Abstract
Human T-cell leukemia virus types 1 and 2 (HTLV-1 and HTLV-2) are closely related retroviruses with nucleotide sequences that are 65% identical. To determine whether their envelope glycoproteins function similarly and to define the molecular determinants of HTLV-2 envelope-mediated functions, we have used pseudotyped viruses and have introduced mutations into regions of the HTLV-2 glycoproteins homologous to those known to be important for HTLV-1 glycoprotein functions. The envelopes of the two viruses could be exchanged with no loss of infectivity, suggesting that the glycoproteins function in broadly similar ways. However, comparative analysis of the HTLV-1 and HTLV-2 glycoproteins showed subtle differences in the structure-function relationships of the two surface glycoprotein (SU) subunits, even though they recognize the same receptor. Indeed, mutations introduced at equivalent positions in the two SU glycoproteins resulted in different phenotypes in the two viruses. The scenario is the opposite for the transmembrane glycoprotein (TM) subunits, in which the functional domains of the two viruses are strictly conserved, confirming the involvement of the TM ectodomain in postfusion events required for full infectivity of the HTLVs. Thus, although they recognize the same receptor, the HTLV-1 and HTLV-2 SU subunits have slightly different ways of transducing the conformational information that primes a common fusion mechanism effected by similar TM subunits.
Human T-cell leukemia virus types 1 and 2 (HTLV-1 and HTLV-2) are closely related retroviruses belonging to the same genus. Their nucleotide sequences are 65% identical. HTLV-1 causes diseases in 1 to 5% of infected individuals (24), whereas HTLV-2 has not been clearly linked with any disease, although rare cases of neuropathy have been described in HTLV-2-infected individuals (14, 17). The in vivo cellular tropisms of the two viruses differ. HTLV-1 is found mostly in the CD4-positive T lymphocytes of infected individuals (27), whereas the main target cells for HTLV-2 are CD8-positive T lymphocytes (16). This difference in the cellular tropism of the two viruses is probably due to postentry events, because the viruses have been shown by in vitro interference assays to interact with the same (32, 33) widely distributed (19, 22, 34) receptor at the surface of human cells.
The entry of the retroviruses into the target cell is ensured by the envelope glycoproteins. These are composed of two subunits, the surface glycoprotein (SU), responsible for attachment of the virus to a cell surface receptor, and the transmembrane glycoprotein (TM), which anchors the SU-TM heterodimer at the surface of the infected cell or virion and performs fusion of the viral envelope with the target cell surface that is required for penetration of the viral core into the cytoplasm. According to the current dynamic model of retroviral fusion, recognition of the cellular receptor by the SU subunit causes conformational changes in the envelope heterodimer which activate the fusion potential of the TM subunit.
The molecular determinants of the HTLV-1 envelope glycoproteins involved in virus entry have been well characterized with neutralizing antibodies or peptides that inhibit fusion or infection (1, 9, 25, 29, 36) and by functional studies of mutated glycoproteins (6, 7, 28). Two domains of the HTLV-1 SU are essential for its function; the first domain is between amino acids 75 and 101 of the SU subunit (which contains 312 amino acids in total), and the second is in a more central region of the SU, between amino acids 181 and 208. The extracellular domain of the HTLV-1 TM glycoprotein contains two functionally distinct regions (28). The amino-terminal part of the TM, which includes a leucine zipper-like motif, is involved in envelope-mediated fusion with the target cell membrane. The other region, amino terminal to the anchorage peptide, is involved in postfusion events required for cell-to-cell transmission of HTLV-1.
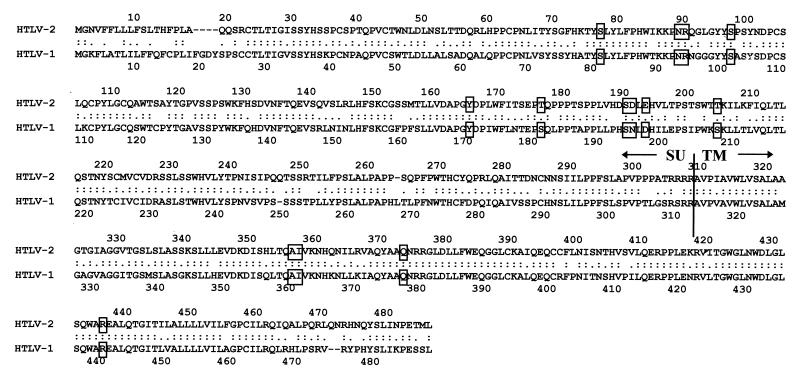
Little is known about the regions of the HTLV-2 glycoproteins involved in their functions, but the sequences of the HTLV-1 and HTLV-2 envelope proteins are very similar (65% conserved residues in the SU part and 79% in the TM part of the molecule [see Fig. 1]), suggesting some functional conservation. Indeed, antibodies directed against the amino-terminal region (amino acids 82 to 97) and the central region (amino acids 186 to 192) of the HTLV-2 SU are neutralizing (25, 35), suggesting that these regions are important for function, as are the homologous domains of the HTLV-1 SU glycoprotein.
