Abstract
Purpose
We report the prevalence and economic cost of skin cancer treatment compared to other cancers overall in the USA from 2012 to 2018.
Methods
Using the Medical Expenditure Panel Survey full-year consolidated data files and associated medical conditions and medical events files, we estimate the prevalence, total costs, and per-person costs of treatment for melanoma and non-melanoma skin cancer among adults aged ≥ 18 years in the USA. To understand the changes in treatment prevalence and treatment costs of skin cancer in the context of overall cancer treatment, we also estimate the prevalence, total costs, and per-person costs of treatment for non-skin cancer among US adults.
Results
During 2012–15 and 2016–18, the average annual number of adults treated for any skin cancer was 5.8 (95% CI: 5.2, 6.4) and 6.1 (95% CI: 5.6, 6.6) million, respectively, while the average annual number of adults treated for non-skin cancers rose from 10.8 (95% CI: 10.0, 11.5) to 11.9 (95% CI: 11.2, 12.6) million, respectively. The overall estimated annual costs rose from $8.0 (in 2012–2015) to $8.9 billion (in 2016–18) for skin cancer treatment and $70.2 to $79.4 billion respectively for non-skin cancer treatment.
Conclusion
The prevalence and economic cost of skin cancer treatment modestly increased in recent years. Given the substantial cost of skin cancer treatment, continued public health attention to implementing evidence-based sun-safety interventions to reduce skin cancer risk may help prevent skin cancer and the associated treatment costs.
Keywords: Skin cancer treatment, Treated prevalence, Treatment costs, Medical Expenditure Panel Survey
Introduction
Skin cancer incidence rates have steadily increased over the past two decades in the USA [1–4]. The majority of skin cancers are non-melanoma skin cancers (NMSC), which are usually nonfatal and treatable, yet the public health impact of NMSC is substantial. In addition to the burden on the healthcare system due to the rising incidence of the two most common forms of NMSC [basal cell carcinomas (BCC) and squamous cell carcinomas (SCC)] [5, 6], there are indirect costs associated with morbidity (e.g., disfigurement, surgical and nonsurgical treatment effects) [7] and loss of productivity including lost workdays or restricted activities [4, 8]. Studies have also shown that individuals diagnosed with NMSC are at increased risk of developing a subsequent incident case of BCC, SCC, or melanoma [9, 10].
Melanoma is the third most common type of skin cancer and accounts for 75% of skin cancer-related deaths [2]. Each year, more than 84,000 new cases and 8,000 deaths from melanoma are reported from central cancer registries [2]. With early detection, 5-year relative survival rate is > 90%, but the effects of treatment and probability of survival can vary by stage of disease [2]. Although melanoma incidence rates are much lower among young adults compared to older adults [11, 12], melanoma remains one of the most common types of cancer among adults in their twenties and thirties [13]. As such, it contributes to significant years of potential life lost and productivity losses from premature deaths that go beyond treatment-related costs [8, 14].
In response to the important public health concerns of skin cancer, in 2014, the Surgeon General released a Call to Action to Prevent Skin Cancer [4], prioritizing efforts to mitigate the increasing trend of skin cancer incidence rates and underscoring the need to address the health and economic challenges of the disease. Subsequently, in 2016, the U.S. Preventive Services Task Force re-evaluated the evidence for skin cancer screening among average-risk population but did not conclude if the balance of benefits outweigh the potential harms of routinely screening for skin cancer via a visual skin examination in adults [6]. In addition, a systematic review evaluating the benefits of screening in reducing melanoma mortality found insufficient evidence of the benefits among average-risk population, and the benefits among population at increased risk have not been determined [6, 15]. However, some organizations such as the American Academy of Dermatology continue to promote initiatives for skin cancer screening like SPOT me® and SPOT Skin Cancer™ [16, 17]. To better understand the benefits of skin cancer screening with the most updated evidence, the U.S. Preventive Services Task Force is currently in the process of updating this review and recommendation [18]. Although the evaluation for the benefits of skin cancer screening is ongoing, some community-wide interventions to reduce UV exposure (e.g., increase the access of shade and sunscreen in the outdoor setting) have been shown to be effective and are recommended by the Community Preventive Services Task Force to reduce skin cancer risk [19, 20].
To inform resource allocation between prevention strategies, it is important to continue to monitor the health outcomes and economic burden of both non-melanoma and melanoma treatment costs at the national level given the high incidence and projected increase in the number of skin cancers [6, 21, 22]. A previous report, using data from 2007 to 2011, estimated that nearly five million people were treated for skin cancer in the USA annually [23]. The purpose of this study is to estimate the treated prevalence and the associated treatment costs with more recent data from 2012 to 2018 in light of organizations re-evaluating the screening guidelines.
