Abstract
Human macrophages express chemokine receptors that act as coreceptors for human immunodeficiency virus type 1 (HIV-1) and are major targets for HIV-1 infection in vivo. The effects of cytokines on HIV-1 infection of macrophages and on the expression of CCR5, the principal coreceptor for macrophage-tropic viruses, have now been investigated. Expression of CCR5 on the surface of freshly isolated human monocytes was virtually undetectable by flow cytometry with the monoclonal antibody 5C7. However, after culture of monocytes for 48 h in serum-free medium, approximately 30% of the resulting macrophages expressed CCR5 and the cells were susceptible to infection by macrophage-tropic HIV-1. Addition of either macrophage colony-stimulating factor (M-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) to the cultures markedly increased both the extent of HIV-1 entry and replication as well as surface expression of CCR5. In contrast, addition of the T-helper 2 (Th2) cell-derived cytokine interleukin-4 (IL-4) or IL-13 prevented the expression of CCR5 induced by culture in medium alone, and IL-4 inhibited virus entry, replication, and cytopathicity under these conditions. IL-4 or IL-13 also prevented the stimulatory effects of M-CSF or GM-CSF on CCR5 expression as well as HIV-1 entry and replication. In addition, IL-4 reversed the increase in CCR5 expression induced by pretreatment of cells with M-CSF. Although IL-10 also inhibits HIV-1 replication in macrophages, it did not suppress surface CCR5 expression induced by colony-stimulating factors. These results indicate that the cytokine environment determines the susceptibility of macrophages to HIV-1 infection by various mechanisms, one of which is the regulation of HIV-1 coreceptor expression.
Macrophages and CD4+ T lymphocytes are the major targets of infection by human immunodeficiency virus type 1 (HIV-1) in vivo (28, 43, 45, 60). The entry of virus into these cells is mediated by interaction of the virus envelope with both CD4 and chemokine receptors. The receptor for stromal cell-derived factor-1 (5), CXC chemokine receptor 4 (CXCR4), has been identified as the coreceptor for T-cell-tropic strains of HIV-1 (21), whereas the CC chemokine receptor CCR5 (48, 52) and, to a lesser extent, CCR3 (29) and CCR2b mediate the binding and entry of macrophage-tropic and dual-tropic primary isolates of HIV-1 (1, 9, 14, 18, 19). The envelope proteins (gp120) of T-cell-tropic and macrophage-tropic viruses have been shown to interact with CXCR4 (32) and CCR5 (59, 61), respectively, in a CD4-dependent manner.
The level of expression of specific chemokine receptors on the cell surface correlates with the susceptibility of cells to HIV-1 infection. Both CXCR4 and CCR5 are differentially expressed and regulated in human T lymphocytes (6). CXCR4 is expressed predominantly on naive T lymphocytes (CD26low CD45RA+ CD45RO−), whereas CCR5 is expressed on activated or memory T cells (CD26high CD45RAlow CD45RO+) (6, 62). The expression of CXCR4 and CCR5 on T cells is up-regulated by exposure to interleukin-2 (IL-2) and phytohemagglutinin (6, 59), whereas CCR5 expression is down-regulated by CD28 costimulation (7). Although lipopolysaccharide rapidly inhibits the expression of CCR2 in human monocytes (55), the regulation of CCR5 expression on monocytes/macrophages and its relation to viral entry have not been characterized.
Cytokines are major host factors in the pathogenesis of HIV-1 infection (20). Patterns of cytokine expression have been linked to a proposed polarization into T-helper 1 (Th1) (cell mediated) or Th2 (humoral) immune responses. Th1 cells are characterized by secretion of IL-2 and gamma interferon, whereas Th2 cells are characterized by secretion of IL-4, IL-5, IL-10, and IL-13. HIV-1 infection affects patterns of cytokine production (24, 34), and cytokines modify the production of HIV-1 from macrophages (31) and T cells (20). Both tumor necrosis factor alpha and IL-1β enhance HIV-1 production through NF-κB-mediated transactivation of the viral long terminal repeat (44). Macrophage colony-stimulating factor (M-CSF) increases the production of HIV-1 (31, 35), while granulocyte-macrophage colony-stimulating factor (GM-CSF) either enhances (31) or in some cases suppresses (17, 35) virus infection. Other cytokines such as the Th2 cytokines IL-4, IL-10, and IL-13 inhibit HIV-1 infection in human primary macrophages (15, 30, 36, 37, 40, 53), and IL-4 blocks virus replication in peripheral blood cultures (46), although the mechanism of inhibition has not been determined.
