Abstract
The varicella zoster virus (VZV) is a latent viral infection and its reactivation has been reported following different conditions such as immunosuppression. This study presents a confirmed case of VZV encephalitis following the first dose administration of the Sinopharm COVID-19 vaccine. A 63-year-old immunocompetent woman who developed VZV encephalitis after first dose administration of Sinopharm COVID-19 vaccine. A final diagnosis of VZV encephalitis was made based on positive CSF PCR results for VZV infection. Treatment was administered with acyclovir and she returned to normal life without any neurological sequelae. In this report, VZV reactivation and VZV encephalitis have been observed after COVID-19 vaccination; however, the results of this report should be considered with some caution, and continued post-vaccine surveillance of adverse events is recommended to explore whether any causal association with VZV reactivation is biologically plausible in this context, or if it is just a coincidence.
Keywords: VZV, COVID-19, Vaccine, Encephalitis, Reactivation, Neurological sequelae
Abbreviations
- COVID-19
coronavirus disease 2019
- RNA
ribonucleic acid
- SARS-CoV-2
severe acute respiratory syndrome coronavirus-2
- WHO
World Health Organization
- ARDS
acute respiratory distress syndrome
- MODS
multiple organ dysfunction syndromes
- RT-PCR
real-time polymerase chain reaction
- IgM
immunoglobulin M
- IgG
immunoglobulin G
- ESR
erythrocyte sedimentation rate
- CRP
C-reactive protein
- IL
interleukin
- TNF
tumor necrosis factor
- PNR
platelet-to-neuophil ratio
- PLR
platelet-to-lymphocyte ratio
- PMR
platelet-to-monocyte ratio
- NLR
neutrophil-to-lymphocyte ratio
- dNLR
derived NLR
- NMR
neutrophil-to-monocyte ratio
- MLR
monocyte-to-lymphocyte ratio
- ELR
eosinophil-to-lymphocyte ratio
- CLR
CRP-to-lymphocyte ratio
- CT
computerized tomography
- CBC
complete blood cell
- WBC
white blood cell
- RBC
red blood cell
- Hb
hemoglobin
- Hct
hematocrit
- MCV
mean corpuscular volume
- MCH
mean corpuscular Hb
- MCHC
mean corpuscular Hb concentration
- PCV
packed cell volume
- RDW
red cell distribution width
- Neut
neutrophil
- Lymph
lymphocyte
- Mono
monocyte
- Eosin
eosinophil
- PLT
platelet
- P-LCR
platelet-large cell ratio
- MPV
mean platelet volume
- PDW
platelet distribution width
- ELISA
enzyme-linked immunosorbent assay
- RBD
receptor binding domain
- ACE2
angiotensin-converting enzyme 2
- NAI
naturally acquired immunity
1. Introduction
Reactivation of latent viral infections has been reported after immunosuppression, including Coronavirus Disease 2019 (COVID-19) patients who received immunomodulatory therapies [1,2]. The development of various vaccine platforms led to a decrease in mortality and morbidity of COVID-19 [3]. However, COVID-19 vaccinations have been associated with some adverse effects, such as reactivation of latent infections [4,5]. Reactivation of the varicella zoster virus (VZV) was reported in some cases following COVID-19 vaccination [4,6]. Here, we report a unique case of VZV encephalitis after COVID-19 vaccine administration.
1.1. Case presentation
A 63-year-old woman came to the emergency department with generalized headache, fatigue, and myalgia. The record of the history revealed that the patient received the first dose of Sinopharm/BBIBPCorV COVID-19 vaccine four days before admission. She had a history of hypertension and anemia (but did not take specific medications). As such, she had a history of chickenpox in childhood, but no history of vaccination for the herpes zoster virus. No prior history of herpes simplex virus (HSV) and COVID-19 infection was reported. On admission, she was conscious and her vital signs were as follows: body temperature: 37 °C, pulse rate: 98 bpm, respiration rate: 22 breaths/min, blood pressure: 110/78 mmHg and arterial oxygen saturation (O2 SAT) was 90 %. On physical examination, there were no abnormal focal neurological deficits. During hospitalization, she had periods of headache, shoulder pain, anorexia, nausea & vomiting, abdominal pain, constipation, back pain, leg pain, and altered mental status (she initially had confusion and later experienced occasional disorientation and delirium).