FIG. 1.
Sequence alignment for the HTLV-2 and HTLV-1 envelope proteins. The HTLV-2 sequence is from Shimotohno et al. (31); the HTLV-1 sequence is from Seiki et al. (30). Square boxes indicate mutations of the HTLV-2 glycoproteins carried out in this study and of the HTLV-1 glycoproteins in our previous studies (7, 28).
To determine whether the two proteins function similarly and to investigate the molecular determinants of HTLV-2 envelope-mediated functions, we used pseudotyped viruses and introduced mutations into the regions of the HTLV-2 glycoproteins corresponding to the regions that are important for HTLV-1 glycoprotein functions.
We first constructed a eucaryotic expression vector of the HTLV-2 envelope glycoproteins (CMV-ENV-2) and verified that it allowed glycoprotein synthesis, syncytium formation, and viral transmission. CMV-ENV-2 contained part of the HTLV-2 genome, including the env, tax, and rex genes, under the control of the simian cytomegalovirus promoter. The proviral sequences, which were derived from the pH6-neo proviral clone (4), obtained from I. S. Y. Chen (University of California at Los Angeles, Los Angeles, Calif.), started at the BamHI site upstream from the env gene (position 5090 in reference 31) and included all the 3′ viral sequences.
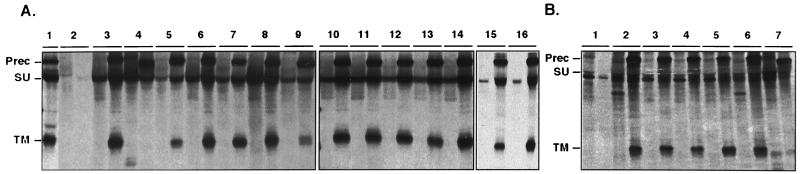
The CMV-ENV-2 construct was used to transiently transfect COS-1 cells, and the glycoproteins were immunoprecipitated from both the lysate and the supernatant, as previously described for HTLV-1 (6). The HTLV-2 envelope precursor (gp62) was synthesized and underwent intracellular maturation similar to that seen in the HTLV-2-infected Mo-2 cell line, yielding the mature SU-gp46 and TM-gp21 subunits (see Fig. 2A).
FIG. 2.
Immunoprecipitation of the HTLV-2 envelope glycoproteins from transfected cells. In each case, the left-hand part of the lane contains the immunoprecipitated glycoproteins from the cell supernatant and the right-hand part contains the immunoprecipitated glycoproteins from the cell lysate. (A) SU mutants. Lane 1, lysate of MO-2 HTLV-2-infected cells; lane 2, negative control; lanes 3, 10, and 15, wild-type envelope positive control (cells transfected with the CMV-ENV-2 construct); lane 4, Gln440-stop (soluble-glycoprotein construct with a stop codon at position 440); lane 5, Ser77-Phe; lane 6, Asn89-Ile; lane 7, Ser97-Leu; lane 8, Tyr166-Ser; lane 9, Thr177-Ile; lane 11, Ser190-Phe; lane 12, Asp191-Val; lane 13, Glu193-Val; lane 14, Thr204-Ile; lane 16, Arg90-Glu. (B) TM mutants. Lane 1, negative control; lane 2, wild-type envelope positive control (cells transfected with the CMV-ENV-2 construct); lane 3, Ala356-Asp; lane 4, Ile357-Arg; lane 5, Gln373-Leu; lane 6, Arg436-Leu; lane 7, Gln440-stop (soluble-glycoprotein construct). Prec, precursor glycoprotein.
We next investigated whether the CMV-ENV-2 expression plasmid led to syncytium formation, using a quantitative assay described for HTLV-1 (7, 28). Transient transfection of COS-LTRLacZ with the CMV-ENV-2 construct, followed by coculture with HeLa-Tat indicator cells, resulted in extensive formation of syncytia. Typical cocultures contained 500 to 1,000 syncytia.