Methods
We used the 2012–18 Medical Expenditure Panel Survey (MEPS), a nationally representative survey containing information on health conditions, health care utilization and costs among the U.S. civilian, non-institutionalized population [24]. The study sample linked data on adults aged ≥ 18 years from full-year consolidated data files, medical conditions files, and medical event files [24]. Full-year consolidated data files included demographic information of the participants; medical conditions files included the information to identify cancer conditions; and medical event files provided data on each medical event and associated expenses by the source of payments for various types of health care services (including office-based visits, hospital outpatient, inpatient stays, home health, emergency room, and prescribed medication purchases) for each participant. See summary in Appendix Table A1.
We linked medical conditions to medical event files to identify medical events associated with NMSC, melanoma skin cancer, and all other (non-skin) cancers for each participant. All other cancers were considered for comparison purpose. From 2012 to 2015, medical conditions files provided Clinical Classification Software (CCS) codes to classify types of cancer, including NMSC (CCS: 23), melanoma (CCS: 22), or all other cancers (CCS: 11–21, 24–45) [23, 25]. CCS codes were defined by grouping ICD-9 condition codes into broader clinically meaningful categories [26, 27]. In 2016, the MEPS changed the classification system to International Classification of Diseases, Tenth Revision (ICD-10) codes in the medical conditions files. Therefore, we used ICD-10 codes C44 and D04 for NMSC; C43 for melanoma; and C00-D49 (all codes for cancer conditions excluding the codes for NMSC and melanoma) for all other cancers in the 2016–18 MEPS datasets [28].
Individuals were classified as being treated for NMSC, melanoma, or all other cancers if they had any medical events associated with the corresponding CCS or ICD-10 codes. For an individual treated for NMSC, melanoma, or all other cancers, treatment costs were calculated by summing up expenses of events associated with the corresponding CCS or ICD-10 codes from all types of payments, including out-of-pocket, private insurance, Medicare, Medicaid, and other miscellaneous sources. Annual total national costs for NMSC, melanoma, and all other cancers were estimated by aggregating treatment costs among all individuals treated for the three cancer conditions.
We stratified the data into two time periods, 2012–15 and 2016–18, to ensure statistical power and precision and also to correspond with changes in the MEPS coding (i.e., the change from CCS to ICD-10 in 2016). Analyses were conducted using the “survey” package in R 4.0.3 to properly account for the MEPS sample design [29, 30].
We reported the average annual number and prevalence of adults with treatment for NMSC, melanoma, and non-skin cancers for the two time periods with 95% confidence intervals to show the uncertainty of these average estimates. In this study, the analysis for non-skin cancers was included to show how the trend observed in skin cancer might differ from the trend in other cancers. Treated prevalence among adults was also estimated by gender and by age group (age 18–64 years and ≥ 65 years). We focused on these two age groups because individuals aged ≥ 65 years were eligible for Medicare. For treatment costs, we reported total annual national costs among U.S. adults, and average and median annual cost per person for NMSC, melanoma, and non-skin cancers. The distributions of treatment costs by source of payment and type of service were reported for NMSC and non-skin cancer, but not for melanoma because of unstable statistical estimates resulted from the small sample sizes in MEPS for melanoma treatment. The p-values reported in the results for cost-associated estimates are based on two sample t-tests to compare differences between the two time periods. All costs were adjusted to 2018 U.S. dollars using the Personal Health Care Expenditure Price Index [31].
Results
The average annual number of U.S. adults treated for skin cancer (either NMSC or melanoma) modestly increased from 5.8 [(95% CI: 5.2, 6.4)] million in 2012–15 to 6.1 [(95% CI: 5.6, 6.6)] million in 2016–18 (p = 0.383). For all other cancers, the increase was from 10.8 [(95% CI: 10.0, 11.5)] million in 2012–15 to 11.9 [(95% CI: 11.2, 12.6)] million in 2016–18 (p = 0.042) (Fig. 1). The prevalence of those treated for NMSC, melanoma, and all other cancers was higher among adults aged ≥ 65 years compared to adults aged 18–64 years. The prevalence of receipt of skin cancer treatment was similar overall for men and women aged ≥ 18 years during the two time periods. However, for age 18–64 years, women more often had received treatment for melanoma [0.3% (95% CI: 0.2%, 0.5%)] than men [0.1%, (95% CI: 0.1%, 0.2%)] in 2016–2018 (p = 0.018); in comparison, for age ≥ 65 years, women had received treatment for melanoma [0.7% (95% CI: 0.5%, 1.0%)] less often than men [1.5% (95% CI: 1.1%, 1.9%)] (p = 0.001). Similarly, for all other cancers, women aged 18–64 years had a higher prevalence of being treated [3.3% (95% CI: 3.0%, 3.6%) in 2012–15; 3.9%, (95% CI: 3.5%, 4.3%) in 2016–18] than men [1.8% (95% CI: 1.6%, 2.1%) in 2012–15; 1.8% (95% CI: 1.6%, 2.1%) in 2016–18].
