Abstract
The development of closed-loop recycling polymers that exhibit excellent performance is of great significance. Sulfur-rich polymers possessing excellent optical, thermal, and mechanical properties are promising candidates for chemical recycling but lack efficient synthetic strategies for achieving diverse structures. Herein, we report a universal synthetic strategy for producing polytrithiocarbonates, a class of sulfur-rich polymers, via the polycondensation of dithiols and dimethyl trithiocarbonate. This strategy has excellent compatibility with a wide range of monomers, including aliphatic, heteroatomic, and aromatic dithiols enabling the synthesis of polytrithiocarbonates with diverse structures. The present synthesis strategy offers a versatile platform for the construction of thermoplastics, elastomers, and vitrimers. Notably, these polytrithiocarbonates can be easily depolymerized via solvolysis into the corresponding monomers, which can be repolymerized to virgin polymers without changing the material properties.
Subject terms: Polymer synthesis, Polymer synthesis, Polymers
Sulfur-rich polymers are promising candidates for chemical recycling but lack efficient synthetic strategies for achieving diverse structures. Here, the authors report a synthesis strategy for polytrithiocarbonates which is compatible with a wide range of monomers enabling diverse structures via polycondensation of dithiols and dimethyl trithiocarbonate.
Introduction
The use of polymers brings great convenience to daily life owing to their low cost, lightweight, and wide range of multifunctionalities1. Over 350 million tons of polymers, of which most are polyolefins, are produced annually. However, their non-degradable features not only result in resource waste but also cause health and environmental issues with the widespread use of vast amounts of polymers2,3. The development of novel polymers capable of chemical recycling to monomers (CRM) is an effective strategy to address these issues. CRM transforms waste polymers directly back into monomers, which reduces the continuous need for monomers and the accumulation of waste plastics4,5. Consequently, an array of polymers capable of CRM has been developed using different recycling methods. For example, polyolefins containing fused rings or polyesters synthesized by the ring-opening polymerization (ROP) of lactones can be depolymerized into the corresponding monomers by heating the bulk materials6–9. Polycarbonates derived from the copolymerization of epoxides and CO2 can also be recycled into the corresponding epoxides in the presence of catalysts10–12. Polyacetals with tensile strengths similar to those of commodity polyolefins and high thermal stabilities have been demonstrated to be capable of CRM with a strong acid catalyst13. In addition, closed-loop recycling of polyethylene-like polycarbonates and polyesters has been achieved by the polycondensation of long-chain diols with carbonates or diesters and recycling via solvolysis14. In this context, achievements made in synthetic strategies have established a CRM platform that enables the straightforward construction of functional polymers with recyclability.
Sulfur-rich polymers, a category of functional polymers, have many attractive features, including a high refractive index15–17, excellent adhesion to heavy metal ions18–20, and electrochemical properties21–23, owing to the incorporation of sulfur atoms into the polymer mainchain24–26. As a representative class of sulfur-rich polymers, polytrithiocarbonates exhibit enhanced properties owing to the high sulfur content of the thiocarbonate groups in the repeated units. In addition, polytrithiocarbonate-containing polymers exhibit anticancer and antimicrobial activities27–29. The copolymerization of episulfides with carbon disulfide (CS2) constitutes a straightforward route for constructing polytrithiocarbonates30,31. However, the resultant polytrithiocarbonates are unrecyclable because of the high thermal stability of the five-membered cyclic trithiocarbonates, which cannot be degraded to episulfides and CS2 or converted into polytrithiocarbonates via ROP32,33. In contrast, the ROP of seven-membered cyclic trithiocarbonates enables the production of aliphatic polytrithiocarbonates. In particular, these polytrithiocarbonates exhibit superior optical, thermal, and mechanical properties compared to the corresponding polycarbonate analogs32. However, the limited number of monomers suitable for polytrithiocarbonate production significantly restricts the development of polymers with tunable properties. Therefore, the development of an efficient approach for the synthesis of recyclable polytrithiocarbonates with diverse structures remains challenging. In this study, we developed a universal synthetic methodology for producing polytrithiocarbonates that are viable for CRM through the polycondensation of dithiols with dimethyl thiocarbonate (DMTC). This strategy is highly compatible with a wide range of monomers, including aliphatic, heteroatomic, and aromatic dithiols. This compatibility enables the synthesis of polytrithiocarbonates with diverse structures, offering a versatile platform for the construction of polytrithiocarbonate-based materials such as thermoplastics, elastomers, and vitrimers (Fig. 1).
Fig. 1. Polycondensation of dithiols and dimethyl trithiocarbonate for synthesizing polytrithiocarbonates.
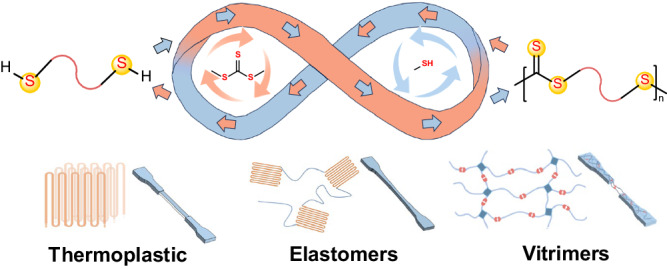
A general synthetic strategy for accessing diverse recyclable sulfur-rich polymers spanning thermoplastics, elastomers, and vitrimers.
