Abstract
Introduction
Highly active antiretroviral therapy (HAART) was piloted in 2002 and was scaled up in 2003 in mainland China. The aim of this study was to evaluate the mortality and its possible predictors based on the long-term initial antiretroviral therapy (ART) cohort among HIV positive children and adolescents.
Methods
This prospective open-labeled multicenter cohort study was conducted from January 2008 to July 2021. The participants were recruited from six representative sites in mainland China. A total of 609 participants with an HIV-positive serostatus and <18 years old were recruited and each participant was informed consent at the time of enrollment. Mortality and annual hazard were calculated, and predictors for death were analyzed using Cox regression models generating hazard ratios (HR).
Results
The results showed that the mortality was 0.721 per hundred person-years, and the annual hazard was less than 0.10 over time. Both CD4+T cell count and CD4+T cell percentage declined in the death group during the follow-up. The Cox regression model showed that the baseline low CD4+T cell count level (Low vs. High: aHR = 8.309, 95% CI: (1.093, 63.135)) and age >5 years old at HIV diagnosis (6–12 vs. 0–5: aHR = 3.140, 95%CI: (1.331, 27.411)); 13–18 vs. 0–5: aHR = 5.451, 95%CI: (1.434, 20.724)) were possible risk factors for death.
Conclusion
The longitudinal cohort study demonstrated the efficacy of China's ART program among HIV-positive children and adolescents which could be beneficial to other countries with limited resources.
Keywords: HIV, Children, Adolescents, Cohort study, Mortality
1. Introduction
Since 2010, new HIV infections among children have declined by 58%, from 310 000 (210 000–490 000) in 2010 to 130 000 (90 000–210 000) in 2022. In 2022, 77% (65–90%) of adults aged 15 years and older living with HIV had access to treatment, as 57% (44–78%) of children aged 0–14 years [1]. The introduction of ART decreased pediatric HIV mortality in developed countries to below 1 per 100 child years [2,3]. An Institution-based retrospective cohort study in Northwest Ethiopia from 2014 to 2018 showed that a total of 251 HIV-positive children on ART were followed up for a total of 60 months, the overall mortality incidence rate in the cohort during the 626 Child-Year-Observation (CYO) was 2.56/100 CYO [4]. Another study on HIV-infected children based on the Thailand Pediatric HIV Observational Database reported that of the 1139 children, the death rate was 1.3 (95% confidence interval (CI) 1.1–1.6) from 2008 to March 2011 [5]. A long-term retrospective cohort analysis of patients aged <12 years at ART initiation in sub-Saharan Africa between 2004 and 2009, and results showed that in 2306 patients with an average follow-up time on ART of 2.3 years (interquartile range 1.5–3.1 years), the overall mortality rate was 2.25 deaths/100 person-years (95% confidence interval CI: 1.84–2.71) [6]. Data on the efficacy of pediatric antiretroviral regimens in the long-term treatment especially for follow-up more than five years of HIV-positive children were limited in China. So, it was necessary to assess the long-term effectiveness, especially on mortality of antiretroviral therapy in children living with HIV.
The implementation of the “Four Free and One Care” policy began in 2003 in China. Declines in mortality and improvements in prognosis in HIV-1-infected adults have been observed as a consequence of the increasing use of highly active antiretroviral therapy (HAART). The national pediatric ART program was launched in the 6 Chinese provinces with the highest HIV prevalence (Henan, Anhui, Hubei, Yunnan, Shanxi, and Guangxi) in 2005. Until 2010, the pediatric ART program expanded to 28 provinces and autonomous regions [7].
Due to the availability of ART drugs being limited for -children in China, a small portion of HIV-positive children were treated using adult formulations by splitting pills. Recommended first-line treatment regimen was zidovudine (AZT) or stavudine (D4T) plus lamivudine (3 TC) plus nevirapine (NVP) or efavirenz (EFV). NVP was preferred for children <3 years or <10 kg. Free CD4+ T cell count and CD4% testing were provided every 6 months for all pediatric ART patients, and follow-up visits were conducted every 3 months [8].
In summary, with the introduction of HAART, it was important to evaluate the survival observed in children after using ART and what factors were associated with the risk for mortality in China.
2. Materials and methods
2.1. Study setting
This study was a prospective open-labeled multicenter cohort study to explore the mortality and annual hazard of initial antiretroviral therapy among HIV-positive children from January 2008 to July 2021 in six representative sites in mainland China. The study sites included the Yunnan Provincial Hospital of Infectious Disease, the Guangxi Zhuang Autonomous Region Center for Disease Control and Prevention, the Shangcai Center for Disease Control and Prevention of Henan Province, The Sixth People's Hospital of Xinjiang Uygur Autonomous Region, The Sixth People's Hospital of Zhengzhou, Guangzhou Eighth People's Hospital, The Second People's Hospital of Yining. The recruitment time was from Jan 2008 to Aug 2011 and from Jan 2013 to Aug 2014, and the follow-up period lasted from April 2008 to July 2021.
