Abstract
Biochemical and structural studies of fragments of the ectodomain of the human immunodeficiency virus type 1 (HIV-1) gp41 transmembrane envelope glycoprotein have demonstrated that the molecular contacts between alpha helices allow the formation of a trimeric coiled coil. By introducing cysteine residues into specific locations along these alpha helices, the normally labile HIV-1 gp160 envelope glycoprotein was converted into a stable disulfide-linked oligomer. Although proteolytic cleavage into gp120 and gp41 glycoproteins was largely blocked, the disulfide-linked oligomer was efficiently transported to the cell surface and was recognized by a series of conformationally dependent antibodies. The pattern of hetero-oligomer formation between this construct and an analogous construct lacking portions of the gp120 variable loops and of the gp41 cytoplasmic tail demonstrates that these oligomers are trimers. These results support the relevance of the proposed gp41 structure and intersubunit contacts to the native, complete HIV-1 envelope glycoprotein. Disulfide-mediated stabilization of the labile HIV-1 envelope glycoprotein oligomer, which has been suggested to possess advantages as an immunogen, may assist attempts to develop vaccines.
Human immunodeficiency virus type 1 (HIV-1) is the etiologic agent of AIDS, which results from the profound depletion of CD4-positive lymphocytes in infected individuals (2, 16, 19).
The entry of HIV-1 into target cells is mediated by the viral envelope glycoproteins. The exterior envelope glycoprotein, gp120, and the transmembrane envelope glycoprotein, gp41, are derived from a gp160 precursor (15). The gp160 glycoprotein results from the addition of N-linked, high-mannose sugar chains to the approximately 845- to 870-amino-acid primary translation product of the env gene in the rough endoplasmic reticulum (15). Oligomers of gp160 form in the endoplasmic reticulum, but the current data do not unambiguously distinguish whether trimers or tetramers constitute this higher-order complex (14, 26, 32, 34). Early results studying cell- or virion-associated HIV-1 envelope glycoproteins suggested the formation of dimers followed by the assembly of dimers into unstable tetramers (14, 32). This interpretation was supported by the analysis of soluble forms of gp160 lacking a membrane-spanning region (34). By contrast, studies of peptide fragments of the gp41 ectodomain, which was shown to be necessary for the oligomerization of soluble forms of gp160, revealed a strong tendency for trimer formation (26). X-ray crystallographic analyses of these gp41 fragments confirm that they are trimeric coiled coils (8, 38).
Following oligomerization, the gp160 glycoprotein is transported to the Golgi apparatus, where cleavage by a cellular protease generates the gp120 and gp41 glycoproteins, which remain associated through noncovalent interactions (15, 24). In mammalian host cells, the addition of complex sugars to selected, probably surface-exposed carbohydrate side chains of the envelope glycoproteins occurs in the Golgi apparatus prior to transport to the cell surface (25).
The mature envelope glycoprotein complex is incorporated from the cell surface into virions, where it mediates virus entry into the host cell. The gp120 exterior envelope glycoprotein binds the CD4 glycoprotein, which serves as a receptor for the virus (10, 23). The binding to CD4 is followed by interaction of the gp120-CD4 complex with one of the chemokine receptors, which are seven-transmembrane G-protein-coupled receptors (1, 9, 11–13, 17). Chemokine receptor interaction is believed to bring the viral envelope glycoprotein complex nearer to the target cell membrane and to trigger additional conformational changes in the envelope glycoproteins (37, 39). These changes are proposed to result in the interaction of the gp41 glycoprotein with the target cell membrane, resulting in fusion of this membrane with the viral membrane. Such a model is consistent with mutagenic analysis. Amino acid changes in the hydrophobic gp41 amino terminus (the fusion peptide), in the amino-terminal half of the ectodomain, or in the transmembrane region all result in fusion-defective envelope glycoproteins (6, 18, 24).
The HIV-1 gp41 ectodomain contains a heptad repeat of hydrophobic residues at the first (a) and fourth (d) positions (Figure 1a), which is the hallmark of a coiled coil (31). Coiled coils are believed to play a central role in influenza virus entry mediated by the hemagglutinin molecule, where the extension of a trimeric coiled coil in the transmembrane HA2 subunit is thought to mark the transition to a fusogenic conformation in this protein (5, 7). Two independent crystal structures of HIV-1 gp41 ectodomain fragments have been obtained, confirming the existence of a trimeric coiled coil that is bound and stabilized by three monomers of a C-terminal helix (8, 38). Because the HIV-1 gp41 glycoprotein is thought to undergo conformational changes, it is uncertain whether the crystallographic structure obtained for the gp41 ectodomain fragments corresponds to that found in the gp160 envelope glycoprotein precursor or represents a fusion-competent conformation.
