Abstract
Microplastics (MPs) have been identified as a major potential threat to the biota and human health. Despite the exponential increase in MP research worldwide, few studies have focused on the extensive Amazon biome. To assess research priorities, the present study reviewed and summarized the available scientific knowledge on MPs in the Amazon, in addition to analyzing population and waste-management data, to evaluate potential sources of MPs in the hydrographic system. Poor sanitation conditions are a main source of MPs for the vast hydrographic basin, and, consequently, for the adjacent ocean. Secondary MPs predominated, mostly fibers (96% of debris), composed of polyamide (32%). Mean MP concentrations ranged from 0.34 to 38.3 particles.individual−1 in biota, 5 to 476,000 particles.m−3 in water, and 492.5 to 1.30848 × 107 particles.m−3 in sediment, values in close comparison with those found in areas profoundly affected by anthropogenic pollution. MPs were widespread in a range of Amazonian environments and species, and negative effects are probably occurring at various ecological levels. However, limited research, methodological constraints, flaws and the lack of standardization, combined with the continental dimensions of the Amazon, hampers the collection of the fundamental knowledge needed to reliably evaluate the impacts and implement effective mitigation measures. There is an urgent need to expand scientific data available for the region, improving local research infrastructure, and training and deploying local researchers.
Keywords: Plastic debris, South atlantic, Pollution, Review, SDG 14
Graphical abstract
Highlights
-
•
Twenty-four articles on microplastic pollution in the Amazon were identified.
-
•
Sampling and processing methods varied considerably among the studies.
-
•
Microplastics were widespread in the river basins analyzed in the Amazon region.
-
•
Secondary microplastics in the form of fibers were dominant.
-
•
Poor sanitation conditions makes the Amazon a potential microplastics hotspot.
1. Introduction
Large-scale production of plastics and lack of adequate management have led to the accumulation of millions of tons of plastic waste in the environment each year, creating one of the currently most serious environmental problems [1]. Our planet has now been exposed to plastic for almost a century, and plastic pollution is widespread in all ecosystems, predominantly in the form of small (1 μm–5 mm), persistent particles, known as microplastics (MPs) [2]. This microscopic debris has the potential to impact virtually all levels of the food chain, including the human species, and exposure to MPs may result in a range of adverse effects, including chemical contamination and even physical harm [[3], [4], [5], [6]].
Due to their ubiquitous presence and potential magnitude of impact, MPs have become a key topic in recent scientific literature [7,8]. However, despite the growing global concerns regarding plastic waste as an environmental, health, and economic problem, until 2019, only about 22.9% of the world's countries have implemented any research on this topic. Europe is responsible for the largest percentage (38%) of available studies on MPs, followed by Asia (36%) and North America (12%) [9]. In contrast, South America has contributed only 7% of the total number of studies, most of these in Brazil, Peru, Argentina, and Colombia over the past decade [10]. Regarding the Amazon biome, research on MPs is emergent, with the first scientific paper published only in 2018 [11].
The extensive Amazon ecosystem supports a rich biological diversity and vital ecosystem services [12]. Given its immense biomass, the region acts as a powerful carbon sink by absorbing and storing significant amounts of carbon dioxide from the atmosphere, and thus plays a crucial role in the potential mitigation of climate change [13]. The Amazon is also a major global source of freshwater, contributing to the hydrological cycle by maintaining and replenishing supplies at a global scale [14,15].
The hydrological connectivity of the Amazon basin allows pollutants to spread readily across long distances, affecting even the most remote and pristine portions of the biome [16]. This connectivity makes the basin extremely vulnerable to pollution by MPs derived from both local and distant sources. MP pollution has been reported in a range of Amazon environments [[17], [18], [19], [20], [21]]. However, few studies have been conducted up to now in the region, and little information is available on the sources, occurrence, and fate of the MPs. In view of this, the present study reviewed the available literature on MPs in the Amazon biome (water, sediments, and biota), complementing this review with a spatial analysis of population density and waste management to evaluate potential MP sources in the region. By combining these approaches, we sought to identify priority areas for research and the potential to advance knowledge of MP pollution patterns in the vast Amazon region. We also sought to evaluate the region as a source of MP pollution for the world's oceans.
2. Methods
2.1. Acquisition and processing of data on population density and waste management
To evaluate the potential sources of MPs in the Amazon basin, we compiled the available data on population density and basic sanitation services in the exorheic hydrographic basins within the biome (Amazon, Tocantins-Araguaia, and West-Northeast Atlantic basins). The population data were obtained from the websites of the Brazilian Institute for Geography and Statistics (IBGE), the National Administrative Department of Statistics (DANE), the Bureau of Statistics (BoS), the General Bureau of Statistics (ABS), the National Institute of Statistics (INE), the National Institute of Statistics and Censuses (INEC), the National Institute of Statistics and Informatics (INEI), and the National Institute of Statistics of Bolivia (INE). The dataset was geoprocessed to generate maps of population density, which were interpolated by geopositioning of the municipalities, through the Inverse Distance Weighted (IDW) interpolation method [22].
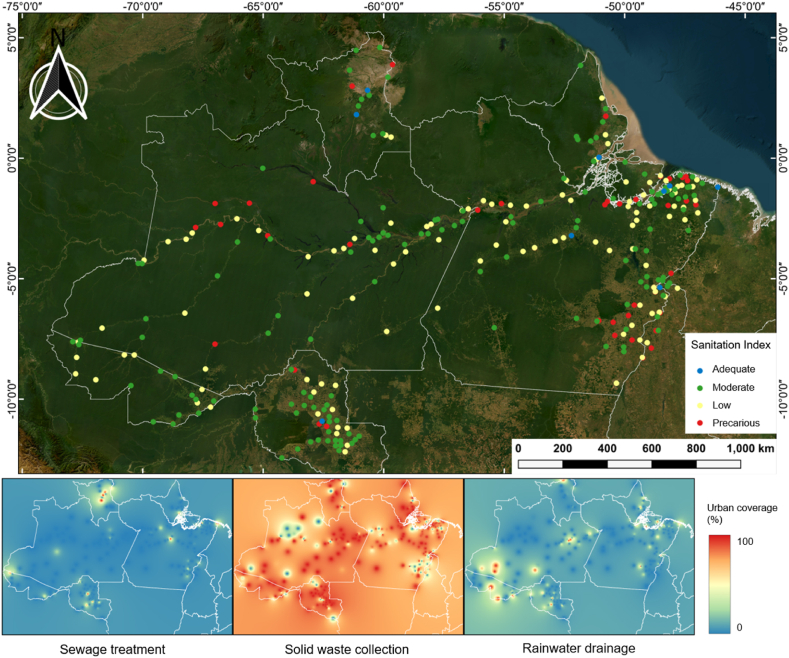
Data on the percentage of urban areas covered by public sewage collection, rainwater-drainage systems, and solid waste disposal systems in the Brazilian Amazon were obtained from the websites of the National Waters and Public Sanitation Agency (ANA) and the National Public Sanitation System (SNIS). Data on basic sanitation for the other countries in the Amazon region were difficult to find and often lacked spatial detail, thus being excluded from the analysis. The resulting dataset was used to produce heat maps to illustrate the coverage of urban services. A Basic Sanitation Index (BSI) was also calculated for all municipalities for which data were available. The cumulative level of basic sanitation was determined by summing the urban service coverage data. To standardize the index, this value was divided by 100, normalizing it as a rational number ranging from 0 to 3. Based on this sanitation index, the public sanitation services of each municipality were classified in one of four possible categories: (i) precarious (BSI = 0), (ii) low (BSI = 0–1), (iii) moderate (BSI = 1–2), and (iv) adequate (BSI >2). The municipalities were geopositioned on a map to generate a thematic map. All maps were produced in QGIS, version 3.10, 64 bits.
2.2. Literature review, search terms, and selection criteria
A comprehensive literature search was conducted to identify published papers on MPs in the Amazon mainland and continental shelf. This search was focused on the principal scientific indexing websites, the Web of Science, Science Direct, and Google Scholar. The literature search was based on a specific set of keywords (see Supplementary Material, Table S1) and analyzed only the white literature (peer-reviewed scientific papers and books) published up until April 2023.
From the search results, publications describing the characteristics and abundance of MP debris found within the geographical limits of the Amazon biome (Fig. 1) were selected. The selection criteria included field studies on biota, sediments, and/or waters. Experimental studies on the effects of MPs on endemic fauna were also considered, in order to assess all available information on the potential impacts of this debris in the biome. Several metric and non-metric variables, including bibliometric information; study-site details; matrix examined; environment analyzed; sampling and processing methods; plastic concentrations, sizes, shapes, colors, and compositions; and other relevant data were extracted from each publication. These data were compiled to generate a database (see Supplementary Material). Descriptive statistics were extracted from the data for the discussion of research patterns and results. The units used to describe the concentration of MPs in sediments varied between units per volume and units per weight. To convert the data obtained to the same units and allow comparison, the sediment density was roughly considered as 1600 kg m−3. After testing the assumptions of homoscedasticity and normality, a Spearman's rank correlation was performed to determine the association between the compiled MP occurrence data and the population and sanitation descriptors.
Fig. 1.