We finally investigated whether our HTLV-2 envelope expression plasmid allowed transmission of the virus in one round of infection, using a quantitative assay modeled on an assay previously designed for HTLV-1 (7). It was based on transcomplementation by the CMV-ENV-2 envelope construct of a defective HTLV-2 provirus in which the envelope gene was replaced by a selectable marker. The indicator provirus used was the SV-HTLV-2-neo plasmid (21), obtained from D. Littman (University of California at San Francisco, San Francisco, Calif.). The HTLV-2 envelope constructs (0.75 μg) and the SV-HTLV-2-neo indicator provirus (0.75 μg) were used to cotransfect COS-1 cells seeded at 3 × 105 cells per 60-mm-diameter dish the previous day. One day after transfection, the cells were treated with 10 μg of mitomycin (Amétycine; Laboratoires Choay, Paris, France) per ml for 3 h at 37°C to prevent growth. The cells were then washed with phosphate-buffered saline, trypsinized, and, along with 4 × 105 B5 cells, used to seed 60-mm-diameter dishes. The cells were cocultured for 2 days and were then transferred to selection medium containing 125 μg of G418 sulfate (Geneticin; Gibco) per ml. G418-resistant colonies were counted after 2 to 3 weeks.
The glycoproteins produced by expression of CMV-ENV-2 allowed HTLV-2 transmission to B5 indicator cells in this assay, routinely giving rise to 500 to 1,000 colonies per 3 × 105 transfected cells after coculture. Use of the supernatants of transfected cells did not result in infection of B5 cells, showing that cell-to-cell transmission of HTLV-2 was more efficient than cell-free transmission in our assay and was the essential mode of infection. Thus, HTLV-1 and HTLV-2 have the same general properties in single-cycle infectivity assays (7).
To test whether the HTLV-1 and HTLV-2 envelope glycoproteins functioned in similar ways, we determined whether effective cell-to-cell transmission would still be allowed if the two proteins were exchanged. For this purpose, we made pseudotypes involving the Gag proteins of one virus and the glycoproteins of the other, using transcomplementation of one indicator provirus in which the env gene was replaced by the neomycin resistance gene by the plasmid expressing the envelope gene of the other virus. The HTLV-1 indicator provirus and the HTLV-1 envelope expression plasmid were the pCS-HTLV-1-neo and the CMV-ENV-1 constructs described previously (7). The HTLV-2 constructs were those described above. Similar numbers of colonies were obtained with all four combinations of constructs, showing that the glycoproteins of one virus can substitute for those of the other virus with no loss of infectivity (data not shown).
We then investigated whether the molecular determinants of SU and TM required for virus entry were identical in HTLV-1 and HTLV-2. We mutated positions in the HTLV-2 glycoproteins homologous to known functional domains of the HTLV-1 glycoproteins (6, 7, 28) (Fig. 1). Oligonucleotide-directed mutagenesis of fragments of the sequence encoding the HTLV-2 envelope protein was performed by the Kunkel method. The mutated env fragments were then inserted into the CMV-ENV-2 expression plasmid, sequenced, and used for transfection. The mutants were named X amino acid position Y, where X and Y are the wild-type and substituted amino acids, respectively, and amino acid position 1 corresponds to the initiator methionine of the HTLV-2 envelope protein.
The molecular determinants of HTLV-2 SU required for virus entry were examined mainly with mutations introduced into two regions of the SU (Fig. 1). The first region was between amino acids 75 and 101 (positions relative to the HTLV-1 envelope protein, with 1 as the initial methionine), known to be important in HTLV-1 for syncytium formation and virus entry. The second region, corresponding to amino acids 181 to 208 in HTLV-1, is also believed to be important for the fusion capacity of the glycoproteins. The intracellular maturation, shedding of SU into the supernatant, fusion capacity, and infectivity of each of the mutated glycoproteins were assessed by quantitative assays, as described above or in references 7 and 28. The results are shown in Fig. 2A and Table 1.
TABLE 1.