FIG. 1.
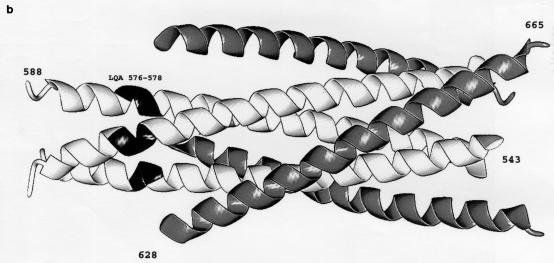
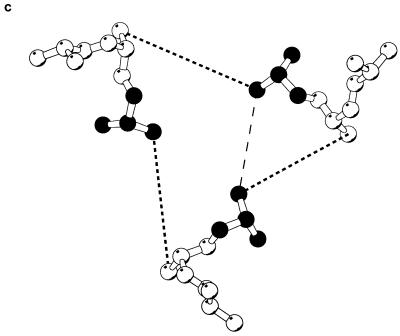
Location of the introduced cysteines in the gp41 ectodomain. (a) Sequence of the coiled-coil region of gp120, with the position along the heptad repeat indicated beneath. (b) X-ray structure of the HIV-1 gp41 coiled coil (38), with the location of the LQA/CCG change shown in black. The amino-terminal α-helical coiled coil is white, and the carboxy-terminal helices are grey. The gp41 residue numbers are indicated. (c) View of the LQA region of the gp41 coiled coil. The perspective is from the threefold symmetry axis of the coiled coil. Only the main chain and Cβ atoms are depicted. The d position leucine is indicated in black. Dark dashed lines are drawn between the Cβ atoms of leucine 576 (d in the heptad repeat) and glutamine 577 (e in the heptad repeat) to indicate where trimeric cross-links might form. The Cβ–Cβ distance is 6.84 Å, outside of the ideal distance for introducing a disulfide bond (5.28 Å) (33, 35). A possible alternative cross-link, between adjacent d position leucines, is shown by a light dashed line.
The lability of HIV-1 envelope glycoprotein oligomers has made it difficult to obtain preparations of molecules that maintain high-order states and has contributed to the uncertainty regarding the number of subunits in the gp160 oligomer. Here we show that the introduction of cysteine residues at particular locations in the gp41 ectodomain helices can result in the formation of disulfide bonds, stabilizing envelope glycoprotein trimers. The results demonstrate the relevance of the available gp41 structures to the complete HIV-1 envelope and imply that at least some of the molecular contacts observed are present before the induction of a fusogenic conformation.
We wished to study whether the introduction of disulfide bonds into the putative sites of contact between the proposed helical coils in the HIV-1 gp41 ectodomain could stabilize the full-length envelope glycoprotein oligomer and allow an analysis of its higher-order state. Since no detailed structure of the HIV-1 gp41 glycoprotein was available at the time this work was initiated, existing dimeric, trimeric, and tetrameric coiled coils (5, 20, 21, 31) were analyzed to predict the optimal positions for placement of cysteine residues. The distance requirements for the formation of intersubunit disulfide bonds were readily met in theoretical dimeric and tetrameric coiled coils (22, 30, 33, 35). In fact, a disulfide bond had been previously introduced into a model dimeric coiled coil by substitution of cysteines at the d position of the helical repeat structure (41). In the case of the hypothetical tetramer, distance requirements for disulfide bond formation could be met by introduction of cysteines at the g and a positions. In the case of the hypothetical trimer, however, no simple substitution of cysteines met the ideal distance requirements for the formation of a disulfide bond. Analysis of trimeric coiled coils for which crystal structures were available suggested that the introduction of glycine residues adjacent to the d and e positions of the helix could provide sufficient backbone flexibility to allow the formation of a stable disulfide bond.
