Abstract
Background
We assessed the effectiveness of sotrovimab vs no early COVID-19 treatment in highest-risk COVID-19 patients during Omicron predominance.
Methods
Retrospective cohort study using the Discover dataset in North West London. Included patients were non-hospitalised, aged ≥12 years and met ≥1 National Health Service highest-risk criterion for sotrovimab treatment. We used Cox proportional hazards models to compare HRs of 28-day COVID-19-related hospitalisation/death between highest-risk sotrovimab-treated and untreated patients. Age, renal disease and Omicron subvariant subgroup analyses were performed.
Results
We included 599 sotrovimab-treated patients and 5191 untreated patients. Compared with untreated patients, the risk of COVID-19 hospitalisation/death (HR 0.50, 95% CI 0.24, 1.06; p=0.07) and the risk of COVID-19 hospitalisation (HR 0.43, 95% CI 0.18, 1.00; p=0.051) were both lower in the sotrovimab-treated group; however, statistical significance was not reached. In the ≥65 years and renal disease subgroups, sotrovimab was associated with a significantly reduced risk of COVID-19 hospitalisation, by 89% (HR 0.11, 95% CI 0.02, 0.82; p=0.03) and 82% (HR 0.18, 95% CI 0.05, 0.62; p=0.007), respectively.
Conclusions
Risk of COVID-19 hospitalisation in sotrovimab-treated patients aged ≥65 years and with renal disease was significantly lower compared with untreated patients. Overall, risk of hospitalisation was also lower for sotrovimab-treated patients, but statistical significance was not reached.
Keywords: COVID-19, Respiratory Infection, Viral infection
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is limited real-world evidence surrounding early, mild-to-moderate COVID-19 treatments, particularly during Omicron subvariant dominance periods, and the UK National Institute for Health and Care Excellence has recommended more evidence is gathered.
WHAT THIS STUDY ADDS
Sotrovimab treatment was associated with a significant reduction in risk of COVID-19 hospitalisation in patients aged ≥65 years and those with renal disease vs an untreated cohort. For the overall cohort, risk of hospitalisation following sotrovimab treatment was also lower compared with the untreated group; however, statistical significance was not achieved. Risk of hospitalisation and/or death was lower for the sotrovimab-treated cohort across all Omicron subvariant periods but did not reach significance for periods 2 (BA.2 peak) and 3 (BA.4/5 emergence).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study begins to fill the evidence gap in relation to early treatments for mild-to-moderate COVID-19, particularly their effectiveness against disease caused by Omicron variants to date.
Introduction
In March 2020, the global spread of SARS-CoV-2 resulted in the declaration of the COVID-19 pandemic by the WHO.1 Some patients are at particularly high risk of severe COVID-19 outcomes, such as those with cancer, renal and liver disease, HIV/AIDS and rare neurological conditions.2 3 In England, early COVID-19 treatment with antivirals or monoclonal antibodies (mAbs) is recommended for people meeting such ‘highest-risk’ criteria, following approval of these drugs by the UK Medicines and Healthcare products Regulatory Agency in late 2021.4–6
Sotrovimab is a dual-action engineered human IgG1κ mAb derived from the parental mAb S309, a potent neutralising mAb directed against a conserved epitope in the SARS-CoV-2 spike protein.7–10 Intravenous sotrovimab 500 mg was shown in COMET-ICE, a randomised clinical trial, to significantly reduce the risk of all-cause >24 hour hospitalisation or death by 79% vs placebo in high-risk patients with mild-to-moderate COVID-19.11 Sotrovimab received conditional marketing authorisation in December 2021 in the UK for use in symptomatic patients with acute COVID-19 (aged ≥12 years and ≥40 kg) who are at increased risk of COVID-19 progression.4 During the study period, National Health Service (NHS) England clinical guidelines recommended sotrovimab as a first-line treatment option,3 and now recommend sotrovimab as a second-line option.12
Since COMET-ICE (August 2020–March 2021),11 new COVID-19 variants of concern have emerged, including Omicron BA.1 and BA.2, which became dominant in January and March 2022, respectively.13–15 In vitro neutralisation assays demonstrated that sotrovimab retained its neutralisation capacity against Omicron BA.1 (3.8-fold IC50 reduction relative to wild-type SARS-CoV-2) but showed a moderate reduction against Omicron BA.2 (15.7-fold reduction).10 A similar reduction in activity was reported for BA.5 (21.6-fold reduction), which was predominant in the UK from July–October 2022.16 17
In the absence of clinical trial data, uncertainty remains regarding how in vitro antibody neutralisation activity translates to clinical effectiveness. Despite emerging real-world evidence demonstrating the effectiveness of sotrovimab vs antivirals during BA.2 and BA.5 predominance, further evidence is critical for providing up-to-date clinical recommendations when considering the evolving variant landscape.18 Our previous paper reports descriptive results for patients treated with sotrovimab, nirmatrelvir/ritonavir or molnupiravir, or patients at highest risk per NHS criteria but who were untreated.19 Here, we assessed the clinical effectiveness of sotrovimab vs no early COVID-19 treatment in highest-risk non-hospitalised patients with COVID-19 in north west London (NWL) from December 2021–July 2022.
Methods
Objectives
The primary objective was to assess the risk of COVID-19-related hospitalisation and/or COVID-19-related death within 28 days of index between patients treated with sotrovimab and highest-risk patients who received no early treatment for COVID-19 (untreated patients).