Results
Polycondensation of dithiols and DTMC
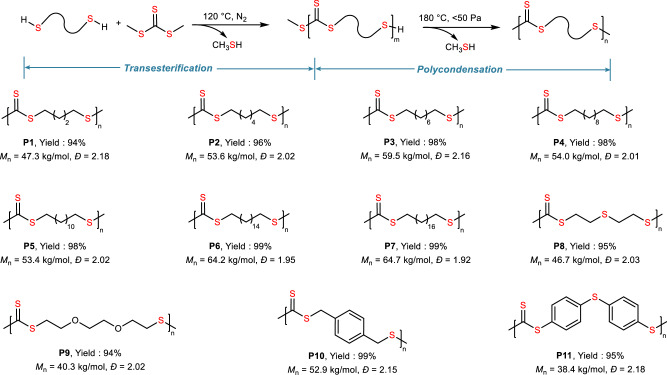
A previous study revealed that the construction of a trithiocarbonate group could be achieved via the coupling of thiols with thiophosgenes34. However, this process requires toxic compounds. We extended this work to construct polytrithiocarbonate via an alternative procedure wherein methyl trithiocarbonate (DMTC)35 is utilized to substitute thiophosgenes for condensation polymerization with dithiols. The polycondensation procedure proceeded by heating the dithiol and DMTC mixture with the catalysis of alkali hydrides to accomplish the transesterification process, followed by the condensation of the polytrithiocarbonate oligomers generated in situ at elevated temperatures under reduced pressure to produce polytrithiocarbonates with high molecular weights (Fig. 2). Using 1,10-decanedithiol as the model monomer, the polycondensation conditions were screened with respect to the reaction temperature for the transesterification process and alkali metal hydride (Supplementary Table 1). The optimal procedure with the transesterification proceeding at 120 °C with potassium hydride and the polycondensation process proceeding at 180 °C afforded the highest efficiency and molecular weights. Using the optimized polycondensation procedure, various dithiols were used to construct polytrithiocarbonates with diverse structures. The C4–C18 aliphatic dithiols can be converted into the corresponding polytrithiocarbonates P1 to P7 with number-average molecular weights (Mn) ranging from 47.3 to 64.7 kg/mol (Fig. 2). The utilization of heteroatomic and aromatic dithiols allows for the incorporation of heteroatoms and aromatic rings into polytrithiocarbonate P8 to P11 with Mns and Đ values ranging from 38.4 to 52.9 kg/mol and 2.02 to 2.18, respectively (Fig. 2). Consequently, a series of polytrithiocarbonates with diverse structures were successfully synthesized in high yields in a facile and efficient manner.
Fig. 2. Synthesis of a variety of polytrithiocarbonates from dithiols and DMTC.
The synthetic procedure contains transesterification and polycondensation processes.
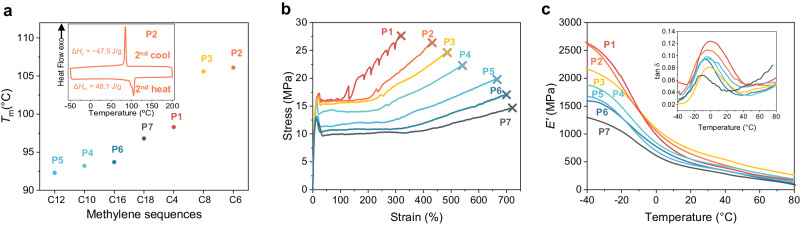
Subsequently, the thermal, mechanical, and gas-barrier properties of the polytrithiocarbonates were investigated (Table 1). Polytrithiocarbonates P1 to P7 all exhibited a crystalline nature with melting (Tm) and crystalline (Tc) temperatures ranging from 92 to 106 °C and 69 to 88 °C, respectively, as characterized by the differential scanning calorimetry (DSC). (Table 1, entries 1 − 7, Fig. 3a and Supplementary Figs. 1−7). Notably, P1 to P3 possess higher Tm than that of P4 to P7, and the highest Tm of 106 °C was observed from P2. The difference in the Tms of these polytrithiocarbonates can be attributed to the different trithiocarbonate group density in the mainchain, as crystalline behavior of polymers is significantly affected by the configuration of methylene sequence in the repeat unit and the stacking ways of function groups36,37. Furthermore, the crystalline characteristics of P1 to P7 were characterized using powder X-ray diffraction (XRD) (Supplementary Fig. 8). As revealed, P4 to P7 possess a similar crystal structure, which are different from P1 to P3. This can be attributed to the higher trithiocarbonate group density of P1 to P3 than that of P4 to P7, resulting in the different stacking ways of polymer mainchain. The reason for the highest Tm of P2 may be attributed to its better crystal structure than other polytrithiocarbonates, as six diffraction peaks were observed for P2. In addition, no glass transition temperatures (Tg) were detected for these polytrithiocarbonates (Fig. 3a). This can be attributed to the high crystallinity nature of these polytrithiocarbonates (Supplementary Fig. 8). The high crystallinity of these polytrithiocarbonates indicates a few of amorphous phase domains existed in the aggregation state of these polytrithiocarbonates, which further results in the wide range of temperature of relaxation behaviors. The relaxation behavior of these polytrithiocarbonates can be observed from the tan δ curves in the DMA analysis, wherein the glass transitions of these polymers span a wide range of temperature (Supplementary Figs 15–21), suggesting longtime relaxation behaviors of these polytrithiocarbonates. In contrast, polytrithiocarbonates containing heteroatoms and aromatic rings (P8 to P11) are amorphous with Tg values ranging from −37 to 66 °C (Table 1, entries 8 − 11, Supplementary Figs. 9−12). In addition, all polytrithiocarbonates possess a 5% weight loss decomposition temperature (Td5%) of over 246 °C, as characterized by thermogravimetric analysis (Supplementary Figs. 13 and 14). Notably, the Td5% increased with an increase in the length of methylene sequences in the dithiols from P1 to P7 (Supplementary Fig. 13). The mechanical performances of the polytrithiocarbonates were further examined via tensile testing using a universal testing system and dynamic mechanical analysis (Supplementary Figs. 15−23). According to the tensile tests, the Young’s modulus (E), ultimate tensile strength (σB), and strain (εB) at break of P1 reached 169 MPa, 27.7 MPa, and 316%, respectively. As the length of methylene sequence in the dithiols increased (P1 to P7), the σB values of the polytrithiocarbonates decreased and the εB values increased (Fig. 3b). The σB and εB values of P8 are much lower than those of P3, suggesting that the incorporation of heteroatoms into the polytrithiocarbonate mainchain weakens both the thermal and mechanical properties, thus leading to a soft material. In contrast, the incorporation of rigid aromatic rings into the polymer mainchain resulted in fragile P10 with E, σB, and εB values of 501 MPa, 20.7 MPa, and 9%, respectively (Table 1, entry 10). In particular, no mechanical performance was detected for P9 and P11 because of their sticky and brittle natures, respectively (Table 1, entries 9 and 11). Additionally, the storage moduli (E’) of P1 to P7 decreased from 2650 to 1292 MPa as the length of the methylene sequence in the dithiols increased (Fig. 3c). The E’ of P8 and P10 are 3423 and 3523 MPa, respectively. Moreover, the gas-barrier properties of the polytrithiocarbonates were tested using oxygen and water vapor transmission rate analyzers. The lowest oxygen transmission rate (O2GTR) and water vapor transmission rate (MVTR) of 1.62 cc/(m2·day) and 0.59 g/(m2·day), respectively, were observed from P1. In contrast, the O2GTR and MVTR of poly(butylene carbonate) (PBC) are 53.04 cc/(m2·day) and 30.55 g/(m2·day)38, respectively, and these values of high-density polyethylene (HDPE) are respective 157.78 cc/(m2·day) and 1.55 g/(m2·day)39, suggesting that P1 has much potential for use as gas-barrier materials. Interestingly, the O2GTR increased with an increase in the length of the methylene sequences in the dithiols. The superior gas-barrier properties of P1 compared with those of other polytrithiocarbonates indicate its highly dense nature. Similarly, polytrithiocarbonates containing longer methylene sequences exhibit higher MVTRs. For P8 and P10, the O2GTR and MVTR were less than 6.06 cc/(m2·day) and 1.75 g/(m2·day), respectively. The O2GTR and MVTR of P9 and P11 were inaccessible because they could not be processed into films using any available procedure.