Fig. 1.
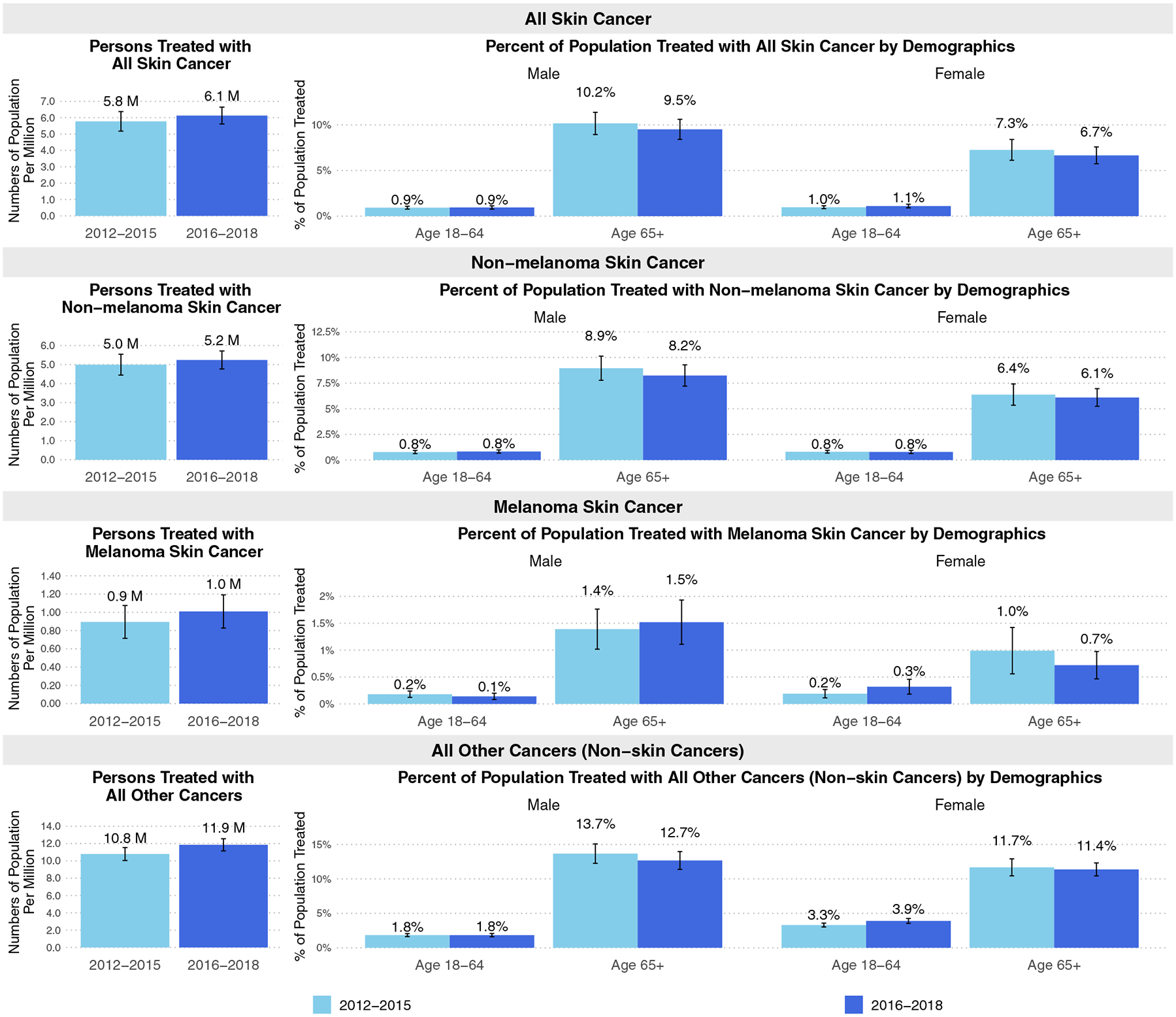
Average annual number of adults treated with skin and non-skin cancers in the USA 2012–18. The values represent the mean estimates, and the vertical bars represent the 95% confidence intervals. M indicates million
The estimated average annual total cost associated with any skin cancer treatment (either NMSC or melanoma) increased about 11.1% from $8.0 to $8.9 billion across the study periods 2012–15 and 2016–18 (p = 0.578) (Table 1). The average annual total cost of treating NMSC increased by nearly 30% from $5.0 billion in 2012–15 to $6.5 billion in 2016–18 (p = 0.073); whereas the average annual total cost of treating melanoma stayed relatively consistent at around $3.0 billion in 2012–15 and $2.5 billion in 2016–18 (p = 0.685). In comparison, the average annual total cost for all other cancers increased about 13.1% from $70.2 to $79.4 billion (p = 0.271). For NMSC, the average annual treatment cost per person increased from $1,010 in 2012–15 to $1,243 in 2016–18 (p = 0.086). As for melanoma, the average annual treatment cost per person was $3,347 in 2012–15 and $2,430 in 2016–18 (p = 0.538). For all other cancers, the average treatment cost per person was $6,507 in 2012–15 and $6,697 in 2016–18 (p = 0.783). By source of payment, Medicare was the largest payer for skin cancer treatment (40.8%) in recent years (2016–18), while private insurance shared the largest cost of all other cancers (40.9%) in the same time period. The proportion of costs paid by Medicare for all other cancers increased by about 21.4% (p = 0.058) between 2012–15 and 2016–18. Overall, office-based visits contributed 64.4% of the costs for skin cancer treatment, 74.8% of NMSC treatment costs, and 29.3% of the costs for all other cancer treatments in 2016–18.
Table 1.
Annual treatment costs for skin cancer and non-skin cancers among adults in the USA 2012–18
| All skin cancer (melanoma or non-melanoma)a | Non-melanoma skin cancer (NMSC) | Melanoma skin cancerc | All other cancers (non-skin cancers) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012–15 | 2016–18 | P-value | 2012–15 | 2016–18 | p-value | 2012–15 | 2016–18 | p-value | 2012–2015 | 2016–2018 | p-value | |
| Sample size from the MEPS | 1,464 | 1,389 | 1,262 | 1,186 | 229 | 235 | 3,718 | 3,132 | ||||
| N (SE) | N (SE) | N (SE) | N (SE) | N (SE) | N (SE) | N (SE) | N (SE) | |||||
| Population size represented | 5,779,547 (304,652) | 6,130,820 (263,331) | 0.383 | 4,995,655 (280,196) | 5,240,821 (240,597) | 0.507 | 895,052 (91,863) | 1,009,154 (92,917) | 0.383 | 10,793,154 (381,095) | 11,857,785 (357,607) | 0.042 |
| Cost estimates | $ (SE) | $ (SE) | $ (SE) | $ (SE) | $ (SE) | $ (SE) | $ (SE) | $ (SE) | ||||
| Total annual national cost in million ($) | 8,031 (1,384) | 8,925 (813) | 0.578 | 5,045 (504) | 6,513 (644) | 0.073 | 2,996b (1,263) | 2,452 (448) | 0.685 | 70,230 (4,836) | 79,408 (6,784) | 0.271 |