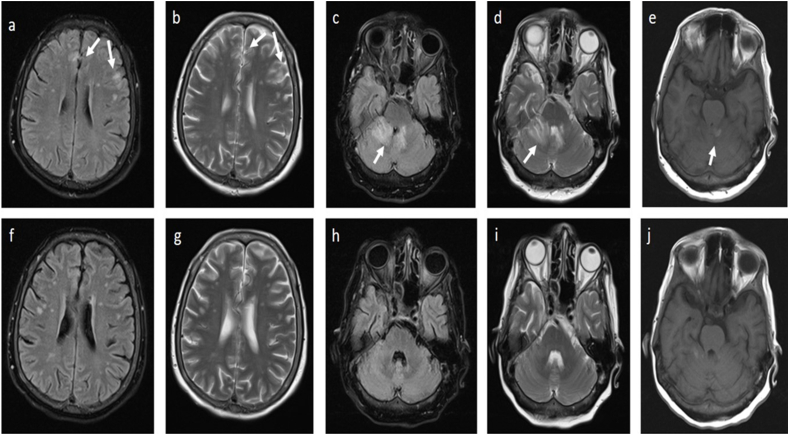
The diagnosis was made by spiral high resolution computed tomography (HRCT) scans of the lung to investigate the possibility of COVID-19 infection. Significant findings on the patient's HRCT scan are increased haziness at the base of both lower lobes, a 5 mm nodule at the base of the right lower lobe, and a prevertebral soft tissue lesion on the right side in favor of extra medullary hematopoiesis. The COVID-19 real-time polymerase chain reaction (PCR) test was negative. Additionally, the results of the primary blood tests showed pancytopenia (WBC: 1800/microliter (Mic), RBC: 344000/Mi, Hb: 10.5 g/dl). Four days after admission, she developed multiple painful vesicular lesions and erythematous patches on the right posterior chest. The patient was examined by a dermatologist and the diagnosis of herpes zoster was confirmed. Due to the worsening of symptoms, especially persistent headache, nausea, and vomiting, brain computed tomography (CT) and neurology consultation were performed. Brain CT showed hypodensity in the cerebellum. Neurological consultation recommended brain magnetic resonance imaging (MRI). Hence, a brain MRI with and without gadolinium was performed. Extensive signal changes in the cortex and cerebellum were reported in MRI, as well as necrotizing lesions, suggesting the diagnosis of encephalitis (Fig. 1, a j). Consequently, the patient received broad-spectrum antibiotics (ceftriaxone and vancomycin), antiviral (acyclovir), corticosteroids (dexamethasone) and a lumbar puncture (LP) procedure. The collected cerebrospinal fluid (CSF) sample was sent to the laboratory for PCR of HSV-1, HSV-2, CMV, VZV, and severe acute respiratory syndrome coronavirus-2 (SARS-COV-2). Subsequently, she was transferred to the ICU (Peymaniyeh hospital, Jahrom, Iran) for further evaluation with the preliminary diagnosis of encephalitis. In the ICU, the patient's delirium worsened and the patient's consciousness occasionally decreased. The CSF analysis demonstrated pleocytosis, a WBC count of 865 per mm3 with neutrophils predominance (neutrophils 62%, lymphocytes 38%), a slightly elevated protein level (60.6 mg/dl, normal range 15–45) and glucose levels (32 mg/dl, normal range 40–75). On day 17 after admission, the CSF PCR result for VZV DNA was positive and the diagnosis of VZV encephalitis was confirmed (Table 1). After 33 days of hospitalization and receiving 28 days of acyclovir (Supplementary Table 1), the patient was discharged from the hospital in good general condition.
Fig. 1.
Post-COVID-19 vaccination magnetic resonance imaging (MRI) suggestive of encephalitis: Flair and T2 hypersignal changes in the cortical and subcortical areas of the frontoparietal lobes (a, b), cerebellar vermis, right cerebellar hemisphere and right middle cerebellar peduncle (c, d) without significant enhancement or restriction. Small focus of T1 hypersignality in the left side of the cerebellar hemisphere suggestive of hemorrhage (e). Post-treatment brain MRI (at 28 days after admission) showed subtle changes in MRI, in which signal changes disappeared (f, g, h, i, j).
Table 1.
Additional laboratory tests which were performed for the patient.
| Test | Results | Normal range |
|---|---|---|
| Blood culture | Negative | – |
| Urine culture | Negative | – |
| FOB | Negative | – |
| HIV ELFA | Negative | – |
| HBSAg ELFA | Negative | – |
| HCV ELFA | Negative | – |
| CA 19-9 | 3.42 U/ml | Up to 37 |
| CA 125(Immulite) (U/ml) | 3.6 U/ml | 0–14 |
| anti-dsDNA IgM antibody | 6.9 | |
| anti-dsDNA IgG antibody | 30.2 | |
| anti-TPO antibodies | 2.3 IU/ml | Up to 31.5 |
FOB: Fecal occult blood.