We have now investigated the roles of growth factors and cytokines in HIV-1 entry and replication, as well as in modulation of the expression of the monocytotropic virus coreceptor CCR5, in human primary macrophages.
Effects of M-CSF, GM-CSF, and IL-4 on HIV-1 entry and replication in macrophages.
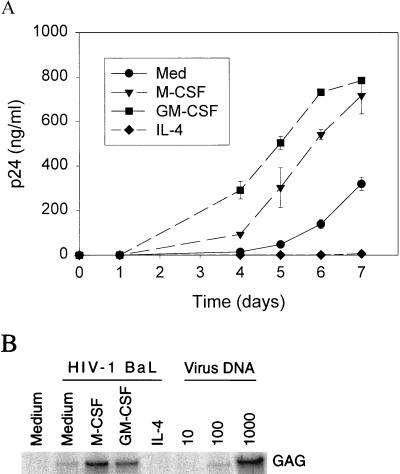
To evaluate further the effects of M-CSF, GM-CSF, and IL-4 on virus replication and entry, we incubated each cytokine at a concentration of 20 ng/ml with monocytes in serum-free cultures before and during infection with HIV-1 BaL. Similar to previous results obtained with serum-containing cultures (30), GM-CSF and M-CSF each increased the production of HIV-1 p24 antigen in our serum-free culture system (Fig. 1A). In contrast to the stimulatory effects of M-CSF and GM-CSF, IL-4 (20 ng/ml) almost completely inhibited p24 antigen release (Fig. 1A) and eliminated HIV-1-induced syncytium formation and cytopathicity (data not shown).
FIG. 1.
Effects of M-CSF, GM-CSF, and IL-4 on HIV-1 replication and entry. Monocytes were isolated by elutriation (26), cultured for 48 h in macrophage-SFM in the absence or presence of M-CSF (20 ng/ml), GM-CSF (20 ng/ml), or IL-4 (20 ng/ml), and then subjected to infection with HIV-1 BaL (2.34 × 107 cell-free virus) (23). (A) At the indicated times after infection, culture supernatants were harvested from cells incubated in the absence or presence of M-CSF, GM-CSF, or IL-4 and were then assayed for p24 antigen by enzyme-linked immunosorbent assay (Coulter, Miami, Fla.). Data are means ± standard deviations of triplicates from a representative experiment. (B) Four hours after infection, lysates were prepared from cells incubated in medium alone or in medium containing the indicated cytokine and were analyzed by semiquantitative PCR for proviral DNA. A control lysate was prepared from cell line 8E5 (22), which contains one copy of the HIV-1 genome per cell. PCR was performed with the gag-specific primers SK38 (5′-ATA ATC CAC CTA TCC CAG TAG GAG AAA T-3′) and SK39 (5′-TTT GGT CCT TGT CTT ATG TCC AGA ATG C-3′), 10 μl of lysate, and Taq polymerase for 30 cycles. The PCR products were detected by hybridization with excess SK19 probe (5′-ATC CTG GGA TTA AAT AAA ATA GTA AGA ATG TAT AGC CCT AC-3′) end labeled with [γ-32P]ATP. Indicated copy numbers (1,000, 100, 10) of control HIV-1 DNA were used as positive controls. Medium, uninfected parallel macrophages treated with medium only. GAG, HIV-1 prototypic gag gene segment amplified by SK18 and SK19 primers.
To determine whether the effects of these cytokines on viral replication and cell fusion reflected an action at the early steps of virus entry, we subjected proviral DNA to semiquantitative PCR amplification 4 h after infection. Both M-CSF and GM-CSF increased, whereas IL-4 reduced, the amount of viral DNA synthesized at this early time point (Fig. 1B). Thus, these cytokines appear to affect HIV-1 infection in human macrophages at an early step of virus entry.
Relation of the effects of cytokines on virus replication to those on expression of CCR5.
The entry of macrophage-tropic HIV-1 into cells depends on the cell surface expression of CD4 and CCR5, although some virus strains can also use CCR2b and CCR3. CCR3 mRNA is not present in either freshly isolated monocytes or cultured macrophages, whereas CCR2b is expressed in fresh monocytes but not in macrophages (42, 55). Therefore, to study the effect of cytokines on HIV-1 coreceptor expression, we focused on the cell surface abundance of CCR5 on monocytes and macrophages.
