and negative binomial hurdle models for analyses. We used latent class analysis (LCA) to explore the extent to which respondents cluster into a set of “health profiles” characterized by their hearing, cognitive status, and health conditions.
Results:
We found that hearing loss is weakly associated with heart disease and diabetes and associated with cerebrovascular disease and falls; cognitive impairment is also associated with cerebrovascular disease and the number of falls. LCA indicates that respondents cluster into a variety of health profiles with a consistent pairing of hearing loss and depression.
Conclusions:
The results are largely consistent with associations reported in epidemiological studies that include age-related hearing loss. Of particular interest in this study is the LCA that suggested that all of the profiles associated with a high likelihood of hearing loss included a high risk of depression. The co-occurrence of these two factors highlights the need to identify and treat hearing loss in older adults, especially as part of the treatment plan for individuals with depressive symptoms.
Over the past 10 years, several large epidemiological data sets have included objective measures of hearing loss and elucidated consistent associations between age-related hearing loss and a host of negative health outcomes related to aging. The directionality of the associations remains unknown; however, several mechanistic pathways have been proposed to explain the relationship between age-related hearing loss and accelerated physical and cognitive declines (Lin & Albert, 2014; Uchida et al., 2019). This study investigates a range of health outcomes and compares groups based on their hearing and cognitive status at baseline in a sample of nursing home–eligible, community-dwelling older adults. This sample is a medically complex group of older adults who are often not included in larger community samples. It is important to understand the health patterns in older adults experiencing multiple chronic conditions.
Age-Related Hearing Loss and Its Associations
Cognitive Decline
The first associations between hearing loss and dementia were reported in the 1980s (Peters et al., 1988; Uhlmann et al., 1986, 1989). Fast forward 30 years and reports from large cross-sectional and longitudinal studies of aging begin to report associations between age-related hearing loss and faster rates of cognitive decline and higher incidence of dementia (Davies et al., 2017; Deal et al., 2017; Lin et al., 2013). With the increasing number of epidemiological studies that include objective assessment of hearing status in the test battery, the strength of these associations has increased (Loughrey et al., 2018). The relationship has proven so consistent that the 2017 Lancet report on dementia risks found that hearing loss in midlife increased the risk of dementia by 9%, the largest potentially modifiable risk factor that begins in adulthood (Livingston et al., 2017, 2020).
There are a number of hypotheses as to why the association between age-related hearing loss and cognitive decline is consistently observed. Two primary concepts include the “common cause” and “cascade” hypotheses (Griffiths et al., 2020; Lin & Albert, 2014; Uchida et al., 2019). As the field increases its understanding of this association, it is important to consider the individuals holistically, including the other comorbidities they may have. While multiple chronic conditions may contribute to the concept of a common pathology underlying both the hearing loss and the cognitive impairment, multiple chronic conditions will also impact the cascade hypothesis in that more health limitations will likely lead to more physical decline and social isolation.
Physical Decline
There are several reports linking age-related hearing loss to declines in physical mobility, including gait speed and measures of strength/frailty (Chen et al., 2015; Martinez-Amezcua et al., 2021; Tian et al., 2021). However, the most important physical function association is likely the relationship between age-related hearing loss and increased falls (Jiam et al., 2016; Kamil et al., 2016). In a systematic review and meta-analysis including 13 studies, hearing loss in older adults was associated with a 2.39 greater odds ratio of falling (Jiam et al., 2016). The exact mechanism of this relationship is unclear. There is likely some shared pathophysiology between age-related hearing and vestibular declines (Gabriel et al., 2022; Viljanen et al., 2009). There is also the more complex pathway of hearing loss leading to withdrawal and thus reducing the amount of social and physical activity in which one engages, which has downstream consequences (Kuo et al., 2021). Mechanistic pathway aside, this association is of critical importance because of the fact that falls are the leading cause of injury-related mortality among older adults and increase health care costs due to emergency department (ED) visits and hospitalization (Riska et al., 2022).
Other Chronic Conditions
Last but not least, age-related hearing loss is associated with several major chronic health conditions, such as diabetes, heart disease, and cerebrovascular disease. These associations are likely related to each other through vascular and metabolic changes happening with age and as a function of health risk factors (e.g., smoking, obesity). In longitudinal cohort studies of aging, there is evidence of increased risk for hearing loss in persons with cardiovascular disease risk factors—both cross-sectionally and longitudinally (Helzner et al., 2011; Mick et al., 2023). The convergence of these chronic conditions highlights a need for a comprehensive approach to care for older adults, such that treatments that enhance function—both social and physical activity—are included as a key component to maintaining health with advancing age (Ghisletta et al., 2023).
Furthermore, hearing loss is associated with reduced health-related quality-of-life ratings, increased reports of social isolation and loneliness, and higher rates of depression (Lawrence et al., 2020; Mick et al., 2014, 2018; Simpson et al., 2015). In a systematic review and meta-analysis that included 35 studies and a sample of over 145,000 individuals, there was a small and significantly greater odds (1.47) of depression in older adults with hearing loss (Lawrence et al., 2020). The authors attribute this association to the fact that older adults with hearing loss experience higher rates of social and emotional loneliness, reduced cognitive function, and increased difficulty with daily activities—all of which contribute to poor psychosocial function and wellness.
Study Population
The current sample includes enrollees at three Programs of All-Inclusive Care for the Elderly (PACE). PACE is a nationwide Medicare/Medicaid beneficiary program that serves as the comprehensive care home for its enrollees (Mui, 2001). To enroll in the program, an individual must be 55 years or older, deemed nursing home eligible by the state, and living in the community at time of enrollment. There are currently 149 PACE programs, operating 273 PACE centers in 32 states and serving over 60,000 older adults (National PACE Association, 2022). The PACE system centers around a Day Health Center where enrollees receive meals, basic medical/rehabilitation care, and social engagement opportunities. Each enrollee has an individualized care plan that determines their schedule for attendance at the Day Health Center based on the level of in-person care they need (e.g., physical therapy or medication administration needs), as well as their social needs to maintain optimal health and to age safely in the community.
The baseline data from this particular sample included 160 individuals from three PACE organizations who participated in on-site hearing tests. The prevalence of hearing loss in that sample was generally consistent with population estimates for hearing loss among older adults (Mamo & Wheeler, 2021). An important characteristic of this sample that we highlighted in the previous report was the high prevalence of other chronic conditions, including diabetes (59%), hypertension (90%), cardiovascular disease (80%), and depression (67%). For comparison, in 2018, the Centers for Medicare and Medicaid Services (CMS) reported much lower prevalence among all of their beneficiaries who were 65 years and older for diabetes (27%), hypertension (60%), ischemic heart disease (29%), and depression (16%; CMS, 2021a). The high prevalence of these chronic conditions is an important reminder that older adults with age-related hearing loss are often managing more than one chronic condition.
In 2018, CMS reported that among dual-eligible (Medicare/Medicaid) individuals 65 years and older, 22% have two to three chronic conditions, 25% have four to five chronic conditions, and 35% have six or more chronic conditions (CMS, 2021b). At the time of our hearing testing in our PACE sample, using only the four conditions included in our previous report (i.e., diabetes, hypertension, cardiovascular disease, and depression), we estimate that 38% of the sample had at least three of the four chronic conditions, and 33% of the sample had all four of those chronic conditions. The PACE sample reflects the health status of a nursing home population of older adults, rather than a general community-dwelling sample of older adults. As such, the lessons learned here are best applied to populations in assisted and nursing care facilities.
The current research objective is to investigate the relationship between hearing loss and cognitive impairment with a variety of indicators of health and physical condition in this sample over time. We hypothesize that PACE participants with hearing loss and cognitive impairment will have a higher burden of other chronic health conditions, physical decline, and health resource utilization compared to PACE participants without hearing loss and cognitive impairment. Outcomes of interest were chosen to reflect the associations reported in the literature between age-related hearing loss and cognitive and physical declines, including issues related to health resource utilization. One goal of this work is to recognize if there are health-related outcomes that are specifically poorer for the hearing loss and cognitively impaired groups such that future intervention work could target broader health-related outcomes as an effect of treating hearing loss in this medically complex group of older adults.
Method
Participants and Setting
Data were collected between February 2019 and February 2022 from three independent PACE organizations in New England. The participants could opt in to be included in the medical chart review component of the study at their time of enrollment in a hearing loss prevalence study. In total, 174 individuals volunteered to do hearing testing and 144 consented to the longitudinal medical chart review. Sixteen of the 174 opted to participate in other aspects of the study but did not want to be included in the longitudinal chart review sample. There were 11 participants whose data were excluded due to lack of capacity to consent.1 Prior to translation of the consent forms,2 there were three volunteers who had insufficient English language proficiency to complete the consent form. The institutional review boards of the University of Massachusetts Amherst and Trinity Health of New England approved this study. Of our final sample of 144 respondents, 22 died and 14 disenrolled over the 3 years of the study. This left us with a final sample of 144 respondents for Year 1, 132 respondents in Year 2, and 108 respondents in Year 3, for a total number of 384 person-year observations.
Procedures and Measures
Hearing Tests
The hearing tests were completed once at the time of enrollment in the study. The hearing tests were composed of otoscopy, questions regarding subjective hearing status and hearing aid use, and pure-tone air-conduction audiometry using clinically validated SHOEBOX audiometers (SHOEBOX Ltd.; Thompson et al., 2015). Participants were not turned away if occluded cerumen was visualized during otoscopy. The hearing tests were administered by members of the research team using a modified automated protocol on SHOEBOX audiometers with RadioEar DD450 circumaural headphones (RadioEar). The automated protocol monitors ambient noise levels during testing and uses an algorithm to detect inconsistent responses. The system then notifies the test administrator if the audiologic thresholds are elevated or inconsistent, allowing the research assistant to reinstruct the participant. Air-conduction testing was collected at octave frequencies 0.5–8 kHz. Thresholds were obtained using behavioral responses such as hand raising or verbal responses dependent on participant preference. Hearing status was determined based on the four-frequency pure-tone average (PTA) of the better hearing ear at 500, 1000, 2000, and 4000 Hz. Hearing loss status was determined as no loss if the PTA was less than or equal to 25 dB HL, mild loss if the PTA was 26–40 dB HL, or moderate/severe if the PTA was greater than 40 dB HL.
Medical Chart Review
As has been noted, 144 participants across the three PACE sites opted in to the longitudinal medical chart review at the time of their hearing test (baseline). Two members of the research team were given limited access annually over the course of a 3-year period to extract the data from the participants' medical charts. The three PACE sites each used a different electronic medical record system. Medical record data from PACE 1 (n = 37) were collected each year between February and April 2019, 2020, and 2021; PACE 2 (n = 46) data collection was from September to October 2019, 2020, and 2021; and PACE 3 (n = 61) data collection was obtained in January–February 2020, 2021, and 2022. The data were extracted annually from the previous 12 months from the date of consent over a 3-year period during the time frames listed above.
