Abstract
Background
Chemotherapy remains the standard-of-care for many patients with locally advanced or metastatic non-small-cell lung cancer (NSCLC), but acquired resistance presents challenges. The aim of this open-label, multicenter phase 2 clinical trial was to determine the efficacy and safety of utidelone, a novel genetically engineered epothilone analog and microtubule-stabilizing agent, as a third- or later-line treatment for locally advanced or metastatic NSCLC.
Methods
Patients who had failed standard second-line treatment (including platinum-containing chemotherapy or targeted therapy) received utidelone (40 mg/m2 via intravenous injection daily, day 1–5) every 21 days. The primary endpoint was the objective response rate (ORR). Secondary endpoints were the duration of response (DoR), progression-free survival (PFS), overall survival (OS), and safety.
Results
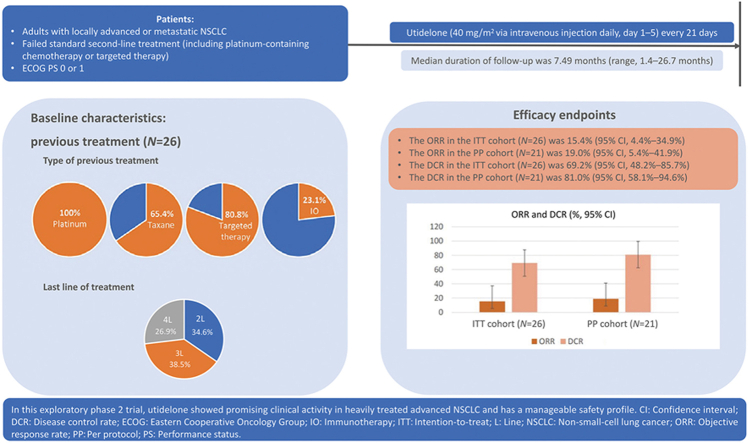
From March 12, 2019 to January 18, 2021, 26 pretreated patients with locally advanced or metastatic NSCLC (100% of patients had received prior platinum and 65.4% patients had received prior taxane treatment) were enrolled (80.8% of patients had adenocarcinoma). At baseline, nine (34.6%) patients had received second-line treatment, 10 (38.5%) patients had received third-line treatment, and seven (26.9%) patients had received fourth- or later-line treatment. By the data cut-off date of August 10, 2021, the median follow-up was 7.49 months (range, 1.4–26.7 months). The ORR was 15.4% (95% confidence interval [CI], 4.4%–34.9%) in the intention-to-treat (ITT) cohort (N = 26) and 19.0% (95% CI, 5.4%–41.9%) in the per-protocol (PP) cohort (N = 21). The disease control rate was 69.2% (95% CI, 48.2%–85.7%) and 81.0% (95% CI, 58.1%–94.6%) in the ITT and PP cohorts, respectively. The median DoR was 4.1 months (95% CI, 3.1–5.1 months) in the ITT cohort. The median PFS was 4.37 months (95% CI, 2.50–5.29 months) in the ITT cohort and 4.37 months (95% CI, 2.50–9.76 months) in the PP cohort. The median OS was not reached, and the 12-month OS rate was 69% (95% CI, 45.1%–84.1%). Grade 3/4 treatment-emergent adverse events occurred in 38.5% of patients, and the most common was peripheral neuropathy (23.1%, all Grade 3), which was manageable with dose modifications.
Conclusions
In this clinical trial, utidelone showed promising efficacy and had a manageable safety profile. Further clinical studies are warranted to confirm its role in NSCLC treatment.
Trial registration
Keywords: Utidelone, Locally advanced or metastatic non-small-cell lung cancer, Efficacy, Platinum- and taxane-refractory
Graphical abstract
Highlights
-
•
A phase 2 clinical trial of utidelone was performed in patients with heavily pretreated, locally advanced or metastatic non-small-cell lung cancer.
-
•
In the intention-to-treat (ITT) cohort, an objective response rate of 15.4% (95% confidence interval [CI], 4.4%–34.9%) and a disease control rate of 69.2% (95% CI, 48.2%–85.7%) was observed.
-
•
A median progression-free survival of 4.37 months (95% CI, 2.50–5.29 months) and a 12-month overall survival rate of 69% (95% CI, 45.1%–84.1%) were observed in the ITT cohort.
-
•
The most common grade 3/4 treatment-emergent adverse event was peripheral neuropathy (23.1%, all Grade 3).
Introduction
In 2020, lung cancer remained the leading cause of cancer-related death worldwide, including in China.1 Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases,2,3 and more than 75% of patients with lung cancer are diagnosed with locally advanced or stage Ⅳ disease.4 Although biomarker identification, targeted therapy, and immunotherapy have substantially improved outcomes for patients with NSCLC in the past decade,2 the prognosis of this malignancy remains dismal, with a 5-year overall survival (OS) rate of 7% for advanced disease in the United States of America.4 A recent prospective study in China analyzed the survival of patients with unresectable stage ⅢB/Ⅳ NSCLC treated according to the choice of their physicians and reported that the median OS was 23.2 months (95% confidence interval [CI], 19.5–25.5 months) and the 1- and 3-year OS rate was 68.9% and 39.0%, respectively.5
Although targeted therapies and/or immunotherapies are part of the standard-of-care for patients with locally advanced or metastatic NSCLC, for many patients, there is either no targetable driver gene alteration or they are not suitable for immunotherapy; for such patients, chemotherapy remains the cornerstone of their treatment.6,7 Furthermore, acquired resistance to targeted therapy and immunotherapy is a substantial problem for patients with locally advanced or metastatic NSCLC, and platinum-based chemotherapy is a suitable option for these patients.6, 7, 8 In addition, microtubule inhibition is an established treatment strategy, and such regimens often also include a taxane.6,7 However, in NSCLC, acquired resistance to standard platinum- and taxane-based regimens is common, which limits their continued use and efficacy.9 Consequently, there is a pressing unmet clinical need for alternative chemotherapeutic agents that can overcome, or are less susceptible to, these resistance mechanisms and have clinical activity in heavily pretreated patients.