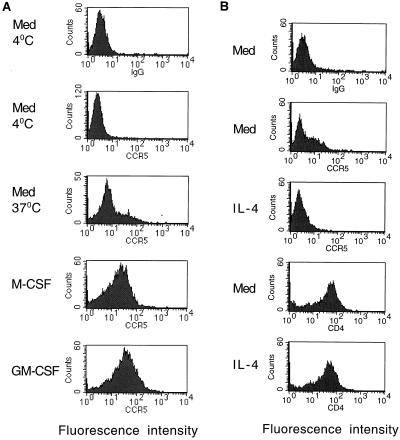
Cell surface expression of CCR5 in monocytes and macrophages was measured by flow cytometry with the monoclonal antibody 5C7. Less than 1% of fresh monocytes expressed CCR5 (Fig. 2A), which is consistent with previously published flow cytometry data (62) and reverse transcription-PCR results (17, 42, 62) indicating very low to absent CCR5 expression in resting monocytes. After culture in macrophage-serum-free medium (SFM) for 2 days, 29% of the cells expressed CCR5. Addition of M-CSF or GM-CSF to the culture increased the number of cells expressing CCR5 to 72 or 86%, respectively. In contrast, IL-4 completely prevented the increase in CCR5 expression induced by incubation of cells in medium alone (Fig. 2B). In the same cultures, the expression of CD4 was not affected by IL-4 treatment. These results indicated that the expression of CCR5 is related to the entry and replication of monocytotropic virus in macrophages cultured in the studied cytokine environments.
FIG. 2.
Regulation of CCR5 expression by M-CSF, GM-CSF, and IL-4. (A) Freshly isolated monocytes were maintained at 4°C in medium (Med) or cultured at 37°C for 48 h in macrophage-SFM alone or in the presence of M-CSF (20 ng/ml) or GM-CSF (20 ng/ml) as indicated. Surface expression of CCR5 was then examined by flow cytometric analysis with monoclonal antibody 5C7 to CCR5 (62) using a FACSortflow cytometer (Becton Dickinson, Sunnyvale, Calif.); analysis was also performed with a mouse isotype-matched immunoglobulin G2a (IgG2a) control antibody (top). (B) Cells were cultured for 48 h at 37°C in medium alone or in the presence of IL-4 (20 ng/ml), after which surface expression of CCR5 and CD4 (Leu-3a) was assessed by flow cytometry; mouse IgG2a was used as an isotype-matched control antibody.
Effects of IL-4 and IL-13 on M-CSF-induced enhancement of HIV-1 infection.
To investigate the interaction of positive and negative effects of different cytokines on HIV-1 infection of macrophages, we next examined the impact of IL-4 and IL-13 on M-CSF-induced enhancement of HIV-1 entry and replication. IL-13 was chosen because it exhibits a subset of the activities of the structurally related IL-4 (64) and it inhibits HIV-1 infection of primary macrophages (36, 37). Seven days after infection of M-CSF-treated macrophages with macrophage tropic viruses HIV-1 BaL, HXB2-168.1, or HXB2-168.3, p24 antigen production in cells cultured in the presence of IL-4 (20 ng/ml) or IL-13 (20 ng/ml) was 0 to 30% of that of cells incubated with M-CSF alone (Fig. 3A). To determine whether the reduced infection in macrophages treated with IL-4 or IL-13 was related to early entry events, we examined proviral DNA synthesis by semiquantitative PCR. Four hours after exposure to HIV-1 BaL in the presence of M-CSF, the amount of proviral DNA in IL-4- or IL-13-treated macrophages was markedly less than that in macrophages cultured with M-CSF alone (Fig. 3B).
FIG. 3.
Inhibition of M-CSF enhancement of HIV-1 entry and replication by IL-4 and IL-13. (A) Monocytes were cultured for 48 h in macrophage-SFM containing M-CSF (20 ng/ml) in the absence or presence of IL-4 (20 ng/ml) or IL-13 (20 ng/ml) and were then subjected to infection with HIV-1 BaL, HXB2-168.1, or HXB2-168.3 isolates (13). Seven days after infection, culture supernatants were harvested and assayed for p24 antigen by enzyme-linked immunosorbent assay. Data are expressed as percentages of p24 production for cells cultured with M-CSF alone and are means ± standard deviations of triplicates from a representative experiment. (B) Four hours after HIV-1 BaL infection of cells treated as described for panel A, cell lysates were prepared and analyzed for proviral DNA by semiquantitative PCR. Indicated copy numbers (500, 50, 5) of control HIV-1 DNA were used as positive controls. No virus, uninfected parallel macrophages treated with medium only as negative control. Gag, HIV-1 prototypic gag gene segment amplified by SK18 and SK19 primers and detected by [γ-32P]ATP-end-labeled SK19 hybridization; Free Probe, SK19 end labeled with [γ-32P]ATP.