The data extracted included relevant demographic and medical history information. One member of the research team extracted the data and used the diagnostic International Classification of Diseases 9th Revision and International Classification of Diseases 10th Revision codes in the participants' charts to record whether each respondent had the following chronic conditions: diabetes, hypertension, cardiovascular disease, atherosclerosis, cerebrovascular disease, depression, cognitive impairment, and frailty. These chronic conditions were of specific interest to the research team due to the epidemiologic and clinical research studies suggestive of correlation between hearing loss and changes in cognitive status in individuals with increased vascular and social risk factors (Lin & Albert, 2014). Frailty was also of interest due to the nature of its relationship with sensory impairments such as hearing and vision loss and the impact it has on age-related clinical conditions including falls and hospitalizations (Zhao et al., 2022).
When extracting diagnostic codes from the medical charts had been completed, we had compiled 850 unique diagnostic codes. To reduce the number of codes included in the analysis script, we referred to the diagnostic categories established by the CMS for their Chronic Conditions Data Warehouse (CMS, 2022) or CMS guidelines for coding (CMS, 2019). Two research assistants independently categorized the entire list of diagnostic codes into the conditions listed above with 91% agreement between the two raters. The first author reviewed the mismatched categories based on the diagnostic codes and resolved the mismatches.
Nursing home placements were coded as a dichotomized yes/no based on current resident status of the participant. Hearing aid use was coded as yes/no/previous based on information gathered from the participant during intake hearing status questionnaire and medical chart information for the subsequent years to determine if there were changes in device usage. Number of falls was recorded within the 12-month period based on documented notes by the PACE staff. All falls were accounted for regardless of whether the fall resulted in hospitalization. Dates of ED visits and dates of hospital admissions and discharges were also extracted from the medical chart based on documentation by the PACE staff. We used this information to calculate the number of ED visits and hospital admissions.
Survey
We also administered a survey at time of enrollment in the study to respondents to collect basic demographic information including date of birth, race and ethnicity, educational attainment, gender, marital status, and smoking status. Seventy of the participants in the current sample opted in to the survey portion of the study. The complete survey results are presented in mixed-methods study of communication at PACE (Mamo et al., 2022). We collected detailed information on respondent race and ethnicity; all but three respondents were White, Black/African American (AA), or Latinx. For our analysis, we combined these three respondents (two Native American, one Asian American) with the Latinx respondents.
Missing Data
Thirteen respondents in the current sample declined to complete the survey. Another 61 participants from one of our PACE sites were not asked to complete the survey; that partner site participated only in the hearing tests and chart review portions of the study due to a high prevalence of non–English-speaking enrollees in their program. We were able to fill in all missing data on race, ethnicity, gender, age, educational attainment, and marital status and on smoking status in most cases from information in the respondents' medical charts during the first chart review. After use of this supplemental source, 27 respondents were missing data for smoking status. Therefore, we included an indicator (0/1) variable for missing data on this measure in our models. This effectively creates an additional category: “missing information on smoking.”3 We have included a table in the Appendix comparing the baseline characteristics of those individuals who completed all 3 years of the study with those of individuals who were lost due to death or attrition after Year 1 or 2. The 36 respondents who did not complete all three waves are slightly older, more likely to be White, and less well educated and have more hearing loss than the sample for whom we have three complete waves of data.
Analytic Strategy
We used the following outcomes for our analysis: heart disease, atherosclerosis, cerebrovascular disease, diabetes, depression, hypertension, living in a nursing home, number of falls in the past year, number of ED visits in the past year, and number of hospital admissions in the past year. Our key independent variables are hearing loss (defined as “no loss,” “mild loss,” or “moderate/severe loss”) and cognitive impairment. We included as covariates whether or not the respondent was frail; the year of the retrospective chart review; and the demographic information collected in the survey: race/ethnicity, age, gender, marital status, and smoking status. Measures of respondent age, cognitive impairment, and frailty are time varying. For the other independent variables, we used the baseline values for each respondent.
For binary outcomes, we used logistic regression models. Because the survey and hearing tests were administered at the date of the first retrospective chart review, our data include 1 year of outcomes that occurred subsequent to the measurement of hearing and collection of demographic information. Therefore, we include up to two annual measurements of the outcomes for each respondent. Because our data include multiple years of data for most respondents, we adjusted the standard errors for clustering of respondent-years within respondents (Hsiao, 2003).4
To facilitate interpretation of the magnitude of statistically significant associations between hearing loss or cognitive impairment and the binary outcomes, adjusted for the set of covariates, we calculated a series of predicted probabilities of each outcome occurring, varying the hearing status and cognitive impairment of the respondent. Using the coefficients from the logistic regression models, we estimated the predicted probability of a chronic condition or nursing home placement (our binary outcomes) for each respondent, first coding all respondents as experiencing hearing loss and then coding all respondents as not experiencing hearing loss. Respondents retained their own values for each of the other covariates. The average (across respondents) of each of the predicted probabilities was then calculated. Standard errors for the predicted probabilities were calculated using the Delta method.5 This approach allows us to compare the predicted probabilities of a chronic condition or nursing home placement occurring for a respondent with hearing loss to those of a respondent with identical values on the covariates who does not experience hearing loss. We followed the same procedure using cognitive impairment.
Ideally, we would have used discrete time hazard models to estimate the relationship between hearing loss, cognitive impairment, and transitions by respondents into the various chronic conditions and to nursing homes. Such a model would include as the sample all respondents who began the study not already experiencing a particular chronic condition. However, a large portion of our sample (> 35% for all chronic conditions except for cerebrovascular and 19% for living in a nursing home) began the study already experiencing these chronic conditions.6 Due to the smaller sizes of the samples eligible for hazard models, convergence issues resulted. We therefore decided to use logistic regression to examine the association between hearing loss, cognitive impairment, and chronic conditions. Because many of the respondents already experienced the chronic conditions before the start of the study, the results from these models cannot be interpreted as evidence of hearing loss or cognitive impairment being a contributing causal factor in the development of other chronic conditions. Rather, these results are meant to illustrate associations only.
For models with count outcomes, we used negative binomial hurdle models. Hurdle models are appropriate for count outcomes where a significant number of respondents have values of 0. For our three count outcomes, the percentages of respondents with values of 0 are 64% (falls), 78% (ED visits), and 80% (hospital admissions). The hurdle model estimates parameters for two equations. Using the outcome “Falls” as an example, the equations are (a) the likelihood of the respondent falling at least once in the past year and (b) the expected count of the number of falls in the past year, conditional upon at least one fall having occurred. The first process is modeled by logistic regression and can also serve as a stand-alone model of whether the respondent fell in the past year. The second process is modeled by a negative binomial model for which respondents with no falls are treated as truncated at 0 (i.e., excluded), creating a count model that is conditional upon at least one event occurring. The two processes together allow for examining the impact of independent variables on the total (unconditional) expected count (Cameron & Trivedi, 2005).7 As with the logistic regression models, we used up to two annual measurements of outcomes for each respondent, and standard errors were adjusted for clustering of respondent-years within respondents.
To facilitate interpretation of significant coefficients from our negative binomial hurdle models, we calculated the impact hearing loss and cognitive impairment had on conditional and unconditional expected counts of the various outcomes using the same process as for predicted probabilities described earlier. The unconditional expected count is simply the expected count of the outcome. The conditional predicted count is the expected count conditional upon at least one event of the outcome occurring in the past year. Standard errors for the predicted counts were calculated using the Delta method.
To reduce the possibility of outliers having undue influence on the models, we “top coded” event counts, such that no respondent had more than a certain number of events included in the model. Specifically, all respondents with more than two hospital admissions were coded as having two hospital admissions. Likewise, all respondents with more than five falls were coded as having five falls, and those with more than three ED visits were coded as having three ED visits.8
Latent Class Analysis
We also estimated models using latent class analysis (LCA). Similar to cluster analysis, LCA identifies groups of respondents based on similar patterns of responses. For instance, one set of respondents might have hearing loss and cognitive impairment and live in nursing homes. Another set might have hearing loss but no cognitive impairment and have experienced multiple falls. However, unlike in cluster analysis, respondents are not actually assigned to groups. Rather, for each respondent, the LCA estimates the probability of being in each group, referred to as latent classes. LCA can be helpful for uncovering patterns between the variables that exist for particular subsets but not all respondents. LCA explores associations and in no way implies causations; therefore, it can be particularly useful when the date of origin of conditions is unknown.
Mathematically, LCA takes the form of a structural equation model with one latent variable that is measured by a set of observed indicators. In LCA, both the latent variable and the observed indicators are categorical rather than continuous. Two sets of parameters are estimated by the model: first, the expected overall probability of being in each latent class and, second, conditional upon (theoretical) membership in each latent class, the expected probability of each indicator having each possible value for that indicator. We say “theoretical” membership because as mentioned earlier, respondents are not actually assigned to classes. Maximum likelihood estimation is used to estimate the parameters of the model (Collins & Lanza, 2010).
The number of classes can be predetermined (confirmatory LCA) or determined as part of the process (exploratory LCA). Because we did not have a theory to suggest a specific number of classes, we used exploratory LCA. For exploratory LCA, the researcher starts by estimating a baseline model with one latent class and continues to expand the number of latent classes in the model until the fit statistics for the model no longer improve or the parameter estimates for the model no longer converge onto specific values. Several fit statistics are typically used to assess the fit of an LCA model: Akaike information criterion (AIC), Bayesian information criterion (BIC), sample size–adjusted BIC, and entropy.9 For the first three aforementioned fit statistics, a lower value indicates a better fitting model. Entropy is a measure of how distinct the latent classes are from each other.10 A value greater than 0.8 suggests a set of distinct latent classes (Tein et al., 2013). Because LCA models do no imply any causal direction, for our LCA models, we used up to three annual measurements for all respondents.
For our LCA, we divided our outcomes into two groups based on what types of measures we expected to hang together.11 Our first LCA model included the following outcomes: falls, ED visits, admission to the hospital, and nursing home status. We also added the covariate frailty to this LCA as we believe that frailty is likely to be especially relevant for falls. Our second LCA used the chronic conditions: heart disease, atherosclerosis, hypertension, diabetes, cerebrovascular disease, and depression. As our primary interest is in the relationships between hearing loss and cognitive impairment with all of the aforementioned outcomes, we included measures of hearing loss and cognitive impairment in both LCA models. For our LCA models, we used all years of data or up to three observations for each respondent.12
Results
Descriptive Statistics
Table 1 shows the descriptive statistics of the respondents. Demographics, smoking status, and hearing thresholds were measured at baseline (Year 1). The sample is predominantly White (46%) and Black (34%), with a sizeable minority of Latinx individuals (17%). The mean age of the sample is 74 years old and contains slightly more women (55%) than men. More than half of the sample has a high school diploma as their highest education (54%). The marital status of the respondents is fairly evenly divided between married, widowed, and divorced/separated (all between 27% and 34%) with a smaller proportion (10%) being never married. Nearly 70% of the sample have hearing loss, and 10%–11% of the sample were current hearing aid users in each of the second and third waves of chart review. Sixty-eight percent of the respondents do not smoke.
Table 1.
