Highlights
-
•
The Brain Health Index combines different magnetic resonance imaging sequences to quantify vascular and non-vascular aspects of brain health.
-
•
We assessed the clinical utility of this novel biomarker in a large-scale cohort.
-
•
The BHI and certain measures of cognition are significantly correlated, as are BHI and risk factors for cognitive decline, both implying good construct validity.
-
•
However, there is not a significant change in BHI over time in a longitudinal sub-cohort.
-
•
The BHI may perform differently in clinical populations, and this is a target for future work.
Keywords: Neuroimaging, structural MRI, aging, Brain Health Index, European Prevention of Alzheimer's Dementia, longitudinal
Abstract
Background
Brain Health Index (BHI) assimilates various MRI sequences, giving a quantitative measure of brain health. To date, BHI validation has been cross-sectional and limited to selected populations. Further large-scale validation and assessment of temporal change is required to understand its clinical utility.
Aim
Assess 1) relationships between variables associated with cognitive decline and BHI 2) associations between BHI and measures of cognition and 3) longitudinal changes in BHI and relationship with cognitive function.
Methods
BHI computation involved Gaussian mixture-model cluster analysis of T1, T2, T2*, and T2 FLAIR MRI data from participants within the European Prevention of Alzheimer's Dementia (EPAD) cohort. Group differences (gender- and health-based) were evaluated using independent samples Welch's t-tests. Relationships between BHI, age and cognitive tests used linear regression. Longitudinal analysis (12/24 months) utilised mixed linear regression models to examine BHI changes, and paired BHI/cognition associations.
Results
Data from N = 1496 predominantly Caucasian participants (50–88 years old, 43.32% male) were used. BHI scores were lower in those with diabetes (p < 0.001, d = 0.419), hypertension (p < 0.001, d = 0.375), hypercholesterolemia (p < 0.001, d = 0.193) and stroke (p < 0.05, d = 0.512). APOE was not significantly related to BHI scores. After correction for age, cross-sectional BHI scores were significantly associated with all measures of cognitive function in males, but only the Four Mountains Test (4MT) in females. Longitudinal change in BHI and cognition were not consistently related.
Conclusions
BHI is a valid marker of cognitive decline and relatively stable over 1-2 year follow-up periods. Further work should assess temporal changes over a longer duration and determine relationships between BHI and cognition in more diverse populations.
1. Background
Pathological changes in dementias tend to be a mix of both structural and neurovascular change [[1], [2], [3], [4]] these represent promising targets for the assessment of brain health and disease. Many magnetic resonance imaging (MRI) approaches to prognostic biomarker development only investigate one possible marker at a time, thereby losing informative data from other MRI sequences which have been collected. An automated image processing measure called the Brain Health Index (BHI) [5] combines T1, T2, T2* and T2 FLAIR sequences to create a single brain mask and provide a score of global brain health. Initial development found BHI to have stronger associations with Addenbrooke's Cognitive Examination Revisited (ACER) than total small vessel disease (SVD) or white matter hyperintensities (WMH) ratings.
Subsequent work by Watt et al. [6] established normative reference values for the BHI in UK Biobank participants (n = 2,990) aged 48-77 years old. Findings also showed lower BHI scores in male participants, and those with known risk factors for brain health, including type 2 diabetes mellitus, hypertension, and smokers, with higher scores for those with lower waist-to-hip ratios and lower pulse pressure. However, issues of generalisability of the UK Biobank are well described, and it remains unclear whether these associations differ in typical populations recruited into dementia-focused research.
An important measure of validity of an imaging biomarker for dementia is to assess relationship with cognitive test scores. These assessments could describe associations with global ‘screening’ tests of cognition e.g. the Mini-Mental State Examination (MMSE) [7], tests that are sensitive to early cognitive change e.g. the, Four Mountains Test (4MT) [8], or more detailed multidomain neuropsychological assessments e.g. the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [9,10]. Many imaging biomarkers have been validated against cognitive scores in cross-sectional analyses only, this is unsatisfactory when the aim of the biomarker is to predict cognitive decline over time. Validation of a biomarker such as BHI must also demonstrate ability to detect neurodegenerative changes longitudinally.
Thus, there is a need for further validation of BHI that includes a population typical of those seen in memory services or dementia research, that allows for comparison against various clinical features and cognitive assessments, and that can chart temporal relationships.
Aims and hypotheses
The primary aims were:
-
1)
To assess association of clinical and demographic features associated with cognitive decline against BHI scores.
-
2)
To describe the association of BHI with general cognitive screening tools, sensitive measure of early cognitive decline and data from multidomain neuropsychological assessments.
-
3)
To perform longitudinal assessments of change in BHI against temporal change in cognitive test results.