Epothilones comprise a class of microtubule-targeting agents with a different mechanism of action from that of taxanes, and were developed to overcome acquired drug resistance.10, 11, 12 The only drug in this class that has been approved by the US Food and Drug Administration (FDA) is ixabepilone, which is used to treat metastatic breast cancer (MBC) after anthracycline and taxane failure.13, 14, 15, 16 However, myelosuppression, hepatic toxicity, and peripheral neuropathy (PN) are common ixabepilone-related toxicities, which often lead to treatment discontinuation.16, 17, 18 Consequently, the development of alternative epothilones with more favorable benefit–risk profiles is desirable. Utidelone is a novel genetically engineered analog of epothilone that was developed by Beijing Biostar Pharmaceuticals, Beijing, China, and can be manufactured at a low cost.19,20 In a phase 3 trial, utidelone in combination with capecitabine significantly prolonged the progression-free survival (PFS) and OS of patients with MBC refractory to both anthracycline- and taxane-based chemotherapy, compared with the survival outcomes achieved with capecitabine alone.20,21 On March 15, 2021, the China National Medical Products Administration (NMPA) approved utidelone for the treatment of patients with MBC who have received at least one previous chemotherapy regimen.22
Based on the efficacy of utidelone for MBC and the manageable toxicities and encouraging activity demonstrated in a phase 1 clinical trial in patients with advanced solid tumors,19 which included four patients with NSCLC, we conducted a phase 2 clinical trial (BG01-1801) of utidelone in patients with locally advanced or metastatic NSCLC who had failed standard second-line treatment (including platinum-based chemotherapy or targeted therapy). Here, we report the efficacy and safety results of this clinical trial.
Methods
Study design and patients
BG01-1801 was an open-label, multicenter phase 2 clinical trial conducted in China to evaluate the efficacy and safety of utidelone in patients with locally advanced or metastatic NSCLC who had received at least two prior systemic regimens, including platinum-containing chemotherapy or targeted therapy (No.NCT03693547).23 The inclusion criteria included an age of 18–70 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, intolerability or failure of the previous standard second-line treatment (including platinum-containing chemotherapy or targeted therapy) for locally advanced or metastatic NSCLC, and an estimated life expectancy of longer than 12 weeks. Patients were required to have measurable disease, no brain metastases, and a < Grade 2 PN within four weeks before enrollment. Further details of the inclusion and exclusion criteria can be found in the Supplementary Material.
Procedures
All patients were administered the recommended dose of utidelone (40 mg/m2 via intravenous injection [iv] daily, on day 1–5) based on a 21-day cycle. Patients received utidelone until disease progression, unacceptable toxicity, voluntary withdrawal, or withdrawal according to the decision of the investigator. Patients were evaluated every two treatment cycles through imaging to assess the objective response, and patients were evaluated as having stable disease (SD) or better continued treatment until disease progression, intolerable toxicity, or death. A safety evaluation was performed during every treatment cycle. Before the administration of utidelone, patients were pretreated with intramuscular or oral diphenhydramine (40 mg), iv dexamethasone (10 mg), and cimetidine (300 mg) to prevent infusion reactions. Follow-up after the end of treatment included an efficacy evaluation every two months until death or the end of the OS follow-up period. Dose adjustments were permitted to manage any toxic effects [Supplementary Material].
Outcomes
The primary endpoint of this clinical trial was the objective response rate (ORR), assessed by investigators according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1,24 defined as the proportion of patients with a complete response (CR) or partial response (PR). Secondary endpoints were the duration of response (DoR; defined as the time from the date of the CR or PR to the date of progressive disease [PD] or death due to any cause, whichever occurred first), PFS (defined as the time from first dose of utidelone treatment to PD or death due to any cause, whichever occurred first, as documented by the investigators), OS (defined as the time from the first dose of utidelone treatment to death due to any cause), and safety. The incidence of treatment-emergent adverse events (TEAEs) and treatment-related adverse events (TRAEs) was assessed by the investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.25
Statistical analysis
Data from all participating hospitals were pooled. All efficacy and safety analyses were performed on the intention-to-treat (ITT) cohort, which referred to all patients who received at least one dose of utidelone. Efficacy analysis was also performed on the per-protocol (PP) cohort, which referred to those who received at least two cycles of utidelone. Data were analyzed using Statistical Analysis System software (SAS Institute, Cary, NC, USA) version 9.4.
The null hypothesis for the ORR of utidelone in this exploratory clinical trial was set at 10% to detect any efficacy signal. This null hypothesis was based on the published ORRs of the microtubule inhibitors ixabepilone (administered daily for five consecutive days), docetaxel, and eribulin when used to treat locally advanced or metastatic NSCLC, which have been reported as 11.6%, 6.0%, and 12.2%, respectively.26, 27, 28 The alternative hypothesis was that the ORR would be ≥ 10%, thus warranting the further development of utidelone as a subsequent-line treatment for locally advanced or metastatic NSCLC. The number of patients included in this small exploratory clinical trial was deemed sufficient to detect an efficacy signal and determine whether a larger clinical trial was warranted.
Unless otherwise noted, for all statistical tests, a 95% CI with a two-sided alpha level of 0.05 was calculated. The 95% CIs for the ORR were calculated using the Clopper–Pearson method. The median DoR, PFS, and OS were estimated using the Kaplan–Meier method, and the corresponding 95% CIs were estimated using the Greenwood formula.