2. Discussion
VZV is a common neurotropic human-limited alpha-herpesvirus [7]. VZV causes varicella or chickenpox, usually in unvaccinated children [7], which presents as a generalized vesicular skin rash [7]. However, the virus remains latent in the sensory ganglia (dorsal root ganglia and cranial nerve ganglia, such as the trigeminal ganglia and the autonomic ganglia in the enteric nervous system (ENS)) [7]. Reactivation of the latent infection has been occurred spontaneously or following various conditions (eg, aging, immune suppression, infections, X-ray radiation, trauma, and malignancy) [7], resulted in localized skin lesions, called herpes zoster or shingles [7]. VZV is the second most common viral agent after HSV in causing encephalitis [8]. Different central nervous system (CNS) disorders, including meningitis, encephalitis, cerebellitis, arteritis, myelitis, vasculitis, and stroke-related syndromes have been reported following herpes zoster, most of which were reported from immunocompromised individuals [9,10]. The most common symptoms after VZV encephalitis were headache, fever, vomiting, altered levels of consciousness, and seizures [9], which were observed in the present case. The diagnosis of VZV encephalitis is based on CSF analysis, PCR, brain CT, and MRI findings [9]. Acyclovir is the choice drug for treatment of VZV [9]. CSF pleocytosis is common in viral encephalitis, which refers to an increase in the number of white blood cells (WBC) (>5 x 10^9/L) [11]. CSF pleocytosis was also observed in the present case.
Various reports of VZV infection [[12], [13], [14]] or VZV encephalitis [[15], [16], [17]] have been reported after COVID-19 infection [2]. As such, cases of HSV encephalitis [18] and VZV meningitis [19] have also been reported after COVID-19 vaccination. The downregulation of natural killer (NK cells) group 2D (NKG2D) ligands (which help prevent autoreactivity of NK cells against host tissues) occurs during HSV and VZV infections, which can contribute to viral latency and evasion from NK cells reactions [18]. However, disruption of this balance can occur during stress-inducing conditions, such as hypoxia and viral infections such as SARS-COV-2 infection, which consequently lead to viral reactivation [18]. It has been suggested that COVID-19 vaccination can initiate cytokine release and immune response cascades, which potentially disrupt the function of CD4+ and CD8+ T cells that triggers VZV reactivation [19]. Furthermore, certain cytokines (such as IL-1, IL-6, TNF-α, and prostaglandins) can be released into the bloodstream, potentially involved in the induction of encephalitis [20]. Post vaccination encephalitis is associated with adjuvant-induced autoimmune/inflammatory syndrome induced by adjuvants (ASIA) [15]. ASIA can present with a wide range of clinical symptoms, including CNS involvements [15].
Although VZV encephalitis has been reported after COVID-19 infection [21], to the best of our knowledge, this is the first report of VZV encephalitis after COVID-19 vaccination.
As VZV encephalitis after COVID-19 vaccination is a very rare and perhaps random event, a causal relationship between these two events cannot be proved based on a single case. It is important to monitor vaccinated people for potential side effects of COVID-19 vaccination. However, healthcare providers should be aware of the initial symptoms of VZV encephalitis after vaccination and take timely interventions to prevent adverse outcomes.
Ethical approval
Informed consent was obtained from the patient for the PUBLICATION of all of their data and/or images. The Ethics Committee of Jahrom University of Medical Sciences approved the case for publication (Ethic code: IR.JUMS.REC.1402.106).
Data availability statement
All data were included in article/supplement.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial or nonprofit sectors.
CRediT authorship contribution statement
Sanaz Rezaeian: Writing – original draft, Methodology, Investigation, Data curation. Fatemeh Rahmanian: Validation, Methodology, Investigation, Data curation. Zohre Rajabpour: Validation, Methodology, Investigation. Ali Taghipour: Validation, Supervision, Methodology, Investigation. Mirza Ali Mofazzal Jahromi: Validation, Methodology, Investigation. Abdolvahab Rahmanian: Validation, Methodology, Investigation. Heshmatollah Shakeri: Validation, Methodology, Investigation. Navid Kalani: Validation, Methodology, Investigation. Maryam Jalali Jahromi: Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Amir Abdoli: Writing – review & editing, Visualization, Validation, Supervision, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Amir Abdoli reports a relationship with Jahrom University of Medical Science that includes: non-financial support. The authors have no competing interests to declare. Other authors have no known competing financial interests or personal relationships that could influence the work reported in this paper.
Acknowledgments
The authors thank the Clinical Research Development Unit of Peymanieh Educational and Research and Therapeutic Center of the Jahrom University of Medical Sciences for supporting this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28703.
Contributor Information
Maryam Jalali Jahromi, Email: jalalimaryam66@yahoo.com.