2. Methods
2.1. Approvals and participant consents
The European Prevention of Alzheimer's Disease (EPAD) Longitudinal Cohort Study (EPAD LCS) was the test dataset. EPAD LCS is registered at www.clinicaltrials.gov Identifier: NCT02804789. This project was secondary analysis of data held by EPAD, with permission given by EPAD via the Alzheimer's Disease Workbench of the Alzheimer's Disease Data Initiative (ADDI; EPAD ID: 200814_GLA_QUT_001). Before partaking in any study-related activities, written informed consent was obtained from either the participant or their legally authorised representative. Reporting of findings followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [11].
2.2. Participant and data selection
EPAD-LCS is a longitudinal, multi-centre, pan-European cohort study, which recruited participants predominantly from various parent cohorts, with the intention of creating a cohort of individuals covering the full range of anticipated likelihood of Alzheimer's disease development. This subject selection process is described at length elsewhere [12].
The current study used the EPAD-LCS-v.IMI dataset (doi: 10.34688/epadlcs_v.imi_20.10.30), which was released in October 2020 and accessed via the Alzheimer's Disease Data Initiative (ADDI) workbench portal: https://portal.addi.ad-datainitiative.org/ after approval of a data access request (www.ep-ad.org/open-access-data/overview). The EPAD LCS was launched in 2015 as a public-private partnership. The primary research goal of the EPAD LCS is to provide a well-phenotyped probability-spectrum population for developing and continuously improving disease models for Alzheimer's disease in individuals without dementia.
The full v.IMI dataset was considered for both cross-sectional and longitudinal aspects of the current study, with slightly different criteria. For the cross-sectional cohort, participants were required to have the full complement of necessary MRI scans (T1, T2, T2 star and FLAIR), core demographic information (age and gender), and RBANS, MMSE and 4MT scores at their baseline visit. The cohort for longitudinal analysis was determined on which participants had the required complement of MRI scans at the first, second and third MRI visits (baseline, 12, and 24 months), as well as all previously mentioned requirements. This selection process is summarised in Fig. A1.
The MRI sequences available include a full complement of scans for BHI computation, as per the work of Dickie et al. [5] – T1, T2, T2 star and T2 FLAIR [13]. The richness of the EPAD cohort additionally enables exploration of lifestyle, genetic and socio-demographic factors which may contribute to BHI scores, thereby further validating the BHI. The genetic data available within EPAD also allows for investigation of the relationship between the APOE e4 allele – the strongest genetic risk factor for dementia [14] – for the first time.
2.3. Derivation of the Brain Health Index
The current study utilised the core sequences from the MRI protocol: T1, FLAIR, T2 and T2*, across three vendors – GE Healthcare, Siemens, and Philips. Vendor-specific parameters for the sequences used are given in Table A1, based on a Table in Lorenzini et al. [13].
Prior to BHI computation, images were registered within-participant using Advanced Normalization Tools (ANTs v2.3.5) [15]. The T1 image underwent rigid body registration to the MNI152 1mm template, and subsequently, the T2 FLAIR, T2, and T2* images were independently aligned to this standardised T1 image using affine registration with 12 points. The resulting images underwent bias field correction using the nonparametric nonuniform intensity normalisation algorithm (N4 Bias Field Correction) [16].
Intracranial volume masks were required for further BHI computation, and the process for their creation is described at length in Watt et al. [6]. In short, to produce custom individual ICV masks for each participant, a generic ICV mask was first created in Mango (Version 4.1, 1531). This generic mask was then used to create custom masks for each participant using diffeomorphic registration in ANTs [15], which were then used to restrict voxels included in BHI computation and avoid inclusion of any skull.
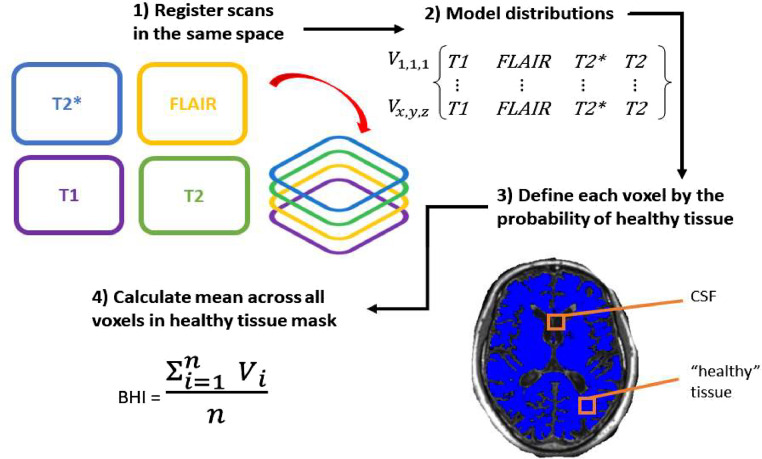
The BHI - described in detail in [5,6] - uses Gaussian mixture model cluster analysis to categorise the ICV voxels of co-registered sequences as either (1)likely normal brain tissue, or (2)likely abnormal tissue or cerebrospinal fluid (CSF). The four co-registered sequences were T1, T2, T2* and FLAIR, and each voxel within the ICV mask is given a value from each sequence. The expectation-maximization algorithm then uses these voxel values to compute the posterior probability of a given voxel being likely either (1) or (2), across all included sequences.(Fig. 1)
Fig. 1.