| Average annual cost per person ($) | 1,389 (230) | 1,456 (122) | 0.797 | 1,010 (81) | 1,243 (109) | 0.086 | 3,347b (1,409) | 2,430 (478) | 0.538 | 6,507 (413) | 6,697 (554) | 0.783 |
| Median annual cost per person ($) | 376 (19) | 386 (26) | 0.756 | 371 (17) | 394 (28) | 0.483 | 381 (41) | 320 (41) | 0.281 | 668 (30) | 534 (28) | 0.001 |
| Percent of total national cost by source of payment | % (SE) | % (SE) | % (SE) | % (SE) | % (SE) | % (SE) | ||||||
| Private | 42.3 (12.5) | 31.9 (3.6) | 0.424 | 35.2 (5.4) | 34.5 (4.5) | 0.921 | – | – | – | 46.5 (4.3) | 40.9 (4.6) | 0.374 |
| Medicare | 36.7 (8.6) | 40.8 (3.6) | 0.660 | 44.6 (2.2) | 42.9 (1.2) | 0.498 | – | – | – | 29.5 (1.8) | 35.8 (2.8) | 0.058 |
| Out-of-pocket | 8.6 (1.8) | 10.7 (0.5) | 0.261 | 9.7 (0.6) | 11.7 (0.5) | 0.010 | – | – | – | 4.9 (0.3) | 4.5 (0.3) | 0.346 |
| Medicaid | 4.0b (2.1) | 9.9b (3.9) | 0.183 | 1.3b (0.9) | 2.6b (1.6) | 0.479 | – | – | – | 7.7 (1.7) | 11.4 (3.4) | 0.330 |
| Other | 8.4 (1.8) | 6.7 (2.0) | 0.528 | 9.2 (1.8) | 8.2b (2.7) | 0.758 | – | – | – | 11.4 (2.4) | 7.4 (1.5) | 0.158 |
| Percent of total national cost by type of service | ||||||||||||
| Office-based medical provider | 58.2 (11.6) | 64.4 (7.8) | 0.657 | 76.2 (2.8) | 74.8 (9.8) | 0.891 | – | – | – | 29.6 (1.1) | 29.3 (2.2) | 0.903 |
| Outpatient department | 13.0 (1.9) | 26.1 (6.4) | 0.050 | 16.2 (3.6) | 18.4b (6.3) | 0.762 | – | – | – | 20.3 (2.2) | 23.1 (2.6) | 0.411 |
| Hospital inpatient | 20.8b (12.9) | 1.6b (1.0) | 0.138 | 2.6b (1.4) | 0.4b (0.4) | 0.131 | – | – | – | 34.5 (3.5) | 23.3 (2.2) | 0.007 |
| Prescription medication | 6.0b (3.0) | 3.4b (0.7) | 0.399 | 3.2 (0.4) | 3.6 (0.8) | 0.655 | – | – | – | 11.1 (2.4) | 18.4 (3.1) | 0.063 |
| Other | 1.9b (0.8) | 4.5b (2.2) | 0.267 | 1.8b (1.0) | 2.8b (1.8) | 0.627 | – | – | – | 4.5 (0.9) | 5.9b (1.9) | 0.505 |
Boldface indicates statistical significance (p < 0.05). Estimates are based on weighted data from the 2012–18 Medical Expenditure Panel Survey. All costs are in 2018 U.S. dollars. Other type of service includes home health and emergency room. Costs by source of payment and type of service are not available for melanoma due to small sample size and unreliable estimates. Because different coding system of medical condition was used in different time period, comparison between two time periods should be made with caution
The sum of the costs associated with cancer treatment between melanoma and non-melanoma skin cancer exceeds the combined estimate of costs associated with skin cancer treatment because some individuals have both melanoma and non-melanoma skin cancer
Estimates with a relative SE > 0.30 are considered unreliable
Because of small sample sizes for estimating the proportion of cost by source of payment and type of service for melanoma skin cancer, the estimates were suppressed (–)
