Abstract
OBJECTIVES
Our goal was to evaluate early and mid-term outcomes of physician-modified endografting for pararenal and thoraco-abdominal aortic aneurysms from 10 Japanese aortic centres.
METHODS
From January 2012 to March 2022, a total of 121 consecutive adult patients who underwent physician-modified endografting for pararenal and thoraco-abdominal aortic aneurysms were enrolled. We analysed early and mid-term postoperative outcomes, including postoperative complications and mortality.
RESULTS
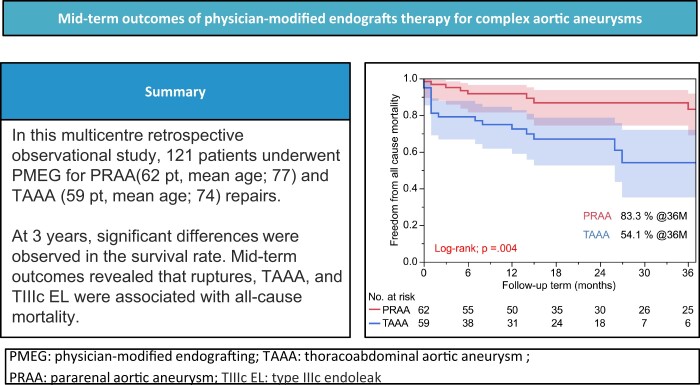
The pararenal and thoraco-abdominal aortic aneurysm groups included 62 (51.2%) and 59 (48.8%) patients, respectively. The overall in-hospital mortality rate was 5.8% (n = 7), with mortality rates of 3.2% (n = 2) and 8.5% (n = 5) in pararenal and thoraco-abdominal aortic aneurysm groups, respectively (P = 0.225). Type IIIc endoleaks occurred postoperatively in 18 patients (14.9%), with a significantly higher incidence (P = 0.033) in the thoraco-abdominal aortic aneurysm group (22.0%, n = 13) than in the other group (8.1%, n = 5). Major adverse events occurred in 7 (11.3%) and 14 (23.7%) patients in pararenal and thoraco-abdominal aortic aneurysm groups (P = 0.074), respectively. The mean follow-up period was 24.2 months. At the 3-year mark, both groups differed significantly in freedom from all-cause mortality (83.3% and 54.1%, P = 0.004), target aneurysm-related mortality (96.8% and 82.7%, P = 0.013) and any reintervention (89.3% and 65.6%, P = 0.002). Univariate and multivariate regression analyses demonstrated that ruptures, thoraco-abdominal aortic aneurysms and postoperative type IIIc endoleaks were associated with an increased risk of all-cause mortality.
CONCLUSIONS
The mid-term outcomes of physician-modified endografting for pararenal and thoraco-abdominal aortic aneurysms were clinically acceptable and comparable with those in other recently published studies. Notably, pararenal and thoraco-abdominal aortic aneurysms represent distinct pathological entities with different postoperative outcomes.
Keywords: Physician-modified endograft, Pararenal aortic aneurysm, Thoracoabdominal aortic aneurysm, Endovascular aneurysm repair, Endoleaks
Endovascular aortic repair (EVAR) or thoracic endovascular aortic repair (TEVAR) has become a widely accepted treatment option for aortic aneurysms [1–2].
INTRODUCTION
Endovascular aortic repair (EVAR) or thoracic endovascular aortic repair (TEVAR) has become a widely accepted treatment option for aortic aneurysms [1–2]. However, complex aortic aneurysms involving pararenal aortic aneurysms (PRAAs) or thoraco-abdominal aortic aneurysms (TAAAs) cannot be ideally treated using off-the-shelf EVAR devices without fenestrations or branches. Commercial fenestrated EVAR or branched EVAR (F/B-EVAR) devices are feasible and effective for complex aortic aneurysms [3]; however, due to socioeconomical and time limitations, physician-modified endografting (PMEG) is widely used to address these challenges [4]. Some previous single and multicentre studies, systematic reviews and meta-analyses have reported acceptable results with the use of PMEG for complex aortic aneurysms [5–10]. However, from a regional distribution viewpoint, PMEG has been indicated sporadically, especially in countries where commercially available F/B-EVAR devices have not been officially introduced. Therefore, in the 2020 Guideline on Diagnosis and Treatment of Aortic Aneurysm and Aortic Dissection [11], there is no clear recommendation for PMEG for complex aortic aneurysms. To the best of our knowledge, no multicentre studies have been reported from any countries like Japan without a history of insurance coverage of F/B-EVAR devices. The goal of this multicentre study was to evaluate the mid-term outcomes of PMEG for repairing PRAAs and TAAAs from 10 Japanese aortic centres.
MATERIALS AND METHODS
Ethical considerations
The study was conducted per the Declaration of Helsinki and approved by the ethics committees of the participating centres (approval no. 332–169). Written informed consent was obtained from all participants or their families.