Flow diagram of the processes involved in the computation of the Brain Health Index.
To assess quality of the computed BHI, participant-specific multi-slice PNG images were created for both ICV and BHI masks using MATLAB 2022a. The ICV mask was overlaid on the participant-specific image and visually assessed for adequate brain coverage without skull inclusion. The BHI mask was overlaid on the participant-specific, bias field-corrected T1 image and visually checked to ensure that it was computed properly and also covered the brain as expected.
2.4. Lifestyle, socio-demographic and genetic information
Socio-demographic data were self-reported at the baseline visit then at each study visit. Physical activity and smoking status were self-reported, with alcohol consumption reported as part of the Healthy Ageing through Internet Counselling in the Elderly (HATICE) questionnaire [17].
TaqMan Genotyping of participant blood samples was used to determine APOE status, with carriers of APOE e defined as those having at least one e4 allele present. Analysis was carried out in a single laboratory at the University of Edinburgh using QuantStudio 12KL Flex. There are separate variables pertaining to biological sex (determined by genetic testing) and gender (self-described) in EPAD. In the full EPAD-LCS-v.IMI dataset – prior to participant identification for the current study – no participant differs across these variables. The gender variable is also more complete in responses. As such, this variable was used in our current work.
2.5. Cognitive testing
To enable a comprehensive and holistic understanding of cognition, three cognitive tests were chosen for comparison with the BHI, differing in their complexity and sensitivity to change.
Firstly, the MMSE, a 30-point questionnaire which assesses various aspects of cognition, including attention, calculation, repetition, complex commands, time and place orientation, and recall [7,18]. The MMSE is currently used extensively within the clinic and research, as it is quick to administer, assessing multiple aspects of memory. However, it is not a suitable stand-alone test for the assessment of those who may develop dementia [19].
The 4MT is a specialist test designed to assess hippocampal function (i.e. visuospatial memory) by testing the capacity of an individual to recognise a location from a novel viewpoint. Specifically, it comprises a computer-generated landscape of four hills, surrounded by a mountain range in the distance. These four hills are of different shapes and sizes. The image is shown for 10 seconds, after which four alternative images are immediately shown, and the participant must select the image matching that which they were just shown. Transient local features – for example weather, lighting, vegetation – vary throughout, and cannot be relied on to identify the correct image [8]. The 4MT is scored out of 15 attempts, with a score of ≤ 8/15 associated with MCI [20]. The 4MT is thought to have greater utility for subtle and early changes, reflecting the function of the hippocampus by using different configurations of computer-generated mountain scenes to assess allocentric spatial memory. As the hippocampus has been shown to be affected early in dementia [21], determination of any relationship between this test and the BHI would provide further support for its clinical utility.
RBANS was used as our multidomain neuropsychological assessment. It comprises twelve subtests, assessing the following five domains: attention, language, immediate and delayed memory, and visuospatial/constructional function [9,10]. Scores can range from 40 to 160, with lower scoring reflective of greater cognitive impairment. Two aspects of RBANS were considered – the total score, which considered the scores from all tests in an overall score, and the the Delayed Memory Index (DMI), which is thought to be most impaired in those with MCI [22]. The RBANS is widely used for the longitudinal tracking of dementia progression and is useful in depicting cognitive variance in those considered cognitively healthy. The DMI section of RBANS has been shown to be that of greatest impairment for individuals with mild cognitive impairment (MCI) [22].
2.6. Statistical analysis
Statistical analyses were performed using various libraries in Python 3 – NumPy [23], SciPy [24], pandas [25], Matplotlib [26], scikit-learn [27] and statsmodels [28] - via Jupyter Notebooks [29]. Analysis was carried out within the Aridhia Workspace (https://www.aridhia.com/). In the cross-sectional cohort, two-tailed independent samples Welch's t-tests were employed to assess possible differences in BHI scores related to gender, and key health conditions reported as part of participants’ medical histories – diabetes, hypertension, hypercholesterolaemia, stroke, head injury, depression, and anxiety. Anxiety, generalised anxiety disorder, and anxiety disorder were grouped together under the umbrella of ‘anxiety’ for the purpose of this analysis, due to small cohort numbers. Linear regression analyses were used to determine relationships between BHI scores and age, and various cognitive test scores (MMSE, 4MT, RBANS total, RBANS DMI). For longitudinal analyses, repeated measure ANOVAs and post-hoc Tukey's and Scheffe's tests were used to assess the change in each cognitive test at all timepoints, as well as BHI at these timepoints. Linear regressions were used to determine the uncorrected relationships between BHI and each cognitive test score. Subsequent mixed linear regression models were used to assess the same relationships, corrected for age and gender, to determine if any significant relationships between given cognitive tests and the BHI remained.