Table 1 shows the mutant HIV-1 envelope glycoproteins (HXBc2 strain) and the observed phenotypes in transfected 293T cells. All of the envelope glycoproteins were defective in proteolytic processing of the gp160 precursor to mature gp120 and gp41 glycoproteins (Table 1). Lack of gp160 proteolytic processing has been associated with two groups of envelope glycoprotein mutants. Members of the first group exhibit major defects in folding and are usually retained in the endoplasmic reticulum. Members of the second group of cleavage-defective HIV-1 envelope glycoprotein mutants appear to exhibit only local conformational or sequence changes near the gp120-gp41 cleavage site. The latter mutants are recognized by conformationally dependent antibodies and are transported from the endoplasmic reticulum through the Golgi apparatus to the cell surface (3, 6, 14, 27). To examine the cell surface expression of the HIV-1 envelope glycoproteins mutants, 293T cells transiently expressing the envelope glycoproteins were incubated with 35S-labeled F105 monoclonal antibody, which is directed against a conformational epitope overlapping the CD4 binding site of gp120 (29). The cells were washed, and the antibody was precipitated with protein-A Sepharose beads. The amount of bound antibody was then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Most of the mutant envelope constructs exhibited little or no detectable surface expression in several experiments (Table 1). We conclude that several of the mutants are inefficiently transported to the cell surface, and are likely to be misfolded.
TABLE 1.
HIV-1 envelope glycoprotein mutants and phenotypes
| Mutant | Heptad position | Proteolytic processing | Higher-order formsa | Surface expressionb | |
|---|---|---|---|---|---|
| 569 | T/C | d | − | − | NDc |
| 583 | V/C | d | − | − | ND |
| 586–587 | YL/CC | ga | − | − | ND |
| 568–570 | LTV/GCC | de | − | − | ND |
| 571–574 | WGIK/GCCG | ga | − | − | − |
| 576–578 | LQA/CCG | de | − | + | + |
| 578–581 | ARIL/GCCG | ga | − | − | − |
| 582–584 | AVE/GCC | de | − | − | − |
| 583–585 | VER/CCG | de | − | − | − |
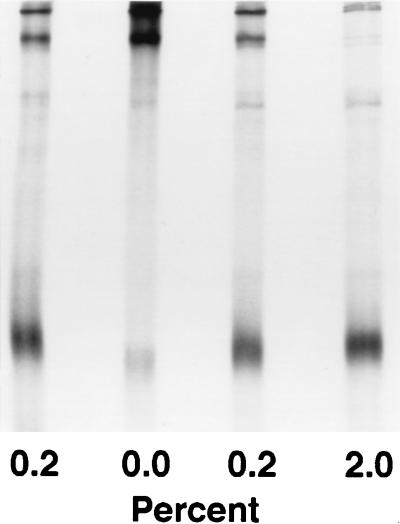
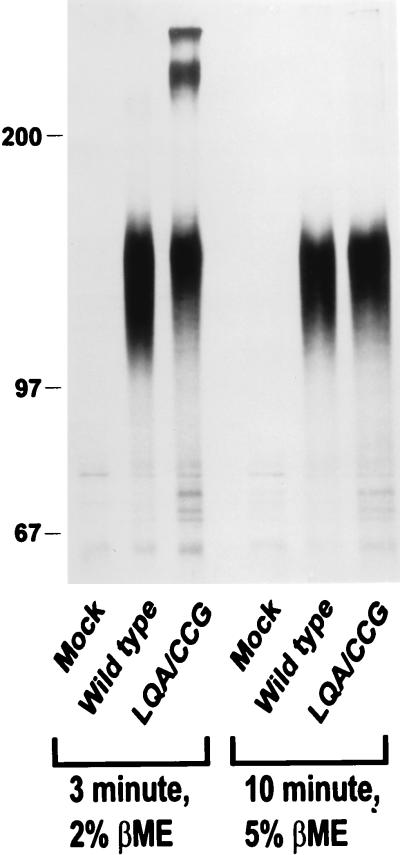
Higher-order forms of the 576–578 LQA/CCG mutant were observed after boiling for 3 min in the presence of 0.2 to 2% β-mercaptoethanol.
Surface expression was assayed by fluorescence-activated cell sorter analysis and by binding of radiolabeled F105 antibody.
ND, not determined.