Results
Patient characteristics
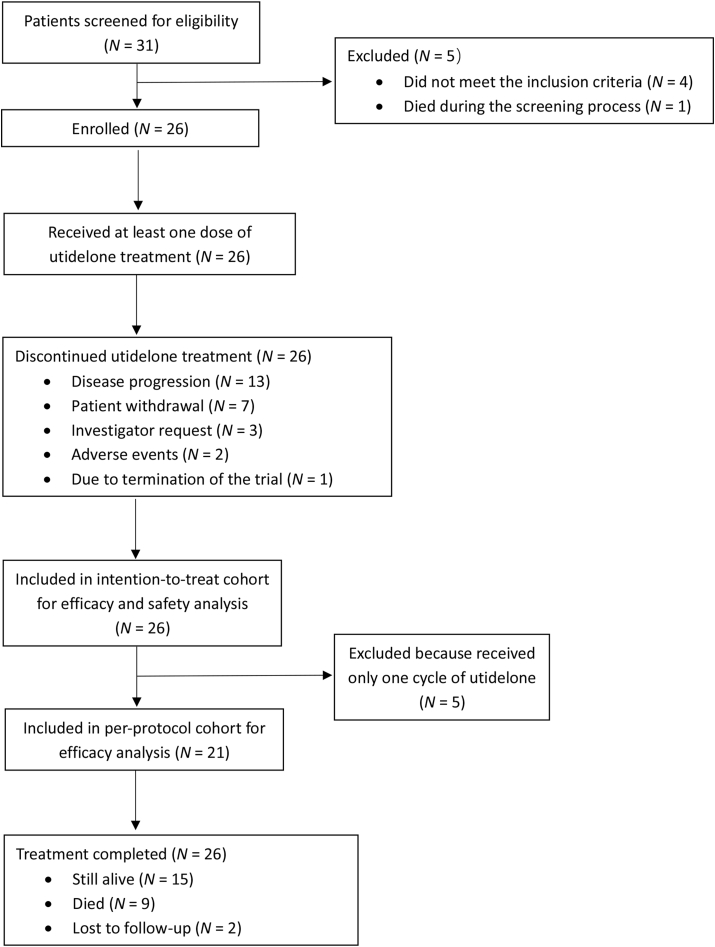
From March 12, 2019 to January 18, 2021, 26 patients with locally advanced or metastatic NSCLC were enrolled and received at least one dose of utidelone (ITT cohort) [Figure 1]. The PP cohort included 21 patients who received at least two cycles of utidelone. The baseline clinical characteristics of patients in the ITT cohort were generally balanced between treatment groups [Table 1]. The patients had a median age of 55 years (range, 39–68 years), and the majority of them had an ECOG PS of 1 (61.5%, 16/26), disease with adenocarcinoma histopathology (80.8%, 21/26), and stage Ⅳ disease (73.1%, 19/26).
Figure 1.
Flowchart of the clinical trail (BG01-1801). N: Number of patients.
Table 1.
Baseline clinical characteristics of patients in the ITT cohort.
| Characteristic | Utidelone (N = 26) |
|---|---|
| Age (years), median (range) | 55 (39–68) |
| Histopathological classification, n (%) | |
| Squamous cell carcinoma | 5 (19.2) |
| Adenocarcinoma | 21 (80.8) |
| Histopathological grade, n (%) | |
| Undifferentiated | 5 (19.2) |
| Poorly differentiated | 6 (23.1) |
| Moderately differentiated | 4 (15.4) |
| Other | 11 (42.3) |
| Clinical stage, n (%) | |
| Stage ⅢA | 1 (3.8) |
| Stage ⅢB | 1 (3.8) |
| Stage Ⅳ | 20 (76.9) |
| Other | 4 (15.5) |
| Metastatic organ/site, n (%) | |
| Lymph nodes | 24 (92.3) |
| Bone | 12 (46.2) |
| Brain | 8 (30.8) |
| Pleura | 4 (15.4) |
| Liver | 2 (7.7) |
| Kidney and adrenal gland | 2 (7.7) |
| Other | 14 (53.8) |
| Number of metastatic sites, n (%) | |
| 1 | 3 (11.5) |
| 2 | 7 (26.9) |
| >2 | 16 (61.5) |
| ECOG PS score, n (%) | |
| 0 | 10 (38.5) |
| 1 | 16 (61.5) |
| Treatment history for NSCLC, n (%) | |
| ≥1 Previous treatment | 26 (100) |
| ≥1 Previous surgery | 8 (30.8) |
| ≥1 Previous radiotherapy | 8 (30.8) |
| ≥1 Previous drug or other treatment | 26 (100) |
| Type of treatment, n (%) | |
| Chemotherapy | 26 (100) |
| Targeted therapy | 21 (80.8) |
| Immunotherapy | 6 (23.1) |
| Other | 1 (3.8) |
| Last treatment line received prior to enrollment, n (%) | |
| 1L | 0 |
| 2L | 9 (34.6) |
| 3L | 10 (38.5) |
| ≥4L | 7 (26.9) |
| Taxane-containing regimens received, n (%) | 17 (65.4) |
| Platinum-containing regimens received, n (%) | 26 (100) |
ECOG: Eastern Cooperative Oncology Group; ITT: Intention-to-treat; L: Line; N: Number of patients; n: Number of patients in each subgroup; NSCLC: Non-small-cell lung cancer; PS: Performance status.
At baseline, nine (34.6%) patients had received second-line treatment for locally advanced or metastatic NSCLC, 10 (38.5%) patients had received third-line treatment, and seven (26.9%) patients had received fourth- or later-line of treatment. In total, 26 (100%) patients had received prior platinum treatment, 17 (65.4%) patients had also received prior taxane treatment, 21 (80.8%) patients had received targeted therapy, and six (23.1%) patients had received immunotherapy. At the data cut-off date of August 10, 2021, the median follow-up was 7.49 months (range, 1.4–26.7months). By the data cut-off date, nine (34.6%) patients were died, 15 patients were still alive, and two were lost to follow-up.