3. Results
Cohort identification and participant attrition for the cross-sectional and longitudinal cohorts are described in Fig. A1. The final cross-sectional cohort is comprised of 1496 participants, and the longitudinal of 197 participants. The demographics of both these cohorts are given in Table 1.
Table 1.
Participant demographics for cross-sectional and longitudinal cohorts.
| Variable | Units | Results | N with available data |
|---|---|---|---|
| Demographics of cross-sectional cohort | |||
| Age | Mean years (SD, range) | 65.5 (7.2, 50.1 – 88.25) | 1496 |
| Gender | M (%M) | 648 (43.32%) | 1496 |
| Ethnicity | Caucasian (%)† | 1181 (98.42%)‡ | 1200 |
| Education | Mean years (mean, SD) | 5 - 32 (14.43, 3.71) | 1496 |
| APOE* | Count (%)* | e3/e3 – 762 (52.23%) e3/e4 – 463 (31.73%) e2/e3 - 124 (8.5%) e4/e4 – 65 (4.46%) e2/e4 – 41 (2.81%) e2/e2 – 4 (0.27%) |
1459 |
| Demographics of longitudinal cohort | |||
| Age at baseline visit | Mean years (SD, range) | 66.31 (6.28, 51.92 – 86) | 197 |
| Gender | M (%M) | 99 (50.25%) | 197 |
| Ethnicity | Caucasian (%)† | 134 (99.26%)§ | 135 |
| Education | Mean years (SD, range) | 13.9 (3.53, 7-25) | 197 |
| APOE* | Count (%)* | E3/e3 – 103 (52.6%) E3/e4 – 57 (29.1%) E2/e3 – 14 (7.1%) |
196 |
Abbreviations: SD, standard deviation
Demographic variable that differs significantly between cross-sectional and longitudinal cohorts.
Percentages given do not include those for whom data was not available.
Of those who reported their ethnicity. Other self-described ethnicities (count): Asian (4); Black (1); British (1); Chinese (1); Hispanic (7); Latin American (2); Mixed Asian (1); Moroccan (1); South East Asian (1).
Of those who reported their ethnicity. Other self-described ethnicities (count): Latin American (1).
3.1. Cross-sectional analyses
3.1.1. Demographic and clinical differences
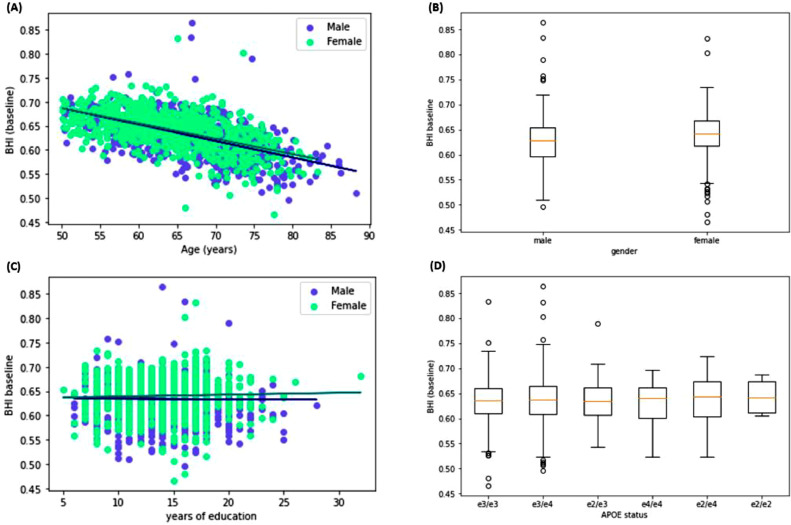
Linear regressions assessing the relationship between age and BHI were computed for both male and female participants and are summarised in Fig. 2(A). Higher age was associated with lower (i.e. worse) BHI scores in the full cross-sectional cohort (R2=0.347, F(1,1494)=793.7, standardised beta= 4.77×10−4, p < 0.001). When gender was considered separately, lower scores with older age were found in males – R2=0.348, F(1 646)=344.2, standardised beta=-4.89×10−4, p < 0.001. This was also the case for female participants – R2=0.322, F(1,848) =403.7, standardised beta=-4.55×10−4, p < 0.001. Results of an independent samples Welch's t-test showed that females had higher BHI scores than males – t=6.968, p < 0.001, Cohen's d = 0.368 (Fig. 2(B)).
