Abstract
Background
Rifampin-resistant tuberculosis is a leading cause of morbidity worldwide; only one-third of persons start treatment, and outcomes are often inadequate. Several trials demonstrate 90% efficacy using an all-oral, 6-month regimen of bedaquiline, pretomanid, and linezolid (BPaL), but significant toxicity occurred using 1200-mg linezolid. After US Food and Drug Administration approval in 2019, some US clinicians rapidly implemented BPaL using an initial 600-mg linezolid dose adjusted by serum drug concentrations and clinical monitoring.
Methods
Data from US patients treated with BPaL between 14 October 2019 and 30 April 2022 were compiled and analyzed by the BPaL Implementation Group (BIG), including baseline examination and laboratory, electrocardiographic, and clinical monitoring throughout treatment and follow-up. Linezolid dosing and clinical management was provider driven, and most patients had linezolid adjusted by therapeutic drug monitoring.
Results
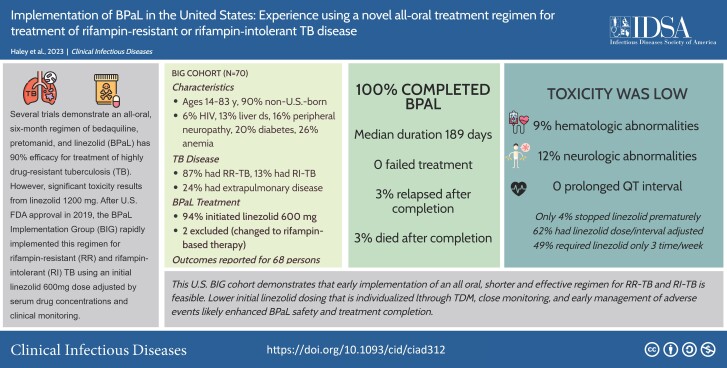
Of 70 patients starting BPaL, 2 changed to rifampin-based therapy, 68 (97.1%) completed BPaL, and 2 of the 68 (2.9%) experienced relapse after completion. Using an initial 600-mg linezolid dose daily adjusted by therapeutic drug monitoring and careful clinical and laboratory monitoring for adverse effects, supportive care, and expert consultation throughout BPaL treatment, 3 patients (4.4%) with hematologic toxicity and 4 (5.9%) with neurotoxicity required a change in linezolid dose or frequency. The median BPaL duration was 6 months.
Conclusions
BPaL has transformed treatment for rifampin-resistant or intolerant tuberculosis. In this cohort, effective treatment required less than half the duration recommended in 2019 US guidelines for drug-resistant tuberculosis. Use of individualized linezolid dosing and monitoring likely enhanced safety and treatment completion. The BIG cohort demonstrates that early implementation of new tuberculosis treatments in the United States is feasible.
Keywords: tuberculosis, drug resistance, bedaquiline, pretomanid, linezolid
An all-oral 6-month regimen of bedaquiline, pretomanid, and linezolid (BPaL) was implemented in the United States to treat rifampin-resistant and rifampin-intolerant tuberculosis. All patients safely completed treatment using close monitoring, linezolid serum drug levels, and early management of adverse events.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/implementation-of-bpal-in-the-united-states-experience-using-a-novel-all-oral-treatment-regimen-for-treatment-of-rifampin-resistant-or-rifampin-intolerant-tb-disease-dee64faf-9909-4a26-ac3a-bf8078a7a4a3
(See the Editorial Commentary by Acuña–Villaorduña and Sinha on pages 1063–4.)
In 2021, an estimated 10.6 million people developed tuberculosis, and 1.6 million died of the disease worldwide [1]. Outcomes are relatively poor among the 450 000 persons with at least rifampin-resistant (RR) tuberculosis, with only one-third starting treatment and a global treatment success rate of approximately 60% [1]. In the United States and its affiliated areas, 618 cases of RR tuberculosis in patients alive at the diagnosis were reported between 2014 and 2018 (Centers for Disease Control and Prevention [CDC], unpublished data). Only 62% completed treatment within 24 months, and 8% died before treatment completion. Of additional concern, persons living with RR tuberculosis face economic, psychological, and social costs [1–3].
Until recently, the US standard of care for patients with RR tuberculosis and rifampin-intolerant tuberculosis included ≥5 drugs in the intensive phase and 4 in the continuation phase, totaling 15–24 months duration [4]. Molecular (genotypic) and culture-based (phenotypic) drug susceptibility testing are used to identify effective drugs [4, 5]. Rifampin-sparing regimens require high pill burden, long duration, high toxicity of “second-line” drugs, complex monitoring, prolonged infectiousness, lengthy respiratory isolation, and profound psychosocial impacts on patients and their families [3, 4, 6].
In August 2019, the US Food and Drug Administration (FDA) approved an all-oral, 6-month regimen of bedaquiline, pretomanid, and linezolid (BPaL) for some patients with drug-resistant pulmonary tuberculosis based on data from the NIX-TB Trial conducted in South Africa [7–9]. Using a linezolid dose of 1200 mg daily, this trial found BPaL to be 90% effective against treatment-intolerant/nonresponsive multidrug-resistant (MDR) tuberculosis (resistant to both rifampin and isoniazid) and extensively drug-resistant tuberculosis (XDR tuberculosis), defined as MDR plus resistance to both fluoroquinolones and injectable agents using pre-2021 definitions from the World Health Organization (WHO) [10]). However, linezolid caused significant hematologic and neurologic toxicity, and >80% of patients experienced an adverse event.
In October 2019, some US tuberculosis physicians began prescribing BPaL using 600 mg of linezolid daily along with therapeutic drug monitoring (TDM). The BPaL Implementation Group (BIG) was convened with the goal of compiling and disseminating clinical information about the US experience with BPaL. We report the real-world management and outcomes of US patients treated with BPaL in 2019–2022.
METHODS
BIG Cohort Development
We collected data on patients who had RR tuberculosis or rifampin-intolerant tuberculosis diagnosed and were treated with BPaL between 14 August 2019 and 30 April 2022, regardless of the anatomic site of disease or indication for BPaL. Patients were managed by their treating clinician, with consultation available from the CDC's tuberculosis Centers of Excellence (https://www.cdc.gov/tb/education/tb_coe/) and local experts. Patients were educated and included in the decision to use the novel BPaL regimen.
BPaL Treatment and Monitoring
Treatment included bedaquiline 400 mg daily for 14 days then 200 mg thrice weekly (TIW), pretomanid 200 mg daily, and linezolid with provider-determined dosing, supervised with directly observed therapy [4, 11]. Providers used existing guidelines and protocols for treatment and monitoring of patients with drug-resistant tuberculosis [4, 7, 12], but management was not standardized.
Before treatment, patients underwent history, physical examination, laboratory testing (including hemogram, human immunodeficiency virus [HIV] serology, pregnancy test, chest radiography, electrocardiography, and blood biochemistry, with magnesium and metabolic, liver panel, and thyroid panels), and visual acuity testing. Patients were typically assessed monthly for treatment response and adverse effects. Providers monitored the QT interval (Fridericia formula QTcF), facilitated in some patients by using a KardiaMobile personal ECG (AliveCor). Chest radiography was usually repeated 2 months after treatment initiation and at the end of therapy. For pulmonary tuberculosis, sputum samples were examined for acid-fast bacilli and cultured for Mycobacterium tuberculosis, normally at least monthly. After discontinuation of BPaL, providers aimed to follow up patients for relapse and resolution of adverse events for ≥2 years.
Laboratory Assessment
Laboratory identification and drug susceptibility testing for M. tuberculosis was performed by the CDC's Division of Tuberculosis Elimination Laboratory Branch (Atlanta, Georgia) and Florida's Bureau of Public Health Laboratories (FLBPHL; Jacksonville). The CDC performed molecular detection of drug resistance using DNA sequencing to detect mutations associated with resistance to rifampin (rpoB), isoniazid (katG, fabG1, and inhA), pyrazinamide (pncA), ethambutol (embB), fluoroquinolones (gyrA and gyrB), and the injectable drugs amikacin (rrs), kanamycin (rrs and eis), and capreomycin (rrs and tlyA) [13]. Phenotypic testing was performed using the indirect agar proportion method, as described elsewhere [14]. The FLBPHL performed molecular detection of drug resistance, using Sanger sequencing to detect mutations associated with linezolid (rrl and rplC) and bedaquiline (atpE or rv0678) resistance in addition to those tested by CDC’s molecular detection of drug resistance (B. Jones, personal communication, May 1, 2023). Phenotypic susceptibilities were determined using a customized Sensititre (Trek Diagnostics System; Thermo Fisher Scientific) broth microdilution plate for first- and second-line drugs.
