Abstract
To investigate the effect of an exercise-based cardiac rehabilitation program on the quality of life (QoL) of patients with chronic Chagas cardiomyopathy (CCC). PEACH study was a single-center, superiority randomized clinical trial of exercise training versus no exercise (control). The sample comprised Chagas disease patients with CCC, left ventricular ejection fraction < 45%, without or with HF symptoms (CCC stages B2 or C, respectively). QoL was assessed at baseline, after three months, and at the end of six months of follow-up using the SF-36 questionnaire. Patients randomized for the exercise group (n = 15) performed exercise training (aerobic, strength and stretching exercises) for 60 min, three times a week, during six months. Patients in the control group (n = 15) were not provided with a formal exercise prescription. Both groups received identical nutritional and pharmaceutical counseling during the study. Longitudinal analysis of the effects of exercise training on QoL, considering the interaction term (group × time) to estimate the rate of changes between groups in the outcomes (represented as beta coefficient), was performed using linear mixed models. Models were fitted adjusting for each respective baseline QoL value. There were significant improvements in physical functioning (β = + 10.7; p = 0.02), role limitations due to physical problems (β = + 25.0; p = 0.01), and social functioning (β = + 19.2; p < 0.01) scales during the first three months in the exercise compared to the control group. No significant differences were observed between groups after six months. Exercise-based cardiac rehabilitation provided short-term improvements in the physical and mental aspects of QoL of patients with CCC.
Trial registration: ClinicalTrials.gov Identifier: NCT02517632; August 7, 2015.
Keywords: Cardiac rehabilitation, Chagas cardiomyopathy, Neglected diseases, Exercise, Heart failure, Quality of life
Subject terms: Cardiomyopathies, Heart failure, Quality of life, Rehabilitation
Introduction
Chagas disease (CD) is a parasitic infection caused by the protozoan Trypanosoma cruzi, which can progress into chronic Chagas cardiomyopathy (CCC) in approximately 30% of chronically infected patients1. CCC is characterized by arrhythmias, thromboembolism, and heart failure (HF), which may cause fatigue, dyspnea, and gradual decline in functional capacity, ultimately decreasing patients’ quality of life (QoL)2.
Patients with CCC typically experience lower QoL compared to healthy individuals3, those with the indeterminate form of CD4,5, and individuals with HF from other etiologies5–7. QoL can reflect the patient’s perception of a disease’s functional impact on different aspects of life3, and has recently emerged as an important health construct associated with hospitalization and mortality8,9. In patients with CCC, QoL has proven to be useful in screening for cardiac dysfunction10 and predicting adverse cardiovascular events11.
Exercise-based cardiac rehabilitation (CR) holds potential value in improving the QoL of patients with different heart diseases12–14. However, little evidence is available addressing the influence of CR on QoL in patients with CCC, as these patients are typically not included in clinical trials15. To the best of our knowledge, only three articles16–18 have examined the impact of exercise on QoL in patients with CCC, yielding divergent results and some methodological limitations. Lima et al. encountered measurement interpretation issues and included patients with preserved functional class16, while Mediano et al. and Mediano et al. used a single-group design with a small sample size17,18. Therefore, the impact of CR on QoL in CCC patients remains uncertain. Evaluating and comprehending these aspects is of paramount importance, considering the clinical complexities of the disease. Understanding QoL allows healthcare providers to tailor treatment plans and interventions to address not only the medical aspects of a condition, but also its impact on patients' overall well-being, resulting in a more effective and comprehensive care.
Thus, we aimed to investigate the effect of an exercise-based CR program on the QoL of patients with CCC. We hypothesized that exercise training would improve both the physical and mental aspects of QoL in these patients.
Methods
Study design
The complete description and the main results for clinical and functional variables of the PEACH study (“Exercise Program in Chagas Heart Disease” in Portuguese) have been previously published19,20. In short, PEACH study was a single-center, superiority randomized parallel-group clinical trial of exercise training versus no exercise training (control), conducted from March 2015 to January 2017 at the Evandro Chagas National Institute of Infectious Diseases (INI) of the Oswaldo Cruz Foundation (Fiocruz). Individuals followed at INI were sequentially recruited to participate in the study. The sample comprised CD patients (confirmed by two distinct serological tests) of both sexes, older than 18 years, diagnosed with CCC, left ventricular ejection fraction (LVEF) < 45%, without or with HF symptoms (CCC stages B2 or C, respectively), New York Heart Association (NYHA) functional class I or II during three months before study enrollment, clinically stable and under optimal medical therapy according to HF guidelines over the prior six weeks before study enrollment. Exclusion criteria were the presence of major comorbidities or limitations that could preclude exercise training, pregnancy, unavailability to attend exercise sessions 3 times a week, practice of regular exercise training at baseline (> 1 week) in the three months prior to the study, smoking, or evidence of associated non-CCC.
Sealed envelopes filled with a computer-generated sequence were used to randomly allocate the eligible patients between the two groups in a 1:1 ratio using WinPepi software. The sequence was generated in blocks and stratified according to CCC stages (B2 and C) by a single researcher who was not involved in the recruitment.
Sample size calculation for the present study considered a significant mean difference between groups of 42.98 in the SF-36 role limitations due to physical problems scale, with a standard deviation of 39.43 points for the control and 34.32 points for the intervention group21. Assuming α = 0.05 and β = 0.20, 26 participants were required (13 in the intervention group and 13 in the control group).