Secondary objectives were to assess the risk of 28-day COVID-19-related hospitalisation and/or COVID-19-related death between sotrovimab-treated and untreated patients among the following subgroups: Omicron subvariant prevalence period (online supplemental figure 1), patients aged <65 and ≥65 years at index and patients with renal dysfunction (‘renal disease’: renal transplant recipients, non-transplant recipients receiving a similar level of immunosuppression to renal transplant recipients; chronic kidney disease stage 4 or 5).
bmjresp-2023-002238supp001.pdf (359.8KB, pdf)
Data source and study design
This retrospective cohort study used data from the Discover dataset, one of Europe’s largest linked longitudinal datasets.20 Discover holds depersonalised coded primary and secondary care data for >2.7 million patients registered with a general practitioner in NWL. The dataset is fed by >400 provider organisations, including >350 general practices, 2 mental health trusts and 2 community trusts.21 Discover’s population has a similar age–sex distribution and comorbidity prevalence to the UK population but is more ethnically diverse.20 The dataset is accessible via Discover-NOW Health Data Research Hub for Real World Evidence through their data science specialists and Information Governance Committee-approved analysts, hosted by Imperial College Health Partners.
In the sotrovimab-treated cohort, index was defined as the date of sotrovimab prescription. Patients in the treated cohort must have had a recorded prescription for sotrovimab within 28 days of COVID-19 diagnosis. In the untreated cohort, index dates were imputed based on the distribution of time to treatment (time between COVID-19 diagnosis and sotrovimab prescription) in the treated cohort (online supplemental figure 2). The baseline period was defined as the 365 days immediately prior to index. Patients were followed up for 28 days from index (acute period), during which outcomes were evaluated.
As there were no sequencing data available, Omicron subvariant dominance period was used as a surrogate.22 Patients were classified into three variant prevalence periods based on diagnosis date: Omicron BA.1/2 emergence: 1 December 2021–12 February 2022 (period 1); BA.2 increasing and at its peak: 13 February 2022–31 May 2022 (period 2); BA.2 falling and BA.4/5 emergence: 1 June 2022–31 July 2022 (period 3) (online supplemental figure 1).
Study population
Eligible patients in both cohorts were aged ≥12 years at index and met ≥1 of the NHS highest-risk criteria for early sotrovimab treatment. At the time of the study, these criteria included Down’s syndrome, solid cancer, haematological diseases (including cancers), renal disease, liver disease, immune-mediated inflammatory diseases, immune deficiencies, HIV/AIDS, solid-organ and stem-cell transplant recipients and rare neurological conditions.2 3 Patients meeting the NHS highest-risk criteria were identified via International Classification of Disease version 10 and Systematized Nomenclature of Medicine (SNOMED) codes appearing in the patient’s records since the first registration in NWL. The SNOMED codes are available in the Supplementary Data (note that due to updates in the highest-risk criteria between this study and the previous descriptive analysis,19 the SNOMED codes used were also updated).
Per the inclusion criteria, patients were non-hospitalised at the time of sotrovimab treatment (ie, patients must not have had an inpatient visit (event from admission to discharge) starting on or before the treatment date, unless the visit was a day case (defined in the NHS as a planned elective admission without a planned overnight stay) or the visit did not incur an overnight stay).
Patients were excluded if they received >1 COVID-19 treatment (sotrovimab, nirmatrelvir/ritonavir, molnupiravir or remdesivir) in an outpatient setting before index or were diagnosed with COVID-19 while hospitalised.
Data analysis
Patient characteristics were recorded, including age, sex, ethnicity, vaccination status and comorbidity history. Cohorts were described in relation to ‘highest-risk’ conditions which made patients eligible for early sotrovimab treatment, as mentioned above, and other high-risk conditions which may increase susceptibility to adverse COVID-19 outcomes (table 1). Continuous variables were summarised using mean, SD, median, IQR and range. Categorical variables were described using frequencies and percentages. Values from ≥1 to <5 were suppressed and are reported as n<5, per our study’s Information Governance and Data Privacy Impact Assessment approvals.
Table 1.
High-risk and highest-risk conditions criteria
| Highest-risk conditions | High-risk conditions |
| Down’s syndrome | Age ≥70 years |
| Solid cancer | Long-term respiratory conditions |
| Haematological disease and stem-cell transplant recipients | Chronic heart disease |
| Advanced renal disease | Chronic kidney disease |
| Liver disease | Chronic liver disease |
| IMID | Chronic neurological condition |
| Immune deficiencies | Diabetes |
| HIV/AIDS | Weakened immune system caused by medical condition or medication |
| Solid organ transplant | Obesity (class III) |
| Rare neurological conditions | Pregnancy |
| Severe respiratory conditions | |
| Rare disease and inborn errors of metabolism |
IMID, immune-mediated inflammatory disease.
Inverse probability of treatment weighting (IPTW) was used to balance baseline characteristics in the treated and untreated cohorts. Weights were derived based on propensity scores, which were further used in weighted Cox regression to adjust for measured confounders between the cohorts. Propensity scores (probability of treatment based on baseline covariates) were obtained using logistic regression or gradient boosting machine models. Propensity score models were used to predict the probability of treatment based on the following covariates: age, gender, time period of COVID-19 diagnosis (ie, Omicron BA.1, BA.2 or BA.5), presence of renal disease (binary), presence of multiple highest-risk conditions (≥2, binary), presence of high-risk conditions (binary), solid-organ transplant (binary), COVID-19 vaccination status (binary), time since vaccination and ethnicity (online supplemental table 1). To obtain an appropriate estimation of the variance of treatment effect and better control type I error rate, inverse probability of treatment weights was stabilised.23 The balance in baseline characteristics between weighted treated and untreated groups was assessed using standardised differences.
Cox proportional hazards models with stabilised weights were performed to assess the HR of COVID-19-related hospitalisation and/or COVID-19-related death among the overall cohort and subgroups (Omicron subvariant prevalence period, age and renal dysfunction). Covariates not balanced after weighting (standardised differences >0.1)24 were included in the Cox proportional hazards model. IPTWs and accordingly doubly robust estimation were performed separately for each Cox model. Analyses were conducted using R V.4.2.1 and the following packages: twang 2.5, cobalt 4.4.1, xtable 1.8–4, survey 4.1–1, stringr 1.4.1, WeightIt 0.13.1, stats 4.2.1, survminer 0.4.9, survival 3.3–1, powerSurvEpi 0.1.3. Data pre-processing was performed using Python 3.9.5 with packages Pandas 1.3.4 and Numpy 1.21.