Linezolid minimum inhibitory concentrations (MICs) ≤ 1 µg/mL were considered susceptible; MICs were not available for bedaquiline or pretomanid when these patients started BPaL. Therapeutic drug monitoring for linezolid using patient blood samples was performed at the University of Florida Infectious Disease Pharmacokinetics Laboratory using liquid chromatography–tandem mass spectrometry (with a Thermo Endura tandem mass spectrometer and a Dionex Ultimate 3000 ultra-high-performance liquid chromatography system. The recommended sampling times were a predose trough followed by 2- and 6-hour postdose samples or, alternatively, 2-, 6-, and 24-hour samples following a single daily dose. If linezolid is given TIW, the recommended trough sampling time is 48 hours after the last dose. Because oral drugs can display delayed absorption for various reasons, 2 postdose samples improve the probability of estimating the maximum plasma concentration. The trough is most closely linked to toxicity. Two or (preferably) 3 samples also allow for a reasonable estimation of area under the curve. Clinicians typically adjusted the linezolid dose and/or dosing interval targeting a predose trough concentration of <2 µg/mL and peak concentration of 12–26 µg/mL 2–6 hours after the dose.
Data Collection and Definitions
The treating teams abstracted data from medical records and securely transmitted data to the University of Florida. The principal investigator verified and categorized data into consistent categories, including demographics, comorbid conditions, tuberculosis disease characteristics, treatment, and monitoring.
Drug resistance was classified using the pre-2021 WHO definitions in place when this cohort was created (MDR, pre-XDR [defined as MDR plus resistance to either fluoroquinolones or injectable agents], and XDR) [10]. Baseline anemia was defined as having a documented diagnosis or hemoglobin level <13.2 µg/dL for men or <11.6 µg/dL for women; thrombocytopenia, as platelet count <150 000/µL, and leukopenia as leukocyte count <4000/µL. Hematologic toxicity was defined by the treating provider as a clinically significant change in hemoglobin, platelet count, or white blood cell count from baseline. Baseline neuropathy required a documented diagnosis; neurologic toxicity was defined as any new or worsened neurologic symptoms during treatment. Culture conversion was defined as having 2 consecutively negative cultures obtained 30 days apart, and treatment failure was defined as lack of culture conversion after 4 months of BPaL or culture reversion to positive with 2 consecutive samples 30 days apart [15]. QT interval prolongation was defined as an absolute QTcF >500 ms or an increase from baseline of >60 ms. BPaL treatment interruption was defined as the number of consecutive days of missing both bedaquiline and pretomanid.
The University of Florida Institutional Review Board (IRB) determined this study to be research exempt from additional review (no. IRB202002323). A data use agreement was enacted between the University of Florida and each contributing site. CDC IRB approval was not required because CDC involvement was limited to assistance with data interpretation and manuscript writing.
RESULTS
Baseline Cohort Characteristics
Seventy patients in 12 states and US territories were included in this cohort. Their median age at diagnosis was 37 years (range, 14–83 years), and their median weight before BPaL treatment was 58.0 kg (range, 40.0–132.7 kg). Most patients were male (n = 46 [65.7%]), non–US-born (n = 63 [90%]), nonwhite (n = 54 [77.9%]), and not Hispanic (n = 59 [84.3%]) (Table 1). Comorbid conditions before BPaL use included anemia (n = 17 [24.2%]), diabetes (n = 28 [20%]), neuropathy (n = 11 [15.7%]), liver disease/alcohol use disorder (n = 9 [12.9%]), renal disease (n = 7 [10%]), hypothyroidism (n = 5 [7.1%]), and HIV infection (n = 4 [5.7%]). Five patients (7.1%) reported prior tuberculosis treatment, and 2 others (2.9%) arrived in the United States on inadequate MDR tuberculosis treatment and were changed to BPaL treatment.
Table 1.
Baseline Patient Characteristics (N = 70)
| Characteristic | Patients, No. (%)a |
|---|---|
| Age, median (range), y | 37 (14–83) |
| Age group | |
| <25 y | 12 (17.4) |
| 25–44 y | 31 (44.3) |
| 45–64 y | 14 (20.0) |
| ≥65 y | 13 (18.6) |
| Male sex | 46 (65.7) |
| Race | |
| White | 16 (22.9) |
| Black | 9 (12.9) |
| Asian | 45 (64.3) |
| Hispanic ethnicity | 11 (15.7) |
| Born outside the United States | 63 (90.0) |
| Baseline comorbid conditions | |
| Baseline weight, median (range), kg | 58.0 (40.0–132.7) |
| HIV infection | 4 (5.7) |
| Diabetes | 14 (20.0) |
| Renal disease | 7 (10.0) |
| Liver disease or alcohol abuse | 9 (12.9) |
| Anemia | 18 (25.7) |
| Neuropathy | 11 (15.7) |
| Immunosuppression | 2 (2.9) |
| Cancer | 2 (2.9) |
| Hypothyroid | 5 (7.1) |
| Tuberculosis disease characteristics | |
| Prior tuberculosis treatment | 5 (7.1) |
| Inadequate MDR tuberculosis treatment on arrival in the United States | 2 (2.9) |
| Drug resistanceb | |
| Rifamycin susceptiblec | 9 (12.9) |
| Rifampin monoresistant | 7 (10.0) |
| MDR | 43 (61.4) |
| Pre-XDR | 10 (14.3) |
| XDR | 1 (1.4) |
| Site of tuberculosis | |
| Pulmonary only | 53 (75.7) |
| Extrapulmonary only | 7 (10.0) |
| Both pulmonary and extrapulmonary | 10 (14.3) |
| Total pulmonary | 63 (90.0) |
| Sites of extrapulmonary tuberculosis | 17 (24.3) |
| Male genitourinary tract | 2 (2.9) |
| Male genitourinary tract and pelvic bone | 1 (1.4) |
| Spine | 2 (2.9) |
| Spine and miliary | 1 (1.4) |
| Intrathoracic adenopathy, 3 ribs and iliac crest | 1 (1.4) |
| Chest wall musculature | 1 (1.4) |
| Chest wall and pleural | 1 (1.4) |
| Peritoneal | 1 (1.4) |
| Mediastinal and hilar adenopathy | 1 (1.4) |
| Cervical lymphadenopathy | 4 (5.7) |
| Cervical lymphadenopathy and pleural | 1 (1.4) |
| Adenopathy, unspecified | 1 (1.4) |
| Cavitation on chest radiograph in patients with pulmonary tuberculosis (n = 63) | 29 (46.0) |
| Positive sputum AFB smear in patients with pulmonary tuberculosis (n = 63) | 34 (54.0) |
| Positive mycobacterial culture, any site | 67 (95.7) |
| Positive sputum culture | 50 (71.4) |
| Linezolid MICs reported (n = 61) | |
| 0.12 µg/mL | 2 (3.3) |
| 0.25 µg/mL | 22 (36.1) |
| 0.5 µg/mL | 30 (49.2) |
| 1.0 µg/mL | 7 (11.5) |
| Molecular detection of drug resistance results reported | 61 (87.1) |
| Reported by the FLBPHLd | 55 (78.6) |
| Reported by the CDC | 33 (47.1) |
Abbreviations: AFB, acid-fast bacilli; CDC, Centers for Disease Control and Prevention; FLBPHL, Florida Bureau of Public Health Laboratories; HIV, human immunodeficiency virus; MDR, multidrug-resistant; MICs, minimum inhibitory concentrations; XDR, extensively drug resistant.
Data represent no. (%) of patients unless otherwise specified.
Using pre-2021 World Health Organization definitions [10], MDR tuberculosis was defined as resistance to both isoniazid and rifampin; pre-XDR tuberculosis, as MDR plus resistance to an injectable or a fluoroquinolone; and XDR tuberculosis, as MDR plus resistance to both an injectable and a fluoroquinolone.
The drug-susceptible tuberculosis category includes 2 patients with initial molecular results suggesting rifampin resistance but with phenotypic results demonstrating susceptibility to rifampin.
Among these 55 patients, atpE and rv0678 failed to amplify for 1 patient each.