Discussion
In recent years (2016–18), an average of 6.1 million adults were treated for skin cancer annually, resulting in an annual total treatment cost of $8.9 billion. In the same period, the average annual number of adults treated for all other cancers was 11.9 million with the associated treatment cost of $79.4 billion yearly. The number of adults aged ≥ 18 years treated for skin cancer (NMSC or melanoma) increased about 6% from 5.8 million in 2012–15 to 6.1 million in 2016–18, and for all other cancers, the increase was about 10% from 10.8 million in 2012–15 to 11.9 million in 2016–18. The increase in the number of adults treated for melanomas was about 13% from 2012–15 to 2016–18.
The overall increase in prevalence and annual costs are modest but notable and consistent with the estimates in Guy et al. [23]. However, caution is warranted when comparing differences across the two time periods (2012–15 and 2016–18) in this study and to that of Guy et al. [23]. This is because of the changes in disease coding, differences in pooled years and the associated changes in the MEPS panel cohorts for those years [32], and the advancements in skin cancer treatment over time [33]. Changes to skin cancer treatment protocols could have influenced our treatment prevalence and cost estimates.
The trends observed in our study reflect the general pattern of skin cancer incidence in the USA and increasing healthcare costs, including cancer care costs. Several studies, reports, and cancer surveillance systems have shown increasing trends in the incidence of NMSC and melanoma skin cancers [1, 2, 7, 21, 34]. Given the lack of systematic, routine data collection on new cases of BCC and SCC in the USA [4, 35], “treated prevalence” from nationally representative survey data adds important information on skin cancer occurrence. Additionally, the factors that affect treatment decisions can be multifactorial in terms of treatment modality, patient preference, provider practice, geriatric considerations or co-occurring conditions, [36] and these factors have not been thoroughly examined for NMSC and melanoma. In our analysis, the highest prevalence of treatment for NMSC, melanoma, and all other (non-skin) cancers was among adults aged ≥ 65 years. This aligns with what is already known about patterns of cancer incidence by age group. Still, given the variety of treatment modalities that are available particularly for treating NMSC, and the wide variations in cost and potentially comparable clinical outcomes, there is a need to more carefully examine the extent to which these decision-influencing factors play a role [7, 37]. These underlying issues, outside the scope of our analysis, may be reflected in our estimates of treatment prevalence and cost estimates of both skin and non-skin cancers.