Measurements
Sociodemographic (age, sex, schooling, and self-reported race), anthropometric, clinical, cardiac function, and maximal progressive cardiopulmonary exercise test (CPET) variables of the eligible patients were obtained during the initial assessment.
Schooling included the years of formal study, stratified into two categories (< 9 years and ≥ 9 years). Self-reported race was recategorized as white and non-white (including black, mulatto, indigenous, and yellow). Anthropometric evaluation consisted of measurements of height and weight, according to Lohman et al.22. Body mass index (BMI) was calculated as the ratio of weight (kg) to height squared (m2) and classified according to the World Health Organization definition23. Clinical variables, such as stage of CCC, NYHA functional class, presence of electrocardiogram abnormalities (ventricular premature beats, sustained and non-sustained ventricular tachycardia, right branch block, left anterior hemiblock, non-specific ventricular repolarization changes, atrial fibrillation, first-degree atrioventricular block, and second-degree atrioventricular block), and medications, were obtained from medical records. Systolic cardiac function was determined by means of left ventricular ejection fraction (LVEF) using the modified Simpson’s rule24. Maximal symptom-limited CPET was performed on a treadmill (Inbramed, Porto Alegre, RS, Brazil) with a ramp protocol and active recovery, using a VO2000 gas analyzer (MedGraphics, St. Paul, MN, USA) connected to a computerized Ergo PC Elite system (Micromed, Brasília, DF, Brazil).
QoL was assessed at baseline, after three months and at the end of follow-up (six months) using the Portuguese version of the Medical Outcomes Study 36-Item Short-form of Health Survey (SF-36) questionnaire25–27. This instrument has good internal consistency, test–retest reliability, and construct validity in Brazilian populations across different age groups and health conditions, including individuals with CCC11,27–32. SF-36 is a generic multidimensional instrument consisting of 36 questions, referring to the four weeks prior to the interview and divided into eight different scales: physical functioning, role limitations due to physical problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems and mental health. These scales define two summary scores: physical component summary (PCS) and mental component summary (MCS). Both PCS and MCS have contributions from all eight scales, but with higher weights for the first four scales in the PCS and for the last four scales in the MCS. The final score ranges from zero (worse QoL) to 100 (best QoL)25,26.
Intervention
Patients randomized for the exercise group performed physical exercise sessions for 60 min, three times a week, for six months. Each session consisted of 30 min of aerobic exercise on a treadmill or cycle ergometer, 20 min of strength training comprising two sets of 12 repetitions for the major muscle groups (sit-ups, push-ups, and pull-ups), and 10 min of stretching exercises. Aerobic exercise intensity was set according to the heart rate at anaerobic threshold obtained during the CPET (90–100% of heart rate at anaerobic threshold in the first month of exercise training and 100–110% of heart rate at anaerobic threshold thereafter)33. The anaerobic threshold values used to determine the target heart rate were derived from the baseline cardiopulmonary exercise tests for training sessions conducted between baseline and the 3rd month, and from the 3-month cardiopulmonary exercise test for training sessions between the 3rd and 6th months. For those patients in which anaerobic threshold was not identified during the CPET (n = 13; 43%), training intensity was prescribed according to the Hellerstein formula [HR = (102 + maximum metabolic equivalents achieved)/1.41)]34, in which the target heart rate ranged from 70% of maximum heart rate obtained in the CPET to the Hellerstein’s formula percentage in the first month, and from the Hellerstein’s formula percentage to 85% of maximum heart rate in the following months. Borg scale was used as an adjuvant of exercise intensity prescription (targeting 2–4 in CR10 Borg scale). The intensity of exercise was controlled by an exercise physiologist that supervised all exercise sessions. All exercise sessions were center-based and carried out in the morning, in an indoor environment with controlled temperature and under a multidisciplinary supervision (including an exercise physiologist and physician). No guidance was given to participants for home-based exercises (outside of the center-based program). Patients in the control group were not provided with a formal exercise prescription.
During the study, patients from both groups underwent monthly appointments with their cardiologist, based on the recommendations of the Brazilian Consensus on CD35. In addition, both groups received identical nutritional and pharmaceutical counseling during the study. Nutritional counseling consisted of general orientation about healthy eating habits, such as reducing the consumption of saturated fatty acids and increasing the intake of poly- and monounsaturated fatty acids, vitamins, and high-fiber carbohydrates. Reductions in sodium consumption and water intake were also stimulated for those patients with HF. Pharmaceutical care consisted of guiding about medication usage, drug dosage, and compliance, added to the monthly distribution of personalized packages, with the pills organized by the time and days that should be taken, according to medical prescription19.
Data analysis
Descriptive analysis consisted of mean and standard deviation for continuous variables and number of observations and percentage for categorical variables. The longitudinal analysis of the effects of exercise-based CR on QoL was performed using mixed linear models. This approach enable us to assess the interaction term (group x time) to estimate the rate of changes between groups in the outcomes. Models were fitted adjusting for each respective baseline QoL value. All participants were considered in the statistical analysis, regardless of compliance or loss to follow-up, characterizing an intention-to-treat analysis. Line graphs were constructed to visually illustrate the crude trajectories of QoL scales during the follow-up in each group.
The Research Electronic Data Capture (REDCap) web application was used for data management and the data analysis was conducted using Stata 13.0. Statistical significance was set at p ≤ 0.05 for all analyses.