Tuberculosis Disease Characteristics
Anatomically, 53 (75.6%) patients had pulmonary tuberculosis, 7 (10.0%) had extrapulmonary tuberculosis, and 10 (14.2%) had both (Table 1). Half of those with pulmonary disease had acid-fast bacilli detected on sputum smear (n = 34, 54.0%), and 29 (46%) had cavitation at radiography. Rifampin monoresistance was reported for 9 patients (12.9%), MDR for 43 (61.4%), pre-XDR for 10 (14.3%), and XDR for 1 patient (1.4%). Three patients (4.2%) with MDR tuberculosis had negative cultures at diagnosis; 1 case was diagnosed based on molecular results, and 2 were close contacts to persons with culture-confirmed MDR tuberculosis. An additional patient inadvertently received rifampin monotherapy for latent tuberculosis infection before initial cultures grew and isoniazid-resistant tuberculosis was diagnosed; this patient was empirically treated with BPaL because subsequent cultures were negative. Seven patients (10%) received BPaL for drug-susceptible tuberculosis because of rifamycin intolerance (Box 1).
Box 1. Reasons for Treatment With Bedaquiline, Pretomanid, And Linezolid Instead of a Rifamycin-Based Regimen in 7 Patients With Rifampin-Susceptible Tuberculosisa.
Anaphylaxis during rifampin treatment for latent tuberculosis infection
Significant drop in hemoglobin level and elevated transaminase levels with fatigue, shortness of breath, and tachycardia during rifamycin treatment
“Intolerant” of rifamycins, pyrazinamide, and fluoroquinolones
Severe cytopenia with fever during rifamycin treatment
Severe gout, pancreatitis, transaminitis, and acute kidney injury (possibly autoimmune) during rifamycin treatment but toleration of BPaL with steroids
Severe neutropenia with both isoniazid and rifamycins
Known resistance to isoniazid, pyrazinamide, and ethambutol but not rifamycins at initial molecular testing; given concern for additional rifampin resistance, BPaL was started while waiting for final phenotypic drug susceptibility testing and was completed even though rifampin was reported susceptible by MIC
Abbreviations: BPaL, bedaquiline, pretomanid, and linezolid; MIC, minimum inhibitory concentration.
aThis box does not include the 2 patients who started BPaL based on initial molecular results and then were changed to a rifampin-based regimen when rifampin susceptibility was determined by phenotypic results.
Linezolid MIC values were reported for 61 (87%) patients, with MICs of 0.12–1.0 µg/mL (Table 1). Among 55 patients with FLBPHL molecular results, no mutations known to be associated with bedaquiline resistance were detected at baseline, and no patients had linezolid resistance-conferring mutations. One patient had a point mutation (Val144Ala; GTG/GCG) in rplC, but the organism was linezolid susceptible at phenotypic testing (MIC, 0.5 µg/mL)
BPaL Treatment and Linezolid Dosing
For 19 patients (27.1%), BPaL was their only tuberculosis treatment regimen (Table 2). Rifamycin-based treatment was the initial regimen for 29 (41.4%). A conventional longer regimen for RR tuberculosis regimen was administered to 33 patients (47.1%) before BPaL. All but 4 patients (94.3%) started BpaL with a linezolid dosage of 600 mg daily; 1 started with 900 mg daily, 2 with 1200 mg daily, and 1 with 600 mg TIW owing to peripheral neuropathy. No patients received other tuberculosis drugs concurrently with BPaL. Two patients changed from BPaL to rifampin-based therapy based on phenotypic susceptibility results and were excluded from subsequent analyses.
Table 2.
Tuberculosis Treatment Characteristics (N = 70)
| Characteristic | Patients, No. (%) |
|---|---|
| Treatment before BPaL | |
| None | 19 (27.1) |
| Rifampin-based regimena | 29 (41.4) |
| Other regimen for rifampin-resistant tuberculosisb | 33 (47.1) |
| Initial BPaL treatment regimen (N = 70) | |
| Initial linezolid dose 600 mg daily | 66 (94.3) |
| Prescribed other tuberculosis drugs simultaneously with BPaL | 0 |
| BPaL stopped after rifampin resistance was excluded by phenotypic drug susceptibility testing | 2 (2.9) |
| Linezolid dosing adjustments before or during BPaL (n = 68)c | |
| Serum drug concentrations obtained for TDM, any reason | 66 (97.1) |
| Dose or frequency adjusted, any reason | 42 (61.8) |
| Adjusted based on TDM | 36 (52.9) |
| Adjusted based on provider decision followed by TDM | 6 (8.8) |
| Trough >2 µg/mL with 600 mg daily | 20 (29.4) |
| Dose or frequency adjusted without symptoms | 14 (20.6) |
| Dose or frequency adjusted with symptoms | 4 (5.7) |
| Dose or frequency not adjusted with symptoms | 2 (2.9) |
| Dose >600 mg required to reach therapeutic range (12–26 µg/mL) | 20 (30.9) |
| Final linezolid dose used during BPaL (n = 68)d | |
| 600 mg daily | 27 (39.7) |
| 600 mg TIW | 21 (30.9) |
| 900 mg daily | 8 (11.8) |
| 900 mg TIW | 10 (14.7) |
| 1200 mg TIW alternating with 600 mg QIW | 1 (1.5) |
| 1200 mg daily | 0 |
| 1200 mg TIW | 1 (1.5) |
Abbreviations: BPaL, bedaquiline, pretomanid, and linezolid; QIW, 4 times weekly (on Tuesday, Thursday, Saturday, and Sunday); TDM, therapeutic drug monitoring; TIW, thrice weekly (on Monday, Wednesday, and Friday).
Rifampin-based regimens include any combination of drugs including rifampin that were used to treat presumed drug-susceptible tuberculosis. The treatment durations for these regimens were not collected. Patients may have received both a rifamycin-based regimen and another regimen for drug resistance before BPaL.
Rifampin-resistant tuberculosis includes resistance to at least rifamycins.
Excludes 2 patients who stopped BPaL after diagnosis of drug-susceptible tuberculosis. Some patients had linezolid started and adjusted before starting BPaL.
This is the linezolid dose and frequency at which the patient completed therapy after potential adjustments based on symptoms or TDM results. A denominator of 68 was used rather than 66 (the number with TDM results) because some patients had linezolid adjusted based on symptoms alone.
Among the remaining 68 patients, TDM was performed in 66 (97.1%) (Table 2). The linezolid dose was changed from 600 mg daily for 42 (61.6%) individuals, based on TDM results in 36 (52.9%) and provider decision in 6 (8.8%). In 20 patients (29.4%), the linezolid trough on 600 mg daily was >2 µg/mL, and 20 patients (29.4%) had serum peak concentrations below the target range of 12–26 µg/mL.
BPaL Treatment Effectiveness
All 68 patients completed their prescribed duration of BPaL, 50 (73.5%) with no treatment interruption (Table 3). No patients were lost to follow-up or died during treatment, and none had failed treatment. Ten (14.7%) had the BPaL duration extended to >39 weeks for bone involvement (7.4%), extensive tuberculosis disease/delayed culture conversion (4.4%) or nonadherence (2.9%). Overall, the median time from the first to the last dose of BPaL was 26.9 weeks (range, 112–325 days). Among 14 patients with pulmonary tuberculosis who received only BPaL and had serial cultures obtained, the median time to culture conversion was 37 days (range, 1–90 days).
Table 3.