Phenotypes of HTLV-2 SU mutantsa
| Envelope glycoproteinb | Precursor cleavage index (%)c | SU in the super- natantc | Syncytium formation index (%)c | Infectivity index (%)c |
|---|---|---|---|---|
| Wild-type HTLV-2 | 100 | + | 100 | 100 |
| Wild-type HTLV-1 | 100 | − | 100 | 100 |
| Ser77-Phe HTLV-2 | 21 | +/− | 7 | 88 |
| Ser81-Phe HTLV-1 | 47 | − | 60 | 81 |
| Asn89-Ile HTLV-2 | 102 | + | 84 | 64 |
| Asn93-Ile HTLV-1 | 87 | − | 103 | 71 |
| Arg90-Glu HTLV-2 | 112 | + | 0 | 0 |
| Arg94-Glu HTLV-1 | 45 | − | 35 | 9 |
| Ser97-Leu HTLV-2 | 200 | + | 0 | 0 |
| Ser101-Leu HTLV-1 | 73 | − | 50 | 6 |
| Tyr166-Ser HTLV-2 | 68 | +++ | 69 | 74 |
| Tyr170-Ser HTLV-1 | 51 | +++ | 30 | 65 |
| Thr177-Ile HTLV-2 | 11 | +/− | 21 | 55 |
| Ser181-Ile HTLV-1 | 43 | − | 20 | 74 |
| Ser190-Phe HTLV-2 | 100 | + | 80 | 54 |
| Ser194-Phe HTLV-1 | 34 | − | 110 | 88 |
| Asp191-Val HTLV-2 | 124 | + | 90 | 72 |
| Asn195-Ile HTLV-1 | 65 | − | 50 | 77 |
| Glu193-Val HTLV-2 | 41 | + | 99 | 72 |
| Asp197-Val HTLV-1 | 49 | − | 10 | 62 |
| Thr204-Ile HTLV-2 | 74 | + | 37 | 97 |
| Ser208-Ile HTLV-1 | 56 | − | 20 | 58 |
Cleavage, syncytium formation, and infectivity indices were calculated as described elsewhere (7, 28). + indicates an estimated amount when SU was immunoprecipitated from the supernatant.
Results for HTLV-1-mutated envelope proteins are from our previous study (7).
Data are the means of at least two independent transfections.
The precursor glycoprotein was cleaved into SU and TM mature products for most mutated HTLV-2 envelope proteins, showing that the intracellular maturation was correctly achieved (Fig. 2A). The tyrosine residue at position 166 in HTLV-2, equivalent to the tyrosine at position 170 of HTLV-1, which is involved in the SU-to-TM association, had the same function in the HTLV-2 envelope heterodimer. Indeed, substitution of this tyrosine in HTLV-2 resulted in the shedding of the SU into the supernatant, as occurs after the Tyr170-Ser mutation in HTLV-1 (Fig. 2A, lane 8). Thus, structural features appear to be conserved in the HTLV-1 and HTLV-2 glycoproteins.
Regarding infectivity, the behavior of the mutated HTLV-2 glycoproteins was similar to that of the HTLV-1 glycoproteins mutated at equivalent positions (Table 1). Two positions in the HTLV-2 SU glycoprotein sequence were essential for cell-to-cell transmission, as were the equivalent positions in the HTLV-1 SU sequence. These were the arginine at position 90 in HTLV-2 (position 94 in HTLV-1) and the serine at position 97 in HTLV-2 (position 101 in HTLV-1).
The scenario became more complicated in comparisons of the fusion capacities of the SU-mutated glycoproteins of HTLV-2 and HTLV-1 (Table 1). Mutations in the region around amino acid 90 in HTLV-2 resulted in phenotypes that were more pronounced than those obtained with similar mutations in HTLV-1, i.e., a large reduction in fusion capacity along with the reduction in infectivity described above. This was the case for the Arg90-Glu and Ser97-Leu mutations. Thus, we obtained no mutated HTLV-2 SU glycoprotein that was capable of fusion but not of cell-to-cell transmission, in contrast to our observations with HTLV-1 mutated glycoproteins (Arg94-Glu or Ser101-Leu).
The reverse occurred for mutations in the region around amino acid 190. Mutations in this region affected the fusion mediated by the HTLV-1 glycoproteins but had no effect on HTLV-2 envelope-mediated fusion. For instance, the glutamate residue at position 193 in HTLV-2 could be replaced by a nonconservative amino acid with no loss of fusion capacity, whereas the equivalent mutation of aspartate 197 in HTLV-1 greatly reduced envelope-mediated fusion. There was also very little loss of fusion capacity for the HTLV-2 Asp191-Val mutant. Thus, the mutations impairing both syncytium formation and virus entry are clearly clustered in the N-terminal region around amino acid 90 in the HTLV-2 SU glycoprotein, suggesting that this domain may be involved in receptor recognition. Taken together, these results show that the functional domains of the SU subunit of HTLV-1 and HTLV-2 are distinct, albeit related.