The relationship between years of education and the BHI was not significant for males (p=0.335) or females (p=0.294; Fig. 2. (C)). However, when this analysis was repeated with age additionally controlled for, this relationship was significant for both males and females. An increase of one year of education corresponds with a decrease of 0.0008 units of the BHI for males (p = 0.036) and a decrease of 0.0007 for females (p = 0.031). The influence of all possible APOE statuses on BHI score was investigated using a one-way ANOVA, which found no difference (F=0.236, p=0.947). Further analysis compared BHI scores across APOE e4 carriers and non-carriers (excluding those with e2/e4 status, due to the protective/deleterious effect) using a Welch's t-test and found no difference (p=0.8; Fig. 2(D)).
Fig. 2.
Key demographic findings in the cross-sectional cohort: (A) Linear regressions assessing the relationship between the age of participants and the BHI; (B) Gender differences in BHI scoring; (C) uncorrected linear regressions assessing the relationship between years of education and BHI scores; and (D) BHI scores by APOE status in the full cross-sectional cohort. In (A) and (C), male participants are depicted in blue, and female participants in green.
Cognition in this cohort is summarised in Table 2. Participants with MCI were compared with those who did not using an independent samples Welch's t-test and were found to be higher in those without MCI (t =3.88, p < 0.001, Cohen's d = 0.565; Fig. 3).
Table 2.
Cognition in complete cross-sectional cohort.
| Variable | Units | Results | N with available data |
|---|---|---|---|
| Reported cognitive decline | None: AD: Mixed dementia: MCI | 1432: 7: 1: 56 | 1496 |
| MMSE | Mean score (SD, range) | 28.55 (1.65, 17 – 30) | 1496 |
| 4MT | Mean score (SD, range) | 8.6 (3.42, 0 -15) | 1463 |
| Total RBANS | Mean score (SD, range) | 102.9 (14.86, 51 – 149) | 1496 |
Fig. 3.
Comparison of BHI scores in the cross-sectional cohort in participants who did and did not report MCI in their medical histories.
Relationships between the BHI and key physiological and morphological measures were also assessed. Summary health and lifestyle information is given in Table A2 for the cross-sectional cohort, and Table A3 for the longitudinal cohort.
Prior to correction for age, male participants showed an increase in BHI score with an increase in BMI (R2=0.01, F(1, 643) = 6.433, p < 0.05), however these were not significantly related within the female cohort. After correction, the relationship between BMI and BHI is no longer significant in male participants (p = 0.767), and remains insignificant for females (p = 0.77). WHR was not associated with the BHI in either males or females (p > 0.05 for both). After correction, this remains true for males (p = 0.4) and females (p = 0.65). Higher pulse pressure was associated with lower BHI scores for both males (R2=0.107, F(1, 643) =77.4, standardised beta=-6.8×10−5, p < 0.001) and females (R2=0.058, F(1, 841) =51.38, standardised beta=-4.1×10−5, p < 0.001). This remains true for male (p = 0.003) but not female (p = 0.941) participants after correction for age.
The associations between certain reported medical histories and the BHI were also assessed using independent samples Welch's t-tests. Participants with diabetes (type 1 or 2) had lower BHI scores than those without diabetes (t=4.47, p < 0.001, Cohen's d = 0.419). Those with hypertension also scored lower on the BHI than those without (t=7.11, p < 0.001, Cohen's d = 0.375), as did those with hypercholesterolaemia (t=3.68, p < 0.001, Cohen's d = 0.193). Those who reported head injuries in their medical histories did not differ in BHI score than those who did not have a history of head injury (p=0.456), however those who had had a stroke had lower BHI scores than those who had not (t=2.738, p < 0.05, Cohen's d = 0.512).
Those who reported a history of depression had higher BHI scores than those who did not (t=2.627, p < 0.01, Cohen's d = -0.153), as did those who reported experiencing ‘anxiety’, ‘generalised anxiety disorder’ or ‘anxiety disorder’ (grouped together under the umbrella of ‘anxiety’ for the purpose of this analysis) compared with those who did not (t=2.44, p < 0.05, Cohen's d = -0.325).
