Abstract
The effect of endogenous interleukin-12 (IL-12) on the influenza virus immune response in BALB/c mice was evaluated. Following primary influenza virus infection, IL-12 mRNA and protein are detected in the lung, with live virus being required for cytokine induction. Endogenous IL-12 contributes to early NK cell-dependent gamma interferon (IFN-γ) production (days 3 and 5) but not late T-cell-dependent IFN-γ secretion (day 7). IL-12 contributes to the inhibition of early virus replication but is not required for virus clearance. IL-12 also modestly contributes to the activation of cytotoxic T lymphocytes. Thus, in this model of experimental influenza virus infection, endogenous IL-12 contributes primarily to the early development and activation of the innate immune response.
Interleukin-12 (IL-12) is a 70-kDa heterodimeric cytokine composed of a 40-kDa heavy chain (p40) and a 35-kDa light chain (p35) (27, 53). IL-12 is produced by monocytes, macrophages, B cells, and dendritic cells primarily in response to bacteria, bacterial products, and intracellular parasites. IL-12 p40, as a monomer or homodimer, is secreted up to a thousandfold excess of the biologically active p70 heterodimer (9). The purpose for the excess secretion of IL-12 p40 is unclear, but recent reports have indicated that p40 homodimers can bind to the IL-12 receptor and inhibit the biological activities of IL-12 in human cells with low efficiency (28) and in mouse cells with much higher efficiency than in human cells (14, 33).
IL-12 is a strong inducer of gamma interferon (IFN-γ) alone or in synergy with other inducers, such as IL-2, phorbol diesters, and anti-CD3 antibodies (7, 27). Other biological activities include enhancement of proliferation (27, 40) and cytotoxicity of activated T and natural killer (NK) cells (27, 43). IL-12 has also been shown to be important in the development of type 1 T helper cells (19, 30, 31).
The correlation between IL-12 production or treatment and virus pathogenesis is still poorly understood, and relatively few published studies have addressed the antiviral activity of IL-12. Although the role of IL-12 in viral infections remains to be defined, its immunomodulatory properties may be important in initiating and/or maintaining an antiviral immune response. Based on evidence that IL-12 may be prominently involved in the host response in an infectious disease state, several investigators have begun to explore the role of IL-12 in viral infections.
The earliest indication that IL-12 might be involved in virus infections was a study in which IL-12 p40 mRNA expression had been determined in several viral infections in vivo including those by lactate dehydrogenase-elevating virus, mouse hepatitis virus, mouse adenovirus, and lymphocytic choriomeningitis virus (8). Herpes simplex virus (HSV) infection in the cornea was shown to induce IL-12 p40 mRNA expression and p40 protein in the cornea and draining lymph nodes within 24 h of infection (26). UV-inactivated virus induced IL-12 p40 mRNA and protein in the cornea, albeit at significantly lower levels than in live virus-infected mice. Culture of corneal cells from naïve mice that had been exposed to HSV in vitro did not result in the induction of IL-12 p40 mRNA. Thus, the cellular source of IL-12 is believed to be infiltrating cells, such as Langerhans cells (16), dendritic cells (26), and neutrophils (17), that have undergone an abortive HSV infection. IL-12 is not detectable during lymphocytic choriomeningitis virus infection, and neutralizing IL-12 antibody has no effect on enhanced NK cell-mediated cytotoxicity in this infection (36, 38). In contrast, murine cytomegalovirus transiently induces the production of IL-12, which is responsible for early NK cell-mediated IFN-γ production and contributes to viral clearance (35, 37). Administration of recombinant IL-12 (rIL-12) with inactivated pseudorabies virus protects against subsequent lethal challenge with infectious virus, possibly by facilitating the induction of neutralizing immunoglobulin G2a (IgG2a) antibodies (45). This effect of IL-12 is at least in part dependent on IFN-γ, as the protective effect of IL-12 is largely abrogated in IFN-γ-receptor knockout (IFN-γ R−/−) mice and administration of IFN-γ in wild-type mice mimics IL-12 activity. An IFN-γ-dependent mechanism is also responsible for the prophylactic effects of IL-12 in lethal encephalomyocarditis virus infection (39). Mice injected with IL-12 prior to infection with a lethal dose of encephalomyocarditis virus survive. However, IL-12 is unable to provide protection in IFN-γ R−/− mice. Thus, IL-12 modulates the immune response during pseudorabies and encephalomyocarditis virus infections through the production of IFN-γ.
Recent studies suggest that IL-12 promotes host recovery during a viral respiratory infection (48). BALB/c mice immunized with inactivated respiratory syncytial virus and rIL-12 have significantly reduced virus titer in lungs following virus challenge. Protection may be related to the production of neutralizing IgG2a antibodies. IL-12 also serves as an adjuvant during vaccination against vesicular stomatitis virus and enhances recovery following virus challenge (4).
In the present study, we analyzed IL-12 induction and involvement in the immune response to influenza virus infection. The immune response to respiratory infection in mice with influenza virus is particularly well characterized and offers a superb model for analyzing the role of IL-12 in the antiviral immune response. Following virus entry and infection in the lung, many cytokines are produced, including IFN-α, IFN-γ, tumor necrosis factor alpha (TNF-α), IL-6, granulocyte-macrophage colony-stimulating factor, and IL-1α and IL-1β (18). These cytokines contribute to the activation of virus-specific CD8+ T cells (1, 10, 29, 49) in the regional mediastinal lymph nodes which then travel via lymph to the lung and lyse virus-infected type 1 alveolar epithelial cells (10). Although cell-mediated immunity may be critical for virus clearance during a primary infection (1, 3), secondary infections are controlled by the production of neutralizing antibodies (2, 20, 21, 23–25, 42). In the present study, we examine the role of endogenous IL-12 in the immune response to primary influenza virus infection in the lung by evaluating cytokine production and generation of cytotoxic T cells (CTLs). In the first few days of infection, IL-12 is produced and contributes to the early IFN-γ production, partially derived from NK cells. Although this early response is important for the resistance to virus infection, as shown by high viral titer when endogenous IL-12 is neutralized, the subsequent production of cytokines and CTL activity later in the infection is independent of endogenous IL-12.
MATERIALS AND METHODS
Antibodies.