Descriptive statistics of sample at baseline (N = 144).
| Characteristic | Percentage/M (SD) | ||
|---|---|---|---|
| Age | 74.0 (8.4) | ||
| Male | 45.1% | ||
| Black | 34.0% | ||
| Latinx | 17.4% | ||
| Other race/ethnicity | 2.1% | ||
| White | 45.5% | ||
| No high school diploma | 34.0% | ||
| High school diploma | 53.5% | ||
| Bachelor's degree | 12.5% | ||
| Widowed | 27.8% | ||
| Married | 33.3% | ||
| Divorced/separated | 28.5% | ||
| Never married | 10.4% | ||
| Smoker | 13.2% | ||
| Nonsmoker | 68.0% | ||
| Smoking missing | 18.8% | ||
| Mild hearing loss | 37.5% | ||
| Moderate/severe hearing loss | 31.9% | ||
|
Medical chart review | |||
|
Year 1
(N = 144) |
Year 2
(N = 132) |
Year 3
(N = 108) |
|
|
Cognitive impairment |
54.2% |
52.3% |
57.4% |
| Frailty | 4.2% | 6.1% | 7.4% |
| Heart disease | 41.7% | 41.7% | 36.1% |
| Atherosclerosis | 35.4% | 35.6% | 34.3% |
| Cerebrovascular disease | 9.7% | 10.6% | 18.5% |
| Hypertension | 54.9% | 50.0% | 50.9% |
| Diabetes | 56.3% | 54.6% | 58.3% |
| Depression | 62.5% | 62.9% | 67.6% |
| Living in a nursing home | 19.4% | 25.8% | 28.7% |
| Number of falls | |||
| 0 | 64.6% | 67.4% | 59.3% |
| 1 | 16.0% | 15.2% | 22.2% |
| 2 | 9.0% | 9.9% | 9.3% |
| 3 | 2.8% | 2.3% | 0.9% |
| 4 | 4.9% | 2.3% | 3.7% |
| 5 or more | 2.8% | 3.0% | 4.6% |
| Number of ED visits | |||
| 0 | 72.2% | 79.6% | 75.9% |
| 1 | 18.1% | 13.6% | 13.9% |
| 2 | 4.9% | 5.3% | 8.3% |
| 3 or more | 4.8% | 1.5% | 1.9% |
| Number of hospital admissions | |||
| 0 | 79.2% | 75.8% | 84.3% |
| 1 | 16.7% | 14.4% | 9.3% |
| 2 or more | 4.2% | 9.8% | 6.5% |
Note. ED = emergency department.
Descriptive statistics for cognitive impairment and the outcome variables are drawn from all 3 years of the study. The sample size is 144 in Year 1, 132 in Year 2, and 108 in Year 3. It is possible for rates of various chronic conditions to actually decline over the 3 years of the study because the sickest respondents were more likely to die during the course of the research. Across the 3 years, 52%–57% of the sample experience cognitive impairment. We see that more than half of the sample has the conditions of diabetes, hypertension, and depression. A sizeable minority (34%–42%) have heart disease and atherosclerosis, with only 10%–19% having cerebrovascular disease (most typically a stroke). About a quarter of the respondents live in a nursing home. We also see from Table 1 that most of the respondents have not experienced a fall in the past year; fewer than 20% have experienced more than one fall. Likewise, less than 30% of the respondents have visited the ED in the past year, and fewer than a quarter have been admitted to the hospital in the past year. On the whole, this is a sample with a high prevalence of chronic health conditions, yet the majority have indications of low health resource utilization (e.g., ED visits and hospitalizations).
Regression Models
Tables 2 –6 show the results from the various regression models. Hearing loss is modeled as a three-category variable: no hearing loss, mild hearing loss, and moderate/severe hearing loss. Each of the two coefficients, mild hearing loss and moderate/severe hearing loss, compares its respective category to the reference category of no hearing loss. We see from Table 2 that compared to no hearing loss, moderate/severe hearing loss is associated with a significantly increased likelihood of falling at least once in the past year. However, there is not a significant difference between mild hearing loss and no hearing loss. In addition, neither category of hearing loss is associated with an increased probability of additional falls beyond the first fall. By contrast, cognitive impairment is not associated with an increased likelihood of the first fall, but for those who fall at least once, cognitive impairment is associated with a significantly increased likelihood of additional falls.
Table 2.
Regression models for number of falls and number of ED visits.
| Characteristic | Falls (yes/no) |
Number of falls |
ED visits (yes/no) |
Number of ED visits |
||||
|---|---|---|---|---|---|---|---|---|
| Coeff. | SE | Coeff. | SE | Coeff. | SE | Coeff. | SE | |
| Cognitive impairment | −0.335 | 0.350 | 0.791* | 0.371 | −0.071 | 0.386 | 0.404 | 0.508 |
| Mild hearing loss | 0.684 | 0.433 | −0.376 | 0.492 | 0.004 | 0.473 | −0.693 | 0.575 |
| Moderate/severe hearing loss | 1.301** | 0.476 | −0.217 | 0.637 | 1.215* | 0.543 | −0.373 | 0.484 |
| Age | −0.046† | 0.027 | 0.019 | 0.036 | −0.047 | 0.029 | −0.027 | 0.028 |
| Male | −0.336 | 0.353 | 0.248 | 0.390 | −0.522 | 0.371 | −0.274 | 0.456 |
| Black | −0.189 | 0.385 | −0.294 | 0.475 | −0.100 | 0.472 | −0.416 | 0.538 |
| Latinx or other race/ethnicity | 0.160 | 0.514 | −0.098 | 0.370 | 0.196 | 0.478 | −0.274 | 0.488 |
| Reference = White | ||||||||
| No high school diploma | 0.135 | 0.410 | −0.088 | 0.509 | 0.603 | 0.428 | 1.229* | 0.515 |
| Bachelor's degree | 0.339 | 0.530 | 0.330 | 0.417 | 0.216 | 0.670 | 2.074* | 0.837 |
| Reference = high school diploma | ||||||||
| Widowed | 0.329 | 0.491 | −0.088 | 0.388 | 0.192 | 0.506 | −0.932† | 0.503 |
| Divorced/separated | 0.178 | 0.453 | 0.050 | 0.385 | 0.344 | 0.488 | −0.977 | 0.637 |
| Single | −0.085 | 0.694 | −0.466 | 0.494 | 0.689 | 0.689 | −1.372 | 1.236 |
| Reference = married | ||||||||
| Smoker | −0.570 | 0.559 | 0.643 | 0.657 | −0.362 | 0.565 | 0.541 | 0.641 |
| Smoking missing | 0.289 | 0.450 | 1.048** | 0.376 | 0.069 | 0.525 | −1.064 | 1.067 |
| Frailty | 0.543 | 0.544 | −0.749 | 0.774 | −0.430 | 0.786 | −14.771*** | 0.991 |
| Wave 3 | 0.457† | 0.234 | −0.281 | 0.247 | 0.337 | 0.311 | 0.090 | 0.241 |
| Constant | 2.121 | 2.042 | −1.451 | 2.614 | 1.569 | 2.148 | 1.941 | 2.111 |
| Observations | 240 | 87 | 240 | 53 | ||||
Note. ED = emergency department; Coeff. = coefficient; SE = standard error.
p < .05.
p < .01.
p < .001.
p < .1.
Table 3.
Regression models for number of hospital admissions and living in a nursing home.
| Characteristic | Hospital admissions (yes/no) |
Number of hospital admissions |
Living in a nursing home |
|||
|---|---|---|---|---|---|---|
| Coeff. | SE | Coeff. | SE | Coeff. | SE | |
| Cognitive impairment | −0.870† | 0.479 | −0.232 | 0.562 | −0.669 | 0.484 |
| Mild hearing loss | 0.906 | 0.577 | 0.125 | 0.425 | 0.338 | 0.510 |
| Moderate/severe hearing loss | 0.853 | 0.710 | −0.189 | 0.474 | 0.369 | 0.707 |
| Age | −0.041 | 0.033 | 0.003 | 0.032 | 0.017 | 0.041 |
| Male | −0.268 | 0.438 | 0.161 | 0.478 | 0.303 | 0.535 |
| Black | 0.587 | 0.504 | 0.395 | 0.410 | −0.933† | 0.530 |
| Latinx or other race/ethnicity | 0.732 | 0.510 | 0.135 | 0.716 | −0.662 | 0.732 |
| Reference = White | ||||||
| No high school diploma | −0.192 | 0.451 | 0.068 | 0.406 | 0.309 | 0.619 |
| Bachelor's degree | −0.135 | 0.636 | 0.424 | 0.561 | 0.607 | 0.649 |
| Reference = high school diploma | ||||||
| Widowed | 1.405* | 0.659 | 0.385 | 0.509 | 0.466 | 0.680 |
| Divorced/separated | 1.152† | 0.657 | 0.218 | 0.597 | −0.130 | 0.602 |
| Single | 0.284 | 0.727 | 0.284 | 0.678 | 0.773 | 0.745 |
| Reference = married | ||||||
| Smoker | −1.081 | 0.717 | −0.614 | 0.763 | −0.773 | 1.062 |
| Smoking missing | 0.940† | 0.485 | −0.215 | 0.457 | 2.175*** | 0.563 |
| Frailty | 0.749 | 0.681 | 0.693 | 0.735 | 0.056 | 0.794 |
| Wave 3 | −0.580† | 0.298 | 0.037 | 0.417 | 0.253† | 0.134 |
| Constant | 0.590 | 2.185 | −1.046 | 2.528 | −2.778 | 3.026 |
| Observations | 240 | 49 | 240 | |||
Note. Coeff. = coefficient; SE = standard error.
p < .05.
p < .001.
p < .1.
Table 4.
Regression models for heart disease, atherosclerosis, and cerebrovascular disease.
| Characteristic | Heart disease |
Atherosclerosis |
Cerebrovascular disease |
|||
|---|---|---|---|---|---|---|
| Coeff. | SE | Coeff. | SE | Coeff. | SE | |
| Cognitive impairment | −0.366 | 0.394 | 0.142 | 0.422 | 1.080* | 0.519 |
| Mild hearing loss | 0.785 | 0.506 | 0.090 | 0.523 | 2.176** | 0.740 |
| Moderate/severe hearing loss | 0.800 | 0.533 | −0.378 | 0.563 | 2.292** | 0.816 |
| Age | −0.033 | 0.031 | −0.023 | 0.031 | −0.023 | 0.039 |
| Male | 0.287 | 0.405 | 0.158 | 0.436 | 0.273 | 0.504 |
| Black | −0.012 | 0.442 | −0.222 | 0.483 | 0.140 | 0.620 |
| Latinx or other race/ethnicity | −0.417 | 0.545 | 0.626 | 0.534 | −0.292 | 0.741 |
| Reference = White | ||||||
| No high school diploma | 0.304 | 0.429 | 0.954* | 0.467 | −0.864 | 0.673 |
| Bachelor's degree | −1.135 | 0.642 | −0.416 | 0.679 | −1.546 | 1.088 |
| Reference = high school diploma | ||||||
| Widowed | 0.735 | 0.557 | 1.019 | 0.561 | −0.297 | 0.775 |
| Divorced/separated | 0.103 | 0.533 | 0.029 | 0.593 | −1.634* | 0.797 |
| Single | 0.021 | 0.696 | 0.610 | 0.676 | −1.242 | 1.092 |
| Reference = married | ||||||
| Smoker | −1.451* | 0.621 | −0.358 | 0.590 | 0.686 | 0.808 |
| Smoking missing | −1.062 | 0.565 | −0.253 | 0.658 | −0.178 | 0.696 |
| Frailty | ||||||
| Wave 3 | −0.152 | 0.144 | −0.038 | 0.137 | 0.759** | 0.265 |
| Constant | 1.854 | 2.337 | 0.397 | 2.380 | −2.065 | 2.796 |
| Observations | 240 | 240 | 240 | |||
Note. Coeff. = coefficient; SE = standard error.
p < .05.
p < .01.