Table 1.
Properties of polytrithiocarbonatesa
| Entry | Polymer | Mnb (kg/mol) | Ðb | Tgc (°C) | Tmc (°C) | Tcc (°C) | Td5%d (°C) | σBe (MPa) | εBe % | Ef (MPa) | E’g (MPa) | O2GTRh cc/(m2·day) | MVTRi g/(m2·day) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P1 | 47.3 | 2.18 | n.d.j | 98.3 | 69.1 | 265 | 27.7 | 316 | 169 | 2650 | 1.62 | 0.59 |
| 2 | P2 | 53.6 | 2.02 | n.d. | 106.1 | 85.5 | 289 | 26.4 | 429 | 268 | 2600 | 18.00 | 2.41 |
| 3 | P3 | 59.5 | 2.16 | n.d. | 105.6 | 85.9 | 297 | 24.6 | 485 | 262 | 2174 | 50.28 | 2.32 |
| 4 | P4 | 54.0 | 2.01 | n.d. | 93.2 | 76.2 | 299 | 22.3 | 546 | 291 | 1880 | 59.37 | 1.81 |
| 5 | P5 | 53.4 | 2.02 | n.d. | 92.3 | 73.4 | 301 | 19.7 | 663 | 201 | 1662 | 108.46 | 2.11 |
| 6 | P6 | 64.2 | 1.95 | n.d. | 93.7 | 76.1 | 302 | 17.0 | 694 | 275 | 1593 | 131.20 | 2.98 |
| 7 | P7 | 64.7 | 1.92 | n.d. | 96.8 | 88.0 | 305 | 14.6 | 718 | 214 | 1292 | 136.27 | 1.66 |
| 8 | P8 | 46.7 | 2.03 | –18.7 | 56.2 | n.d. | 251 | 6.9 | 449 | 63 | 3423 | 4.29 | 1.06 |
| 9 | P9 | 40.3 | 2.02 | –37.2 | n.d. | n.d. | 292 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 10 | P10 | 52.9 | 2.15 | 14.4 | 114.3 | n.d. | 246 | 20.7 | 9 | 501 | 3523 | 6.06 | 1.75 |
| 11 | P11 | 38.4 | 2.18 | 66.1 | n.d. | n.d. | 258 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
aPolymerization performed at 120 °C and 70000 Pa for 2.0 h, followed by the polycondensation at 150 °C and 2000 Pa for 2.0 h and 180 °C and 50 Pa until 2.0 h after the Weissenberg phenomenon.
bThe molecular weights and dispersities of the polymers were determined by gel permeation chromatography equipped with a triple detection array, including a differential refractive index (RI) detector, a two-angle light scattering (LS) detector, and a fourbridge capillary viscometer at 150 °C using 1,2,4-trichlorobenzene as the eluent.
cThe glass transition temperature (Tg), melting temperature (Tm), and crystalline temperature (Tc) were determined using differential scanning calorimetry.
dTemperature at a molecular weight loss of 5% determined by thermogravimetric analysis.
eThe breaking strength (σB) and elongation at break (εB) were determined by uniaxial tensile elongation testing.
fYoung’s modulus (E) calculated as the slope from 0% to 1% strain.
gStorage modulus at −40 °C determined by dynamic mechanical analysis.
hOxygen permeation rate determined using an oxygen transmission rate analyzer.
iWater vapor transmittance determined using a water vapor transmission rate analyzer.
jNot determined.
Fig. 3. Thermal and mechanical properties of P1 to P7.
a The Tm values of the aliphatic polytrithiocarbonates. b Stress–strain curves. c Storage moduli.