Patients without evidence of ≥1 of the NHS highest-risk criteria for early treatment were excluded from the main analysis. However, we conducted an exploratory analysis to identify patients who met government criteria for ‘shielding’ during the early phase of the pandemic (please refer to the Supplementary Data for full methods and results of this analysis).
Compliance with ethics guidelines
This study complied with the Helsinki Declaration’s ethical standards and was approved by the Discover Data Research Access Group. Ethics approval for the use of the Discover Platform for research was obtained from the West Midlands, Solihull HRA research ethics committee (reference 18/WM/0323, Integrated Research Application System project ID 253449). This study complied with applicable patient privacy laws. Data were aggregated and counts <5 were suppressed. No direct patient contact or primary collection of individual patient data occurred. Study results were in tabular form and aggregate analyses that omit patient identification; therefore, informed consent, ethics committee or institutional review board approval were not required. Any publications do not include patient identifiers.
Patient and public involvement
Patients were not directly involved in the study design due to its retrospective nature; however, the results will be widely available through open access.
Results
Patient demographics and baseline characteristics
The analysis included 5790 patients, 599 (10.3%) of whom were treated with sotrovimab and 5191 (89.7%) who were eligible highest-risk untreated patients (table 2). A total of 2946 patients were diagnosed during period 1 (173 sotrovimab-treated, 2773 untreated), 1978 were diagnosed during period 2 (285 sotrovimab-treated, 1693 untreated) and 866 were diagnosed during period 3 (141 sotrovimab-treated, 725 untreated).
Table 2.
Patient characteristics
| Characteristic | Sotrovimab (n=599) | Untreated (n=5191) |
| Age | ||
| Mean (SD) | 57.4 (15.6) | 52.5 (17.6) |
| Median (Q1–Q3) | 58 (46–70) | 53 (40–65) |
| Age group ≥65 years, n (%) | 211 (35.2) | 1302 (25.1) |
| Female sex, n (%) | 303 (50.6) | 2459 (47.4) |
| Ethnicity, n (%) | ||
| White | 309 (54.3) | 2556 (49.2) |
| Asian/Asian British | 136 (23.9) | 1167 (22.5) |
| Black/Black British | 69 (12.1) | 768 (14.8) |
| Mixed | 18 (3.2) | 189 (3.6) |
| Other | 37 (6.5) | 277 (5.3) |
| Unknown | 30 (5.0) | 234 (4.5) |
| Vaccination status, n (%) | ||
| Fully* | 560 (93.5) | 4536 (87.4) |
| Not fully | 39 (6.5) | 655 (12.6) |
| Time since last vaccination (days) | ||
| Mean (SD) | 131.6 (90.3) | 127.2 (89.3) |
| Median (Q1–Q3) | 112 (66–165) | 97 (49–167) |
| Time to treatment (days) | ||
| Mean (SD) | 1.9 (1.6) | 1.8 (1.6) |
| Median (Q1–Q3) | 1 (1–2) | 1 (1–2) |
| Highest-risk conditions, n (%) | ||
| Down’s syndrome | 6 (1.0) | 138 (2.7) |
| Solid cancer | 72 (12.0) | 487 (9.4) |
| Haematological disease and stem-cell transplant recipients | 71 (11.9) | 400 (7.7) |
| Renal disease | 254 (42.4) | 1094 (21.1) |
| Liver disease | 41 (6.8) | 487 (9.4) |
| IMID | 45 (7.5) | 512 (9.9) |
| Immune deficiencies | 75 (12.5) | 1250 (24.1) |
| HIV/AIDS | 60 (10.0) | 1149 (22.1) |
| Solid-organ transplant | 72 (12.0) | 244 (4.7) |
| Rare neurological conditions | 81 (13.5) | 797 (15.4) |
| Number of highest-risk conditions, n (%) | ||
| 1 | 446 (74.5) | 3887 (74.9) |
| 2 | 129 (21.5) | 1252 (24.1) |
| 3+ | 24 (4.0) | 52 (1.0) |
| High-risk conditions, n (%) | ||
| Age ≥70 years | 150 (25.0) | 943 (18.2) |
| Long-term respiratory conditions | 134 (22.4) | 1116 (21.5) |
| Chronic heart disease | 336 (56.1) | 1810 (34.9) |
| Chronic kidney disease | 155 (25.9) | 769 (14.8) |
| Chronic liver disease | 60 (10.0) | 572 (11.0) |
| Diabetes | 169 (28.2) | 1024 (19.7) |
| Weakened immune system caused by medical condition or medication | 122 (20.4) | 780 (15.0) |
| Obesity (class III) | 14 (2.3) | 94 (1.8) |
| Pregnancy | 8 (1.3) | 265 (5.1) |
| Rare disease and inborn errors of metabolism | 8 (1.3) | 265 (5.1) |
| Number of high-risk conditions, n (%) | ||
| 0 | 103 (17.2) | 1617 (31.2) |
| 1 | 123 (20.5) | 1177 (22.7) |
| 2–3 | 263 (43.9) | 1705 (32.8) |
| 4+ | 110 (18.4) | 692 (13.3) |
*Minimum complete vaccination schedule plus at least 1 booster.
IMID, immune-mediated inflammatory disease.