Ethical considerations
All participants received information about the goals and procedures of the study and voluntarily agreed to participate by means of signing a written informed consent. The study was performed in accordance to the resolution 466/2012 of the Brazilian National Council of Health and was approved by the Institutional Research Ethics Committee (CAAE: 38038914.6.0000.5262) in February, 2015. The clinical trial was registered at ClinicalTrials.gov (NCT02517632).
Results
A flow chart of participant inclusion and follow-up is depicted in Fig. 1. Losses to follow-up during the six months were restricted to one participant in the control group that died between 3- and 6- month visits from non-CCC related cause. Exercise group achieved 80% of attendance to training sessions during the first three months and an overall compliance of 74% at the end of six months of follow-up.
Figure 1.
Flow chart of screening, randomization, and follow-up of participants included in the study.
Sample characteristics
Baseline characteristics of the patients included in each arm are shown in Table 1. The overall mean age was 59.8 (± 10.0) years, most were men (66.7%), with the majority presenting stage C of CCC (73.3%). In relation to baseline QoL assessments, patients presented lower scores in the physical scales compared to the mental scales, with the overall mean summary scores equal to 43.0 (± 9.8) for PCS and 53.0 (± 11.7) for MCS.
Table 1.
Baseline characteristics of participants included in the study (n = 30).
| Variable | Control (n = 15) | Exercise (n = 15) |
|---|---|---|
| Age (years) | 60.7 (10.6) | 57.9 (9.5) |
| Sex | ||
| Women | 6 (40.0%) | 4 (26.7%) |
| Men | 9 (60.0%) | 11 (73.3%) |
| Schooling | ||
| < 9 years | 13 (86.7%) | 12 (80.0%) |
| ≥ 9 years | 2 (13.3%) | 3 (20.0%) |
| Race | ||
| White | 4 (26.7%) | 8 (53.3%) |
| Non-white | 11 (73.3%) | 7 (46.7%) |
| Clinical form of CCC | ||
| B2 (without heart failure) | 4 (26.7%) | 4 (26.7%) |
| C (with heart failure) | 11 (73.3%) | 11 (73.3%) |
| LVEF (%) | 30.9 (7.0) | 32.3 (8.7) |
| VO2 peak (ml kg−1 min−1) | 15.4 (6.3) | 17.6 (4.7) |
| BMI (kg/m2) | 25.6 (4.3) | 25.1 (6.2) |
| Hypertension | 1 (6.7%) | 0 (0.0%) |
| Diabetes mellitus | 2 (13.3%) | 3 (20.0%) |
| Dyslipidemia | 4 (26.7%) | 5 (33.3%) |
| Stroke | 0 (0.0%) | 5 (33.3%) |
| Electrocardiogram abnormalities | 12 (80%) | 10 (66.7%) |
| Cardiac device | 9 (60.0%) | 5 (33.3%) |
| Medicationsa | ||
| Beta-blocker | 14 (93.3%) | 15 (100%) |
| ACEI or ARB | 14 (93.3%) | 14 (93.3%) |
| Diuretics | 10 (66.7%) | 12 (80.0%) |
| Aldosterone antagonist | 8 (53.3%) | 7 (46.7%) |
| Amiodarone | 2 (13.3%) | 4 (26.7%) |
| Anticoagulants | 7 (46.7%) | 7 (46.7%) |
| SF-36 QoL scales and summaries | ||
| Physical functioning | 60.7 (26.2) | 71.0 (26.5) |
| Role limitations due to physical problems | 58.3 (46.0) | 61.7 (46.2) |
| Bodily pain | 59.8 (19.9) | 87.1 (19.0) |
| General health perceptions | 53.6 (18.1) | 70.8 (23.2) |
| Physical component summary | 39.5 (9.3) | 46.6 (9.3) |
| Vitality | 60.0 (29.4) | 73.7 (22.8) |
| Social functioning | 85.2 (21.9) | 81.0 (25.8) |
| Role limitations due to emotional problems | 64.4 (42.7) | 66.7 (45.4) |
| Mental health | 76.8 (23.8) | 85.6 (15.5) |
| Mental component summary | 52.4 (12.4) | 53.7 (11.3) |
aMedications: Beta-blocker: carvedilol; Angiotensin-converting enzyme inhibitors: enalapril and captopril; Angiotensin receptor blockers: losartan; Diuretics: furosemide and hydrochlorothiazide; Aldosterone antagonist: spironolactone; Anticoagulants: warfarin.
CCC chronic Chagas cardiomyopathy, LVEF left ventricular ejection fraction, VO2 peak oxygen intake at peak exercise, BMI body mass index, ACEI angiotensin-converting enzyme inhibitors, ARB angiotensin receptor blockers, SF-36 Medical Outcomes Study 36-Item Short-form of Health Survey, QoL quality of life.