Outcomes for Treatment With Bedaquiline, Pretomanid, and Linezolid (n = 68)a
| Outcome | Patients, No. (%)b |
|---|---|
| Completed prescribed course of BPaL | 68 (100) |
| Completed 26 wk of BPaL | 55 (80.9) |
| Completed <26 wk of BPaL | 3 (4.4) |
| Rifampin-intolerant drug-susceptible tuberculosis; treatment included 70 d of rifampin-based therapy followed by 112 d of BPaL (total, 26 wk) | 1 (1.5) |
| Rifampin-intolerant drug-susceptible tuberculosis; treatment included 3 mo of rifampin-based therapy followed by 165 d of BPaL (total, >26 wk) | 1 (1.5) |
| Completed 24 wk owing to bedaquiline prescription error | 1 (1.5) |
| Completed >26 wk of BPaL | 10 (14.7) |
| Tuberculosis involving bone | 5 (7.4) |
| Significant burden of disease or culture conversion >60 d from start of BPaL | 3 (4.4) |
| Nonadherence/prolonged treatment interruption | 2 (2.9) |
| Time from first to last BPaL dose, median (range), d | 188.5 (112–325) |
| Treatment interruption during BPaL (any)c | 18 (26.5) |
| Treatment interruption during BPaL, consecutive days | |
| <7 d | 4 (5.9) |
| 7–13 d | 6 (8.8) |
| 14–20 d | 2 (2.9) |
| 21–27 d | 3 (4.4) |
| ≥28 d | 2 (2.9) |
| Not reported | 1 (1.5) |
| Time to culture conversion, median (range), d (n = 14)d | 37 (1–90) |
| Hematologic and neurologic events during BPaL (n = 68) | |
| Linezolid discontinued before completion of full BPaL regimen | 3 (4.4) |
| Occurrence of both hematologic and neurologic events requiring linezolid change or discontinuation | 1 (1.5) |
| Occurrence of only hematologic events requiring linezolid change or discontinuation | 3 (4.4) |
| Occurrence of only hematologic events not requiring linezolid change or discontinuation | 2 (2.9) |
| Occurrence of only neurologic symptoms requiring linezolid change or discontinuation | 3 (4.4) |
| Neurologic symptoms not requiring change or discontinuation of linezolid | 5 (7.4) |
| Elevated liver enzyme levels (>5 times ULN)e | 2 (2.9) |
| Lactic acidosis during BPaL treatment | 0 |
| Other symptoms not requiring change in BPaL regimen (n = 68) | |
| Gastrointestinal (nausea, vomiting, diarrhea or abdominal discomfort) | 14 (20.6) |
| Rash or pruritis | 8 (11.8) |
| Elevated liver enzyme levels (>3 times ULN) | 7 (10.3) |
| Anxiety or panic attack | 4 (5.9) |
| Fatigue | 3 (4.4) |
| Hair loss | 2 (2.9) |
| Black hairy tongue | 1 (1.5) |
| Yellow-brown teeth discoloration | 1 (1.5) |
| Dactylitis and tremor | 1 (1.5) |
| QTc interval >500 ms or increase of >60 ms | 0 (0) |
| Duration of follow-up after completion of BPaL treatment without recurrent tuberculosis (n = 68)f | |
| ≥6 mo | 55 (80.9) |
| ≥12 mo | 36 (52.9) |
| ≥24 mo | 19 (27.9) |
| Lost to follow-up after treatment without any follow-up | 2 (2.9) |
| Lost to follow-up after the 6-mo posttreatment visit | 3 (4.4) |
| Died after completion of BPaL treatment (n = 68)g | 2 (2.9) |
| Relapse after completion of full BPaL regimen (n = 68)h | 2 (2.9) |
Abbreviations: BPaL, bedaquiline, pretomanid, and linezolid; QTc, QT interval; ULN, upper limit of normal.
Of the initial 70 patients, 2 discontinued BPaL when drug-susceptible tuberculosis was confirmed.
Data represent no. (%) of patients unless otherwise specified.
BPaL treatment interruption was defined as missing doses of bedaquiline and pretomanid and does not include holding linezolid for a few days before changing dosing frequency.
Culture conversion from date of initial positive tuberculosis culture to date of first consecutively negative culture was calculated in patients with only pulmonary disease who had no tuberculosis treatment before BPaL and had a documented sputum culture conversion (2 consecutively negative cultures taken 30 days apart).
Liver enzyme levels were elevated to >5 times the ULN in 2 patients. The first patient was a 24 years old male who reported no liver disease but was taking ethambutol, pyrazinamide, moxifloxacin, and clofazimine at U.S. entry (had stopped high-dose isoniazid, prothionamide, and bedaquiline one-month prior) and had a total bilirubin 1.3 g/dL (normal range 0.2-1.0 mg/dL) and alanine transaminase (ALT) 69 units/dL (normal range <64 units/L) prior to starting BPaL. Three weeks after the start of BPaL treatment, the patient was asymptomatic with ALT and aspartate transaminase (AST) levels of 186 and 372 µg/mL, respectively. BPaL treatment was held 1 week and then restarted with ALT and AST levels of 82 and 45 µg/mL, respectively, and he completed 26 weeks of BPaL without further laboratory or clinical abnormalities. The second patient, a 26 years old female, had type I diabetes but no known liver disease. Prior to BPaL, she was treated with levaquin, rifabutin, pyrazinamide, ethambutol, and linezolid for about 8 weeks. Laboratory values one month prior to BPaL included AST 25 U/L, ALT 74 U/L, bilirubin 0.26 mg/dL and alkaline phosphatase 99 IU/L (normal ranges not provided) One month after BPaL treatment initiation, she became critically ill with COVID-19, requiring prolonged hospitalization, with peak ALT and AST levels of 450 and 141 µg/mL, respectively. BPaL treatment was held for 8 weeks and then restarted with normal AST and ALT levels; a 26-week course of BPaL treatment was completed.
The denominator of n = 68 excludes the 2 patients who changed from to rifampin-based tuberculosis therapy. Five patients have not had their 6-month follow-up visit yet, 2 died after completion of BPaL treatment, precluding follow-up, and 1 had a relapse and remains on therapy.
One patient died after treatment completion with no evidence of tuberculosis relapse, and 1 died after relapse occurred but before tuberculosis treatment was restarted.
Relapse is considered to occur when a patient has completed tuberculosis treatment without declaration of treatment failure and subsequently receives a diagnosis of tuberculosis requiring repeated treatment, with evidence indicating that the recurrence is due to the same strain recorded for the baseline specimen. The first of 2 patients with relapse had extensive cavitary pulmonary disease resistant to rifampin, ethambutol, and fluoroquinolones and had no human immunodeficiency virus (HIV) or diabetes. This patient showed clinical improvment with culture conversion at 90 days and completed 26 weeks of BPaL treatment, with directly observed therapy (DOT). Culture-confirmed relapse occurred approximately 6 months after completion of BPaL treatment. The minimum inhibitory concentrations (MICs) both before treatment and after relapse were 0.12 ug/mL, 0.5 µg/mL, and 0.125 µg/mL, respectively (ie, no MIC increase for BPaL drugs). Similarly, samples before treatment and after relapse showed no linezolid-associated mutations (rplC or rrl) or bedaquiline atpE mutations. Retrospectively, both samples had a detectable bedaquiline Pro48Leu rv0678 mutation which has unknown clinical significance [16]. The patient is being treated with BPaL and pyrazinamide and continues to be closely monitored.
The second patient with relapse was an inmate in a correctional facility at diagnosis. The patient was an alcoholic with past cocaine use, had no HIV or diabetes, and had cavitary tuberculosis resistant to isoniazid, rifampin, pyrazinamide, and ethambutol. The patient was transferred to the hospital, treated with a second-line regimen for 6 months, and acquired new fluoroquinolone resistance. The linezolid MIC increased from 0.5 to 1.0 µg/mL before culture conversion occurred at 84 days. The patient was discharged to home, started BPaL (linezolid, 600 mg/d) for 3 weeks, and then was lost to follow-up, followed by reincarceration and detoxification with a 3-week treatment interruption. The patient completed 14 weeks of BPaL treatment while incarcerated, then 9 weeks in the community, for a total of 26 weeks (all by DOT). Seven months later, this patient was hospitalized with respiratory distress requiring mechanical ventilation, bilateral cavitary pneumonia, and bloody stools; the patient did not report recent tuberculosis diagnosis or treatment and sputum smears were acid-fast bacilli (AFB) negative. The patient’s condition improved during treatment with linezolid, piperacillin-tazobactam, and high-dose steroids. Mycobacterium tuberculosis grew in the admission sputum culture after 8 weeks, by which time the patient’s respiratory status had deteriorated. Repeated sputum, urine, and stool samples were AFB positive. The patient experienced respiratory arrest and died in the hospital before antituberculosis therapy could be initiated. Molecular detection of drug resistance on relapse isolate indicated 2 rv0678 frame shift mutations and a bedaquiline critical concentration (CC) of 1 µg/mL; the linezolid MIC was unchanged at 1.0 µg/mL. Prerelapse isolate testing for bedaquiline resistance is pending at the time of writing.