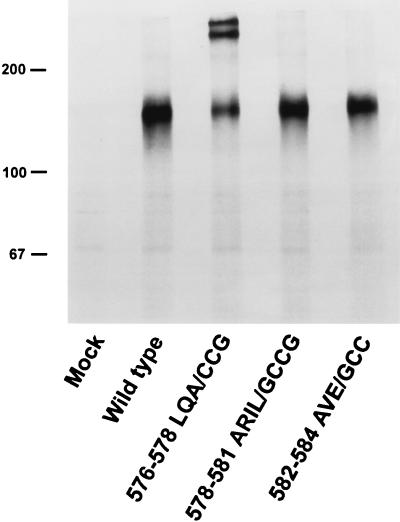
The wild-type and mutant envelope glycoproteins were precipitated from lysates of transfected cell by using specific anti-gp41 or anti-gp120 monoclonal antibodies. One mutant, 576–578 LQA/CCG (hereafter referred to as LQA/CCG), was notable for the existence of two high-molecular-weight forms evident on polyacrylamide gels even after boiling or gentle reduction (up to 2% β-mercaptoethanol) (Fig. 2 through 4). The same high-molecular-weight forms were observed when 10 mM iodoacetamide was present in the buffers used for cell lysis and sample preparation (Fig. 4). Upon boiling the mutant protein in higher concentrations of β-mercaptoethanol, the high-molecular-weight bands disappeared, with a concomitant increase in the amount of the 160,000-molecular-weight form (Fig. 3 and 4). These results are consistent with the formation of higher-order, disulfide-linked structures for the mutant gp160 envelope glycoprotein. The cysteines introduced at residues 576 and 577 of this envelope glycoprotein mutant were predicted to form intersubunit disulfide bonds between the d and e positions of a trimeric coiled coil (Fig. 1). The conservative substitution of glycine for alanine at position f of the helix (residue 578) was designed to increase the flexibility of the protein backbone in this region. Experiments performed with another mutant identical to LQA/CCG but lacking the alanine-to-glycine substitution at position 578 indicated that this substitution was not absolutely required for the stabilization of the observed higher-order forms (data not shown).
FIG. 2.
Immunoprecipitation of HIV-1 envelope glycoprotein variants. Plasmids encoding the wild-type HIV-1 envelope glycoproteins and three of the mutant envelope glycoproteins described in Table 1 were transfected into COS-1 cells. Cell lysates were immunoprecipitated with the anti-gp41 antibody D61, and the precipitates were boiled in 2% β-mercaptoethanol for 3 min before being analyzed on an SDS–8% polyacrylamide gel.
FIG. 4.
Formation of the LQA/CCG higher-order forms in the presence of iodoacetamide. Lysates of 293T cells expressing the LQA/CCG construct were immunoprecipitated with the anti-CD4 binding site antibody F105 and boiled for 3 min with the indicated percentage of β-mercaptoethanol in the presence (left lane) or absence (other lanes) of 10 mM iodoacetamide. In the experiment in the left lane, iodoacetamide was present during cell lysis and throughout the sample preparation.
FIG. 3.
Analysis of wild-type and LQA/CCG envelope glycoproteins. Lysates were immunoprecipitated with the anti-gp41 antibody D61 and boiled in either 2 or 5% β-mercaptoethanol (βME) for 3 or 10 min, as indicated, before being analyzed on an SDS–8% polyacrylamide gel.
The LQA/CCG mutant was cleavage defective when synthesized in transfected COS-1 or HeLa cells and exhibited impaired proteolytic processing when produced in 293T cells, compared with the wild-type HIV-1 envelope glycoproteins (data not shown). To determine whether impaired cleavage was the result of a global folding defect, cell surface expression and recognition by a panel of monoclonal antibodies was examined. In contrast to the other mutants examined, the LQA/CCG mutant was expressed on the surface of transfected cells, at levels slightly lower than those of HIV-1 envelope glycoproteins containing amino acid changes at the proteolytic cleavage site (3) (Fig. 5). This cleavage-defective mutant has been previously shown to be expressed on the cell surface at a level comparable to that of wild-type HIV-1 envelope glycoprotein (3). Moreover, the higher-order forms of the LQA/CCG mutant were precipitated by a number of monoclonal antibodies that recognize discontinuous epitopes on the HIV-1 gp120 envelope glycoprotein (29). These include the F105 antibody, which recognizes the CD4 binding site, and the 17b antibody, which recognizes a CD4-induced epitope (Fig. 6). The LQA/CCG mutant was also precipitated by the T4 antibody, which exclusively recognizes oligomeric gp140, as well as the gp41 antibodies T3 and D61 (Fig. 6 legend) (4). The F105 antibody recognized the 483 V/C and 582–584 AVE/CCG mutants with lower efficiency than it recognized LQA/CCG or the wild-type envelope glycoprotein (data not shown). Together, these results suggest that the LQA/CCG mutant does not exhibit global defects in folding or transport.
FIG. 5.
