Abstract
Introduction
Epilepsy is a common neurological disorder characterised by recurrent seizures. Almost half of patients who have an unprovoked first seizure (UFS) have additional seizures and develop epilepsy. No current predictive models exist to determine who has a higher risk of recurrence to guide treatment. Emerging evidence suggests alterations in cognition, mood and brain connectivity exist in the population with UFS. Baseline evaluations of these factors following a UFS will enable the development of the first multimodal biomarker-based predictive model of seizure recurrence in adults with UFS.
Methods and analysis
200 patients and 75 matched healthy controls (aged 18–65) from the Kingston and Halifax First Seizure Clinics will undergo neuropsychological assessments, structural and functional MRI, and electroencephalography. Seizure recurrence will be assessed prospectively. Regular follow-ups will occur at 3, 6, 9 and 12 months to monitor recurrence. Comparisons will be made between patients with UFS and healthy control groups, as well as between patients with and without seizure recurrence at follow-up. A multimodal machine-learning model will be trained to predict seizure recurrence at 12 months.
Ethics and dissemination
This study was approved by the Health Sciences and Affiliated Teaching Hospitals Research Ethics Board at Queen’s University (DMED-2681-22) and the Nova Scotia Research Ethics Board (1028519). It is supported by the Canadian Institutes of Health Research (PJT-183906). Findings will be presented at national and international conferences, published in peer-reviewed journals and presented to the public via patient support organisation newsletters and talks.
Trial registration number
Keywords: Epilepsy, Magnetic Resonance Imaging, Electroencephalography, Cognition, Machine Learning
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Our proposed multimodal biomarker-based predictive model of seizure recurrence after unprovoked first seizure integrates behavioural, electroencephalography and MRI data.
The multicentre nature of this study allows for preliminary assessment of the model in two demographically and culturally distinct groups of Canadian patients.
However, the study is only recruiting from the Canadian population, which may limit generalisability.
Since recruitment is based on the occurrence of clinical events and is contingent on factors impacting first seizure clinic capacity (eg, changes in staffing or wait lists), delays to completion may occur.
Introduction
Background and rationale
Epilepsy, first seizure and recurrence risk
Epilepsy is a disorder of the brain characterised by an ‘enduring predisposition to generate epileptic seizures’,1 manifesting as at least two unprovoked seizures >24 hours apart or one unprovoked seizure with a >60% risk of recurrence.2 The prevalence of active epilepsy is around 1% but up to 10% of the population will experience a single seizure at some point in their lives.3 4 Seizures may be provoked by toxic/metabolic disturbances, trauma or stroke but most cases are unprovoked first seizure (UFS).
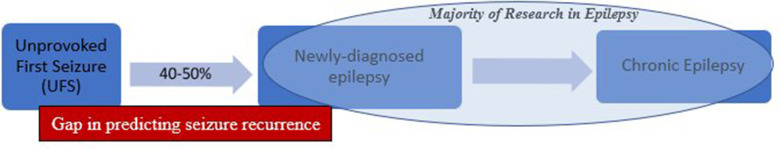
Following a UFS and in the absence of treatment, 40%–50% of these individuals will have further seizures within 2 years and thus be diagnosed with epilepsy (figure 1).5 6 Most recurrence (~40%) occurs in the first year. Evidence-based guidelines identify four clinical factors that increase the risk of recurrence following UFS7 including epileptiform abnormalities on electroencephalography (EEG), a remote symptomatic cause (eg, brain tumour on neuroimaging), abnormal neurological examination and a first seizure during sleep.
Figure 1.
Progression from UFS to epilepsy.
Antiseizure medications may be offered to individuals with identified risk factors. However, most patients with UFS have normal examination, EEG and brain imaging at presentation8 9 (figure 1).
Current challenges in clinical decision-making
In patients with UFS and no adverse prognostic factors, typical clinical practice is to defer treatment until after a second event. This approach is associated with morbidity through increased risk of accidents (eg, falls, motor vehicle accidents), carries implications for driving privileges and can also have profound psychosocial impact (eg, impact on employment, education and mental health). On the other hand, while early treatment after a UFS can reduce the risk of seizure recurrence by around 35% in the short term,7 it is associated with medication side effects in up to 31% of patients.7 Hence, offering treatment to patients at low risk of recurrence may mean unnecessary treatment with its associated adverse effects. The ability to determine individual recurrence risk after UFS would help determine whether early treatment is warranted and whether the potential benefits outweigh the risks.10
Epilepsy as a network disorder and limitations in the literature
Epilepsy is increasingly conceptualised as a network disorder11 with seizures sustained by microstructural or biochemical disturbances in normal-appearing brain tissue outside of the presumed seizure focus.12 Widespread changes in structural and functional brain connectivity, and behavioural manifestations of these changes including cognitive dysfunction13 14 and mood disturbance, may yield biomarkers for clinical outcomes.
Most research to date has focused on individuals with chronic or newly diagnosed epilepsy (NDE) (figure 1), but there is an increasing evidence that these changes are detectable prior to the time of formal diagnosis. Despite this, the population with UFS remains significantly underexplored.