Using the same principles as those used for SU mutagenesis, we substituted amino acids of the HTLV-2 TM at positions predicted by our HTLV-1 work to be crucial for function (28). We introduced mutations in the N-terminal part of the ectodomain, which is important for fusion, and a mutation in the C-terminal part of the TM, known to be involved in the postfusion events required for cell-to-cell transmission of the virus (Fig. 1).
The phenotypes obtained for the HTLV-2 glycoproteins with mutations in the TM subunit were the same as those previously described for HTLV-1 (Fig. 2B and Table 2), both in terms of the fusion capacity of the mutated glycoproteins and their ability to allow cell-to-cell transmission of the virus. Thus, the functional domains of the TM subunit are conserved in HTLV-1 and HTLV-2. In particular, the ectodomain of the TM glycoprotein of HTLV-2 is involved in postfusion events required for full infectivity, as is that of HTLV-1 (28).
TABLE 2.
Phenotypes of HTLV-2 TM mutantsa
| Envelope glycoproteinb | Precursor cleavage index (%)c | Syncytium formation index (%)c | Infectivity index (%)c |
|---|---|---|---|
| Wild-type HTLV-2 | 100 | 100 | 100 |
| Wild-type HTLV-1 | 100 | 100 | 100 |
| Ala356-Asp HTLV-2 | 74 | 0 | 0 |
| Ala360-Glu HTLV-1 | 85 | 0 | 0 |
| Ile357-Arg HTLV-2 | 55 | 0 | 0 |
| Ile361-Arg HTLV-1 | 81 | 0 | 0 |
| Gln373-Leu HTLV-2 | 77 | 12 | 10 |
| Gln377-Leu HTLV-1 | 82 | 0 | 22 |
| Arg436-Leu HTLV-2 | 44 | 130 | 0 |
| Arg440-Leu HTLV-1 | 58 | 207 | 0 |
Cleavage, syncytium formation, and infectivity indices were calculated as described elsewhere (7, 28). The amount of SU immunoprecipitated from the supernatant was similar to that for the wild type for all mutated glycoproteins.
Results for HTLV-1-mutated envelope proteins are from our previous study (28).
Data are the means of at least two independent transfections.
In this study, we first determined whether the glycoproteins of the HTLV-1 and HTLV-2 retroviruses could be exchanged without either virus losing infectivity. The great similarity in the glycoprotein sequences of the two viruses, which suggested that such an exchange might be possible, led MacClure et al. to propose that their env genes resulted from some kind of copy choice that had occurred in the past during a period of coinfection by these viruses (23). The cell-to-cell transmission obtained when the viral core of one virus was enveloped with the glycoproteins of the other was similar to that obtained with its own glycoproteins. This result showed that there are functional similarities between the two glycoproteins, given that the two envelope expression plasmids produced similar amounts of glycoprotein (data not shown) and that the two viruses use the same receptor (32, 33). Our in vitro infectivity assay showed that either of the HTLV envelopes mediated the transmission of either of the HTLV cores exclusively via cell-to-cell contact, consistent with the in vivo data showing that neither of the viruses can be transmitted by cell-free body fluids (2, 10, 18).
We next investigated whether the HTLV-2 and HTLV-1 envelope glycoproteins had similar molecular determinants for fusion and cell-to-cell transmission. In the transmission process, the SU ensures binding to a cellular receptor and the TM carries out fusion between membranes. Two domains of the HTLV-1 SU have been identified by mutagenesis studies as critical for the envelope-mediated functions, and they are also the targets of neutralizing antibodies. These regions are located between amino acids 75 and 101 and amino acids 181 and 208 of the HTLV-1 SU glycoprotein. The introduction of mutations into the equivalent domains of the HTLV-2 SU glycoprotein resulted in phenotypes that were different from but related to those obtained with HTLV-1. Mutations of the arginine residue at position 94 and of the serine residue at position 101 in HTLV-1 abolished cell-to-cell transmission, although the mutated glycoproteins were capable of fusion. This result led us to believe that the SU might be involved in postfusion events required for infectivity (7). This was not the case for HTLV-2, in which equivalent mutations at positions 90 and 97 abolished the fusion process and, consequently, cell-to-cell transmission, implicating this domain in receptor recognition rather than in postfusion events. Moreover, the phenotypes resulting from two other mutations introduced into the 77 to 97 region of the HTLV-2 SU showed that this domain was more important for fusion in HTLV-2 than was the corresponding 75 to 101 region in HTLV-1.