Objective response rate
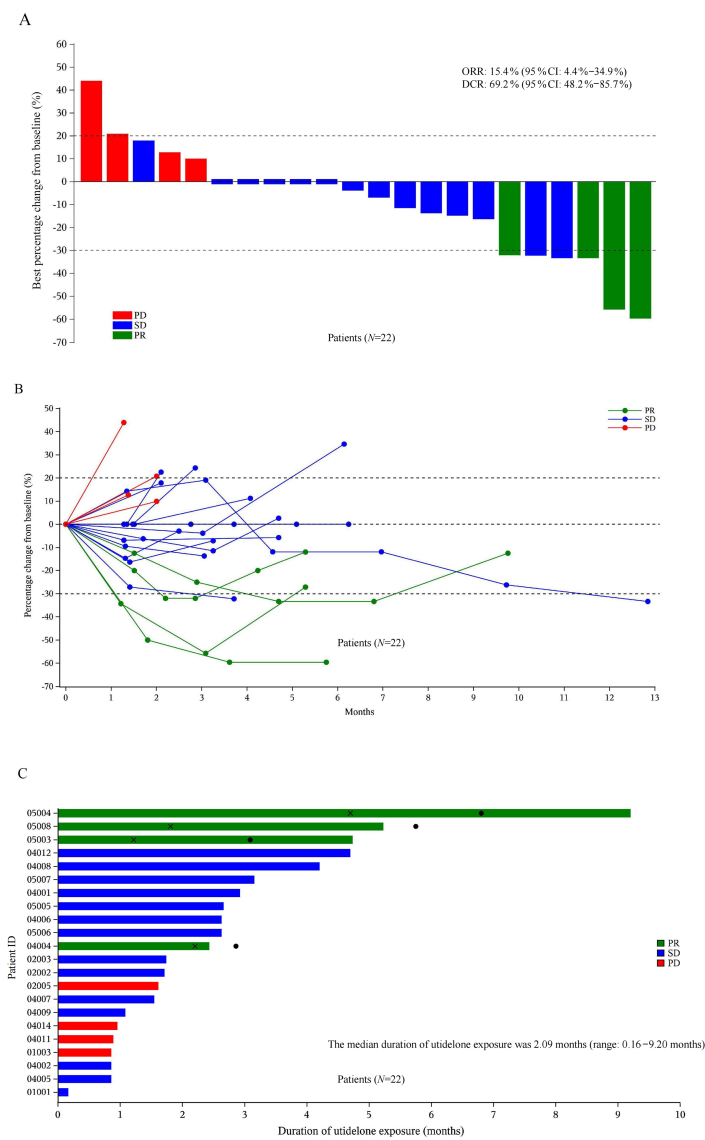
Efficacy was evaluated in both the ITT (N = 26) and PP (N = 21) cohorts, with ORRs determined to be 15.4% (95% CI, 4.4%–34.9%) and 19.0% (95% CI, 5.4%–41.9%), respectively [Table 2]. The median DoR in the ITT cohort was 4.1 months (95% CI, 3.1–5.1 months). Furthermore, tumor shrinkage was observed in 54.5% (12/22) of patients in the ITT cohort [Figure 2A] and 57.1% (12/21) of patients in the PP cohort [Supplementary Figure 1A]. The investigator-assessed percentage change in tumor size from baseline at different time points in the ITT and PP cohort is shown in Figure 2B and Supplementary Figure 1B, respectively.
Table 2.
Best objective responses to utidelone in NSCLC patients.
| Parameters | ITT cohort (N = 26) n (%) | PP cohort (N = 21) n (%) |
|---|---|---|
| Best objective response evaluation | ||
| Complete response | 0 | 0 |
| Partial response | 4 (15.4) | 4 (19.0) |
| Stable disease | 14 (53.8) | 13 (61.9) |
| Progressive disease | 4 (15.4) | 4 (19.0) |
| Not evaluable | 4 (15.4) | 0 |
| Objective response rate, 95% CI |
4 (15.4) 4.4–34.9 |
4 (19.0) 5.4–41.9 |
| Disease control rate, 95% CI |
18 (69.2) 48.2–85.7 |
17 (81.0) 58.1–94.6 |
The 95% CIs were calculated based on the Clopper–Pearson method. CI: Confidence interval; ITT: Intention-to-treat; N: Number of patients; NSCLC: Non-small-cell lung cancer; PP: Per-protocol.
Figure 2.
Efficacy of utidelone for patients with NSCLC in the ITT cohort. Waterfall plot of the best percentage change in the investigator-assessed size of target tumor lesions from baseline in the ITT cohort (A), spider plot of the change in the investigator-assessed tumor size over time in the ITT cohort (B) and swimmer plot of the ITT cohort (C). The dashed line at 20% represents the boundary for PD determination, and the dashed line at −30% represents the boundary for PR determination. Black crosses indicate the timepoint when the objective response was first observed, and the black dots indicate the timepoint when the objective response ended. Note: N = 22, as the best ORR was not evaluable for four of the patients, as per the study protocol. CI: Confidence interval; DCR: Disease control rate; ID: Identification; ITT: Intention-to-treat; N: Number of patients; NSCLC: Non-small-cell lung cancer; ORR: Objective response rate; PD: Progressive disease; PR: Partial response; SD: Stable disease.
In the ITT cohort, the disease control rate (DCR) was 69.2% (95% CI, 48.2%–85.7%); four patients achieved a PR and 14 patients had SD [Table 2]. In the PP cohort, the DCR was 81.0% (95% CI, 58.1%–94.6%). In the subgroups of patients who had received two, three, four, or more lines of prior treatment and in those who had received prior taxane or targeted therapy, the ORRs ranged from 11.1% to 20% and the DCRs ranged from 57.1% to 82.4% [Supplementary Table 1].
Utidelone exposure
All 26 patients in the safety cohort received a median of 3.0 cycles of utidelone treatment (range, 1.0–10.0 cycles). The median duration of utidelone exposure was 2.09 months (range, 0.16–9.20 months) [Figure 2C] and 2.43 months (range, 0.85–9.20 months) [Supplementary Figure 1C] in the ITT and PP cohort, respectively. A general trend could be seen indicating a better response with a longer exposure to utidelone.