Patients and study design
In this multicentre, retrospective, observational study, pre- and post-procedural data on PMEG for PRAAs and TAAAs, excluding dissected aneurysms, were prospectively collected from 10 Japanese centres between January 2012 and March 2022. In these centres, the surgical criteria did not deviate from global standards, with interventions recommended for aneurysms meeting either size-specific criteria (>5.5 or 4.5 cm for the fusiform or saccular type) [12], those that were symptomatic or those with rapidly expanding sizes (>5 mm over 6 months). Each centre formed a multidisciplinary team to evaluate the patients’ general conditions for elective surgery, classifying patient comorbidities according to the Society for Vascular Surgery/American Association for Vascular Surgery reporting standards [13]. Patient eligibility for PMEG adhered to the following criteria: (i) American Society of Anaesthesiologists score [14] of ≥ 3 or inapplicability of conventional open repair for anatomical reasons or comorbidities; and (ii) considerable involvement of visceral vessels. The appropriateness of the PMEG indication in each case was further retrospectively evaluated by all participating researchers, including T.S., H.M., Y.I., K.H., N.H., K.Y., N.K., Y.K., H.H., T.U., Y.M. and N.K. The patients underwent baseline clinical examinations, laboratory tests and imaging, with subsequent follow-ups at 6–8 weeks, 6 months and 12 months postoperatively and annually thereafter. Imaging examinations at each follow-up interval included computed tomography angiography (CTA) for sac behaviour assessment and renovisceral branch patency assessment. In patients in whom CTA was contraindicated, CT without contrast was used, and duplex ultrasound was used to assess branch patency. Technical success was defined as successful deployment of PMEG devices with completed branch reconstructions according to preoperative planning. Study end-point data collected from the participating centres also included in-hospital mortality, incidence of postoperative endoleaks, major adverse events —such as all-cause death at 30 days or within hospital stay, myocardial infarction, acute kidney injury, new-onset dialysis, respiratory failure, bowel ischaemia requiring resection, paraplegia, and major stroke—and freedom from all-cause mortality, aneurysm-related mortality and secondary interventions. Decisions for reintervention were based on discussions within each centre, considering factors such as occurrence of persistent type II endoleaks associated with AAA growth > 5 mm, presence or imminence of type I or IIIc endoleaks, relevant migration, graft occlusion, rupture or infection. Sac behaviour was categorized as growth (increase of ≥5 mm in maximum-minimum diameter), shrinkage (decrease of ≥5 mm) or stable (<5-mm change).
Stent graft modification
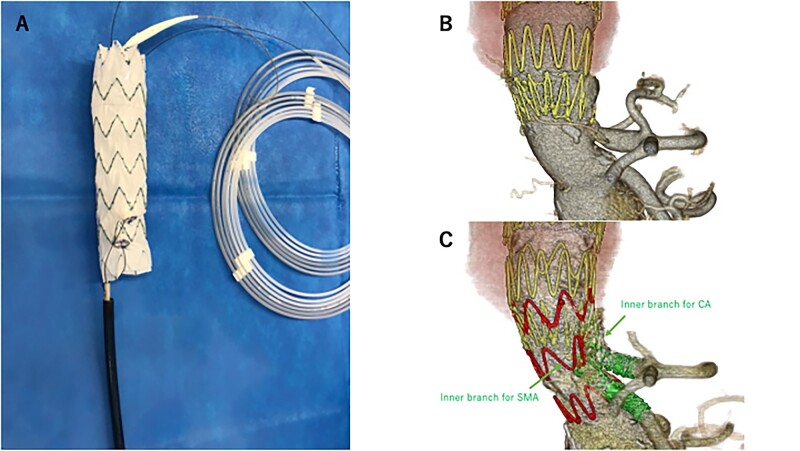
Each centre independently determined the specific device to be modified and the modes of renovisceral branch reconstruction, including scallop or fenestration without a bridging stent or a bridging stent with either fenestration, cuffed fenestration or directional or inner branch. Generally, fenestration was selected if a proximal length of 5 mm or more could be secured; otherwise, branch was selected. The inner branch [15–19] was indicated and used in 5 centres (T.S., K.Y., N.K., Y.K. and T.U.). Because this study was retrospective in nature, the method of creating scallops, fenestrations or branches was not standardized. All patients underwent preoperative high-resolution CTA. Device sizing and PMEG planning were performed based on 3-dimensional D measurements using either of these dedicated three-dimensional vascular imaging workstations, including Aquarius (TeraRecon, Foster City, CA, USA) and OsiriX Imaging (Pixmeo SARL, Bernex, Switzerland) software packages. Centreline lumen reconstructions were used to determine the aortic diameter at the proximal and distal landing zones and the distance between the proximal edge of the stent graft and the clock position of the centre of the fenestration. Some centres (T.S., H.M. and Y.M.) used three-dimensional models to confirm the preprocedural design of modifications [20]. Stent grafts of sufficient length were selected to ensure a minimum of 15-mm proximal and distal landing zones in the healthy aorta. The stent graft was modified on a back table before anaesthesia was administered. A fenestration was created in predetermined locations by burning the Dacron fabric via electrocautery. A snare catheter or coil was used as a marker for the fenestration, and 5–0 ethibond or proline sutures were used for fixation. The diameter-reducing tie technique was not performed routinely in this study because we were not familiar with it, and diameter-reducing ties can complicate the orientation of target vessels and fenestrations. The inner branches were created by cutting Endurant leg (Medtronic, Santa Rosa, CA, USA) or Viabahn stent grafts (W. L. Gore and Associates, Flagstaff, AZ, USA) and were attached to the fenestration using running sutures (Fig. 1). The modified stent graft was manually re-sheathed. In some cases, precannulation techniques were used to facilitate the procedure.
Figure 1:
An 82-year-old man with an enlarged thoraco-abdominal aortic aneurysm due to a type Ib endoleak underwent a physician-modified endovascular graft with inner branches. (A) The physician-modified endovascular graft with inner branches for coeliac artery and superior mesenteric artery Zenith alpha thoracic stent graft back-table preparation. (B) Preoperative computed tomography scan. (C) Postoperative computed tomography scan. CA: celiac artery; SMA: superior mesenteric artery.