Synthesis of polytrithiocarbonate-based thermoplastic elastomer
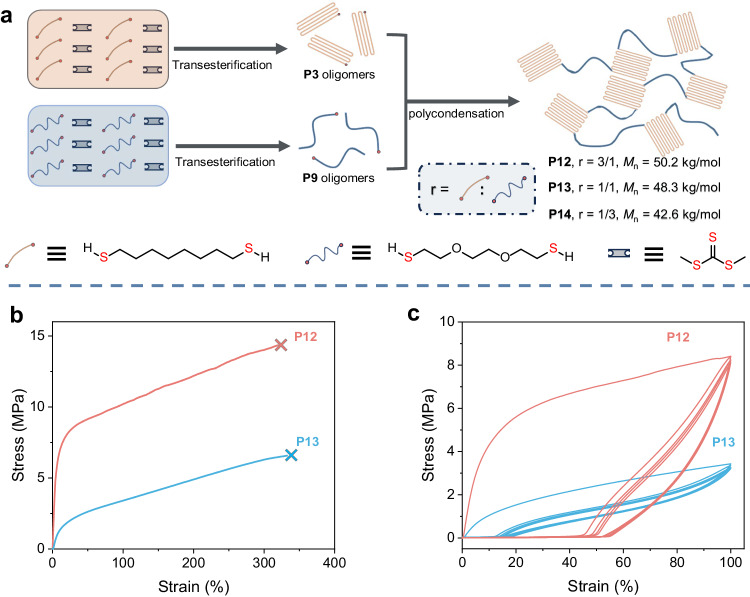
With these diverse polytrithiocarbonates that exhibit distinct thermal and mechanical properties containing “hard” and “soft” materials in hand, the application of these polytrithiocarbonates to construct thermoplastic elastomers was explored. The “hard” P3 oligomer and “soft” P9 oligomer were used to construct multiblock copolymers with an alternating “hard” and “soft” structure (Fig. 4a). In particular, the adjustable feed ratio of dithiols P3 oligomer to P9 oligomer allows attaining P12, P13, and P14 with a tunable “hard” to “soft” ratio, wherein the molar ratio of 3 to 9 in P12, P13, and P14 are 3:1, 1:1, and 1:3, respectively (Supplementary Table 2). A melting peak at 79 °C, together with a crystalline peak at 53 °C, is observed for P12, and only a melting peak at 38 °C is observed for P13, as characterized by DSC (Supplementary Figs. 24 and 25). In contrast, only a glass transition peak at −46 °C is observed for P14, which presented as a sticky polymer exhibiting no mechanical performance (Supplementary Fig. 26). In addition, mechanical property characterization revealed that P12 possesses σB and εB values of 14.3 MPa 320%, respectively (Fig. 4b). The increase in the P9 oligomer content in the multiblock copolymer results in the decrease in tensile strength but an increase in elongation of P13 (σB = 6.6 MPa, εB = 332%) (Supplementary Table 2, entries 1 and 2). Additionally, the storage moduli (E’) at 25 °C of P12 to P13 decreased from 182 to 13 MPa as the content of P9 oligomer increased from 25% to 50% (Supplementary Figs. 27 and 28). No yield points were detected for P12 and P13, suggesting their elastomeric nature, which was further supported by the results obtained from cyclic tensile tests. As shown in Fig. 4c, hysteresis is observed for P12 during the first cycle, together with a residual strain of 44% in the second cycle. In contrast, the hysteresis and residual strain (11%) of P13 both decreased, suggesting a higher elasticity than P12. Notably, no obvious accumulation of permanent deformation was observed for these two elastomers, and almost no decay in the tensile strengths at the maximum strain point during continuous cycles was detected40. These results indicate the excellent fatigue resistance of these two elastomers, which was further supported by the rapidly diminishing and stabilized hysteresis within five cycles41,42.
Fig. 4. Synthesis and characterization of polytrithiocarbonate-based elastomers.
a Schematic of the synthesis of copolymers P12, P13, and P14. b Stress–strain curves of P12 and P13. c Cyclic tensile tests of the P12 and P13 for five cycles in successive loading-unloading cycles at a strain of 100% under a deformation rate of 20 mm/min. P14 is a sticky solid with a low Tg of −46 °C and it is difficult to characterize its mechanical properties.
Synthesis and characterization of polytrithiocarbonate-based vitrimer containing S–S bonds
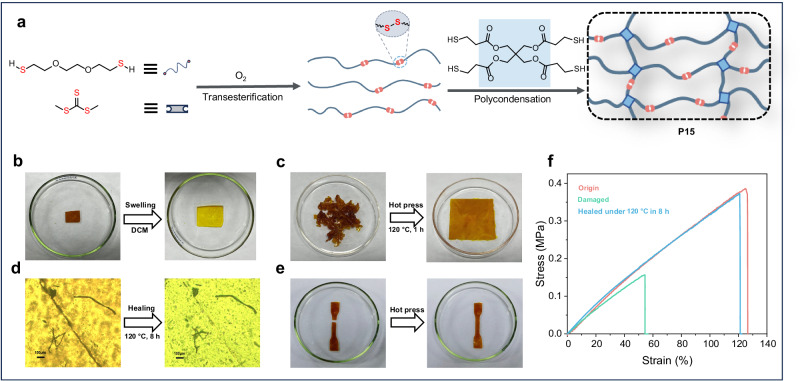
The introduction of disulfide bonds, a typical dynamic covalent bond43, into the polymer mainchain endows polymers with dynamic features, thus allowing them to be widely applied in materials science44 as self-healing materials45–47, drug delivery systems48,49, and vitrimers50–52. In this study, the disulfide bond was expected to be formed via the oxidation of thiols by introducing a small amount of oxygen into the reaction system during the transesterification process. Consequently, an array of disulfide-containing polytrithiocarbonates derived from various dithiols was obtained (Supplementary Figs. 29−36). Notably, with dithiol 4 as the model monomer, the modulation of disulfide linkage content was achieved over a wide range from 1% to 18% by controlling the amount of air introduced (Supplementary Fig. 37). Subsequently, a vitrimer (P15) is envisioned to be formed by capitalizing on the polytrithiocarbonates derived from dithiol 9 with a disulfide linkage content of 18% to copolymerize with tetradentate thiol (Supplementary Fig. 38), that is pentaerythritol tetrakis (3-mercapto-propionate) (Fig. 5a). The swelling test of P15 in dichloromethane indicated its crosslinked nature (Fig. 5b). In addition, the broken fragment of P15 can be reprocessed by hot pressing at 120 °C under 10 MPa for 1 h (Fig. 5c). This can be attributed to exchange reactions between nearby disulfide bonds, which lead to network topology rearrangements. A dumbbell-shaped P15 sample with a scratch was placed at 120 °C without pressure for 8 h. Subsequently, the scratch almost completely disappeared, as observed under an optical microscope (Fig. 5d). In addition, this vitrimer sample was capable of self-healing even when subjected to more serious damage (Fig. 5e). To evaluate the mechanical properties after self-healing, the mechanical properties of the original sample and damaged sample before and after healing were further characterized using tensile tests. As shown in Fig. 5f, the damaged P15 sample possesses a σB and εB of 0.16 MPa and 53%, respectively. In contrast, after self-healing, the damaged sample possesses comparable mechanical performances (σB = 0.37 MPa, εB = 121%) to that of the original sample (σB = 0.38 MPa, εB = 128%).