Effects of IL-4 and IL-13 on CCR5 expression induced by M-CSF or GM-CSF.
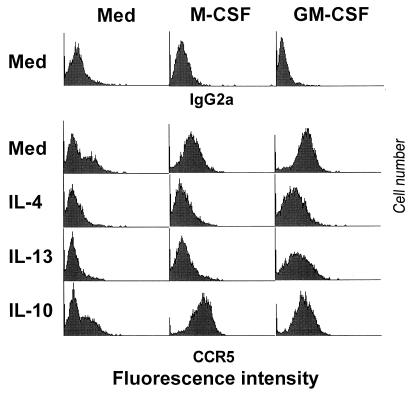
We next examined the effects of IL-4 and IL-13 on the M-CSF- and GM-CSF-induced increases in CCR5 expression on macrophages. IL-4 and IL-13 prevented not only the increase in CCR5 expression normally apparent during culture in medium alone for 48 h but also the up-regulation of CCR5 expression by M-CSF and GM-CSF (Fig. 4). Titration experiments showed that a concentration of 2 ng/ml was sufficient for IL-4 to inhibit completely the M-CSF-induced increase in CCR5 expression (data not shown).
FIG. 4.
Effects of IL-4 and IL-13 on up-regulation of CCR5 expression by M-CSF and GM-CSF. Monocytes were cultured for 48 h in macrophage-SFM in the presence of the indicated combinations of cytokines. Surface expression of CCR5 was then examined by flow cytometry; mouse IgG2a was used as an isotype-matched control antibody (upper). Fluorescence intensity is presented on a logarithmic scale.
Effect of IL-10 on CCR5 expression.
IL-10, like IL-4 and IL-13, is a Th2 cytokine that is produced by monocytes and inhibits HIV-1 infection of macrophages (8, 30, 37, 53). We therefore examined the effect of IL-10 on CCR5 expression. In contrast to IL-4 and IL-13, IL-10 (30 ng/ml) did not downmodulate CCR5 expression on macrophages incubated in the absence or presence of M-CSF or GM-CSF (Fig. 4) and actually increased CCR5 expression in the presence of M-CSF. IL-10 was active on these cells as shown by suppression of HLA-DR expression (data not shown). These results are consistent with a previous study showing that IL-10 does not affect viral entry in human primary macrophages (30).
Effect of IL-4 on cells already expressing CCR5 in response to M-CSF.
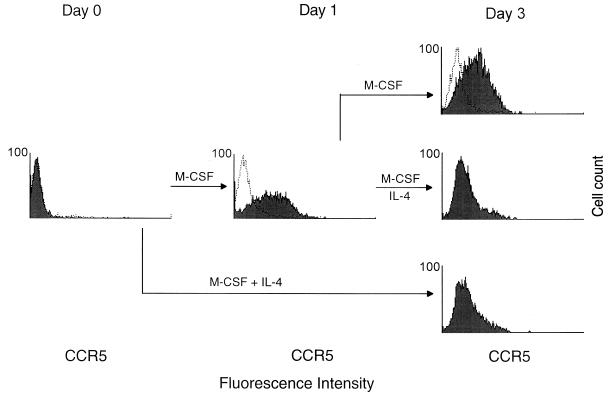
Given that IL-4 prevented the M-CSF-induced increase in CCR5 expression in macrophages, we next investigated the effect of IL-4 in cells in which coreceptor expression had already been induced by M-CSF. Monocytes were incubated with M-CSF for 1 day and then in the additional presence of IL-4 for 2 days (Fig. 5). CCR5 expression was then evaluated by flow cytometry and compared with that of cells cultured continuously with M-CSF alone or with M-CSF plus IL-4. CCR5 expression was increased after exposure of cells to M-CSF for 1 day, and this increase was prevented by addition of IL-4 at the beginning of the culture. The M-CSF-induced increase in CCR5 expression was also completely reversed after addition of IL-4 at the end of day 1.