Statistical analysis
Variables and end points were analysed for the entire cohort, and the cohort was categorized into 2 groups based on the extent of the anatomical aneurysm: the PRAA and TAAA groups. Data were reported using the Society for Vascular Surgery reporting standards for F-BEVAR [21]. Kaplan–Meier estimates were used to report time-dependent outcomes for up to 3 years, and differences were determined using the log-rank test. Normally distributed variables are expressed as mean ± standard deviation. Variables with skewed data are expressed as median and interquartile range. Pearson’s χ2 or Fisher’s exact test was used to analyse categorical variables. Differences between continuous variables were tested using the Wilcoxon rank-sum test, the Mann–Whitney U test or the two-sided Student t-test. Cox proportional hazards regression analysis was performed to identify risk factors for overall survival. Statistical significance was set at P-values < 0.05. Analyses were performed using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline characteristics
The anatomical classification of aneurysms and the baseline characteristics of patients are summarized in Tables 1 and 2. This study enrolled a total of 121 patients, with 62 (51.2%) and 59 (48.8%) patients in the PRAA and TAAA groups, respectively. In the TAAA group, the extent of the aneurysm was classified as follows: extent I (3.3%), extent II (1.7%), extent III (19.0%), extent IV (14.0%) and extent V (14.0%). The mean age of the entire cohort was 75.6 ± 7.6 years. Patients in the PRAA group were older than those in the TAAA group (77.1 ± 7.7 vs 74.0 ± 7.2 years, P = 0.022). There were 100 males (82.6%) in the entire cohort, with the male proportion being higher in the PRAA group than in the TAAA group (90.3 vs 74.6%, P = 0.024). The median maximum-minimum aneurysmal diameter was 57.9 ± 12.7 mm (PRAA: 55.9 ± 11.9 vs TAAA: 60.1 ± 13.2 mm, P = 0.069). Overall, 18 procedures (14.9%) were performed for rupture: 7 (11.3%) in the PRAA group and 11 (18.6%) in the TAAA group.
Table 1:
Baseline characteristics of 121 patients treated using physician-modified endografting for pararenal aortic aneurysm and thoraco-abdominal aortic aneurysm.
| Variable | All patients (n = 121) | PRAA (n = 62) | TAAA (n = 59) | P-value |
|---|---|---|---|---|
| Age (years) | 75.6 ± 7.63 | 77.1 ± 7.74 | 74.0 ± 7.23 | 0.022 |
| Male sex | 100 (82.6) | 56 (90.3) | 44 (74.6) | 0.024 |
| Hypertension | 114 (94.2) | 58 (93.5) | 56 (94.9) | 0.749 |
| Diabetes mellitus | 21 (17.4) | 12 (19.4) | 9 (15.3) | 0.555 |
| Dyslipidaemia | 60 (49.6) | 29 (46.8) | 31 (52.5) | 0.530 |
| Coronary artery disease | 39 (32.2) | 24 (38.7) | 15 (25.4) | 0.119 |
| Cerebrovascular disease | 28 (23.1) | 13 (21.0) | 15 (25.4) | 0.566 |
| Peripheral artery disease | 19 (15.7) | 9 (14.5) | 10 (16.9) | 0.716 |
| COPD | 45 (37.2) | 22 (35.5) | 23 (39.0) | 0.694 |
| CKD (eGFR < 59) | 62 (51.2) | 31 (50.0) | 31 (52.5) | 0.782 |
| Smoking | 90 (74.4) | 45 (72.6) | 45 (76.3) | 0.645 |
| Antithrombotic therapy | 81 (66.9) | 45 (72.6) | 36 (61.0) | 0.252 |
| Previous aortic surgery | 36 (29.8) | 14 (22.6) | 22 (37.3) | 0.079 |
| ASA score ≥ 3 | 77 (63.6) | 36 (58.1) | 41 (69.5) | 0.448 |
| Maximum–minimum diameter of aneurysm (mm) | 57.9 ± 12.7 | 55.9 ± 11.9 | 60.1 ± 13.2 | 0.069 |
| Rupture | 18 (14.9) | 7 (11.3) | 11 (18.6) | 0.262 |
Continuous variables are presented as mean ± standard deviation and categorical variables as number of patients (%).
ASA: American Society of Anesthesiologists; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; PRAA: pararenal aortic aneurysm; TAAA: thoraco-abdominal aortic aneurysm.
Table 2:
Procedural details.
| Variable | Overall (n = 121) | PRAA (n = 62) | TAAA (n = 59) | P-value |
|---|---|---|---|---|
| Modified main device | ||||
| EVAR device | 45 (37.2) | 42 (67.7) | 3 (5.1) | |
| Zenith Flex | 28 | 26 | 2 | |
| Zenith alpha abdominal | 7 | 6 | 1 | |
| Endurant | 10 | 10 | 0 | |
| TEVAR device | 76 (62.8) | 20 (32.3) | 56 (94.9) | |
| Zenith Tx2 | 20 | 4 | 16 | |
| Zenith alpha thoracic | 29 | 14 | 15 | |
| Talent | 1 | 0 | 1 | |
| Valiant Captivia | 4 | 2 | 2 | |
| Valiant Navion | 2 | 0 | 4 | |
| Relay Plus | 18 | 0 | 18 | |
| Main device delivery system (Fr) | 20.7 ± 2.44 | 19.5 ± 2.01 | 21.9 ± 2.21 | <0.001 |
| Target vessels (n) | 342 | 160 | 182 | |
| Vessels per patient | 2.87 ± 1.05 | 2.58 ± 1.03 | 3.08 ± 1.00 | 0.008 |
| Type of incorporation per vessel | <0.001 | |||
| Fenestrations | 271 | 150 | 121 | |
| Branches | 71 | 10 | 61 | |
| Bridging stents | ||||
| Stent graft | 189 | 96 | 93 | |
| Bare metal stent | 43 | 29 | 14 | |
| Cerebrospinal fluid drainage | 0 | 0 | 0 | |
| Customization time, min | 60.4 ± 23.2 | 54.4 ± 16.0 | 67.9 ± 29.1 | 0.010 |
| Contrast volume, mL | 163.6 ± 78.2 | 150.2 ± 61.7 | 179.4 ± 92.2 | 0.058 |
| Fluoroscopy time, min | 120.7 ± 77.1 | 121.2 ± 62.0 | 120.3 ± 90.9 | 0.950 |
| Total radiation dose, mGy | 4124 ± 3911 | 4281.7 ± 3175.4 | 3964.0 ± 4563.0 | 0.661 |
| Total procedure time, min | 325.1 ± 139.9 | 295.5 ± 104.2 | 356.2 ± 164.9 | 0.018 |
| Estimated blood loss, mL | 372.0 ± 428.5 | 300.1 ± 254.4 | 470.0 ± 578.0 | 0.074 |
Continuous variables are presented as mean ± standard deviation and categorical variables as numbers of patients (%).