Studying patients following UFS and before medication is commenced removes the confounding factor of antiseizure medication use on cognition and brain networks present in most studies.15 Furthermore, baseline evaluation of multiple factors indicative of neurological network dysfunction will enable the development of the first multimodal approach that can be applied to prediction of seizure recurrence at the earliest stages of epilepsy. We will incorporate the following three domains to predict seizure recurrence:
Domain 1: neuropsychological comorbidity—cognitive dysfunction
Cognitive dysfunction is evident at all stages of epilepsy, relates to seizure frequency and severity and may predict clinical course.13 14 Up to 80% of individuals with chronic epilepsy have cognitive impairment16 and approximately 40% of treatment-naive patients with new-onset epilepsy have cognitive dysfunction in at least two domains.17
Cognitive dysfunction may already be present following a first seizure. A recently published study from our Halifax First Seizure Clinic (HFSC) reported cognitive dysfunction in at least one cognitive domain in 56% of patients with NDE and UFS.18 Within the UFS subgroup, the prevalence of cognitive dysfunction in at least one domain was 41.2%. Individuals with UFS who were subsequently diagnosed with epilepsy were significantly more likely to demonstrate cognitive dysfunction at presentation than those who did not.
Domain 2: psychiatric comorbidity—depression and anxiety
Depression and anxiety are common psychiatric comorbidities of epilepsy, with prevalence ranging from 40% to 69% and from 31% to 65%, respectively, depending on the setting and method of evaluation.19 20 Although mood and anxiety disorders are often considered a consequence of epilepsy, there is evidence of bidirectional relationship between these psychiatric conditions and seizure control.21
Patients presenting to the HFSC have a significantly higher prevalence of both depression and anxiety compared with controls.22 Depression and a history of suicide attempts each significantly increase the risk of UFS.23 Individuals with UFS who were later diagnosed with epilepsy had an increased rate of depression compared with controls, while those without further seizures did not.19 Anxiety is highly prevalent in UFS and associated with an increased risk of seizure recurrence.24
Domain 3: alterations in brain structure and connectivity
Subtle brain network disturbances associated with seizures can also be explored using anatomical scans and measures of structural and functional connectivity derived from MRI and EEG.
Anatomical changes
In patients with epilepsy, multicentre studies reveal widespread altered subcortical volumes and cortical thinning.25 Thalamic atrophy has been documented in patients scanned within a week of first seizure,26 and hippocampal atrophy has been observed in newly diagnosed focal epilepsy.27 The integrity of the thalamus and thalamocortical connectivity are key in both focal28 and generalised epilepsy,29 so may yield early biomarkers of epilepsy. Measures of hippocampal volume and diffusion parameters can distinguish participants with and without seizure recurrence in early disease.30
Structural connectivity
Structural integrity is primarily studied via diffusion-weighted imaging. Maps of structural connectivity can be analysed with graph theory to assess changes in brain networks.31 32 Network metrics such as characteristic path length, small-worldness and global efficiency differ between subjects with focal epilepsy and controls.33 A reduction in network efficiency and bilateral alterations in network connectivity is also observed in patients with newly diagnosed focal epilepsy.34 However, no studies explore structural connectivity in relation to seizure recurrence in the UFS population.
Functional connectivity: MRI
Resting-state functional MRI (rsfMRI) combines high spatial and temporal resolution to provide an index of functional brain connectivity. In newly diagnosed focal epilepsy, altered functional connectivity is observed within the frontoparietal attentional network.35 Alterations in fractional amplitude of low frequency fluctuations (fALFF) can differentiate patients with new-onset epilepsy from those with first seizure.36 No studies specifically address seizure recurrence after UFS.
Functional connectivity: EEG
EEG provides a complementary means to assess functional connectivity with exceptional temporal resolution. A decision tree-based machine-learning classifier applied to network metrics from baseline EEG classified children referred with suspected epilepsy as having epilepsy or not with much greater sensitivity (96%) and specificity (95%) than the presence of interictal discharges.37
Multiple papers have found evidence to support the presence of different network connectivity patterns after the UFS in those later diagnosed with epilepsy versus controls, including decreased alpha and beta band connectivity38 and increased theta band connectivity.39 Applying machine learning to combined functional connectivity and frequency-based features can help diagnose epilepsy,40 and machine learning applied to combined EEG and rsfMRI data demonstrates greater accuracy in predicting seizure recurrence than the clinical impression alone.41 EEG features including phase lag index, coherence and synchronisation likelihood were the most discriminatory.
Summary of studies
There is ample evidence that cognitive dysfunction, mood disturbance, anatomical, structural and functional brain network disruptions are present at the early stages of epilepsy and may be predictive of clinical course. Although there has been limited research examining these factors and their relation to seizure recurrence in the UFS population, preliminary data from the literature and from our research strongly suggest prognostic value of these variables and their utility in a multimodal prediction approach.
Aims of this study
Our goal is to examine the baseline behavioural and neuroanatomical characteristics of adult patients with UFS and to develop the first multimodal biomarker-based predictive model of seizure recurrence in this population.
We aim:
To determine the prevalence and nature of cognitive dysfunction and mood disturbance in adults with UFS, and to determine how these factors differ between those with (UFS-r) and without (UFS-nr) seizure recurrence.