FIG. 5.
Reversal of the M-CSF-induced increase in CCR5 expression in monocytes by IL-4. Monocytes were cultured for 1 day in the presence of M-CSF and then for an additional 2 days with M-CSF alone (top) or with M-CSF plus IL-4 (middle). Alternatively, cells were cultured in the presence of M-CSF and IL-4 for 3 days (bottom). Cell surface expression of CCR5 was examined by flow cytometry with antibody 5C7 (filled histogram) at the indicated times; mouse IgG2a (open histogram) was used as an isotype-matched control antibody. Fluorescence intensity is presented on a logarithmic scale.
We have determined the effects of several cytokines on the expression of CCR5 as well as on HIV-1 entry and replication in human primary macrophages. Our results indicate that cytokines affect early steps in viral entry through modulation of CCR5 expression.
Our demonstration of the lack of expression of CCR5, the primary coreceptor for monocytotropic viruses, on freshly isolated monocytes may partially explain the absence of infected peripheral blood monocytes in the circulation of HIV-1-positive individuals and the resistance of freshly isolated blood monocytes to HIV-1 infection (50, 54). Transcripts encoding other chemokine receptors, including CCR3, a coreceptor for HIV-1 in microglial cells (27), are not detectable by reverse transcription-PCR in freshly isolated blood monocytes (42, 55). Although CCR2b mRNA is detectable in fresh monocytes by such analysis (42, 55), this coreceptor may not function to mediate HIV-1 infection in monocytes and it is not targeted by the virus isolates used in the present study. The abundance of CCR2b mRNA also decreases markedly during the maturation of monocytes into macrophages (42).
After culture in SFM for 48 h, 29% of monocytes expressed CCR5 and the cells became infectable by macrophage-tropic HIV-1. Thus, our results indicate that monocyte differentiation into macrophages, such as that which occurs during migration of monocytes into tissues, is accompanied by the expression of CCR5 and the development of susceptibility to infection by macrophage-tropic HIV-1. Similar results were reported recently by Di Marzio et al. (17). Our data are consistent with CCR5 being the primary coreceptor for infection by monocytotropic virus, as was indicated previously in a report that cells isolated from individuals with a mutation in the CCR5 gene were resistant to monocytotropic virus infection (12, 47). We have also observed a high level of expression of CXCR4 on monocytes/macrophages, and this expression was increased by culture (for up to 2 weeks) but the cells were not susceptible to infection by T-cell-line-tropic virus, as determined by p24 production. Whether the expressed CXCR4 receptors are defective in mediating virus entry or whether other cell surface molecules such as heparan sulfate (51) are also required remains to be determined.
GM-CSF and M-CSF enhanced viral entry and replication in human primary macrophages and markedly increased expression of CCR5. GM-CSF is secreted by monocytes in response to stimulation with gp120 from certain strains of HIV-1 (10), and M-CSF has been shown to be secreted after virus infection in vitro (25). However, a progressive impairment in the ability of CD4+ T cells to secrete GM-CSF has been described for HIV-1-infected individuals (4, 49), and this impairment may in turn limit coreceptor expression in vivo. Other groups have reported that GM-CSF inhibits HIV replication in macrophages at an undefined dose (35) and suppresses expression of a marker gene carried by an HIV envelope pseudotyped virus at a high dose of recombinant GM-CSF but not at lower doses of the cytokine (17). Differences in experimental systems, including culture conditions and dose of GM-CSF used to activate cells, may account for these divergent results.
IL-4 or IL-13 not only prevented the expression of CCR5 and inhibited HIV-1 infection in monocytes/macrophages cultured in medium alone, but they also blocked the increase in CCR5 expression induced by GM-CSF or M-CSF as well as M-CSF stimulation of HIV-1 entry. Although the Th2 cytokine IL-10 has previously been shown to inhibit HIV replication (8, 30, 37, 53), a recent report showed that IL-10 increased HIV-1 replication and enhanced CCR5 expression (57). We found that IL-10 did not downmodulate or stimulate CCR5 expression in macrophages directly but instead slightly enhanced CCR5 surface expression when added together with M-CSF (see Fig. 4).