Zenith Flex (Cook Medical, Inc, Bloomington, IN, USA); Zenith alpha abdominal (Cook Medical, Bloomington, IN, USA); Endurant (Medtronic, Santa Rosa, CA, USA); Zenith TX2 (Cook Medical, Bloomington, IN, USA); Zenith alpha thoracic (Cook Medical, Bloomington, IN, USA); Talent (Medtronic, Santa Rosa, CA, USA); Valiant Captivia (Medtronic, Santa Rosa, CA, USA); Valiant Navion (Medtronic, Santa Rosa, CA, USA); Relay Plus (Terumo Aortic, Sunrise, FL, USA).
EVAR: endovascular aneurysm repair; PRAA: pararenal aortic aneurysm; TAAA: thoraco-abdominal aortic aneurysm; TEVAR: thoracic EVAR.
Procedural details
All procedures were performed—with the patient under general anaesthesia—in either an operating room equipped with a C-arm or a hybrid endovascular room (Table 2). An EVAR device was often used (67.7%) in the PRAA group. The TEVAR devices were often used in the TAAA group (94.9%). Compared with the PRAA group, the TAAA group used larger main device delivery systems (P < 0.001), with a larger number of target vessels per patient (P = 0.008). No cerebrospinal fluid drainage was performed in either group. The TAAA group had significantly longer customization and total procedural times than the PRAA group (P = 0.010 and P = 0.018, respectively). Contrast volume (PRAA vs TAAA: 150.2 ± 61.7 vs 179.4 ± 92.2 ml, P = 0.058), fluoroscopy time (121.2 ± 62.0 vs 120.3 ± 90.9 min, P = 0.950) and total radiation dose (4281.7 ± 3175.4 vs 3064.0 ± 4563.0 mGy, P = 0.661) were similar between the groups.
Postoperative outcomes
The postoperative outcomes are summarized in Table 3. Two in-hospital deaths occurred in the PRAA group and 5 occurred in the TAAA group. In-hospital death was observed mainly in rupture cases (1 PRAA and 3 TAAAs). The technical success rates were 93.5% and 98.3% in the PRAA and TAAA groups, respectively, whereas the technical success rates per vessel were 97.5% in the PRAA group and 99.5% in the TAAA group. The incidence of endoleaks at completion was not significantly different between the groups. Postoperative type IIIc endoleaks in the chronic phase were observed more frequently in the TAAA group than in the PRAA group (22.0 vs 8.1%, P = 0.033). Major adverse events occurred in 7 patients in the PRAA group and in 14 patients in the TAAA group (11.3 vs 23.7%, P=0.074). Spinal cord injury occurred predominantly in 9 patients in the TAAA group (0 vs 9 cases, P=0.002), with 8 cases of paraparesis (6.6%) and 1 of paraplegia (0.8%). The overall mean hospital length of stay was longer in the TAAA group than in the PRAA group (29.9 ± 61.5 and 11.1 ± 8.8 days, P=0.024).
Table 3:
In-hospital postoperative outcomes.
| Variable | Overall (n = 121) | PRAA (n = 62) | TAAA (n = 59) | P-value |
|---|---|---|---|---|
| Major adverse event | 21 (17.4) | 7 (11.3) | 14 (23.7) | 0.074 |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| New-onset dialysis | 5 (4.1) | 3 (4.8) | 2 (3.4) | 0.691 |
| Stroke | 4 (3.3) | 2 (3.2) | 2 (3.4) | 0.960 |
| Respiratory failure | 5 (4.1) | 2 (3.2) | 3 (5.1) | 0.613 |
| Bowel ischaemia | 2 (1.6) | 2 (3.2) | 0 (0) | 0.159 |
| Spinal cord injury | 9 (7.4) | 0 (0) | 9 (15.3) | 0.002 |
| Paraparesis | 8 (6.6) | 0 (0) | 8 (13.6) | 0.004 |
| Paraplegia | 1 (0.8) | 0 (0) | 1 (1.7) | 0.322 |
| In-hospital mortality | 7 (5.8) | 2 (3.2) | 5 (8.5) | 0.225 |
| Length of hospital stay, days | 20.3 ± 44.2 | 11.1 ± 8.8 | 29.9 ± 61.5 | 0.024 |
| ICU stay, days | 2.8 ± 5.0 | 1.4 ± 2.4 | 4.2 ± 6.4 | 0.024 |
| Postoperative device migration | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Endoleaks before discharge | ||||
| Ia+b | 6 (5.0) | 3 (4.8) | 3 (5.1) | 0.951 |
| II | 9 (7.4) | 4 (6.5) | 5 (8.5) | 0.676 |
| IIIc | 18 (14.9) | 5 (8.1) | 13 (22.0) | 0.033 |
| IV | 1 (0.8) | 1 (1.6) | 0 (0) | 0.321 |
Continuous variables are presented as mean ± standard deviation and categorical variables as number of patients (%).
ICU: intensive care unit; PRAA: pararenal aortic aneurysm; TAAA: thoraco-abdominal aortic aneurysm.
Follow-up outcomes
Table 4 summarizes the follow-up outcomes. The PRAA group had a significantly longer follow-up period than the TAAA group (30.5 ± 27.9 vs 17.7 ± 24.8 months, P=0.009). The TAAA group required secondary interventions more frequently than the PRAA group (6.5 vs 23.7%, P = 0.008). Most re-treatments were performed for type IIIc endoleaks (TAAA group, 9 cases; PRAA group, 2 cases); moreover, covered stent deployment was performed in 5 cases, stent grafting in 4 cases, coiling for aneurysm sac in 1 case and open repair in 1 case. Sac shrinkage was significantly more common in the PRAA group than in the TAAA group (67.7 vs 40.7%, P=0.008). The estimated 36-month freedom from all-cause mortality (Fig. 2) was 83.3% and 54.1% in the PRAA and TAAA groups, respectively (P = 0.004). The overall freedom from target aneurysm-related mortality was 90.2%, with rates of 96.8% in the PRAA group and 82.7% in the TAAA group at 36 months (P = 0.013) (Fig. 3). Furthermore, freedom from secondary interventions at 36 months was 89.3% and 65.6% in the PRAA and TAAA groups, respectively (P = 0.002). Table 5 shows the associations of baseline characteristics and postoperative outcomes with all-cause mortality. Univariate and multivariate Cox regression analyses revealed that ruptures, TAAAs and postoperative type IIIc endoleaks were associated with an increased risk of all-cause mortality. Table 6 shows the associations of baseline characteristics/postoperative outcomes with target aneurysm-related mortality. In the univariate and multivariate Cox regression analyses, ruptures and TAAAs were significantly associated with an increased risk of target aneurysm-related mortality.