Effect of physical exercise training on quality of life
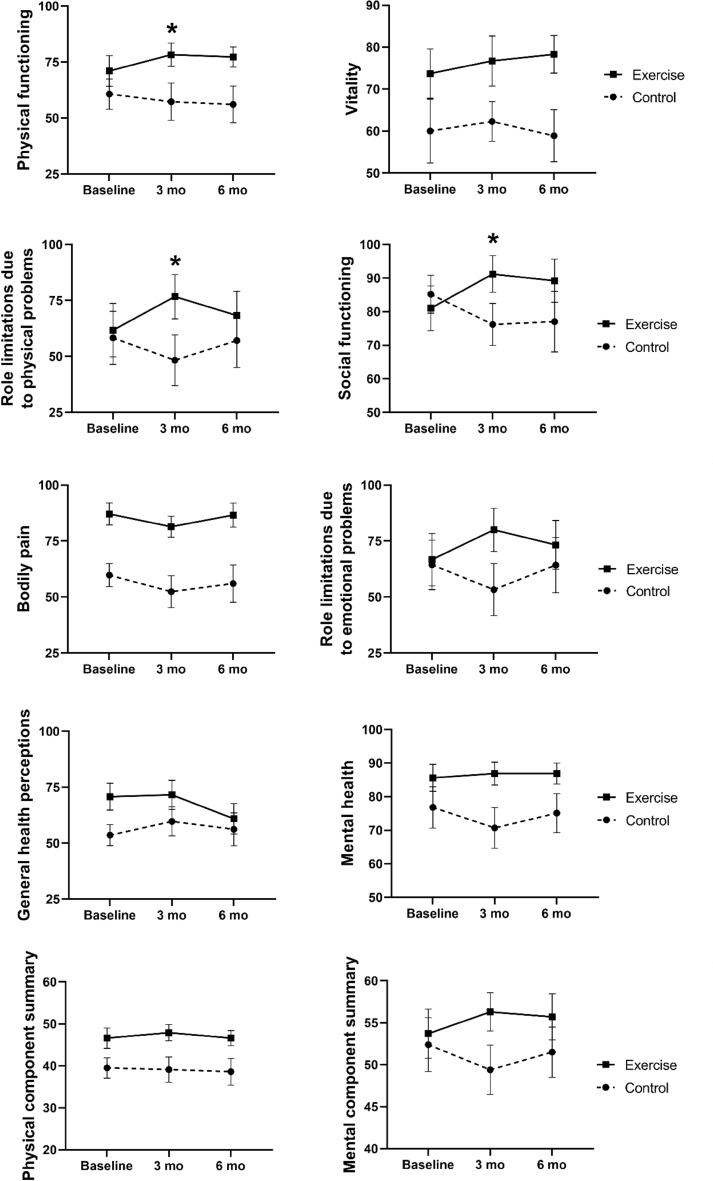
The effects of physical exercise during the follow-up are depicted in Table 2, while Fig. 2 illustrates the crude trajectories for QoL scales during the follow-up in each group.
Table 2.
Crude means (standard deviation) and adjusted beta values for QoL at 3 and 6- month of follow-up.
| SF-36 QoL scales | 3 months Control n = 15 (100%) Exercise n = 15 (100%) |
6 months Control n = 14 (93.3%) Exercise n = 15 (100%) |
||||
|---|---|---|---|---|---|---|
| Mean (sd) | β | pa | Mean (sd) | β | pa | |
| Physical functioning | ||||||
| Control | 57.3 (32.1) | + 10.7 | 0.02 | 56.1 (30.6) | + 13.6 | 0.08 |
| Exercise | 78.3 (20.1) | 77.3 (17.4) | ||||
| Role limitations due to physical problems | ||||||
| Control | 48.3 (43.8) | + 25.0 | 0.01 | 57.1 (45.4) | + 2.9 | 0.85 |
| Exercise | 76.7 (38.3) | 68.3 (41.7) | ||||
| Bodily pain | ||||||
| Control | 52.4 (27.5) | + 4.3 | 0.62 | 56.0 (31.3) | + 7.2 | 0.48 |
| Exercise | 81.4 (18.1) | 86.6 (20.9) | ||||
| General health perceptions | ||||||
| Control | 59.7 (25.2) | − 5.3 | 0.40 | 56.2 (27.5) | − 11.0 | 0.20 |
| Exercise | 71.6 (25.2) | 60.9 (26.3) | ||||
| Physical component summary | ||||||
| Control | 39.1 (11.7) | + 1.7 | 0.47 | 38.6 (11.9) | + 0.6 | 0.84 |
| Exercise | 47.9 (7.4) | 46.6 (7.1) | ||||
| Vitality | ||||||
| Control | 62.3 (18.4) | + 0.7 | 0.13 | 58.9 (23.1) | + 7.6 | 0.88 |
| Exercise | 76.7 (23.2) | 78.3 (17.4) | ||||
| Social functioning | ||||||
| Control | 76.2 (24.1) | + 19.2 | < 0.01 | 77.0 (33.7) | + 16.7 | 0.14 |
| Exercise | 91.2 (21.1) | 89.2 (24.9) | ||||
| Role limitations due to emotional problems | ||||||
| Control | 53.3 (45.1) | + 24.4 | 0.09 | 64.3 (46.2) | + 10.4 | 0.59 |
| Exercise | 80.0 (37.4) | 73.3 (42.2) | ||||
| Mental health | ||||||
| Control | 70.7 (23.3) | + 7.5 | 0.09 | 75.1 (21.6) | + 7.2 | 0.23 |
| Exercise | 86.9 (13.2) | 86.9 (12.1) | ||||
| Mental component summary | ||||||
| Control | 49.4 (11.4) | + 5.6 | 0.06 | 51.5 (11.2) | + 4.7 | 0.28 |
| Exercise | 56.3 (8.8) | 55.7 (10.6) | ||||
aLinear mixed models including the interaction between time, group, and time x group adjusted for baseline values.
SF-36 Medical Outcomes Study 36-Item Short-form of Health Survey, QoL quality of life.
Estimates in [bold] are statistically significant
Figure 2.
Estimated mean changes from baseline for quality of life.