Patients aged ≥65 years accounted for 35.2% (n=211/599) of the sotrovimab-treated group and 25.1% (n=1302/5191) of untreated patients. A high percentage of sotrovimab-treated patients had renal disease (42.4%, n=254/599 vs 21.1%, n=1094/5191 of untreated patients), while lower percentages were reported for other highest-risk comorbidities (table 2). A high percentage of sotrovimab-treated patients had high-risk comorbidities such as chronic heart disease (56.1%, n=336/599), chronic kidney disease (25.9%, n=155/599) and diabetes (28.2%, n=169/599). Among untreated patients, 34.9% (n=1810/5191) had chronic heart disease, 14.8% (n=769/5191) had chronic kidney disease and 19.7% (n=1024/5191) had diabetes. The proportion of patients categorised as fully vaccinated (minimum complete vaccination schedule plus ≥1 booster) was 93.5% (n=560/599) in the sotrovimab group and 87.4% (n=4356/5191) in the untreated group. Of the patients treated with sotrovimab, 94 (15.7%) received an additional COVID-19 treatment during the acute follow-up period (molnupiravir, n=57; nirmatrelvir/ritonavir, n=31; remdesivir, n=6).
Clinical outcomes
After weighting, the time period of COVID-19 diagnosis covariate remained unbalanced between cohorts and was included in all weighted Cox proportional hazards models (online supplemental table 2).
In the sotrovimab-treated cohort, all-cause and COVID-19-related hospitalisations were experienced by 7.2% (n=43/599) and 1.2% (n=7/599) of patients, respectively. Fewer than 5 patients died within 1 month of index (table 3). In the untreated cohort, all-cause and COVID-19-related hospitalisations were experienced by 5.2% (n=270/5191) and 1.7% (n=90/5191) of patients, respectively. Within 1 month of index, 22 patients (0.4%) died (table 3).
Table 3.
HRs for IPTW weighted* Cox proportional hazards for study outcomes
| Clinical outcomes | Sotrovimab | Untreated |
| Overall cohort, n | 599 | 5191 |
| Compound (hospitalisation or death) | ||
| HR | 0.50 | |
| 95% CI | 0.24, 1.06 | |
| P value | 0.07 | |
| COVID-19-related hospitalisation | ||
| Patients with event, n (%) | 7 (1.2) | 90 (1.7) |
| HR | 0.43 | |
| 95% CI | 0.18, 1.00 | |
| P value | 0.051 | |
| Death | ||
| Patients with event, n (%) | <5 | 22 (0.4) |
| HR | 0.71 | |
| 95% CI | 0.16,3.20 | |
| P value | 0.65 | |
| Patients aged <65 years, n | 388 | 3803 |
| Compound (hospitalisation or death) | ||
| HR | 0.79 | |
| 95% CI | 0.34, 1.85 | |
| P value | 0.58 | |
| COVID-19-related hospitalisation | ||
| Patients with event, n (%) | 6 (1.5) | 47 (1.2) |
| HR | 0.70 | |
| 95% CI | 0.28, 1.75 | |
| P value | 0.44 | |
| Death | ||
| Patients with event, n (%) | <5 | <5 |
| HR | 1.98 | |
| 95% CI | 0.21, 18.18 | |
| P value | 0.55 | |
| Patients aged ≥65 years, n | 211 | 1302 |
| Compound (hospitalisation or death) | ||
| HR | 0.25 | |
| 95% CI | 0.06, 1.12 | |
| P value | 0.07 | |
| COVID-19-related hospitalisation | ||
| Patients with event, n (%) | <5 | 43 (3.3) |
| HR | 0.11 | |
| 95% CI | 0.02, 0.82 | |
| P value | 0.03 | |
| Death | ||
| Patients with event, n (%) | <5 | 19 (1.5) |
| HR | 0.55 | |
| 95% CI | 0.07, 4.05 | |
| P value | 0.55 | |
| Patients without renal disease, n | 345 | 4097 |
| Compound (hospitalisation or death) | ||
| HR | 0.55 | |
| 95% CI | 0.21, 1.47 | |
| P value | 0.24 | |
| COVID-19-related hospitalisation | ||
| Patients with event, n (%) | <5 | 53 (1.3) |
| HR | 0.55 | |
| 95% CI | 0.19, 1.62 | |
| P value | 0.28 | |
| Death | ||
| Patients with event, n (%) | <5 | 9 (0.2) |
| HR | 0.54 | |
| 95% CI | 0.07, 4.27 | |
| P value | 0.56 | |
| Patients with renal disease, n | 254 | 1094 |
| Compound (hospitalisation or death) | ||
| HR | 0.28 | |
| 95% CI | 0.09, 0.89 | |
| P value | 0.031 | |
| COVID-19-related hospitalisation | ||
| Patients with event, n (%) | <5 | 37 (3.4) |
| HR | 0.18 | |
| 95% CI | 0.05, 0.62 | |
| P value | 0.007 | |
| Death | ||
| Patients with event, n (%) | <5 | 13 (1.2) |
| HR | 0.51 | |
| 95% CI | 0.07, 3.89 | |
| P value | 0.52 | |
*IPTW included patient age, gender, time period of index, presence of renal disease, presence of multiple highest-risk conditions, vaccination status, days since last vaccination and ethnicity. Cox proportional hazards additionally adjusted for multiple highest-risk conditions, age and ethnicity.
IPTW, inverse probability of treatment weighting.