Table 4:
Follow-up outcomes.
| Variable | Overall (n = 121) | PRAA (n = 62) | TAAA (n = 59) | P-value |
|---|---|---|---|---|
| Follow-up time, month | 24.2 ± 27.1 | 30.5 ± 27.9 | 17.7 ± 24.8 | 0.009 |
| Secondary intervention | 18 (14.9) | 4 (6.5) | 14 (23.7) | 0.008 |
| Sac behaviour | ||||
| Shrinkage | 66 (54.5) | 42 (67.7) | 24 (40.7) | 0.003 |
| Stable | 47 (38.8) | 16 (25.8) | 31 (52.5) | 0.003 |
| Growth | 8 (6.6) | 4 (6.5) | 4 (6.8) | 0.942 |
PRAA: pararenal aortic aneurysm; TAAA: thoraco-abdominal aortic aneurysm.
Figure 2:
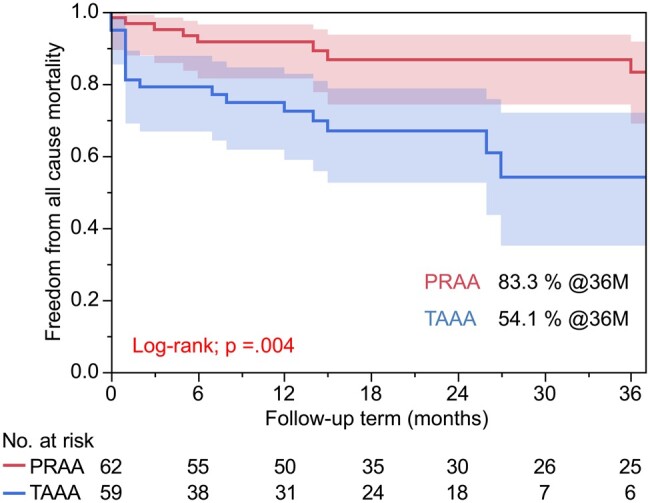
Estimated 36-month freedom from all-cause mortality in the pararenal aortic aneurysm and thoraco-abdominal aortic aneurysm groups. PRAA: pararenal aortic aneurysm; TAAA: thoraco-abdominal aortic aneurysm.
Figure 3:
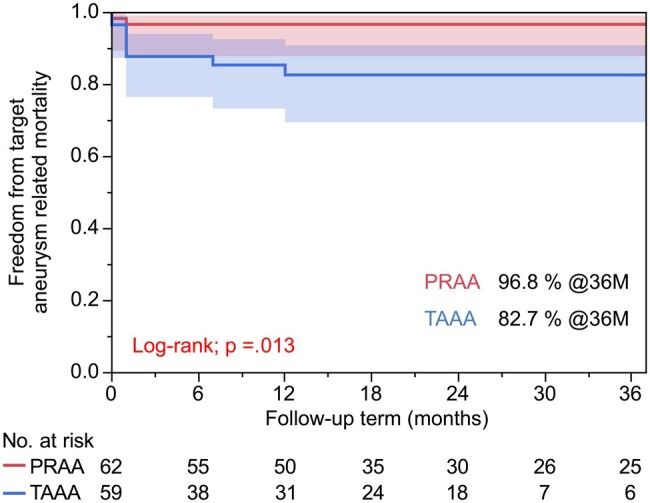
Estimated 36-month freedom from target aneurysm-related mortality in the pararenal aortic aneurysm and thoraco-abdominal aortic aneurysm groups. PRAA: pararenal aortic aneurysm; TAAA: thoraco-abdominal aortic aneurysm.
Table 5:
Selected results of univariate and multivariate analysis for all-cause mortality.
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P- value | |
| Male | 1.28 (0.50–4.37) | 0.643 | ||
| Age | 1.03 (0.98–1.08) | 0.298 | ||
| CKD | 1.56 (0.75–3.31) | 0.232 | ||
| Rupture | 2.97 (1.32–6.23) | 0.005 | 2.85 (1.23–6.23) | 0.011 |
| ASA score ≥3 | 2.04 (0.91–5.19) | 0.104 | ||
| TAAA | 2.93 (1.34–6.40) | 0.007 | 2.34 (1.07–5.42) | 0.038 |
| Previous aortic surgery | 1.34 (0.57–2.94) | 0.476 | ||
| Postoperative TIIIc ELs | 3.03 (1.18–6.86) | 0.012 | 3.33 (1.26–7.88) | 0.005 |
AAA: abdominal aortic aneurysm; ASA: American Society of Anesthesiologists; CI: confidence interval; CKD: chronic kidney disease; HR: hazard ratio; TIIIc Els: type IIIc endoleaks; TAAA: thoracoabdominal aortic aneurysm.
Table 6:
Selected results of univariate and multivariate analysis for target aneurysm-related mortality.