2.2. Participants and data collection
Participants were recruited by the following inclusion criteria: (1) having an HIV-positive serostatus. (2) being <18 years old when first identified as HIV positive. (3) initiated standard Chinese first-line regimen followed by the Chinese National ART guidelines from the three original drug classes [8,9]. The ART regimens combined with two nucleotide reverse transcriptase inhibitors (NRTIs) plus one non-NRTIs (NNRTIs) or one protease inhibitors (PIs). The specific antiretroviral regimens combination was (d4T/AZT/ABC) +3TC+(NVP/EFV/LPV/r) *. (see details in Supplement materials) (4) signed informed consent voluntarily by children's parents or other guardians at the time of enrollment.
The epidemiological and demographic characteristics (Age, gender, WHO clinical stages, disclosure, ART sites, infection route, ethnicity, and ART regimens) were obtained from a web-based clinical data management system. The HIV-1 viral load in plasma was quantified using a Standard Amplicor HIV Monitor assay, version 1.5 (Roche Diagnostics, Indianapolis, IN, USA), with a limit of detection of 40 copies/ml. The CD4+ T cell count was measured by a standard flow cytometry technique with a TruCOUNT tube in routinely equipped laboratories (BD Biosciences, San Jose, CA, USA).
2.3. Definitions
According to the UNAIDS guidelines and CDC recommendations, the participants were divided into three age groups (0–5 years old, 6–12 years old, and 13–18 years old) [10,11]. The participants who only had the baseline viral load were defined as a loss to follow-up. The primary outcome was all-cause mortality during the follow-up. The virological failure was defined as having more than twice HIV-1 viral load (VL) testing data and two consecutive VL > 200 copies/mL after at least 6 months of ART. CD4+ T cell counts were set into three level groups based on the CDC recommendations, including Low, Medium, and High [10] (see details in Table 1).
Table 1.
Demographic information on HIV + children who initial ART [n (%); M (Q25, Q75)].
| Variables | Social information | Variables | Clinical information |
|---|---|---|---|
| Total | 592 | ||
| Age at diagnosis (years old) | 4.44(2.50,6.66) | WHO clinical stages | |
| ∼5 | 340 (58.22) | I | 239 (40.37) |
| ∼12 | 217 (37.16) | II | 97 (16.39) |
| ∼18 | 27 (4.62) | III | 196 (33.11) |
| Age at ART initiation (years old) | 6.31(4.20,9.26) | IV | 60 (10.14) |
| ∼5 | 198 (33.45) | ART regimens | |
| ∼12 | 331 (55.91) | AZT+3TC + LPV/r | 253 (42.74) |
| ∼18 | 63 (10.64) | D4T+3TC + LPV/r | 16 (2.70) |
| Ethnicity | AZT+3TC + EFV/NVP | 174 (29.39) | |
| Han | 431 (72.80) | D4T+3TC + EFV/NVP | 26 (4.39) |
| Others | 161 (27.20) | ABC+3TC + LPV/r | 123 (20.78) |
| Gender | Baseline CD4+T cell count levela | ||
| Male | 332 (56.08) | Low | 219 (37.69) |
| Female | 260 (43.92) | Medium | 228 (39.07) |
| ART Sites | High | 135 (23.23) | |
| CDC-based | 181 (30.58) | Baseline CD4+T cell percentage (%) | |
| Hospital-based | 411 (69.42) | <15 | 172 (29.05) |
| Disclosure | ≥15 | 420 (70.95) | |
| Yes | 113 (19.09) | Baseline VL (copies/ml) | |
| No | 479 (80.91) | <1000 | 39 (6.59) |
| Infection route | <100000 | 160 (27.03) | |
| Maternal-infant | 561 (94.76) | ≥100000 | 393 (66.39) |
| Others (blood/sexual/others) | 31 (5.24) | Period of infection to art (days) | 235.0 (34.0, 1141.0) |
| ≤14 | 69 (12.17) | ||
| >14 | 498 (87.83) |
| Level | Age at ART initiation |
|
|---|---|---|
| ≤ 5 years old | >5 years old | |
| Low | <500 | <200 |
| Medium | 500–1000 | 200–500 |
| High | >1000 | >500 |
CD4+T cell count level definition.
Ethical statement
This study was approved by the ethics committee of Beijing Ditan Hospital of Capital Medical University (Approval number: 2019-037-002). All participants or their legal guardians were informed consent at the time of enrolment.
2.4. Statistical analysis
Statistical analysis was performed by the “survival” package and “ggplot2” package from R studio 4.2.1 and plots were also produced by Prism 9.1.1. Data were presented as absolute and relative frequencies. All-cause mortality and annual hazard were calculated, and risk factors for death were analyzed using univariate and multivariate stepwise Cox regression models generating hazard ratios (HR). The 95% confidence interval (CI) was used to estimate the precision of the HR. Statistical significance was defined as a two-tailed P value of <0.05.
*:
①d4T: Stavudine; ②AZT: Zidovudine; ③ABC: Abacavir; ④3TC: Lamivudine; ⑤NVP: Nevirapine;
⑥EFV: Efavirenz; ⑦LPV/r: Lopinavir/Ritonavir.