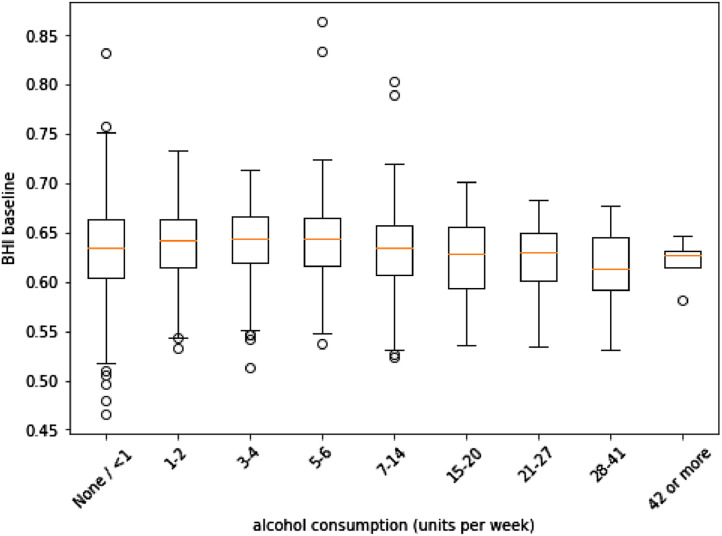
A one-way ANOVA assessed differences in BHI by merit of smoking status, with no difference found (p = 0.153). A one-way ANOVA assessing BHI scoring based on units of alcohol consumed highlighted a difference on this basis (F = 3.076, p < 0.005). A follow-up Tukey's test clarified that this difference was only found between those who consumed 5 to 6 units per week, having higher scores than those who consumed 28 to 41 units per week (95% CI: 0.000 to 0.057, p < 0.05). Comparisons can be seen in Fig. 4.
Fig. 4.
BHI scores by alcohol consumption (units per week) in the full cross-sectional cohort.
3.1.2. Cognitive testing
For all cognitive test analyses in the cross-sectional cohort, participants were grouped by gender due to the finding of significant differences in BHI scores between male and female participants. Results within the cross-sectional cohort are given in Table A4.
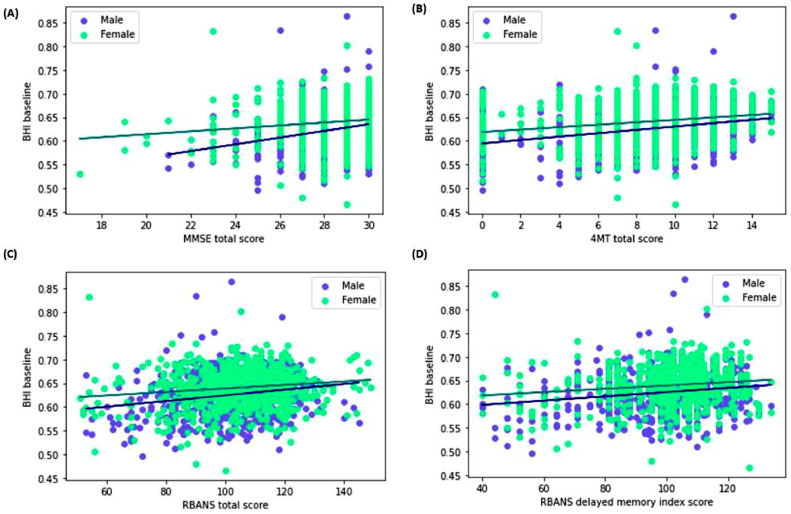
BHI score increased with higher MMSE scores in both males (R2 0.064, F(1,646)=44.04, standardised beta=4.615×10−3, p < 0.001) and females (R2=0.019, F(1,846)=16.25, standardised beta=1.782×10−3, p < 0.001). When corrected for age, this remains significant for males (p = 0.001), but not for females (p = 0.601). Linear regressions assessing the relationship between examiner-recorded 4MT scores and BHI found higher 4MT scores were associated with higher BHI scores in both male (R2=0.088, F(1, 632) =60.77, standardised beta=9.95×10−4, p < 0.001) and female (R2=0.047, F(1,827) =40.55, standardised beta=7.9×10−4, p < 0.001) participants. These remain significant after correcting for age (males: p < 0.001; females: p = 0.027).
Increasing RBANS total score was associated with an increased BHI score for both male (R2=0.041, F(1,646) =27.38, standardised beta=4.1×10−5, p < 0.001) and female participants (R2=0.02, F(1,846) =17.07, standardised beta=2.5×10−5, p < 0.001). Results remain significant for males (p = 0.001), but not for females (p = 0.095) after correcting for age. Linear regressions focusing specifically on the DMI score within RBANS were also carried out, and also found to be significant for males (R2=0.03, F(1,646) =20.28, standardised beta=2.7×10−5, p < 0.001) and females (R2=0.019, F(1,846)=16.13, standardised beta=2.3×10−5,p < 0.001). After correction for age, males remain significant (p = 0.004) but females do not (p = 0.082). Uncorrected findings are depicted in Fig. 5 below.
Fig. 5.
Uncorrected linear regressions depicting the relationship between BHI scores and (A) MMSE; (B) 4MT; (C) RBANS total; and (D) RBANS DMI scores. Male participants are depicted in blue, and female participants in green.