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Male | 2.41 (0.46–44.40) | 0.400 | ||
| Age | 2.23 (0.09–65.30) | 0.627 | ||
| CKD | 1.34 (0.42–4.56) | 0.616 | ||
| Rupture | 3.92 (1.14–12.42) | 0.021 | 4.42 (1.27–14.34) | 0.021 |
| ASA score ≥3 | 7.10 (1.34–131.44) | 0.063 | ||
| TAAA | 5.44 (1.42-35.51) | 0.029 | 4.71 (1.21-30.97) | 0.048 |
| Previous aortic surgery | 0.89 (0.20–3.09) | 0.867 | ||
| Postoperative TIIIc ELs | 2.74 (0.60–9.57) | 0.138 | 3.35 (0.72–12.06) | 0.113 |
ASA: American Society of Anesthesiologists; CI: confidence interval; CKD: chronic kidney disease; HR: hazard ratio; IIIc Els: type IIIc endoleaks; TAAA: thoracoabdominal aortic aneurysm.
DISCUSSION
This multicentre retrospective observational study demonstrated that the procedural success and postoperative results of F/B-EVAR in Japan reached acceptable levels, despite being performed as PMEG. Key procedural accuracy and safety metrics—such as overall F/B ostial matching and absence of procedural mortality in non-rupture cases—closely paralleled the outcomes observed in other PMEG reports [4, 5]. The mid-term results in the present study—including the low levels of sac size increase and favourable freedom from aneurysm-related mortality, except those in rupture cases—were comparable with findings of both PMEG and industry-made F/B-EVAR device studies. These findings indicate the non-inferiority of our PMEG practice while also highlighting country-specific structural issues, which warrant careful consideration and potential remodelling.
In Japan, industry-made stent grafts were approved and introduced in 2006. By introducing refined versions of devices and the necessary ancillary equipment, the challenges experienced with earlier generations in terms of the use of EVAR devices were not observed domestically. Additionally, a national committee for stent graft management, which comprised representatives from relevant academic associations, was established to ensure efficacy and safety. Currently, more than 80% of AAAs in Japan are treated with EVAR, and the usage of industry-made EVAR and TEVAR devices in our country is among the highest worldwide. F/B-EVAR has been accepted as an option for high-risk patients with PRAAs and TAAAs. However, even in F/B-EVAR-advanced countries, PMEG has become widespread mainly because of the prolonged access time to industry-made devices. Meanwhile, PMEG has been widely used in countries where the introduction of industry-made devices and the aforementioned ancillary devices have been delayed because of a lack of F/B-EVAR available options.
At 36 months, patients in the PRAA group had higher freedom from all-cause mortality, aneurysm-related mortality and secondary interventions than did patients in the TAAA group. Ruptures and postoperative type IIIc endoleaks were associated with all-cause mortality, whereas ruptures and TAAAs were associated with target aneurysm-related mortality. TAAAs and PRAAs represented separate and distinct pathological entities in terms of postoperative outcomes. Postoperative type IIIc endoleaks occurred more frequently in the TAAA group than in the PRAA group with a significant difference, contributing to the differences in clinical outcomes. The anatomical characteristics of the renovisceral branches differ between PRAAs and TAAAs, with those in PRAAs arising from narrower aortic segments and those in TAAAs from wider segments. Studies have highlighted that type IIIc endoleaks from directional branches still represent one of the leading causes of reintervention after branched endovascular repair using an off-the-shelf or custom-made device [22]. The gap distance between the endograft-reinforced fenestration and the target artery origin at the aortic wall has been associated with an increased risk of target vessel instability and the related endoleaks (Ic and IIIc) [23]. Moreover, manufacturing of the graft may affect type IIIc endoleaks because the procedures for the modifications were not standardized. For the treatment of TAAA, the use of PMEG might be restricted to emergency cases or to countries where commercially available F/B-EVAR devices have not been officially introduced. Therefore, to ensure F/B-EVAR effectiveness, innovative solutions for enhanced durability and accessibility are required to address target vessel instability in complex aneurysms. One potential solution to mitigate the risk of type IIIc endoleaks in the acute postoperative period is the implementation of physician-modified inner-branched endovascular repair [15–19]. However, the accessibility of this approach varied across centres, as demonstrated in this study, where 5 of the 10 centres utilized it. Another potential solution for target vessel instability is the utilization of potential dedicated covered stents for bridging stents, capable of accommodating the anatomical changes caused by postoperative remodelling. In the future, covered stents may provide better stability and compatibility with the dynamic nature of the vascular system after procedures [24].
For the treatment of complex aortic aneurysms, open repair and hybrid repair are widely accepted treatments [11, 25–27]. During the same period as this study, 242 open repairs and 41 hybrid repairs were performed for the treatment of complex aortic aneurysms in 10 Japanese centres. However, these procedures have a certain degree of morbidity and mortality. The relatively recent development of endovascular approaches to this problem has shown improved short-term morbidity and reasonable durability [28]. In this study, PMEG was used in patients in whom open repair was not amenable because of anatomical reasons or comorbidities. The low mortality rate in these difficult patients seems to be acceptable.
In this 10-centre cohort, the technical success rates per patient (95.9%) and vessel (98.5%) appeared to be acceptable. However, the relatively longer fluoroscopy time (121.2 ± 62.0 min) observed in PRAA procedures in comparison with that noted in a large-volume, single-centre study (103 ± 49 min) [4] may be attributed to its nascence in our countries. Additionally, our series included more than 10% of emergency cases, and the procedures for these cases are often affected by the necessity of performing other life-saving medical procedures concurrently. Some institutions had to use C-arms for these emergency and complicated procedures instead of hybrid operation suites. The limited accessibility of ancillary devices and imaging facilities, which might have significantly reduced procedural time and radiation dose, may also reflect the nascent stage of the adoption of the F/B-EVAR device in our country. The availability of F/B-EVAR devices and improvements in the reimbursement process should make the use of bare metal stents or limb extensions of EVAR devices obsolete. Despite these limitations, the short-term technical successes and in-hospital mortality were similar to those in a high-volume, single-centre study [4] and a systematic review [10].