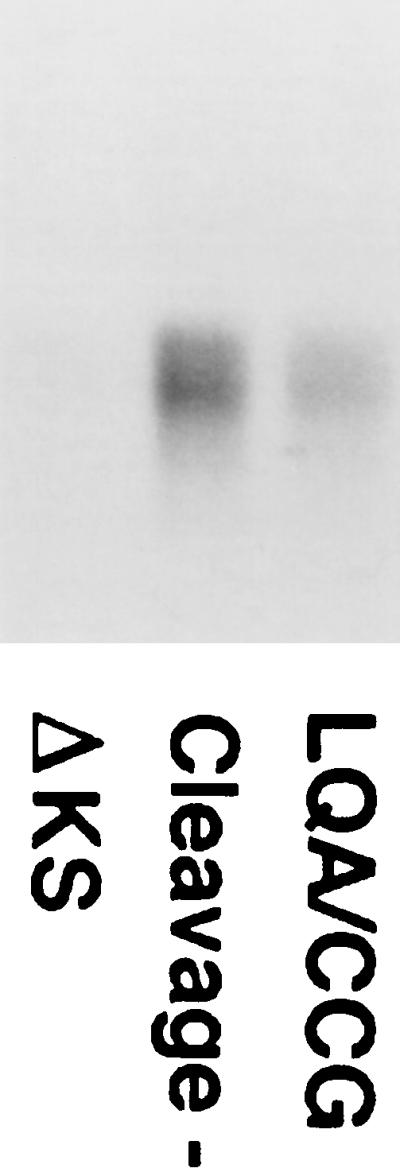
Cell surface expression of the LQA/CCG envelope glycoproteins. 293T cells were transfected with a control plasmid or with plasmids expressing a mutant HIV-1 envelope glycoprotein with amino acid changes at the proteolytic cleavage site (3) or LQA/CCG envelope glycoproteins. The transfected cells were incubated with radiolabeled F105 antibody for 2 h, washed, and lysed. The antibody was precipitated and analyzed by SDS-PAGE. The antibody heavy chain is shown.
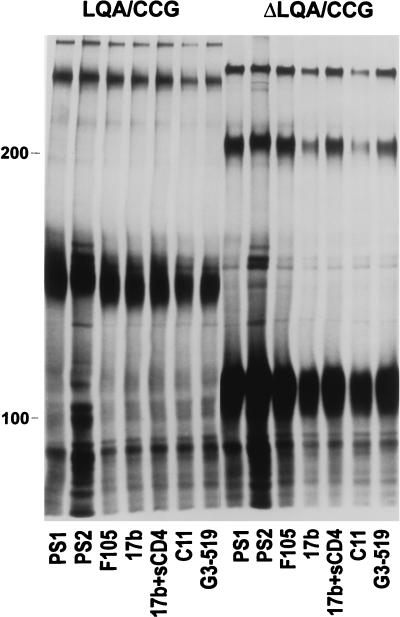
FIG. 6.
Precipitation of LQA/CCG and ΔLQA/CCG envelope glycoproteins with antibodies. 293T cells expressing the LQA/CCG and the ΔLQA/CCG envelope glycoproteins were lysed in Nonidet P-40 buffer. Cell lysates were precipitated with HIV-1-infected patient sera (PS1, PS2), the F105 antibody, the 17b antibody in the presence or absence of soluble CD4, the C11 antibody, or the G3-519 antibody (28). The A32 antibody and the D61, T3, and T4 anti-gp41 antibodies (4) all recognized both monomeric and higher-order forms of LQA/CCG and ΔLQA/CCG envelope glycoproteins (data not shown). The 110.4 antibody, directed against the third variable (V3) loop of gp120, also recognized the LQA/CCG glycoproteins (Fig. 7, lane 1:1, αV3). The LQA/CCG and ΔLQA/CCG glycoproteins were not precipitated by monoclonal antibodies against unrelated proteins (data not shown).