Delimitation of the Amazon biome and collection sites of the microplastic studies identified in the region. The images were acquired by the BaseMap tool of the QGis 3.16.5 software, and obtained through the QuickMapServices 0.19.16 plugin, data source: ESRI Satellite.
3. Results and discussion
3.1. The amazon basin as a MP pollution hotspot
Only 2.6% of the 313 municipalities analyzed in the Brazilian Amazon region were classified as adequate in terms of their basic sanitation services, while 34.8% were classified as low and 15% as precarious (Fig. 2). Solid waste disposal systems were a more satisfactory urban service, with a mean availability of 76% among the different municipalities, although sewage collection (6.2%) and rainwater drainage systems (11.7%) showed notably low mean percentages of urban service. Anthropogenic litter input is highly aggravated by the inadequacy of basic services such as waste management [[23], [24], [25]]. The poor sanitation conditions found in most of the Amazon region thus represent a major potential source of input of plastics and MPs for the world's largest fluvial system.
Fig. 2.
Basic sanitation index (BSI) and percentage of urban coverage served by sanitation services in the municipalities of the Brazilian Amazon region. The BSI was determined by summing the urban service coverages and diving the obtained value by 100 to normalizing the index as a rational number from 0 to 3. The BSI was categorized as follows: Precarious (BSI = 0), Low (BSI > 0–1), Moderate (BSI > 1–2), and Adequate (BSI >2). The images were acquired by the BaseMap tool of the QGis 3.16.5 software, and obtained through the QuickMapServices 0.19.16 plugin, data source: ESRI Satellite. Data on public sewage collection, rainwater-drainage systems, and solid-waste disposal systems obtained from the websites of the Brazilian National Waters and Public Sanitation Agency (ANA) and the National Public Sanitation System (SNIS).
Population density in the Amazon region tends to be highest along the river margins, with peaks on the Amazon (181 inhabitants.km−2) and Tocantins (2512 inhabitants.km−2) rivers (Fig. 3). This scenario, together with the unregulated urbanization of the region [26], its poor sanitation conditions and extensive landscapes, makes the Amazon basin a potential MP hotspot for the world's oceans. This conclusion is supported by a recent estimate that identifies the Amazon River as the world's second most-polluted river [27].
Fig. 3.
Population density (number per km2) in the Amazon biome. Images acquired by the BaseMap tool of the QGis 3.16.5 software and obtained through the QuickMapServices 0.19.16 plugin. Population data obtained from the websites of the Brazilian Institute for Geography and Statistics (IBGE), the Colombian National Administrative Department of Statistics (DANE), the Guyanese Bureau of Statistics (BoS), the Surinamese General Bureau of Statistics (ABS), the Venezuelan National Institute of Statistics (INE), the Ecuadorian National Institute of Statistics and Censuses (INEC), the Peruvian National Institute of Statistics and Informatics (INEI), and the National Institute of Statistics of Bolivia (INE).
Furthermore, fishing is a major commercial and subsistence activity in numerous communities along the Amazon coast [28], where it can represent both a marine- and a land-based source of plastics for the environment if not managed adequately. Fisheries equipment evolved with the introduction of plastic polymers, and fishing gear is now made primarily of plastic. Large amounts of these plastic objects invade the aquatic environment when accidently lost, deliberately discarded, or after the natural wear and tear of long-term use [29]. In general, fisheries activities tend to be correlated with higher amounts of MPs in the environment [[30], [31], [32]].
Moreover, certain natural landscapes of the Amazon region are also prominent tourist destinations. The local tourism industry has developed irregularly and lacks adequate organization, often disregarding the socio-environmental context of the region. A mass influx of tourists can temporarily increase the population of communities located on the Amazon coast by as much as sixfold [18,33], far exceeding the local carrying capacity. The impacts generated by mass tourism include pollution by sewage and littering, overuse of water resources, and unregulated urbanization [34]. These factors combine to increase the release of pollutants, in particular MPs, into the environment [[35], [36], [37], [38]].
3.2. Literature review
A total of 399.670 publications were retrieved from the literature search (16.561 from Web of Science, 125.356 from Science Direct, and 257.753 from Google Scholar). Among these, 24 published scientific papers on MPs were identified in the Amazon biota (66% of the studies), water and/or sediments (17% each). No studies on nano-plastics (<1 μm) in the natural environment were located during the review. The articles were published over a span of five and a half years, from 2018 to 2023, although one-third were released within the past six months (Fig. 4A), reflecting the extremely recent development of this field of research in the Amazon region. Most papers were published in the English language except for one in Spanish. The majority were published in peer-reviewed international journals with a high impact factor (7.9 ± 3.3 on average), such as Environmental Pollution (20.8% of the studies), Marine Pollution Bulletin and Science of the Total Environment (16.7% each).
Fig. 4.
The distribution of the 23 published papers on microplastic (MP) pollution in the Amazon identified in the present study, according to A: year of publication; B: sector of the basin; C: matrix analyzed; D: research topic; E: reported MP shapes; and F: polymer composition. ABS: Acrylonitrile Butadiene Styrene; EVA: Ethylene-Vinyl Acetate; HDPE: High-Density Polyethylene; PET: Polyethylene Terephthalate; PVC: Polyvinyl Chloride.
Most of the studies were conducted in the Brazilian Amazon (19 publications), followed by Peru (2), and Ecuador and Guyana (1 each). The middle Amazon basin was most studied (40% of the papers), followed by the lower (35%) and upper (15%) basins and the continental shelf (10%). Fish represented 54% of the matrices, while water and sediment corresponded to 17% each. Cnidarians, insects, and crustaceans each comprised 4% of the matrices (Fig. 4B and C).
These papers describe the baseline of MP pollution in the Amazon (Table 1), exploring the ingestion of MP by aquatic fauna, its presence in the abiotic environment, and the physiological effects of exposure to it (Fig. 4D). A plurality of publications (46%) assessed the ingestion of MPs, mostly in freshwater (6 studies) or estuarine fish (3). Ingestion of MPs was also reported in the freshwater shrimp Macrobrachium amazonicum [39] and the coastal sea anemone Bunodosoma cangicum; the latter was proposed as a potential biomonitor species for MP contamination [40].
Table 1.
Summary of the laboratory procedures and mean microplastic (MP) concentrations reported in 21 studies of MPs in the Amazon environment or its biota identified here. Peer-reviewed scientific papers on MP pollution in the Amazon, published between January 2018 and April 2023.
| Matrix | Sampling method | Processing | Analysis | Contamination control | Mean MP concentration | Reference |
|---|---|---|---|---|---|---|
| Biota | ||||||
| Coastal fish (3 spp.) | Purchased from fisherman | Necropsy | Microscopy | Cleaning of worksurfaces and instruments, use of vinyl gloves, cotton laboratory clothing, use of closed shoes | – | [53] |
| River fish (16 spp.) | – | Necropsy | FTIR spectroscopy | Laminar flow, filtration of solutions, petri dishes to airborne contamination | 2.09 MPs.individual−1 | [77] |
| River fish (11 spp.) | Purchased from local market | Necropsy | Microscopy | Use of metal or glass instruments, cleaning of worksurfaces, cotton laboratory clothing, petri dishes to airborne contamination | 0.34 ± 1.19 MPs.individual−1 | [55] |
| River fish (29 spp.) | Bottom trawl (5 mm mesh) | Necropsy, digestion (30% H2O2, 75 °C, 48 h), filtration (0.45 μm mesh) | FTIR spectroscopy | Cleaning and covering of worksurfaces and instruments, cotton laboratory clothing, avoidance of plastic instruments, petri dishes to airborne contamination, reduced personnel in workstation | 1.8 ± 1.6 MPs.individual−1 | [49] |
| Phylloicus elektoros | Experiment | – | – | – | – | [48] |
| Macrobrachium amazonicum | – | Necropsy, digestion (30 and 40% H2O2, room temperature, 96 h), flotation (36 g.100 ml−1 NaCl), filtration (5 μm mesh) | FTIR spectroscopy, FT-Raman | Cleaning of worksurfaces and instruments, cotton laboratory clothing, use of metal instruments, use of latex gloves, blank control | 5.45 ± 4.07 and 3.20 ± 2.64 MPs.individual−1 | [39] |
| Symphysodon aequifasciatus | Experiment | Necropsy, digestion (10% KOH) | Fluorescence confocal microscopy | – | – | [47] |
| S. aequifasciatus | Experiment | Necropsy, digestion (10% KOH, 30 °C, 12 h) | Fluorescence microscopy | – | – | [46] |
| Bunodosoma cangicum | Active searching | Necropsy | FTIR spectroscopy | Cleaning of worksurfaces and instruments, petri dishes to airborne contamination, avoidance of plastic instruments, reduced personnel in workstation, filtration of solutions | 1.6 ± 1.5 MPs.individual−1 | [40] |
| Estuarine fish (46 spp.) | Bottom trawl | Necropsy | FTIR spectroscopy | Cleaning of worksurfaces and instruments, petri dishes to airborne contamination | 1.2 ± 5 MPs.individual−1 | [17] |
| Hypanus guttatus | Purchased from fisherman | Necropsy | FTIR spectroscopy | Cleaning and covering of instruments, use of fume hood, natural fiber laboratory clothing, maintenance of doors and windows closed in workstation, petri dishes to airborne contamination | 2.4 ± 1.7 MPs.individual−1 | [52] |