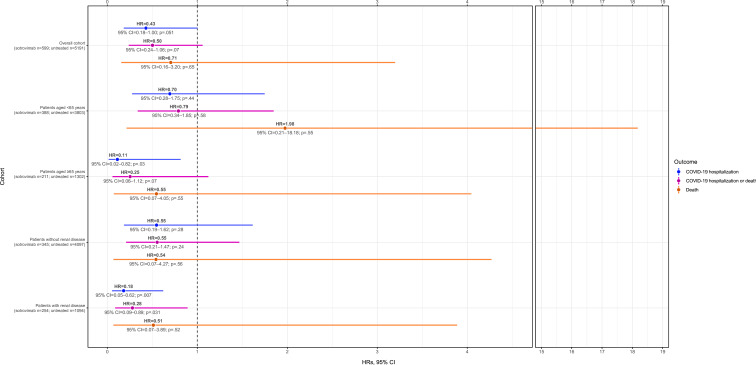
IPTW HRs for COVID-19 hospitalisation or death, COVID-19 hospitalisation and death are shown in figure 1 and table 3. Compared with no treatment, the risk of COVID-19 hospitalisation was 57% lower (HR 0.43, 95% CI 0.18, 1.00; p=0.051) in the sotrovimab-treated group. Similarly, the risk of COVID-19 hospitalisation or death was 50% lower (HR 0.50, 95% CI 0.24, 1.06; p=0.07) in the sotrovimab-treated group. The event rate for death was too low for conclusions to be drawn.
Figure 1.
IPTW Cox proportional HRs for COVID-19 hospitalisation and/or death in sotrovimab-treated compared with untreated patients (entire cohort, n=5790). IPTW, inverse probability of treatment weighting.
Subgroup analyses
Among patients aged <65 years, COVID-19-related hospitalisations were experienced by 1.5% (n=6/388) of those treated with sotrovimab and 1.2% (n=47/3803) of those untreated. Fewer than 5 patients in each cohort died within 1 month of index (table 3). The IPTW HR of COVID-19 hospitalisation or death was 0.79 (95% CI 0.34, 1.85; p=0.58) for sotrovimab vs no treatment (figure 1). The IPTW HRs for COVID-19 hospitalisation and death in patients aged <65 years were 0.70 (95% CI 0.28, 1.75; p=0.44) and 1.98 (95% CI 0.21, 18.18; p=0.55), respectively (figure 1; table 3). The event rate for death was too low to draw conclusions.
Among patients aged ≥65 years, COVID-19-related hospitalisations were experienced by fewer than 5 of the 211 sotrovimab-treated patients and 3.3% (n=43/1302) of untreated patients. Deaths within 1 month of index were reported for fewer than 5 sotrovimab-treated patients and 1.5% (n=19/1302) of untreated patients (table 3). Sotrovimab treatment was associated with a statistically significant 89% reduction in the risk of COVID-19 hospitalisation vs no treatment (HR 0.11, 95% CI 0.02, 0.82; p=0.03) (figure 1). IPTW HRs for composite COVID-19 hospitalisation or death and death alone were 0.25 (95% CI 0.06, 1.12; p=0.07) and 0.55 (95% CI 0.07, 4.05; p=0.55), respectively (figure 1; table 3).
Among patients without renal disease, none of the IPTW HRs were statistically significant, although all were <1 (figure 1). However, sotrovimab treatment was associated with a statistically significant 72% reduction in the risk of COVID-19 hospitalisation or death (HR 0.28, 95% CI 0.09, 0.89; p=0.031) among patients with renal disease vs no treatment (figure 1; table 3). The risk of COVID-19 hospitalisation was also significantly lower, by 82%, following sotrovimab treatment compared with the untreated group (HR 0.18, 95% CI 0.05, 0.62; p=0.007). As above, the event rate for death was too low for conclusions to be drawn.
In period 1, sotrovimab treatment was associated with a statistically significant 75% risk reduction in COVID-19 hospitalisation or death vs no treatment (HR 0.25, 95% CI 0.07, 0.89; p=0.032) (table 4). In periods 2 and 3, IPTW HRs of COVID-19 hospitalisation or death were 0.53 (95% CI 0.14, 2.00; p=0.35) and 0.78 (95% CI 0.23, 2.69; p=0.69), respectively, for sotrovimab-treated patients compared with the untreated group (table 4).
Table 4.
HRs of treated vs untreated for IPTW weighted* Cox proportional hazards for study outcomes
| Clinical outcomes | Period 1† (n=2946) |
Period 2 ‡ (n=1978) |
Period 3§ (n=866) |
| Compound (hospitalisation or death) | |||
| HR | 0.25 | 0.53 | 0.78 |
| 95% CI | 0.07, 0.89 | 0.14, 2.00 | 0.23, 2.69 |
| P value | 0.032 | 0.35 | 0.69 |
| Hospitalisation | |||
| HR | – | 0.51 | 0.60 |
| 95% CI | – | 0.09, 2.72 | 0.14, 2.62 |
| P value | – | 0.43 | 0.49 |
| Death | |||
| HR | – | 0.59 | 1.04 |
| 95% CI | – | 0.07, 4.75 | 0.11, 9.68 |
| P value | – | 0.62 | 0.97 |
Cox models could not be solved for hospitalisation and death in period 1 (likely due to multicollinearity).
*IPTW included patient age, gender, time period of index, presence of renal disease, presence of multiple highest-risk conditions, vaccination status, days since last vaccination and ethnicity. Cox proportional hazards additionally adjusted for multiple highest-risk conditions, age and ethnicity.
†Patients with an index date of 1 December 2021–12 February 2022.
‡Patients with an index date of 13 February 2022–31 May 2022.
§Patients diagnosed between 1 June 2022 and 31 July 2022.
IPTW, inverse probability of treatment weighting.
