The development of chimeric antigen receptors (CARs) has undergone remarkable evolution. Starting from first-generation CARs, which lacked co-stimulatory signalling domains and exhibited limited efficacy due to inadequate signalling strength and durability, subsequent developments involved leveraging the intrinsic structural modularity of T-cell receptors (TCRs) and closely mimicking the mechanisms of T cell activation upon ligand binding. Second-generation (2G) CAR designs incorporated one co-stimulatory domain to augment and sustain T-cell activation, most notably CD28-CD3ζ and 4-1BB-CD3ζ. Building upon these advancements, third-generation CARs further refined this architecture by integrating multiple co-stimulatory domains (1). Given this extensive development, immunotherapy using CAR-engineered T-cells has achieved remarkable success in treating haematological malignancies. However, effectiveness in combating solid tumours such as small-cell lung cancer (SCLC) has been notably limited thus far (1). SCLC represents approximately 15% of lung cancers and is characterized by a rapid rate of proliferation, a strong propensity for early metastasis, and an unfavourable prognosis (2). In fact, SCLC has a notably elevated mortality rate compared to other prevalent solid tumours. Analysing data from the US Surveillance, Epidemiology, and End Results (SEER) registry spanning from 1983 to 2012, while there was a slight enhancement in the 5-year survival rate, the median survival remained a mere 7 months (2).
Over the past decade, there has been a significant evolution in first-line treatment approaches for SCLC. Immunotherapies which amplify T-cell activity against cancer cells through the blockade of CTLA-4, PD-1, or PD-L1, have demonstrated positive effects in patients with SCLC (3,4). Moreover, a phase II clinical trial (ALTER 0303 trial: NCT02388919) has shown potential benefits of anlotinib, a multi-targeted tyrosine kinase inhibitor, in terms of progression-free survival and overall survival in patients with advanced SCLC (5).
However, despite the elevated tumour mutation burden observed in this tumour type, responsiveness to T-cell checkpoint blockade is restricted to approximately 10–12% of patients (4,6). The limited efficacy of immunotherapies against SCLC may be attributed to multiple mechanisms including lowered tumour cell surface expression of major histocompatibility complex (MHC) class I molecules (7), failure of antigen presentation and substantial heterogeneity within individual tumours (8,9).
Delta-like ligand 3 (DLL3) is an inhibitory Notch ligand which has emerged as an attractive tumour-specific target that is overexpressed on the cell surface of SCLC cells (10). By contrast, expression in normal tissues is largely restricted to intracellular membranes, most notably the Golgi apparatus (1). Various strategies for targeting DLL3 are currently under investigation, both in the pre-clinical and clinical settings. These include the exploration of antibody-drug conjugates (ADCs), T-cell engager molecules, and CAR-based therapies. Ovalpituzumab tesirine (Rova-T) is an ADC targeting DLL3 and consists of the humanized anti-DLL3 monoclonal antibody SC16LD6.5 conjugated to a pyrrolobenzodiazepine (PBD) dimer toxin that induces DNA damage (8). The phase 3 MERU trial was designed to assess Rova-T as a first-line maintenance therapy for SCLC, but has been concluded prematurely. Disappointingly the trial revealed no survival benefit for patients treated with Rova-T compared to those receiving a placebo (11). A noteworthy advancement in bispecific T-cell engager (BiTE) technology, has led to the development of tarlatamab (AMG 757) (12). This molecule is designed to engage both T-cells and DLL3-expressing cancer cells simultaneously. In patients with relapsed/refractory (R/R) SCLC, it demonstrated manageable safety with encouraging response durability, both in a median duration of response at 12.3 months and in a median overall survival at 13.2 months (13). The positive clinical pharmacology profile of tarlatamab has provided support for the development of CAR-T therapy targeting DLL3 in SCLC. The first clinical report of CAR T-cell therapy targeting DLL3 involves a 4-1BB-containing 2G product known as AMG 119. In a report of five patients with R/R SCLC, AMG 119 has been demonstrated to be well tolerated at the doses tested, with no dose-limiting toxicities and at least one partial response achieved (14,15).
In principle, the efficacy of CAR T-cell immunotherapy of SCLC could be enhanced by implementing approaches that actively reshape the immunosuppressive tumour microenvironment (TME) (16). One such strategy entails the modification of CAR T-cells to release pro-inflammatory cytokines such as interleukin (IL)-12 or members of the IL-1 superfamily (17), including IL-18. These constructs are variously referred to as fourth-generation CARs, armoured CARs or TRUCKs (T-cell redirected for universal cytokine-mediated killing). Jaspers et al. have investigated this approach in the context of DLL3-specific CAR T-cell immunotherapy of SCLC (17).