The receptors for IL-4 and IL-13 share the IL-4 receptor α chain (CD124) (2, 41). Both IL-4 and IL-13 induce the tyrosine phosphorylation of the kinase JAK1 and 4PS, a 170-kDa protein associated with phosphatidylinositol 3-kinase, and they induce expression of the LSK tyrosine kinase in human monocytes (39) and STAT6 (IL-4 STAT) in human colon carcinoma cell lines (38). It is possible that both IL-4 and IL-13 may regulate CCR5 expression by a common pathway and at the transcription level.
It is possible that IL-4 and IL-13 suppress CCR5 expression on macrophages as a result of ligand-induced endocytosis. The chemokines stromal cell-derived factor-1α and RANTES (regulated on activation, normal T expressed and secreted) induce rapid endocytosis of their specific receptors (3). However, in our culture system, neither IL-4 nor IL-13 induced the release of the CCR5 ligands RANTES, MIP-1α, and MIP-1β from human monocytes/macrophages in the absence or presence of M-CSF (unpublished data). In fact, both IL-4 and IL-13 inhibit lipopolysaccharide-induced MIP-1α secretion (16) and Staphylococcus aureus-induced MIP-1β production.
Increased expression of IL-4 in HIV-1-seropositive individuals has been detected (33, 46). The abundance of IL-13 has also been shown to be greater than that of IL-4 in peripheral blood mononuclear cells and lymph nodes of such individuals (63). It is not clear whether the T-helper phenotype shifts from Th1 to Th2 or from Th1 to Th0 in HIV-1-infected individuals (34). However, both Th2 and Th0 T cells, as well as CD8+ cells, produce IL-4 and IL-13 (33, 58). CD8+ T cells also produce RANTES, MIP-1α, and MIP-1β, all of which are capable of blocking CCR5-mediated entry of macrophage-tropic virus into CD4+ T cells (11).
The regulation of the expression of CCR5 by IL-4 and IL-13, the blocking of virus coreceptors by chemokines, and the production of as-yet-unidentified suppressor factors are likely important host determinants in the control of HIV-1 infection. The combination of IL-4 and IL-13 with CCR5 antagonists (56) may prove therapeutically beneficial for HIV-1-infected individuals. In this regard, treatment of patients with Kaposi’s sarcoma with IL-4 has been associated with moderate decreases in HIV viral load (35a).
Acknowledgments
We thank V. Calvert for the preparation of human primary monocytes. Monoclonal antibody 5C7 was obtained through the AIDS Research and Reference Reagent Program of the Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Aman M J, Tayebi N, Obiri N I, Puri R K, Modi W S, Leonard W J. cDNA cloning and characterization of the human interleukin 13 receptor alpha chain. J Biol Chem. 1996;271:29265–29270. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- 3.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagnara G P, Zauli G, Re M C, Furlini G, Giovannini M, Ranieri S, Brizzi M F, La Placa M. Impaired GM-CSF production by cultured light density mononuclear cells and T lymphocytes correlates with the number of circulating CFU-gm in HIV-1 seropositive subjects. Int J Cell Cloning. 1991;9:239–250. doi: 10.1002/stem.5530090308. [DOI] [PubMed] [Google Scholar]
- 5.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 6.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll R G, Riley J L, Levine B L, Feng Y, Kaushal S, Ritchey D W, Bernstein W, Weislow O S, Brown C R, Berger E A, June C H, St. Louis D C. Differential regulation of HIV-1 fusion cofactor expression by CD28 costimulation of CD4+ T cells. Science. 1997;276:273–276. doi: 10.1126/science.276.5310.273. [DOI] [PubMed] [Google Scholar]
- 8.Chang J, Naif H M, Li S, Jozwiak R, Ho-Shon M, Cunningham A L. The inhibition of HIV replication in monocytes by interleukin 10 is linked to inhibition of cell differentiation. AIDS Res Hum Retroviruses. 1996;12:1227–1235. doi: 10.1089/aid.1996.12.1227. [DOI] [PubMed] [Google Scholar]
- 9.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 10.Clouse K A, Cosentino L M, Weih K A, Pyle S W, Robbins P B, Hochstein H D, Natarajan V, Farrar W L. The HIV-1 gp120 envelope protein has the intrinsic capacity to stimulate monokine secretion. J Immunol. 1991;147:2892–2901. [PubMed] [Google Scholar]