To determine the nature of the higher-order forms observed for the LQA/CCG mutant, a variant of this mutant was created. This variant, ΔV1/V2/V3 (tail−) 576–578 LQA/CCG (hereafter referred to as ΔLQA/CCG), is identical to the LQA/CCG mutant except that it lacks the V1/V2 and V3 gp120 loops and a large portion of the gp41 cytoplasmic tail. Specifically, residues 128 to 194 and 298 to 303 were replaced by glycine-alanine-glycine linkers. In addition, the gp41 cytoplasmic tail was truncated after residue 713 by the introduction of a stop codon into the env gene. These deletions have been shown not to compromise the proper folding or transport of HIV-1 envelope glycoproteins; rather, the deletions appear to promote efficient surface expression (40). Fluorescence-activated cell sorter analysis indicated that the ΔLQA/CCG glycoprotein was expressed on the cell surface as efficiently as a construct with the same variable loop deletions but lacking the LQA/CCG change and more efficiently than either wild-type envelope or LQA/CCG envelope glycoproteins lacking these deletions (data not shown). The ΔLQA/CCG glycoproteins were recognized by a panel of antibodies that recognize conformation-dependent epitopes on the gp120 and gp41 glycoproteins (Fig. 6). The ΔLQA/CCG envelope glycoprotein precursor migrated with an apparent molecular mass of 110 kDa, presumably a monomer, and again with two apparently higher-order forms resistant to boiling and gentle reduction. The smaller of these higher-order forms migrated slightly slower than the 200-kDa marker protein, suggesting that it represents a dimer of the ΔLQA/CCG protein (Fig. 6 and 7, lanes LQA/CCG and LQA/CCG). The larger of the two higher-order forms of the ΔLQA/CCG protein migrated similarly to the smaller of the two higher-order forms of the LQA/CCG protein. This is consistent with the expected molecular mass of approximately 330 kDa for a ΔLQA/CCG trimer and an expected molecular mass of 320 kDa for an LQA/CCG dimer.
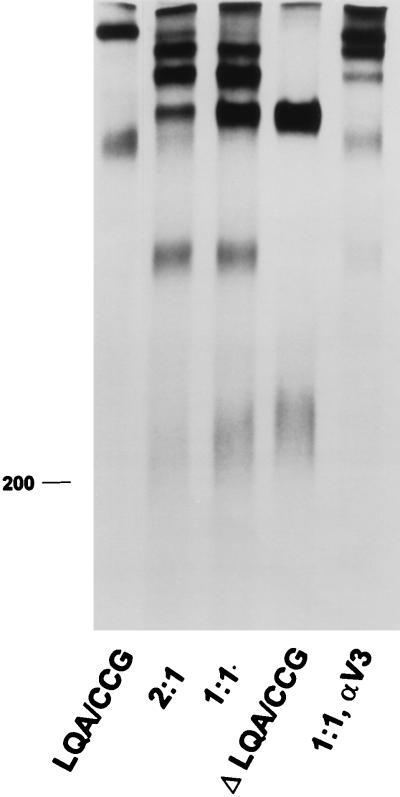
FIG. 7.
Formation of hetero-oligomers between LQA/CCG and ΔLQA/CCG envelope glycoproteins. Serum from an HIV-1-infected individual was used to precipitate lysates of 293T cells transfected with plasmids encoding LQA/CCG and ΔLQA/CCG envelope glycoproteins. In lane 2:1, plasmids expressing the LQA/CCG and ΔLQA/CCG envelope glycoproteins were transfected at a 2:1 ratio, while in lane 1:1, the LQA/CCG- and ΔLQA/CCG-expressing plasmids were transfected in equal amounts. In lane 1:1, αV3, the same cell lysates as those used for the experiment in lane 1:1 were used for precipitation by the anti-V3 loop antibody 110.4.
To provide additional information about the number of subunits in the observed higher-order forms, the LQA/CCG and ΔLQA/CCG proteins were expressed in the same cells by cotransfection of their respective expressor plasmids. We anticipated that these two proteins would form hetero-oligomers and that the pattern of bands formed would allow a determination of the number of subunits in the assembled oligomers. For example, if the oligomer were a trimer, one would expect to observe two different species of heterotrimers of 380 and 430 kDa in addition to the 480- and 330-kDa homotrimers. In addition to the monomers and 220- and 320-kDa homodimers, a heterodimer of 270 kDa would be expected. Markedly different patterns of hetero-oligomers would be observed if the assembled oligomer were a tetramer.