To determine changes in structural and functional brain networks (using MRI and EEG) following UFS compared with controls, and in patients with UFS-r and UFS-nr.
To develop a multimodal predictive model that combines clinical information with the identified significant biomarkers (from aims 1–2) to predict 12-month risk of seizure recurrence after UFS.
Methods and analysis
Patient recruitment
Research centres
We will recruit patients with UFS from two Canadian epilepsy centres that have clinics specifically dedicated to evaluation and treatment of UFS and NDE.
The HFSC is part of a comprehensive, academically driven programme providing clinical and counselling services to adult patients (ages 18+) from across Atlantic Canada (population 2.3 million). Between July and December 2021, HFSC received 654 referrals, with 244 (37%) classified as UFS. Recruitment estimates for this site based on two recent studies are approximately 40 participants per year with a full-time research coordinator.
The Kingston First Seizure Clinic was established as part of the comprehensive services provided through the provincial government-designated District Epilepsy Centre in Kingston and has a catchment area comprising the whole of South-Eastern Ontario (population 500 000). The clinic assesses over 120 patients per year and is targeted specifically at those with first seizure, with patients with new-onset epilepsy seen in other clinics.
Inclusion & exclusion criteria
This study will include adult patients with UFS between the ages of 18 and 65 years. Individuals over the age of 65 will not be included to reduce the probability of including individuals with early dementia. We will also exclude individuals who, on assessment during their first clinic appointment, are determined to have non-epileptic events, prior seizure events or diagnosis of epilepsy (eg, based on abnormal CT or EEG), provoked seizure (eg, medication, substance misuse, metabolic), acute symptomatic seizures, an existing prescription for antiseizure drugs, significant CNS comorbidity that may affect cognition and brain networks (eg, progressive neurological disorder, MS), previous neurosurgery or contraindication to MRI. We will also include a sample of age, sex and education-matched healthy controls with the same exclusion criteria.
Sample size calculation
A pilot study conducted at the HFSC (P.I. A. Omisade) informs the sample size calculation for this multicentre study. The pilot represents the first study examining multimodal biomarkers of seizure recurrence following untreated UFS (n=15 to date) and treated new-onset epilepsy (NOE, n=14).
Sample size estimates are based on group comparisons between the UFS-r and UFS-nr subgroups. Group sizes of 55 (UFS-r) and 72 (UFS-nr) are sufficient for cognitive impairment, anxiety/depression and resting state fMRI data (see online supplemental material).
bmjopen-2024-086153supp001.pdf (52.5KB, pdf)
For other EEG and MRI-derived network and connectivity measures, we rely on literature. A protocol for a prospective observational cohort study of seizure recurrence in patients with NDE using these data as predictors gives an estimate of 72 patients (24 with seizure recurrence, 48 without) and 48 controls using a very stringent significance level of 0.001, power of 90% and effect side estimates based on their previous studies.42
A sample size of 150 patients with UFS (of whom ~60 will experience a recurrence) and 75 healthy controls is sufficient to detect changes in all metrics for which we have pilot data and allows up to 12 variables in the predictive model based on the rule-of-thumb √sample size predictors. A further 50 patients (25% of the total) will form an independent replication dataset.
Planned study visits
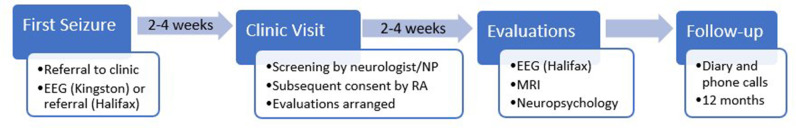
Patients will be seen in the First Seizure Clinics by a neurologist or nurse–practitioner within 2–4 weeks of the seizure event (figure 2). The initial visit will involve a standard clinical assessment, review of inclusion and exclusion criteria, and, if appropriate, referral for the research study. The informed consent discussion will be conducted by a research assistant following the clinic visit. Participants will undergo cognitive screening assessment, MRI and EEG (if not already done) within 2–4 weeks of the initial clinic visit (figure 2).
Figure 2.
Individual patient study participation timeline. EEG, electroencephalography, NP, nurse practitioner, RA, research assistant.
Seizure recurrence will be monitored by a diary provided to each participant with instructions to contact the research team in the event of a seizure. A member of the research team will follow up with participants by telephone at 3, 6, 9 and 12 months following the initial seizure. The primary outcome will be seizure recurrence at 12 months.
Healthy control participants will complete the same neuropsychological battery, MRI scans and EEG protocols to evaluate baseline level of impairment in a healthy population and for the group comparisons of UFS.
Clinical variables
The following information will be documented at the time of the initial first seizure clinic visit: age, sex, gender, time between seizure and clinic visit (in days), first seizure arising from sleep (yes/no), comorbid neurological or psychiatric conditions (yes/no), substance use (yes/no and types of substances), medications and abnormal findings on neuroimaging (yes/no) if completed prior to the clinic visit.
Neuropsychological assessment procedures
The cognitive screening battery is detailed in table 1. Participants will complete mood questionnaires at the same time using the Hospital Anxiety and Depression Scale,43 which generates separate scores for symptoms of depression and anxiety.