Table 6.
Regression models for hypertension, diabetes, and depression.
| Characteristic | Hypertension |
Diabetes |
Depression |
|||
|---|---|---|---|---|---|---|
| Coeff. | SE | Coeff. | SE | Coeff. | SE | |
| Cognitive impairment | −0.584 | 0.402 | −0.426 | 0.404 | 0.434 | 0.426 |
| Mild hearing loss | −0.706 | 0.493 | 1.032† | 0.555 | 0.394 | 0.506 |
| Moderate/severe hearing loss | −0.660 | 0.551 | 0.496 | 0.545 | 0.579 | 0.616 |
| Age | 0.027 | 0.031 | −0.043 | 0.040 | −0.056† | 0.030 |
| Male | 0.354 | 0.393 | 0.305 | 0.428 | −0.749† | 0.400 |
| Black | 0.411 | 0.451 | 1.593*** | 0.471 | −0.771 | 0.482 |
| Latinx or other race/ethnicity | 1.067* | 0.516 | 1.881** | 0.622 | −0.781 | 0.553 |
| Reference = White | ||||||
| No high school diploma | 0.288 | 0.452 | 0.461 | 0.483 | −0.123 | 0.486 |
| Bachelor's degree | 0.332 | 0.627 | 1.141† | 0.686 | −0.431 | 0.634 |
| Reference = high school diploma | ||||||
| Widowed | −0.429 | 0.530 | 0.023 | 0.529 | 0.811 | 0.556 |
| Divorced/separated | −0.954† | 0.563 | −0.302 | 0.558 | 0.061 | 0.520 |
| Single | −0.275 | 0.756 | 0.067 | 0.721 | −0.979 | 0.793 |
| Reference = married | ||||||
| Smoker | 1.292* | 0.606 | −1.057 | 0.653 | −0.256 | 0.675 |
| Smoking missing | −0.155 | 0.559 | −0.141 | 0.545 | 0.009 | 0.520 |
| Frailty | −0.497 | 0.734 | ||||
| Wave 3 | 0.033 | 0.129 | 0.167 | 0.139 | 0.290* | 0.129 |
| Constant | −1.613 | 2.275 | 2.025 | 2.779 | 5.002* | 2.277 |
| Observations | 240 | 240 | 240 | |||
Note. Coeff. = coefficient; SE = standard error.
p < .05.
p < .01.
p < .001.
p < .1.
Table 2 also shows that relative to no hearing loss, moderate/severe hearing loss is associated with a significantly increased likelihood of visiting the ED at least once in the past year. There is no significant difference between mild hearing loss and no hearing loss, and neither category of hearing loss is associated with an increased likelihood of additional ED visits during the year. Cognitive impairment is not associated with an increased likelihood of ED visits. Table 3 shows that hearing loss is not associated with hospital admissions or living in a nursing home. Likewise, cognitive impairment is not associated with the likelihood of living in a nursing home. There was a tendency for cognitive impairment to be negatively associated with the likelihood of at least one hospital admission, but this pattern did not reach statistical significance (p < .05). There was no significant association with additional hospital admissions beyond the first.
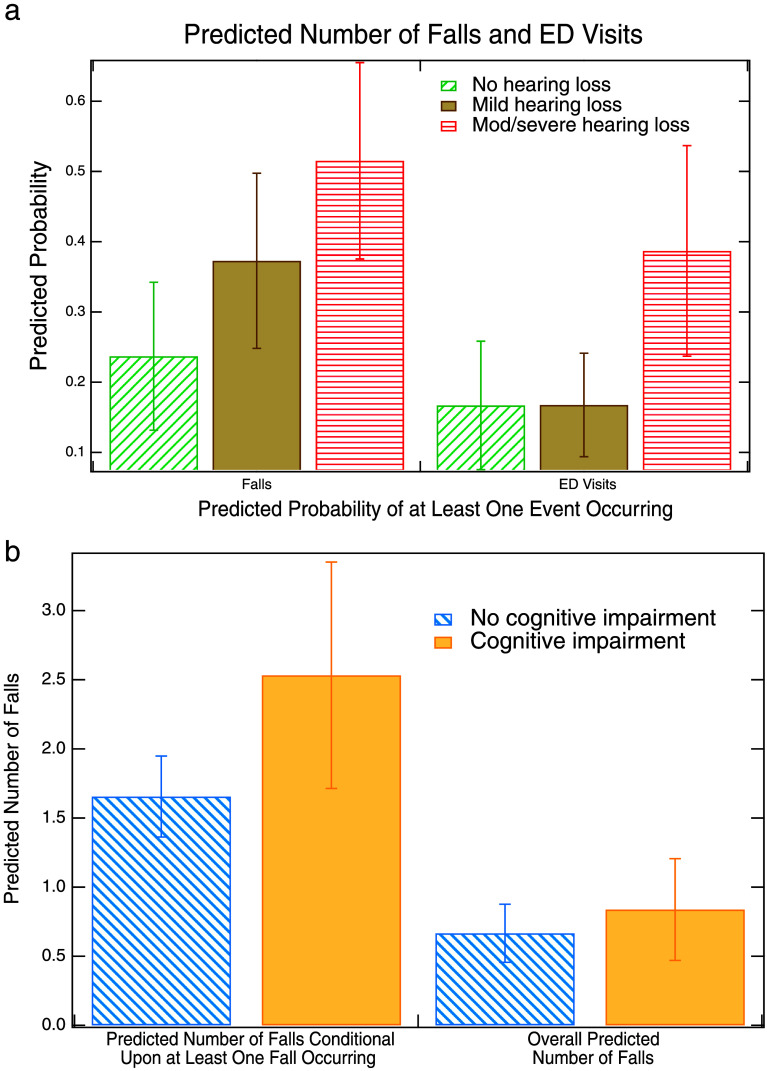
Figures 1a and 1b illustrate the magnitude of the relationships between hearing loss and cognitive impairment with falls and ED visits. We see from Figure 1a that the predicted probability of falling at least once in the past year is 24% for individuals without hearing loss, while it is 37% for individuals with mild hearing loss and 52% for individuals with moderate/severe hearing loss.13 Figure 1a also shows that the likelihood of having at least one ED visit in the past year is 17% for both individuals with no hearing loss and those with mild hearing loss. However, this risk rises to 39% for those with moderate/severe hearing loss. Figure 1b illustrates that the predicted number of falls for individuals who have fallen at least once (predicted number of falls conditional upon at least one fall occurring) is 1.7 for individuals without cognitive impairment and 2.6 for individuals with cognitive impairment. The second set of bars in Figure 1b (overall predicted number of falls) shows the impact of cognitive impairment on the number of falls for the entire sample (both those who have fallen and those who have not). Here, the difference between individuals with and without cognitive impairment is much smaller: 0.66 falls for those without cognitive impairment and 0.82 falls for individuals who have cognitive impairment.14 The overall predicted number of falls takes into account both the coefficient for cognitive impairment in the model of falling at least once (which was not statistically significant) and the corresponding coefficient for the model of the number of falls conditional upon falling at least once (which was statistically significant). In this case, the difference between cognitively impaired and not cognitively impaired for the overall predicted number of falls is not statistically significant.15
Figure 1.
(a) Predicted probabilities of at least one fall occurring based on hearing loss status and (b) predicted number of falls based on cognitive status. Figure 1b shows overall predicted number of falls and number of falls conditional upon at least one fall occurring. Error bars represent 95% confidence intervals around the point estimates. Hearing loss is based on four-frequency pure-tone average (0.5–4 kHz) in the better hearing ear: no loss (25 dB HL or less), mild loss (26–40 dB HL), and moderate/severe loss (> 40 dB HL). Cognitive impairment is based on medical chart codes. Predicted probabilities and predicted counts are adjusted for age, gender, race/ethnicity, education level, marital status, smoking status, frailty, and wave of the study. ED = emergency department.
Tables 4–6 show the results from the logistic regression models of comorbidities. We see from Table 4 that compared to individuals with no hearing loss, those who have both mild and moderate/severe hearing loss have an increased risk of cerebrovascular disease.16 There is also a trend toward a statistically significant association (p < .1) between mild hearing loss and diabetes, although individuals with moderate/severe hearing loss do not have an increased risk of diabetes.17 Table 4 shows no statistically significant relationship between hearing loss and atherosclerosis, hypertension, or depression.
Table 4 also does not show a significant association between hearing loss and heart disease. However, the heart disease coefficients for mild hearing loss and moderate/severe hearing loss are very similar in magnitude. We estimated a supplemental model (as shown in Table 5) where we combined mild and moderate/severe hearing loss into one category of hearing loss. In this model, there is a trend toward statistical significance (p < .1) for the association between overall hearing loss and heart disease. We explored the technique of collapsing the hearing loss categories for all of the other outcomes, but no additional associations became statistically significant or showed a trend toward statistical significance. As has been noted, the coefficients for mild and moderate/severe hearing loss are very similar in the model of heart disease. They differ by less than 2%, and not surprisingly, chi-square tests indicate that the difference between them is not statistically significant. Therefore, we believe it would be justifiable to simply use the model presented in Table 5 for heart disease. In order to present our models in a consistent, unified manner, we have chosen not to do this. However, due to the trend toward statistical significance in the relationship between hearing loss and heart disease in Table 5, we will include this association in our visual illustrations of the magnitude of effects.
Table 5.
Regression models for heart disease with binary indicator of hearing loss.
| Characteristic | Heart disease |
|
|---|---|---|
| Coeff. | SE | |
| Cognitive impairment | −0.366 | 0.393 |
| Hearing loss | 0.790† | 0.457 |
| Age | 0.289 | 0.400 |
| Male | −0.033 | 0.031 |
| Black | −0.013 | 0.443 |
| Latinx or other race/ethnicity | −0.418 | 0.540 |
| Reference = White | ||
| No high school diploma | 0.307 | 0.423 |
| Bachelor's degree | −1.137† | 0.636 |
| Reference = high school diploma | ||
| Widowed | 0.735 | 0.556 |
| Divorced/separated | 0.101 | 0.526 |
| Single | 0.020 | 0.696 |
| Reference = married | ||
| Smoker | −1.451* | 0.622 |
| Smoking missing | −1.062† | 0.565 |
| Wave 3 | −0.153 | 0.144 |
| Constant | 1.841 | 2.301 |
| Observations | 240 | |
Note. Coeff. = coefficient; SE = standard error.
p < .05.
p < .1.