This study has several limitations. First, it is a retrospective observational study, and the analyses were exploratory in nature. Moreover, the 95% confidence intervals were not adjusted for multiple comparisons, and the inferences drawn from them may not be reproducible. Second, the number of cases at each facility was small. Additionally, the procedures for the modifications and indications were not standardized. Third, the study results may need to be confirmed in other ethnic groups. Fourth, the angiograms and computed tomography scans were site-reported without core laboratory validation. However, acceptable operative results were obtained in our multicentre study in Japan, where commercial F/B-EVAR stent grafts and ancillary devices have not been officially introduced. Nevertheless, this study provides valuable insights into the application of F/B-EVAR technologies for the treatment of complex aortic aneurysms.
CONCLUSION
This multicentre observational study confirmed the reproducibility of acceptable PMEG results in repairing complex aneurysms in Japan where commercially available F/B-EVAR devices have not been officially introduced. Postoperative type IIIc endoleaks are associated with all-cause mortality and represent a universally important issue that needs to be addressed. For the treatment of TAAA, the use of PMEG might be restricted to emergency cases or to countries where commercially available F/B-EVAR devices have not been officially introduced.
ACKNOWLEDGMENTS
The authors thank Hiroki Uchiyama, MD, PhD and Kei Mukawa, MD for their cooperation in enrolling patients in this study.
Glossary
ABBREVIATIONS
- B-EVAR
Branched EVAR
- CTA
Computed tomography angiography
- EVAR
Endovascular aneurysm repair
- F-EVAR
Fenestrated EVAR
- PMEG
Physician-modified endografting
- PRAAs
Pararenal aortic aneurysms
- TAAA
Thoraco-abdominal aortic aneurysm
- TEVAR
Thoracic EVAR
Contributor Information
Tsuyoshi Shibata, Department of Cardiovascular Surgery, Sapporo Medical University, 291, Minami 1-jo Nishi 16-chome, Chuo-ku, Sapporo, Hokkaido, 060-8543, Japan.
Hiroshi Mitsuoka, Department of Cardiovascular Surgery, Shizuoka City Shizuoka Hospital, Shizuoka, Japan.
Yutaka Iba, Department of Cardiovascular Surgery, Sapporo Medical University, 291, Minami 1-jo Nishi 16-chome, Chuo-ku, Sapporo, Hokkaido, 060-8543, Japan.
Kenichi Hashizume, Department of Cardiovascular Surgery, Saiseikai Utsunomiya Hospital, Utsunomiya, Japan.
Norio Hongo, Department of Radiology, Oita University, Oita, Japan.
Kiyomitsu Yasuhara, Department of Cardiovascular Surgery, Isesaki Municipal Hospital, Isesaki, Japan.
Noriaki Kuwada, Department of Cardiovascular Surgery, Kawasaki Medical School, Kurashiki, Japan.
Yoshiaki Katada, Department of Radiology, Tokyo Medical University Ibaraki Medical Center, Ibaraki, Japan.
Hitoki Hashiguchi, Department of Cardiovascular Surgery, Hokkaido Prefectural Kitami Hospital, Kitami, Japan.
Takeshi Uzuka, Department of Cardiovascular Surgery, Sunagawa City Medical Center, Sunagawa, Japan.
Yuta Murai, Department of Cardiovascular Surgery, Shizuoka Medical Center, Shizuoka, Japan.
Tomohiro Nakajima, Department of Cardiovascular Surgery, Sapporo Medical University, 291, Minami 1-jo Nishi 16-chome, Chuo-ku, Sapporo, Hokkaido, 060-8543, Japan.
Junji Nakazawa, Department of Cardiovascular Surgery, Sapporo Medical University, 291, Minami 1-jo Nishi 16-chome, Chuo-ku, Sapporo, Hokkaido, 060-8543, Japan.
Nobuyoshi Kawaharada, Department of Cardiovascular Surgery, Sapporo Medical University, 291, Minami 1-jo Nishi 16-chome, Chuo-ku, Sapporo, Hokkaido, 060-8543, Japan.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
FUNDING
The authors received no financial support for the research, authorship or publication of this article.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest with respect to the research, authorship or publication of this article.
REFERENCES
- 1. Grassi V, Trimarchi S, Weaver F, de Beaufort HWL, Azzizzadeh A, Upchurch GR Jr,. GREAT participants et al. Endovascular repair of descending thoracic aortic aneurysms-a mid-term report from the Global Registry for Endovascular Aortic Treatment (GREAT). Eur J Cardiothorac Surg 2022;61:357–64. [DOI] [PubMed] [Google Scholar]
- 2. Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, Dutch Randomized Endovascular Aneurysm Management (DREAM)Trial Group et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004;351:1607–18. [DOI] [PubMed] [Google Scholar]
- 3. Gallitto E, Faggioli G, Pini R, Mascoli C, Ancetti S, Fenelli C. et al. Endovascular repair of thoraco-abdominal aortic aneurysms by fenestrated and branched endografts. Eur J Cardiothorac Surg 2019;56:993–1000. [DOI] [PubMed] [Google Scholar]
- 4. Chait J, Tenorio ER, Hofer JM, DeMartino RR, Oderich GS, Mendes BC.. Five-year outcomes of physician-modified endografts for repair of complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg 2023;77:374–85.e4. [DOI] [PubMed] [Google Scholar]
- 5. Starnes BW. Physician-modified endovascular grafts for the treatment of elective, symptomatic, or ruptured juxtarenal aortic aneurysms. J Vasc Surg 2012;56:601–7. [DOI] [PubMed] [Google Scholar]
- 6. Azuma T, Yokoi Y, Hayakawa N, Domoto S, Niinami H.. Multibranched endovascular repair using a modified endograft with hydrogel-reinforced fenestrations. Eur J Cardiothorac Surg 2022;62:ezac042. [DOI] [PubMed] [Google Scholar]
- 7. Stephen E, Joseph G, Sen I, Chacko S, Premkumar P, Varghese L. et al. A novel cautery instrument for on-site fenestration of aortic stent-grafts: a feasibility study of 18 patients. J Endovasc Ther 2013;20:638–46. [DOI] [PubMed] [Google Scholar]