Monoclonal rat anti-mouse cytokine antibodies used were C17.8 (IgG2a) (anti-IL-12 p40 antibody), C17.15 (IgG2a) (anti-IL-12 p40), C15.1 (IgG1) (anti-IL-12 p40), C15.6 (IgG1) (anti-IL-12 p40), AN18 (IgG1) (anti-IFN-γ), and XMG1.6 (IgG1) (anti-IFN-γ). AN18 was kindly provided by Gianni Garotta (Human Genomics, Rockville, Md.). XMG1.6 was kindly provided by Alan Sher (National Institute of Allergy and Infectious Diseases, Bethesda, Md.). All of the other antimouse antibodies were produced and characterized in our laboratory.
Rabbit anti-asialo-GM1 (αAsGM1) was obtained from Wako Chemicals (Richmond, Va.). Normal rabbit serum was obtained from Cedar Lane (Hornby, Ontario, Canada). Normal rat Igs were obtained from Sigma (St. Louis, Mo.).
Recombinant cytokines.
Murine cytokines TNF-α, IFN-γ, and IL-12 were used as standards in cytokine assays. TNF-α was obtained from Genzyme (Cambridge, Mass.). IL-12 was kindly provided by Stan Wolf (Genetics Institute, Cambridge, Mass.). IFN-γ was kindly provided by Gianni Garotta.
Animals.
Five- to seven-week-old female inbred BALB/c mice (H-2d) were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). Animals were maintained in a specific-pathogen-free environment. The animal colony was screened regularly for the presence of murine pathogens (including respiratory viruses) and consistently tested negative.
Virus.
Influenza virus strain A/Puerto/Rico/8/34 H1N1 (PR8) was grown in the allantoic cavity (2) of 10-day-old embryonated hen’s eggs and stored as infectious allantoic fluid at −70°C. Virus stock was shown to be endotoxin free (EF) by E. toxate (Limulus) assay (Sigma). Virus titer was determined by agglutination of chicken erythrocytes (SPAFAS, Preston, Conn.) and is expressed as hemagglutinating units (HAU) (2). PR8 virus purified by sucrose gradient centrifugation (2) was inactivated by exposure to a UV lamp (Mineralight Lamp, San Gabriel, Calif.). The petri dish containing the virus was approximately 10 cm in distance from a UV lamp (125 W) and was exposed to a wavelength of 254 nm for 15 min. Loss of infectivity was ascertained by Madin-Darby canine kidney (MDCK) cell infectivity assay.
Virus infection of BALB/c mice.
Mice were administered an intraperitoneal (i.p.) injection of 200 μl of a combination of xylazine (140 mg) (Haver/Mobey, Shawnee, Kans.) and ketamine (0.7 mg) (Fort Dodge, Fort Dodge, Iowa) diluted 1:10 in EF-phosphate-buffered saline (PBS). After the mice were anesthetized, 20 μl of EF-PBS containing 1 HAU of PR8 virus (105 50% tissue culture infective doses) per ml was administered by intranasal inoculation by applying a sterile pipette tip (Rainin, Woburn, Mass.) to the outer nares and slowly injecting the virus into the upper respiratory tract.
Antibody and cytokine administration.
All antibodies used in vivo were purified on a protein G column (Pharmacia, Piscataway, N.J.). Mice were injected i.p. with 100 μg of purified IL-12-neutralizing antibody (C17.8) per 200 μl on days −1, 2, and 5 of PR8 infection. Control animals were injected with normal rat Ig.
Bronchoalveolar lavage (BAL).
Mice were sacrificed between 24 h and 10 days postinfection. The thoracic cavity was exposed, and a 25-gauge needle attached to a 1-ml syringe was inserted into the trachea immediately posterior to the larynx. The entire respiratory tract was lavaged with 1 ml of EF-RPMI 1640 medium three times in a reproducible manner. The BAL fluid was then transferred to 1.5-ml microcentrifuge tubes, and cells and cellular debris were pelleted at 100 × g for 5 min. The resultant supernatants were aliquoted into sterile microcentrifuge tubes and stored at −70°C until analysis.
Cytokine measurement.
Murine IFN-γ and IL-12 p40 were detected by a two-site radioimmunoassay (RIA) with the monoclonal antibody pairs AN18-XMG1.6 and C17.15-C15.6, respectively (55). Murine TNF-α was detected by enzyme-linked immunosorbent assay (ELISA) with capture antibody-horseradish peroxidase-conjugated monoclonal antibody pairs provided by Genzyme. These immunoassays had a sensitivity of ≤30 pg/ml.
Murine IL-12 p70 was measured in the BAL fluid by a modification of the antibody capture assay (12) with 2D6 cells (32) (kindly provided by Hiromi Fujiwara, Osaka, Japan) as indicator cells. Briefly, 96-well flat-bottom plates (Linbro, Aurora, Ohio) were coated overnight at 4°C with 15 μg each of C15.1 (rat IgG1) and C15.6 (rat IgG1) anti-murine IL-12 p40 monoclonal antibodies per ml in carbonate-bicarbonate buffer, pH 9.5. The plates were washed three times with sterile EF-PBS and blocked with 1% bovine serum albumin (BSA) diluted in EF-PBS for 1 h at 37°C. After the plates were washed, 100 μl of BAL fluid samples or murine rIL-12 standard dilutions were added to the wells (in triplicate), and the plates were incubated overnight at 4°C. After the plates were washed three times with sterile EF-PBS, 105 2D6 cells were added per well. Following an overnight incubation at 37°C, the cells were labeled with 1 μCi of [3H]thymidine (ICN, Costa Mesa, Calif.) per well for 16 h at 37°C and 5% CO2. The cells were harvested onto glass fiber filter paper, and incorporation of [3H]thymidine into cells was measured by a beta scintillation counter (Packard, Meriden, Conn.). Sample counts were compared against the standard curve, and IL-12 p70 concentrations were expressed as picograms per milliliter. The assay had a sensitivity of ≤3 pg/ml.
Analysis of IL-12 p40 mRNA.
Lung tissue not utilized for collection of BAL fluid was homogenized in a sterile Pyrex glass tissue grinder (Fisher Scientific, Fair Lawn, N.J.) containing Ultraspec (Biotex, Houston, Tex.) to extract total RNA for RNase protection assay. Twenty micrograms of sample RNA was assayed for p40 mRNA with a murine p40 riboprobe containing the sequence between nucleotide positions 35 and 280 of the published murine p40 cDNA sequence (46). It was cloned into the PCR II/TA cloning vector (Invitrogen, San Diego, Calif.). For RNase protection, it was linearized with EcoRI and the antisense riboprobe was transcribed with T7 RNA polymerase (Promega, Madison, Wis.). The transcript was 275 bases long, and the protected RNA was 245 bases. Each RNA sample was also simultaneously assayed for cyclophilin (Amersham, Arlington Heights, Ill.) as an internal control for equal RNA usage. The p40 signal from each sample was corrected against the internal control. Quantitation of the p40 mRNA content in each sample was achieved by comparing the signal strength to that of a ribroprobe standard curve with known amounts of RNA subjected to the same treatment as the RNA samples.