Our analysis showed that total treatment costs were about $6.5 billion annually for NMSC and about $2.5 billion annually for melanoma, and that Medicare was the largest payer of treatment services. These findings are not surprising as Medicare provides health insurance coverage for older adults who are most at increased risk for skin cancer [11, 12], and has long covered the cost of skin cancer treatment. One study from 2003 reported NMSC to be the fifth most costly cancer to Medicare based on data from the Medicare Current Beneficiary Survey [37]. As healthcare costs correlate with rising incidence in cancer cases, melanoma treatment costs are projected to triple by 2030 [21].
Although our study highlights the substantial costs of skin cancer treatment, it also provides an opportunity to emphasize the importance of prevention efforts [38] and careful follow-up of persons with a history of any kind of skin cancer. Several studies have reported an increased risk of second primary cancers among individuals diagnosed with non-melanoma skin cancers and those with in situ or invasive melanoma [9, 10, 38]. Studies have shown the risk to be higher by about 20–60% [9]. As such, our findings point to the importance of ongoing efforts to implement and scale up evidence-based strategies to reduce skin cancer risk. For example, communities across the country have implemented community-based prevention programs and campaigns to raise sun-safety awareness, promote use of sun protection, and increase the availability of shade and sunscreen in public outdoor spaces [39]. Evidence from Australia suggests that combination of preventive strategies for skin cancer at the individual and community levels can reduce skin cancer risk with a high return on investment [20].
The current study has limitations. First, the sample of individuals diagnosed with cancer in the MEPS sample is relatively small [32]. The proportion of adults aged ≥ 18 years treated for cancer in our MEPS sample (2016–18) was about 1.6% for all skin cancers and about 3.9% for all other cancers, representing about 2.5% for all skin cancer and about 4.8% for all other cancers in the population aged ≥ 18 years. However, MEPS data have been used in other studies to estimate the national prevalence and healthcare utilization of cancer treatment in the USA because MEPS provides nationally representative samples every year [23, 32, 40]. Second, the MEPS survey is subject to measurement errors and potential recall bias of cancer treatment and cost estimates [24, 41]. For example, the medical conditions were self-reported by survey respondents and in some cases, one family member was the respondent on behalf of all family members [41]. Additionally, if a respondent had multiple health conditions during the survey period, there is the potential for recall bias and misclassification associated with healthcare utilization for the associated conditions [41]. Given the potential for these biases, our prevalence and treatment costs are likely to be underestimations of the actual costs [41]. Third, MEPS does not include clinical variables, such as detailed healthcare services received, stage at diagnosis, type of treatments received by a patient, and survival time [32]; therefore, we were unable to account for these in our estimates. Last, cancer conditions were defined based on the CCS and ICD-10 codes in the MEPS. Therefore, skin examination such as benign lesions, skin cancer pre-cursers (actinic keratoses), or lesions classified uncertain behavior were not included in the study.
In summary, this study shows that the prevalence and cost of skin cancer treatment remains substantial, affecting 6.1 million U.S. adults with total treatment cost of $8.9 billion annually. These estimates suggest that melanoma morbidity and corresponding treatment costs have continued to increase in the U.S. These findings underscore the continued need for implementation of evidence-based prevention strategies and a better understanding of the potential benefits of routine screening for high-risk populations. Future research can investigate how skin cancer treatment costs change with other important factors such as the year and stage of diagnosis or focus on estimating costs for precancerous lesions.
Acknowledgments
We would like to thank Ray Kuntz at the Agency for Healthcare Research and Quality for administrative help and technical support.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Appendix
See Table A1.
Table A1.
Information extracted from different types of MEPS data files
| Data files | Information used in analysis |
|---|---|
| Household full-year consolidated data file | Person ID |
| Age | |
| Sex | |
| Survey weight | |
| Household appendix to MEPS Event file | Person ID |
| Medical condition ID | |
| Medical event ID | |
| Medical conditions file | Person ID |
| Medical condition ID | |
| CCS code (2012–15) | |
| ICD-10 code (2016–18) | |
| Medical event file | |
| Prescribed medicines files | Person ID |
| Hospital inpatient stays files | Medical event ID |
| Emergency room visits files | Sum of payments |
| Outpatients visits files | Amount paid by Medicaid |
| Office-based medical provider visits files | Amount paid by Medicare |
| Amount paid by self or family | |
| Home health files | Amount paid by private insurance |
Footnotes
Conflict of interest All authors are employees at the Centers for Disease Control and Prevention (CDC) and have no conflicts of interest or financial disclosures. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Data availability
The data that support the findings of this study are available from the Agency for Healthcare Research and Quality but restrictions apply to the availability of these data due to the detailed information of cancer condition, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Agency for Healthcare Research and Quality.