Table 1.
Neuropsychological test battery
| Cognitive domain | Tests/scores |
| General intelligence (IQ) | WASI-II Vocabulary and Matrix Reasoning Sub-Scales |
| Attention and working memory | WMS-IV Digit Span Total Score |
| WMS-IV Symbol Span Total Score | |
| Processing speed | Symbol Digit Modalities Test—Oral |
| Trails A-Oral | |
| Executive function | DKEFS Verbal Fluency-Switching Subtest |
| DKEFS Color-Word Interference—Interference Subtest | |
| Trails B—Oral | |
| Memory | Rey Auditory Verbal Learning Test (Immediate Recall Trial 5, Long Delay Free Recall) |
| Aggie Figural Learning Test (as above) | |
| Language | WASI-II Vocabulary |
| DKEFS Verbal Fluency (Letter, Semantic) | |
| Boston Naming Test | |
| Visuospatial/visuoconstruction | WASI-II Matrix Reasoning |
| Taylor Complex Figure Copy |
IQ, WASI-II, Wechsler Abbreviated Scale of Intelligence 2nd edition;57 WMS-III, Wechsler Memory Scales 3rd edition,58 Symbol Digit Modalities Test,59 Trails A and B: Trail Making Test,60 DKEFS: Delis-Kaplan Executive Function Scales,61 Rey Auditory Verbal Learning Test,62 Aggie Figural Fluency,63 Boston Naming Test,64 Taylor Complex Figure Copy.65
Neuroimaging protocol
Neuroimaging in Kingston will take place on the 3T Siemens Magnetom Prisma Fit scanner located in the Centre for Neuroscience Studies at Queen’s University. The protocol employs sequences adapted from the Human Connectome Project (http://www.humanconnectomeproject.org/) and diffusion imaging is based on recommendations from DSI Studio (http://dsi-studio.labsolver.org/Manual/b-table-for-qbi-dsi-and-gqi-scans). The established protocol includes the following acquisitions:
Structural scans including a 3D T1-weighted MPRAGE (0.8 mm isotropic, 7 min), 3D T2-weighted SPACE (0.8 mm isotropic, 6 min) and a T2-weighted FLAIR scan (1 mm, 6 min); a 2D T2-weighted sequence with high in-plane resolution will be added to enable hippocampal assessments and in accordance with the HARNESS protocol.44
rsf-MRI (2 mm isotropic, 8×Multiband, 800 ms temporal resolution, acquired in two phase-encoding directions with additional field map, 15 min).
Diffusion-weighted imaging (1.5 mm isotropic, 4×Multiband, acquired with 185 diffusion-weighting directions over two shells in opposite phase encoding directions (98 directions in AP and 99 directions in PA), maximum b-value of 3000 s/mm2, with a reverse phase-encode non-diffusion-weighted scan for distortion correction, 12 min).
A harmonised sequence will be implemented on the 3T GE MR750 scanner located in Halifax with a 32-channel Nova Medical coil and be validated with two human volunteers scanned at both sites. Image quality will be assessed using MRIQC (poldracklab.github.io/mriqc/). Any biases in quantitative metrics between the two sites will be corrected using ComBat, an algorithm first described in genomics that derives a batch-specific transformation to express all data in a common space removing any batch effects using an empirical Bayes framework.45 In this case, the ‘batches’ are the two centres. This approach has been validated in neuroimaging studies46 47 and used in prior multicentre epilepsy neuroimaging studies as part of the ENIGMA Consortium.48
EEG protocol
A routine EEG will be acquired using standard electrode placement according to the 10–20 International System and a sampling rate of at least 500 Hz and recorded for 30 min including hyperventilation and photic stimulation as standard activation procedures (assuming no contraindication). In Kingston, all EEG recordings will take place in Kingston Health Sciences Centre prior to the clinical assessment and in Halifax, they will take place 2–4 weeks following the initial clinic visit at the QEII Health Sciences Centre (as per local policies). If patients had completed an EEG elsewhere in Nova Scotia prior to the clinic visit, the EEG will be read by the epileptologists in Halifax.
Data analysis
Primary outcome
The primary outcome is seizure recurrence by 12 months. Participants will form three groups: healthy controls, participants with UFS and no seizure recurrence by 12 months (UFS-nr) and those with UFS and seizure recurrence (UFS-r). Initial analyses will compare all participants with UFS to healthy controls to determine baseline differences. Subsequent analyses will compare the UFS-nr and UFS-r cohorts to identify discriminatory variables for the multimodal predictive model.
Neuropsychology
Individual assessments will be scored using published demographically corrected norms to produce individual standard scores for each task that will be converted to Z-scores. Both individual domain-specific and global Z-scores (ie, average Z-score across entire battery) will be used for further analyses.
Neuroimaging
Neuroimaging data will be stored using the Brain Imaging Data Structure specification49 to facilitate subsequent processing.
Anatomical MRI comprising T1-weighted images will be processed with FreeSurfer to yield volumes of key structures, such as the thalamus and maps of cortical thickness. Group comparisons will be performed as documented above to identify key changes.