| Stream fish (14 spp.) | Hand nets (3 mm mesh) | Necropsy, digestion (H2O2, room temperature), filtration (0.2 μm mesh) | Microscopy | Petri dishes to airborne contamination, cleaning and covering of instruments, filtration of solutions, cotton field and laboratory clothing, use of metal instruments, fixation liquid analysis, cotton cover on microscope | 5.6 ± 3.8 MPs.individual−1 | [50] |
| Potamotrygon leopoldi | Longline and cast net | Necropsy | FTIR spectroscopy | Cotton laboratory clothing, reduced personnel in workstation, non-use of air conditioning, cleaning and covering of instruments, filtration of solutions, use of fume hood, petri dishes to airborne contamination | 3.8 ± 1.6 MPs.individual−1 | [51] |
| River fish (15 spp.) | Purchased from local market | Necropsy, digestion (10 Mol.L−1 NaOH, 120 h), sieving (0.075 μm mesh) | Microscopy | Cleaning of worksurfaces, blank control | 38.3 MPs.individual−1 | [54] |
| S. aequifasciatus | Experiment | Lyophilization, digestion (HNO3, 70 °C, 2 h) | Fluorescence spectroscopy | – | – | [11] |
| S. aequifasciatus | Experiment | – | – | – | – | [45] |
| Sediment | ||||||
| River sediment | Van Veen grab | Sieving (63 μm mesh), digestion (H2O2, room temperature, 24 h), flotation (1.70 kg.L−1 ZnCl2), filtration (18 μm mesh) | Microscopy | Use of non-plastic instruments | 417–8178 MP kg−1 | [19] |
| Beach sediment | Hand trowel | Sieving (2000 and 500 μm meshes) | Microscopy | Not described | 987–761 MP kg−1 | [44] |
| Beach sediment | Excavation of quadrants | Sieving (250, 500 and 5000 μm meshes), flotation (1.15 kg.L−1 NaCl), filtration | Microscopy | Plastic cover on microscope, petri dishes to airborne contamination | 492.5 MP m−3 | [18] |
| Beach sediment | Excavation of quadrants | Sieving (300 μm mesh), flotation (1.2 kg.L−1 NaCl), filtration (2 μm mesh) | Microscopy | Not described | 3040–20,300 MP m−3 | [43] |
| Water | ||||||
| Artificial pond water | Horizontal and vertical plankton net trawl | Sieving (6 mm mesh) | Microscopy | Cotton laboratory clothing, petri dishes to airborne contamination, cleaning of work instruments, reduced personnel in workstation, use of nitrile masks and gloves | 20,000–476,000 MP m−3 | [42] |
| Surface river water | Plankton net trawl (300 μm mesh) | – | Microscopy | Petri dishes to airborne contamination, cotton laboratory clothing, use of metal instruments | 3.2 ± 5.85 MP m−3 | [41] |
| Surface marine water | Metal bucket and plankton net (45 μm mesh) | Filtration (0.47 μm mesh) | FTIR spectroscopy | Cotton laboratory clothing, cleaning of worksurfaces and instruments, use of non-plastic instruments, blank control, use of still-air box coupled with vacuum pump | 4772 ± 2761–2672 ± 1167 MP m−3 | [20] |
| Surface river and stream water | Water pump and plankton net (55 μm mesh) | Drying (45 °C), filtration (0.7 μm mesh), sieving (53 μm mesh), digestion (30% H2O2, 40 °C, 100 rpm), flotation (1.8 kg.L−1 NaI) | FTIR spectroscopy | Use of non-plastic instruments, cleaning of instruments, cotton laboratory and field clothing, use of positive pressure room with filtered air input, use of laminar flow, filtration of solutions, blank control | 5–74,550 MP m−3 | [21] |
Environmental screening (i.e., water and sediment screening) for MPs was documented in eight papers (33% of the studies). Half of these papers addressed water contamination, while the remainder focused on sediment contamination. The presence of MPs was reported in the waters of the Amazon continental shelf [20], as well as in samples from freshwater sources in the Amazon basin, including the Amazon, Negro, Tapajós, and Tocantins rivers and in urban streams [21,41]. The presence of these pollutants was also investigated in manmade freshwater fish-farming enclosures [42]. MPs were quantified in sediments from the beds of the Negro, Solimões, and Amazon rivers [19], sandy estuarine beaches [18,43], and a river beach [44].
A total of five experimental studies (21% of the papers) were conducted on the Amazon fauna. Four investigated the physiological effects and cumulative impacts of MPs and other environmental pressures on the endemic ornamental blue discus fish, Symphysodon aequifasciatus [11,[45], [46], [47]]. The other tested the effects of MPs and climate change on the aquatic insect Phylloicus elektoros [48].
3.3. Methods for sampling and processing MPs
The procedures employed for sampling and processing MPs, as well as quality control, are critical factors in any study, because these particles may be found suspended in the air in any anthropogenic environment, including research facilities. The use of inadequate analytical protocols or a lack of quality-control and quality-assurance procedures can cause extensive background contamination [10], leading to inaccurate results. In general, the sampling and processing methods employed for the analysis of MPs have varied widely in studies of the biota, water, and sediments of the Amazon region (Table 1).
Fish have been collected by bottom trawls [17,49], hand nets [50], longlines and cast nets [51], or purchased from fishermen [52,53] or in local markets [54,55]. Invertebrates were collected manually by active searching [39,40]. Specimens have been processed by necropsy of the organs and, in some but not all cases, by digestion, flotation, sieving, and filtration of the samples. Most of the studies on MP ingestion by Amazonian biota involved polymer analyses (7 of 11 papers), including Fourier Transform Infra-Red (FTIR) and FT-Raman spectroscopy. Background contamination was minimized using specific procedures in all studies that assessed the presence of MPs in the biota, ranging from basic precautions to strict protocols (see Supplementary Material).
The experimental studies on S. aequifasciatus examined the effects of MPs composed of different plastic polymers (polyethylene, polystyrene, and polyamide), both alone and combined with other stressors [11,[45], [46], [47]]. The experiments assessed parameters such as accumulation of MPs and cadmium post-exposure predatory performance and swimming behavior, and intestinal microbiota, as well as biomarkers of neurotransmission, digestion, energy production, oxidative stress, and the response of the immune system. Firmino et al. [48] experimentally investigated the effects of MPs, both alone and combined with increasing temperatures and carbon dioxide concentrations, on the aquatic insect P. elektoros, based on the survival and food consumption of individuals.
Different studies obtained water samples for analysis using plankton nets (45, 55, and 300 μm mesh), by surface trawling [41,42], filtering surface-water samples collected in a metal bucket [20], or with a water pump [21]. The samples were analyzed using stereomicroscopy, in natura [41] or after passing the samples through metal sieves [42] or vacuum filtration [20]. In some cases, the samples underwent additional processing by sieving, digestion, and flotation [21]. Two of the studies included FTIR spectroscopy. Background contamination was avoided by specific procedures in all the studies (see Supplementary Material).
Samples of beach sediment were collected from the substrate surface [44] or from different depth strata [18,43]. Gerolin et al. [19] collected sediment samples from riverbeds with a Van Veen grab. Samples were processed by sieving [44] or by sieving followed by flotation and filtration [18,43]; only one study used digestion [19]. None of the studies conducted polymer analyses. Only Martinelli and Monteiro [18] and Gerolin et al. [19] described some type of contamination control.
3.4. Limitations and challenges for sampling and processing MPs in the amazon
The Amazon's vastness and complex aquatic systems, coupled with the limited availability of material and financial resources, impose substantial logistical challenges to MP research, and contribute to disparities and flaws in methodological design. The studies found here used 17 different schemes to classify the particles, which introduces a significant complication for comparability of the data. Studies on biota, employed the most standardized methods for extraction and analysis of the particles (see Supplementary Material). Furthermore, some environmental studies did not replicate their samples, which also limits assessment of the variability and reliability of their results [18,20,21,44].
The mesh sizes employed to collect water samples varied considerably among studies, ranging between 45 μm and 300 μm. Similarly, sieve meshes used in sediments processing ranged from 63 μm to 500 μm. Notably, many environments of the amazon biome are characterized by high levels of organic matter [56], which can obstruct the finest meshes, and complicate the digestion, sieving, and filtration processes of MP, thus requiring adaptation of the methodological procedures.
The lower limit of detection reported for the size of MPs in the Amazon region was 55 μm, which is important since the level of contamination may be underestimated by a lack of data for smaller particles. Nevertheless, this is probably a common pattern worldwide in studies of this type, where the smaller the particles analyzed, the sparser the data (e.g., Gigault et al. [57]).
Most studies employed NaCl solutions for density sample separation, an inexpensive method considered optimal for separating low-density plastic particles [58]. High-density solutions such as ZnCl2 are effective for separating a wide range of plastic particles, although only Gerolin et al. [19] used this technique. In particular, the use of a high-torque mechanical agitator during sediment density separation may have fragmented the MPs, leading to an overestimate of their abundance [43].