- 11.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 12.Connor R I, Paxton W A, Sheridan K E, Koup R A. Macrophages and CD4+ T lymphocytes from two multiply exposed, uninfected individuals resist infection with primary non-syncytium-inducing isolates of human immunodeficiency virus type 1. J Virol. 1996;70:8758–8764. doi: 10.1128/jvi.70.12.8758-8764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jong J J, Goudsmit J, Keulen W, Klaver B, Krone W, Tersmette M, de Ronde A. Human immunodeficiency virus type 1 clones chimeric for the envelope V3 domain differ in syncytium formation and replication capacity. J Virol. 1992;66:757–765. doi: 10.1128/jvi.66.2.757-765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 15.Denis M, Ghadirian E. Interleukin 13 and interleukin 4 protect bronchoalveolar macrophages from productive infection with human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1994;10:795–802. doi: 10.1089/aid.1994.10.795. [DOI] [PubMed] [Google Scholar]
- 16.de Waal, Malefyt R, Figdor C G, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, Dang W, Zurawski G, de Vries J E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- 17.Di Marzio P, Tse J, Landau N R. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res Hum Retroviruses. 1998;14:129–138. doi: 10.1089/aid.1998.14.129. [DOI] [PubMed] [Google Scholar]
- 18.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 19.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 20.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 21.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 22.Folks T M, Powell D, Lightfoote M, Koenig S, Fauci A S, Benn S, Rabson A, Daugherty D, Gendelman H E, Hoggan M D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986;164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 24.Graziosi C, Pantaleo G, Gantt K R, Fortin J P, Demarest J F, Cohen O J, Sekaly R P, Fauci A S. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 25.Gruber M F, Weih K A, Boone E J, Smith P D, Clouse K A. Endogenous macrophage CSF production is associated with viral replication in HIV-1-infected human monocyte-derived macrophages. J Immunol. 1995;154:5528–5535. [PubMed] [Google Scholar]
- 26.Hayes M P, Wang J, Norcross M A. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 27.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 28.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 29.Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany H L, Murphy P M, Yoshie O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. 1996;271:7725–7730. doi: 10.1074/jbc.271.13.7725. [DOI] [PubMed] [Google Scholar]
- 30.Kootstra N A, van’t Wout A, Huisman H G, Miedema F, Schuitemaker H. Interference of interleukin-10 with human immunodeficiency virus type 1 replication in primary monocyte-derived macrophages. J Virol. 1994;68:6967–6975. doi: 10.1128/jvi.68.11.6967-6975.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyanagi Y, O’Brien W A, Zhao J Q, Golde D W, Gasson J C, Chen I S. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988;241:1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 32.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 33.Maggi E, Manetti R, Annunziato F, Cosmi L, Giudizi M G, Biagiotti R, Galli G, Zuccati G, Romagnani S. Functional characterization and modulation of cytokine production by CD8+ T cells from human immunodeficiency virus-infected individuals. Blood. 1997;89:3672–3681. [PubMed] [Google Scholar]
- 34.Maggi E, Mazzetti M, Ravina A, Annunziato F, de Carli M, Piccinni M P, Manetti R, Carbonari M, Pesce A M, del Prete G. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994;265:244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda S, Akagawa K, Honda M, Yokota Y, Takebe Y, Takemori T. Suppression of HIV replication in human monocyte-derived macrophages induced by granulocyte/macrophage colony-stimulating factor. AIDS Res Hum Retroviruses. 1995;11:1031–1038. doi: 10.1089/aid.1995.11.1031. [DOI] [PubMed] [Google Scholar]
- 35a.Miles, S. Personal communication.