Functional MRI will be preprocessed using fMRIPrep50 and the brain will be parcellated into regions using the Desikan-Killiany atlas in FreeSurfer. Matrices of functional connectivity will be determined using fALFF in different frequency bands. Network metrics such as clustering coefficient, characteristic path length and small-worldness51 will be derived from the resulting connectivity graphs using Brain Connectivity Toolbox (https://sites.google.com/site/bctnet/) and compared between groups. Secondary analyses will include seed-to-voxel analyses of specific cognitive networks, such as the default mode network.35
Diffusion-weighted MRI will be preprocessed with QSIprep (https://qsiprep.readthedocs.io) and tractography will be used to generate matrices of structural connectivity. The connectivity matrices will be analysed with Brain Connectivity Toolbox and network metrics compared between groups. Key metrics will include characteristic path length, small-worldness and global efficiency.33
The functional and structural connectomes will also be analysed with Network-Based Statistics, a toolbox to robustly identify which parts of the connectome differ between groups and to identify potential factors to include in the machine-learning-based multimodal prognostication model.34
Electroencephalography
EEG data will be anonymised and exported from the hospital system and subsequently imported into EEGLAB (https://sccn.ucsd.edu/eeglab/index.php). Standard automated preprocessing will be used to remove artefacts52 and the EEG will be converted to an average reference montage, excluding the channels that commonly contain artefact (eg, eye blinking artefact in Fp1/Fp2). Using artefact-free epochs of EEG data, a band-pass filter will be used to split the data into commonly used frequency bands including delta (2–4 Hz), theta (4–8 Hz), lower alpha (8–10.5 Hz), upper alpha (10.5–13 Hz), lower beta (13–20 Hz), higher beta (20–30 Hz) and gamma (30–45 Hz). For each frequency band, functional connectivity will be assessed between each channel using Phase Lag Index,53 coherence and synchronisation likelihood54 and subsequently averaged across each channel. Group comparisons will be performed to identify potential factors for the prognostication model.
Predictive model
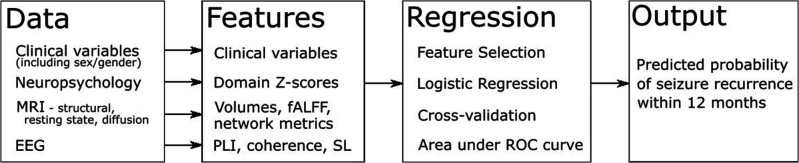
We will build a multivariate prediction model for seizure recurrence using binomial logistic regression with L2 regularisation to avoid overfitting.
Quantitative metrics derived from each modality will comprise the potential feature set (figure 3), and the output will be the predicted probability of seizure recurrence within 12 months. Only features demonstrating the most significant differences between groups with and without seizure recurrence and lacking significant correlation with other such features (Pearson’s r<0.7) will be retained aiming for a maximum of 12 features in the final model (based on the rule of thumb of features).
Figure 3.
Modelling approach. EEG, electroencephalography; fALFF, fractional amplitude of low frequency fluctuations; PLI, phase lag index; ROC, receiver operating characteristics; SL, synchronisation likelihood.
For validation, we will apply stratified 10-fold cross-validation, with inner folds used for model building and hyperparameter tuning through grid search and outer folds used for unbiased test sets. Performance will be assessed using the area under the receiver operating characteristics curve. Sensitivity, specificity, positive and negative predictive values will also be determined. After building our model, we will perform an independent validation using 50 subjects (25%) never included in model building.
Timeline
This study will be conducted over a 5-year period beginning in August 2023. Following set-up and harmonisation, data collection will occur over 30 months ending July 2026 with a further 12 months of follow-up. Data analysis and knowledge translation will start when 12-month follow-up data become available.
Comparison to other studies
A recent protocol seeks to investigate seizure recurrence after UFS in 100 participants in the UK, with the majority having conventional MRI studies and only a minority undergoing advanced MRI sequences.55 While serum biomarkers are also included, we instead include a comprehensive neuropsychological assessment. Further, we include a control population for comparison and a larger sample size to enable the development of a predictive model.
A second study (SWISS FIRST) in Switzerland is prospectively recruiting patients presenting with a possible first seizure in Switzerland, and thus does not have the same rigorous exclusion criteria to ensure that only those with UFS as diagnosed by a neurologist are included.56 No specific follow-up is planned outside routine clinical care. Analysis will include morphometry and functional connectivity from MRI, and spike maps and microstates from EEG to predict recurrence.
Patient and public involvement
The identification and development of the research questions and outcomes have been informed by close collaboration with local epilepsy charities. Epilepsy South-Eastern Ontario (Kingston) works closely with Kingston Health Sciences Centre in providing support and counselling to patients, including those experiencing their first seizure. The Epilepsy Association of the Maritimes (Halifax) has worked closely with Nova Scotia Health for 40 years and notes frequent calls from people who have had a first seizure and are thus wondering if they will have another and if so, when. Both organisations have committed to disseminate research findings via education sessions, newsletters and social media feeds.
Ethics and dissemination
Ethical approval
This study was approved by the Health Sciences and Affiliated Teaching Hospitals Research Ethics Board at Queen’s University (DMED-2681-22) and the Nova Scotia Research Ethics Board (1028519). It is registered with ClinicalTrials.gov with identifier NCT05724719.