Survival
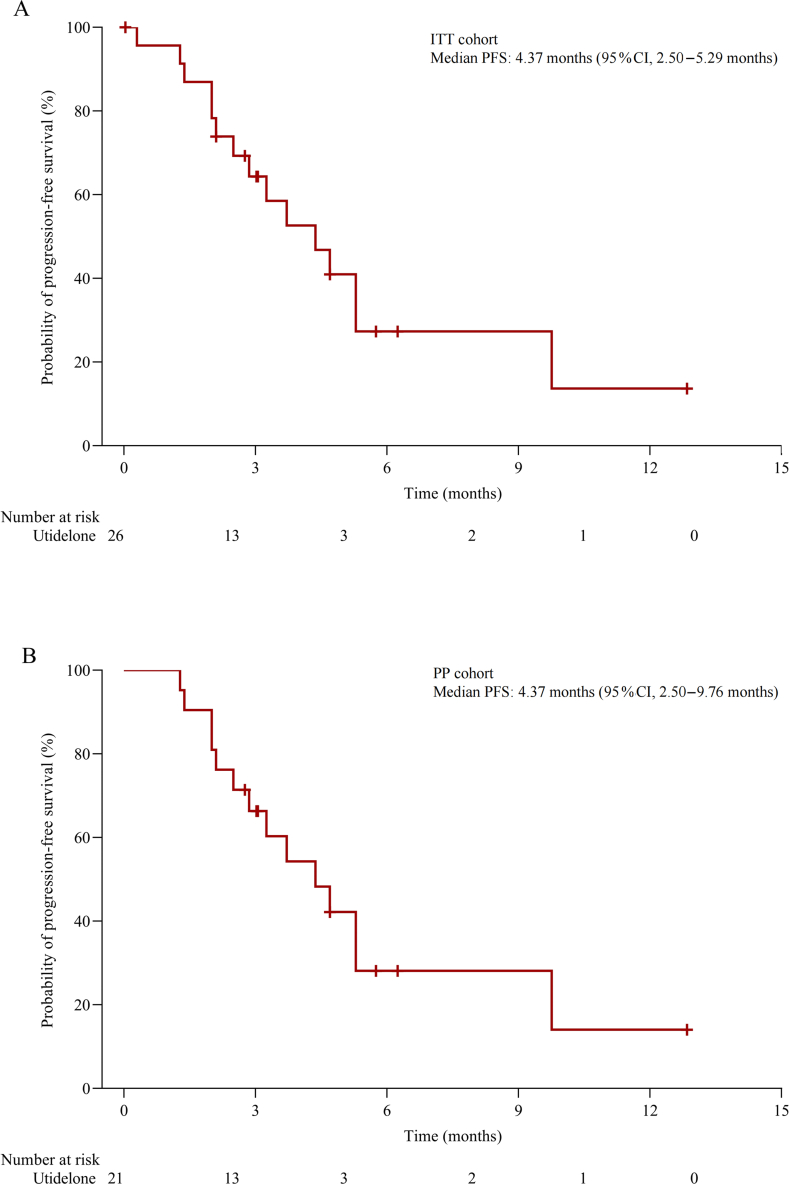
The median PFS was 4.37 months (95% CI, 2.50–5.29 months) in the ITT cohort [Figure 3A] and 4.37 months (95% CI, 2.50–9.76 months) in the PP cohort [Figure 3B]. The median OS was not reached, the 12-month OS rate was 69.0% (95% CI, 45.1%–84.1%) [Supplementary Figure 2A] and 71.0% (95% CI, 42.7%–87.1%) [Supplementary Figure 2B] in the ITT and PP cohort, respectively.
Figure 3.
PFS of patients with NSCLC treated with utidelone. Kaplan–Meier plots of PFS in the ITT cohort (A) and PP cohort (B). Data cut-off date: August 10, 2021. CI: Confidence interval; ITT: Intention-to-treat; NSCLC: Non-small-cell lung cancer; PFS: Progression-free survival; PP: Per-protocol.
Safety profile
All 26 patients in the ITT cohort were included in the safety cohort, and all patients experienced TEAEs; 38.5% (10/26) of the patients had Grade 3/4 TEAEs. The most common TEAEs of any grade, reported in >25% of patients, were PN (80.8%), insomnia (38.5%), anemia (34.6%), alopecia (34.6%), lymphopenia (30.8%), neutropenia (30.8%), and decreased appetite (26.9%). Common Grade 3/4 TEAEs (in >5% of patients) were PN (23.1%, all Grade 3), lymphopenia (7.7%), elevated γ-glutamyltransferase (7.7%), hyponatremia (7.7%), and pain in an extremity (7.7%) [Table 3].
Table 3.
TEAE (incidence ≥10%) in the safety cohort.
| Preferred term |
Utidelone (N = 26) n (%) |
|
|---|---|---|
| All grades | ≥ Grade 3 | |
| Peripheral neuropathy | 21 (80.8) | 6 (23.1) |
| Insomnia | 10 (38.5) | 0 |
| Anemia | 9 (34.6) | 1 (3.8) |
| Alopecia | 9 (34.6) | 0 |
| Lymphopenia | 8 (30.8) | 2 (7.7) |
| Neutropenia | 8 (30.8) | 1 (3.8) |
| Decreased appetite | 7 (26.9) | 0 |
| Leukopenia | 6 (23.1) | 1 (3.8) |
| Hypoalbuminemia | 6 (23.1) | 0 |
| Hyponatremia | 6 (23.1) | 2 (7.7) |
| Hypoesthesia | 6 (23.1) | 0 |
| Pain in extremity | 6 (23.1) | 2 (7.7) |
| Hypertriglyceridemia | 6 (23.1) | 0 |
| γ-Glutamyltransferase increase | 5 (19.2) | 2 (7.7) |
| Alanine aminotransferase increase | 5 (19.2) | 1 (3.8) |
| Asthenia | 5 (19.2) | 0 |
| Diarrhea | 4 (15.4) | 0 |
| Hypercholesterolemia | 4 (15.4) | 0 |
| Constipation | 3 (11.5) | 0 |
| Hypochloremia | 3 (11.5) | 0 |
| Nausea | 3 (11.5) | 0 |
| Hyperglycemia | 3 (11.5) | 0 |
| Aspartate aminotransferase increase | 3 (11.5) | 1 (3.8) |
| Blood alkaline phosphatase increase | 3 (11.5) | 1 (3.8) |
| Pulmonary embolism | 0 | 1 (3.8) |
| Bilirubin conjugated increase | 0 | 1 (3.8) |
N: Number of patients; n: Number of patients in each subgroup; TEAE: Treatment-emergent adverse event.
The dose of utidelone was reduced for nine (34.6%) patients, treatment was interrupted for ten (38.5%) patients and two (7.7%) patients discontinued the treatment, owing to TEAEs [Table 4]. The most common TEAE was PN, which was mainly sensory PN. However, PN could be managed with a dose reduction, by prolonging the dosing interval, with a treatment interruption, or through symptomatic management using adjunctive treatments.
Table 4.
Dose adjustments of utidelone due to TEAE (safety cohort).
| Preferred term | Utidelone Safety cohort (N = 26) n (%) |
|---|---|
| TEAE leading to dose reduction | 9 (34.6) |
| Peripheral neuropathy | 5 (19.2) |
| Pain in extremity | 2 (7.7) |
| Hyponatremia | 1 (3.8) |
| Epilepsy | 1 (3.8) |
| Hypoesthesia | 1 (3.8) |
| TEAE leading to dose interruption | 10 (38.5) |
| Peripheral neuropathy | 5 (19.2) |
| Alanine aminotransferase increase | 1 (3.8) |
| Intestinal obstruction | 1 (3.8) |
| Epilepsy | 1 (3.8) |
| Hypoesthesia | 1 (3.8) |
| Anal pain | 1 (3.8) |
| Insomnia | 1 (3.8) |
| Aspartate aminotransferase increase | 1 (3.8) |
| Pain in extremity | 1 (3.8) |
| TEAE leading to dose discontinuation | 2 (7.7) |
| Hypoesthesia | 1 (3.8) |
| Perianal abscess | 1 (3.8) |
N: Number of patients; n: number of patients in each subgroup; TEAE: Treatment-emergent adverse event.