BPaL Treatment Adverse Effects
Four patients with baseline anemia required a blood transfusion during linezolid treatment; the linezolid dosage was changed from 600 mg daily to TIW (Table 4), and 1 patient discontinued linezolid at week 23 of BPaL. Three had a linezolid trough concentration >2 µg/mL, and 1 did not have TDM. One of these patients with a high linezolid trough also reported blurry vision that resolved with transfusion and change to TIW linezolid. Two other patients experienced a decrease in hemoglobin during BPaL; both had low linezolid trough concentrations, and the linezolid dose/frequency was not changed.
Table 4.
Hematologic and Neurologic Events During Treatment With Bedaquiline, Pretomanid, and Linezolid (n = 68)
| Event and Patient Descriptions | Linezolid Trough Concentration |
|---|---|
| Both hematologic and neurologic events requiring linezolid change or discontinuation (n = 1) | |
| 69 y female with diabetes, breast cancer (treatment unknown), baseline hemoglobin 10.6 g/dL, and peripheral neuropathy (in fingers and toes); reported blurry vision and received transfusion after 13 d of linezolid 600 mg/d; high serum trough concentration; linezolid changed to 600 mg TIW; no further transfusions or symptoms and full BPaL regimen completed | 11.6 µg/mL |
| Only hematologic events requiring linezolid change or discontinuation (n = 3) | |
| 83 y female, with diabetes, untreated hypothyroidism, and baseline hemoglobin 8.0 g/dL; required transfusions before and 10 d after starting linezolid at 600 mg/d; platelet counts “decreasing”; high serum trough concentration; linezolid changed from 600 mg/d to 600 mg TIW; no further transfusions or symptoms and full BPaL regimen completed | 9.96 µg/mL |
| 70 y male with baseline gout; admitted with transaminitis, pancreatitis, and anemia on rifampin, isoniazid, pyrazinamide, and ethambutol; after improvement, treatment was changed to BPaL (linezolid, 600 mg/d); in 5th week of BPaL, readmission with recurrent transaminitis, pancreatitis, and anemia requiring 1-unit transfusion of RBCs; steroids given for possible autoimmune etiology; with high trough concentration, linezolid changed to 600 mg TIW; around wk 23, linezolid was discontinued owing to hemoglobin level of 6.9 g/dL; bedaquiline and pretomanid treatment completed | 2.9 µg/mL |
| 63 y male with alcoholic cirrhosis, oxygen-dependent lung disease, and baseline anemia with hemoglobin level 8 g/dL, required transfusion before starting linezolid 600 mg/d and again 1 mo after starting; dosage changed to linezolid 600 mg TIW through completion of BPaL regimen | Not done |
| Only hematologic events not requiring linezolid change or discontinuation (n = 2) | |
| 76 y male; linezolid changed empirically from 600 mg/d to 600 mg TIW after 12 d, owing to baseline untreated diabetes and renal disease; low linezolid trough at 48 h; later in therapy, hemoglobin decreased from baseline of 15.4 to 12.3 g/dL and platelet count decreased from 173 109/L to 97 × 109/L, then stabilized; BPaL regimen completed without further linezolid changes | 0.39 µg/mL |
| 58 y male with diabetes and chronic hepatitis B; linezolid 900 mg/d started 5 mo before bedaquiline and pretomanid; hemoglobin level of 14.2 g/dL 6 mo after linezolid initiation; linezolid trough was trace; 2 mo later, provider documented “anemia”; linezolid was continued at 900 mg/d, and 26 wk of BPaL treatment was completed | Trace |
| Only neurologic symptoms requiring linezolid change or discontinuation (n = 3) | |
| 76 y male with diabetes, stage 3 chronic kidney disease, and baseline peripheral neuropathy; reported blurry vision after starting linezolid 600 mg/d, but vision examination and Isahara test results were unchanged; vision resolved with change to 600 mg TIW; full BPaL regimen completed | 9.3 µg/mL |
| 81 y female with diabetes, hypothyroidism, and vitamin B12 deficiency; started linezolid 600 mg/d; discontinued linezolid at 12 wk for worsened neuropathy despite 1-wk trial of 600 mg TIW; completed bedaquiline and pretomanid treatment | 1.13 µg/mL |
| 50 y female with smoking-related chronic lung disease, hypothyroidism, and opioid use disorder; developed persistent hand numbness and discontinued linezolid 600 mg/d at 24 wk without trial of 600 mg TIW; completed bedaquiline and pretomanid treatment | 0.3 µg/mL |
| Neurologic symptoms not requiring change or discontinuation of linezolid (n = 5) | |
| 18 y female reported new numbness in toes; linezolid continued at 600 mg/d; symptoms resolved after completion of BPaL regimen | 3.3 µg/mL |
| 50 y female with baseline anxiety; reported transient tingling in face and scalp and intermittent numbness/tingling in eyes and fingers; symptoms resolved; linezolid continued at 600 mg/d until completion of BPaL treatment | 2.4 µg/mL |
| 25 y male; no symptoms on 600 mg/d but reported numbness and tingling in 2 toes approximately 10 wk after linezolid was increased to 900 mg/d; symptoms persisted throughout treatment but resolved after BPaL completion | 1.5 and 2.03 µg/mLa |
| 37 y male with HIV; linezolid increased from 600 to 1200 mg TIW based on TDM, with linezolid trough of 0.1 µg/mL at 48 h; reported arm numbness and weakness that resolved by the end of BPaL treatment | 0.1 µg/mL |
| 56 y female with vitamin B12 deficiency; experienced 2 d of tingling in fingertips when gardening, which never recurred; completed BPaL treatment with linezolid 600 mg/d | 1.3 µg/mL |
Abbreviations: BPaL, bedaquiline, pretomanid, and linezolid; DOT, directly observed therapy; HIV, human immunodeficiency virus; RBCs, red blood cells; TDM, therapeutic drug monitoring; TIW, thrice weekly (Monday, Wednesday, and Friday).
With regard to neurologic events, 2 patients discontinued linezolid prematurely for worsening peripheral neuropathy despite trough concentrations <2 µg/mL; bedaquiline and pretomanid were completed. One patient experienced neurologic symptoms and had a linezolid trough concentration >2 µg/mL; symptoms resolved with a change from linezolid 600 mg daily to TIW and the patient completed a full course of BPaL. Transient numbness and tingling of extremities were also reported in 5 patients with varying trough concentrations but did not require linezolid dose or frequency adjustment (Table 4). Other minor adverse effects included gastrointestinal symptoms (n = 14 [20.6%]), rashes (n = 8 [11.8%]), and anxiety (n = 4 [5.9%]). In 7 patients (10.3%), serum aspartate aminotransaminase and/or alanine aspartate aminotransaminase levels increased to >3 times the upper limit of normal (40 µg/mL), and 2 had a level >5 times the upper limit of normal (Table 3). None had prolonged QTcF interval or lactic acidosis.
Follow-up After BPaL Completion
At the time of writing, 55 of 68 patients (80.9%) who completed BPaL had ≥6 months of follow-up without relapse, 36 (52.9%) had ≥12 months, and 19 (27.9%) had ≥24 months. Two patients (2.9%) were lost to follow-up after BPaL treatment completion, and 3 (4.4%) were lost to follow-up after 6 months of follow-up. Of the remaining 65, all but 2 (96.9%) are still alive; 2 experienced a relapse of tuberculosis disease (Table 3).
DISCUSSION
We describe a cohort of 70 US patients treated with BPaL for RR or rifampin-intolerant tuberculosis disease under program conditions. Preliminary data on early outcomes in 16 of these patients have been reported elsewhere, but this in-depth review of detailed clinical courses for additional patients with longer follow-up provides more robust information for clinical use of this new regimen [17, 18]. All patients completed bedaquiline and pretomanid, with only 3 stopping linezolid prematurely. An initial 600-mg daily linezolid dose, use of TDM, careful monitoring for effectiveness and toxicity, and supportive care contributed to this success. The median BPaL duration of 27 weeks was less than half the recommended duration for traditional regimens in 2019 US guidelines for drug-resistant tuberculosis [4].
Concerns about bone marrow suppression, peripheral neuropathy, and optic neuritis may hinder uptake of BPaL and other linezolid-containing regimens. Linezolid has a narrow therapeutic window. It inhibits protein synthesis and growth by disrupting bacterial mitochondria but can similarly poison human mitochondria. Suppression of adenosine triphosphate synthesis in bone marrow precursor cells leads to myelosuppression, one of linezolid's most predictable toxic effects [19]. Although the exact mechanism of neurologic injury is less clear, linezolid-induced neurotoxicity is also likely mediated via mitochondrial dysfunction [20, 21]. Both linezolid's efficacy and its toxicity are concentration and duration dependent, with higher trough concentrations increasing mitochondrial dysfunction [22, 23].