IL-18 is synthesized as an inactive precursor known as pro-IL-18. Activation occurs when the N-terminal pro-peptide of pro-IL-18 is removed by caspase 1 cleavage. Mature IL-18 then binds to target cell receptors, initiating signalling via MyD88. This pro-inflammatory cytokine acts as a powerful stimulus to both innate and adaptive immune responses. It enables the recruitment of tumour-infiltrating T-cells of both αβ and γδ subtypes (18), natural killer cells (19), dendritic cells (DCs) (19), and (anti-tumour) M1-polarized macrophages, while reducing pro-tumour regulatory T-cells and immunosuppressive M2-polarized macrophages (20).
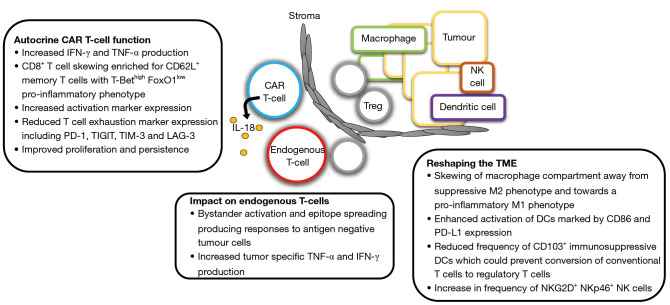
Armouring CAR T-cell systems based on IL-18 generally involve the expression of the constitutively active form of this cytokine. This is achieved by replacing the IL-18 pro-peptide with a signal peptide, directing this protein for export via the secretory pathway. Armouring of CAR T-cells to release active IL-18 can reproducibly potentiate anti-tumour activity (20,21), even in the absence of lymphodepletion (22). This is accompanied by favourable modulation of the TME (17,20,22) and amplification of endogenous immune surveillance via epitope spreading (22) (Figure 1). On a note of caution however, biologically active IL-18 has been linked to a number of inflammatory pathologies (23). In keeping with this, CAR T-cells that constitutively release IL-18 have caused toxicity in immune-competent mouse models (21).
Figure 1.
Mechanisms that promote tumour clearance by IL-18 secreting CAR T-cells. CAR, chimeric antigen receptor; IFN, interferon; TNF, tumour necrosis factor; IL, interleukin; Treg, regulatory T cell; NK, natural killer; DC, dendritic cell.
A particular strength of the study by Jaspers et al. is the evaluation of the IL-18 armouring technology in both human and mouse T-cells, respectively, leveraging the choice between CD28 and 4-1BB co-stimulatory domains. Individually, this provides clinical relevance and enables the study of the immunological consequences of the therapy. Having selected DLL3-specific CAR candidates with 4-1BB as a co-stimulatory domain to promote CAR-T cell persistence and enhance memory formation, they first set up an immune-competent mouse model of metastatic SCLC. In this context, non-armoured CAR T-cells achieved dose-dependent anti-tumour activity. Efficacy was further potentiated by pre-conditioning with cyclophosphamide, in keeping with the known importance of lymphodepleting chemotherapy in boosting CAR T-cell expansion in vivo (24). Next, they evaluated IL-12 or IL-18 armoured CAR T-cells in this model, omitting preconditioning chemotherapy and employing a modest dose of two million CAR T-cells to compare the efficacy of these approaches. Release of IL-18 by the CAR T-cells led to a sharp increase in serum levels of both interferon (IFN)-γ and tumour necrosis factor (TNF)-α for 3 days after treatment, although it is unclear if any toxicity ensued. Only IL-18-secreting CAR T-cells were detected in the blood and effectively shrank SCLC tumours over a period of approximately 15 days, resulting in prolonged survival compared to the control or IL-12 armoured DLL3 CAR T-cell group.
IL-18 armouring significantly increased liver-infiltrating CAR T cells by four-fold on day 3, primarily CD8+ cells, with enhanced activation shown by IFN-γ and/or TNF-α expression. This effect was observed in both CAR T cells and to a lesser extent in CAR- bystander T-cells, demonstrating the presence of epitope spreading. Moreover, secretion of IL-18 by the CAR T-cells enhanced the migration of tumour-specific CD4+ T-cells (on day 3) and CD8+ T-cells (on days 3 and 6).
To potentiate the efficacy of this approach, tumour-bearing mice received cyclophosphamide conditioning 1 day before a low dose of 0.5×106 armoured CAR T-cells. This led to a substantial increase in the anti-tumour response with some complete responses observed. Next, tumour re-challenge was performed in disease-free mice. Although complete responses were not achieved, investigators observed a delay in tumour outgrowth compared to treatment naïve mice. Importantly, when tumour re-challenge was undertaken with DLL3 knockout tumour cells, a small but significant delay in tumour outgrowth was also observed. Together, these data suggest that, not only had CAR T-cells persisted in the mice, but that bystander CAR− T-cells had also acquired anti-tumour activity. As previously reported (22), such epitope spreading is an important effect of IL-18-armoured CAR T-cells since it facilitates responses against tumour cells that do not express or have downregulated the CAR target.