Dosage:
ABC: 8 mg/kg, twice a day (maximum 300 mg each time)
AZT:180∼240 mg/m body surface area, once every 12 h (for infants over 6 weeks old)
3TC:4 mg/kg twice daily (for infants over 30 days old)
NVP:160∼200 mg/m body surface area, once a day for 14 days, and then once every 12 h.
EFV:15 mg/kg once daily before going to bed (EFV is not intended for children weighing less than 10 kg or younger than 3 years of age)
In pediatric who are using d4T, the dose is 1 mg/kg, once every 12 h (the maximum cannot exceed 30mg/time), but d4T should be gradually replaced with AZT or ABC.
3. Results
3.1. Demographic information
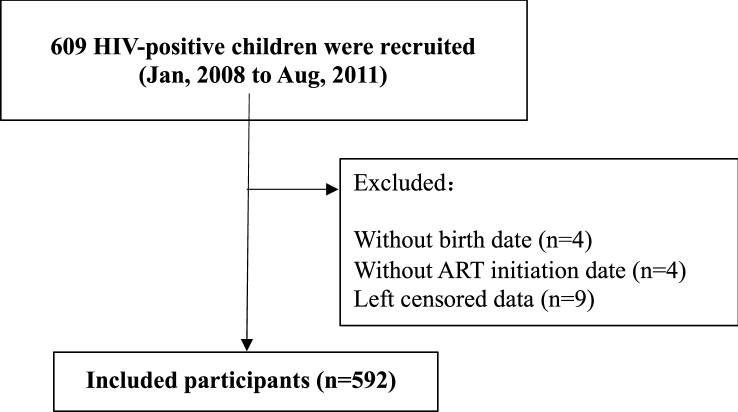
The study recruited 609 participants and included 592 in the study ultimately (Fig. 1 displayed the flow chart of the study). Among the participants, both genders occupied nearly one-half (Males occupied 56.08%). The 561 (94.76%) participants were transmitted with maternal-infant. The median age at HIV diagnosis was 4.44 (2.50, 6.66) years old, and the median age at ART initiation was 6.31 (4.20, 9.26) years old. The WHO clinical stage I occupied 40.37% at baseline. 66.39% with the viral load ≥100000 copies/ml, and the Low CD4+T cell counted 37.69% in the baseline. The median period from diagnosis to ART initiation was 235.0 (34.0, 1141.0) days, and the period >14 days occupied 87.83% (Table 1).
Fig. 1.
Flowchart of the study.
3.2. Mortality and hazard
The median follow-up time was 6.22 (2.07–8.99) person-years in total participants, and the longest follow-up time was 13.43 person-years. The results showed that the total loss to follow-up percentage was 17.90% (106/592). There were 26 deaths during the follow-up.
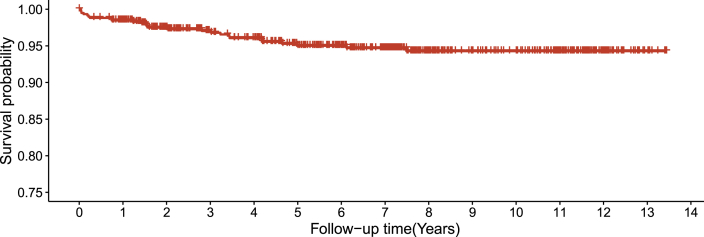
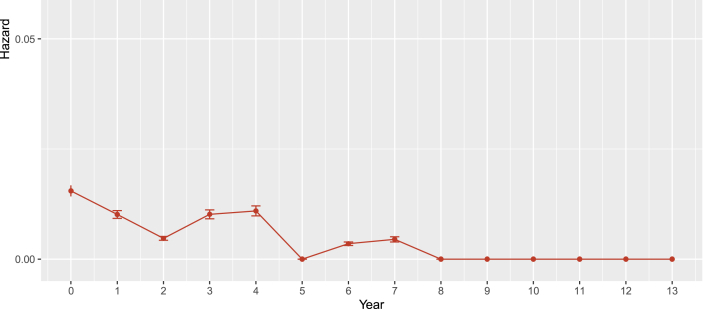
The mortality was 0.721 (0.443, 0.998) per hundred person-years, and the proportion of death was 4.39% (26/592). Eleven of 26 deaths experienced virological failure, and seven of them with >100000 baseline viral load. Fig. 2, Fig. 3 displayed the survival probability and annual hazard, which showed that the HIV-positive children who were on ART had above 0.90 survival probability over time. The annual hazard of death was greatest at an early stage of ART initiation and decreased gradually.
Fig. 2.
Survival analysis among children living with HIV.
Fig. 3.
Annual hazard of mortality among children living with HIV.