3.2. Longitudinal
Key summary scores from the longitudinal cohort are given in Table 3.
Table 3.
Key summary scores from the longitudinal sub-cohort.
| Timepoint |
||||
|---|---|---|---|---|
| Units | Baseline | 12 months | 24 months | |
| BHI | Mean (SD, range) | 0.645 (0.038, 0.523 – 0.803) | 0.643 (0.036, 0.516 – 0.733) | 0.64 (0.036, 0.507 – 0.726) |
| MMSE* | 28.67 (1.49, 19 – 30) | 28.55 (1.6, 22 – 30) | 28.7 (1.57, 18 – 30) | |
| 4MT | 8.8 (2.73, 0 -15) | 9.3 (2.92, 0 -15) | 7.49 (4.83, 0 -15) | |
| RBANS total | 104.5 (11.98, 67 – 134) | 100.17 (13.97, 59 – 141) | 106.94 (13.46, 66 – 139) | |
| RBANS DMI | 103.9 (12.74, 68 – 130) | 103.4 (13.75, 56 – 134) | 107.55 (14.12, 48 – 134) | |
For 12- and 24-month timepoints, data was available for n = 197, but longitudinal analysis only used 196 participants for assessment of MMSE as only n = 196 were available at baseline. This participant was kept in the cohort due to their data availability for other cognitive tests.
Repeated measures ANOVAs were used to determine whether the BHI and cognitive test results changed longitudinally, before any correction was carried out. BHI scores decreased over time (F(2,392)=5.8938, p < 0.005), however a post-hoc Tukey's test to determine which timepoints significantly differed from each other was not significant for any pairwise comparison. A further Scheffe's test to corroborate this result also found no significant changes in score between any measured timepoints.
There was no significant difference in MMSE score over time. Assessment of the 4MT by repeated measures ANOVA found a change over time (F(2,392)=17.37, p < 0.001). A subsequent Tukey's test found that there was no difference in score between baseline and 12 months, but that there was a decrease in score between 12 and 24 months (95% CI:0.95 to 2.664, p < 0.001). It was also lower at 24 months than baseline (95% CI:0.457 to 2.172, p < 0.005). RBANS total score changed over time (F(2,392)=43.3907, p < 0.001). Post-hoc Tukey's testing highlighted a decrease between baseline and 12 months (95% CI: 1.214 to 7.446, p < 0.005), and an increase between 12 months and 24 months (95% CI:-9.888 to -3.655, p < 0.001). However, there was no significant difference between baseline and 24 months. RBANS DMI changed over time (F(2,392)=14.5226, p < 0.001). Post-hoc Tukey's testing showed no significant difference between scores at baseline and 12 months, but an increase between 12 months and 24 months (95% CI:-7.36 to -0.945, p < 0.01). Scores were higher at 24 months than baseline (95% CI: -6.852 to -0.437, p < 0.05).
Mixed linear regression models were then employed to assess the aforementioned relationships, additionally controlling for age and gender. Education and APOE were not controlled for due to a lack of significant relationships with BHI score identified in the cross-sectional cohort analysis. Models computed to assess the relationship between RBANS total and BHI scores did not converge at any of the considered timepoints, and therefore findings cannot be reliably reported. This was also the case for RBANS total at 12 months, and 4MT at baseline. Results are summarised in Table 4.
Table 4.
Results of mixed linear regression model analyses. Statistically significant findings are denoted as: * p < 0.05, ** p < 0.01, *** p < 0.001. Abbreviations: N.S., not significant.
| Model convergence? | Gender | Age | Cognitive test | |
|---|---|---|---|---|
| MMSE | ||||
| Baseline | Yes | ** | *** | N.S. |
| 12 months | Yes | * | *** | N.S |
| 24 months | Yes | ** | *** | N.S |
| 4MT | ||||
| Baseline | No | Cannot be reliably reported due to lack of model convergence | ||
| 12 months | Yes | * | *** | N.S. |
| 24 months | Yes | ** | *** | N.S. |
| RBANS total | ||||
| Baseline | No | Cannot be reliably reported due to lack of model convergence | ||
| 12 months | No | |||
| 24 months | No | |||
| RBANS DMI | ||||
| Baseline | Yes | ** | *** | N.S. |
| 12 months | No | Cannot be reliably reported due to lack of model convergence | ||
| 24 months | Yes | ** | *** | N.S. |
4. Discussion
Using two sub-studies we validated the BHI biomarker, a cross-sectional study of 1496 participants, which utilised the rich data within EPAD to assess BHI against risk factors for dementia and cognitive tests, and a longitudinal sub-study of 197 participants to investigate changes in the relationship between BHI and cognitive tests over time.