Table 4 also shows that cognitive impairment is associated with a statistically significant increase in the likelihood of having cerebrovascular disease. However, there are no significant associations between cognitive impairment and any other chronic condition. As has been discussed in the Analytic Strategy section, because many individuals entered the study already having heart disease, diabetes, and cerebrovascular disease, we cannot make causal inferences about whether hearing loss or cognitive impairment leads to heart disease, cerebrovascular disease, or diabetes. Rather, our data suggest that there are associations between hearing loss and/or cognitive impairment and these comorbidities.
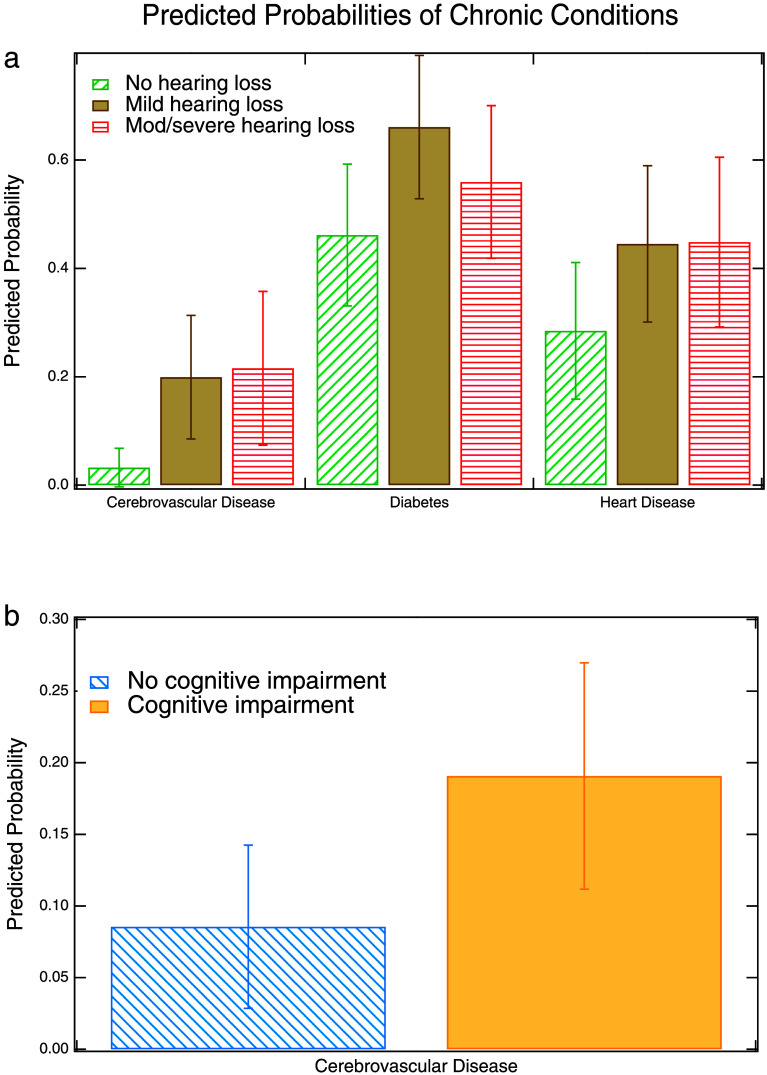
Figure 2a illustrates the magnitude of the relationships between hearing loss with cerebrovascular disease, diabetes, and heart disease. We see that the predicted probability of cerebrovascular disease is 3% for those without hearing loss compared to 20% for those with mild hearing loss and 22% for those with moderate/severe hearing loss. The predicted probability of having heart disease is 28% for those without hearing loss compared to 45% for those with either mild or moderate/severe hearing loss. Experiencing hearing loss is thus associated with a nearly 20-percentage point increase in the likelihood of having each of these two comorbidities. Finally, for diabetes, the corresponding percentages are 46% for those without hearing loss, 66% for those with mild hearing loss, and 56% for those with moderate/severe hearing loss. Figure 2b illustrates the relationship between cognitive impairment and cerebrovascular disease. We see that the predicted probability of having cerebrovascular disease is 9% for those without cognitive impairment compared to 19% for those with cognitive impairment.
Figure 2.
Predicted probabilities of chronic health conditions based on (a) hearing status or (b) cognitive status. Error bars represent 95% confidence intervals around the point estimates. Hearing loss is based on four-frequency pure-tone average (0.5–4 kHz) in the better hearing ear: no loss (25 dB HL or less), mild loss (26–40 dB HL), and moderate/severe loss (> 40 dB HL). Cognitive impairment is based on medical chart codes. Predicted probabilities are adjusted for age, gender, race/ethnicity, education level, marital status, smoking status, frailty, and wave of the study.
Latent Class Analysis
Our first LCA model explores profiles based on the relationships between cognitive impairment, hearing loss, frailty, number of falls, number of ED visits, number of hospital admissions, and living in a nursing home. Table 7 shows the fit statistics for this model estimated with one to five latent classes. The model with three latent classes has the best (lowest) values for both the AIC and sample size–adjusted BIC. In addition, the entropy measure (0.862) suggests a distinct set of latent classes. The entropy value is also highest for the model with three latent classes. The only measure that suggests a different number of latent classes is the BIC, for which the two-class model has the lowest value. Taken all together, the fit statistics suggest that the three-class model has the best fit.
Table 7.
Measures of fit for latent class analysis of hearing, cognition, and physical condition.
| Number of classes | AIC | BIC | Sample size–adjusted BIC | Entropy |
|---|---|---|---|---|
| 1 | 3937.677 | 3996.937 | 3949.344 | |
| 2 | 3837.523 | 3959.993 | 3861.634 | 0.784 |
| 3 | 3822.618 | 4008.298 | 3859.174 | 0.862 |
| 4 | 3828.409 | 4077.300 | 3877.410 | 0.679 |
| 5 | 3833.280 | 4145.380 | 3894.725 | 0.832 |
Note. AIC = Akaike information criterion; BIC = Bayesian information criterion.
Table 8 shows the expected probability of being in each latent class as well as the predicted probabilities of each value for each indicator, conditional upon membership in that latent class. Below are the profiles of each of the three latent classes, based on the results presented in Table 8.
Table 8.
Latent classes of hearing, cognition, and physical condition.
| Predicted probability of class membership | Class 1 | Class 2 | Class 3 |
|---|---|---|---|
| 0.796 | 0.084 | 0.120 | |
| Cognitive impairment | |||
| No | 0.454 | 0.326 | 0.558 |
| Yes | 0.546 | 0.674 | 0.442 |
| Hearing loss | |||
| None | 0.348 | 0.000 | 0.385 |
| Mild | 0.427 | 0.275 | 0.208 |
| Moderate/severe | 0.225 | 0.725 | 0.407 |
| Number of falls | |||
| 0 | 0.796 | 0.000 | 0.056 |
| 1 | 0.132 | 0.734 | 0.066 |
| 2 | 0.043 | 0.101 | 0.426 |
| 3 | 0.018 | 0.000 | 0.051 |
| 4 | 0.009 | 0.165 | 0.132 |
| 5 or more | 0.002 | 0.000 | 0.268 |
| Number of ED visits | |||
| 0 | 0.883 | 0.301 | 0.243 |
| 1 | 0.080 | 0.566 | 0.353 |
| 2 | 0.033 | 0.066 | 0.236 |
| 3 or more | 0.004 | 0.067 | 0.168 |
| Number of hospital admissions | |||
| 0 | 0.860 | 0.422 | 0.617 |
| 1 | 0.110 | 0.335 | 0.186 |
| 2 or more | 0.030 | 0.243 | 0.197 |
| Frailty | |||
| No | 0.956 | 0.731 | 1.000 |
| Yes | 0.044 | 0.269 | 0.000 |
| Lives in nursing home | |||
| No | 0.821 | 0.887 | 0.245 |
| Yes | 0.179 | 0.113 | 0.755 |
Note. Hearing loss based on better ear pure-tone average at 500, 1000, 2000, and 4000 Hz: none (< 25 dB HL), mild (26–40 dB HL), and moderate-severe (> 40 dB HL). ED = emergency department.
Latent Class 1: Expected probability of class membership: 0.80. This latent class is characterized by cognitive impairment (55%) and hearing loss (65%), particularly mild hearing loss (43%). This latent class also has low risk (< 20%) of frailty, a fall, an ED visit, a hospital admission, or living in a nursing home.
Latent Class 2: Expected probability of class membership: 0.08. This latent class is characterized by cognitive impairment (67%) and moderate/severe hearing loss (73%). The likelihood of no hearing loss for this class is essentially nonexistent (< 0.05%). Although this latent class is unlikely to be frail, the class is at high (73%) risk for one fall. This class has 70% likelihood of at least one ED visit and greater than 50% likelihood of a hospital admission, although the risk of living in a nursing home is very low (11%).
Latent Class 3: Expected probability of class membership: 0.12. This class is characterized by hearing loss (62%), particularly moderate/severe hearing loss (41%), but has a less than 50% likelihood of having cognitive impairment. In marked contrast to the other two classes, this class has a high risk (over 75%) of living in a nursing home. While their likelihood of frailty is essentially nonexistent (< 0.05%), this class has a high likelihood (over 85%) of experiencing more than one fall and a 75% likelihood of visiting the ED at least once. However, they have a less than 50% chance of having been admitted to the hospital.
To summarize, two classes are characterized by both hearing loss and cognitive impairment. Of these, the class with greater risk of hearing loss and cognitive impairment has experienced a variety of issues including a fall, an ED visit, and a hospital admission. The class characterized by (comparatively) less risk of hearing loss and cognitive impairment has few physical issues and little use of hospital services. The third class, which is characterized by hearing loss but less than 50% risk of cognitive impairment, has the highest risk of falls and ED visits and is the most likely to live in a nursing home.
Our second latent class model explores profiles based on the relationships between cognitive impairment, hearing loss, heart disease, atherosclerosis, cerebrovascular disease, hypertension, diabetes, and depression. Table 9 shows the fit statistics for this model. We estimated models containing up to seven classes. Estimation of the seven-class model was problematic. While the model technically did converge, the matrix of variances of the parameter estimates was not positive definite, which is akin to having a variance with a value less than 0. This is frequently an indication that the model is not identified, meaning that a unique estimate does not exist for every parameter. This is common when the model has too many parameters, which can be directly related to the number of latent classes.
Table 9.
Measures of fit for latent class analysis of hearing, cognition, and comorbidities.
| Number of classes | AIC | BIC | Sample size–adjusted BIC | Entropy |
|---|---|---|---|---|
| 1 | 4249.367 | 4284.923 | 4256.367 | |
| 2 | 4208.916 | 4283.978 | 4223.694 | 0.610 |
| 3 | 4197.074 | 4311.643 | 4219.630 | 0.620 |
| 4 | 4190.991 | 4345.066 | 4221.324 | 0.685 |
| 5 | 4177.390 | 4370.971 | 4215.501 | 0.795 |
| 6 | 4168.448 | 4401.536 | 4214.338 | 0.817 |
| 7 | Model estimation issues. | |||
Note. AIC = Akaike information criterion; BIC = Bayesian information criterion.