References
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F (2012) A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 166(5):1069–1080. 10.1111/j.1365-2133.2012.10830.x [DOI] [PubMed] [Google Scholar]
- 2.U.S. Cancer Statistics Working Group (2021) U.S. Cancer Statistics Data Visualizations Tool, based on 2020 submission data (1999–2018) U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. www.cdc.gov/cancer/dataviz [Google Scholar]
- 3.Islami F, Ward EM, Sung H et al. (2021) Annual report to the nation on the status of cancer, part 1: national cancer statistics. JNCI J Natl Cancer Inst djab131. 10.1093/jnci/djab131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services (2014) The Surgeon General’s Call to Action to Prevent Skin Cancer. Office of the Surgeon General, Washington (DC) [PubMed] [Google Scholar]
- 5.Guy GP, Ekwueme DU, Tangka FK, Richardson LC (2012) Melanoma Treatment Costs: a systematic review of the literature, 1990–2011. Am J Prev Med 43(5):537–545. 10.1016/j.amepre.2012.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wernli KJ, Henrikson NB, Morrison CC, et al. (2016) Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet] Agency for Healthcare Research and Quality (US), Rockville (MD). http://www.ncbi.nlm.nih.gov/books/NBK379854/ [PubMed] [Google Scholar]
- 7.Thomson J, Hogan S, Leonardi-Bee J et al. (2020) Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev. 10.1002/14651858.CD003412.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy GP, Ekwueme DU (2011) Years of potential life lost and indirect costs of melanoma and non-melanoma skin cancer: a systematic review of the literature. Pharmacoeconomics 29(10):863–874. 10.2165/11589300-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 9.Rees JR, Zens MS, Gui J et al. (2014) Non melanoma skin cancer and subsequent cancer risk. PLoS ONE 9(6):e99674. 10.1371/journal.pone.0099674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duarte AF, Sousa-Pinto B, Haneke E, Correia O (2018) Risk factors for development of new skin neoplasms in patients with past history of skin cancer: a survival analysis. Sci Rep 8(1):15744. 10.1038/s41598-018-33763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulson KG, Gupta D, Kim TS et al. (2020) Age-specific incidence of melanoma in the United States. JAMA Dermatol 156(1):57. 10.1001/jamadermatol.2019.3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holman DM, Freeman MB, Shoemaker ML (2018) Trends in melanoma incidence among non-hispanic whites in the United States, 2005 to 2014. JAMA Dermatol 154:361. 10.1001/jamadermatol.2017.5541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleyer A, Viny A, Barr R (2006) Cancer in 15- to 29-year-olds by primary site. Oncologist 11(6):590–601. 10.1634/theoncologist.11-6-590 [DOI] [PubMed] [Google Scholar]
- 14.Ekwueme DU, Guy GP, Li C et al. (2011) The health burden and economic costs of cutaneous melanoma mortality by race/ethnicity–United States, 2000 to 2006. J Am Acad Dermatol 65(5):S133.e1–S133.e12. 10.1016/j.jaad.2011.04.036 [DOI] [PubMed] [Google Scholar]
- 15.Wernli KJ, Henrikson NB, Morrison CC et al. (2016) Screening for skin cancer in adults: updated evidence report and systematic review for the US preventive services task force. JAMA 316(4):436. 10.1001/jama.2016.5415 [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Dermatology Association (2022) Free Skin Cancer Screenings – Skin Cancer Screening Program. https://www.aad.org/public/public-health/skin-cancer-screenings#. Accessed 6 Jan 2022
- 17.American Academy of Dermatology and American Academy of Dermatology Association (2020) SPOT me® Skin Cancer Screening – 2020 Program Guidelines
- 18.U.S. Preventive Services Task Force (2022) Screening for Skin Cancer. https://uspreventiveservicestaskforce.org/uspstf/draft-update-summary/skin-cancer-screening-1. Accessed 4 Feb 2022
- 19.Guide to Community Preventive Services (2020) CPSTF Findings for Cancer Prevention and Control. https://www.thecommunityguide.org/content/task-force-findings-cancer-prevention-and-control. Accessed 14 Oct 2021
- 20.Shih ST, Carter R, Heward S, Sinclair C (2017) Economic evaluation of future skin cancer prevention in Australia. Prev Med 99:7–12. 10.1016/j.ypmed.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 21.Guy GP, Thomas CC, Thompson T et al. (2015) Vital signs: melanoma incidence and mortality trends and projections - United States, 1982–2030. MMWR Morb Mortal Wkly Rep 64(21):591–596 [PMC free article] [PubMed] [Google Scholar]