There were significant improvements in physical functioning (β = + 10.7; p = 0.02), role limitations due to physical problems (β = + 25.0; p = 0.01) and social functioning (β = + 19.2; p < 0.01) scales during the first three months in the exercise group compared to the control group. However, no significant differences were observed between groups after six months of follow-up.
Discussion
The main findings of the present study consisted of short-term improvements in some physical and mental aspects of QoL. These results are in accordance with previous studies that suggested a beneficial influence of exercise training on QoL of patients with CCC16,18. In addition, the relatively high compliance with the exercise protocol used in the present study in comparison to previous studies in the literature suggests that CR is a feasible strategy and should be encouraged as a coadjuvant in the treatment of CCC36–38.
The influence of exercise training on QoL among patients with other cardiovascular diseases has been previously investigated in the literature. A meta-analysis of randomized controlled trials conducted by Dallas et al. included 5,786 patients with HF and showed significant improvements on physical and mental QoL scales following exercise training (− 0.82, 95% CI − 1.02 to − 0.62; p = 0.00001), regardless of the instrument used to measure QoL14. On the other hand, Quittan et al. used the SF-36 questionnaire to compare the effects of three months of aerobic exercise training (intervention group) or usual daily living activities (control group) in the QoL of HF patients. Similar to our results, they found significant improvements in physical functioning and role limitations due to physical problems scales39.
Considering patients with CCC, the studies conducted so far presented conflicting results. Mediano et al. concluded that an 8-month exercise-based CR program improved the physical scales of QoL in a sample of patients with severe CCC18. In opposition, the study conducted by Lima et al. found unchanged physical QoL aspects following exercise training16. This surprising lack of improvement observed in the latter study may be explained by the fact that 63% of the patients did not present any kind of functional limitations (NYHA class I), with the remaining 37% presenting only mild limitations (NYHA class II)16. On the other hand, a greater percentage of patients included in our study referred some degree of functional limitations, representing a population that can benefit more from the improvements in functional capacity promoted by physical exercise training20.
Social functioning scale usually reflects the impact of physical health or emotional issues on social activities25. Interestingly, it was the only mental scale that presented a significant improvement following exercise training, although role limitations due to emotional problems, mental health, and MCS reached borderline p-values after three months of follow-up (p < 0.10). This result may be related to improvements on functional capacity due to exercise training20, which could enable patients to engage in a greater number of social activities. In addition, participation in a CR program, with regular social support from the CR team and other patients, appears to be beneficial for the patient's social functioning by itself40. Our results are partially in line with those observed by Lima et al., that also found improvements in QoL mental scales (vitality, role limitations due to emotional problems, and mental health, but not in social functioning)16. Conversely, Mediano et al. found no positive influence of an 8-month exercise-training program on any QoL mental scales in patients with severe CCC18. Studies including patients with heart diseases from other etiologies did not show significant effects of physical exercise on the social functioning scale21,39. Patients with CCC may experience distinct psychosocial challenges, including stigma, anxiety, and depression, which could affect their social functioning differently than patients with other more prevalent heart diseases. Sociocultural differences among patient populations with different heart diseases may also influence their perceptions of and responses to CR programs, potentially impacting their social functioning outcomes36,37,41–43.
The short-term improvements (3 months) on QoL obtained in the exercise training group in comparison to controls were not sustained up to the end of the study follow-up (6 months), except for the borderline change on physical functioning scale after six months of follow-up (p = 0.08). This unexpected result can be partially explained by the potential influence of the nutritional and pharmaceutical interventions offered to the control group throughout the study period, which may have positively impacted their QoL, as previously demonstrated44. In addition, the multidisciplinary care provided during each physical exercise session, which allowed a close observation of potential clinical decompensation that were treated immediately, may have influenced the result45. Moreover, untrained individuals tend to present optimistic expectations when starting a physical exercise program. Thus, the level of interest may be higher in the first three months, decreasing later, which was reflected in the reduction in the perception of QoL at the end of the six months of follow-up46.
The present study has limitations and strengths. First, considering that exercise training is a behavioral intervention, masking the study participants as well as the CR team was not possible. The greater frequency of interactions between participants in the exercise training group and CR health professionals may have influenced the differences observed in QoL outcomes between the groups. However, it is important to acknowledge that in a clinical trial focusing on physical exercise within a cardiac rehabilitation context, mitigating such influences is always challenging. Nevertheless, participants from both groups attended monthly appointments with their cardiologist, in addition to receiving identical nutritional and pharmaceutical counseling during the study. These efforts were made to ensure parity in non-exercise aspects of care. Secondly, despite SF-36 is the most widely used instrument to evaluate QoL, this instrument is not validated for use in CD population, which may result in a less accurate assessment of QoL and increased risk of nondifferential error. Moreover, the SF-36 questionnaire may not evaluate specific cognitive features associated with physical activity, such as self-efficacy, which has been demonstrated to affect QoL changes related to physical activity47,48. The lack of evaluation of total physical activity levels during the study (besides attendance rates to training sessions) may also be ackowledged as a limitation, making difficult the identification of participants that changed their physical activity habits during the study, especially in the control group, which may have driven our results to the null hypothesis. Besides that, the sample was composed of patients regularly followed in a national reference center for treatment of infectious diseases, which may limit the external validity. The sample size was calculated based only on primary outcome, which prevented us from conducting subgroup analysis. However, the study design with strict patient´s follow-up and the relatively long-term duration of the study are potential strengths.