The units used to report the concentration of MPs in sediments also vary among studies. While some authors report concentrations as the number of units per volume [18,43], others report the number per weight [19,44], thus introducing difficulties in the comparison of different studies.
A crucial factor in MP research is the reliable identification of polymers, because organic materials such as cellulose can be mistaken for plastic particles. Only about half (9 of 19) of the publications on the occurrence of MPs in the natural environment employed state-of-the-art techniques, such as FTIR and FT-Raman, to identify the polymers. Studies that do not conduct FITR or FT-Raman should at least adopt some appropriate method, such as chemical digestion, to distinguish plastics from natural compounds, although only six studies used chemical digestion prior to quantifying and classifying the MPs. Studies that did not use any analytical procedure to confirm the composition of the particles should use the term “potential MPs” when referring to the observed debris.
The use of quality-control protocols in both the field and laboratory is also essential for a reliable analysis of MPs. Only two of the papers identified here mentioned no steps to avoid background contamination [43,44]. Again, the protocols were not standardized in any way, and involved not only different procedures, but also varying levels of stringency in the control of potential contamination. There was also a general lack of contamination control in the field, with only three studies mentioning any steps to avoid background contamination during sampling [21,40,50].
Overall, these limitations indicate the need for harmonization and standardization of sampling, processing, and analytical methods used to study MPs, as well as the protocols used to control background contamination. In this context, we encourage researchers to apply methodological guidelines provided by international organizations, including the Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection [2] and the United Nations Environment Programme [59]. Future studies would also benefit from the parameters outlined in our database (see Supplementary Material), which should not only enable implementation of more accurate measurements, but also guarantee the reliability of comparisons between studies. Moreover, the implementation of local scientific meetings and workshops to discuss MP pollution in the Amazon is recommended to promote the improvements in methodological standardization.
3.5. MP environmental loads and effects
The studies identified in this review show that pollution by MPs is widespread across the Amazon basin, being detected in environments ranging from small rivers in the vicinity of the Andes to the Amazon continental shelf. A wide range of shapes and polymers were recorded in the Amazon region (Fig. 4E and F). However, few data concerning measurement of the effects of these pollutants were available.
In general, secondary microplastics in the form of fibers predominated in the matrices analyzed, comprising 96% of the reported debris. This outcome aligns with the global pattern, as fibers are the most commonly identified shape of MPs in the scientific literature [25,60]. The most common polymers identified in the Amazon region were polyamide (PA, 32%), polyethylene terephthalate (PET, 16%) and high-density polyethylene (HDPE, 13%). PA is primarily employed in synthetic textile products, representing most MP fibers released by domestic effluents, especially wastewater from washing machines [61,62]. Its prevalence corroborates the hypothesis that poor sanitation conditions in the Amazon are the major source of MPs for the biome. Additionally, PA is also commonly used in the production of fishing nets and ropes, suggesting the prominence of fishing-related activities as another MP sources in the region. Moreover, PET is mainly used in the manufacture of bottles and strapping, and HDPE in the production of plastic bags and storage containers [2].
Mean concentrations of MP ranged from 5 to 476,000 particles.m−3 in the waters and from 492.5 to 13,084.8 × 103 particles.m−3 in the sediments of the Amazon region, with the highest environmental concentrations found in the waters and sediments of the Negro River, middle Amazon basin. These MP concentrations place the Amazon region in close comparison with areas profoundly affected by anthropogenic pollution, such as the Yellow River (MP concentrations in water: 380,000–1,392,000 particles.m−3, and sediment: 69,712–984,000 particles.m−3) and the Yangtze River in China (water: 480–21,520 particles.m−3, and sediment: 57,216–5096.5 × 103 particles.m−3) [[63], [64], [65]].
Microplastic was reported in a total of 85 Amazonian species, including 83 fish, one cnidarian and one crustacean. In Amazon's fish, MP concentrations ranged from 0.34 to 38.3 particles.individual−1, with a mean frequency of occurrence of 65.5% in the specimens analyzed. Elsewhere, lower frequencies of occurrence have been found in fish from highly polluted rivers such as the Mississippi (12%) and the Thames (33%) [66,67]. The frequency of MP occurrence in Amazon fish was also higher than those found in marine fish from the Yellow Sea (34% [68]), North Sea (2.6% [69]), and off the Portuguese coast (19.8% [70]).
Regarding Amazon invertebrates, the native shrimp Macrobrachium amazonicum showed mean MP concentrations of 5.45 ± 4.07 and 3.20 ± 2.64 particles.individual−1 [39]. MP concentrations in these shrimps were only slightly lower than those found for shrimp collected in areas surrounded by petrochemical industries, such as the northern Bay of Bengal (Penaeus monodon: 6.60 ± 2 MPs.individual−1 and Metapenaeus monoceros: 7.80 ± 2 MPs.individual−1 [71]) and the Musa estuary in the Persian Gulf (Penaeus indicus: 21.8 MPs.individual−1 and Penaeus semisulcatus: 7.8 MPs.individual−1 [72]).
In the sea anemone Bunodosoma cangicum, MPs were reported in a mean concentration of 0.8 particles.g−1 of wet weight (or 1.6 ± 1.5 particles.individual−1) [40]. These values are notably lower than those discovered in Actinia equina along the north coast of Spain (35.7 particles.g−1 [73]), and comparable to sea anemones from the family Actiniidae collected in the Chukchi Sea (0.04–0.94 particles.g−1 [74]).
A positive correlation was found between the frequency of occurrence of MP reported in the studies and population density of the study area (Spearman's rho = 0.4745356, S = 50,068, p = 0.000006), highlighting the impact of population distribution on debris input. Linear regression analysis indicates that 10.7% of the variance in the MP frequency of occurrence can be explained by population density (R2 = 0.1074, F = 9.747, p = 0.00249) (Fig. 5). No significant correlation was found between MP frequency of occurrence and sanitation index (Spearman's rho = −0.06213969, S = 101,205, p = 0.5768).
Fig. 5.
Scatter plot between population density and microplastic frequency of occurrence (FO) reported in literature. Shaded area indicates 95% confidence interval for the linear regression. R2 = 0.1074, F = 9.747, p = 0.00249, y = 52.50 + 0.22x.
In the upper Amazon basin, Lucas-Solis et al. [44] reported high MP concentrations in beach sediments along the Misahualli River, a small affluent of an early tributary of the Amazon River. Mean MP concentrations were 1579.2 × 103 particles.m−3 of dry sediment for plastics measuring 0.5–2.0 mm, and 1217.6 × 103 particles.m−3 for plastics measuring 2–5 mm, with most particles classified as fibers (97%). Studies in Iquitos found MPs in gills and internal organs of commercial fish species from local markets, with a notable increase in MP frequency of occurrence and concentration over one year period. Chota-Macuyama and Mendoza [55] found MPs in one out of 11 fish species, with a total frequency of occurrence of 12% and a mean abundance of 0.34 (±1.19) particles.individual−1. One year later, Rojas et al. [54] found MPs in 15 fish species from the same area, with items occurring in 100% of the specimens analyzed, in a mean abundance of 38.3 particles.individual−1. Fibers predominated in both cases, comprising more than 80% of the MPs. However, only Chota-Macuyama and Mendoza [55] determined the polymeric composition of the particles, by digestion (NaOH 10 mol.L−1 for 120 h), which may account for the difference of one order of magnitude from the results of Rojas et al. [54]. Additionally, Iquitos is a large urban center (146,853 inhabitants, 24.76 inhabitants.km−2) that continually suffers a process of unregulated urbanization, resulting in the rapid proliferation of periurban settlements exposed to poor sanitation conditions along the margins of the rivers that surround the city [75,76]. The progress of this process contributes to the accelerated increase of environmental pollution and may account to the observed discrepancies between different years.
In the middle Amazon basin, sediments contained only fibers, of 8 different colors, whereas the water samples contained MPs in the shape of fragments, fibers, films, beads and foams, of 10 different colors. Mean MP concentrations in the sediments varied from 667,200 particles.m−3 in the Amazon River to 13,084.8 × 103 particles.m−3 in the Negro River [19]. In the water of the middle basin, mean values varied from 0.25 particles.m−3 in the Tapajós River [41] to 170,666.7 particles.m−3 in the Madeira River basin [42]. Furthermore, a total of 32 plastic polymers have been reported from the water of this region [21,41].
MP was reported in the digestive tract of 38 fish species of the middle amazon basin, including the endemic Xingu River ray (Potamotrygon leopoldi) [49,51,77]. The detected debris were classified as fibers, spheres, foam, and hard and soft fragments, in 14 different colors. A total of 17 different polymers were identified in the organism. Mean MP concentrations varied from 1.8 (±1.6) to 3.8 (±1.6) particles.individual−1. MP was also recorded in the in a commercially valuable native shrimp (Macrobrachium amazonicum) in the area. Particles were identified in the digestive tract, cephalothorax, and abdomen of 89% of the shrimp specimens, with a mean concentration ranging from 3.2 (±2.64) particles.individual−1 in a rural portion of the floodplain to 5.45 (±4.07) particles.individual−1 in an urban area. Blue polypropylene fibers were the predominant type of MP found in the shrimp [39].