For patients with linezolid trough concentrations <2 µg/mL, toxicity may also be influenced by genetic variations in human mitochondria as well as clinical risk factors that increase risk of mitochondrial damage despite the lower linezolid concentrations [19, 24–26]. In this cohort, the 4 patients requiring blood transfusion had baseline anemia, and the 4 reporting neuropathy requiring discontinuation of linezolid or extension of the dosing interval had other risk factors, including baseline neuropathy, diabetes, thyroid disease, vitamin B12 deficiency, and opioid abuse. Thus, toxicity may be minimized by closely monitoring high-risk patients and using TDM to guide linezolid exposure. This strategy of linezolid dosing and monitoring is consistent with an established high-quality, patient-centered precision medicine approach frequently used in the United States [5, 27–30].
While an alternative strategy is to decrease the daily linezolid dose from 600 to 300 mg when toxicity or a high serum trough level is detected, we preferred the 600-mg TIW approach based on pharmacokinetic data. High trough values reflect slow clearance. Extending the dosing interval directly addresses slow clearance, and this should allow linezolid concentrations at the mitochondria to fall to zero. Using the higher dose of 600 mg TIW also produces higher maximum plasma concentrations than 300 mg daily. This would favor a higher concentration gradient driving drug into the mycobacterial-laden lesions. Head-to-head comparison of these strategies has not been performed, to our knowledge.
Receiving 600 mg of linezolid daily, adjusted by clinical symptoms and TDM, our patients experienced less linezolid-associated hematologic and neurologic toxicity than patients receiving 1200 mg daily in both NIX-TB and ZeNix Trials [7, 31]. With high tolerability, there were few prolonged interruptions, and 100% completed BPaL treatment much more quickly compared with the prior MDR tuberculosis standard of care [4]. While the long-term efficacy of this approach remains to be seen, only 2 relapses have been reported thus far, and follow-up continues. The availability of drug susceptibility testing for patients in this cohort was important, and broader availability of both molecular and phenotypic testing to evaluate for both baseline and acquired resistance to BPaL agents will be critical [5, 27]. To date, half of this cohort (36 patients) remain tuberculosis free 1 year after completion of BPaL treatment, and a quarter (18 patients) successfully completed 2 years of follow-up. The use of a collaborative entity, BIG, enabled broad dissemination of challenges and successes encountered by early BPaL adopters and offered a platform for rapidly advancing clinical expertise and scale-up of this novel regimen across the United States.
Recent evidence further supports linezolid dosing of 600 mg daily when combined with bedaquiline and pretomanid [7, 31, 32]. ZeNix, a multinational randomized controlled clinical trial, addressed this directly [31]. With a factorial design, the study compared daily linezolid at 1200 mg for 26 or 9 weeks and 600 mg for 26 or 9 weeks, combined with bedaquiline and pretomanid. The overall risk-benefit ratio favored linezolid at 600 mg for 26 weeks, based on lower toxicity and fewer dose modifications coupled with rare bacteriological failure (in 1 of 45 participants) [31]. In May 2022, WHO endorsed BPaL with or without moxifloxacin (BPaLM) for RR tuberculosis, recommending linezolid 600 mg daily throughout treatment and allowing dose reduction for toxicity or poor tolerability [33].
However, uniform dosing throughout treatment may not be the most effective, safest approach to maximize treatment completion. In our study, based on TDM or toxic effects, 30% of patients required linezolid dosing of >600 mg daily, and half changed to TIW dosing. Despite evidence that TDM decreases the time to culture conversion and enhances treatment success for drug-susceptible tuberculosis, most providers do not obtain serum drug concentrations for their patients [27, 34, 35]. Challenges include a paucity of laboratories specialized for TDM, lack of funding, and technical challenges with obtaining and shipping multiple blood samples to the few laboratories performing these assays [36, 37]. Collective efforts by tuberculosis providers, programs, and policymakers to optimize capacity for TDM for individualized drug dosing has the potential to increase the safe, relapse-free cure [4, 5, 27, 37–39].
Despite the advantages of BPaL, it was FDA approved only for patients with highly drug-resistant pulmonary disease [8]. The BIG cohort expanded BPaL treatment to any patient with rifamycin resistance or intolerance and to patients with extrapulmonary tuberculosis, populations not included in trials [7, 31, 32]. Current US guidelines for RR tuberculosis contain no explicit recommendations for treating extrapulmonary disease or rifampin-intolerant drug-susceptible tuberculosis [4, 33]. The ability of BPaL to sterilize extrapulmonary tissues has not been determined in clinical trials, and the optimal duration for various forms of extrapulmonary tuberculosis remains uncertain. Despite the paucity of data, WHO recommendations were updated in December 2022 to endorse the use of the BPaLM/BPaL regimen for all forms of extrapulmonary disease except tuberculosis involving the central nervous system and osteoarticular and disseminated (miliary) tuberculosis [40]. Results from the BIG cohort are reassuring, and we aim to follow up these patients closely and report on long-term outcomes in the future.
Our study has limitations inherent to any retrospective observational study, including missing data, inadequate details on adverse events, and lack of standardized patient evaluation, treatment, monitoring, or follow-up. Consistency was gained by using a single laboratory to perform TDM, but not all serum samples for linezolid concentrations were obtained with standardized timing. The optimal timing for TDM is 2 weeks after linezolid is initiated and at the time of any adverse event; preferably, TDM also is repeated after any change in dose or dosing frequency. Another limitation is that many patients in our cohort had received treatment with other first- or second-line tuberculosis medications before BPaL, which could also have affected treatment outcomes. Because our study describes real-world practice, these findings are still useful for informing US clinical practices using this new regimen. A strength of our study was the diversity of the patients with respect to race, comorbid conditions, age, and clinical care under routine tuberculosis program conditions, making our findings more generalizable to the United States.
Three years since FDA approval, BPaL has transformed treatment for RR or intolerant tuberculosis in the United States. The findings from this study confirm the current WHO recommendations to use an initial linezolid daily dose of 600 mg rather than 1200 mg. Notably, the addition of personalized drug dosing with close monitoring and early management of adverse effects likely enhanced safety and treatment completion. Support to local providers by BPaL-experienced tuberculosis expert consultants was also likely influential. The BIG cohort demonstrates that with collaborative efforts among providers and public health programs, early implementation of new tuberculosis treatments is feasible, serving as a model for future innovations.
Contributor Information
Connie A Haley, Southeastern National Tuberculosis Center, Division of Infectious Diseases and Global Medicine, Department of Medicine in the College of Medicine, University of Florida, Gainesville, Florida, USA; Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Marcos C Schechter, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Georgia State Tuberculosis Program, Atlanta, Georgia, USA.
David Ashkin, Southeastern National Tuberculosis Center, Division of Infectious Diseases and Global Medicine, Department of Medicine in the College of Medicine, University of Florida, Gainesville, Florida, USA.
Charles A Peloquin, Translational Research, College of Pharmacy and Emerging Pathogens Institute, University of Florida, Gainesville, Florida, USA.
J Peter Cegielski, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Barbara B Andrino, Fairfax County Health Department, Annandale, Virginia, USA.
Marcos Burgos, New Mexico Department of Health, Santa Fe, New Mexico, USA; University of New Mexico School of Medicine, Albuquerque, New Mexico, USA; New Mexico Veterans Affairs Health Care System, Albuquerque, New Mexico, USA.
Lori A Caloia, Louisville Metro Department of Public Health and Wellness, Louisville, Kentucky, USA; Humana Healthy Horizons in Kentucky, Louisville, Kentucky, USA.
Lisa Chen, Curry International Tuberculosis Center, University of California, San Francisco, California, USA.
Angel Colon-Semidey, Puerto Rico Department of Health, San Juan, Puerto Rico, USA.
Malini B DeSilva, Saint Paul–Ramsey County Public Health, Saint Paul, Minnesota, USA; HealthPartners Institute, Bloomington, Minnesota, USA.
Shireesha Dhanireddy, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA.
Susan E Dorman, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina, USA; South Carolina Department of Health and Environmental Control, Greenville, South Carolina, USA.