To conclude, the main finding of the present study is that exercise-based CR provided short-term improvements in the physical and mental aspects of QoL of patients with CCC. These results are of great clinical meaning since CR is a simple and low-cost tool to improve patients' QoL, especially in endemic areas. Future studies examining the dose–response relationship between exercise and QoL and potential influence of different lifestyle intervention strategies (isolated or combined) on long-term QoL responses are necessary to support clinical recommendations for patients with CCC.
Author contributions
F.S.N.S.M., P.S.S., G.M.S.S., A.S.S., R.M.S., M.T.H., A.M.H.M., P.E.A.A.B., and M.F.F.M. contributed to the study conception and design and in data collection and treatment. Data analysis was performed by M.C.V. and M.F.F.M. The first draft of the manuscript was written by M.C.V. and M.F.F.M. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pérez-Molina JA, Molina I. Chagas disease. Lancet. 2018;391(10115):82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- 2.Saraiva RM, Mediano MFF, Mendes FSNS, Sperandio da Silva GM, Veloso HH, Sangenis LHC, da Silva PS, Mazzoli-Rocha F, Sousa AS, Holanda MT, Hasslocher-Moreno AM. Chagas heart disease: An overview of diagnosis, manifestations, treatment, and care. World J. Cardiol. 2021;13(12):654–675. doi: 10.4330/wjc.v13.i12.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira BG, Abreu MN, Abreu CD, Rocha MO, Ribeiro AL. Health-related quality of life in patients with Chagas disease. Rev. Soc. Bras. Med. Trop. 2011;44(2):150–156. doi: 10.1590/s0037-86822011005000002. [DOI] [PubMed] [Google Scholar]
- 4.Gontijo ED, Guimarães TN, Magnani C, Paixão GM, Dupin S, Paixão LM. Qualidade de vida dos portadores de doença de Chagas. Rev. Méd. Minas Gerais. 2009;19(4):281–286. [Google Scholar]
- 5.Pelegrino VM, Dantas RA, Ciol MA, Clark AM, Rossi LA, Simões MV. Health-related quality of life in Brazilian outpatients with Chagas and non-Chagas cardiomyopathy. Heart Lung. 2011;40(3):e25–e31. doi: 10.1016/j.hrtlng.2010.05.052. [DOI] [PubMed] [Google Scholar]
- 6.Paz LFA, Medeiros CA, Martins SM, Bezerra SMMDS, Oliveira Junior W, Silva MBA. Quality of life related to health for heart failure patients. Rev. Bras. Enferm. 2019;72:140–146. doi: 10.1590/0034-7167-2018-0368. [DOI] [PubMed] [Google Scholar]
- 7.Olivera MJ, Fory JA, Buitrago G. Comparison of health-related quality of life in outpatients with Chagas and matched non-Chagas chronic heart failure in Colombia: A cross-sectional analysis. Am. J. Trop. Med. Hyg. 2021;104(3):951–958. doi: 10.4269/ajtmh.20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of serial Kansas city cardiomyopathy questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: A secondary analysis of 2 randomized clinical trials. JAMA Cardiol. 2017;2(12):1315–1321. doi: 10.1001/jamacardio.2017.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, Abbate A. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019;73(17):2209–2225. doi: 10.1016/j.jacc.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 10.Ávila MR, Figueiredo PHS, Lima VP, Silva WT, Vianna MVA, Fernandes LHC, de Azevedo ACA, Lima MMO, de Carvalho Bastone A, do Carmo Pereira Nunes M, Mediano MFF, da Costa Rocha MO, Costa HS. Accuracy of health-related quality of life in identifying systolic dysfunction in patients with Chagas cardiomyopathy. Trop. Med. Int. Health. 2021;26(8):936–942. doi: 10.1111/tmi.13590. [DOI] [PubMed] [Google Scholar]
- 11.Costa HS, Lima MMO, Figueiredo PHS, Chaves AT, Nunes MCP, da Costa Rocha MO. The prognostic value of health-related quality of life in patients with Chagas heart disease. Qual. Life Res. 2019;28(1):67–72. doi: 10.1007/s11136-018-1980-7. [DOI] [PubMed] [Google Scholar]
- 12.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O'Connor CM, Weinfurt KP, Investigators HF-ACTION. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal HM, Lough F, Rees K, Singh S, Taylor RS. Exercise-based rehabilitation for heart failure: Systematic review and meta-analysis. Open Heart. 2015;2(1):e000163. doi: 10.1136/openhrt-2014-000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallas K, Dinas PC, Chryssanthopoulos C, Dallas G, Maridaki M, Koutsilieris M, Philippou A. The effects of exercise on VO2 peak, quality of life and hospitalization in heart failure patients: A systematic review with meta-analyses. Eur. J. Sport Sci. 2021;21(9):1337–1350. doi: 10.1080/17461391.2020.1846081. [DOI] [PubMed] [Google Scholar]
- 15.Bocchi EA. Exercise training in Chagas’ cardiomyopathy: Trials are welcome for this neglected heart disease. Eur. J. Heart Fail. 2010;12(8):782–784. doi: 10.1093/eurjhf/hfq124. [DOI] [PubMed] [Google Scholar]