In the lower Amazon basin, MPs have been found to be widespread in the sediments of estuarine sandy beaches. This debris was detected in strata up to 80 cm deep, with mean concentrations ranging from 492.5 to 20,300 particles.m−3 across different beaches [18,43]. At least 95% of the particles were fibers, although fragments and pellets were also found. Blue, transparent, black, red, green, white, and brown particles were reported.
Water from the lower basin contained mean MP concentrations varying from 9 particles.m−3 in the right branch of the Amazon River estuary to 3095 particles.m−3 in urban streams. Fibers, films, fragments and glitter, of 10 different colors, were reported from the samples. Moreover, polyester, polyethylene (PE), polystyrene (PS), polypropylene (PP), acrylic, Polyvinyl Chloride (PVC), alkyd varnish, and PA were detected in the waters of this area [21].
Ribeiro-Brasil et al. [50] recorded MPs in 14 fish species from 12 streams in the basins of the Guamá and Acará-Capim rivers in the lower Amazon basin. MPs were detected in the gills and digestive tracts of all but one of the fish. On average, 5.6 (±3.8) particles were found per individual. Only fibers and fragments were detected in this study, with most MPs reported as fibers (93.5%).
MPs have also been detected in invertebrates from the lower Amazon basin. Morais et al. [40] reported MPs in the gastrovascular cavity of the sea anemone Bunodosoma cangicum collected from intertidal beach rocks on the right margin of the Marajó Bay estuary. MPs were documented in 75.6% of the specimens, with a mean of 1.6 (±1.5) particles.individual−1. Fibers (84% of the particles), fragments (12%), and films (4%), of 8 different colors were reported in the organism. MP composition was identified as PET (44.7%), PP (18.4%), PA (10.5%), polyurethane (PU; 10.5%), PE (7.9%), acrylonitrile butadiene styrene (ABS; 2.6%), PS (2.6%), and rayon (2.6%).
On the Amazon continental shelf, Queiroz et al. [20] recorded MPs in 100% of the water samples collected, with a mean concentration of 4772 (±2761) particles.m−3 in the rainy season and 2672 (±1167) particles.m−3 in the dry season. Fibers predominated during the rainy season (58% of the total MPs), and fragments (53%) in the dry season. The MPs were blue (28%), transparent (25%), or yellow (16%), composed of PA (22%), PU (11%), ABS (8%), PET (7%), ethylene–vinyl acetate (EVA, 6%), PE (3%), and PVC and PP (1% each). The high MP concentrations recorded may have been related to the presence of manmade cellulose fibers, which were counted together with the MPs, and the lack of digestion steps used.
Regarding the fauna of the Amazon continental shelf, Pegado et al. [17,52] and Alfred et al. [53], reported MPs in 14 fish species from the Amazon River estuary, three commercial fish species off the coast of Guyana, and in the longnose stingray (Hypanus guttatus) in the Gulf of Maranhão. MP occurred in 28% of the analized specimens and mean abundances varied from 1.2 (±5) to 2.4 (±1.7) particles.individual−1. MPs included pellets, microbeads, films, fibers, foam, and fragments. The identified colors were transparent, blue, red, green, yellow, white, black, silver, and orange. Particles were composed by PE, PA, rayon, PET, ABS, PP, and a blend of PET and styrene butadiene rubber (SBR).
Experimental studies on the endemic fish S. aequifasciatus, revealed that MPs can decrease acetylcholinesterase (AChE) activity, negatively impacting its predation performance. Digestive enzyme activity also decreased by exposure to MP, particularly at elevated temperatures. Exposure to MPs alone, and alongside cadmium (Cd), resulted in increased oxidative stress. MP Also reduced the rate of weight gain, altered several neurotransmitter levels, and affected gut and gill microbial communities. Furthermore, exposure to nanoplastic has also reduced the fish's swimming and predation performance [11,[45], [46], [47]].
The exposure of the endemic aquatic insect P. elektoros to MP increased the orgasnism's mortality risk by approximately sevenfold. Furthermore, the combined effects of climate-change factors (increases in temperature and carbon dioxide) and MP exposure significantly reduced P. elektoros feeding, although MPs alone had no negative effects on the insect feeding [48].
3.6. Remarks for future research
Despite the overall limitations and the paucity of published papers, the available data indicate a widespread MP contamination in the environments and organisms of the Amazon region. All analyzed samples of sediment and water contained MPs, as well as half of the examined specimens. This scenario raises a number of concerns and highlights the urgent need to consolidate scientific capacity in countries of the Amazon region, aiming to improve the understanding of MP pollution patterns, which will, in turn, be essential to guide the implementation of effective measures to mitigate the impacts of these pollutants.
The existing gap in the literature on MP pollution in the Amazon basin is still far from being filled. Current knowledge is nowhere nearly sufficient to draw reliable inferences on the source dynamics, spatial distribution, temporal variability, trophic transfer, and ecological effects of MPs in the region. There is thus a clear need to highlight the specific context and constraints to high-quality research in the developing countries of South America. Research in this region is typically limited by material and financial resources, as well as a lack of qualified personnel, research programs, and institutional support. Budgets are also usually far too limited to allow significant scientific progress. However, despite these obstacles, some countries, such as Brazil, still have succeeded in producing important research, often through international partnerships.
While most of the studies identified here were conducted in the Brazilian Amazon, it is crucial to broaden efforts to encompass other regions within the Amazon biome, including Bolivia, Colombia, Venezuela, Suriname, and French Guiana, where no studies whatsoever were found. Another shortfall in the available literature is that all the studies identified here were conducted in aquatic environments, leaving a significant gap regarding contamination of the terrestrial sediments and biota. Furthermore, the biological studies focused on only four organism groups – fish, cnidarians, crustaceans, and insects –, with no studies on mollusks, annelids, echinoderms, amphibians, turtles, birds, reptiles, or mammals. It will be crucial to address these research gaps in order to better assess the risks associated with MP pollution for the local communities. This research should also contribute to the development of appropriate legislation and regulations to prevent MPs from entering the trophic web and ultimately reaching the human species.
No studies on the possible MP contamination of human populations in the Amazon were found. Local traditional and indigenous communities in this biome have a profound connection with the natural environment and rely on natural resources for subsistence and cultural practices. From the observed patterns of MP pollution, these communities are doubtless exposed to significant levels of contamination, with increasing risks to their health. It is of the utmost importance to direct research efforts toward a better understanding of the level of exposure of these communities to MPs, and the potential health hazards.
Investigation of the occurrence of MPs and identification of their composition are essential steps in understanding the extent of MP pollution in the Amazon region. A comprehensive understanding of the primary sources and dispersal patterns will be essential for the development of effective mitigation strategies to reduce emissions of these pollutants. To our knowledge, the present study is the first to evaluate the potential primary sources of MPs in the region. Future research should consider a more systematic investigation of sources such as wastewater discharge, runoff, industrial activities, landfills, and others.
Another pattern observed here is the general lack of experimental studies, which can provide valuable insights for assessment of the persistence, accumulation, and potential biological and ecological impacts of MPs. These data are essential for modeling and predicting the long-term consequences of MP pollution on biodiversity, which will also be essential for the development of effective conservation and management strategies.
Ultimately, we strongly encourage implementation of local scientific meetings and workshops to discuss the growing problem of MPs in the Amazon region and coordinate research and management efforts. Any such initiative should aim to bring together regional and international experts in the field, to address the environmental problems and health risks associated with MP contamination in the biome from biological, physical, and chemical perspectives, as well as determining priority areas for research and establishing a collaborative network for the Amazon region as a whole. Additionally, recommended short-, medium- and long-term management measures to MP pollution in the Amazon are provided in Table 2.
Table 2.
Recommended short-, medium- and long-term management measures to microplastic (MP) pollution in the Amazon biome.