Felicia F Dworkin, New York City Department of Health and Mental Hygiene, Bureau of Tuberculosis Control, New York, New York, USA.
Heidi Hammond-Epstein, Southeastern National Tuberculosis Center, University of Florida, Gainesville, Florida, USA.
Alice V Easton, New York City Department of Health and Mental Hygiene, Bureau of Tuberculosis Control, New York, New York, USA.
James T Gaensbauer, Department of Pediatrics and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Bijan Ghassemieh, Public Health—Seattle & King County, Seattle, Washington, USA; Department of Medicine, University of Washington, Seattle, Washington, USA.
Maria E Gomez, Southeastern National Tuberculosis Center, University of Florida, Gainesville, Florida, USA.
David Horne, Pulmonary, Critical Care and Sleep Medicine, Harborview Medical Center, University of Washington, Seattle, Washington, USA.
Supriya Jasuja, Cook County Department of Public Health, Forest Park, Illinois, USA.
Betsy A Jones, Bureau of Public Health Laboratories, Florida State Tuberculosis Program, Jacksonville, Florida, USA.
Leonard J Kaplan, Division of Infectious Diseases, Department of Medicine, NorthShore University HealthSystem, Evanston, Illinois, USA.
Asharaf Edward Khan, Jefferson County Department of Health, Birmingham, Alabama, USA.
Elizabeth Kracen, Public Health—Seattle & King County, Seattle, Washington, USA; Department of Medicine, University of Washington, Seattle, Washington, USA.
Sarah Labuda, Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, Puerto Rico Department of Health, San Juan, Puerto Rico, USA.
Karen M Landers, Alabama Department of Public Health, Montgomery, Alabama, USA.
Alfred A Lardizabal, Global Tuberculosis Institute, Rutgers University, Newark, New Jersey, USA.
Maria T Lasley, Southeastern National Tuberculosis Center, University of Florida, Gainesville, Florida, USA.
David M Letzer, Medical College of Wisconsin, Milwaukee, Wisconsin, USA.
Vinicius K Lopes, Sheboygan County Health and Human Services, Sheboygan, Wisconsin, USA; Southern California Infectious Diseases Associates, Inc., Newport Beach, California, USA.
Ronald J Lubelchek, Cook County Department of Public Health, Forest Park, Illinois, USA; Division of Infectious Diseases, John H. Stroger, Jr. Hospital of Cook County, Chicago, Illinois, USA; Department of Medicine, Rush University Medical Center, Chicago, Illinois, USA.
C Patricia Macias, Health Transformation Program NorthShore University, Chicago, Illinois, USA; The International Union Against Tuberculosis and Lung Disease, Paris, France.
Aimee Mihalyov, Louisville Metro Department of Public Health and Wellness, Louisville, Kentucky, USA.
Elizabeth Ann Misch, Division of Infectious Disease, Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA.
Jason A Murray, Emergency Medicine, Saint Elizabeth Healthcare System, Edgewood, Kentucky, USA; Northern Kentucky Health Department, Florence, Kentucky, USA.
Masahiro Narita, Public Health—Seattle & King County, Seattle, Washington, USA; Department of Medicine, University of Washington, Seattle, Washington, USA.
Diana M Nilsen, New York City Department of Health and Mental Hygiene, Bureau of Tuberculosis Control, New York, New York, USA.
Megan J Ninneman, Jackson Memorial Hospital, Miami, Florida, USA.
Lynne Ogawa, Saint Paul–Ramsey County Public Health, Saint Paul, Minnesota, USA.
Alawode Oladele, Dekalb County Tuberculosis Program, Decatur, Georgia, USA.
Melissa Overman, South Carolina Department of Health and Environmental Control, Greenville, South Carolina, USA.
Susan M Ray, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA; Georgia State Tuberculosis Program, Atlanta, Georgia, USA.
Kathleen A Ritger, Chicago Department of Public Health, Chicago, Illinois, USA.
Marie-Claire Rowlinson, Bureau of Public Health Laboratories, Florida State Tuberculosis Program, Jacksonville, Florida, USA; Wadsworth Center, New York State Department of Health, Albany, New York, USA.
Nadya Sabuwala, Minnesota Department of Health, Saint Paul, Minnesota, USA.
Thomas M Schiller, Winnebago County Health Department, Rockford, Illinois, USA.
Lawrence E Schwartz, Tacoma-Pierce County Health Department, Tacoma, Washington, USA.
Christopher Spitters, Division of Allergy and Infectious Diseases, Department of Medicine, University of Washington, Seattle, Washington, USA; Snohomish County Health Department, Everett, Washington, USA; Washington State Department of Health, Shoreline, Washington, USA.
Douglas B Thomson, Barren River District Health Department, Bowling Green, Kentucky, USA.
Rene Rico Tresgallo, Department of Medicine, University of Miami, Jackson Memorial Hospital, Miami, Florida, USA.
Patrick Valois, Bureau of Public Health Laboratories, Florida State Tuberculosis Program, Jacksonville, Florida, USA.
Neela D Goswami, Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
for the BPaL Implementation Group:
Rocio Agraz-Lara, Amina Ahmed, Ana Alvarez, Lisa Armitage, Pennan Barry, Robert Belknap, John Bernardo, Mary Bravo, Sarah Brode, Elizabeth Burden, Joseph Burzynski, Caralee Caplan-Shaw, Ken Castro, Terry Chorba, William Connors, Victoria Cook, Andrea Cruz, Charles Daley, Shom Dasgupta, Sonia Dhingra, Thomas Dobbs, Ellen Elmore, Frank Erwin, Vincent Escuyer, Christina Fiske, Beth Gadkowski, German Henestroza, Julie Higashi, Shereen Katrak, Chris Keh, Amanda Khalil, Lilian Kigonya, Michael Lauzardo, Sapna Morris, Sonal Munsiff, Scott Nabity, Margaret Oxtoby, Amee Patrawalla, Allison Phillips, Ann Raftery, Caitlin Reed, Brian Rock, Kelly Russo, Harleen Sahini, Paul Saleeb, Roberto Santos, Barbara Seaworth, Joanna Shaw-KaiKai, Jeff Starke, Jason Stout, Wesley Stubblefield, Zelalem Temesgen, Keziah Thomas, Jeffrey Tornheim, Caryn Upton, Daniel Urbine, Shu-hua Wang, Jon Warkentin, Risa Webb, John Wilson, Johnathan Wortham, and Salinia Yu, Claudia Altman, Irfan Hafiz, Deepa Prabhakar, and William Bowler
Notes
Acknowledgments. Additional BPaL Implementation Group members at the time this manuscript was submitted include Rocio Agraz-Lara, Amina Ahmed, Ana Alvarez, Lisa Armitage, Pennan Barry, Robert Belknap, John Bernardo, Mary Bravo, Sarah Brode, Elizabeth Burden, Joseph Burzynski, Caralee Caplan-Shaw, Ken Castro, Terry Chorba, William Connors, Victoria Cook, Andrea Cruz, Charles Daley, Shom Dasgupta, Sonia Dhingra, Thomas Dobbs, Ellen Elmore, Frank Erwin, Vincent Escuyer, Christina Fiske, Beth Gadkowski, German Henestroza, Julie Higashi, Shereen Katrak, Chris Keh, Amanda Khalil, Lilian Kigonya, Michael Lauzardo, Sapna Morris, Sonal Munsiff, Scott Nabity, Margaret Oxtoby, Amee Patrawalla, Allison Phillips, Ann Raftery, Caitlin Reed, Brian Rock, Kelly Russo, Harleen Sahini, Paul Saleeb, Roberto Santos, Barbara Seaworth, Joanna Shaw-KaiKai, Jeff Starke, Jason Stout, Wesley Stubblefield, Zelalem Temesgen, Keziah Thomas, Jeffrey Tornheim, Caryn Upton, Daniel Urbine, Shu-hua Wang, Jon Warkentin, Risa Webb, John Wilson, Johnathan Wortham, and Salinia Yu.