Analysis of viral titer.
Lungs were aseptically removed from animals at various intervals after infection, rinsed in sterile PBS, and stored at −70°C until assay. At the time of assay, individual lungs were disrupted with Dounce homogenizers in 2 ml of ice-cold PBS containing 0.1% BSA. The extracts were centrifuged for 10 min at 750 × g to pellet cell debris, and the supernatants were diluted serially 10-fold in Iscove’s medium-BSA (Isc-BSA). A total of 100 μl of a freshly trypsinized suspension of 5 × 105 MDCK cells per ml in Iscove’s modified Dulbecco’s medium–0.01% BSA (Isc-BSA; Gibco BRL, Grand Island, N.Y.) was added to wells of flat-bottomed 96-well microtiter plates and followed by addition of 50 μl of lung extract dilutions to six replicate wells. The cultures were incubated overnight at 37°C in humidified air–5% CO2. A total of 50 μl of trypsin (2.5% trypsin; Whittaker Bioproducts, Inc., Walkersville, Md.: freshly diluted 1/750 in Isc-BSA) was then added to each well, and the cultures were further incubated as described above. After 3 days of incubation, the culture supernatants were tested for the presence of viral hemagglutinin activity by mixing 25 μl of supernatant with 25 μl of a 1% suspension of chicken erythrocytes. Lung virus titers are expressed as dilutions of lung extract at which 50% of the MDCK cultures revealed virus growth (44).
In vivo depletion of NK cells.
PR8-infected BALB/c mice were injected i.p. on days −1, 2, and 5 postinfection with either αAsGM1 diluted 1:10 in PBS, 200 μl i.p. (Wako Chemicals), or normal rabbit serum (Cedar Lane), and BAL samples were collected on days 5 and 7 postinfection for cytokine analysis. To determine if the dose of αAsGM1 was effective in neutralizing NK cells, noninfected mice treated with αAsGM1 or rabbit serum were injected i.p. with 100 μg of poly(I-C) (Sigma) 18 h prior to spleen removal and splenocyte NK cell activity was measured by 51Cr release with the NK cell-sensitive YAC-1 cell line (American Type Culture Collection, Rockville, Md.) as target.
Cell-mediated cytotoxicity assay.
Two million splenocytes, isolated from 10-day virus-infected mice, were cultured for 4 days with 106 syngeneic irradiated (50 Gy) splenocytes from normal mice infected with PR8 in vitro. Cytotoxic activity was determined with 51Cr-labeled P815 target cells that had been infected with PR8 virus (5,000 HAU/106 cells) in vitro overnight at 37°C. Cultured cells were incubated with targets in 96-well round-bottom microtiter plates (Costar, Cambridge, Mass.) for 4 h at 37°C and 5% CO2. The assay was performed with triplicate samples with four different effector/target cell ratios. Following incubation, 50 μl of cell-free supernatant was removed from each well for gamma scintillation counting. The percentage of specific activity was calculated as follows:
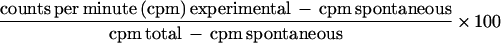 |
Statistical analysis.
Statistical significance was determined by Student’s t test.
RESULTS
IL-12 p40 mRNA expression in the lungs of PR8-infected mice.
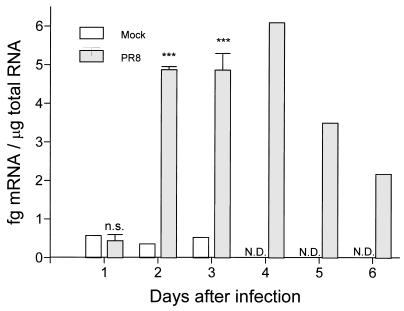
Lung tissue from BALB/c mice, infected intranasally with a sublethal dose (1 HAU/ml) of PR8 virus, was collected daily for 6 days and lung tissue from mock-infected mice was collected daily for 3 days to test for the presence of IL-12 p40 mRNA by RNase protection assay. There was minimal detectable p40 mRNA during the initial 24 h of infection (Fig. 1). However, by 48 h postinfection, the PR8-infected mice had significantly more IL-12 p40 mRNA in their lungs than did control mice. The IL-12 p40 mRNA concentration remained elevated up to day 4 postinfection and decreased over the next 2 days.
FIG. 1.
Detection of IL-12 p40 mRNA in the lungs of BALB/c mice infected with PR8 virus. Lung tissue samples from four PR8-infected mice were collected daily on days 1, 2, and 3 postinfection, and lung tissue samples from two PR8-infected mice were collected on days 4, 5, and 6 postinfection. On days 1, 2, and 3, lung tissue was collected from mock-infected mice. Each sample was simultaneously assayed for cyclophilin as an internal control for equal RNA usage. The p40 signal was corrected against the internal control. Quantitation of the p40 mRNA content in each sample was achieved by comparing the signal strength to that of a riboprobe standard curve with known amounts of RNA subjected to the same treatment as the RNA samples. Values are the means ± standard errors of samples. ∗∗∗, P < 0.005. n.s., not significant by Student’s t test. N.D., not detectable.
Cytokine production in the lung.