Next, the authors focussed on the myeloid compartment within the SCLC TME. Analysis of CAR T-cell-treated mice revealed that IL-18 armouring had reprogrammed myeloid cells in the liver, steering them toward a more pro-inflammatory phenotype. Specifically, they found increased number of CD11b+ Gr1− macrophages and CD11c+ MHC-II+ DCs at that location. Furthermore, there was a decrease in the anti-inflammatory “M2-like” macrophages with a CD206+ MHC-IIlo phenotype. They also demonstrated elevated expression on both macrophages and DCs of the activation marker, CD86, consistent with localized activation of antigen-presenting cells in the liver.
Investigators next switched species to evaluate human IL-18 armoured CAR T-cells. In this context, they replaced the 4-1BB co-stimulatory domain with CD28 to prioritize robust initial activation and proliferation of CAR T-cells in a xenograft mouse model. As expected, IFN-γ production by these cells was increased and CAR T-cell proliferation was further enhanced when IL-18 armoured CAR T-cells were cultured with DLL3+ target cells. When tested in a range of SCLC xenograft models, IL-18-armoured CAR T-cells consistently demonstrated enhanced anti-tumour activity when compared to non-armoured counterparts. This was accompanied by increased CAR T-cell number at the site of disease, with CD8+ T cell skewing, enhanced activation, maintained memory marker expression and reduced exhaustion. To further enhance therapeutic efficacy, the investigators capitalized on the elevated levels of PD-L1 on tumour cells induced by IL-18. They combined a suboptimal dose of 0.3×106 IL-18-armoured CAR T-cells with an anti-PD-1 antibody infusion twice weekly in xenograft SCLC models, resulting in a further improvement in disease control.
Moreover, the persistent expression of DLL3 on tumour cells following treatment could suggest the possibility that repeated CAR T-cell infusion perhaps combined with PD-L1 blockade could benefit patients with residual disease or those experiencing relapse after initial CAR T-cell therapy. Combined DLL3 CAR T-cell therapy with PD-L1 blockade is further supported by the fact that anti-PD-L1 therapy has been approved for use in SCLC. However, careful consideration should be given to additional immune escape mechanisms, for example pertaining to the TME, or additional changes in tumour antigenic profile over time.
In conclusion, DLL3 is emerging as a highly attractive target for CAR T-cell immunotherapy of SCLC and the study of Jaspers et al. reinforces the tractability of this target. Ongoing CAR T-cell clinical trials directed against this target are listed in Table 1. It should also be noted that Novartis have recently licensed DLL3-specific CARs from Legend Biotech, signalling the involvement of large pharma in their future development (https://www.fiercebiotech.com/biotech/novartis-pays-legend-100m-upfront-give-solid-tumor-car-t-t-charge-treatment, accessed November 20th, 2023).
Table 1. Ongoing CAR T-cell clinical trials directed against DLL3 (https://www.clinicaltrials.gov/, assessed 20th November 2023).
| Disease | Sponsor | Notes | Identifier |
|---|---|---|---|
| SCLC | Tianjin Medical University Cancer Institute and Hospital | Multicentre phase I dose escalation study of CAR-NK cells | NCT05507593 |
| SCLC | Legend Biotech USA | Multicentre phase I CAR T-cell study | NCT05680922 |
| SCLC | Amgen | Single centre phase I CAR T-cell study: AMG 119. Listed as suspended on November 20th, 2023 | NCT03392064 |
CAR, chimeric antigen receptor; DLL3, delta-like ligand 3; SCLC, small-cell lung cancer; NK, natural killer.
Armouring strategies involving IL-18 appear to be more promising than predecessors based on IL-12, owing to greater safety while maintaining multifaceted beneficial actions on host cells, the TME and endogenous immune surveillance. Although there are conceptual safety concerns arising from autocrine IL-18 stimulation of CAR T-cells, these have not yet materialised in clinical studies involving this technology (25). Nonetheless, development of technologies that incorporate inducible IL-18 production or which restrict activity of this pro-inflammatory cytokine to the TME could provide an additional safety margin for this approach, allowing for more aggressive dosing regimens (20).
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-793/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-793/coif). C.M.H. and R.M. are employees of Leucid Bio Ltd. J.M. is the founder, Chief Scientific Officer and shareholder of Leucid Bio Ltd. The authors have no other conflicts of interest to declare.