- 22.Weir HK, Thompson TD, Stewart SL, White MC (2021) Cancer Incidence Projections in the United States Between 2015 and 2050. Prev Chronic Dis 18:210006. 10.5888/pcd18.210006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guy GP, Machlin SR, Ekwueme DU, Yabroff KR (2015) Prevalence and costs of skin cancer treatment in the US, 2002–2006 and 2007–2011. Am J Prev Med 48(2):183–187. 10.1016/j.amepre.2014.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medical Expenditure Panel Survey. Rockville, MD: Agency for Healthcare Research and Quality. https://meps.ahrq.gov/mepsweb/data_stats/download_data_files.jsp. Accessed 15 Jan 2021 [Google Scholar]
- 25.Healthcare Cost and Utilization Project (HCUP) Clinical Classifications Software (CCS) for ICD-9-CM. Agency for Healthcare Research and Quality, Rockville MD. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed 15 Jan 2021 [Google Scholar]
- 26.Elixhauser A, Steiner C, Whittington C, McCarthy E (1999) Clinical Classifications for Health Policy Research: Hospital Inpatient Statistics, 1995. Agency for Health Care Policy and Research, Rockville [Google Scholar]
- 27.Davis-Ajami ML, Lu ZK, Wu J (2019) Multiple chronic conditions and associated health care expenses in US adults with cancer: a 2010–2015 Medical Expenditure Panel Survey study. BMC Health Serv Res 19:981. 10.1186/s12913-019-4827-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization (2004) ICD-10: international statistical classification of diseases and related health problems: tenth revision, 2nd ed. World Health Organization. https://apps.who.int/iris/handle/10665/42980. [Google Scholar]
- 29.Lumley T (2004) Analysis of complex survey samples. J Stat Softw 9(8):1–19 [Google Scholar]
- 30.R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 31.Using the appropriate price indices for analyses of health care expenditures or income across multiple years. Agency for Healthcare Research and Quality. https://meps.ahrq.gov/about_meps/Price_Index.shtml. Accessed 15 Jan 2021 [Google Scholar]
- 32.Yabroff KR, Dowling E, Rodriguez J et al. (2012) The medical expenditure panel survey (MEPS) experiences with cancer survivorship supplement. J Cancer Surviv 6(4):407–419. 10.1007/s11764-012-0221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curti BD, Faries MB (2021) Recent advances in the treatment of melanoma. N Engl J Med 384(23):2229–2240. 10.1056/NEJMra2034861 [DOI] [PubMed] [Google Scholar]
- 34.Gershenwald JE, Guy GP (2016) Stemming the rising incidence of melanoma: calling prevention to action. JNCI J Natl Cancer Inst 108(1):dvj381. 10.1093/jnci/djv381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Cancer Statistics Working Group (2020) Data Visualizations Tool Technical Notes – 2020 Submission Diagnosis Years 1999–2018. https://www.cdc.gov/cancer/uscs/pdf/uscs-data-visualizations-tool-technical-notes-2017-508.pdf. Accessed 7 Jan 2022
- 36.Linos E, Chren M, Stijacic Cenzer I, Covinsky KE (2016) Skin cancer in U.S. elderly adults: does life expectancy play a role in treatment decisions? J Am Geriatr Soc 64(8):1610–1615. 10.1111/jgs.14202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Housman TS, Feldman SR, Williford PM et al. (2003) Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol 48(3):425–429. 10.1067/mjd.2003.186 [DOI] [PubMed] [Google Scholar]
- 38.Balamurugan A, Rees JR, Kosary C et al. (2011) Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol 65(5):S69.e1–S69. e9. 10.1016/j.jaad.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 39.Division of Cancer Prevention and Control, Centers for Disease Control and Prevention (2021) Skin Cancer Prevention Success Stories. https://www.cdc.gov/cancer/skin/success-stories/index.htm. Accessed 14 Oct 2021
- 40.Halpern MT, Yabroff KR (2008) Prevalence of outpatient cancer treatment in the United States: estimates from the medical panel expenditures survey (MEPS). Cancer Invest 26(6):647–651. 10.1080/07357900801905519 [DOI] [PubMed] [Google Scholar]
- 41.Machlin SR, Soni A, Zhengyi F (2010) Understanding and analyzing MEPS household component medical condition data. Technical report, AHRQ. https://www.meps.ahrq.gov/survey_comp/MEPS_condition_data.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Agency for Healthcare Research and Quality but restrictions apply to the availability of these data due to the detailed information of cancer condition, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Agency for Healthcare Research and Quality.