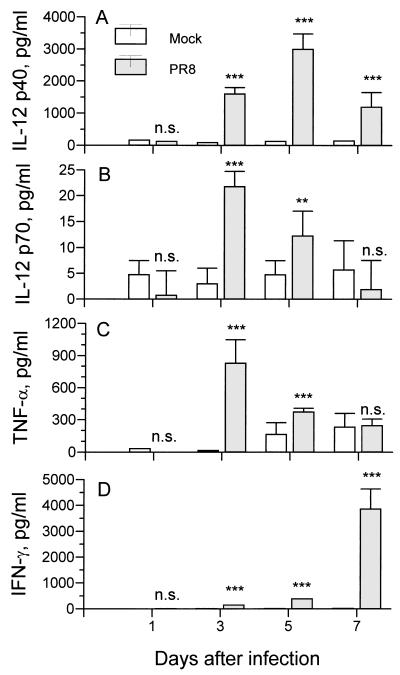
Mice infected with PR8 virus were sacrificed on days 1, 3, 5, and 7 postinfection, and the lungs were lavaged three times with 1 ml of EF RPMI 8866 medium. The BAL fluid samples were individually assayed for IL-12 p40 by RIA (Fig. 2A). Mock-infected mice were given an equivalent amount of noninfectious allantoic fluid intranasally. The concentrations of IL-12 p40 and p70 in the BAL fluid of mock-infected mice remained low during the entire collection period. IL-12 p40 and p70 were very low or not detectable in the initial 24 h of virus infection, consistent with the lack of p40 mRNA in the lungs 24 h postinfection. But by day 3 postinfection, over 1 ng of IL-12 p40 per ml was produced, and peak levels were reached on day 5 with almost 4 ng/ml present in the BAL fluid of virus-infected mice. The biologically active p70 heterodimer was assayed by antibody capture biological assay. There was an increase in IL-12 p70, with maximal levels at day 3 after infection, 2 days earlier than the peak levels of IL-12 p40 (Fig. 2B).
FIG. 2.
Cytokine production in BAL fluid of BALB/c mice infected with PR8 virus. (A) IL-12 p40 was measured by RIA. Data are from 15 BAL fluid samples from PR8-infected mice and 10 BAL fluid samples from mock-infected mice (five separate experiments). (B) IL-12 p70 was measured by capture assay. Eight BAL fluid samples from PR8-infected mice and four BAL fluid samples from mock-infected mice were collected at each time point (two separate experiments). (C) TNF-α was measured by ELISA. Data are from six BAL fluid samples from PR8-infected mice and three BAL fluid samples from mock-infected mice (three separate experiments). (D) IFN-γ was measured by RIA. Data are from 15 BAL fluid samples from PR8-infected mice and 10 BAL fluid samples from mock-infected mice (five separate experiments). Values are means ± standard errors of samples from individual mice. ∗∗, P < 0.01; ∗∗∗, P < 0.005. n.s., not significant by Student’s t test of the comparison between groups treated with noninfectious allantoic fluid and with PR8 virus.
BAL fluids collected on days 1, 3, 5, and 7 postinfection were also analyzed for the presence of IFN-γ by RIA and for TNF-α by ELISA. Virus infection induced TNF-α production by day 3 postinfection, with a rapid decline thereafter (Fig. 2C). IFN-γ was significantly detectable in virus-infected mice compared to mock-infected mice as early as day 3 postinfection, reached peak levels at day 7 (Fig. 2D), and was very low by day 9 postinfection (data not shown).
Productive virus infection is required for cytokine induction.
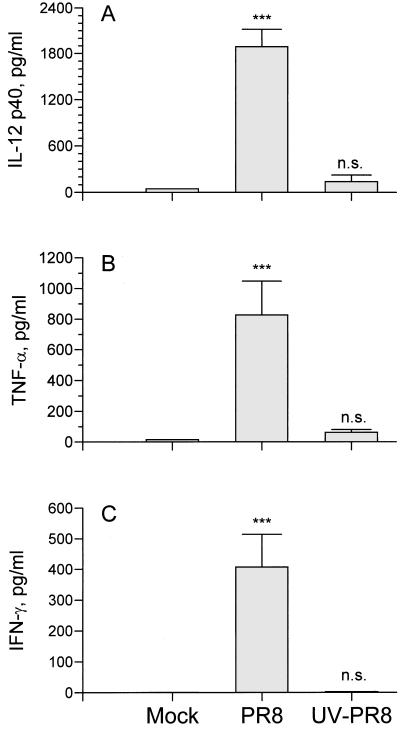
The observation that there is a delay in IL-12 production following intranasal infection with PR8 suggests that the presence of viral antigen is not enough to induce the release of IL-12 and that, rather, active virus replication may be required. To determine if live virus is required for induction of IL-12 production, a total of six mice were given 20 μl of 106 HAU of UV-irradiated purified PR8 virus per ml intranasally and BAL fluid was collected every 24 h for 3 days. Data in Fig. 3 show the concentrations of cytokines in BAL fluids collected from six mice on day 3. The concentration of IL-12 p40 (Fig. 3A) was less than 200 pg/ml in mice infected with UV-treated virus and was comparable to that of the mock-infected mice. This is in sharp contrast to the more than 2 ng of IL-12 p40 per ml measured in live virus-infected mice. Thus, active virus replication appears to be required for IL-12 production. Production of cytokines, such as TNF-α (Fig. 3B) and IFN-γ (Fig. 3C), during influenza virus infection also requires live replicating virus; however, a high dose of UV-irradiated PR8 virus transiently induced a detectable level of TNF-α that quickly returned to baseline at 48 h postinoculation (data not shown). IFN-α was detected in the BAL fluid of UV-inactivated virus, although at concentrations two to four times lower than that found in BAL fluid from mice infected with live virus (data not shown).
FIG. 3.
Live virus is required for the production of proinflammatory cytokines. BALB/c mice were infected intranasally with 20 μl of 106 HAU of purified PR8 virus (per ml) that had been exposed to UV light. BAL fluid was collected 3 days after infection. (A) IL-12 p40 was measured by RIA. (B) TNF-α was measured by ELISA. (C) IFN-γ was measured by RIA. Experiments included a total of six mice (three separate experiments). Values are means ± standard errors of samples. ∗∗∗, P < 0.005. n.s., not significant by Student’s t test of the comparison between groups treated with noninfectious allantoic fluid and with PR8 virus.
IL-12-dependent and -independent IFN-γ production.
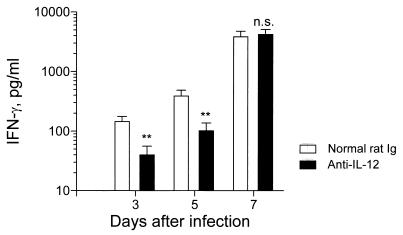
Because IL-12 is a potent inducer of IFN-γ, we determined if endogenous IL-12 plays a role in the production of IFN-γ. A total of 45 mice (three mice per group per experiment) in five separate experiments were injected i.p. with 100 ng of IL-12-neutralizing antibody on day −1 of infection and, every 3 days thereafter, received two additional injections. Control mice were injected with normal rat Igs. On days 3 and 5 postinfection, IL-12-neutralizing antibody significantly reduced, although it did not completely eliminate, IFN-γ production (Fig. 4). However, on day 7 there was no difference in IFN-γ production between anti-IL-12 antibody-treated virus-infected mice and control antibody-treated infected mice. The in vivo bioactivity of the neutralizing antibody for the duration of the experiment was confirmed by the inability to detect IL-12 p40 in PR8-infected mice treated with anti-IL-12 antibody (data not shown). Thus, there appears to be a dichotomy in IFN-γ production, in that early secretion in the lung is partly IL-12 dependent but that, by day 7 of infection, IFN-γ is IL-12 independent.