The results of coexpressing the LQA/CCG and ΔLQA/CCG proteins in 293T cells are shown in Fig. 7, lanes 2:1 and 1:1. By varying the ratios of the cotransfected plasmids, the pattern of intensity of the observed bands was altered, helping to confirm the identity of the proteins in each band. The LQA/CCG and ΔLQA/CCG proteins were transfected alone in the experiments in the equivalent lanes. In lane 2:1, the LQA/CCG and ΔLQA/CCG mutants were expressed by using a 2:1 ratio of plasmids encoding these constructs. In lane 1:1, equal amounts of each plasmid were transfected. The pattern of bands corresponds precisely to that expected for a trimer. The density of the heterotrimeric forms reflects that expected from the relative expression of each of the mutants present in the transfected cell. The identity of the components in each band was further confirmed by precipitating the lysate shown in lane 1:1 with an antibody, 110.3, against the gp120 V3 loop (Fig. 7, lane 1:1, αV3). As expected, this antibody recognized only oligomeric forms proposed to contain the LQA/CCG protein. The decreasing order of efficiency with which the 110.3 antibody precipitated the 480-, 430-, 380-, and 330-kDa proteins is consistent with the proposed content of 3, 2, 1, and 0 LQA/CCG monomers, respectively, in the trimer. We conclude that the LQA/CCG and ΔLQA/CCG proteins form disulfide bonds to stabilize a trimer.
We have shown that the introduction of cysteines at a specific location in the HIV-1 gp41 coiled coil stabilizes dimeric and trimeric forms of a cleavage-defective gp160 glycoprotein. This glycoprotein was expressed efficiently on the cell surface and was precipitated by antibodies that recognize conformation-dependent gp120 epitopes (29, 36). Thus, the impaired cleavage of the LQA/CCG mutant does not appear to result from global misfolding or inefficient transport along the secretory pathway. The cleavage defect could reflect a subtle conformational alteration in the envelope glycoprotein region recognized by the cellular protease or could suggest that a degree of flexibility at the cleavage site is necessary for efficient proteolytic processing and is not present in the LQA/CCG mutant. Similarly, the use of disulfide-linked envelope glycoprotein oligomers that exhibit efficient proteolytic processing may be helpful in understanding the conformational changes that occur during viral fusion. The construction of such molecules represents a future objective.
These studies were initiated in the absence of information about the HIV-1 gp41 coiled-coil structure and without certain knowledge of the oligomeric state of the complete HIV-1 envelope glycoproteins. Recently, crystal structures for the gp41 helical coiled coils have been obtained, demonstrating a trimeric structure (8, 38). The proximity of residues 576 and 577, the positions of the cysteine substitutions in the LQA/CCG mutant, in these structural models (Fig. 1c) suggests that at least some of the intersubunit molecular contacts defined for the isolated gp41 peptides are present during the assembly of the full-length envelope glycoprotein precursor. A more complete understanding of the conformational changes relevant to the fusion process will require additional detailed information about intersubunit and intrasubunit gp41 contacts in the context of the gp160 precursor and in a fusion-active state.
Dimers as well as trimers of the mutant were apparently stabilized by the formation of disulfide bonds. The dimer form of the mutant was less abundant than the trimer and was more sensitive to disruption by boiling or mild reduction. Stable dimers could represent intermediates in the assembly or disassembly of the trimer. Alternatively, the dimer could result from the formation of an alternative disulfide bond between the cysteines in the d positions, excluding the possibility of forming the three d–e disulfide bonds presumably present in the trimer (Fig. 1c).
These studies identify one strategy for stabilizing the HIV-1 envelope glycoprotein oligomer through intersubunit disulfide bonds. This strategy may be useful in producing stable trimers for structural or vaccine studies, where the lability of these higher-order forms has been problematic. It has been suggested that soluble forms of the HIV-1 envelope glycoproteins oligomers might have advantages over monomeric gp120 preparations as immunogens, since the former are more likely to mimic the native envelope glycoprotein spike on virions (4). Disulfide cross-linking of the HIV-1 envelope glycoprotein trimer could stabilize otherwise labile neutralization epitopes specific for the oligomer, mask biologically irrelevant epitopes exposed on the gp120 or gp160 monomer but buried on the functional oligomer, and lengthen the half-life of the intact vaccine construct in the body. With the availability of a crystallographic model of the gp41 exterior domain, the disulfide cross-linking strategy could be applied to other regions of the coiled coil.
Acknowledgments
We thank Lorraine Rabb and Yvette McLaughlin for manuscript preparation and Christine Naugle for graphics assistance.