Five (19.2%) patients experienced serious adverse events (SAEs) in this clinical trial, as follows: PN (one patient; Grade 3), intestinal obstruction (one patient; Grade 2), epileptic seizure (one patient; Grade 2), perianal abscess (one patient; Grade 1), and pulmonary embolism (one patient; Grade 3). The PN and intestinal obstruction SAEs were considered treatment-related. There were no deaths related to TEAEs.
Subsequent anticancer treatment
Of the ITT cohort, 53.8% (14/26) of the patients were administered at least one subsequent anticancer treatment after the discontinuation of utidelone, which included targeted therapy (26.9%), other chemotherapy (15.4%), immunotherapy and radiotherapy (7.7%), and other treatments (3.8%).
Discussion
Despite many targeted agents being approved in the past decade for locally advanced or metastatic NSCLC, there is an urgent unmet need for treatment regimens that offer an alternative option for heavily pretreated patients who are either not suitable for, or have acquired resistance to, targeted agents, immunotherapy, and/or chemotherapy regimens. This clinical trial demonstrated the promising clinical activity and manageable safety profile of utidelone. This is the first clinical trial of an epothilone agent to show efficacy for heavily pretreated patients with platinum- and/or taxane-resistant locally advanced or metastatic NSCLC.
As the primary endpoint, the ORR for utidelone was 15.4% in the ITT cohort and 19.0% in the PP cohort, with a DCR of 69.0% and 71.0% in the ITT and PP cohort, respectively. Responses were achieved even in the heavily pretreated patients, including those who received prior targeted therapy. Although the number of patients in each subgroup was small, the data are encouraging and should be examined closely in future clinical studies. In the ITT cohort, the median PFS in this clinical trial was 4.37 months (95%CI: 2.50–5.29 months), the 12-month OS rate was 69.0% (95%CI: 45.1%–84.1%), and the median OS was not reached. Although it is not possible to compare the outcomes of this clinical trial directly with those of clinical trials of other subsequent-line therapies in different patient cohorts, it is interesting to note that the efficacy outcomes are in the same range as those observed for docetaxel in clinical trials of patients with locally advanced or metastatic NSCLC who had failed platinum-containing regimens. For example, in the TAX320 NSCLC Study Group trial, the ORR for docetaxel was 6.7%, the median OS was 5.7 months, and the 1-year OS was 32%.29 In more recent phase 3 clinical trials of immunotherapy agents in patients with NSCLC who had failed a platinum-containing regimen and appropriate targeted therapies, the docetaxel control arms achieved ORRs of 9%–15% and DCRs of 43%–56%.27, 28, 29, 30 In these immunotherapy clinical trails, the median PFS for docetaxel was 2.8–4.2 months, the median OS was 6.0–9.7 months, and the 1-year OS rates were 24%–39%.30, 31, 32, 33, 34
The efficacy of utidelone in patients with locally advanced or metastatic NSCLC also appears promising when considering the data reported for other novel microtubule-targeting agents evaluated for locally advanced or metastatic NSCLC treatment. For example, a phase 2 clinical trial of ixabepilone, as a second-line treatment, reported ORRs of 12%–14%, depending on the dose of ixabepilone used, and a DCR of 48%, a median PFS of 5.3–5.8 months, and a median OS of 7.3–8.3 months, but the patients were not as heavily pretreated as those in this clinical trial and, as targeted therapy and immunotherapy options were not available when it was conducted, patients had not received these drugs in their first-line regimens.26 Another phase 2 clinical trial of ixabepilone plus carboplatin in treatment-naïve patients with advanced NSCLC reported a median PFS of 5.3 months and a median OS of 13 months, but the efficacy of this combination for a heavily pretreated cohort of patients remains unknown.35 Eribulin, an inhibitor of microtubule dynamics, failed to demonstrate a superior OS compared with the investigator's choice of chemotherapy in a phase 3 clinical trial in patients with advanced nonsquamous NSCLC who had been heavily pretreated; the ORR was 12.2% for the eribulin group and 15.2% for the chemotherapy group, and the median OS for both treatment groups was 9.5 months.28
Utidelone was well tolerated in this clinical trial of pretreated patients and the common TEAEs were similar to those noted in clinical trials of utidelone for patients with MBC.20,21 In the phase 3 clinical trial of utidelone plus capecitabine vs. capecitabine alone, to treat MBC, the profiles and incidences of the most common AEs, including palmar-plantar erythrodysesthesia and hematological and gastrointestinal toxicities, were similar between the two groups.21 Furthermore, the patient age, disease stage, and treatment history were similar in this phase 2 clinical trial for patients with locally advanced or metastatic NSCLC and the phase 3 clinical trial for patients with MBC.20,21
Based on the literature, utidelone is the only microtubule inhibitor, to date, to be associated with a low incidence of myelosuppression (3.8% for Grade 3/4 neutropenia in comparison to that with other microtubule inhibitors: 67.3% for docetaxel,27 38.6% for nab-paclitaxel,36 48.7% for eribulin,28 and 17.0% for ixabepilone26 in lung cancer. In addition, no febrile neutropenia was reported with utidelone in this clinical trial, whereas this AE was reported with docetaxel, nab-paclitaxel, and ixabepilone.27,28,36 To confirm these observations pertaining to the AE profile of utidelone, head-to-head comparisons with other microtubule inhibitors will be necessary. Platinum-based regimens are associated with considerable toxicity, with major AEs that include hematological toxicities, nephrotoxicities, and nausea and vomiting.37 Docetaxel is particularly associated with PN, myelosuppression (including neutropenia and febrile neutropenia), arthralgias, myalgias, and skin reactions.38 For both ixabepilone and eribulin, an incidence of Grade 3/4 neutropenia of approximately 45%–49% has been reported in clinical trials in patients with advanced or metastatic NSCLC.26,28
Consistent with PN being a common AE of microtubule inhibitors, including eribulin, paclitaxel, and ixabepilone, it was the prominent AE associated with utidelone in this clinical trial, occurring as a Grade 3/4 AE in 23.1% of patients. However, it could be managed with a dose delay, dose reduction, or symptomatic treatment, generally resulting in resolution of this AE from Grade 3 to baseline in approximately three weeks. Similarly, in the phase 3 clinical trial of utidelone plus capecitabine in patients with MBC pretreated with anthracycline and taxane, 81% of patients experienced any-grade PN and 22% of patients developed Grade 3 PN. All the Grade 3 PN in the phase 3 clinical trial in MBC were resolved in approximately 3.6 weeks.20 In this phase 2 clinical trial for patients with locally advanced or metastatic NSCLC, there were no discontinuations of utidelone owing to PN. By contrast, PN was the most frequent TRAE leading to discontinuation in the utidelone plus capecitabine group (16% vs. 0% in the capecitabine alone group) in the phase 3 clinical trial of utidelone for MBC.20
In phase 1 and phase 2 clinical trials of utidelone for solid tumors, iv administration once every 21 days and iv administration daily on day 1–5, every 21 days, were evaluated.19,39 We selected the 5-day dosing schedule every 21 days for further development as it resulted in a similar safety profile but better efficacy than the once-every-21-day schedule. An oral formulation of utidelone is also in development to enable its more convenient administration and to reduce the incidence of PN. Limitations of this clinical trail include it being a single-arm study with a small sample size, which precluded any subgroup analyses; a randomized controlled clinical trail in a larger patient cohort would be needed to address this.