Discussion
This study assessed the effectiveness of sotrovimab vs no early COVID-19 treatment in non-hospitalised, highest-risk patients with COVID-19 in NWL, using data from one of Europe’s largest longitudinal datasets. We previously reported descriptive results for a similar cohort, which were used to confirm the feasibility of this comparative effectiveness analysis.19
There was evidence that sotrovimab treatment was associated with a significant reduction in the risk of COVID-19 hospitalisation in vulnerable groups vs no treatment. An 89% decrease in the risk of COVID-19-related hospitalisation was observed for sotrovimab-treated patients aged ≥65 years (p=0.03). Furthermore, decreases in the risk of both COVID-19 hospitalisation or death and COVID-19 hospitalisation were observed for patients with renal disease (72% risk reduction [p=0.031] and 82% risk reduction [p=0.007], respectively), who are at especially high risk of severe COVID-19 outcomes.25 Similarly, a study of patients on renal replacement therapy found that sotrovimab was associated with a 65% lower risk of 28-day COVID-19-related hospitalisation and/or death than molnupiravir (HR 0.35, 95% CI 0.17, 0.71; p=0.004).26
In the overall cohort, the risk of hospitalisation following sotrovimab treatment was reduced by 57% (p=0.051), and the risk of COVID-19 hospitalisation or death by 50% (p=0.07), although these values did not reach statistical significance. Validation of these results on a larger scale would be valuable. Our results are similar to those of a recent US retrospective analysis of patients diagnosed with COVID-19 during the Delta and early Omicron waves, which reported that sotrovimab was associated with 55% lower risk of 30-day all-cause hospitalisation or mortality vs no mAb treatment (p<0.001).27 Additionally, a further US study reported a 70% risk reduction in 30-day hospitalisation or mortality among sotrovimab-treated patients vs no treatment during BA.1 predominance.28 In COMET-ICE, risk of all-cause >24 hour hospitalisation or death was reduced by 79% for sotrovimab vs placebo.11 However, our study was a real-world study, and more likely to include an older population with more comorbidities and greater ethnic diversity. Furthermore, COMET-ICE was conducted while the original ‘wild type’ variant was predominant, rather than the Omicron variants predominant during this study.
For sotrovimab-treated patients, we also report a reduced risk of hospitalisation or death during BA.1 predominance and non-significant trends for reduced risk during BA.2 and BA.5, possibly not significant due to low event rate and small sample size for this comparison. Harman et al previously reported low proportions of hospitalisations between sequencing-confirmed Omicron BA.1 and BA.2 cases treated with sotrovimab.29 Another retrospective cohort study using Hospital Episode Statistics data in England reported low levels of COVID-19-attributable hospitalisations and deaths in patients presumed to be treated with sotrovimab (based on NHS data showing that 99.98% of COVID-19-mAb-treated individuals received sotrovimab during the study period), with no significant differences in hospitalisation rates during Omicron BA.1, BA.2 or BA.5 predominance.30 Zheng et al used the OpenSAFELY platform to evaluate the comparative effectiveness of sotrovimab and molnupiravir for preventing severe COVID-19 outcomes from 16 December 2021–10 February 2022. They reported that 0.96% of sotrovimab-treated patients experienced 28-day COVID-19-attributable hospitalisation or death, vs 2.05% of molnupiravir-treated patients. In Cox proportional hazards models, sotrovimab was associated with a lower risk than molnupiravir (HR 0.54, 95% CI 0.33, 0.88; p=0.01).31 A further Zheng et al study reported similar risk of severe COVID-19 outcomes between patients treated with sotrovimab and nirmatrelvir/ritonavir during Omicron BA.2 and BA.5 predominance in the UK (HR 0.89, 95% CI 0.48, 1.63; p=0.698).32 In another OpenSAFELY study that emulated target trials, HRs for 28-day COVID-19 hospitalisation or death were 0.76 (95% CI 0.66, 0.89) during BA.1 predominance and 0.92 (95% CI 0.79, 1.06) during BA.2 predominance for sotrovimab-treated vs untreated patients.33
It is very challenging to conduct randomised controlled trials in the context of a constantly evolving variant landscape. In the absence of such data, information from in vitro neutralisation assays has been used to guide decisions on the effectiveness of sotrovimab (and other mAbs) against emerging variants.34 35 These assays have shown that the neutralising activity of sotrovimab against Omicron subvariants is reduced compared with wild-type virus; however, there are no models available that can reliably correlate this in-vitro data to clinical outcomes. The totality of available evidence, including data from real-world studies such as ours, should be considered when deciding on treatment options for COVID-19.
As with sotrovimab, real-world studies have assessed the effectiveness of other available treatments during periods when Omicron subvariants have been predominant. A study in Greece among patients aged ≥65 years reported that both molnupiravir and nirmatrelvir/ritonavir significantly reduced the risk of hospitalisation and death compared with no oral antiviral therapy.36 The study included a period when Omicron subvariants BA.1, BA.2 and BA.5 successively predominated (December 2021 to July 2022). In another study, conducted in Hong Kong, nirmatrelvir/ritonavir but not molnupiravir was associated with a reduced risk of hospitalisation compared with no oral antiviral use.37 Patients included in this study attended outpatient clinical between 16 February 2022 and 31 March 2022. Another study from Hong Kong, conducted over a similar period (22 February 2022 to 31 March 2022), reported that both antivirals were associated with a reduced risk of hospital admission and mortality from all causes, as compared with a control group who did not receive any antiviral treatment.38
In our previous descriptive study, ~40% of sotrovimab-treated patients did not have evidence of a highest-risk condition that made them eligible for treatment.19 This was unexpected: as a high-cost specialist drug, sotrovimab is only for NHS use among specifically defined patients. We therefore performed an exploratory analysis to investigate if we were missing a criterion for identifying eligible patients that might instead be captured in the shielding code (see Supplemental Data). By including patients eligible for ‘shielding’ without documented evidence in the database of ≥1 of the NHS highest-risk criteria for early treatment, our exploratory analysis may have included more patients who had a better prognosis than the main analysis. While including only patients with unequivocal evidence of highest-risk criteria reduced the sample size for the main analysis, the untreated cohort used in the exploratory analysis may be less comparable to the sotrovimab-treated cohort.