- 16.Lima MM, Rocha MO, Nunes MC, Sousa L, Costa HS, Alencar MC, Britto RR, Ribeiro AL. A randomized trial of the effects of exercise training in Chagas cardiomyopathy. Eur. J. Heart Fail. 2010;12(8):866–873. doi: 10.1093/eurjhf/hfq123. [DOI] [PubMed] [Google Scholar]
- 17.Mediano MFF, Mendes FSNS, Pinto VL, Silva GM, Silva PS, Carneiro FM, Sangenis LH, Saraiva RM, Xavier SS, Brasil PE, Hasslocher-Moreno AM, Sousa AS. Cardiac rehabilitation program in patients with Chagas heart failure: A single-arm pilot study. Rev. Soc. Bras. Med. Trop. 2016;49(3):319–328. doi: 10.1590/0037-8682-0083-2016. [DOI] [PubMed] [Google Scholar]
- 18.Mediano MFF, Mendes FSNS, Pinto VLM, Silva PSD, Hasslocher-Moreno AM, Sousa AS. Reassessment of quality of life domains in patients with compensated Chagas heart failure after participating in a cardiac rehabilitation program. Rev. Soc. Bras. Med. Trop. 2017;50(3):404–407. doi: 10.1590/0037-8682-0429-2016. [DOI] [PubMed] [Google Scholar]
- 19.Mendes FSNS, Sousa AS, Souza FC, Pinto VL, Silva PS, Saraiva RM, Xavier SS, Veloso HH, Holanda MT, Costa AR, Carneiro FM, Silva GM, Borges JP, Tibiriça E, Pinheiro RO, Lara FA, Hasslocher-Moreno AM, Brasil PE, Mediano MF. Effect of physical exercise training in patients with Chagas heart disease: Study protocol for a randomized controlled trial (PEACH study) Trials. 2016;17(1):433. doi: 10.1186/s13063-016-1553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendes FSNS, Mediano MFF, de Castro E, Souza FC, da Silva PS, Carneiro FM, de Holanda MT, Saraiva RM, Xavier SS, Americano do Brasil PEA, de Sousa AS. Effect of physical exercise training in patients with Chagas heart disease (from the PEACH STUDY) Am J. Cardiol. 2020;125(9):1413–1420. doi: 10.1016/j.amjcard.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Pozehl B, Duncan K, Krueger S, VerMaas P. Adjunctive effects of exercise training in heart failure patients receiving maximum pharmacologic therapy. Prog. Cardiovasc. Nurs. 2003;18(4):177–183. doi: 10.1111/j.0889-7204.2003.02414.x. [DOI] [PubMed] [Google Scholar]
- 22.Lohman, T. G., Roche, A. F. & Martorel, R. Anthropometric stardization reference manual. Human Kinectis (1988).
- 23.World Health Organization. Obesity: Preventing and managing the global epidemic: report of a WHO consultation. WHO (2000). [PubMed]
- 24.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Ware, J. E., Kosinski, M., & Keller, S. D. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Health Assessment Lab (1994).
- 27.Ciconelli RM, Ferraz MB, Santos W, Meinão I, Quaresma MR. Tradução para a língua portuguesa e validação do questionário genérico de avaliação de qualidade de vida SF-36 (Brasil SF-36) Rev. Bras. Reumatol. 1999;39(3):143–150. [Google Scholar]
- 28.Contopoulos-Ioannidis DG, Karvouni A, Kouri I, Ioannidis JP. Reporting and interpretation of SF-36 outcomes in randomised trials: Systematic review. BMJ (Clin. Res. Ed.) 2009;338:a3006. doi: 10.1136/bmj.a3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima MG, Barros MB, César CL, Goldbaum M, Carandina L, Ciconelli RM. Health related quality of life among the elderly: A population-based study using SF-36 survey. Cad. Saude Publica. 2009;25(10):2159–2167. doi: 10.1590/s0102-311x2009001000007. [DOI] [PubMed] [Google Scholar]
- 30.Laguardia J, Campos MR, Travassos CM, Najar AL, Anjos LA, Vasconcellos MM. Psychometric evaluation of the SF-36 (v.2) questionnaire in a probability sample of Brazilian households: results of the survey Pesquisa Dimensões Sociais das Desigualdades (PDSD), Brazil, 2008. Health Qual. Life Outcomes. 2011;9:61. doi: 10.1186/1477-7525-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulugeta H, Sinclair PM, Wilson A. Health-related quality of life of people with heart failure in low- and middle-income countries: A systematic review and meta-analysis. Qual. Life Res. 2023 doi: 10.1007/s11136-023-03563-2.Advanceonlinepublication. [DOI] [PubMed] [Google Scholar]
- 32.Soloveva A, Gale CP, Han NT, Hurdus B, Aktaa S, Palin V, Mebrahtu TF, Van Spall H, Batra G, Dondo TB, Bäck M, Munyombwe T. Associations of health-related quality of life with major adverse cardiovascular and cerebrovascular events for individuals with ischaemic heart disease: Systematic review, meta-analysis and evidence mapping. Open Heart. 2023;10(2):e002452. doi: 10.1136/openhrt-2023-002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalho T, Milani M, Ferraz AS, Silveira ADD, Herdy AH, Hossri CAC, Silva CGSE, Araújo CGS, Rocco EA, Teixeira JAC, Dourado LOC, Matos LDNJ, Emed LGM, Ritt LEF, Silva MGD, Santos MAD, Silva MMFD, Freitas OGA, Nascimento PMC, Stein R, Meneghelo RS, Serra SM. Brazilian Cardiovascular Rehabilitation Guideline—2020. Diretriz Brasileira de Reabilitação Cardiovascular—2020. Arq. Bras. Cardiol. 2020;114(5):943–987. doi: 10.36660/abc.20200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellerstein HK, Franklin BA. Rehabilitation of Coronary Patient. John Wiley; 1978. [Google Scholar]