| Management Proposal | Description | Expected Impact |
|---|---|---|
| Short-Term | ||
| Waste management improvement | Immediate improvements in waste collection, recycling, and disposal systems. | Decrease plastic and MP input to the environment. |
| Implementation of awareness campaigns | Launch educational campaigns and actions targeting communities, industries, and stakeholders to raise awareness about the sources, impacts and mitigation of MP pollution. | Increase public participation in the prevention and mitigation of MP pollution. |
| Funding of science and research inniatitives | Through stakeholders and institutions engagement, promote increase in fundings to allow research on plastic and microplastic pollution and mitigation | Increase in quality and quantity of data and publications for the Amazon |
| Policy enforcement | Strengthen enforcement of existing environmental regulations on waste management and water treatment. | Reinforce existing measures on plastic pollution, by applying the laws and regulations already approved by local states. |
| Medium-Term | ||
| Infrastructure improvement | Improve sewage and waste treatment facilities to include technologies capable of filtering MPs, especially in urban centers. | Reduce the input of MPs from urban runoff into the waterways. |
| Building awareness, education and societal participation | Foster educational programs in schools, universities and the third sector, to actively engage the different sectors of society for effective mitigation of plastic and MP pollution | Stablishment of permanent programs to raise public awareness and consequently the reduction of plastic pollution |
| Research and monitoring | Expand comprehensive studies on distribution, sources, sinks and effects of MPs. Establish regular monitoring programs of MP pollution. Development of bio-plastics derived from local materials and biomass | Improve knowledge for the academic community and data for informed decision-making. |
| Long-Term | ||
| Policy and regulation development | Develop and implement regulations especificaly targeting the prevetion and the mitigation of plastic and MP pollution (e.g. banning of single use, disposable plastics). | Systematic reduction in plastic and MP pollution from urban sources. |
| Implementation of sustainable economic practices | Promote and support sustainable practices and plastic-free alternative materials in industries such as fishing, agriculture, and tourism | Long-term decrease in MP pollution sources. |
| Restoration initiatives | Initiate projects to restore affected habitats and investigate methods to reduce and recicle MPs from the environment safely. | Rehabilitation of environment and reduction in MP impacts. |
| Sustainable Urban Planning | Integrate MP pollution control into urban planning and development policies to manage future growth sustainably. | Prevent future MP pollution by considering environmental impacts in urban development. |
| Economic Incentives | Implement taxes and subsidies to encourage reduction in plastic use and enhance recycling efforts. Introduce regulations for industries to adopt bio-based polymers. | Systematic reduction in MP pollution. |
4. Conclusions
This review has revealed the emergence of the problem of MPs in the Amazon biome, and the urgent need to improve current knowledge of this growing impact. Despite the methodological flaws and intrinsic limitations of the studies identified here, the available data clearly reveal the ubiquity of MPs in the surface waters, sediments, and living organisms of the whole Amazon basin. Secondary MPs, in the shape of fibers, were predominant, while the presence of pellets is still limited, although the debris analyzed in the different studies included an ample range of size, shapes, colors, and polymer composition. The potential long-term impacts of these pollutants nevertheless remain unclear. Consolidating scientific capacity will be the key to the evolution of MP research in the countries of the Amazon region, in both the quantity and quality of published data. Building networks, enhancing the exchange of personnel and information among institutions, implementing intensive training and workshops, and establishing international partnerships will be effective strategies, not only for the advancement of MP research in the Amazon region, but also the development and consolidation of management efforts.
Capsule
Even considering their specific limitations and intrinsic flaws, the studies identified in this review revealed the ubiquity of microplastics in the water, sediments, and biota of the diverse environments of the Amazon biome.
Consent for publication
The authors unanimously agree to submit this manuscript for possible publication.
Funding
The English review of the manuscript was financed by the Pro-Rectorate of Research and Post Graduation of the Federal University of Pará (PROPESP/UFPA grant no. 23073.067710/2023–66). LMSM was funded by the Brazilian Coordination for Higher Education Personnel Training (CAPES; DS grant no. 88887.601154/2021–00). MOS was funded by National Council for Scientific and Technological Development (CNPq; grants no. 442337/2020–5, 313518/2020–3), Alexander Von Humboldt-Stiftung, Fulbright Commission, FUNCAP (PELD and Chief-Scientist Program), I-plastics (JPI Oceans Consortium – Grant PCI2020-112059), and CAPES (CAPES-PRINT, CAPES-AVH). TG was funded by “Detetives do Plástico: Abordagem Integrada para a Avaliação da Poluição por Plásticos na Costa Semiárida do Ceará” (FUNCAP # UNI-0210-00136.01.00/23), and CNPq (#308528/2022-0). JEMFand LMSM were supported by the United Nations Environment Program, through the Gulf and Caribbean Fisheries Institute (UNEP, GCFI; grant no. #SSFA/2021/3581). JEMF, LMSM and NF were supported by the Research Support and Development Foundation (FADESP, grant no. M1-32QCL-000009).
Data availability statement
Data were included in the supplementary material.
CRediT authorship contribution statement
L.M.S. Morais: Writing – original draft, Visualization, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. A.F.S. Queiroz: Visualization, Investigation, Formal analysis, Data curation. B.K.F. Brito: Investigation, Data curation. N. Fenzl: Writing – review & editing, Project administration, Funding acquisition. M.O. Soares: Writing – review & editing. T. Giarrizzo: Writing – original draft, Supervision, Resources, Methodology, Conceptualization. J.E. Martinelli Filho: Writing – original draft, Supervision, Resources, Project administration, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the editor and the reviewers for their valuable comments on the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28851.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Barnes D.K., Galgani F., Thompson R.C., Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B. 2009;364:1985–1998. doi: 10.1098/rstb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GESAMP . In: Reports and Studies. Kershaw P.J., Turra A., Galgani F., editors. 2019. Guidelines for the monitoring and assessment of plastic litter and microplastics in the ocean. IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection. GESAMP No. 99, 130 pp. ISSN: 1020–4873. [Google Scholar]
- 3.Wright S.L., Thompson R.C., Galloway T.S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Fossi M.C., Marsili L., Baini M., Giannetti M., Coppola D., Guerranti C., Panti C. Fin whales and microplastics: the mediterranean sea and the sea of cortez scenarios. Environ. Pollut. 2016;209:68–78. doi: 10.1016/j.marpolbul.2016.05.077. [DOI] [PubMed] [Google Scholar]
- 5.Auta H.S., Emenike C.U., Fauziah S.H. Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions. Environ. Int. 2017;102:165–176. doi: 10.1016/j.envint.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Doyle D., Sundh H., Almroth B.C. Microplastic exposure in aquatic invertebrates can cause significant negative effects compared to natural particles - a meta-analysis. Environ. Pollut. 2022;315 doi: 10.1016/j.envpol.2022.120434. [DOI] [PubMed] [Google Scholar]
- 7.Hale R.C., Seeley M.E., La Guardia M.J., Mai L., Zeng E.Y. A global perspective on microplastics. J. Geophys. Res. Oceans. 2020;125 doi: 10.1029/2018JC014719. [DOI] [Google Scholar]
- 8.Koelmans A.A., Redondo-Hasselerharm P.E., Nor N.H.M., de Ruijter V.N., Mintenig S.M., Kooi M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022;7:138–152. doi: 10.1038/s41578-021-00411-y. [DOI] [Google Scholar]
- 9.Ajith N., Arumugam S., Parthasarathy S., Manupoori S., Janakiraman S. Global distribution of microplastics and its impact on marine environment – a review. Environ. Sci. Pollut. Res. 2020;27:25970–25986. doi: 10.1007/s11356-020-09015-5. [DOI] [PubMed] [Google Scholar]
- 10.Grillo J.F., Rebolledo A.G., Sabino M.A., Ramos R. Microplastics in Latin America and the Caribbean: on the adoption of reporting standards and quality assurance and quality control protocols. Environ. Adv. 2022;8 doi: 10.1016/j.envadv.2022.100236. [DOI] [Google Scholar]