The authors also acknowledge Claudia Altman, Irfan Hafiz, Deepa Prabhakar, and William Bowler, US public health staff who tirelessly provide care for patients with tuberculosis and their families; the Southeastern National Tuberculosis Center; the Global Tuberculosis Institute at Rutgers; the Heartland National Tuberculosis Center; the Curry International Tuberculosis Center; the Division of Tuberculosis Elimination, Centers for Disease Control and Prevention; the Florida Department of Health Bureau of Public Health Laboratories, the New York City Department of Health Wadsworth Laboratory; the Johns Hopkins Hospital Medical Mycobacteriology; the Virginia Tuberculosis Foundation; and the TB Alliance.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This article does not overlap substantially with any articles already published or accepted for publication. Preliminary findings from some patients in this report are broadly referenced in a brief letter to the editor and in a case report [17,18] (both included in the Supplementary Materials), but the current manuscript provides substantially more detailed clinical information on individual patients and includes a much larger cohort with longer follow-up.
References
- 1. World Health Organization . Global tuberculosis report 2022. Geneva, Switzerland: World Health Organization, 2022. Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022. Accessed 15 March 2023. [Google Scholar]
- 2. Tanimura T, Jaramillo E, Weil D, Raviglione M, Lönnroth K. Financial burden for tuberculosis patients in low- and middle-income countries: a systematic review. Eur Respir J 2014; 43:1763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomas BE, Shanmugam P, Malaisamy M, et al. Psycho-socio-economic issues challenging multidrug resistant tuberculosis patients: a systematic review. PLoS One 2016; 11:e0147397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis: an official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med 2019; 200:e93–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar K, Kon OM. Personalised medicine for tuberculosis and non-tuberculous mycobacterial pulmonary disease. Microorganisms 2021; 9:2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lan Z, Ahmad N, Baghaei P, et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet Respir Med 2020; 8:383–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med 2020; 382:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration . Center for Drug Evaluation and Research: application number 212862Orig1s000. 2019. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212862Orig1s000Lbl.pdf, Accessed 15 March 2023.
- 9. Centers for Disease Control and Prevention . Provisional CDC guidance for the use of pretomanid as part of a regimen [bedaquiline, pretomanid, and linezolid (BPaL)] to treat drug-resistant tuberculosis disease. 2022. Available at: https://www.cdc.gov/tb/topic/drtb/bpal/default.htm. Accessed 15 March 2023.
- 10. Viney K, Linh NN, Gegia M, et al. New definitions of pre-extensively and extensively drug-resistant tuberculosis: update from the World Health Organization. Eur Respir J 2021; 57:2100361. [DOI] [PubMed] [Google Scholar]
- 11. Burzynski J, Mangan JM, Lam CK, et al. In-person vs electronic directly observed therapy for tuberculosis treatment adherence: a randomized noninferiority trial. JAMA Netw Open 2022; 5:e2144210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curry International TB Center. Drug-resistant tuberculosis: a survival guide for clinicians, 3rd edition/2022 updates. Available at: https://www.currytbcenter.ucsf.edu/products/view/drug-resistant-tuberculosis-survival-guide-clinicians-3rd-edition. Accessed 15 March 2023.
- 13. Centers for Disease Control and Prevention. User guide: Molecular Detection of Drug Resistance (MDDR) in Mycobacterium tuberculosis complex by DNA sequencing (Version 3.0). Available at: https://www.cdc.gov/tb/topic/laboratory/mddr-user-guide.htm. Accessed 15 March 2023. February 2023.
- 14. Campbell PJ, Morlock GP, Sikes RD, et al. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother 2011; 55:2032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization . Definitions and reporting framework for tuberculosis—2013 revision: updated December 2014 and January 2020. Geneva, Switzerland: World Health Organization, 2013:1–47. [Google Scholar]
- 16. World Health Organization . Catalogue of mutations in Mycobacterium tuberculosis complex and their association with drug resistance. Geneva, Switzerland: World Health Organization, 2021. [Google Scholar]
- 17. Goswami ND, Ashkin D, Haley CA; BAM Project Team . Pretomanid in the treatment of patients with tuberculosis in the United States. N Engl J Med 2022; 387:850–2. [DOI] [PubMed] [Google Scholar]
- 18. Haley CA, Macias P, Jasuja S, et al. Novel 6-month treatment for drug-resistant tuberculosis, United States. Emerg Infect Dis 2021; 27:332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oehadian A, Santoso P, Menzies D, Ruslami R. Concise clinical review of hematologic toxicity of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis: role of mitochondria. Tuberc Respir Dis (Seoul) 2022; 85:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Vriese AS, Coster RV, Smet J, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis 2006; 42:1111–7. [DOI] [PubMed] [Google Scholar]
- 21. Bano S, Nawaz A, Numan A, Hassan MA, Shafique MBA. A case report and literature review of the outcome of linezolid-induced optic and peripheral neuropathy in patients with multidrug-resistant pulmonary TB. Front Neurol 2022; 13:908584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song T, Lee M, Jeon HS, et al. Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. EBioMedicine 2015; 2:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown AN, Drusano GL, Adams JR, et al. Preclinical evaluations to identify optimal linezolid regimens for tuberculosis therapy. mBio 2015; 6:e01741-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu X, Aoki M, Osa S, et al. Safety of linezolid in patients with decreased renal function and trough monitoring: a systematic review and meta-analysis. BMC Pharmacol Toxicol 2022; 23:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cattaneo D, Gervasoni C, Cozzi V, Castoldi S, Baldelli S, Clementi E. Therapeutic drug management of linezolid: a missed opportunity for clinicians? Int J Antimicrob Agents 2016; 48:728–31. [DOI] [PubMed] [Google Scholar]
- 26. Rao GG, Konicki R, Cattaneo D, et al. Therapeutic drug monitoring can improve linezolid dosing regimens in current clinical practice: a review of linezolid pharmacokinetics and pharmacodynamics. Ther Drug Monit 2020; 42:83–92. [DOI] [PubMed] [Google Scholar]
- 27. Lange C, Aarnoutse R, Chesov D, et al. Perspective for precision medicine for tuberculosis. Front Immunol 2020; 11:566608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alffenaar JWC, Stocker SL, Forsman LD, et al. Clinical standards for the dosing and management of TB drugs. Int J Tuberc Lung Dis 2022; 26:483–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horter S, Daftary A, Keam T, et al. Person-centred care in TB. Int J Tuberc Lung Dis 2021; 25:784–7. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization . End TB strategy. Geneva, Switzerland: World Health Organization; 2015. Available at: https://www.who.int/publications/i/item/WHO-HTM-TB-2015.19. Accessed 15 March 2023.
- 31. Conradie F, Bagdasaryan TR, Borisov S, et al. Bedaquiline-Pretomanid-linezolid regimens for drug-resistant tuberculosis. N Engl J Med 2022; 387:810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nyang'wa BT, Berry C, Kazounis E, et al. A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N Engl J Med 2022; 387:2331–43. [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization . Rapid communication: key changes to the treatment of drug-resistant tuberculosis. Geneva, Switzerland: World Health Organization, 2022. Available at: https://www.who.int/publications/i/item/WHO-UCN-TB-2022-2. Accessed 15 March 2023. [Google Scholar]
- 34. Peloquin C. The role of therapeutic drug monitoring in mycobacterial infections. Microbiol Spectr 2017; 5:1–8. [DOI] [PubMed] [Google Scholar]
- 35. Heysell SK, Moore JL, Peloquin CA, Ashkin D, Houpt ER. Outcomes and use of therapeutic drug monitoring in multidrug-resistant tuberculosis patients treated in Virginia, 2009-2014. Tuberc Respir Dis (Seoul) 2015; 78:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Margineanu I, Akkerman O, Cattaneo D, et al. Practices of therapeutic drug monitoring in tuberculosis: an international survey. Eur Respir J 2022; 59:2102787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haley C, Rowlinson MC, Ashkin D. Tuberculosis therapy: “in pursuit of perfection”. Clin Infect Dis 2021; 73:e3529–30. [DOI] [PubMed] [Google Scholar]
- 38. Dookie N, Ngema SL, Perumal R, Naicker N, Padayatchi N, Naidoo K. The changing paradigm of drug-resistant tuberculosis treatment: successes, pitfalls, and future perspectives. Clin Microbiol Rev 2022; 35:e0018019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bolhuis MS, Akkerman OW, Sturkenboom MGG, et al. Linezolid-based regimens for multidrug-resistant tuberculosis (TB): a systematic review to establish or revise the current recommended dose for TB treatment. Clin Infect Dis 2018; 67:S327–35. [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization . WHO consolidated guidelines on tuberculosis. Module 4: treatment—drug-resistant tuberculosis treatment. 2022. update. Available at: https://www.who.int/publications/i/item/9789240063129. Accessed 15 March 2023.