3.3. Risk factors
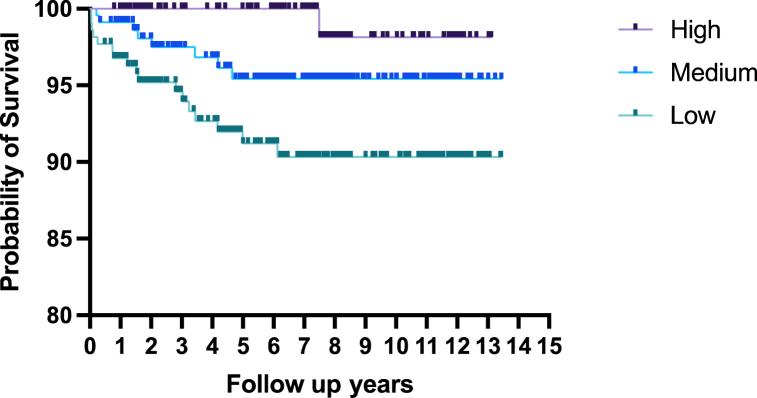
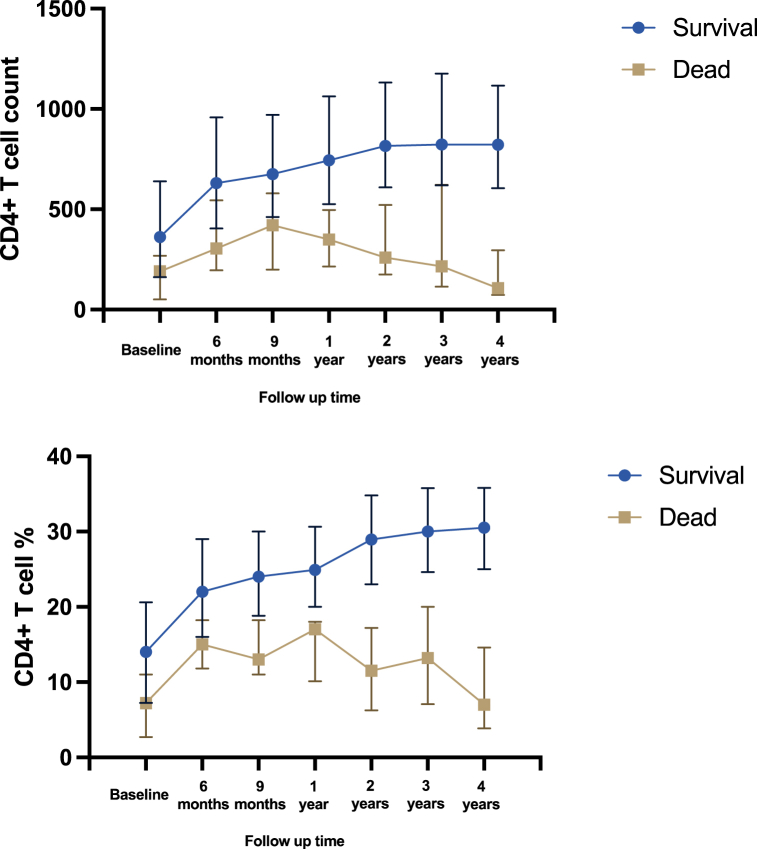
For the 13-year follow-up overall, the differences in total mortality were found between the different groups from baseline CD4+ T cell level (Low vs. High: aHR = 8.309, 95%CI: (1.093, 63.135)). There was a link between baseline lower CD4+T cell levels and mortality. The cumulative hazard of death through the entire follow-up period in different baseline CD4+T cell levels was shown in Fig. 4. During the follow-up, there was a short-term increase in CD4+T cell count and then a gradual decline to a lower level in the death group, while the survival group increased gradually after ART (Fig. 5).
Fig. 4.
Cumulative hazard of mortality among children living with HIV in different CD4+ T cell levels.
Fig. 5.
CD4+ T cell count/CD4+ T cell % of different status among children living with HIV during the follow up.
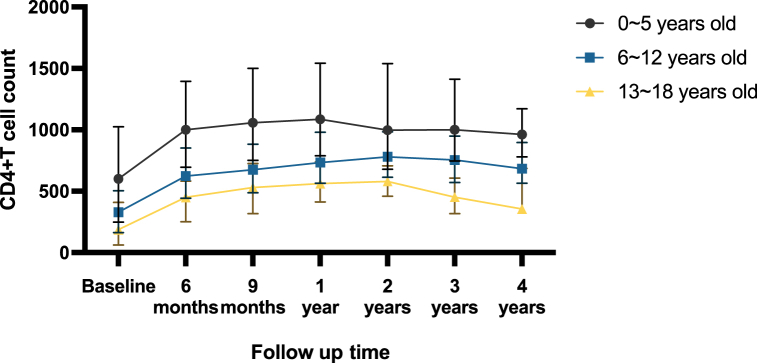
The differences in total mortality were also found between the different age groups at HIV diagnosis (6–12 vs. 0–5: aHR = 3.140, 95%CI: (1.331, 27.411)); 13–18 vs. 0–5: aHR = 5.451, 95%CI: (1.434, 20.724)). Age >5 years old when diagnosed with HIV was associated with mortality. At the same time, Longitudinal trends of CD4+ T cell levels among different age groups at ART initiation over the follow-up period showed a higher hazard of death among adolescents especially 13–18 years old (Fig. 6).