Our results are compatible with data showing that the N-terminal regions of the HTLV-1 and HTLV-2 SU glycoproteins contain homologous neutralizing determinants which are type specific (25). Thus, although these domains are crucial for envelope-mediated functions in both viruses and, accordingly, contain epitopes which are targets of neutralizing antibodies, they are not equivalent, either structurally or functionally. The same was true for the 177 to 204 region (region 181 to 208 in HTLV-1), in which mutations only slightly diminished fusion for HTLV-2 compared to that for HTLV-1 and which represents a type-specific epitope (5, 15, 20). These differences in the structure-function relationships of the HTLV-1 and HTLV-2 SU glycoproteins are puzzling given that the two retroviruses use the same receptor at the surface of human cells (32, 33). As there is no binding assay for the HTLVs, we cannot discriminate between receptor recognition and postbinding events. However, we suspect that the HTLV-1 and HTLV-2 SU subunits activate fusion via distinct conformational changes.
Whereas the SU subunits of the envelope heterodimers in HTLV-1 and HTLV-2 behaved differently, the TM subunits functioned in the same way. The retroviral TM glycoproteins have more clearly defined domains than do the SU glycoproteins, because their sequences are analogous to that of the previously crystallized HA2 glycoprotein of the influenza virus (13) and because the atomic structures of the Moloney murine leukemia virus and human immunodeficiency virus type 1 TM ectodomains have recently been determined (3, 12, 37). The region adjacent to the N-terminal fusion peptide contains the leucine zipper-like motif found in all retroviral TM glycoproteins (8), which is probably involved in the extension of the fusion peptide toward the target cell membrane (37). We have shown that this motif is essential for efficient HTLV-1 glycoprotein-mediated fusion (28), as is the case for human immunodeficiency virus type 1 (11, 38) and, as is shown here and elsewhere (26), for HTLV-2.
The most-C-terminal region of the ectodomain of the HTLV-1 TM glycoprotein has a remarkable property (28) which is also a characteristic of the HTLV-2 TM. Although glycoproteins with mutations in this domain are competent for fusion, they do not allow cell-to-cell transmission of HTLV-1 or HTLV-2. Our results with HTLV-1 led us to suggest that the C-terminal part of the TM ectodomain is involved in postfusion events required for infection, although the exact nature of these postfusion events remains unknown. Entry of the viral core into the cytoplasm of the target cell may require additional conformational changes involving the TM ectodomain. The integrity of this domain is required for HTLV-2 transmission, as was previously shown for HTLV-1, further supporting and extending this idea.
Our functional analyses therefore suggest that, although they recognize the same receptor at the surface of target cells, the HTLV-1 and HTLV-2 SU subunits have slightly different ways of transducing the conformational information used to prime a common mechanism of fusion carried out by similar TM subunits.
The sequences of the HTLV-1 and HTLV-2 envelope proteins are 65% conserved in the SU part and 79% conserved in the TM part of the molecule. Reverse transcriptase is the protein that differs least from one retrovirus to another (23). Thus, two retroviruses’ having sequences for another protein that are more similar than those of their reverse transcriptases cannot be due to ordinary divergence. Since the reverse transcriptase sequences of HTLV-1 and HTLV-2 are only 65% identical, the TM subunits of their envelope proteins undoubtedly have an anomalous degree of similarity. Evidence for an anomalous pattern for the SU subunit is, however, less clear, as emphasized by differences in its functioning in HTLV-1 and HTLV-2 that are shown in our work. Thus, if copy choice occurred in the past during a period of coinfection by ancestors of these two human retroviruses, as has been suggested previously (23), it may have involved only the part encoding the TM subunit rather than the entire length of the env gene.
Acknowledgments
Arielle R. Rosenberg and Lélia Delamarre contributed equally to this work.
We are grateful to Jennifer Richardson for reading the manuscript. We also thank Y. Coste (CRTS, Montpellier, France) for providing sera from HTLV-2-infected individuals.
This work was supported by grants from the Association Nationale pour la Recherche sur le SIDA (Paris, France) and from the Association pour la Recherche sur le Cancer (Villejuif, France) as well as by equipment grants from the Ligue Départementale des Yvelines (Versailles, France) and the Fondation pour la Recherche Médicale (Paris, France).