Data governance and confidentiality
The Kingston site will store written files, including consent forms and cognitive data, in a locked filing cabinet accessible only to GW. MRI and EEG data will be stored on a secure server in the Centre for Neuroscience Studies. Deidentified study participant ID’s will be assigned to make data non-identifiable for data analysis. The master linking log will be securely saved on a password-protected server in the Centre for Neuroscience Studies at Queen’s University, separate from the Data Collection/Capture Sheet. Deidentified data will be stored electronically on a secure password protected server in the Centre for Neuroscience Studies and in a web-based database hosted by the Faculty of Health Sciences (RedCap).
The Halifax site will enter deidentified data directly on the Kingston based RedCap, and Halifax will have a separate linking log/database for identifiable data elements held locally. Data transferred to another site will be deidentified, transferred with SFTP and encrypted. All data transfer will be covered by a data transfer agreement to/from Halifax.
After the storage period of 5 years beyond the end of the study, deidentified data will be archived indefinitely in the Queen’s University’s Institutional Repository as per the Canadian Institutes of Health Research requirements and Queens’ University Policy. Confidentiality will be protected to the extent permitted by applicable laws.
Dissemination
We will present results in peer-reviewed journals and at epilepsy-related national and international conferences (eg, American Epilepsy Society, Canadian League Against Epilepsy, International Epilepsy Congress) and local events.
Findings will be presented to people with epilepsy and lay audience members in the Epilepsy Association of the Maritimes newsletter, via Epilepsy South-Eastern Ontario and supported with relevant talks via these patient support organisations.
Data set
All code and algorithms will be made available as open source on repositories such as GitHub.
Supplementary Material
Footnotes
Contributors: The initial draft of the protocol was jointly developed by GW and AO. KB, LBL, DB, JG, KI, MS, GS, BW and SW reviewed and provided feedback on the protocol. BCB and KBG-R performed a literature search to update the protocol following funding, and BCB prepared the protocol for submission.
Funding: This work was supported by the Canadian Institutes of Health Research (CIHR) grant number PJT-183906.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League against epilepsy (ILAE) and the International Bureau for epilepsy (IBE). Epilepsia 2005;46:470–2. 10.1111/j.0013-9580.2005.66104.x [DOI] [PubMed] [Google Scholar]
- 2. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–82. 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 3. Annegers JF, Hauser WA, Lee JR, et al. Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935-1984. Epilepsia 1995;36:327–33. 10.1111/j.1528-1157.1995.tb01005.x [DOI] [PubMed] [Google Scholar]
- 4. Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia 1993;34:453–68. 10.1111/j.1528-1157.1993.tb02586.x [DOI] [PubMed] [Google Scholar]
- 5. Berg AT. Risk of recurrence after a first unprovoked seizure. Epilepsia 2008;49 Suppl 1:13–8. 10.1111/j.1528-1167.2008.01444.x [DOI] [PubMed] [Google Scholar]
- 6. Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology 1991;41:965–72. 10.1212/wnl.41.7.965 [DOI] [PubMed] [Google Scholar]
- 7. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults: report of the guideline development subcommittee of the American Academy of neurology and the American epilepsy society. Neurology 2015;84:1705–13. 10.1212/WNL.0000000000001487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crocker CE, Pohlmann-Eden B, Schmidt MH. Role of neuroimaging in first seizure diagnosis. Seizure 2017;49:74–8. 10.1016/j.seizure.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 9. Rizvi S, Ladino LD, Hernandez-Ronquillo L, et al. Epidemiology of early stages of epilepsy: risk of seizure recurrence after a first seizure. Seizure 2017;49:46–53. 10.1016/j.seizure.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 10. Foster E, Carney P, Liew D, et al. First seizure presentations in adults: beyond assessment and treatment. J Neurol Neurosurg Psychiatry 2019;90:1039–45. 10.1136/jnnp-2018-320215 [DOI] [PubMed] [Google Scholar]
- 11. Bernhardt BC, Bonilha L, Gross DW. Network analysis for a network disorder: the emerging role of graph theory in the study of epilepsy. Epilepsy Behav 2015;50:162–70. 10.1016/j.yebeh.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 12. Richardson MP. Large scale brain models of epilepsy: dynamics meets connectomics. J Neurol Neurosurg Psychiatry 2012;83:1238–48. 10.1136/jnnp-2011-301944 [DOI] [PubMed] [Google Scholar]
- 13. Hermann BP, Seidenberg M, Dow C, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol 2006;60:80–7. 10.1002/ana.20872 [DOI] [PubMed] [Google Scholar]