- 11.Wen B., Zhang N., Jin S.R., Chen Z.Z., Gao J.Z., Liu Y., Xu Z. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquat. Toxicol. 2018;195:67–76. doi: 10.1016/j.aquatox.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Albert J.S., Carnaval A.C., Flantua S.G., Lohmann L.G., Ribas C.C., Riff D., Carrillo J.D., Fan Y., Figueiredo J.J., Guayasamin J.M., Hoorn C., Melo G.H., Nascimento N., Quesada C.A., Ulloa C.U., Val P., Arieira J., Encalada A.C., Nobre C.A. Human impacts outpace natural processes in the Amazon. Science. 2023;379:eabo5003. doi: 10.1126/science.abo5003. [DOI] [PubMed] [Google Scholar]
- 13.Araujo E.C.G., Sanquetta C.R., Dalla Corte A.P., Pelissari A.L., Orso G.A., Silva T.C. Global review and state-of-the-art of biomass and carbon stock in the Amazon. J. Environ. Manag. 2023;331 doi: 10.1016/j.jenvman.2023.117251. [DOI] [PubMed] [Google Scholar]
- 14.Papa F., Frappart F., Güntner A., Prigent C., Aires F., Getirana A.C., Maurer R. Surface freshwater storage and variability in the Amazon basin from multi‐satellite observations, 1993–2007. J. Geophys. Res. Atmos. 2013;118(11) doi: 10.1002/2013JD020500. 951–11, 965. [DOI] [Google Scholar]
- 15.Baker J.C., Cintra B.B., Gloor M., Boom A., Neill D., Clerici S., Leng M.J., Helle G., Brienen R.J. The changing amazon hydrological cycle–inferences from over 200 Years of tree‐ring oxygen isotope data. J. Geophys. Res. Biogeosci. 2022;127 doi: 10.1029/2022JG006955. [DOI] [Google Scholar]
- 16.Castello L., McGrath D.G., Hess L.L., Coe M.T., Lefebvre P.A., Petry P., Arantes C.C. The vulnerability of Amazon freshwater ecosystems. Conserv. Lett. 2013;6:217–229. doi: 10.1111/conl.12008. [DOI] [Google Scholar]
- 17.Pegado T., Schmid K., Winemiller K.O., Chelazzi D., Cincinelli A., Dei L., Giarrizzo T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018;133:814–821. doi: 10.1016/j.marpolbul.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Martinelli Filho J.E., Monteiro R.C.P. Widespread microplastics distribution at an Amazon macrotidal sandy beach. Mar. Pollut. Bull. 2019;145:219–223. doi: 10.1016/j.marpolbul.2019.05.049. [DOI] [PubMed] [Google Scholar]
- 19.Gerolin C.R., Pupim F.N., Sawakuchi A.O., Grohmann C.H., Labuto G., Semensatto D. Microplastics in sediments from Amazon rivers. Brazil. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141604. [DOI] [PubMed] [Google Scholar]
- 20.Queiroz A.F.S., da Conceição A.S., Chelazzi D., Rollnic M., Cincinelli A., Giarrizzo T., Martinelli Filho J.E. First assessment of microplastic and artificial microfiber contamination in surface waters of the Amazon Continental Shelf. Sci. Total Environ. 2022;839 doi: 10.1016/j.scitotenv.2022.156259. [DOI] [PubMed] [Google Scholar]
- 21.Rico A., Redondo-Hasselerharm P.E., Vighi M., Waichman A.V., de Souza Nunes G.S., de Oliveira R., Singdahl-Larsen C., Hurley R., Nizzetto L., Schell T. Large-scale monitoring and risk assessment of microplastics in the Amazon River. Water Res. 2023;232 doi: 10.1016/j.watres.2023.119707. [DOI] [PubMed] [Google Scholar]
- 22.Kearney K.M., Harley J.B., Nichols J.A. Inverse distance weighting to rapidly generate large simulation datasets. J. Biomech. 2023;158 doi: 10.1016/j.jbiomech.2023.111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton A.A., Dixon S.J. Microplastics: an introduction to environmental transport processes. Wiley Interdiscip. Rev. Water. 2018;5:e1268. doi: 10.1002/wat2.1268. [DOI] [Google Scholar]
- 24.Fries A.S., Coimbra J.P., Nemazie D.A., Summers R.M., Azevedo J.P.S., Filoso S., Newton M., Gelli G., Oliveira R.C.N., Pessoa M.A.R., Dennison W.C. Guanabara Bay ecosystem health report card: Science, management, and governance implications. Reg. Stud. Mar. Sci. 2019;25 doi: 10.1016/j.rsma.2018.100474. [DOI] [Google Scholar]
- 25.Orona-Návar C., García-Morales R., Loge F.J., Mahlknecht J., Aguilar-Hernández I., Ornelas-Soto N. Microplastics in Latin America and the Caribbean: a review on current status and perspectives. J. Environ. Manag. 2022;309 doi: 10.1016/j.jenvman.2022.114698. [DOI] [PubMed] [Google Scholar]
- 26.Becker B.K. Geopolítica da Amazônia. Estud. Avançados. 2005;19:71–86. doi: 10.1590/S0103-40142005000100005. [DOI] [Google Scholar]
- 27.Giarrizzo T., Andrade M.C., Schmid K., Winemiller K.O., Ferreira M., Pegado T., Chelazzi D., Cincinelli A., Fearnside P.M. Amazonia: the new frontier for plastic pollution. Front. Ecol. Environ. 2019;17:309–310. doi: 10.1002/fee.2071. [DOI] [Google Scholar]
- 28.Castello L., McGrath D.G., Arantes C.C., Almeida O.T. Accounting for heterogeneity in small-scale fisheries management: the Amazon case. Mar. Pol. 2013;38:557–565. doi: 10.1016/j.marpol.2012.09.001. [DOI] [Google Scholar]
- 29.Lusher A., Hollman P., Mendoza-Hill J. FAO Fisheries and Aquaculture Technical Paper; Rome: 2017. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; p. 147.http://www.fao.org/3/i7677en/i7677en.pdf [Google Scholar]
- 30.Dowarah K., Devipriya S.P. Microplastic prevalence in the beaches of Puducherry, India and its correlation with fishing and tourism/recreational activities. Mar. Pollut. Bull. 2019;148:123–133. doi: 10.1016/j.marpolbul.2019.07.066. [DOI] [PubMed] [Google Scholar]
- 31.Chen B., Fan Y., Huang W., Rayhan A.S., Chen K., Cai M. Observation of microplastics in mariculture water of Longjiao Bay, southeast China: influence by human activities. Mar. Pollut. Bull. 2020;160 doi: 10.1016/j.marpolbul.2020.111655. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W., Zhang S., Zhao Q., Qu L., Ma D., Wang J. Spatio-temporal distribution of plastic and microplastic debris in the surface water of the Bohai Sea, China. Mar. Pollut. Bull. 2020;158 doi: 10.1016/j.marpolbul.2020.111343. [DOI] [PubMed] [Google Scholar]
- 33.Santos T.M.T., Petracco M., Venekey V. Effects of vehicle traffic and trampling on the macrobenthic community of Amazonian macrotidal sandy beaches. J. Mar. Biol. Assoc. U. K. 2022;102:285–307. doi: 10.1017/S0025315422000480. [DOI] [Google Scholar]
- 34.Davenport J., Davenport J.L. The impact of tourism and personal leisure transport on coastal environments: a review. Estuar. Coast Shelf Sci. 2006;67:280–292. doi: 10.1016/j.ecss.2005.11.026. [DOI] [Google Scholar]
- 35.Retama I., Jonathan M.P., Shruti V.C., Velumani S., Sarkar S.K., Roy P.D., Rodríguez-Espinosa P.F. Microplastics in tourist beaches of Huatulco Bay, Pacific coast of southern Mexico. Mar. Pollut. Bull. 2016;113:530–535. doi: 10.1016/j.marpolbul.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 36.Garcés-Ordóñez O., Díaz L.F.E., Cardoso R.P., Muniz M.C. The impact of tourism on marine litter pollution on Santa Marta beaches, Colombian Caribbean. Mar. Pollut. Bull. 2020;160 doi: 10.1016/j.marpolbul.2020.111558. [DOI] [PubMed] [Google Scholar]
- 37.Chen M.C., Chen T.H. Spatial and seasonal distribution of microplastics on sandy beaches along the coast of the Hengchun Peninsula, Taiwan. Mar. Pollut. Bull. 2020;151 doi: 10.1016/j.marpolbul.2019.110861. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L., Liu J., Xie Y., Zhong S., Gao P. Occurrence and removal of microplastics from wastewater treatment plants in a typical tourist city in China. J. Clean. Prod. 2021;291 doi: 10.1016/j.jclepro.2021.125968. [DOI] [Google Scholar]
- 39.dos Anjos Guimarães G., de Moraes B.R., Ando R.A., Sant'Anna B.S., Perotti G.F., Hattori G.Y. Microplastic contamination in the freshwater shrimp Macrobrachium amazonicum in Itacoatiara, Amazonas, Brazil. Environ. Monit. Assess. 2023;195:434. doi: 10.1007/s10661-023-11019-w. [DOI] [PubMed] [Google Scholar]
- 40.Morais L.M.S., Sarti F., Chelazzi D., Cincinelli A., Giarrizzo T., Martinelli Filho J.E. The sea anemone Bunodosoma cangicum as a potential biomonitor for microplastics contamination on the Brazilian Amazon coast. Environ. Pollut. 2020;265 doi: 10.1016/j.envpol.2020.114817. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira L.S., Oliveira-Junior J., Cajado R.A., Silva F.K., Zacardi D.M. Ichthyoplankton and plastic waste drift in a river in the Amazon Basin, Brazil. Front. Environ. Sci. 2023;11 doi: 10.3389/fenvs.2023.1068550. [DOI] [Google Scholar]
- 42.Dantas Filho J.V., Pedroti V.P., Santos B.L.T., de Lima Pinheiro M.M., de Mira Á.B., da Silva F.C., de Vargas Schons S. First evidence of microplastics in freshwater from fish farms in Rondônia state, Brazil. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novaes G.O.D., Moura Monteiro S.D., Rollnic M. Microplastics on the fluvio-estuarine beaches of cotijuba island, Pará River estuary (Brazil) J. Coast Res. 2020;95:780–784. doi: 10.2112/SI95-152.1. [DOI] [Google Scholar]
- 44.Lucas-Solis O., Moulatlet G.M., Guamangallo J., Yacelga N., Villegas L., Galarza E., Capparelli M.V. Preliminary assessment of plastic litter and microplastic contamination in freshwater depositional areas: the case study of Puerto Misahualli, Ecuadorian Amazonia. Bull. Environ. Contam. Toxicol. 2021;107:45–51. doi: 10.1007/s00128-021-03138-2. [DOI] [PubMed] [Google Scholar]