FIG. 4.
Endogenous IL-12 is required for IFN-γ production in the lungs during the early phase of influenza virus infection. PR8-infected mice were injected with normal rat Ig (100 ng) or anti-IL-12 antibody (100 ng) on days −1, 2, and 5 of infection. BAL fluid was collected on days 3, 5, and 7 postinfection and assayed for the presence of IFN-γ by RIA. Experiments included a total of 45 mice (three mice per group per experiment in five separate experiments). Values are means ± standard errors of samples. ∗∗, P < 0.01. n.s., not significant by Student’s t test.
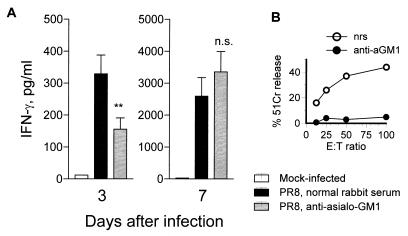
IFN-γ is produced primarily by T cells and NK cells (50). To determine if NK cells are involved in IFN-γ production during primary influenza virus infection, a total of 18 mice (three separate experiments) were injected i.p. with αAsGM1 antibody on day −1 and, every 3 days thereafter, received two additional injections. Control mice were injected with normal rabbit serum. BAL fluid was collected on days 3 and 7, and IFN-γ was assayed by RIA. Virus-infected mice treated with αAsGM1 antibody produced significantly less IFN-γ than did virus-infected control mice on day 3 postinfection (Fig. 5). However, by day 7 postinfection, mice in both groups produced comparable amounts of IFN-γ. The effectiveness of the αAsGM1 treatment in depleting NK cells was supported by the lack of killing of YAC-1 targets by splenocytes from poly(I-C)- and αAsGM1-treated mice in a 51Cr release assay (Fig. 5B). The data suggest that NK cells participate in IL-12-dependent IFN-γ production. At day 7 of infection, T cells are likely to be the IL-12-independent IFN-γ producers.
FIG. 5.
Effect of NK cell depletion on IFN-γ production. One day prior to infection with PR8 virus and 4 days postinfection, a total of 18 (three separate experiments) BALB/c mice were injected i.p. with 200 μl of αAsGM1 rabbit antimouse polyclonal antibody diluted 1:10 in PBS. Eighteen control mice were injected with normal rabbit serum (nrs). BAL fluid was collected on days 3 and 7 postinfection and assayed for the presence of IFN-γ by RIA. (B) To confirm in vivo depletion of NK cells by αAsGM1, noninfected mice treated with αAsGM1 or normal rat serum were injected i.p. with 100 μg of poly(I-C) 18 h prior to spleen removal and splenocyte NK cell activity was measured by 51Cr release with the NK cell-sensitive YAC-1 cell line. Values are means ± standard errors of samples. ∗∗, P < 0.01. n.s., not significant by Student’s t test of the comparison between groups treated with normal rabbit serum and with αAsGM1 serum. E:T ratio, effector/target cell ratio.
Effect of IL-12 on virus titer.
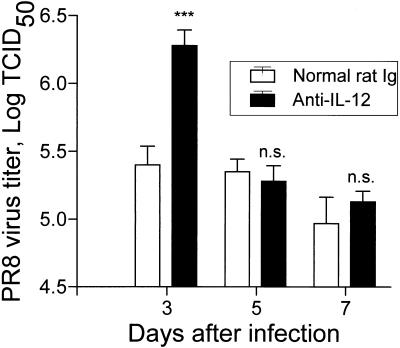
Influenza virus shedding in the lung lasts up to 7 to 10 days after infection (1, 10). To test whether endogenous IL-12 was affecting viral multiplication in vivo, lung tissues from PR8-infected mice treated with anti-IL-12 or the control normal rat Igs were assayed for virus titer. Treatment with anti-IL-12 on day −1 and day 2 of infection significantly increased the virus titer at day 3 postinfection compared to that for PR8-infected mice treated with control antibody (Fig. 6). However, at days 5 and 7 postinfection, the virus titer was not significantly different between the anti-IL-12-treated and the control mice (Fig. 6), and both groups cleared the virus completely by day 9 postinfection (data not shown).
FIG. 6.
Effect of endogenous IL-12 on virus titer. Lung tissue was collected from individual PR8-infected mice treated with normal rat Ig or anti-IL-12 at 1, 2, and 5 days. Virus titer was determined as described in Materials and Methods. Experiments included three mice per time point per experiment for three separate experiments. Values are means ± standard errors of samples. ∗∗∗, P < 0.005. n.s., not significant by Student’s t test of the comparison between groups treated with normal rat Ig and with PR8 virus. TCID50, 50% tissue culture infective dose.
IL-12 and virus-specific cell-mediated cytotoxicity.
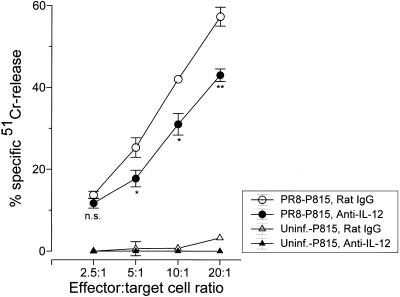
To determine the effect of endogenous IL-12 on virus-specific cell-mediated immunity, we measured the virus-specific CTL activity in virus-infected mice treated with IL-12-neutralizing antibody (Fig. 7). Splenic leukocytes from day 10 infected mice treated with anti-IL-12 were assayed for the ability to kill PR8-infected P815 target cells by a standard 51Cr release assay. Blocking endogenous IL-12 resulted in a modest (twofold) but statistically significant decrease in CTL activity in virus-infected mice compared to that in infected mice treated with an irrelevant antibody (Fig. 7). The data suggest that endogenous IL-12 contributes to the activation of virus-specific CTLs and that the loss of IL-12 can result in a less vigorous cell-mediated immune response.