We see from Table 9 that based on the AIC and sample size–adjusted BIC, the six-class model has the best fit. However, the difference in the sample size–adjusted BIC between the five- and six-class models is very small. The six-class model is also the only model to achieve the desired entropy value of greater than 0.8, although the entropy value for the five-class model is very close (0.795). The BIC is not very useful for determining fit for this model as it suggests that a two-class model is preferred, which is very out of step with the other fit statistics.
Although the six-class model has slightly better fit, we chose to use the five-class model as we felt the interpretation of this model was clearer. In particular, the six-class model included more cases where the likelihood of a specific comorbidity occurring for a particular class was slightly above or below 50%, limiting meaningful interpretation. In addition, in the six-class model, the comorbidities did not divide as neatly across classes. Thus, we felt that in terms of interpretation, the five-class model has more “distinct” classes. Finally, the six-class model included three latent classes with expected probabilities lower than 0.1 as compared to only one such latent class for the five-class model.
Table 10 shows the expected probability of being in each latent class as well as the predicted probabilities of each value for each indicator, conditional upon membership in that latent class. Below are the profiles of each of the five latent classes, based on the results presented in Table 10.
Table 10.
Latent classes of hearing, cognition, and comorbidities: five-class model.
| Predicted probability of class membership | Class 1 | Class 2 | Class 3 | Class 4 | Class 5 |
|---|---|---|---|---|---|
| 0.044 | 0.330 | 0.197 | 0.142 | 0.287 | |
| Cognitive impairment | |||||
| No | 0.000 | 0.597 | 0.150 | 0.327 | 0.637 |
| Yes | 1.000 | 0.403 | 0.850 | 0.673 | 0.363 |
| Hearing loss | |||||
| None | 0.082 | 0.268 | 0.157 | 0.247 | 0.574 |
| Mild | 0.000 | 0.451 | 0.571 | 0.25 | 0.317 |
| Moderate/severe | 0.918 | 0.281 | 0.271 | 0.502 | 0.109 |
| Heart disease | |||||
| No | 1.000 | 0.32 | 0.822 | 0.000 | 1.000 |
| Yes | 0.000 | 0.68 | 0.178 | 1.000 | 0.000 |
| Atherosclerosis | |||||
| No | 1.000 | 0.459 | 0.696 | 0.584 | 0.811 |
| Yes | 0.000 | 0.541 | 0.304 | 0.416 | 0.189 |
| Cerebrovascular disease | |||||
| No | 0.551 | 0.829 | 0.871 | 0.834 | 1.000 |
| Yes | 0.449 | 0.171 | 0.129 | 0.166 | 0.000 |
| Hypertension | |||||
| No | 0.021 | 0.348 | 1.000 | 0.559 | 0.303 |
| Yes | 0.979 | 0.652 | 0.000 | 0.441 | 0.697 |
| Diabetes | |||||
| No | 0.658 | 0.000 | 0.396 | 1.000 | 0.657 |
| Yes | 0.342 | 1.000 | 0.604 | 0.000 | 0.343 |
| Depression | |||||
| No | 0.000 | 0.275 | 0.250 | 0.356 | 0.588 |
| Yes | 1.000 | 0.725 | 0.750 | 0.644 | 0.412 |
Note. Hearing loss based on better ear pure-tone average at 500, 1000, 2000, and 4000 Hz: none (< 25 dB HL), mild (26–40 dB HL), and moderate-severe (> 40 dB HL).
Latent Class 1: Expected probability of class membership: 0.04. This latent class is characterized by an extremely high (> 99.95%) likelihood of cognitive impairment, a very high (> 90%) chance of moderate/severe hearing loss and hypertension, and an extremely high (> 99.95%) risk of depression.
Latent Class 2: Expected probability of class membership: 0.33. This class is characterized by hearing loss (73%), particularly mild hearing loss (45%), but has only about a 40% chance of having cognitive impairment. This class is characterized by several comorbidities including heart disease (68%), atherosclerosis (54%), hypertension (65%), diabetes (> 99.95%), and depression (73%).
Latent Class 3: Expected probability of class membership: 0.20. This class is also characterized by hearing loss (84%), especially mild hearing loss (57%), but also has an 85% chance of cognitive impairment. This class has a 75% risk of depression.
Latent Class 4: Expected probability of class membership: 0.14. This class is characterized by hearing loss (75%), especially moderate-to-severe hearing loss (50%), and has nearly a 70% chance of experiencing cognitive impairment. This class has a very high risk of heart disease (> 99.95%) and a substantial risk of depression (64%).
Latent Class 5: Expected probability of class membership: 0.29. This class has only just above a 40% chance of hearing loss and less than a 40% chance of cognitive impairment. This class has a 70% risk of hypertension.
To summarize, of the five classes, there are three characterized by a high likelihood of cognitive impairment and hearing loss. Of these, one class is at risk for hypertension and depression, another is at risk for diabetes and depression, and a third is at risk for heart disease and depression. While hearing loss and cognitive impairment cluster with depression across the board, there are three sets of risk profiles, each emphasizing one physical health condition. The class characterized by hearing loss but not cognitive impairment actually has the greatest risk of comorbidities; this class is at risk for all comorbidities except cerebrovascular disease. Finally, the one class characterized by neither hearing loss nor cognitive impairment is characterized primarily by risk for hypertension.
Discussion
Consistent with the literature from large epidemiological studies, older adults with hearing loss fared more poorly on a number of age-related health outcomes than other older adults in the sample. Notable conditions for which adults in the sample with hearing loss were more likely to experience included heart disease, diabetes, and cerebrovascular disease. Interestingly, among the comorbid chronic conditions included in the model, only cerebrovascular disease was significantly more probable when analyzing persons with and without cognitive impairment. Among the physical mobility and health resource utilization variables, falls and ED visits were more likely among those with moderate/severe hearing loss. Furthermore, while hearing loss was associated with the likelihood of having one fall, cognitive impairment was associated with the likelihood of experiencing additional falls.
An important finding in this sample comes from the LCA that included all of the comorbid chronic conditions included in our regression models. Notably, the only profile in the sample that was not likely to experience depression was the group with good hearing and a low likelihood of cognitive impairment. All four profiles with hearing loss were also positive for depression. Moreover, the latent class model with the comorbidities is an interesting reflection of patterns observed in the epidemiological literature. For example, the group (33% of the sample) with hearing loss and without cognitive impairment was likely to have most of the comorbidities included in this study—heart disease, atherosclerosis, hypertension, diabetes, and depression. When considering the group with hearing loss and cognitive impairment (39% of the sample), they split into three profiles, each having one of the key chronic conditions and all of them having depression.
This pattern suggests that when someone has hearing loss, they should be screened for depression and vice versa. While this analysis does not allow for determining which condition occurred first, it does suggest that there is frequent co-occurrence. We know that a major sequelae of hearing loss is social withdrawal, and when that overlaps with depression, the negative consequences are likely to be exacerbated (Mahmoudi et al., 2019). Treating hearing loss is not a treatment for depression, but it has been found to improve quality-of-life ratings for adults in general (Ferguson et al., 2017) and so should be included in protocols to address depressive symptoms in older adults. While screening for depression is outside the scope of practice for speech-language pathologists and audiologists, communication professionals who work with primary care and rehabilitation teams can increase awareness of the association between hearing loss and depression to provide more holistic person-centered care.
Overall, the results from this sample are similar to those reported from epidemiological population surveys. Unique to this community sample though is that these participants have all been deemed nursing home eligible. Therefore, among a group of older adults experiencing relatively poor health, we are still able to distill that persons with hearing loss are more likely to have other chronic conditions. Due to the nature of our medical chart data extraction, it is not possible to tell what came first in this sample—hearing loss versus the comorbid chronic conditions tracked in this data set. Nevertheless, the associations between hearing loss and other chronic conditions highlight the need to identify and address hearing loss in this sample of nursing home–eligible adults. Especially, as highlighted above, the consistent profile of hearing loss and depression in the LCA points to the functional impact of untreated hearing loss.18
There is a consistent trend in the literature that individuals with hearing loss have higher health resource utilization and, as such, higher medical costs (Deardorff et al., 2019; Reed et al., 2019; Wells et al., 2019). We observed more falls over 3 years among PACE participants with hearing loss at baseline. This is critically important in capitated health systems because falls often come with the high cost of ED care and hospital admissions. They are the leading cause of injury and accidental death in older adults. The underlying mechanism linking age-related hearing loss and an increased odds of falls is not well understood. However, there is some evidence of parallel vestibular perceptual declines in persons with age-related hearing decline (Gabriel et al., 2022). An examination of claims data finds no benefit of hearing aid use on falls reduction (Riska et al., 2022). Nevertheless, with a known connection between the two conditions and the comprehensive care structure of the PACE program, there is the potential that improved identification of hearing loss in their population could serve as an impetus for falls risk mitigation to be added to individual care plans sooner.
Another interesting aspect of the current sample is that it has more racial/ethnic diversity than many clinical samples. Thus, while a relatively small sample, it is an important contribution to increasing representation in our research literature regarding age-related hearing loss and healthy aging (46% White/non-Hispanic, 35% Black/AA, 16% Latinx). The only significant difference in the health conditions as a function of race/ethnicity was that both the Black/AA and Latinx groups were more likely to have diabetes than the White participants. Similar patterns are seen in population studies of older adults. And, yet, the comprehensive care nature of the PACE system may alleviate some of the other common health disparities that have been observed in community samples.
Limitations
There are several aspects of this study that limit the generalizability of the data. First, as with all medical chart reviews, codes extracted from the medical chart do not provide any context from which to interpret the health condition(s) of the individual. Second, the older adults in this sample, while primarily community dwelling, are all nursing home eligible, which places them in a generally unwell category, which is reflected in the high prevalence of multiple chronic conditions in this sample. Due to the high prevalence of chronic conditions, it is difficult to tease apart the impact of hearing loss; that said, it is critical to consider the treatment needs and options for older adults whose hearing loss is not treated as an important-to-treat condition among their many other chronic health diagnoses. In addition, since hearing was assessed only once, it is possible that hearing status changed over the course of the study for some respondents. This could potentially obscure associations between hearing loss and the various outcomes. Third, as discussed in the Analytic Strategy section, many of our respondents already suffered from chronic conditions and/or lived in nursing homes at the start of the study. Thus, we cannot disentangle which came first—hearing loss and cognitive impairment or chronic conditions and nursing home status. Therefore, the findings for the relationships between hearing loss and cognitive impairment and these outcomes should be interpreted as descriptive and associative rather than evidence of causal relationships. Finally, our relatively small sample size limits the power to detect statistically significant associations between hearing loss, cognitive impairment, and our outcomes. We are confident that the statistically significant findings indeed reflect true relationships; it may be the case that some of the associations that did not reach significance are underpowered rather than unimportant.