Fig. 5. Synthesis and characterization of polytrithiocarbonate-based vitrimer containing S–S bonds.
a Schematic of the synthesis of vitrimer. b Swelling test of the vitrimer in dichloromethane for 12 h. c The remodeling procedure of the vitrimer. d Optical microscopic images of the vitrimer before and after healing. e The healing procedure of the dumbbell-shaped vitrimer by hot pressing. f The stress–strain curves of the original, damaged, and healed vitrimer.
Closed-loop recycling of polytrithiocarbonates
Recycling of polymers is an efficient strategy for reducing the negative effects of waste polymeric materials. Previous studies have demonstrated that sulfur-containing polymers53–55, including polythioesters, polydisulfides, and polytrithiocarbonates, are viable for CRM. For the long methylene sequence-containing polytrithiocarbonates, the trithiocarbonate linkages in the mainchain can be readily broken using nucleophiles. In this context, long-chain polytrithiocarbonates are anticipated to be amenable to quantitative chemical recycling through reverse transesterification catalyzed by alkali hydroxides in methyl mercaptan (MeSH) by capitalizing on the in-chain functional groups as breakpoints (Fig. 6a).
Fig. 6. Chemical recycling of polytrithiocarbonates.
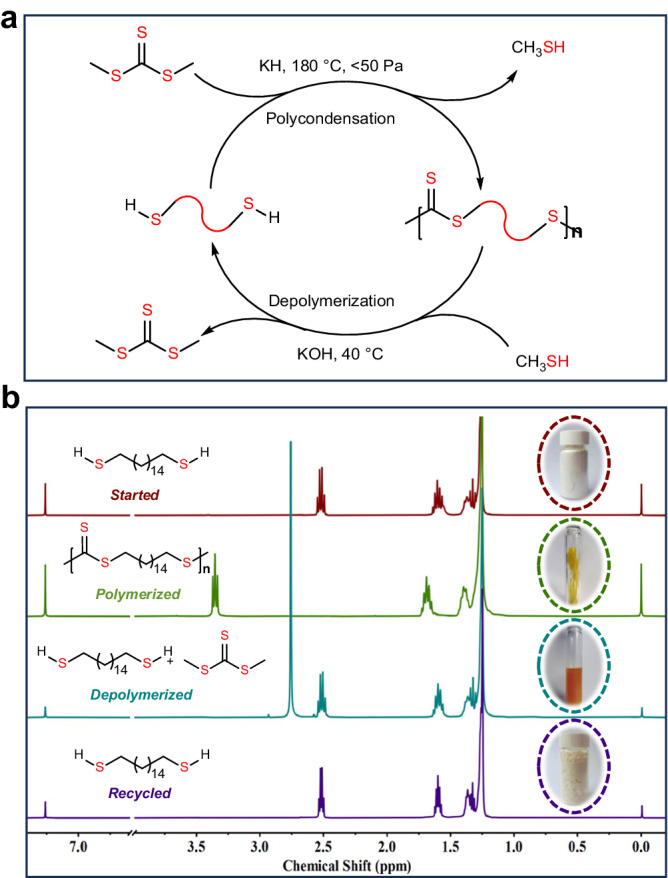
a Schematic of the chemical recycling of polytrithiocarbonates. b Overlay of the 1H NMR spectra in CDCl3 of the closed-loop recycling of P6.
For example, the yellow solid P6 changed into red-orange after being treated with potassium hydroxide in MeSH at 50 °C for 24 h (Fig. 6b). Purified 1,16-hexadecanedithiol and DMTC were recovered in 97% yield after recrystallization. Notably, it was available to obtain P6 directly from the mixture before separation through polycondensation manner. Many types of polymers are mixed during the practical sorting of mixed waste streams. Thus, the recycling of polytrithiocarbonates from polyolefin waste is desirable. To this end, pieces of commercial polypropylene (PP) (plastic solvent cap), HDPE (solvent bottle cap), and P4 were subjected to the depolymerization procedure described above (Supplementary Fig. 39). Once the depolymerization procedure finished, PP and HDPE pieces were filtered out in their original unaltered states, whereas P4 was completely depolymerized, affording 1,10-decanedithiol and DMTC in 96% yield. These results indicate that the obtained polytrithiocarbonates are viable for CRM via a reverse transesterification procedure and afford purified DMTC and dithiol monomers in high yields, thus establishing the closed-loop life cycles of polytrithiocarbonates.
Discussion
In summary, we developed an efficient strategy for chemically recyclable sulfur-rich polymers via the polycondensation of dithiols and DMTC. This method is compatible with a broad range of dithiols and produces polytrithiocarbonates with diverse structures. Additionally, this synthetic procedure enables the construction of polytrithiocarbonates with enriched architectures covering multiblock structures and dynamic networks, from which thermoplastic elastomers and vitrimers can be accessed using different dithiols. Moreover, the obtained polytrithiocarbonates were viable for CRM via reverse transesterification, thus endowing them with a promising application potential. Research on the application of polytrithiocarbonates in the fabrication of composites is ongoing.