Amir Abdoli, Email: a.abdoli25@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Abdoli A., Taghipour A., Mofazzal Jahromi M.A., Eftekharian F., Sahraei R., Sanie M.S. Latent viral infections as neglected risk factors for long COVID. Lancet Glob Health. 2024;12(2):e197. doi: 10.1016/S2214-109X(24)00010-X. [DOI] [PubMed] [Google Scholar]
- 2.Abdoli A., Falahi S., Kenarkoohi A. COVID-19-associated opportunistic infections: a snapshot on the current reports. Clin Expe Med. 2022;22:327–346. doi: 10.1007/s10238-021-00751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreadakis Z., Kumar A., Román R.G., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discover. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 4.Katsikas Triantafyllidis K., Giannos P., Mian I.T., Kyrtsonis G., Kechagias K.S. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines. 2021;9(9):1013. doi: 10.3390/vaccines9091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatty A.L., Peyser N.D., Butcher X.E., Cocohoba J.M., Lin F., Olgin J.E., et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw. Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruki T., Ishikane M., Suzuki T., Ujiie M., Katano H., Ohmagari N. A case of varicella zoster virus meningitis following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. Int. J. Infect. Dis. 2021;113:55–57. doi: 10.1016/j.ijid.2021.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershon A.A., Breuer J., Cohen J.I., Cohrs R.J., Gershon M.D., Gilden D., et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.16. PubMed PMID: 27188665; PubMed Central PMCID: PMCPMC5381807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciancia S., Crisafi A., Fontana I., De Fanti A., Amarri S., Iughetti L. Encephalitis due to herpes zoster without rash in an immunocompetent 12-year-old girl: case report and review of the literature. BMC Pediatr. 2020;20:1–5. doi: 10.1186/s12887-020-02244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lizzi J., Hill T., Jakubowski J. Varicella zoster virus encephalitis. Clinical Practice and Cases in Emergency Medicine. 2019;3(4):380. doi: 10.5811/cpcem.2019.8.43010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdoli A., Taghipour A., Pirestani M., Mofazzal Jahromi M.A., Roustazadeh A., Mir H., et al. Infections, inflammation, and risk of neuropsychiatric disorders: the neglected role of “co-infection”. Heliyon. 2020;6(12) doi: 10.1016/j.heliyon.2020.e05645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellul M., Solomon T. Acute encephalitis–diagnosis and management. Clin. Med. 2018;18(2):155. doi: 10.7861/clinmedicine.18-2-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furer V., Zisman D., Kibari A., Rimar D., Paran Y., Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology. 2021;60(SI):SI90–S95. doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santovito L.S., Pinna G. A case of reactivation of varicella–zoster virus after BNT162b2 vaccine second dose? Inflamm. Res. 2021;70(9):935–937. doi: 10.1007/s00011-021-01491-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tessas I., Kluger N. Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021;35(10):e620–2. doi: 10.1111/jdv.17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J.-J., Tseng H.-P., Lin C.-L., Hsu R.-F., Lee M.-H., Liu C.-H. Acute encephalitis after COVID-19 vaccination: a case report and literature review. Hum. Vaccines Immunother. 2022;18(5) doi: 10.1080/21645515.2022.2082206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S.Y., Chen H.H., Liu P.Y., Shi Z.Y., Lin Y.H., Tsai C.A., et al. Case report of acute encephalitis following the AstraZeneca COVID‐19 vaccine. Int. J. Rheum. Dis. 2022;25(8):950–956. doi: 10.1111/1756-185X.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mörz M. A case report: multifocal necrotizing encephalitis and myocarditis after BNT162b2 mRNA vaccination against COVID-19. Vaccines. 2022;10(10):1651. doi: 10.3390/vaccines10101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moslemi M., Ardalan M., Haramshahi M., Mirzaei H., Sani S.K., Dastgir R., et al. Herpes simplex encephalitis following ChAdOx1 nCoV-19 vaccination: a case report and review of the literature. BMC Infect. Dis. 2022;22(1):1–4. doi: 10.1186/s12879-022-07186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruki T., Ishikane M., Suzuki T., Ujiie M., Katano H., Ohmagari N. A case of varicella zoster virus meningitis following BNT162b2 mRNA COVID-19 vaccination in an immunocompetent patient. Int. J. Infect. Dis. 2021;113:55–57. doi: 10.1016/j.ijid.2021.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J.J., Tseng H.P., Lin C.L., Hsu R.F., Lee M.H., Liu C.H. Acute encephalitis after COVID-19 vaccination: a case report and literature review. Hum Vaccin Immunother. 2022;18(5) doi: 10.1080/21645515.2022.2082206. PubMed PMID: 35700455; PubMed Central PMCID: PMCPMC9621012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel P., Undavia A., Choudry R., Zhang Y., Prabhu A.M. COVID-19 associated with concomitant varicella zoster viral encephalitis. Neurology: Clin. Pract. 2021;11(2):e219–e221. doi: 10.1212/CPJ.0000000000000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data were included in article/supplement.