Conclusions
The current data set adds to a growing literature about the multifaceted associations between age-related hearing loss and other chronic conditions, as well as health resource utilization. This sample is particularly unique because it represents a fairly unwell community sample; they are nursing home eligible but not yet institutionalized. They do not represent a typical epidemiological sample, nor do they represent a typical clinical sample. This investigation offers a glimpse into the complex care needs of older adults experiencing a high burden of multiple chronic conditions.
As we move forward with an emphasis on holistic care planning, especially for older adults, there is evidence to support prioritizing identification and treatment of hearing loss in the broader scheme of comprehensive care (Pichora-Fuller et al., 2015). Particularly noteworthy in this sample was the LCA that identified a likelihood of depression for all of the profiles that included hearing loss, and the only profile without a high likelihood of depression was the group without hearing loss or cognitive impairment. Our data cannot determine the direction of that relationship; nevertheless, given the propensity of hearing loss to exacerbate social isolation, it is worth mitigating the impacts of hearing loss as part of the care management for those with or at risk for depression. Notably, even in the face of high rates of multimorbidity, the relationship between hearing loss and depression is persistent.
While randomized controlled trials are needed to understand the protective nature of hearing aids, multiple population-based studies have observed delayed cognitive decline, reduced risk of depression, and reduced injuries from falls among hearing aid users (Mahmoudi et al., 2019). Given the potentially modifiable impacts of age-related hearing loss, there continues to be an urgent need to investigate whether treatment mitigates the negative effects of hearing loss. Future studies should explore more accessible service delivery options to better identify and treat hearing loss for older adults experiencing multiple chronic conditions, as well as examine the broad health impacts of addressing hearing loss in this complex clinical population.
Author Contributions
Sara K. Mamo: Conceptualization (Lead), Data curation (Supporting), Formal analysis (Supporting), Funding acquisition, Investigation (Supporting), Methodology (Equal), Project administration (Lead), Software (Supporting), Supervision, Validation (Equal), Visualization (Lead), Writing – original draft (Lead), Writing – review & editing (Lead). Jessica Pearlman: Conceptualization (Supporting), Data curation (Supporting), Formal analysis (Lead), Methodology (Equal), Software (Lead), Validation (Equal), Visualization (Supporting), Writing – original draft (Supporting), Writing – review & editing (Supporting). Kara A. Wheeler: Conceptualization (Supporting), Data curation (Lead), Investigation (Lead), Project administration (Supporting), Validation (Equal), Writing – original draft (Supporting), Writing – review & editing (Supporting). Programs of All-Inclusive Care for the Elderly staff: Resources.
Data Availability Statement
A de-identified data set used in this article is available upon reasonable request from the corresponding author, Sara K. Mamo.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communication Disorders Grant K23DC016855 (awarded to Sara K. Mamo). The authors gratefully acknowledge the contributions of Katie Garrity, Alissya Silva, and Ally Tracchi to the literature review and data coding. The authors offer their sincerest thanks to the staff and participants at the Program of All-Inclusive Care for the Elderly centers who collaborated on this project, providing support and space to do this work.
Appendix
Descriptive Statistics of Sample at Baseline by Attrition Status
| Characteristic | Percentage/M (SD) |
Percentage/M (SD) |
|---|---|---|
| Completed 3 waves (N = 108) |
Died/left before Wave 2 or 3 (n = 36) |
|
| Age | 72.7 (7.3) | 78.0 (10.3) |
| Male | 46.3% | 41.7% |
| Race/ethnicity | ||
| Black | 38.0% | 22.2% |
| Latinx | 18.5% | 13.9% |
| Other race/ethnicity | 2.8% | 0.0% |
| White | 40.7% | 63.9% |
| Education | ||
| No high school diploma | 29.6% | 47.2% |
| High school diploma | 57.4% | 41.7% |
| Bachelor's degree | 13.0% | 11.1% |
| Marital status | ||
| Widowed | 25.9% | 33.3% |
| Married | 32.4% | 36.1% |
| Divorced/separated | 32.4% | 16.7% |
| Never married | 9.3% | 13.9% |
| Smoking status | ||
| Smoker | 13.0% | 13.9% |
| Nonsmoker | 67.6% | 69.4% |
| Smoking missing | 19.4% | 16.7% |
| Hearing status | ||
| Mild hearing loss | 40.7% | 27.8% |
| Moderate/severe hearing loss | 24.1% | 55.6% |
Funding Statement
This work was supported by National Institute on Deafness and Other Communication Disorders Grant K23DC016855 (awarded to Sara K. Mamo).
Footnotes
Due to the high prevalence of cognitive impairment anticipated in this participant population, we included a capacity to consent assessment for all potential research participants.
The consent forms were translated into Spanish, Portuguese, and Haitian Creole to accommodate the multilingual participant population at one of the three PACE sites.
If information is not included on all variables, respondents would be dropped automatically from all models during statistical analysis. Some strategy must be used to address missing data. Additional options are to use the average of the variable(s) with missing data (not applicable to categorical variables) or to use multiple imputation. Multiple imputation techniques are designed for single-equation models so they would not be appropriate for the negative binomial hurdle models used for several of our outcomes (Allison, 2002).
When estimating regression models with observations as more than one time point per individual, the unit of analysis is the person-year. Because 2 years of data were used for the regression analysis, the number of observations for the regressions is 240, as shown in Tables 2–6.
The Delta method is a technique to estimate standard errors for functions of estimated parameters, such as regression model coefficients. The predicted probabilities calculated here are functions of regression model coefficients (Cameron & Trivedi, 2005).
We were able to estimate a hazard model for cerebrovascular disease. However, in order to be consistent with the other outcomes, we report results from the logistic regression model for this outcome. Results from the hazard model are available upon request. The smaller sample sizes of the hazard models were exacerbated by what is termed “perfect prediction”—when everyone with a specific value on a categorical independent variable has the same value on the outcome. Such cases are dropped from the model as the best fitting model will allow for a parameter of infinitely small or large size for the variable, which all cases with one value have the same value on the outcome. Hence, these observations do not influence other parameters. Our hazard models suffered from high levels of perfect prediction, resulting in even smaller samples.
The formula for conditional expected count is as follows: E(yi|yi > 0) = exp(Xiβ)/[1 − (1 + αexp(Xiβ))−1/α], where α is the dispersion parameter. The formula for unconditional expected count is as follows: p(count ≥ 1) × E(yi|yi > 0), where p(count ≥ 1) is calculated using the (logistic regression) coefficients from the equation for the likelihood of the outcome occurring at least once in the past year. Since any probability is ≥ 0, a positive beta in the second (negative binomial) equation indicates an increase in both the expected and unexpected counts.
More than 99% of respondents had two or fewer hospital admissions. Likewise, 98% of respondents experienced five or fewer falls, and 98% of respondents made three or fewer trips to the ED.
AIC, BIC, and sample size–adjusted BIC are all based on the log likelihood statistic for the model, and each makes adjustments according to the number of parameters and the sample size. Sample size–adjusted BIC is somewhat of a misnomer since all three statistics make various adjustments for sample size. The formula for AIC is (−2 × log likelihood) + p, where p is the number of parameters. The formula for BIC is (−2 × log likelihood) + (p × ln(N)), where N is the sample size and ln means natural log. The formula for sample size–adjusted BIC replaces N with N × (n + 2)/24).
We used a normalized measure of entropy used that produces values between 0 and 1.
We initially tried a model with all 13 variables. However, convergence issues resulted for more than three classes even with 2,000 iterations. This is not uncommon when the number of parameters in an LCA gets large. Models with a large number of parameters also tend to hinder clear interpretation of the latent classes.
When estimating LCA models with observations at more than one time point per individual, the unit of analysis is the person-year. All 3 years of data were used in the LCA, and therefore, the number of observations is 384.
Chi-square tests show that the difference between mild and moderate/severe hearing loss is not statistically significant for either falls or ED visits.
The overall unconditional predicted number of falls is also smaller for both groups because this value (which is essentially an average) includes all the people who have 0 falls.
A bootstrap method was used to calculate the standard error and confidence interval for the difference between overall predicted number of falls simulating cognitive impairment and overall predicted number of falls simulating no cognitive impairment.
Chi-square tests show that the difference in risk for cerebrovascular disease between mild and moderate/severe hearing loss is not statistically significant.
Chi-square tests show that the difference in risk for diabetes between mild and moderate/severe hearing loss is not statistically significant.
We performed a sensitivity analysis, excluding the 11% of our sample who reported current hearing aid use. For this restricted sample, the patterns were consistent with those based on the full sample, though the estimates were less precise due to the smaller sample size. We also do not have any details about the consistency of hearing aid use from those who reported having hearing aids. Therefore, we present results for the entire sample, including respondents who use hearing aids.