REFERENCES
- 1.Baba E, Nakamura M, Tanaka Y, Kuroki M, Itoyama Y, Nakano S, Niho Y. Multiple neutralizing B-cell epitopes of human T-cell leukemia virus type 1 (HTLV-1) identified by human monoclonal antibodies. J Immunol. 1993;151:1013–1024. [PubMed] [Google Scholar]
- 2.Canavaggio M, Leckie G, Allain J P, Steaffens J W, Laurian Y, Brettler D, Lee H. The prevalence of antibody to HTLV-I/II in United States plasma donors and in United States and French hemophiliacs. Transfusion. 1990;30:780–782. doi: 10.1046/j.1537-2995.1990.30991048781.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen I S Y, McLaughlin J, Golde D W. Long terminal repeats of human T-cell leukemia virus II genome determine target cell specificity. Nature. 1984;309:276–279. doi: 10.1038/309276a0. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y M A, Zhang X Q, Dahl C E, Samuel K P, Schooley R T, Essex M, Papas T S. Delineation of type-specific regions on the envelope glycoproteins of human T cell leukemia viruses. J Immunol. 1991;147:2368–2376. [PubMed] [Google Scholar]
- 6.Delamarre L, Pique C, Pham D, Tursz T, Dokhélar M C. Identification of functional regions in the human T-cell leukemia virus type 1 SU glycoprotein. J Virol. 1994;68:3544–3549. doi: 10.1128/jvi.68.6.3544-3549.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delamarre L, Rosenberg A R, Pique C, Pham D, Dokhélar M C. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J Virol. 1997;71:259–266. doi: 10.1128/jvi.71.1.259-266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delwart E L, Mosialos G, Gilmore T. Retroviral envelope glycoproteins contain a “leucine zipper”-like repeat. AIDS Res Hum Retroviruses. 1990;6:703–706. doi: 10.1089/aid.1990.6.703. [DOI] [PubMed] [Google Scholar]
- 9.Desgranges C, Souche S, Vernant J C, Smadja D, Vahlne A, Horal P. Identification of novel neutralization-inducing regions of the human T cell lymphotropic virus type I envelope glycoproteins with human HTLV-I-seropositive sera. AIDS Res Hum Retroviruses. 1994;10:163–173. doi: 10.1089/aid.1994.10.163. [DOI] [PubMed] [Google Scholar]
- 10.Donegan E, Lee H, Operskalski E A, Shaw G M, Kleinman S H, Busch M P, Stevens C E, Schiff E R, Nowicki M J, Hollingsworth C G, Mosley J W The Transfusion Safety Study Group. Transfusion transmission of retroviruses: human T-lymphotropic virus types I and II compared with human immunodeficiency virus type 1. Transfusion. 1994;34:478–483. doi: 10.1046/j.1537-2995.1994.34694295061.x. [DOI] [PubMed] [Google Scholar]
- 11.Dubay J W, Roberts S J, Brody B, Hunter E. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J Virol. 1992;66:4748–4756. doi: 10.1128/jvi.66.8.4748-4756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fass D, Harrison S C, Kim P S. Retrovirus envelope domain at 1.7 Å resolution. Nat Struct Biol. 1996;3:465–469. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 13.Gallaher W R, Ball J M, Garry R F, Griffin M C, Montelaro R C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989;5:431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- 14.Harrington W J, Jr, Sheremata W, Hjelle B, Dube D K, Bradshaw P, Foung S K, Snodgrass S, Toedter G, Cabral L, Poiesz B. Spastic ataxia associated with human T-cell lymphotropic virus type II infection. Ann Neurol. 1993;33:411–414. doi: 10.1002/ana.410330416. [DOI] [PubMed] [Google Scholar]
- 15.Horal P, Hall W W, Svennerholm B, Lycke J, Jeansson S, Rymo L, Kaplan M H, Vahlne A. Identification of type-specific linear epitopes in the glycoproteins gp46 and gp21 of human T-cell leukemia virus type I and type II using synthetic peptides. Proc Natl Acad Sci USA. 1991;88:5754–5758. doi: 10.1073/pnas.88.13.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ijichi S, Ramundo M B, Takahashi H, Hall W W. In vivo cellular tropism of human T cell leukemia virus type II (HTLV-II) J Exp Med. 1992;176:293–296. doi: 10.1084/jem.176.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson S, Lehky T, Nishimura M, Robinson S, McFarlin D E, Dhib-Jalbut S. Isolation of HTLV-II from a patient with chronic, progressive neurological disease clinically indistinguishable from HTLV-I-associated myelopathy/tropical spastic paraparesis. Ann Neurol. 1993;33:392–396. doi: 10.1002/ana.410330411. [DOI] [PubMed] [Google Scholar]