There are some limitations to this study. One explanation for the lack of statistically significant benefit of sotrovimab in the overall cohort is that the sample size and composite endpoint event rates were too small as hospitalisation rates decreased, particularly in later months39 (online supplemental table 3). Although significant efforts were made to account for confounding factors in the analysis, the influence of unidentified and unmeasured confounders (ie, baseline COVID-19 severity and symptoms) cannot be excluded. In the untreated cohort, health-seeking behaviours (or lack thereof) may have confounded results. Vaccination rate among the untreated cohort was significantly lower, and we lack detail on time interval between symptom onset and formal diagnosis, and hence treatment initiation. As is common to database analyses, our reporting of patient characteristics and comorbidities is dependent on accurate recording by healthcare practitioners; missing data cannot be ruled out. Identification of an appropriate control may also have impacted results; we observed a large number of sotrovimab-treated patients had no highest-risk conditions that were used to identify controls. In addition, the Discover dataset is restricted to NWL so it was not possible to evaluate subnational geographical trends, and our results may not be generalizable to other populations. Finally, the likely SARS-CoV-2 variant was defined by an ecological proxy rather than being confirmed by sequencing.
In conclusion, this study compared the effectiveness of sotrovimab with no early treatment in non-hospitalised, highest-risk patients with COVID-19 in NWL from December 2021–July 2022. Sotrovimab treatment was associated with a significant reduction in risk of COVID-19 hospitalisation in patients aged ≥65 years and those with renal disease vs the untreated cohort. For the overall cohort, risk of hospitalisation following sotrovimab treatment was also lower compared with the untreated group; however, statistical significance was not achieved. Risk of hospitalisation and/or death was lower for the sotrovimab-treated cohort across all time periods but did not reach significance for periods 2 and 3. Further research with a larger sample size should be considered.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables, grammatical editing and referencing) was provided by Kathryn Wardle of Apollo, OPEN Health Communications, in accordance with Good Publication Practice (GPP) 3 guidelines ( www.ismpp.org/gpp-2022 ), and was funded by GSK and Vir Biotechnology, Inc.
Footnotes
Contributors: MD, MJY, VP, BL, DCG, JDW, SY, BFP, HJB, TK and SJB designed the study; ERG performed inverse probability of treatment weighted survival analysis; MJY performed descriptive analysis; MD, ERG, MJY, BL, DCG, JDW, SY, BFP, EJL, WK, TK and SJB interpreted results. MD is responsible for the overall content as the guarantor. All authors took part in drafting, revising or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding: This study was supported by GSK in collaboration with Vir Biotechnology, Inc (study number 219543).
Competing interests: MD, DCG, EJL, WK and HJB are employees of, and/or shareholders in, GSK. VP was an employee of GSK at the time of the study and is now an employee of KVM Analytics. ERG, MJY, JDW, SY, BFP and TK are (or were at time of study) employees of Imperial College Health Partners, which received funding from GSK and Vir Biotechnology to conduct the study. BL is an employee of OPEN Health, which received funding from GSK and Vir Biotechnology, Inc, to conduct the study. A consultancy fee was paid to SJB’s institutional account.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The Discover data that support the findings of this study are available from Imperial College Health Partners via approval from the Discover Data Research Access Group (DRAG) under certain restrictions.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. World Health Organization . Coronavirus disease (COVID-19) pandemic. Available: https://www.who.int/europe/emergencies/situations/covid-19 [Accessed 5 Jul 2023].
- 2. Department of Health and Social Care . Higher-risk patients eligible for COVID-19 treatments: independent advisory group report. Available: https://www.gov.uk/government/publications/higher-risk-patients-eligible-for-covid-19-treatments-independent-advisory-group-report [Accessed 5 Jul 2023].
- 3. Department of Health and . Interim clinical commissioning policy: Antivirals or Neutralising Monoclonal antibodies for non-hospitalised patients with COVID-19. Available: https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=104044 [Accessed 5 Jul 2023].
- 4. Medicines and Healthcare products Regulatory Agency . Summary of product characteristics for Xevudy. Available: https://www.gov.uk/government/publications/regulatory-approval-of-xevudy-sotrovimab/summary-of-product-characteristics-for-xevudy [Accessed 5 Jul 2023].
- 5. Medicines and Healthcare products Regulatory Agency . First oral antiviral for COVID-19, Lagevrio (Molnupiravir), approved by MHRA. available at. n.d. Available: https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra#:~:text=and%20licensing%20guidance-,First%20oral%20antiviral%20for%20COVID%2D19%2C%20Lagevrio%20(molnupiravir),review%20of%20the%20available%20evidence
- 6. Medicines and Healthcare products Regulatory Agency . Oral COVID-19 antiviral, Paxlovid, approved by UK regulator. Available: https://www.gov.uk/government/news/oral-covid-19-antiviral-paxlovid-approved-by-uk-regulator [Accessed 5 Jul 2023].
- 7. Gaudinski MR, Coates EE, Houser KV, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med 2018;15:e1002493. 10.1371/journal.pmed.1002493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ko S-Y, Pegu A, Rudicell RS, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 2014;514:642–5. 10.1038/nature13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinto D, Park Y-J, Beltramello M, et al. Cross-neutralization of SARS-Cov-2 by a human Monoclonal SARS-Cov antibody. Nature 2020;583:290–5. 10.1038/s41586-020-2349-y [DOI] [PubMed] [Google Scholar]
- 10. Cathcart AL, Havenar-Daughton C, Lempp FA, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-Cov-2. bioRxiv [Preprint]. 10.1101/2021.03.09.434607 [DOI]
- 11. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of Sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2022;327:1236–46. 10.1001/jama.2022.2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Institute for Health and Care Excellence . Final draft guidance: Therapeutics for people with COVID-19. Available: https://www.nice.org.uk/guidance/ta878/documents/final-appraisal-determination-document [Accessed 5 Jul 2023].
- 13. Mendiola-Pastrana IR, López-Ortiz E, Río de la Loza-Zamora JG, et al. SARS-Cov-2 variants and clinical outcomes: a systematic review. Life (Basel) 2022;12:170. 10.3390/life12020170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . Weekly epidemiological update on COVID-19. 2022. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-january-2022 [Accessed 5 Jul 2023].