References
- Allison, P. D. (2002). Missing data. SAGE. 10.4135/9781412985079 [DOI] [Google Scholar]
- Cameron, A. C., & Trivedi, P. (2005). Microeconometrics: Methods and applications. Cambridge University Press. 10.1017/CBO9780511811241 [DOI] [Google Scholar]
- Centers for Medicare and Medicaid Services. (2019). ICD-10-CM official guidelines for coding and reporting FY 2019. https://www.cms.gov/Medicare/Coding/ICD10/Downloads/2019-ICD10-Coding-Guidelines-.pdf
- Centers for Medicare and Medicaid Services. (2021a). Chronic condition charts: 2018. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Chartbook_Charts
- Centers for Medicare and Medicaid Services. (2021b). Multiple chronic conditions. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/MCC_Main
- Centers for Medicare and Medicaid Services. (2022). Chronic conditions data warehouse. HealthATP. https://www2.ccwdata.org/web/guest/condition-categories-chronic
- Chen, D. S., Betz, J., Yaffe, K., Ayonayon, H. N., Kritchevsky, S., Martin, K. R., Harris, T. B., Purchase-Helzner, E., Satterfield, S., Xue, Q. L., Pratt, S., Simonsick, E. M., Lin, F. R., & for the Health ABC study . (2015). Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. The Journals of Gerontology: Series A, Biological Sciences and Medical Sciences, 70(5), 654–661. 10.1093/gerona/glu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, L., & Lanza, S. (2010). Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. Wiley. [Google Scholar]
- Davies, H. R., Cadar, D., Herbert, A., Orrell, M., & Steptoe, A. (2017). Hearing impairment and incident dementia: Findings from the English Longitudinal Study of Ageing. Journal of the American Geriatrics Society, 65(9), 2074–2081. 10.1111/jgs.14986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal, J. A., Betz, J., Yaffe, K., Harris, T., Purchase-Helzner, E., Satterfield, S., Pratt, S., Govil, N., Simonsick, E. M., Lin, F. R., & Health ABC Study Group . (2017). Hearing impairment and incident dementia and cognitive decline in older adults: The Health ABC study. The Journals of Gerontology: Series A, Biological Sciences and Medical Sciences, 72(5), 703–709. 10.1093/gerona/glw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deardorff, W. J., Liu, P. L., Sloane, R., Van Houtven, C., Pieper, C. F., Hastings, S. N., Cohen, H. J., & Whitson, H. E. (2019). Association of sensory and cognitive impairment with healthcare utilization and cost in older adults. Journal of the American Geriatrics Society, 67(8), 1617–1624. 10.1111/jgs.15891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, M. A., Kitterick, P. T., Chong, L. Y., Edmondson-Jones, M., Barker, F., Hoare, D. J., & Cochrane ENT Group . (2017). Hearing aids for mild to moderate hearing loss in adults. Cochrane Database of Systematic Reviews, 2017(9), Article CD012023. 10.1002/14651858.CD012023.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel, G. A., Harris, L. R., Gnanasegaram, J. J., Cushing, S. L., Gordon, K. A., Haycock, B. C., Pichora-Fuller, M. K., & Campos, J. L. (2022). Vestibular perceptual thresholds in older adults with and without age-related hearing loss. Ear and Hearing, 43(2), 420–435. 10.1097/aud.0000000000001118 [DOI] [PubMed] [Google Scholar]
- Ghisletta, P., Dahle, C. L., & Raz, N. (2023). Age-related hearing loss, cognitive performance, and metabolic risk in healthy adults: A seven-year longitudinal study. The Journals of Gerontology: Series B, Psychological Sciences and Social Sciences, 78(3), 409–420. 10.1093/geronb/gbac148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, T. D., Lad, M., Kumar, S., Holmes, E., McMurray, B., Maguire, E. A., Billig, A. J., & Sedley, W. (2020). How can hearing loss cause dementia? Neuron, 108(3), 401–412. 10.1016/j.neuron.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner, E. P., Patel, A. S., Pratt, S., Sutton-Tyrrell, K., Cauley, J. A., Talbott, E., Kenyon, E., Harris, T. B., Satterfield, S., Ding, J., & Newman, A. B. (2011). Hearing sensitivity in older adults: Associations with cardiovascular risk factors in the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society, 59(6), 972–979. 10.1111/j.1532-5415.2011.03444.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao, C. (2003). Analysis of panel data (2nd ed.). Cambridge University Press. 10.1017/CBO9780511754203 [DOI] [Google Scholar]
- Jiam, N. T.-L., Li, C., & Agrawal, Y. (2016). Hearing loss and falls: A systematic review and meta-analysis. The Laryngoscope, 126(11), 2587–2596. 10.1002/lary.25927 [DOI] [PubMed] [Google Scholar]
- Kamil, R. J., Betz, J., Powers, B. B., Pratt, S., Kritchevsky, S., Ayonayon, H. N., Harris, T. B., Helzner, E., Deal, J. A., Martin, K., Peterson, M., Satterfield, S., Simonsick, E. M., Lin, F. R., & for the Health ABC study . (2016). Association of hearing impairment with incident frailty and falls in older adults. Journal of Aging and Health, 28(4), 644–660. 10.1177/0898264315608730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, P. L., Di, J., Ferrucci, L., & Lin, F. R. (2021). Analysis of hearing loss and physical activity among US adults aged 60-69 years. JAMA Network Open, 4(4), Article e215484. 10.1001/jamanetworkopen.2021.5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, B. J., Jayakody, D. M. P., Bennett, R. J., Eikelboom, R. H., Gasson, N., & Friedland, P. L. (2020). Hearing loss and depression in older adults: A systematic review and meta-analysis. The Gerontologist, 60(3), e137–e154. 10.1093/geront/gnz009 [DOI] [PubMed] [Google Scholar]
- Lin, F. R., & Albert, M. (2014). Hearing loss and dementia – Who is listening? Aging & Mental Health, 18(6), 671–673. 10.1080/13607863.2014.915924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F. R., Yaffe, K., Xia, J., Xue, Q. L., Harris, T. B., Purchase-Helzner, E., Satterfield, S., Ayonayon, H. N., Ferrucci, L., Simonsick, E. M., & Health ABC Study Group . (2013). Hearing loss and cognitive decline in older adults. JAMA Internal Medicine, 173(4), 293–299. 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413–446. 10.1016/s0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., Ballard, C., Banerjee, S., Burns, A., Cohen-Mansfield, J., Cooper, C., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Larson, E. B., Ritchie, K., Rockwood, K., Sampson, E. L., … Mukadam, N. (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673–2734. 10.1016/s0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- Loughrey, D. G., Kelly, M. E., Kelley, G. A., Brennan, S., & Lawlor, B. A. (2018). Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: A systematic review and meta-analysis. JAMA Otolaryngology–Head & Neck Surgery, 144(2), 115–126. 10.1001/jamaoto.2017.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi, E., Basu, T., Langa, K., McKee, M. M., Zazove, P., Alexander, N., & Kamdar, N. (2019). Can hearing aids delay time to diagnosis of dementia, depression, or falls in older adults? Journal of the American Geriatrics Society, 67(11), 2362–2369. 10.1111/jgs.16109 [DOI] [PubMed] [Google Scholar]
- Mamo, S. K., & Wheeler, K. A. (2021). The combined burden of hearing loss and cognitive impairment in a group care setting for older adults. Journal of Speech, Language, and Hearing Research, 64(2), 328–336. 10.1044/2020_jslhr-20-00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo, S. K., Wheeler, K. A., Plano Clark, V. L., & Jacelon, C. S. (2022). A mixed methods study of hearing loss, communication, and social engagement in a group care setting for older adults. Perspectives of the ASHA Special Interest Groups, 7(2), 592–609. 10.1044/2021_PERSP-21-00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Amezcua, P., Powell, D., Kuo, P. L., Reed, N. S., Sullivan, K. J., Palta, P., Szklo, M., Sharrett, R., Schrack, J. A., Lin, F. R., & Deal, J. A. (2021). Association of age-related hearing impairment with physical functioning among community-dwelling older adults in the US. JAMA Network Open, 4(6), Article e2113742. 10.1001/jamanetworkopen.2021.13742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick, P., Kawachi, I., & Lin, F. R. (2014). The association between hearing loss and social isolation in older adults. Otolaryngology—Head and Neck Surgery, 150(3), 378–384. 10.1177/0194599813518021 [DOI] [PubMed] [Google Scholar]
- Mick, P., Parfyonov, M., Wittich, W., Phillips, N., Guthrie, D., & Pichora-Fuller M. K. (2018). Associations between sensory loss and social networks, participation, support, and loneliness: Analysis of the Canadian Longitudinal Study on Aging. Canadian Family Physician, 64(1), e33–e41. [PMC free article] [PubMed] [Google Scholar]
- Mick, P. T., Kabir, R., Pichora-Fuller, M. K., Jones, C., Moxham, L., Phillips, N., Urry, E., & Wittich, W. (2023). Associations between cardiovascular risk factors and audiometric hearing: Findings from the Canadian Longitudinal Study on Aging. Ear and Hearing. Advance online publication. 10.1097/aud.0000000000001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui, A. C. (2001). The Program of All-Inclusive Care for the Elderly (PACE): An innovative long-term care model in the United States. Journal of Aging & Social Policy, 13(2–3), 53–67. 10.1300/j031v13n02_05 [DOI] [PubMed] [Google Scholar]
- National PACE Association. (2022). National PACE Association. https://www.npaonline.org/
- Peters, C. A., Potter, J. F., & Scholer, S. G. (1988). Hearing impairment as a predictor of cognitive decline in dementia. Journal of the American Geriatrics Society, 36(11), 981–986. 10.1111/j.1532-5415.1988.tb04363.x [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller, M. K., Mick, P., & Reed, M. (2015). Hearing, cognition, and healthy aging: Social and public health implications of the links between age-related declines in hearing and cognition. Seminars in Hearing, 36(3), 122–139. 10.1055/s-0035-1555116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, N. S., Altan, A., Deal, J. A., Yeh, C., Kravetz, A. D., Wallhagen, M., & Lin, F. R. (2019). Trends in health care costs and utilization associated with untreated hearing loss over 10 years. JAMA Otolaryngology–Head & Neck Surgery, 145(1), 27–34. 10.1001/jamaoto.2018.2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riska, K. M., Peskoe, S. B., Kuchibhatla, M., Gordee, A., Pavon, J. M., Kim, S. E., West, J. S., & Smith, S. L. (2022). Impact of hearing aid use on falls and falls-related injury: Results from the Health and Retirement Study. Ear and Hearing, 43(2), 487–494. 10.1097/aud.0000000000001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, A. N., Simpson, K. N., & Dubno, J. R. (2015). Health-related quality of life in older adults: Effects of hearing loss and common chronic conditions. Healthy Aging Research, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tein, J. Y., Coxe, S., & Cham, H. (2013). Statistical power to detect the correct number of classes in latent profile analysis. Structural Equation Modeling, 20(4), 640–657. 10.1080/10705511.2013.824781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, G. P., Sladen, D. P., Borst, B. J. H., & Still, O. L. (2015). Accuracy of a tablet audiometer for measuring behavioral hearing thresholds in a clinical population. Otolaryngology–Head and Neck Surgery, 153(5), 838–842. 10.1177/0194599815593737 [DOI] [PubMed] [Google Scholar]
- Tian, R., Almeida, O. P., Jayakody, D. M. P., & Ford, A. H. (2021). Association between hearing loss and frailty: A systematic review and meta-analysis. BMC Geriatrics, 21(1), Article 333. 10.1186/s12877-021-02274-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida, Y., Sugiura, S., Nishita, Y., Saji, N., Sone, M., & Ueda, H. (2019). Age-related hearing loss and cognitive decline—The potential mechanisms linking the two. Auris Nasus Larynx, 46(1), 1–9. 10.1016/j.anl.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Uhlmann, R. F., Larson, E. B., & Koepsell, T. D. (1986). Hearing impairment and cognitive decline in senile dementia of the Alzheimer's type. Journal of the American Geriatrics Society, 34(3), 207–210. 10.1111/j.1532-5415.1986.tb04204.x [DOI] [PubMed] [Google Scholar]
- Uhlmann, R. F., Larson, E. B., Rees, T. S., Koepsell, T. D., & Duckert, L. G. (1989). Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA, 261(13), 1916–1919. 10.1001/jama.1989.03420130084028 [DOI] [PubMed] [Google Scholar]
- Viljanen, A., Kaprio, J., Pyykko, I., Sorri, M., Pajala, S., Kauppinen, M., Koskenvuo, M., & Rantanen, T. (2009). Hearing as a predictor of falls and postural balance in older female twins. Journal of Gerontology: Series A, Biological Sciences and Medical Sciences, 64(2), 312–317. 10.1093/gerona/gln015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells, T. S., Wu, L., Bhattarai, G. R., Nickels, L. D., Rush, S. R., & Yeh, C. S. (2019). Self-reported hearing loss in older adults is associated with higher emergency department visits and medical costs. INQUIRY: The Journal of Health Care Organization, Provision, and Financing, 56, Article 46958019896907. 10.1177/0046958019896907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y., Ding, Q., Lin, T., Shu, X., Xie, D., Gao, L., & Yue, J. (2022). Combined vision and hearing impairment is associated with frailty in older adults: Results from the West China Health and Aging Trend study. Clinical Interventions in Aging, 17, 675–683. 10.2147/cia.S362191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A de-identified data set used in this article is available upon reasonable request from the corresponding author, Sara K. Mamo.