Fig. 6.
CD4+ T cell count by different age group at ART initiation among children living with HIV during the follow up.
Adjusted hazard ratios (95% CI) for baseline predictors of total deaths were shown in Table 2.
Table 2.
Risk baseline factors for mortality analyzed by Cox regression model.
| Variable | Mortality (95%CI)) | HR (95% CI) | P | aHR (95% CI) | P |
|---|---|---|---|---|---|
| Age at diagnosis (years old) | |||||
| ∼5 | 0.045(0.110, 0.608) | 1.0 | 1.0 | ||
| ∼12 | 0.081(0.600, 1.831) | 3.070 (1.301, 7.245) | 0.010 | 3.140 (1.331, 7.411) | 0.009 |
| ∼18 | 0.944(-0.373, 6.037) | 5.528 (1.454, 21.012) | 0.012 | 5.451 (1.434, 20.724) | 0.013 |
| Age at ART initiation (years old) | |||||
| ∼5 | 0.072(0.087, 0.782) | 1.0 | |||
| ∼12 | 0.051(0.304, 1.027) | 1.338 (0.508, 3.522) | 0.555 | ||
| ∼18 | 0.367(0.666, 4.476) | 4.139 (1.377, 12.441) | 0.011 | ||
| ART site | |||||
| CDC based | 0.072(0.322, 1.253) | 1.0 | |||
| Hospital based | 0.045(0.335, 1.022) | 0.691 (0.317, 1.508) | 0.354 | ||
| Baseline Disclosure | |||||
| Yes | 0.185(0.831, 3.231) | 3.502 (1.605, 7.642) | 0.002 | ||
| No | 0.033(0.217, 0.696) | 1.0 | |||
| WHO clinical stages | |||||
| I | 0.077(0.048, 0.727) | 1.0 | |||
| II | 0.179(0.014, 1.416) | 1.911 (0.513, 7.118) | 0.334 | ||
| III | 0.073(0.276, 1.177) | 2.193 (0.749, 6.419) | 0.152 | ||
| IV | 0.262(0.475, 3.19) | 5.490 (1.742, 17.304) | 0.003 | ||
| ART regimens | |||||
| AZT+3TC + LPV/r | 0.070(0.265, 1.131) | 1.0 | |||
| D4T+3TC + LPV/r | 1.017(-0.785, 4.851) | 3.532 (0.774, 16.127) | 0.103 | ||
| AZT+3TC + EFV/NVP | 0.076(0.188, 1.035) | 1.034 (0.408, 2.624) | 0.943 | ||
| D4T+3TC + EFV/NVP | 0.458(-0.44, 1.356) | 0.857 (0.109, 6.701) | 0.883 | ||
| ABC+3TC + LPV/r | 0.182(0.112, 1.708) | 1.139 (0.389, 3.335) | 0.813 | ||
| Baseline CD4+T cell count | |||||
| Low | 0.121(-0.116, 0.358) | 10.964 (1.459, 82.390) | 0.020 | 8.309 (1.093, 63.135) | 0.041 |
| Medium | 0.075(0.185, 1.018) | 4.977 (0.622, 39.800) | 0.130 | 4.146 (0.416, 33.318) | 0.181 |
| High | 0.074(0.659, 1.854) | 1.0 | 1.0 | ||
| Baseline CD4+T cell percentage (%) | |||||
| <15 | 0.090(0.404, 1.57) | 1.0 | |||
| ≥15 | 0.040(0.297, 0.906) | 0.574 (0.264, 1.250) | 0.162 | ||
| Baseline VL (copies/ml) | |||||
| <1000 | 0.433(-0.171, 2.768) | 1.0 | |||
| <100000 | 0.105(0.126, 1.132) | 0.499 (0. 124, 1.997) | 0.326 | ||
| ≥100000 | 0.041(0.368, 1.035) | 2.941 (0.171, 1.994) | 0.391 | ||
| Period from diagnosis to ART (days) | |||||
| ≤14 | 0.226(0.140, 2.121) | 1.0 | |||
| >14 | 0.033(0.376, 0.963) | 0.567 (0.212, 1.512) | 0.257 | ||
4. Discussion
Antiretroviral therapy for HIV-positive children and adolescents faced severe resource limitations in China. The implementation of the policy of antiviral treatment among HIV-positive children began in 2005 in China, and the choice of antiviral drugs for children was limited, which still lag behind developed countries.
Our study was a long-term cohort study of children living with HIV who initiated ART in China, aiming to know the survival outcome of antiretroviral therapy. The mortality rate in this study was less than 1 per 100 person-years, and the hazard of death was less than 0.05 during the follow-up. Previous studies on the mortality of children on ART ranged from 1.8 to 2.3 per 100 person-years in Asia and South America [[12], [13], [14]]. A Retrospective cohort study with a total of 221 children who were initiated on HAART from 2005 to 2009 and followed up until 2013 conducted in Cameroon showed that 9.9% of children (n = 22) died over a follow-up period of 755 child-years (mortality of 2.9 per 100 child-years) [15]. A nationwide cohort study in 2010 in China of HIV-positive pediatrics found that mortality was greatest within the first 6 months of treatment (7.5 per 100 person-years) and that by 12 months, it had stabilized to a new level (approximately 1.0 per 100 person-year) [7].