- 18.Kleinman S, Swanson P, Allain J P, Lee H. Transfusion transmission of human T-lymphotropic virus types I and II: serologic and polymerase chain reaction results in recipients identified through look-back investigations. Transfusion. 1993;33:14–18. doi: 10.1046/j.1537-2995.1993.33193142303.x. [DOI] [PubMed] [Google Scholar]
- 19.Krichbaum-Stenger K, Poiesz B J, Keller P, Ehrlich G, Gavalchin J, Davis B H, Moore J L. Specific adsorption of HTLV-I to various target human and animal cells. Blood. 1987;70:1303–1311. [PubMed] [Google Scholar]
- 20.Lal R B, Rudolph D L, Kaplan J E, Hjelle B, Levine P H, Coligan J E, Viscidi R P. Identification of immunodominant epitopes in envelope glycoprotein of human T lymphotropic virus type II. Virology. 1992;186:274–279. doi: 10.1016/0042-6822(92)90081-y. [DOI] [PubMed] [Google Scholar]
- 21.Landau N R, Page K A, Littman D R. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q X, Camerini D, Xie Y, Greenwald M, Kuritzkes D R, Chen I S Y. Syncytium formation by recombinant HTLV-II envelope glycoprotein. Virology. 1996;218:279–284. doi: 10.1006/viro.1996.0192. [DOI] [PubMed] [Google Scholar]
- 23.McClure M A, Johnson M S, Feng D F, Doolittle R F. Sequence comparisons of retroviral proteins: relative rates of change and general phylogeny. Proc Natl Acad Sci USA. 1988;85:2469–2473. doi: 10.1073/pnas.85.8.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy E L, Hanchard B, Figueroa J P, Gibbs W N, Lofters W S, Campbell M, Goedert J J, Blattner W A. Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int J Cancer. 1989;43:250–253. doi: 10.1002/ijc.2910430214. [DOI] [PubMed] [Google Scholar]
- 25.Palker T J, Riggs E R, Spragion D E, Muir A J, Scearce R M, Randall R R, McAdams M W, McKnight A, Clapham P R, Weiss R A, Haynes B F. Mapping of homologous, amino-terminal neutralizing regions of human T-cell lymphotropic virus type I and II gp46 envelope glycoproteins. J Virol. 1992;66:5879–5889. doi: 10.1128/jvi.66.10.5879-5889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon B, Chen I S Y. Identification of a domain within the human T-cell leukemia virus type 2 envelope required for syncytium induction and replication. J Virol. 1998;72:1959–1966. doi: 10.1128/jvi.72.3.1959-1966.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson J H, Edwards A J, Cruickshank J K, Rudge P, Dalgleish A G. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. 1990;64:5682–5687. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg A R, Delamarre L, Pique C, Pham D, Dokhélar M C. The ectodomain of the human T-cell leukemia virus type 1 TM glycoprotein is involved in postfusion events. J Virol. 1997;71:7180–7186. doi: 10.1128/jvi.71.10.7180-7186.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagara Y, Inoue Y, Shiraki H, Jinno A, Hoshino H, Maeda Y. Identification and mapping of functional domains on human T-cell lymphotropic virus type 1 envelope proteins by using synthetic peptides. J Virol. 1996;70:1564–1569. doi: 10.1128/jvi.70.3.1564-1569.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimotohno K, Takahashi Y, Shimizu N, Gojobori T, Golde D W, Chen I S Y, Miwa M, Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc Natl Acad Sci USA. 1985;82:3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sommerfelt M A, Weiss R A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 33.Sommerfelt M A, Williams B P, Clapham P R, Solomon E, Goodfellow P N, Weiss R A. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988;242:1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- 34.Sutton R E, Littman D R. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J Virol. 1996;70:7322–7326. doi: 10.1128/jvi.70.10.7322-7326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka Y, Tanaka R, Hoshino H. Identification of a novel neutralization epitope on envelope gp46 antigen of human T-cell-leukemia virus-type-II (HTLV-II) Int J Cancer. 1994;59:655–660. doi: 10.1002/ijc.2910590513. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka Y, Zeng L, Shiraki H, Shida H, Tozawa H. Identification of a neutralization epitope on the envelope gp46 antigen of human T cell leukemia virus type I and induction of neutralizing antibody by peptide immunization. J Immunol. 1991;147:354–360. [PubMed] [Google Scholar]
- 37.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 38.Wild C, Dubay J W, Greenwell T, Baird T, Jr, Oas T G, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci USA. 1994;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]