References
- 1.Maher J. Chimeric Antigen Receptor (CAR) T-Cell Therapy for Patients with Lung Cancer: Current Perspectives. Onco Targets Ther 2023;16:515-32. 10.2147/OTT.S341179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021;7:3. 10.1038/s41572-020-00235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022;7:100408. 10.1016/j.esmoop.2022.100408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ready NE, Ott PA, Hellmann MD, et al. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J Thorac Oncol 2020;15:426-35. 10.1016/j.jtho.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 5.Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. 10.1001/jamaoncol.2018.3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac Oncol 2020;15:618-27. [DOI] [PubMed] [Google Scholar]
- 7.Doyle A, Martin WJ, Funa K, et al. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med 1985;161:1135-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart CA, Gay CM, Xi Y, et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat Cancer 2020;1:423-36. 10.1038/s43018-019-0020-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian Y, Zhai X, Han A, et al. Potential immune escape mechanisms underlying the distinct clinical outcome of immune checkpoint blockades in small cell lung cancer. J Hematol Oncol 2019;12:67. 10.1186/s13045-019-0753-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136. 10.1126/scitranslmed.aac9459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson ML, Zvirbule Z, Laktionov K, et al. Rovalpituzumab Tesirine as a Maintenance Therapy After First-Line Platinum-Based Chemotherapy in Patients With Extensive-Stage-SCLC: Results From the Phase 3 MERU Study. J Thorac Oncol 2021;16:1570-81. 10.1016/j.jtho.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 12.Giffin MJ, Cooke K, Lobenhofer EK, et al. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin Cancer Res 2021;27:1526-37. 10.1158/1078-0432.CCR-20-2845 [DOI] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Champiat S, Lai WV, et al. Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. J Clin Oncol 2023;41:2893-903. 10.1200/JCO.22.02823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou D, Byers LA, Sable B, et al. Clinical Pharmacology Profile of AMG 119, the First Chimeric Antigen Receptor T (CAR-T) Cell Therapy Targeting Delta-Like Ligand 3 (DLL3), in Patients with Relapsed/Refractory Small Cell Lung Cancer (SCLC). J Clin Pharmacol 2024;64:362-70. 10.1002/jcph.2346 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Tacheva-Grigorova SK, Sutton J, et al. Allogeneic CAR T Cells Targeting DLL3 Are Efficacious and Safe in Preclinical Models of Small Cell Lung Cancer. Clin Cancer Res 2023;29:971-85. 10.1158/1078-0432.CCR-22-2293 [DOI] [PubMed] [Google Scholar]
- 16.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol 2020;17:147-67. 10.1038/s41571-019-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaspers JE, Khan JF, Godfrey WD, et al. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J Clin Invest 2023;133:e166028. 10.1172/JCI166028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markowitz GJ, Yang P, Fu J, et al. Inflammation-Dependent IL18 Signaling Restricts Hepatocellular Carcinoma Growth by Enhancing the Accumulation and Activity of Tumor-Infiltrating Lymphocytes. Cancer Res 2016;76:2394-405. 10.1158/0008-5472.CAN-15-1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong JL, Berk E, Edwards RP, et al. IL-18-primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment. Cancer Res 2013;73:4653-62. 10.1158/0008-5472.CAN-12-4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chmielewski M, Abken H. CAR T Cells Releasing IL-18 Convert to T-Bet(high) FoxO1(low) Effectors that Exhibit Augmented Activity against Advanced Solid Tumors. Cell Rep 2017;21:3205-19. 10.1016/j.celrep.2017.11.063 [DOI] [PubMed] [Google Scholar]
- 21.Hu B, Ren J, Luo Y, et al. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep 2017;20:3025-33. 10.1016/j.celrep.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avanzi MP, Yeku O, Li X, et al. Engineered Tumor-Targeted T Cells Mediate Enhanced Anti-Tumor Efficacy Both Directly and through Activation of the Endogenous Immune System. Cell Rep 2018;23:2130-41. 10.1016/j.celrep.2018.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canna SW, de Jesus AA, Gouni S, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 2014;46:1140-6. 10.1038/ng.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bechman N, Maher J. Lymphodepletion strategies to potentiate adoptive T-cell immunotherapy - what are we doing; where are we going? Expert Opin Biol Ther 2021;21:627-37. 10.1080/14712598.2021.1857361 [DOI] [PubMed] [Google Scholar]
- 25.Svoboda J, Gerson JN, Landsburg DJ, et al. Interleukin-18 secreting autologous anti-CD19 CAR T-cells (huCART19-IL18) in patients with non-Hodgkin lymphomas relapsed or refractory to prior CAR T-cell therapy. Blood 2022;140:4612-4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as