CD4+ T cell count, WHO clinical stage, which reflected the level of immunity, and age at diagnosis or initial treatment may affect the survival outcome. Previous studies showed a significant reduction in mortality with early ART initiation, within the first 12 weeks of life, accompanied with a greater than 90% probability of survival into adulthood [16]. In our study, the age at ART initiation did not show statistical significance for the mortality outcome. The possible reason was that the children or adolescents included in our study were those who survived despite not initiating ART within one year of birth. One retrospective cohort analysis of 2224 children who were registered with the government ART centers in Mumbai from 2004 to 2019 showed that the hazard ratio for mortality was higher in children who registered with the ART center in the first year of life compared with those who in children older than 1 year of age [14].
The CD4+ T cell level in our study was divided according to the standards of UNAIDS and USA CDC. The recovery of immune level was judged by CD4+ T cell count and percentage. The standard suggested that the CD4+ T cell count took precedence over the CD4+ T cell percentage, and the percentage was considered only if the count was missing. The results showed that the initial treatment baseline CD4+ T cell level had an impact on the survival outcome, and low CD4+ T cell led to high mortality. Among those whose outcome was death, it showed that CD4+ T cell level rose after a period of antiviral treatment but eventually gradually declined to a lower level and led to death. Our study findings were consistent with previous studies. One study on baseline characteristics and association with early mortality among HIV-positive patients in Nigeria showed that the factors associated with early mortality included male sex, HIV encephalopathy, and low CD4+T cell count (<50 cells) [17]. Another study on 12-month mortality in antiretroviral-treated children based on the West African Database from 2000 to 2008 also reported mortality was associated with advanced clinical stage, CD4 percentage <15% at ART initiation and year (>2005) of ART initiation [18].
It should be noted that in our cohort, many participants were in the late WHO clinical stage or had low CD4 levels at the time of initiation of treatment, which was associated with a high risk of death. The advanced disease stage increased mortality risk has also been reported in many countries in Africa. In one study conducted in Asia from 2001 to 2016, it found that the WHO stage 3 or 4 was associated with higher mortality [19]. Until 2017, WHO recommended that all people living with HIV should be offered rapid initiation of ART, defined as within seven days of a positive HIV diagnosis, provided there were no contraindications [20].
Our results also showed that diagnosed HIV positive between 12 and 18 years old have a higher hazard of death. Some previous studies have also focused on the survival of HIV-positive children in different age groups. A retrospective cohort study was carried out in eight health facilities in two regions of Ethiorom between 2005 and 2013. They categorized participants as adolescents (ages 10–14 and 15–19) and children. The results showed that of the 2058 participants studied, mortality hazard was significantly higher among younger adolescents (aHR = 2.8 [1.4, 5.4]) and older adolescents (aHR = 2.3 [1.1, 4.9]) compared with children [21]. The possible explanation for our study was that the group aged 12–18 in this study had a low CD4+ T cell level when diagnosed with HIV, so the lower immune level caused a higher death hazard. Such a low immunity status at the time of diagnosis suggested that children in this age group were being delayed detection and treatment. A study in China from 2011 to 2015 showed that the proportion of late diagnosis in all counted cases was 41.7% (2949/7073) [22]. At the same time, the sample size for the 12–18 age group was small, which led to a higher proportion of death and may impact the study results. Another important possible reason may be related to medication adherence. Adolescents may not take their medication on time because they were rebellious as they enter adolescence or because of the inconvenience of going to school. A longitudinal retrospective study in low-resource settings made use of routinely collected data for all 27 229 clinic adults and pediatric patients from 2008 to Feb 2017. The study compared rates of virological suppression, adherence, and defaulting among children, adolescents, and adults. The results showed that younger and older adolescents (ages 10–14 years and 15–19 years respectively) were less likely to achieve virological suppression compared to adults, and young children (ages 0–4 years), older children (ages 5–9 years), and younger adolescents were less adherent to ART compared to adults respectively [23].
This study also reported that HIV disclosure was associated with higher mortality in the univariable analysis, which was inconsistent with previous studies. Some studies reported disclosure improved retention in care and reduced mortality in HIV-positive children by disclosing at school age [24,25]. The possible reason was that age between 12 and 18 group had a higher proportion of disclosure than other age groups in our study, while age between 12 and 18 in our study tended to be in the late stage of HIV or in the lower immune level, which might result in a higher hazard of death.