Method
Materials
Iodomethane was purchased from Energy Chemical (Beijing, China), 1,4-butanedithiol, 1,6-hexanedithiol, 1,8-octanedithiol, 1,10-decanedithiol, 1,12-dibromododecane, 1,16-dibromohexadecane, bis(2-mercaptoethyl) sulfide, 1,4-benzenedimethanethiol and 4,4’-thiobisbenzenethiol were purchased from Aladin Reagent (Shanghai, China), 1,18-dibromooctadecane were purchased from Leyan Chemical (Shanghai, China). All other reagents for making monomers were purchased from Damao Chemical.
Characterization methods
NMR
1H and 13C NMR spectra were recorded on a Varian INOVA-400 MHz type (1H, 400 MHz; 13C, 100 MHz) spectrometer. Their peak frequencies were referenced versus an internal standard (TMS) shifts at 0 ppm for 1H NMR and against the solvent, CDCl3 at 77.4 ppm for 13C NMR, respectively.
Gel permeation chromatography (GPC)
Molecular weight and molecular weight distribution of the polymers with low solubility at room temperature were determined by gel permeation chromatography (GPC) with the PL-GPC220 equipped with a triple detection array, including a differential refractive index (RI) detector, a two-angle light scattering (LS) detector, and a fourbridge capillary viscometer at 150 °C using 1,2,4-trichlorobenzene as the eluent.
Differential scanning calorimetry (DSC)
The analysis of DSC was carried out with a METTLER TOLEDO DSC 3 thermal analyzer. Conditions: under N2 atmosphere, the heating and cooling were at a rate of –10 and 10 K/min. Glass transition temperatures (Tg) were determined from the second heating cycle.
Thermo-gravimetric analysis (TGA)
Thermo-gravimetric analyses of the resulted polymers were measured on a Mettler-Toledo TGA/SDTA851e.
Dynamic mechanical analysis (DMA)
A rectangular spline for the Dynamic Mechanical Properties Test with a scale of 16 mm × 3.5 mm × 0.2 mm was obtained by cutting the film. The test was performed at small tension film mode, 0.1% strain, 1 Hz, 3.0 °C min−1 on a Mettler-Toledo DMA/SDTA 1+.
Uniaxial tensile elongation testing
The test was performed at a strain rate of 5 mm/min on an INSTRON 6800.
Water vapor permeability
The moisture vapor transmission rate was determined at 37.8 °C with gas at 90% RH and 10 SCCM, following ASTM F1249 standard.
Oxygen permeation analyzer
The oxygen transmission rate was determined at 23.0 °C under 100% oxygen concentration, following ASTM D3985 standard.
Polarizing optical microscope (POM)
Optical microscope images were obtained on Zeiss Axioscope 5 polarizing microscope equipped with a Linkam hot stage.
Wide angle X-ray diffraction (WAXD)
Powder X-ray diffraction data were collected on an EMPYREAN diffractometer with Cu KR radiation (λ = 1.54056 Å) over the 2θ range of 5–60 ° with a scan speed of 0.3333 °/s and a step size of 0.02 ° at room temperature. Crystallinity values of polymers were determined by deconvolution of WAXS diffractograms in MDI JADE 6.5 software.
Supplementary information
Acknowledgements
W.-M.R. and T.-J.Y. acknowledge support from the National Natural Science Foundation of China (NSFC, 22171037 and 22101040). T.-J.Y. acknowledges the support from the China Postdoctoral Science Foundation (BX2021050 and 2021M690517). X.-B.L. acknowledges the support from the National Key Research and Development Program of China (2021YFA1501704). Gratitude is expressed to the Fundamental Research Funds for the Central Universities (DUT22LAB609).
Author contributions
W.-M.R. conceived the project. W.-M.R. and T.-J.Y. directed the research. J.-Z.Z. carried out experiments and collected the overall data. J.-Z.Z and B.-H.R. analyzed data. J.-Z.Z and T.-J.Y. wrote the original manuscript draft, X.-B.Lu and W.-M.R. revised and finalized the manuscript. All co-authors discussed the results and commented on the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that the data supporting this study are available within the paper and the Supplementary information File. All other data is available from the authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-47382-6.
References
- 1.Zhang X, Fevre M, Jones GO, Waymouth RM. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 2018;118:839–885. doi: 10.1021/acs.chemrev.7b00329. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann M, Tekman MB, Gutow L. Sea change for plastic pollution. Nature. 2017;544:297–297. doi: 10.1038/544297a. [DOI] [PubMed] [Google Scholar]
- 3.Rochman CM, Hoellein T. The global odyssey of plastic pollution. Science. 2020;368:1184–1185. doi: 10.1126/science.abc4428. [DOI] [PubMed] [Google Scholar]
- 4.Coates GW, Getzler YDYL. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020;5:501–516. doi: 10.1038/s41578-020-0190-4. [DOI] [Google Scholar]
- 5.Jehanno C, et al. Critical advances and future opportunities in upcycling commodity polymers. Nature. 2022;603:803–814. doi: 10.1038/s41586-021-04350-0. [DOI] [PubMed] [Google Scholar]
- 6.Hong M, Chen EY. Completely recyclable biopolymers with linear and cyclic topologies via ring-opening polymerization of gamma-butyrolactone. Nat. Chem. 2016;8:42–49. doi: 10.1038/nchem.2391. [DOI] [PubMed] [Google Scholar]
- 7.Zhu JB, Watson EM, Tang J, Chen EY. A synthetic polymer system with repeatable chemical recyclability. Science. 2018;360:398–403. doi: 10.1126/science.aar5498. [DOI] [PubMed] [Google Scholar]
- 8.Sathe D, et al. Olefin metathesis-based chemically recyclable polymers enabled by fused-ring monomers. Nat. Chem. 2021;13:743–750. doi: 10.1038/s41557-021-00748-5. [DOI] [PubMed] [Google Scholar]