FIG. 7.
Effect of endogenous IL-12 on CTL activity. The spleens of PR8-infected mice treated with neutralizing anti-IL-12 antibody (C17.8) or control antibody (rat IgG) were collected at 10 days postinfection, and splenocytes from individual mice were cultured with irradiated splenocytes infected with PR8 virus in vitro as stimulators. After 4 days, the cultured cells were tested as effector cells against 51Cr-labeled PR8-infected P815 target cells at the indicated effector/target cell ratios in a 51Cr release cytotoxicity assay. Experiments included four mice per experimental condition (two separate experiments). Values are means ± standard errors of samples. ∗, P < 0.05; ∗∗, P < 0.1. n.s., not significant by Student’s t test of the comparison between groups treated with normal rat Ig and with PR8 virus.
DISCUSSION
Because IL-12 is centrally involved in many bacterial and parasitic immune responses, we wanted to determine if IL-12 was important for the development and maintenance of an antiviral immune response. Several investigators have focused on the effect of IL-12 treatment on the antiviral immune response (5, 13, 36–39, 45, 48) in the mouse experimental system and have found IL-12 to be protective and to mediate its effect through the production of IFN-γ. However, there are only a few published reports of endogenous IL-12 protein production in a murine virus infection (26, 37). Because the in vitro and in vivo characteristics of IL-12 suggest that it may be a key factor in the regulation of the host immune response against virus infection, we were interested in determining what function IL-12 may play in influenza virus-specific immunity and have utilized the murine influenza virus infection model to address this issue. The murine influenza virus infection model is very well characterized, and much is known about the cellular and humoral immune response that develops during influenza virus infection. However, less is known about the cytokines involved in generating inflammation at the site of infection. This is especially true for the early phase of the infection, prior to the appearance of CTLs. Our goal was to better define this cascade of events by studying the effect of endogenous IL-12 on the development and maintenance of the anti-influenza virus immune response.
We have observed IL-12 p40 mRNA expression in the lungs 48 h after intranasal influenza virus infection and detected both p40 and p70 proteins in the BAL fluids of BALB/c mice at day 3 postinfection. Because monocytes and macrophages are the major producer cells for IL-12, it is likely that the sources of IL-12 in the lung are activated resident alveolar macrophages and monocytes that infiltrate the lung during infection. IL-12 p70 continues to be produced in the lung through day 5, although the biologically inactive p40 is still detectable in the BAL fluid at day 7 postinfection. The significance of the prolonged secretion of p40 is unclear, although some studies have indicated that the p40 homodimer may act in an inhibitory manner to block binding of p70 to the IL-12 receptor (14).
In order to explore IL-12 induction during influenza virus infection, we asked whether live replicating virus was an important factor. BALB/c mice infected with UV-inactivated influenza virus produced neither IL-12 nor TNF-α at 3 days postinoculation in the lung, although a short burst of TNF-α production was seen at 24 h postinoculation. However, IFN-α, a cytokine which has been reported to be released upon stimulation with UV-inactivated noninfectious virus (41), was produced upon inoculation of UV-irradiated virus, although at lower levels than in infected animals. There is no clear explanation as to why production of IL-12 and TNF-α requires live virus. Both cytokines are produced in monocytes and macrophages, and it is likely that IL-12 and TNF-α are released from the same producer cells. Virus replication may trigger release of factors that activate gene transcription, increase mRNA stability, or activate the translation processes. Nain et al. (34) have suggested that inhibition of transcriptional repressors, synthesis of viral gene products, or location of those products within the cytoplasm may induce cytokine production.
To determine if secretion of IL-12 translates into an active role in the influenza immune response, a number of biological activities were examined, namely, cytokine induction, virus clearance, and cytolytic responses. Influenza virus infection induces a cascade of cytokine mRNA (6) and proteins (18) in the lungs and mediastinal lymph nodes, including IL-1α/β, IL-2, IL-4, IL-5, IL-6, IL-10, IFN-γ, TNF-α, granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, macrophage colony-stimulating factor, leukotriene B, and, based on this study, IL-12.
IFNs in general and IFN-γ in particular have been shown to be especially important in regulating the influenza immune response through numerous biological activities, such as inhibition of virus replication via induction of enzymes that block replicative machinery (22), activation of alveolar macrophages (51), and increased expression of major histocompatibility complex class II and adhesion molecules on antigen-presenting cells (11, 54), and their role in the development of CTLs in vitro (52, 56). To determine a functional role for IL-12 in influenza virus infection, its effect on IFN-γ induction was examined. Following infection of BALB/c mice with PR8 virus, IFN-γ was produced in the lung within 3 days of infection and continued through day 7. Treatment with IL-12-neutralizing antibody significantly blocked IFN-γ production during the first 5 days of infection. However, after 5 days of infection, IL-12 was no longer required for IFN-γ production, which markedly increased on day 7 postinfection. These data are suggestive of an IL-12-dependent and IL-12-independent source of IFN-γ during influenza virus infection. There are two possible mechanisms to explain this dichotomy in IFN-γ production. (i) There may be a population of cells that initially produce IFN-γ in response to IL-12 but later in the infection lose the ability to respond to IL-12, through downregulation of the IL-12 receptor or via a disruption in the IL-12 receptor signaling pathway during virus infection, or (ii) there are two separate IFN-γ-producing populations; one is responsive to IL-12 and the other is not. IL-12-dependent and -independent IFN-γ production has also been described for murine cytomegalovirus infection (35–37), where IL-12 is responsible for early (day 3) NK cell-mediated IFN-γ production but not late (day 7) T-cell-mediated IFN-γ production. During influenza virus infection, the local population of NK cells contributes to early pulmonary immunity (47). These cells may become activated following release of IL-12 from alveolar macrophages and monocytes and become one of the sources of early IL-12-dependent IFN-γ production. However, by day 7 postinfection, virus-specific T cells that have migrated into the lower respiratory tract from the regional mediastinal lymph nodes are killing virus-infected cells as well as releasing IFN-γ to inhibit further virus replication (10). By this time, IL-12 is no longer being released into the pulmonary milieu and the virus-specific effector cells do not require IL-12 for IFN-γ production. Therefore, a possible explanation for the IL-12-dependent and -independent IFN-γ production is that IFN-γ produced early in the virus infection comes from NK cells and possibly T cells stimulated with endogenous IL-12. However, during the later stages of virus infection IFN-γ is being produced by antigen-specific T cells that do not require IL-12. This was further confirmed by showing that IFN-γ was significantly decreased on day 3 postinfection in the BAL fluid from αAsGM1 antibody-treated mice compared to that in BAL fluid from virus-infected mice treated with irrelevant antibody. However, by day 7 postinfection, there was no difference in IFN-γ production between treatment groups.