- 14. Pohlmann-Eden B, Aldenkamp A, Baker GA, et al. The relevance of neuropsychiatric symptoms and cognitive problems in new-onset epilepsy - current knowledge and understanding. Epilepsy Behav 2015;51:199–209. 10.1016/j.yebeh.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 15. Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord 2011;4:385–407. 10.1177/1756285611417920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Helmstaedter C, Aldenkamp AP, Baker GA, et al. Disentangling the relationship between epilepsy and its behavioral comorbidities - the need for prospective studies in new-onset epilepsies. Epilepsy Behav 2014;31:43–7. 10.1016/j.yebeh.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 17. Witt JA, Helmstaedter C. Should cognition be screened in new-onset epilepsies? A study in 247 untreated patients. J Neurol 2012;259:1727–31. 10.1007/s00415-012-6526-2 [DOI] [PubMed] [Google Scholar]
- 18. Jackson-Tarlton CS, Whatley BP, Kasheke GDS, et al. A prospective pilot study of cognitive impairment and mood in adults with first seizure, new-onset epilepsy, and newly diagnosed epilepsy at time of initial seizure presentation. Epilepsy Behav 2020;112:107359. 10.1016/j.yebeh.2020.107359 [DOI] [PubMed] [Google Scholar]
- 19. Scott AJ, Sharpe L, Thayer Z, et al. How frequently is anxiety and depression identified and treated in hospital and community samples of adults with epilepsy. Epilepsy Behav 2021;115:107703. 10.1016/j.yebeh.2020.107703 [DOI] [PubMed] [Google Scholar]
- 20. Gurgu RS, Ciobanu AM, Danasel RI, et al. Psychiatric comorbidities in adult patients with epilepsy (a systematic review). Exp Ther Med 2021;22:909. 10.3892/etm.2021.10341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thapar A, Kerr M, Harold G. Stress, anxiety, depression, and epilepsy: investigating the relationship between psychological factors and seizures. Epilepsy Behav 2009;14:134–40. 10.1016/j.yebeh.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 22. Lane C, Crocker C, Legg K, et al. Anxiety and depression in adult first seizure presentations. Can J Neurol Sci 2018;45:144–9. 10.1017/cjn.2017.285 [DOI] [PubMed] [Google Scholar]
- 23. Hesdorffer DC, Hauser WA, Olafsson E, et al. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol 2006;59:35–41. 10.1002/ana.20685 [DOI] [PubMed] [Google Scholar]
- 24. Baldin E, Hauser WA, Pack A, et al. Stress is associated with an increased risk of recurrent seizures in adults. Epilepsia 2017;58:1037–46. 10.1111/epi.13741 [DOI] [PubMed] [Google Scholar]
- 25. Whelan CD, Altmann A, Botía JA, et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain 2018;141:391–408. 10.1093/brain/awx341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perani S, Tierney TM, Centeno M, et al. Thalamic volume reduction in drug-naive patients with new-onset genetic generalized epilepsy. Epilepsia 2018;59:226–34. 10.1111/epi.13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leek NJ, Neason M, Kreilkamp BAK, et al. Thalamohippocampal atrophy in focal epilepsy of unknown cause at the time of diagnosis. Eur J Neurol 2021;28:367–76. 10.1111/ene.14565 [DOI] [PubMed] [Google Scholar]
- 28. Bernhardt BC, Bernasconi N, Kim H, et al. Mapping thalamocortical network pathology in temporal lobe epilepsy. Neurology 2012;78:129–36. 10.1212/WNL.0b013e31823efd0d [DOI] [PubMed] [Google Scholar]
- 29. McGill ML, Devinsky O, Wang X, et al. Functional neuroimaging abnormalities in idiopathic generalized epilepsy. Neuroimage Clin 2014;6:455–62. 10.1016/j.nicl.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmidt MH, Crocker CE, Abdolell M, et al. Toward individualized prediction of seizure recurrence: hippocampal neuroimaging features in a cohort of patients from a first seizure clinic. Epilepsy Behav 2021;122:108118. 10.1016/j.yebeh.2021.108118 [DOI] [PubMed] [Google Scholar]
- 31. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186–98. 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- 32. Vaughan DN, Rayner G, Tailby C, et al. MRI-negative temporal lobe epilepsy: a network disorder of neocortical Connectivity. Neurology 2016;87:1934–42. 10.1212/WNL.0000000000003289 [DOI] [PubMed] [Google Scholar]
- 33. Park KM, Lee BI, Shin KJ, et al. Progressive topological disorganization of brain network in focal epilepsy. Acta Neurol Scand 2018;137:425–31. 10.1111/ane.12899 [DOI] [PubMed] [Google Scholar]
- 34. Kreilkamp BAK, McKavanagh A, Alonazi B, et al. Altered structural connectome in non-lesional newly diagnosed focal epilepsy: relation to pharmacoresistance. Neuroimage Clin 2021;29:102564. 10.1016/j.nicl.2021.102564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alonazi BK, Keller SS, Fallon N, et al. Resting-state functional brain networks in adults with a new diagnosis of focal epilepsy. Brain Behav 2019;9:e01168. 10.1002/brb3.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gupta L, Janssens R, Vlooswijk MCG, et al. Towards prognostic biomarkers from BOLD fluctuations to differentiate a first epileptic seizure from new-onset epilepsy. Epilepsia 2017;58:476–83. 10.1111/epi.13658 [DOI] [PubMed] [Google Scholar]