- 9.Li X-L, Clarke RW, Jiang J-Y, Xu T-Q, Chen EYX. A circular polyester platform based on simple gem-disubstituted valerolactones. Nat. Chem. 2022;15:278–285. doi: 10.1038/s41557-022-01077-x. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Zhou H, Guo JZ, Ren WM, Lu XB. Completely recyclable monomers and polycarbonate: approach to sustainable polymers. Angew. Chem. Int. Ed. 2017;56:4862–4866. doi: 10.1002/anie.201701438. [DOI] [PubMed] [Google Scholar]
- 11.Singer FN, Deacy AC, McGuire TM, Williams CK, Buchard A. Chemical recycling of poly(cyclohexene carbonate) using a Di‐MgII catalyst. Angew. Chem. Int. Ed. 2022;61:e202201785. doi: 10.1002/anie.202201785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuire TM, Deacy AC, Buchard A, Williams CK. Solid-state chemical recycling of polycarbonates to epoxides and carbon dioxide using a heterodinuclear Mg(II)Co(II) catalyst. J. Am. Chem. Soc. 2022;144:18444–18449. doi: 10.1021/jacs.2c06937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abel BA, Snyder RL, Coates GW. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science. 2021;373:783–789. doi: 10.1126/science.abh0626. [DOI] [PubMed] [Google Scholar]
- 14.Haussler M, Eck M, Rothauer D, Mecking S. Closed-loop recycling of polyethylene-like materials. Nature. 2021;590:423–427. doi: 10.1038/s41586-020-03149-9. [DOI] [PubMed] [Google Scholar]
- 15.Griebel JJ, et al. New infrared transmitting material via inverse vulcanization of elemental sulfur to prepare high refractive index polymers. Adv. Mater. 2014;26:3014–3018. doi: 10.1002/adma.201305607. [DOI] [PubMed] [Google Scholar]
- 16.Sun Z, Huang H, Li L, Liu L, Chen Y. Polythioamides of high refractive index by direct polymerization of aliphatic primary diamines in the presence of elemental sulfur. Macromolecules. 2017;50:8505–8511. doi: 10.1021/acs.macromol.7b01788. [DOI] [Google Scholar]
- 17.Kim DH, et al. One-step vapor-phase synthesis of transparent high refractive index sulfur-containing polymers. Sci. Adv. 2020;6:eabb5320. doi: 10.1126/sciadv.abb5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo M, Zhang X-H, Du B-Y, Wang Q, Fan Z-Q. Regioselective and alternating copolymerization of carbonyl sulfide with racemic propylene oxide. Macromolecules. 2013;46:5899–5904. doi: 10.1021/ma401114m. [DOI] [Google Scholar]
- 19.Tian T, Hu R, Tang BZ. Room temperature one-step conversion from elemental sulfur to functional polythioureas through catalyst-free multicomponent polymerizations. J. Am. Chem. Soc. 2018;140:6156–6163. doi: 10.1021/jacs.8b02886. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, et al. Sulfur conversion to multifunctional poly(o-thiocarbamate)s through multicomponent polymerizations of sulfur, diols, and diisocyanides. J. Am. Chem. Soc. 2021;143:3944–3950. doi: 10.1021/jacs.1c00243. [DOI] [PubMed] [Google Scholar]
- 21.Ji X, Lee KT, Nazar LF. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009;8:500–506. doi: 10.1038/nmat2460. [DOI] [PubMed] [Google Scholar]
- 22.Manthiram A, Fu Y, Chung SH, Zu C, Su YS. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014;114:11751–11787. doi: 10.1021/cr500062v. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, et al. Sulfur-based aqueous batteries: electrochemistry and strategies. J. Am. Chem. Soc. 2021;143:15475–15489. doi: 10.1021/jacs.1c06923. [DOI] [PubMed] [Google Scholar]
- 24.Kausar A, Zulfiqar S, Sarwar MI. Recent developments in sulfur-containing polymers. Polym. Rev. 2014;54:185–267. doi: 10.1080/15583724.2013.863209. [DOI] [Google Scholar]
- 25.Yue T-J, Wang L-Y, Ren W-M. The synthesis of degradable sulfur-containing polymers: precise control of structure and stereochemistry. polym. chem. 2021;12:6650–6666. doi: 10.1039/D1PY01065D. [DOI] [Google Scholar]
- 26.Zhang, X.-H. & Theato, P. Sulfur-Containing Polymers From Synthesis to Functional Materials (Wiley-VCH, Weinheim, 2021).
- 27.Dehmel F, et al. Trithiocarbonates—Exploration of a new head group for HDAC inhibitors. Bioorganic Med. Chem. Lett. 2007;17:4746–4752. doi: 10.1016/j.bmcl.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 28.Rzayev ZM, Turk M, Soylemez EA. Bioengineering functional copolymers. XXI. Synthesis of a novel end carboxyl-trithiocarbonate functionalized poly(maleic anhydride) and its interaction with cancer cells. Bioorg. Med. Chem. 2012;20:5053–5061. doi: 10.1016/j.bmc.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 29.Mabkhot YN, et al. Synthesis and biological screening of a novel enaminone-grafted trithiocarbonate: a potential anticancer and antimicrobial agent. Med. Chem. Res. 2020;29:954–961. doi: 10.1007/s00044-020-02535-2. [DOI] [Google Scholar]
- 30.Nakano K, Tatsumi G, Nozaki K. Synthesis of sulfur-rich polymers: copolymerization of episulfide with carbon disulfide by using [PPN]Cl/(salph)Cr(lll)Cl system. J. Am. Chem. Soc. 2007;129:15116–15117. doi: 10.1021/ja076056b. [DOI] [PubMed] [Google Scholar]
- 31.Silvano S, et al. Synthesis of sulfur-rich polymers: copolymerization of cyclohexene sulfide and carbon disulfide using chromium complexes. Macromolecules. 2020;53:8837–8846. doi: 10.1021/acs.macromol.0c01555. [DOI] [Google Scholar]
- 32.Zhao J-Z, et al. Recyclable sulfur-rich polymers with enhanced thermal, mechanical, and optical performance. Macromolecules. 2022;55:8651–8658. doi: 10.1021/acs.macromol.2c01628. [DOI] [Google Scholar]