This work was supported by grants to Joseph Sodroski from the National Institutes of Health (AI 24755 and AI 39420) and by a Center for AIDS Research grant to the Dana-Farber Cancer Institute (AI 28691). Dana-Farber Cancer Institute is also a recipient of a Cancer Center grant from the National Institutes of Health (CA 06516). Richard Wyatt was a fellow of the American Foundation for AIDS Research. This work was made possible by gifts from the late William McCarty-Cooper, from the G. Harold and Leila Y. Mathers Charitable Foundation, and from the Friends 10.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Barré-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 3.Bosch V, Pawlita M. Mutational analysis of the human immunodeficiency virus type 1 env gene product proteolytic cleavage site. J Virol. 1990;64:2337–2344. doi: 10.1128/jvi.64.5.2337-2344.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broder C C, Earl P L, Long D, Abedon S T, Moss B, Doms R W. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure: oligomer-specific and -sensitive monoclonal antibodies. Proc Natl Acad Sci USA. 1994;91:11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 6.Cao J, Bergeron L, Helseth E, Thali M, Repke H, Sodroski J. Effects of amino acid changes in the extracellular domain of the human immunodeficiency virus type 1 gp41 envelope glycoprotein. J Virol. 1993;67:2747–2755. doi: 10.1128/jvi.67.5.2747-2755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr C M, Kim P S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- 8.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 9.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 10.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 11.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 13.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 14.Earl P L, Doms R W, Moss B. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci USA. 1990;87:648–652. doi: 10.1073/pnas.87.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earl P L, Moss B, Doms R W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991;65:2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fauci A S, Macher A M, Longo D L, Lane H C, Rook A H, Masur H, Gelmann E P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 17.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 18.Freed E O, Myers D J, Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci USA. 1990;87:4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 20.Harbury P B, Kim P S, Alber T. Crystal structure of an isoleucine-zipper trimer. Nature. 1994;371:80–83. doi: 10.1038/371080a0. [DOI] [PubMed] [Google Scholar]
- 21.Harbury P B, Zhang T, Kim P S, Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science. 1993;262:1401–1407. doi: 10.1126/science.8248779. [DOI] [PubMed] [Google Scholar]
- 22.Hazes B, Dijkstra B W. Model building of disulfide bonds in proteins with known three-dimensional structure. Protein Eng. 1988;2:119–125. doi: 10.1093/protein/2.2.119. [DOI] [PubMed] [Google Scholar]
- 23.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 24.Kowalski M, Potz J, Basiripour L, Dorfman T, Goh W C, Terwilliger E, Dayton A, Rosen C, Haseltine W, Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987;237:1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- 25.Leonard C K, Spellman M W, Riddle L, Harris R J, Thomas J N, Gregory T J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265:10373–10382. [PubMed] [Google Scholar]
- 26.Lu M, Blacklow S C, Kim P S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 27.McCune J M, Rabin L B, Feinberg M B, Lieberman M, Kosek J C, Reyes G R, Weissman I L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988;53:55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- 28.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore J P, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muskal S M, Holbrook S R, Kim S H. Prediction of the disulfide-bonding state of cysteine in proteins. Protein Eng. 1990;3:667–672. doi: 10.1093/protein/3.8.667. [DOI] [PubMed] [Google Scholar]
- 31.O’Shea E K, Klemm J D, Kim P S, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 32.Pinter A, Honnen W J, Tilley S A, Bona C, Zaghouani H, Gorny M K, Zolla-Pazner S. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J Virol. 1989;63:2674–2679. doi: 10.1128/jvi.63.6.2674-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiter Y, Brinkmann U, Jung S H, Pastan I, Lee B. Disulfide stabilization of antibody Fv: computer predictions and experimental evaluation. Protein Eng. 1995;8:1323–1331. doi: 10.1093/protein/8.12.1323. [DOI] [PubMed] [Google Scholar]
- 34.Schawaller M, Smith G E, Skehel J J, Wiley D C. Studies with crosslinking reagents on the oligomeric structure of the env glycoprotein of HIV. Virology. 1989;172:367–369. doi: 10.1016/0042-6822(89)90142-6. [DOI] [PubMed] [Google Scholar]
- 35.Sowdhamini R, Srinivasan N, Shoichet B, Santi D V, Ramakrishnan C, Balaram P. Stereochemical modeling of disulfide bridges. Criteria for introduction into proteins by site-directed mutagenesis. Protein Eng. 1989;3:95–103. doi: 10.1093/protein/3.2.95. [DOI] [PubMed] [Google Scholar]
- 36.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 38.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 39.Wu L, Gerard N P, Wyatt R, Choe H, Paralin C, Ruffing N, Borsetti A, Cardosa A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interactions of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;184:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou N E, Kay C M, Hodges R S. Disulfide bond contribution to protein stability: positional effects of substitution in the hydrophobic core of the two-stranded alpha-helical coiled-coil. Biochemistry. 1993;32:3178–3187. doi: 10.1021/bi00063a033. [DOI] [PubMed] [Google Scholar]