- 35.Dias JCP, Ramos AN, Jr, Gontijo ED, Ostermayer AL, Shikanai-Yasuda MA, Coura JR, Torres RM, Melo JRC, Almeida EA, Oliveira W, Jr, Silveira AC, Rezende JM, Pinto FS, Ferreira AW, Rassi A, Fragata Filho AA, Sousa AS, Correia D, Jansen AM, Andrade GMQ, Britto C, Pinto AYN, Rassi A, Jr, Campos DE, Abad-Franch F, Santos SE, Chiari E, Hasslocher-Moreno AM, Moreira EF, Marques DSO, Silva EL, Marin-Neto JA, Galvão LMC, Xavier SS, Valente SAS, Carvalho NB, Cardoso AV, Costa VM, Vivaldini SM, Oliveira SM, Valente VC, Lima MM, Alves RV. 2nd Brazilian consensus on Chagas Disease, 2015. Rev. Soc. Bras. Med. Trop. 2016;49:3–60. doi: 10.1590/0037-8682-0505-2016. [DOI] [PubMed] [Google Scholar]
- 36.Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ (Clin. Res. Ed.) 2015;351:h5000. doi: 10.1136/bmj.h5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, Brawner CA, Whooley MA, Chang T, Stolp H, Schieb L, Wright J. Tracking cardiac rehabilitation participation and completion among medicare beneficiaries to inform the efforts of a national initiative. Circ. Cardiovasc. Qual. Outcomes. 2020;13(1):e005902. doi: 10.1161/CIRCOUTCOMES.119.005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor RS, Dalal HM, McDonagh STJ. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat. Rev. Cardiol. 2022;19(3):180–194. doi: 10.1038/s41569-021-00611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quittan M, Sturm B, Wiesinger GF, Pacher R, Fialka-Moser V. Quality of life in patients with chronic heart failure: a randomized controlled trial of changes induced by a regular exercise program. Scand. J. Rehabil. Med. 1999;31(4):223–228. doi: 10.1080/003655099444399. [DOI] [PubMed] [Google Scholar]
- 40.Candelaria D, Randall S, Ladak L, Gallagher R. Health-related quality of life and exercise-based cardiac rehabilitation in contemporary acute coronary syndrome patients: A systematic review and meta-analysis. Qual. Life Res. 2020;29(3):579–592. doi: 10.1007/s11136-019-02338-y. [DOI] [PubMed] [Google Scholar]
- 41.Uchoa E, Firmo JO, Dias EC, Pereira MS, Gontijo ED. Signos, significados e ações associados à doença de Chagas [Signs, meanings, and actions associated with Chagas disease] Cad. Saude Publica. 2002;18(1):71–79. doi: 10.1590/s0102-311x2002000100008. [DOI] [PubMed] [Google Scholar]
- 42.Magnani C, Oliveira BG, Gontijo ED. Representações, mitos e comportamentos do paciente submetido ao implante de marcapasso na doença de Chagas [Representations, myths, and behaviors among Chagas disease patients with pacemakers] Cad. Saude Publica. 2007;23(7):1624–1632. doi: 10.1590/s0102-311x2007000700013. [DOI] [PubMed] [Google Scholar]
- 43.Silva WT, Ávila MR, Oliveira LFF, Figueiredo PHS, Lima VP, Bastone AC, Costa FSMD, Mediano MFF, Costa HS, Rocha MODC. Prevalence and determinants of depressive symptoms in patients with Chagas cardiomyopathy and predominantly preserved cardiac function. Rev. Soc. Bras. Med. Trop. 2020;53:e20200123. doi: 10.1590/0037-8682-0123-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambela MDC, Mediano MFF, Carneiro FM, Ferreira RR, Waghabi MC, Mendes VG, Oliveira LS, de Holanda MT, de Sousa AS, da Costa AR, Xavier SS, da Silva GMS, Saraiva RM. Impact of pharmaceutical care on the quality of life of patients with heart failure due to chronic Chagas disease: Randomized clinical trial. Br. J. Clin. Pharmacol. 2020;86(1):143–154. doi: 10.1111/bcp.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collado-Mateo D, Lavín-Pérez AM, Peñacoba C, Del Coso J, Leyton-Román M, Luque-Casado A, Gasque P, Fernández-Del-Olmo MÁ, Amado-Alonso D. Key factors associated with adherence to physical exercise in patients with chronic diseases and older adults: An umbrella review. Int. J. Environ. Res. Public Health. 2021;18(4):2023. doi: 10.3390/ijerph18042023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones F, Harris P, Waller H, Coggins A. Adherence to an exercise prescription scheme: the role of expectations, self-efficacy, stage of change and psychological well-being. Br. J. Health Psychol. 2005;10:359–378. doi: 10.1348/135910704X24798. [DOI] [PubMed] [Google Scholar]
- 47.McAuley E, Szabo A, Gothe N, Olson EA. Self-efficacy: Implications for physical activity, function, and functional limitations in older adults. Am. J. Lifestyle Med. 2011 doi: 10.1177/1559827610392704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Z, Zhu D, Jiang Y, Lin Y, Yang Y, Luan W. Cross-sectional study on the SF-36, the general self-efficacy, the social support, and the health promoting lifestyle of the young elderly in a community in Shanghai, China. Ann. Palliat. Med. 2021;10(1):518–529. doi: 10.21037/apm-20-2462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.