- 33.Wang X, et al. DFT-supported mechanistic understanding of the ring-opening polymerization of cyclic trithiocarbonates mediated by organic base. Chem. Res. Chin. Univ. 2023;39:772–776. doi: 10.1007/s40242-023-3034-0. [DOI] [Google Scholar]
- 34.El‐Hewehi Z. Umsetzungsprodukte von chlorierten Benzyl‐chloriden und ihre Verwendbarkeit als Schädlingsbekämpfungsmittel. Prakt. Chem. 1962;16:201–206. doi: 10.1002/prac.19620160314. [DOI] [Google Scholar]
- 35.Arzehgar Z, Ahmadi H. A convenient one-pot method for the synthesis of symmetrical dialkyl trithiocarbonates using NH4OAc under mild neutral conditions. J. Chin. Chem. Soc. 2019;66:303–306. doi: 10.1002/jccs.201800062. [DOI] [Google Scholar]
- 36.Tasaki M, et al. Crystal structure analyses of arylate polyesters with long methylene segments and their model compounds on the basis of 2-D X-ray diffractions and infrared progression bands. Polymer. 2014;55:1228–1248. doi: 10.1016/j.polymer.2014.01.024. [DOI] [Google Scholar]
- 37.Kaner P, et al. Kinetic control of chlorine packing in crystals of a precisely substituted polyethylene. Toward advanced polyolefin materials. Macromolecules. 2013;47:236–245. doi: 10.1021/ma401541r. [DOI] [Google Scholar]
- 38.Lu Y, et al. Kilogram-scale production of biodegradable poly(butylene carbonate): molecular weight dependence of physical properties and enhanced crystallization by nucleating agent. J. Polym. Environ. 2022;31:1510–1524. doi: 10.1007/s10924-022-02689-7. [DOI] [Google Scholar]
- 39.Seong DM, Lee H, Kim J, Chang JH. High oxygen and water-vapor transmission rate and in vitro cytotoxicity assessment with illite-polyethylene packaging films. Materials. 2020;13:2382. doi: 10.3390/ma13102382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong W, et al. An ultra-low hysteresis, self-healing and stretchable conductor based on dynamic disulfide covalent adaptable networks. J. Mater. Chem. A. 2022;10:2012–2020. doi: 10.1039/D1TA08737A. [DOI] [Google Scholar]
- 41.Sun N, et al. Preparation and characterization of lignin-containing self-healing polyurethane elastomers with hydrogen and disulfide bonds. Ind. Crops. Prod. 2021;174:114178. doi: 10.1016/j.indcrop.2021.114178. [DOI] [Google Scholar]
- 42.Li M, et al. Superstretchable, yet stiff, fatigue-resistant ligament-like elastomers. Nat. Commun. 2022;13:2279–2287. doi: 10.1038/s41467-022-30021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakma P, Konkolewicz D. Dynamic covalent bonds in polymeric materials. Angew. Chem.Int. Ed. 2019;58:9682–9695. doi: 10.1002/anie.201813525. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Q, Qu DH, Feringa BL, Tian H. Disulfide-mediated reversible polymerization toward intrinsically dynamic smart materials. J. Am. Chem. Soc. 2022;144:2022–2033. doi: 10.1021/jacs.1c10359. [DOI] [PubMed] [Google Scholar]
- 45.Kim SM, et al. Superior toughness and fast self‐healing at room temperature engineered by transparent elastomers. Adv. Mater. 2017;30:1705145. doi: 10.1002/adma.201705145. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Urban MW. Self-healing polymers. Nat. Rev. Mater. 2020;5:562–583. doi: 10.1038/s41578-020-0202-4. [DOI] [Google Scholar]
- 47.Li B, Cao PF, Saito T, Sokolov AP. Intrinsically self-healing polymers: from mechanistic insight to current challenges. Chem. Rev. 2023;123:701–735. doi: 10.1021/acs.chemrev.2c00575. [DOI] [PubMed] [Google Scholar]
- 48.Du S, Liew SS, Li L, Yao SQ. Bypassing endocytosis: direct cytosolic delivery of proteins. J. Am. Chem. Soc. 2018;140:15986–15996. doi: 10.1021/jacs.8b06584. [DOI] [PubMed] [Google Scholar]
- 49.Lu J, Wang H, Tian Z, Hou Y, Lu H. Cryopolymerization of 1,2-dithiolanes for the facile and reversible grafting-from synthesis of protein-polydisulfide conjugates. J. Am. Chem. Soc. 2020;142:1217–1221. doi: 10.1021/jacs.9b12937. [DOI] [PubMed] [Google Scholar]
- 50.Van Zee NJ, Nicolaÿ R. Vitrimers: permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020;104:101233. doi: 10.1016/j.progpolymsci.2020.101233. [DOI] [Google Scholar]
- 51.Huang S, et al. Covalent adaptable liquid crystal networks enabled by reversible ring-opening cascades of cyclic disulfides. J. Am. Chem. Soc. 2021;143:12543–12551. doi: 10.1021/jacs.1c03661. [DOI] [PubMed] [Google Scholar]
- 52.Shi C, Zhang Z, Scoti M, Yan XY, Chen EY. Endowing polythioester vitrimer with intrinsic crystallinity and chemical recyclability. ChemSusChem. 2023;16:e202300008. doi: 10.1002/cssc.202300008. [DOI] [PubMed] [Google Scholar]
- 53.Shelef O, Gnaim S, Shabat D. Self-immolative polymers: an emerging class of degradable materials with distinct disassembly profiles. J. Am. Chem. Soc. 2021;143:21177–21188. doi: 10.1021/jacs.1c11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plummer CM, Li L, Chen Y. Ring-opening polymerization for the goal of chemically recyclable polymers. Macromolecules. 2023;56:731–750. doi: 10.1021/acs.macromol.2c01694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narmon AS, van Slagmaat CAMR, De Wildeman SMA, Dusselier M. Sustainable polythioesters via thio(no)lactones: monomer synthesis, ring-opening polymerization, end-of-life considerations, and industrial perspectives. ChemSusChem. 2023;16:e202202276. doi: 10.1002/cssc.202202276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting this study are available within the paper and the Supplementary information File. All other data is available from the authors upon request.