- 36.Montaner L J, Doyle A G, Collin M, Herbein G, Illei P, James W, Minty A, Caput D, Ferrara P, Gordon S. Interleukin 13 inhibits human immunodeficiency virus type 1 production in primary blood-derived human macrophages in vitro. J Exp Med. 1993;178:743–747. doi: 10.1084/jem.178.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montaner L I, Gordon S. TH2 downregulation of macrophage HIV-1 replication. Science. 1995;267:538–539. doi: 10.1126/science.7824955. [DOI] [PubMed] [Google Scholar]
- 38.Murata T, Noguchi P D, Puri R K. IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: similarities between IL-4 and IL-13 signaling. J Immunol. 1996;156:2972–2978. [PubMed] [Google Scholar]
- 39.Musso T, Varesio L, Zhang X, Rowe T K, Ferrara P, Ortaldo J R, O’Shea J J, McVicar D W. IL-4 and IL-13 induce Lsk, a Csk-like tyrosine kinase, in human monocytes. J Exp Med. 1994;180:2383–2388. doi: 10.1084/jem.180.6.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naif H M, Li S, Ho-Shon M, Mathijs J M, Williamson P, Cunningham A L. The state of maturation of monocytes into macrophages determines the effects of IL-4 and IL-13 on HIV replication. J Immunol. 1997;158:501–511. [PubMed] [Google Scholar]
- 41.Obiri N I, Debinski W, Leonard W J, Puri R K. Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common gamma chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J Biol Chem. 1995;270:8797–8804. doi: 10.1074/jbc.270.15.8797. [DOI] [PubMed] [Google Scholar]
- 42.Oravecz T, Pall M, Roderiquez G, Gorrell M D, Ditto M, Nguyen N Y, Boykins R, Unsworth E, Norcross M A. Regulation of the receptor specificity and function of the chemokine RANTES by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med. 1997;186:1865–1872. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 44.Osborn L, Kunkel S, Nabel G J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 46.Poli G, Fauci A S. Role of cytokines in the pathogenesis of human immunodeficiency virus infection. In: Aggarwal B B, Puri R K, editors. Human cytokines: their role in disease and therapy. Cambridge, Mass: Blackwell Science; 1995. pp. 421–449. [Google Scholar]
- 47.Rana S, Besson G, Cook D G, Rucker J, Smyth R J, Yi Y, Turner J D, Guo H H, Du J G, Peiper S C, Lavi E, Samson M, Libert F, Liesnard C, Vassart G, Doms R W, Parmentier M, Collman R G. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the delta ccr5 mutation. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raport C J, Gosling J, Schweickart V L, Gray P W, Charo I F. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 49.Re M C, Zauli G, Furlini G, Giovannini M, Ranieri S, Ramazzotti E, Vignoli M, La Placa M. GM-CSF production by CD4+ T-lymphocytes is selectively impaired during the course of HIV-1 infection. A possible indication of a preferential lesion of a specific subset of peripheral blood CD4+ T-lymphocytes. Microbiologica. 1992;15:265–270. [PubMed] [Google Scholar]
- 50.Rich E A, Chen I S, Zack J A, Leonard M L, O’Brien W A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Invest. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roderiquez G, Oravecz T, Yanagishita M, Bou-Habib D C, Mostowski H, Norcross M A. Mediation of human immunodeficiency virus type 1 binding by interaction of cell surface heparan sulfate proteoglycans with the V3 region of envelope gp120-gp41. J Virol. 1995;69:2233–2239. doi: 10.1128/jvi.69.4.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 53.Saville M W, Taga K, Foli A, Broder S, Tosato G, Yarchoan R. Interleukin-10 suppresses human immunodeficiency virus-1 replication in vitro in cells of the monocyte/macrophage lineage. Blood. 1994;83:3591–3599. [PubMed] [Google Scholar]
- 54.Schuitemaker H, Kootstra N A, Koppelman M H, Bruisten S M, Huisman H G, Tersmette M, Miedema F. Proliferation-dependent HIV-1 infection of monocytes occurs during differentiation into macrophages. J Clin Invest. 1992;89:1154–1160. doi: 10.1172/JCI115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sica A, Saccani A, Borsatti A, Power C A, Wells T N, Luini W, Polentarutti N, Sozzani S, Mantovani A. Bacterial lipopolysaccharide rapidly inhibits expression of C-C chemokine receptors in human monocytes. J Exp Med. 1997;185:969–974. doi: 10.1084/jem.185.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 57.Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, Polentarutti N, Sica A, Locati M, Mackay C, Wells T, Biswas P, Vicenzi E, Poli G, Mantovani A. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439–444. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Street N E, Mosmann T R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991;5:171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- 59.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 60.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 61.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 62.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectibility by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zou W, Dulioust A, Fior R, Durand-Gasselin I, Boue F, Galanaud P, Emilie D. Increased T-helper-type 2 cytokine production in chronic HIV infection is due to interleukin (IL)-13 rather than IL-4. AIDS. 1997;11:533–534. [PubMed] [Google Scholar]
- 64.Zurawski G, de Vries J E. Interleukin 13 elicits a subset of the activities of its close relative interleukin 4. Stem Cells. 1994;12:169–174. doi: 10.1002/stem.5530120204. [DOI] [PubMed] [Google Scholar]