Virus-specific CD4+ and CD8+ CTLs mediate their antiviral protective effects in vivo by direct cytolysis of virus-infected cells (15, 29). To determine the mechanism by which endogenous IL-12 may be affecting early virus immunity, the effect of IL-12 on the virus-specific cell-mediated immune response was examined. There was a modest but significant decrease in the major histocompatibility complex-restricted cytolytic activity of splenic leukocytes from anti-IL-12-treated mice compared to that for control mice. Thus, endogenous IL-12 is helpful in developing and maintaining an influenza virus-specific T-cell-mediated immune response, although its effect appears modest and not essential for efficient antiviral immunity.
Based on our observations, IL-12 appears to be important primarily in early activation of the immune response during primary influenza virus infection through (i) the early induction of IFN-γ, (ii) the inhibition of early virus replication, and (iii) the contribution to the activation of CTLs. However, as the immune response progresses and becomes antigen driven, IL-12 is no longer required in the pulmonary environment for mechanisms instrumental in mediating virus clearance, namely, activation of effector T cells and secretion of neutralizing antibodies. As a result, infected BALB/c mice are able to recover from influenza virus infection in the absence of IL-12.
ACKNOWLEDGMENT
This work was supported by Public Health Service grants AI34412, CA10815, CA20833, and CA32898.
REFERENCES
- 1.Ada G L, Jones P D. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Barrette T, Inglis S C. Growth, purification and titration of influenza viruses. In: Mahy B W, editor. Virology, a practical approach. Oxford, United Kingdom: IRL Press; 1991. pp. 119–150. [Google Scholar]
- 3.Bender B S, Croghan T, Zhang L, Small P A., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi Z, Barna M, Komatsu T, Reiss C S. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi Z, Quandt P, Komatsu T, Barna M, Reiss C S. IL-12 promotes enhanced recovery from vesicular stomatitis virus infection of the central nervous system. J Immunol. 1995;155:5684–5689. [PubMed] [Google Scholar]
- 6.Carding S R, Allan W, McMickle A, Doherty P C. Activation of cytokine genes in T cells during primary and secondary murine influenza pneumonia. J Exp Med. 1993;177:475–482. doi: 10.1084/jem.177.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan S H, Perussia B, Gupta J W, Kobayashi M, Pospísil M, Young H A, Wolf S F, Young D, Clark S C, Trinchieri G. Induction of IFN-γ production by NK cell stimulatory factor (NKSF): characterization of the responder cells and synergy with other inducers. J Exp Med. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutelier J P, Van Broeck J, Wolf S F. Interleukin-12 gene expression after viral infection in the mouse. J Virol. 1995;69:1955–1958. doi: 10.1128/jvi.69.3.1955-1958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste-Amezaga M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (NKSF/IL-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty P, Allan C, Eichelberger W M. Roles of αβ and γδ T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 11.Fellous M, Nir U, Wallach D, Merlin G, Rubenstein M, Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci USA. 1982;79:3082. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gately M K, Chizzonite R. Measurement of human and mouse interleukin 12. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. Vol. 1. New York, N.Y: John Wiley & Sons; 1992. pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Giese N A, Morse H C., III In vivo treatment with interleukin 12 protects mice from immune abnormalities observed during murine acquired immunodeficiency syndrome (MAIDS) J Exp Med. 1994;180:2199–2208. doi: 10.1084/jem.180.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familletti P C, Gubler U, Presky D H, Stern A S, Gately M K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 15.Graham M B, Daulton D K, Giltinan D, Braciale V L, Stewart T A, Braciale T J. Response to influenza infection in mice with a targeted disruption in the interferon γ gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendricks R L, Janowica M, Tumpey T M. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J Immunol. 1992;148:2522–2529. [PubMed] [Google Scholar]
- 17.Hendricks R L, Tumpey T M. Contribution of virus and immune factors to herpes simplex virus type-1 induced corneal pathology. Investig Ophthalmol Vis Sci. 1990;31:1929–1939. [PubMed] [Google Scholar]
- 18.Hennet T, Ziltener H J, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149:932–939. [PubMed] [Google Scholar]
- 19.Hsieh C, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Listeria-induced Th1 development in αβ-TCR transgenic CD4+ T cells occurs through macrophage production of IL-12. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 20.Hyland L. Respiratory virus infection of mice provokes a permanent humoral immune response. J Virol. 1994;67:6083–6086. doi: 10.1128/jvi.68.9.6083-6086.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasaki T, Nozima T. Defense mechanisms against primary influenza virus infection in mice. I. The roles of interferon and neutralizing antibodies and thymus dependence of interferon and antibody production. J Immunol. 1977;118:256–263. [PubMed] [Google Scholar]
- 22.Joklik W K. Interferons. In: Fields B N, Kniper D N, editors. Fields virology. 2nd ed. New York, N.Y: Raven Press; 1990. pp. 383–410. [Google Scholar]
- 23.Jones P D. Influenza virus-specific antibody-secreting cells in the murine lung during primary influenza virus infection. J Virol. 1986;60:614–619. doi: 10.1128/jvi.60.2.614-619.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones P D. Persistence of influenza virus-specific antibody-secreting cells and B-cell memory after primary murine influenza virus infection. Cell Immunol. 1987;109:53–64. doi: 10.1016/0008-8749(87)90291-7. [DOI] [PubMed] [Google Scholar]
- 25.Jones P D. Influenza-specific antibody-secreting cells and B cell memory in the murine lung after immunization with wild-type, cold-adapted variant and inactivated influenza virus. Vaccine. 1987;5:244–248. doi: 10.1016/0264-410x(87)90109-5. [DOI] [PubMed] [Google Scholar]