In conclusion, this clinical trial demonstrated the encouraging efficacy and manageable safety profile of utidelone for patients with locally advanced or metastatic NSCLC who have failed standard second-line treatment.
Funding
None.
Authors contribution
Yuankai Shi: design, writing, revision of clinical trial protocol, collection of data, analysis, and interpretation of data, drafting, and review of the manuscript, and approval of the final version before submission; Li Tang: design, writing, revision of clinical trial protocol, drafting and review of the manuscript, and approval of the final version before submission; Gongyan Chen: collection of data, analysis, and interpretation of data, review of the manuscript, and approval of the final version before submission; Yanqiu Zhao: collection of data, analysis, and interpretation of data, review of the manuscript, and approval of the final version before submission; Jing Zhao: collection of data, analysis and interpretation of data, review of the manuscript, and approval of the final version before submission; and Lin Lin: collection of data, analysis and interpretation of data, review of the manuscript, and approval of the final version before submission.All investigators had access to the data and agreed to be accountable for the accuracy and integrity of the data and analyses. The corresponding author had full access to all clinical trial data and had final responsibility for the decision to submit the manuscript for publication.
Ethics statement
This clinical trial was compliant with the Declaration of Helsinki and was performed in accordance with Good Clinical Practice guidelines. The protocol was approved by the ethics committees of each of the four participating hospitals. The approval numbers are 18–105/1683, AF-06-1.3, 2019082219, and KS2018480. Written informed consent was obtained from each patient before enrollment.
Data availability statement
The data collected for this trial can be made available to others in a de-identified form after all primary and secondary endpoints have been published and in the presence of a data transfer agreement. Data can be made available from the corresponding author upon reasonable request.
Conflict of interest
Li Tang is an employee of Beijing Biostar Pharmaceuticals and is the inventor of several patents owned by Beijing Biostar Pharmaceuticals (formerly Beijing Biostar Technologies). All other authors declare no competing interests. Beijing Biostar Pharmaceuticals Co., Ltd., Beijing, China had no role in data collection, analysis, or interpretation. The statistical analyses for this trial were performed by a data management group (Nanjing CR Medicon Pharmaceutical Technology Co., Ltd.). We declare that we have no financial and personal relationships with Beijing Biostar Pharmaceuticals Co., Ltd., and Nanjing CR Medicon Pharmaceutical Technology Co., Ltd., that can inappropriately influence our work.
Acknowledgments
We thank all the patients and their families; their participation and support made this clinical trial possible. Our appreciation is extended to all staff in the participating centers and also to the staff at Nanjing CR Medicon Pharmaceutical Technology Co., Ltd., for their role in data management and statistical analysis. We thank Ezzie Hutchinson, EKH Medical Communications Ltd, for editorial assistance with this manuscript. We also thank Dr. Tongji Xie (National Cancer Centre/National Clinical Research Centre for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, China) for manuscript editing assistance. The authors would like to thank Li Tang from the Beijing Biostar Pharmaceuticals Co., Ltd., for paticipating in organizing this clincial trail.