- 37. van Diessen E, Otte WM, Braun KPJ, et al. Improved diagnosis in children with partial epilepsy using a multivariable prediction model based on EEG network characteristics. PLoS One 2013;8:e59764. 10.1371/journal.pone.0059764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koo GE, Jeong HT, Youn YC, et al. Is functional connectivity after a first unprovoked seizure different based on subsequent seizures and future diagnosis of epilepsy J Epilepsy Res 2022;12:62–7. 10.14581/jer.22011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Douw L, de Groot M, van Dellen E, et al. Functional connectivity' is a sensitive predictor of epilepsy diagnosis after the first seizure. PLoS One 2010;5:e10839. 10.1371/journal.pone.0010839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matos J, Peralta G, Heyse J, et al. Diagnosis of epilepsy with functional connectivity in EEG after a suspected first seizure. Bioengineering 2022;9:690. 10.3390/bioengineering9110690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drenthen GS, Jansen JFA, Gommer E, et al. Predictive value of functional MRI and EEG in epilepsy diagnosis after a first seizure. Epilepsy Behav 2021;115:107651. 10.1016/j.yebeh.2020.107651 [DOI] [PubMed] [Google Scholar]
- 42. de Bézenac C, Garcia-Finana M, Baker G, et al. Investigating imaging network markers of cognitive dysfunction and pharmacoresistance in newly diagnosed epilepsy: a protocol for an observational cohort study in the UK. BMJ Open 2019;9:e034347. 10.1136/bmjopen-2019-034347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes 2003;1:29. 10.1186/1477-7525-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang I, Bernasconi A, Bernhardt B, et al. MRI essentials in epileptology: a review from the ILAE imaging taskforce. Epileptic Disord 2020;22:421–37. 10.1684/epd.2020.1174 [DOI] [PubMed] [Google Scholar]
- 45. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007;8:118–27. 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- 46. Fortin J-P, Parker D, Tunç B, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017;161:149–70. 10.1016/j.neuroimage.2017.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu M, Linn KA, Cook PA, et al. Statistical harmonization corrects site effects in functional connectivity measurements from multi‐site fmri data. Human Brain Mapping 2018;39:4213–27. 10.1002/hbm.24241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sisodiya SM, Whelan CD, Hatton SN, et al. The ENIGMA-epilepsy working group: mapping disease from large data SETS. Hum Brain Mapp 2020;43:113–28. 10.1002/hbm.25037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gorgolewski KJ, Auer T, Calhoun VD, et al. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci Data 2016;3:160044. 10.1038/sdata.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Esteban O, Markiewicz CJ, Blair RW, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 2019;16:111–6. 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–69. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 52. Pedroni A, Bahreini A, Langer N. Automagic: standardized preprocessing of big EEG data. Neuroimage 2019;200:460–73. 10.1016/j.neuroimage.2019.06.046 [DOI] [PubMed] [Google Scholar]
- 53. Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel eeg and meg with diminished bias from common sources. Human Brain Mapping 2007;28:1178–93. 10.1002/hbm.20346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stam CJ, van Dijk BW. Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data SETS. Physica D: Nonlinear Phenomena 2002;163:236–51. 10.1016/S0167-2789(01)00386-4 [DOI] [Google Scholar]
- 55. Adan GH, de Bézenac C, Bonnett L, et al. Protocol for an observational cohort study investigating biomarkers predicting seizure recurrence following a first unprovoked seizure in adults. BMJ Open 2022;12:e065390. 10.1136/bmjopen-2022-065390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jin BZ, De Stefano P, Petroulia V, et al. Diagnosis of epilepsy after first seizure. introducing the SWISS FIRST study. Clin Transl Neurosci 2020;4:2514183X2093944. 10.1177/2514183X20939448 [DOI] [Google Scholar]
- 57. Wechsler D. WASI II: Wechsler Abbreviated Scale of Intelligence. 2nd Ed. Texas: Pearson, 2011. [Google Scholar]
- 58. Wechsler D. Wechsler Memory Scale. 3rd Ed (WMS–III). Technical and Interpretive Manual. Pearson. Texas, 2003. [Google Scholar]
- 59. Smith A. Symbol Digit Modalities Test: SDMT. California: Testzentrale, 2000. [Google Scholar]
- 60. Heaton RK, et al. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, Florida: Psychological Assessment Resources, Inc, 2004. [Google Scholar]
- 61. Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (DKEFS). San Antonio, Texas: Pearson, 2001. [Google Scholar]
- 62. Schmidt M. Rey Auditory and Verbal Learning Test. A Handbook. California: Western Psychological Association, 1996. [Google Scholar]
- 63. Majdan A, Sziklas V, Jones-Gotman M. Performance of healthy subjects and patients with resection from the anterior temporal lobe on matched tests of verbal and visuoperceptual learning. J Clin Exp Neuropsychol 1996;18:416–30. 10.1080/01688639608408998 [DOI] [PubMed] [Google Scholar]
- 64. Landis T, Regard M, Graves R, et al. Semantic paralexia: a release of right hemispheric function from left hemispheric control. Neuropsychologia 1983;21:359–64. 10.1016/0028-3932(83)90022-2 [DOI] [PubMed] [Google Scholar]
- 65. Taylor LB. Localisation of cerebral lesions by psychological testing. Clin Neurosurg 1969;16:269–87. 10.1093/neurosurgery/16.cn_suppl_1.269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2024-086153supp001.pdf (52.5KB, pdf)