4.1. Limitations
This study was subject to limitations. The observed 17.90% loss to follow-up could potentially lead to an underestimation of the total deaths, but given the extensive 13-year observation period, some loss to follow-up was inevitable. On the other hand, the deaths counted for mortality in this study were all-cause deaths that might not have been entirely due to factors related to HIV infection or treatment, such as accidents. In addition, there was a certain bias in the population included in this study. This population experienced a long time from diagnosis to ART initiation and survived an early period without antiviral treatment. At the same time, the risk factors analysis merely focused on the baseline characteristics effects on mortality, which might be incomprehensive. Nevertheless, the present study underscores the importance for researchers to pay attention to the impact of baseline characteristics on death outcomes and emphasize the urgency of initiating rapid treatment.
5. Conclusion
This study was a prospective longitudinal cohort, based on the survival analysis showed a decline in mortality, which demonstrated the efficacy of China's ART program among HIV-positive children and adolescents. The risk factors for mortality included baseline low CD4+ T Cell count level and age >5 years old when diagnosed with HIV.
These findings offered valuable insights that could be beneficial to other countries with limited resources. Emphasizing the enhancement of immune levels and prioritizing early ART for children emerges as a critical takeaway.
Declarations
The study protocol was reviewed and approved by the Institutional Ethics Committee at the Beijing Ditan Hospital Capital Medical University in China (Approval number: 2019-037-002). Participants or their legal guardians were informed consent at the time of enrolment.
Funding
This study was supported by the Thirteen-Fifth Key Project, Ministry of Science and Technology of China (2018ZX10302-102); Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20191802); Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX202126); John C. Martin Foundation(2017-G14).
Data availability statement
The data associated with this study has not been deposited into a publicly available repository.
The authors are unable or have chosen not to specify which data has been used.
CRediT authorship contribution statement
Hanxi Zhang: Writing – review & editing, Writing – original draft, Methodology, Formal analysis. Xiaojie Lao: Writing – review & editing, Methodology. Huiqin Li: Investigation. Hongyan Lu: Investigation. Yuewu Cheng: Investigation. Yuxia Song: Investigation. Qingxia Zhao: Investigation. Jinfeng Chen: Investigation. Fuxiu Ye: Investigation. Hongxin Zhao: Writing – review & editing. Fujie Zhang: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge all physicians and laboratory staff of each research center who have been involved in clinical data collection, laboratory testing and treatment for HIV. We also thank Yao Wang, Fangning Chen, for managing the database and assisting in the transport of specimens.
References
- 1.UNAIDS . _FactSheet_en. 2023. https://www.unaids.org/en/resources/fact-sheet [Google Scholar]
- 2.Judd A., Doerholt K., Tookey P.A., Sharland M., Riordan A., Menson E., Novelli V., Lyall E.G., Masters J., Tudor-Williams G., Duong T., Gibb D.M., Collaborative HIV Paediatric Study (CHIPS), & National Study of HIV in Pregnancy and Childhood (NSHPC) Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996-2006: planning for teenage and adult care. Clin. Infect. Dis. : an official publication of the Infectious Diseases Society of America. 2007;45(7):918–924. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 3.Brady M.T., Oleske J.M., Williams P.L., Elgie C., Mofenson L.M., Dankner W.M., Van Dyke R.B., Pediatric AIDS Clinical Trials Group219/219C Team Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. Journal of acquired immune deficiency syndromes (1999) 2010;53(1):86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biyazin Y., Wondwossen K., Wubie A.B., Getachew M., Gebremichael B. Survival and predictors of mortality among HIV-positive children on antiretroviral therapy in public hospitals. Journal of pharmaceutical policy and practice. 2022;15(1):48. doi: 10.1186/s40545-022-00448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phongsamart W., Hansudewechakul R., Bunupuradah T., Klinbuayaem V., Teeraananchai S., Prasithsirikul W., Kerr S.J., Akarathum N., Denjunta S., Ananworanich J., Chokephaibulkit K. Long-term outcomes of HIV-infected children in Thailand: the Thailand pediatric HIV observational database. Int. J. Infect. Dis.: IJID: official publication of the International Society for Infectious Diseases. 2014;22:19–24. doi: 10.1016/j.ijid.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Kabue M.M., Buck W.C., Wanless S.R., Cox C.M., McCollum E.D., Caviness A.C., Ahmed S., Kim M.H., Thahane L., Devlin A., Kochelani D., Kazembe P.N., Calles N.R., Mizwa M.B., Schutze G.E., Kline M.W. Mortality and clinical outcomes in HIV-infected children on antiretroviral therapy in Malawi, Lesotho, and Swaziland. Pediatrics. 2012;130(3):e591–e599. doi: 10.1542/peds.2011-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y., Li C., Sun X., Mu W., McGoogan J.M., He Y., Cheng Y., Tang Z., Li H., Ni M., Ma Y., Chen R.Y., Liu Z., Zhang F. Mortality and treatment outcomes of China's National Pediatric antiretroviral therapy program. Clin. Infect. Dis. 2013 Mar;56(5):735–744. doi: 10.1093/cid/cis941. Epub 2012 Nov 21. PMID: 23175558; PMCID: PMC3657487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinese National ART Guidelines (The Second Version) People's Medical Publishing House; 2008. [Google Scholar]