- 8. Yang G, Zhang M, Zhang Y, Du X, Qiao T, Li X. et al. Endovascular repair of postdissection aortic aneurysms using physician-modified endografts. Ann Thorac Surg 2021;112:1201–8. [DOI] [PubMed] [Google Scholar]
- 9. Tong YH, Yu T, Zhou MJ, Liu C, Zhou M, Jiang Q. et al. Use of 3D printing to guide creation of fenestrations in physician-modified stent-grafts for treatment of thoracoabdominal aortic disease. J Endovasc Ther 2020;27:385–93. [DOI] [PubMed] [Google Scholar]
- 10. Gouveia E Melo R, Fernández Prendes C, Caldeira D, Stana J, Rantner B, Wanhainen A. et al. Systematic review and meta-analysis of physician modified endografts for treatment of thoraco-abdominal and complex abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2022;64:188–99. [DOI] [PubMed] [Google Scholar]
- 11. Ogino H, Iida O, Akutsu K, Chiba Y, Hayashi H, Ishibashi-Ueda H, Japanese Circulation Society, the Japanese Society for Cardiovascular Surgery, the Japanese Association for Thoracic Surgery and the Japanese Society for Vascular Surgery Joint Working Group et al. JCS/JSCVS/JATS/JSVS 2020 guideline on diagnosis and treatment of aortic aneurysm and aortic dissection. Circ J 2023;87:1410–621. [DOI] [PubMed] [Google Scholar]
- 12. Karthaus EG, Tong TML, Vahl A, Hamming JF, Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing Dutch Society of Vascular Surgery, the Steering Committee of the Dutch Surgical Aneurysm Audit and the Dutch Institute for Clinical Auditing Saccular abdominal aortic aneurysms: patient characteristics, clinical presentation, treatment, and outcomes in the Netherlands. Ann Surg 2019;270:852–8. [DOI] [PubMed] [Google Scholar]
- 13. Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 2002;35:1048–60. [DOI] [PubMed] [Google Scholar]
- 14. Dripps RD. New classification of physical status. Anesthesiology 1963;24:111. [Google Scholar]
- 15. D'Oria M, Mirza AK, Tenorio ER, Kärkkäinen JM, DeMartino RR, Oderich GS.. Physician-modified endograft with double inner branches for urgent repair of supraceliac para-anastomotic pseudoaneurysm. J Endovasc Ther 2020;27:124–9. [DOI] [PubMed] [Google Scholar]
- 16. Shibata T, Kawaharada N, Yasuhara K, Naraoka S.. Physician-modified stent graft with inner branches for treating ruptured thoracoabdominal aortic aneurysm. Eur J Cardiothorac Surg 2022;61:952–4. [DOI] [PubMed] [Google Scholar]
- 17. Shibata T, Iba Y, Nakajima T, Hosaka I, Kawaharada N.. Pararenal aortic aneurysm repair using a physician-modified stent-graft with inner branches. J Vasc Surg Cases Innov Tech 2022;8:356–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torrealba J, Panuccio G, Kölbel T, Gandet T, Heidemann F, Rohlffs F.. Physician-modified endograft with inner branches for the treatment of complex aortic urgencies. J Endovasc Ther 2022;29:697–704. [DOI] [PubMed] [Google Scholar]
- 19. Shibata T, Iba Y, Nakajima T, Nakazawa J, Ohkawa A, Hosaka I. et al. Initial outcomes of physician-modified inner branched endovascular repair in high-surgical-risk patients. J Endovasc Ther 2023;15266028231169183. [DOI] [PubMed] [Google Scholar]
- 20. Mitsuoka H, Terai Y, Miyano Y, Naitou T, Tanai J, Kawaguchi S. et al. Preoperative planning for physician-modified endografts using a three-dimensional printer. Ann Vasc Dis 2019;12:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oderich GS, Forbes TL, Chaer R, Davies MG, Lindsay TF, Mastracci T, Writing Committee Group et al. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J Vasc Surg 2021;73:4S–52S. [DOI] [PubMed] [Google Scholar]
- 22. Gennai S, Simonte G, Mattia M, Leone N, Isernia G, Fino G. et al. Analysis of predisposing factors for type III endoleaks from directional branches after branched endovascular repair for thoracoabdominal aortic aneurysms. J Vasc Surg 2023;77:677–84. [DOI] [PubMed] [Google Scholar]
- 23. Chait J, Tenorio ER, Mendes BC, Barbosa Lima GB, Marcondes GB, Wong J. et al. Impact of gap distance between fenestration and aortic wall on target artery instability following fenestrated-branched endovascular aortic repair. J Vasc Surg 2022;76:79–87.e4. [DOI] [PubMed] [Google Scholar]
- 24. de Niet A, Post RB, Reijnen MMPJ, Zeebregts CJ, Tielliu IFJ.. Geometric changes over time in bridging stents after branched and fenestrated endovascular repair for thoracoabdominal aneurysm. J Vasc Surg 2019;70:702–9. [DOI] [PubMed] [Google Scholar]
- 25. Coselli JS, LeMaire SA, Preventza O, de la Cruz KI, Cooley DA, Price MD. et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2016;151:1323–37. [DOI] [PubMed] [Google Scholar]
- 26. Kondov S, Frankenberger L, Siepe M, Keyl C, Staier K. et al. Results of open thoracoabdominal aortic replacement in patients unsuitable for or after endovascular repair with remaining disease components. Interactive CardioVascular and Thoracic Surg 2022;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shuto T, Wada T, Miyamoto S, Kamei N, Hongo N, Mori H. et al. Ten-year experience of the thoraco-abdominal aortic aneurysm treatment using a hybrid thoracic endovascular aortic repair. Interact CardioVasc Thorac Surg 2018;26:951–6. [DOI] [PubMed] [Google Scholar]
- 28. Liu T, Zhao J, Sun J, Wu K, Wang W.. Comparison of efficiency and safety of open surgery, hybrid surgery and endovascular repair for the treatment of thoracoabdominal aneurysms: a systemic review and network meta-analysis. Front Cardiovasc Med 2023;10:1257628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.