- 45.Wen B., Jin S.R., Chen Z.Z., Gao J.Z., Liu Y.N., Liu J.H., Feng X.S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus) Environ. Pollut. 2018;243:462–471. doi: 10.1016/j.envpol.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 46.Huang J.N., Wen B., Xu L., Ma H.C., Li X.X., Gao J.Z., Chen Z.Z. Micro/nano-plastics cause neurobehavioral toxicity in discus fish (Symphysodon aequifasciatus): insight from brain-gut-microbiota axis. J. Hazard Mater. 2022;421 doi: 10.1016/j.jhazmat.2021.126830. [DOI] [PubMed] [Google Scholar]
- 47.Huang J.N., Zhang Y., Xu L., He K.X., Wen B., Yang P.W., Ding J.Y., Li J.Z., Ma C.H., Gao J.Z., Chen Z.Z. Microplastics: a tissue-specific threat to microbial community and biomarkers of discus fish (Symphysodon aequifasciatus) J. Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127751. [DOI] [PubMed] [Google Scholar]
- 48.Firmino V.C., Martins R.T., Brasil L.S., Cunha E.J., Pinedo-Garcia R.B., Hamada N., Juen L. Do microplastics and climate change negatively affect shredder invertebrates from an amazon stream? An ecosystem functioning perspective. Environ. Pollut. 2023;321 doi: 10.1016/j.envpol.2023.121184. [DOI] [PubMed] [Google Scholar]
- 49.Costa I.D., Costa L.L., da Silva Oliveira A., de Carvalho C.E.V., Zalmon I.R. Microplastics in fishes in amazon riverine beaches: influence of feeding mode and distance to urban settlements. Sci. Total Environ. 2023;863 doi: 10.1016/j.scitotenv.2022.160934. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro-Brasil D.R.G., Torres N.R., Picanço A.B., Sousa D.S., Ribeiro V.S., Brasil L.S., de Assis Montag L.F. Contamination of stream fish by plastic waste in the Brazilian Amazon. Environ. Pollut. 2020;266 doi: 10.1016/j.envpol.2020.115241. [DOI] [PubMed] [Google Scholar]
- 51.Trindade P.A., Brabo L.D., Andrades R., Azevedo-Santos V.M., Andrade M.C., Candore L., Cabigliera S.B., Chelazzi D., Cincinelli A., Jeffres C.A., Giarrizzo T. First record of plastic ingestion by a freshwater stingray. Sci. Total Environ. 2023;880 doi: 10.1016/j.scitotenv.2023.163199. [DOI] [PubMed] [Google Scholar]
- 52.Pegado T., Brabo L., Schmid K., Sarti F., Gava T.T., Nunes J., Giarrizzo T. Ingestion of microplastics by Hypanus guttatus stingrays in the western atlantic ocean (Brazilian amazon coast) Mar. Pollut. Bull. 2021;162 doi: 10.1016/j.marpolbul.2020.111799. [DOI] [PubMed] [Google Scholar]
- 53.Alfred S., Ram M., Lakenarine R., Hemraj D., Maharaj G. Occurrence and characteristics of microdebris in commercial fish species of Guyana, South America. Mar. Pollut. Bull. 2022;182 doi: 10.1016/j.marpolbul.2022.114021. [DOI] [PubMed] [Google Scholar]
- 54.Rojas R.R., Arango-Mora C., Nolorbe-Payahua C., Medina M., Vasquez M., Flores J., Murayari F., Vásquez C., Almeida V.D., Ramos W., Rios Isern E., Marapara del Aguila J., Castro C.J., del Águila J., Dias Jamara F., Vasconcelos-Souza M. Microplastic occurrence in fish species from the Iquitos region in Peru, western Amazonia. Acta Amazonica. 2023;53:65–72. doi: 10.1590/1809-4392202201212. [DOI] [Google Scholar]
- 55.Chota-macuyama W., Mendoza J.C. Primer registro de ingestión de microplásticos por un pez de importancia comercial en la ciudad de Iquitos, Amazonía Peruana. Folia Amaz. 2020;29:179–188. doi: 10.24841/fa.v29i2.521. [DOI] [Google Scholar]
- 56.Constantino I.C., Teodoro G.C., Moreira A.B., Paschoal F.M., Trindade W.G., Bisinoti M.C. Distribution of metals in the waters and sediments of rivers in central Amazon Region, Brazil. J. Braz. Chem. Soc. 2019;30:1906–1915. doi: 10.21577/0103-5053.20190100. [DOI] [Google Scholar]
- 57.Gigault J., ter Halle A., Baudrimont M., Pascal P.-Y., Gauffre F., Phi T.-L., El Hadri H., Grassl B., Reynaud S. Current opinion: what is a nanoplastic? Environ. Pollut. 2018;235:1030–1034. doi: 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 58.Wagner M., Lambert S. vol. 58. Springer; Cham: 2018. Freshwater microplastics. (Handbook of Environmental Chemistry). [Google Scholar]
- 59.United Nations Environment Programme (UNEP) Monitoring plastics in rivers and lakes. Guidelines for the Harmonization of Methodologies. 2020 Nairobi No. 01, 108 pp. ISBN: 978-92-807-3819-3. [Google Scholar]
- 60.Wu P., Huang J., Zheng Y., Yang Y., Zhang Y., He F., Chen H., Quan G., Yan J., Li T., Gao B. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019;184 doi: 10.1016/j.ecoenv.2019.109612. [DOI] [PubMed] [Google Scholar]
- 61.Acharya S., Rumi S.S., Hu Y., Abidi N. Microfibers from synthetic textiles as a major source of microplastics in the environment: a review. Textil. Res. J. 2021;91:2136–2156. doi: 10.1177/00405175219912. [DOI] [Google Scholar]
- 62.Sillanpää M., Sainio P. Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. Pollut. Res. 2017;24:19313–19321. doi: 10.1007/s11356-017-9621-1. [DOI] [PubMed] [Google Scholar]
- 63.Hu L., Chernick M., Hinton D.E., Shi H. Microplastics in small waterbodies and tadpoles from Yangtze River delta, China. Environ. Sci. Technol. 2018;52:8885–8893. doi: 10.1021/acs.est.8b02279. [DOI] [PubMed] [Google Scholar]
- 64.Han M., Niu X., Tang M., Zhang B.T., Wang G., Yue W., Kong X., Zhu J. Distribution of microplastics in surface water of the lower Yellow River near estuary. Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.135601. [DOI] [PubMed] [Google Scholar]
- 65.Liu R.P., Li Z.Z., Liu F., Dong Y., Jiao J.G., Sun P.P., El-Wardany R.M. Microplastic pollution in Yellow River, China: current status and research progress of biotoxicological effects. China Geol. 2021;4:585–592. doi: 10.31035/cg2021081. [DOI] [Google Scholar]
- 66.Horton A.A., Jürgens M.D., Lahive E., van Bodegom P.M., Vijver M.G. The influence of exposure and physiology on microplastic ingestion by the freshwater fish Rutilus rutilus (roach) in the River Thames, UK. Environ. Pollut. 2018;236:188–194. doi: 10.1016/j.envpol.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 67.Gad A.K., Toner K., Benfield M.C., Midway S.R. Microplastics in mainstem Mississippi River fishes. Front. Environ. Sci. 2023;10 doi: 10.3389/fenvs.2022.1065583. [DOI] [Google Scholar]
- 68.Sun X., Li Q., Shi Y., Zhao Y., Zheng S., Liang J., Tian Z. Characteristics and retention of microplastics in the digestive tracts of fish from the Yellow Sea. Environ. Pollut. 2019;249:878–885. doi: 10.1016/j.envpol.2019.01.110. [DOI] [PubMed] [Google Scholar]
- 69.Foekema E.M., De Gruijter C., Mergia M.T., van Franeker J.A., Murk A.J., Koelmans A.A. Plastic in North Sea fish. Environ. Sci. Technol. 2013;47:8818–8824. doi: 10.1021/es400931b. [DOI] [PubMed] [Google Scholar]
- 70.Neves D., Sobral P., Ferreira J.L., Pereira T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015;101:119–126. doi: 10.1016/j.marpolbul.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Hossain M.S., Rahman M.S., Uddin M.N., Sharifuzzaman S.M., Chowdhury S.R., Sarker S., Chowdhury M.S.N. Microplastic contamination in penaeid shrimp from the northern Bay of bengal. Chemosphere. 2020;238 doi: 10.1016/j.chemosphere.2019.124688. [DOI] [PubMed] [Google Scholar]
- 72.Abbasi S., Soltani N., Keshavarzi B., Moore F., Turner A., Hassanaghaei M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere. 2018;205:80–87. doi: 10.1016/j.chemosphere.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 73.Janssens L., Garcia-Vazquez E. Dangerous microplastics in topshells and anemones along the north coast of Spain. Mat. Pollut. Bull. 2021;173 doi: 10.1016/j.marpolbul.2021.112945. [DOI] [PubMed] [Google Scholar]
- 74.Fang C., Zheng R., Hong F., Jiang Y., Chen J., Lin H., Bo J. Microplastics in three typical benthic species from the Arctic: occurrence, characteristics, sources, and environmental implications. Environ. Res. 2021;192 doi: 10.1016/j.envres.2020.110326. [DOI] [PubMed] [Google Scholar]
- 75.Hubbard B., Sarisky J., Gelting R., Baffigo V., Seminario R., Centurion C. A community demand-driven approach toward sustainable water and sanitation infrastructure development. Int. J. Hyg Environ. Health. 2011;214:326–334. doi: 10.1016/j.ijheh.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Instituto Nacional de Estadística e Informática (INEI) - Perú . 2018. Censos Nacionales 2017: XII de Población, VII de Vivienda y III de Comunidades Indígenas.https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1561/ Acessed: 08 november 2023. [Google Scholar]
- 77.Andrade M.C., Winemiller K.O., Barbosa P.S., Fortunati A., Chelazzi D., Cincinelli A., Giarrizzo T. First account of plastic pollution impacting freshwater fishes in the Amazon: ingestion of plastic debris by piranhas and other serrasalmids with diverse feeding habits. Environ. Pollut. 2019;244:766–773. doi: 10.1016/j.envpol.2018.10.088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were included in the supplementary material.