- 9.Chinese National ART Guidelines (The Third Version) People's Medical Publishing House; 2012. [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Revised surveillance case definition for HIV infection--United States, 2014. MMWR. Recommendations and reports: morbidity and mortality weekly report. Recommendations and reports. 2014;63(RR-03):1–10. [PubMed] [Google Scholar]
- 11.Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/adolescents-and-young-adults-hiv
- 12.Nguyen R.N., Ton Q.C., Luong M.H., Le L.H.L. Long-term outcomes and risk factors for mortality in a cohort of HIV-infected children receiving antiretroviral therapy in vietnam. HIV AIDS (Auckl) 2020;12:779–787. doi: 10.2147/HIV.S284868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teeraananchai S., Kerr S.J., Puthanakit T., et al. Attrition and mortality of children receiving antiretroviral treatment through the universal coverage health program in Thailand. J. Pediatr. 2017;188:210–216.e1. doi: 10.1016/j.jpeds.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Acharya S., Palkar A., Sayed A.P., Setia M.S. Retrospective cohort analysis of survival of children living with HIV/AIDS in Mumbai, India. BMJ Open. 2021;11(9) doi: 10.1136/bmjopen-2021-050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Njom Nlend A.E., Loussikila A.B. Predictors of mortality among HIV-infected children receiving highly active antiretroviral therapy. Med. Maladies Infect. 2017;47(1):32–37. doi: 10.1016/j.medmal.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Somi G., Majigo M., Manyahi J., Nondi J., Agricola J., Sambu V., Todd J., Rwebembera A., Makyao N., Ramadhani A., Matee M. Pediatric HIV care and treatment services in Tanzania: implications for survival. BMC Health Serv. Res. 2017;17(1):540. doi: 10.1186/s12913-017-2492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinyemi J.O., Adesina O.A., Kuti M.O., Ogunbosi B.O., Irabor A.E., Odaibo G.N., Olaleye D.O., Adewole I.F. Temporal distribution of baseline characteristics and association with early mortality among HIV-positive patients at University College Hospital, Ibadan, Nigeria. Afr. J. AIDS Res.: AJAR. 2015;14(3):201–207. doi: 10.2989/16085906.2015.1052526. [DOI] [PubMed] [Google Scholar]
- 18.Ekouevi D.K., Azondekon A., Dicko F., Malateste K., Touré P., Eboua F.T., Kouadio K., Renner L., Peterson K., Dabis F., Sy H.S., Leroy V., IeDEA pediatric West Africa Working Group pWADA 12-month mortality and loss-to-program in antiretroviral-treated children: the IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000-2008. BMC Publ. Health. 2011;11:519. doi: 10.1186/1471-2458-11-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartlett A.W., Truong K.H., Songtaweesin W.N., et al. Characteristics, mortality, and outcomes at transition for adolescents with perinatal HIV infection in Asia. AIDS. 2018;32(12):1689–1697. doi: 10.1097/QAD.0000000000001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. 2017 https://www.who.int/publications/i/item/WHO-HIV-2017.18 [PubMed] [Google Scholar]
- 21.Jerene D., Abebe W., Taye K., Ruff A., Hallstrom I. Adolescents living with HIV are at higher risk of death and loss to follow up from care: analysis of cohort data from eight health facilities in Ethiopia. PLoS One. 2019 Oct 17;14(10) doi: 10.1371/journal.pone.0223655. PMID: 31622391; PMCID: PMC6797201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin L., Cheng X.L., Qin Y.L., et al. Analysis on late diagnosis and related factors among newly diagnosed HIV/AIDS cases in Anhui province from 2011 to 2015. Chin. J. Prev. Med. 2018;52(4):415–418. doi: 10.3760/cma.j.issn.0253-9624.2018.04.015. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 23.Chakakala-Chaziya J., Patson N., Samuel V., Mbotwa J., Buonsenso D., Chisale M., Phiri E., O'Hare B. A comparison of clinical outcomes among people living with HIV of different age groups attending queen Elizabeth central hospital outpatient ART Clinic in Malawi. Front. Med. 2023;10 doi: 10.3389/fmed.2023.1175553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cluver L.D., Hodes R.J., Toska E., Kidia K.K., Orkin F.M., Sherr L., Meinck F. 'HIV is like a tsotsi. ARVs are your guns': associations between HIV-disclosure and adherence to antiretroviral treatment among adolescents in South Africa. AIDS (Lond.) 2015;29(Suppl 1):S57–S65. doi: 10.1097/QAD.0000000000000695. [DOI] [PubMed] [Google Scholar]
- 25.Ngeno B., Waruru A., Inwani I., Nganga L., Wangari E.N., Katana A., Gichangi A., Mwangi A., Mukui I., Rutherford G.W. Disclosure and clinical outcomes among young adolescents living with HIV in Kenya. J. Adolesc. Health: official publication of the Society for Adolescent Medicine. 2019;64(2):242–249. doi: 10.1016/j.jadohealth.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with this study has not been deposited into a publicly available repository.
The authors are unable or have chosen not to specify which data has been used.