Managing Editor: Peng Lyu
Footnotes
Given his role as Editor in Chief, Prof. Yuankai Shi had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Peng Lyu.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpt.2023.10.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N., Forjaz G., Mooradian M.J., et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.About lung cancer. American Cancer Society; Atlanta: 2022. https://www.cancer.org/content/dam/CRC/PDF/Public/8703.00.pdf [Last accessed on 2023 March 31] [Google Scholar]
- 4.Surveillance, epidemiology, and end results: 2011–2017. SEER Registry Data. National Cancer Institute; Bethesda: 2023. https://seer.cancer.gov/statfacts/html/lungb.html [Last accessed on 2023 March 31] [Google Scholar]
- 5.Shi Y., Zhang X., Wu G., et al. Treatment strategy, overall survival and associated risk factors among patients with unresectable stage IIIB/IV non-small cell lung cancer in China (2015–2017): a multicentre prospective study. Lancet Reg Health West Pac. 2022;23 doi: 10.1016/j.lanwpc.2022.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical practice guidelines in oncology. Non-small cell lung cancer. V2. 2023. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 Last accessed on 2023 March 31] [Google Scholar]
- 7.Chinese Association for Clinical Oncologists, Medical Oncology Branch of Chinese International Exchange, Promotion Association for Medical and Healthcare Clinical practice guideline for stage IV primary lung cancer in China (2023 edition) [in Chinese] Natl Med J Chin. 2023;45:1–30. doi: 10.3760/cma.j.cn112152-20221009-00687. [DOI] [PubMed] [Google Scholar]
- 8.Hardin C., Shum E., Singh A.P., Perez-Soler R., Cheng H. Emerging treatment using tubulin inhibitors in advanced non-small cell lung cancer. Expet Opin Pharmacother. 2017;18:701–716. doi: 10.1080/14656566.2017.1316374. [DOI] [PubMed] [Google Scholar]
- 9.Sève P., Dumontet C. Chemoresistance in non-small cell lung cancer. Curr Med Chem Anticancer Agents. 2005;5:73–88. doi: 10.2174/1568011053352604. [DOI] [PubMed] [Google Scholar]
- 10.Bollag D.M., McQueney P.A., Zhu J., et al. Epothilones, a new class of microtubule-stabilizing agents with a Taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 11.Giannakakou P., Gussio R., Nogales E., et al. A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc Natl Acad Sci U S A. 2000;97:2904–2909. doi: 10.1073/pnas.040546297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee F.Y., Borzilleri R., Fairchild C.R., et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- 13.Fda Drug Approvals . 2007. Ixempra prescribing information. Silver Spring: FDA.https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022065s002lbl.pdf [Last accessed on 2023 March 31] [Google Scholar]
- 14.Thomas E., Tabernero J., Fornier M., et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007;25:3399–3406. doi: 10.1200/JCO.2006.08.9102. [DOI] [PubMed] [Google Scholar]
- 15.Perez E.A., Lerzo G., Pivot X., et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007;25:3407–3414. doi: 10.1200/JCO.2006.09.3849. [DOI] [PubMed] [Google Scholar]
- 16.Thomas E.S., Gomez H.L., Li R.K., et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 17.Sparano J.A., Vrdoljak E., Rixe O., et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–3263. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rak Tkaczuk K.H.R. Ixabepilone as monotherapy or in combination with capecitabine for the treatment of advanced breast cancer. Breast Cancer. 2011;5:1–14. doi: 10.4137/BCBCR.S5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang P., Sun M., Qiu R., Tang L., Dou G., Xu B. Phase I clinical and pharmacokinetic study of UTD1, a genetically engineered epothilone analog in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;68:971–978. doi: 10.1007/s00280-011-1571-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P., Sun T., Zhang Q., et al. Utidelone plus capecitabine versus capecitabine alone for heavily pretreated metastatic breast cancer refractory to anthracyclines and taxanes: a multicentre, open-label, superiority, phase 3, randomised controlled trial. Lancet Oncol. 2017;18:371–383. doi: 10.1016/S1470-2045(17)30088-8. [DOI] [PubMed] [Google Scholar]
- 21.Xu B., Sun T., Zhang Q., et al. Efficacy of utidelone plus capecitabine versus capecitabine for heavily pretreated, anthracycline- and taxane-refractory metastatic breast cancer: final analysis of overall survival in a phase III randomised controlled trial. Ann Oncol. 2021;32:218–228. doi: 10.1016/j.annonc.2020.10.600. [DOI] [PubMed] [Google Scholar]
- 22.China approves utidelone as primary new drug of epothilone class for breast cancer. PharmArch; 2021. https://www.pharmarch.com/china-approves-Utidelone-as-primary-new-drug-of-epothilone-class-for-breast-cancer/ [Last accessed on 2023 March 31] [Google Scholar]
- 23.gov Clinicaltrials. National Institutes of Health; Bethesda: 2018. NCT03693547. Clinical study of utidelone injection in patients with advanced non-small cell lung cancer (NSCLC)https://clinicaltrials.gov/ct2/show/NCT03693547 [Last accessed 2023 March 31] [Google Scholar]
- 24.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.CTCAE 4.03. Common Terminology criteria for adverse events (CTCAE). Version 4.0. National Cancer Institute; Bethesda: 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf [Last accessed on 2023 March 31] [Google Scholar]
- 26.Vansteenkiste J., Lara P.N., Jr., Le Chevalier T., et al. Phase II clinical trial of the epothilone B analog, ixabepilone, in patients with non small-cell lung cancer whose tumors have failed first-line platinum-based chemotherapy. J Clin Oncol. 2007;25:3448–3455. doi: 10.1200/JCO.2006.09.7097. [DOI] [PubMed] [Google Scholar]
- 27.Fossella F.V. Docetaxel in second-line treatment of non-small-cell lung cancer. Clin Lung Cancer. 2002;3(Suppl 2):S23–S28. doi: 10.3816/clc.2002.s.010. [DOI] [PubMed] [Google Scholar]
- 28.Katakami N., Felip E., Spigel D.R., et al. A randomized, open-label, multicenter, phase 3 study to compare the efficacy and safety of eribulin to treatment of physician's choice in patients with advanced non-small cell lung cancer. Ann Oncol. 2017;28:2241–2247. doi: 10.1093/annonc/mdx284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fossella F.V., DeVore R., Kerr R.N., et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 non-small cell lung cancer study group. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 30.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 33.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fehrenbacher L., Spira A., Ballinger M., et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 35.Edelman M.J., Schneider C.P., Tsai C.M., et al. Randomized phase II study of ixabepilone or paclitaxel plus carboplatin in patients with non-small-cell lung cancer prospectively stratified by beta-3 tubulin status. J Clin Oncol. 2013;31:1990–1996. doi: 10.1200/JCO.2012.45.3282. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H., Taima K., Morimoto T., et al. A single-arm phase II study of nab-paclitaxel for patients with chemorefractory non-small cell lung cancer. BMC Cancer. 2017;17:683. doi: 10.1186/s12885-017-3684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Addario G., Pintilie M., Leighl N.B., Feld R., Cerny T., Shepherd F.A. Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol. 2005;23:2926–2936. doi: 10.1200/JCO.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Cella D., Peterman A., Hudgens S., Webster K., Socinski M.A. Measuring the side effects of taxane therapy in oncology: the functional assessment of cancer therapy-taxane (FACT-taxane) Cancer. 2003;98:822–831. doi: 10.1002/cncr.11578. [DOI] [PubMed] [Google Scholar]
- 39.Zhang P., Tong Z., Tian F., et al. Phase II trial of utidelone as monotherapy or in combination with capecitabine in heavily pretreated metastatic breast cancer patients. J Hematol Oncol. 2016;9:68. doi: 10.1186/s13045-016-0297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected for this trial can be made available to others in a de-identified form after all primary and secondary endpoints have been published and in the presence of a data transfer agreement. Data can be made available from the corresponding author upon reasonable request.