- 15. World Health Organization . Weekly epidemiological update on COVID-19. 2022. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---22-march-2022 [Accessed 5 2023].
- 16. Park Y-J, Pinto D, Walls AC, et al. Imprinted antibody responses against SARS-Cov-2 Omicron sublineages. Science 2022;378:619–27. 10.1126/science.adc9127 [DOI] [PubMed] [Google Scholar]
- 17. UK Health Security Agency . SARS-Cov-2 variants of concern and variants under investigation in England: technical briefing 49. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1129169/variant-technical-briefing-49-11-january-2023.pdf [Accessed 5 2023].
- 18. Drysdale M, Gibbons DC, Singh M, et al. Real-world effectiveness of Sotrovimab for the treatment of SARS-Cov-2 infection during Omicron BA.2 Subvariant predominance: a systematic literature review. Infection 2024;52:1–17. 10.1007/s15010-023-02098-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel V, Yarwood MJ, Levick B, et al. Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving Sotrovimab, oral Antivirals or no treatment in England. medRxiv [Preprint]. 10.1101/2022.11.28.22282808 [DOI] [PubMed]
- 20. Bottle A, Cohen C, Lucas A, et al. How an electronic health record became a real-world research resource: comparison between London’s whole systems integrated care database and the clinical practice research Datalink. BMC Med Inform Decis Mak 2020;20:71. 10.1186/s12911-020-1082-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Discover-NOW . Our Dataset: linked data. Available: https://discover-now.co.uk/the-data [Accessed 5 2023].
- 22. UK Health Security Agency . SARS-Cov-2 variants of concern and variants under investigation in England: technical briefing 45. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1115071/Technical-Briefing-45-9September2022.pdf [Accessed 5 2023].
- 23. Xu S, Ross C, Raebel MA, et al. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health 2010;13:273–7. 10.1111/j.1524-4733.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hippisley-Cox J, Khunti K, Sheikh A, et al. Risk prediction of COVID-19 related death or hospital admission in adults testing positive for SARS-Cov-2 infection during the Omicron wave in England (Qcovid4): cohort study. BMJ 2023;381:e072976. 10.1136/bmj-2022-072976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng B, Campbell J, Carr EJ, et al. Comparative effectiveness of Sotrovimab and Molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients on kidney replacement therapy: observational cohort study using the Opensafely-UKRR linked platform and SRR database. Clinical Kidney Journal 2023;16:2048–58. 10.1093/ckj/sfad184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng MM, Reyes C, Satram S, et al. Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-Cov-2 Delta and Omicron waves in the USA. Infect Dis Ther 2023;12:607–21. 10.1007/s40121-022-00755-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young-Xu Y, Korves C, Zwain G, et al. Effectiveness of sotrovimab in preventing COVID-19-related hospitalizations or deaths among U.S. veterans. Open Forum Infect Dis 2023;10:ofad605. 10.1093/ofid/ofad605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harman K, Nash SG, Webster HH, et al. Comparison of the risk of hospitalisation among BA.1 and BA.2 COVID-19 cases treated with sotrovimab in the community in England. Influenza Other Respir Viruses 2023;17:e13150. 10.1111/irv.13150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel V, Levick B, Boult S, et al. Characteristics and outcomes of COVID-19 patients presumed to be treated with sotrovimab in Nhs hospitals in England. medRxiv [Preprint]. 10.1101/2023.02.08.23285654 [DOI] [PMC free article] [PubMed]
- 31. Zheng B, Green ACA, Tazare J, et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: observational cohort study with the opensafely platform. BMJ 2022;379:e071932. 10.1136/bmj-2022-071932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng B, Tazare J, Nab L, et al. Comparative effectiveness of Nirmatrelvir/Ritonavir versus Sotrovimab and Molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised high-risk patients during Omicron waves: observational cohort study using the Opensafely platform. Lancet Reg Health Eur 2023;34:100741. 10.1016/j.lanepe.2023.100741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tazare J, Nab L, Zheng B, et al. Effectiveness of Sotrovimab and Molnupiravir in community settings in England across the Omicron Ba.1 and Ba.2 Sublineages: emulated target trials using the OpenSAFELY platform. Epidemiology [Preprint]. 10.1101/2023.05.12.23289914 [DOI]
- 34. Bruel T, Hadjadj J, Maes P, et al. Serum neutralization of SARS-Cov-2 Omicron Sublineages BA.1 and BA.2 in patients receiving Monoclonal antibodies. Nat Med 2022;28:1297–302. 10.1038/s41591-022-01792-5 [DOI] [PubMed] [Google Scholar]
- 35. Case JB, Mackin S, Errico JM, et al. Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-Cov-2 Omicron lineage strains. Nat Commun 2022;13:3824. 10.1038/s41467-022-31615-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paraskevis D, Gkova M, Mellou K, et al. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir as treatments for COVID-19 in patients at high risk. J Infect Dis 2023;228:1667–74. 10.1093/infdis/jiad324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yip TC-F, Lui GC-Y, Lai MS-M, et al. Impact of the use of oral antiviral agents on the risk of hospitalization in community Coronavirus disease. Clinical Infectious Diseases 2023;76:e26–33. 10.1093/cid/ciac687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wai AK-C, Chan CY, Cheung AW-L, et al. Association of molnupiravir and nirmatrelvir-ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health West Pac 2023;30:100602. 10.1016/j.lanwpc.2022.100602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Office for National Statistics . Coronavirus (COVID-19) latest insights: hospitals. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/hospitals [Accessed 5 2023].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-002238supp001.pdf (359.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The Discover data that support the findings of this study are available from Imperial College Health Partners via approval from the Discover Data Research Access Group (DRAG) under certain restrictions.