- 26.Kanangat S, Thomas J, Gangappa S, Babu J S, Rouse B T. Herpes simplex virus type 1-mediated up-regulation of IL-12 (p40) mRNA expression. Implications in immunopathogenesis and protection. J Immunol. 1996;156:1110–1116. [PubMed] [Google Scholar]
- 27.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–846. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling P, Gately M K, Gubler U, Stern A S, Lin P, Hollfelder K, Su C, Pan Y C, Hakimi J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–127. [PubMed] [Google Scholar]
- 29.Lukacher A E, Morrison L A, Braciale V L, Braciale T J. T lymphocyte function in recovery from experimental viral infection: the influenza model. In: Steinman R M, North R J, editors. Mechanisms of host resistance to infectious agents, tumors and allografts. New York, N.Y: Rockefeller Press; 1986. pp. 233–254. [Google Scholar]
- 30.Manetti R, Gerosa F, Giudizi M G, Biagiotti R, Parronchi P, Piccinni M, Sampognaro S, Maggi E, Romagnani S, Trinchieri G. Interleukin-12 induces stable priming for interferon-γ (IFN-γ) production during differentiation of human T helper (Th) cells and transient IFN-γ production in established Th2 cell clones. J Exp Med. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manetti R, Parronchi P, Giudizi M G, Piccinni M-P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (NKSF/IL-12) induces Th1-type specific immune responses and inhibits the development of IL-4 producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maruo S, Toyo-oka K, Oh-hora M, Tai X, Iwata H, Takenaka H, Yamada S, Ono S, Hamaoka T, Kobayashi M, Wysocka M, Trinchieri G, Fujiwara H. IL-12 produced by antigen-presenting cells induces IL-2-independent proliferation of T helper cell clones. J Immunol. 1996;156:1748–1755. [PubMed] [Google Scholar]
- 33.Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rüde E, Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 34.Nain M, Hinder F, Gong J, Schmidt H, Bender A, Sprenger H, Gemsa D. Tumor necrosis factor-alpha production of influenza A virus-infected macrophages and potentiating effect of lipopolysaccharides. J Immunol. 1990;145:1921–1928. [PubMed] [Google Scholar]
- 35.Orange J S, Biron C A. Characterization of early IL-12, IFN-alpha/beta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 36.Orange J S, Salazar-Mather T P, Opal S M, Spencer R L, Miller A H, McEwen B S, Biron C A. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orange J S, Wang B, Terhorst C, Biron C A. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orange J S, Wolf S F, Biron C A. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994;152:1253–1264. [PubMed] [Google Scholar]
- 39.Ozmen L, Aguet M, Trinchieri G, Garotta G. The in vivo antiviral activity of interleukin-12 is mediated by gamma interferon. J Virol. 1995;69:8147–8150. doi: 10.1128/jvi.69.12.8147-8150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perussia B, Chan S, D’Andrea A, Tsuji K, Santoli D, Pospisil M, Young D, Wolf S, Trinchieri G. Natural killer cell stimulatory factor or IL-12 has differential effects on the proliferation of TCRαβ+, TCRγδ+ T lymphocytes and NK cells. J Immunol. 1992;149:3495–3502. [PubMed] [Google Scholar]
- 41.Peschke T, Bender A, Nain M, Gemsa D. Role of macrophage cytokines in influenza A virus infections. Immunobiology. 1993;189:340–355. doi: 10.1016/s0171-2985(11)80365-7. [DOI] [PubMed] [Google Scholar]
- 42.Renegar K B, Small P A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 43.Robertson M J, Soiffer R J, Wolf S F, Manley T J, Donahue C, Young D, Herrmann S H, Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175:779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherle P A, Palladino G, Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J Immunol. 1992;148:212–217. [PubMed] [Google Scholar]
- 45.Schijns V E, Haagmans B L, Horzinek M C. IL-12 stimulates an antiviral type 1 cytokine response but lacks adjuvant activity in IFN-gamma-receptor-deficient mice. J Immunol. 1995;155:2525–2532. [PubMed] [Google Scholar]
- 46.Schoenhaut D S, Chua A O, Wolitzky A G, Quinn P M, Dwyer C M, McComas W, Familletti P C, Gately M K, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 47.Stein-Streilein J, Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986;136:1435–1441. [PubMed] [Google Scholar]
- 48.Tang Y W, Graham B S. Interleukin-12 treatment during immunization elicits a T helper cell type 1-like immune response in mice challenged with respiratory syncytial virus and improves vaccine immunogenicity. J Infect Dis. 1995;172:734–738. doi: 10.1093/infdis/172.3.734. [DOI] [PubMed] [Google Scholar]
- 49.Taylor P M, Askonas B A. Influenza nucleoprotein specific cytotoxic T cell clones are protective in vivo. Immunology. 1986;58:417. [PMC free article] [PubMed] [Google Scholar]
- 50.Trinchieri G, Perussia B. Immune interferon: a pleiotropic lymphokine with multiple effects. Immunol Today. 1985;6:131–136. doi: 10.1016/0167-5699(85)90080-5. [DOI] [PubMed] [Google Scholar]
- 51.Wells M A, Albrecht P, Ennis F A. Recovery from a viral respiratory infection. I. Influenza pneumonia in normal and T-deficient mice. J Immunol. 1981;126:1036–1041. [PubMed] [Google Scholar]
- 52.Wille A, Gessner A, Lother H, Lehmann-Grube F. Mechanisms of recovery from acute virus infection. VIII. Treatment of lymphocytic choriomeningitis virus-infected mice with interferon-gamma monoclonal antibody blocks generation of virus-specific cytotoxic T lymphocytes and virus elimination. Eur J Immunol. 1989;19:1283–1288. doi: 10.1002/eji.1830190720. [DOI] [PubMed] [Google Scholar]
- 53.Wolf S F, Temple P A, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick R M, Kelleher K, Herrmann S H, Clark S C, Azzoni L, Chan S H, Trinchieri G, Perussia B. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 54.Wong G H, Clark-Lewis I, Harris A W, Schrader J W. Effect of cloned interferon-gamma on expression of H-2 and Ia antigens on cell lines of hemopoietic, lymphoid, epithelial, fibroblastic and neuronal origin. Eur J Immunol. 1984;14:52–56. doi: 10.1002/eji.1830140110. [DOI] [PubMed] [Google Scholar]
- 55.Wysocka M, Kubin M, Vieira L Q, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 56.Yamada Y K, Meager A, Yamada A, Ennis F A. Human interferon alpha and gamma production by lymphocytes during the generation of influenza virus specific cytotoxic T lymphocytes. J Gen Virol. 1986;67:2325–2334. doi: 10.1099/0022-1317-67-11-2325. [DOI] [PubMed] [Google Scholar]