Within the cohort of subjects with a full complement of required scans, feasibility of BHI was evidenced, with most scans resulting in a successful computation of BHI. Mean BHI scores throughout are broadly comparable to our prior work [6]. Female participants had significantly higher BHI scores than male participants, as was also the case in our prior work [6], and may be linked to male individuals having larger brains with poorer perfusion than their female counterparts [30,31]. Scores decreased significantly with age, which was expected and has been shown previously [6]. After correction for age, increased years of education were significantly associated with a decline in BHI score, which was unexpected. Taken together with our findings from our prior work – which considered education as degree/no degree, rather than years of experience and found that males with degrees had higher BHI scores that those who did not have a degree [6] – the relationship between BHI and education requires further investigation.
Variables pertaining to medical histories were also considered, and associations with vascular risk factors were demonstrated and reassuring for the BHI, giving the established links between vascular health and dementia [[32], [33], [34], [35]]. Those who reported MCI in their medical histories evidenced significantly lower BHI scores that those who did not. Studies have suggested that brain changes can be seen at this stage of cognitive decline [36].
In some instances, expected associations were not demonstrated, examples including WHR and BMI [37], which is an aspect of brain health that the BHI is designed to measure. Smoking status and units of alcohol consumed did not significantly associate with BHI. The risk that smoking poses to brain health is well-established [38], and this finding was contrary to our own prior work using UK Biobank [6]. Some of these findings may reflect sample size issues, distribution of disease and how data were coded, for example EPAD participants who are non-drinkers are grouped along with those consuming small volumes of alcohol (<1 unit per week). This may negate associations as increasing data suggest that any alcohol exposure can be harmful to brain health [39].
An unexpected finding of this study was that participants with depression and/or anxiety had significantly higher BHI scores than those who did not. Depression has been associated with neuroinflammation [40,41], some loss of brain volume [42,43], and long-term damage to the hippocampus [44]. However, it has been suggested some of this neurological change may be reversible [45,46]. It has been suggested that people with depression sometimes evidence better cognition, and that those who are healthier are more likely to attend assessment [47,48]. Nevertheless, there is a wealth of research which has shown a relationship between mood disorders and poor cognition [[49], [50], [51], [52]], and as such the current finding is counterintuitive to what we may expect. Further research may enable elucidation of whether this is a legitimate finding or is a spurious result due to, for example, a confounding variable which has not been accounted for in the current work.
One of the strongest risk factors for dementia is the presence of the APOE e4 allele [14], but no significant differences in BHI scores due to genetic complement of APOE were seen. Whilst risk and the e4 allele do not correlate identically in every population [[53], [54], [55], [56]], much of the research was carried out in Caucasian populations, who represent the vast majority of participants herein. Current evidence suggests APOE e4 mostly has influences in later-life and the current cohort is probably too young to exhibit these [[57], [58], [59]]. The incorporation of multiple MRI phenotypes in the BHI may also obscure specific influences which may be seen if assessed individually. Research has shown APOE interacts with amyloid-beta [60] which was not assessed within the current study but may provide further explanation unexpecting findings between APOE and BHI scores.
The key focus of the cross-sectional cohort within this study was to assess the relationships between BHI scores and multiple cognitive tests. It was hypothesised that individuals with higher cognitive test scores would have higher BHI scores, and after correction for age this was found to be the case in all cognitive tests assessed for male participants, but only the 4MT for females. This test focuses on the hippocampus, a known region of early change in dementia [21], thereby providing support for the utility of BHI as a marker for early brain changes. Whilst cognitive assessments focused on global cognition and memory, other key domains of cognition relevant to neurocognitive disorders – such as executive function - were not investigated. This is a key limitation in understanding the relationship between BHI and cognition and should be addressed in subsequent work.
The longitudinal sub-cohort was investigated to determine any changes in the BHI, as well as its relationship to cognitive scores over time. It was hypothesised that BHI scores would decrease with time, and that this alteration in brain health would be reflected in decreased MMSE, RBANS and 4MT scores. However, many of the changes that were found were too small for any significant differences to be captured. This may be a product of sample size, or length of follow-up, and has implications for design of biomarker-based research studies.
Limitations
The longitudinal sub-study cohort size is modest in size (n = 197) and follow-up over only two years and three timepoints may not be sufficient to demonstrate definitive change. Future work would benefit from following a cohort over a longer period and at more timepoints.
Currently, reference values for BHI in younger adults are unknown, and would further aid interpretation of these findings in older adult cohorts. It should be acknowledged that some data used in this study is self-report data, which can have various issues. Participants may exaggerate or understate their responses or respond in a manner they deem to be socially desirable [61]. This is particularly pertinent to smoking and alcohol consumption, making the basis of these specific results somewhat unreliable.
The vast majority of participants in the current cohort are Caucasian, which is also true of EPAD as a whole. This is a common problem in neuroimaging [62] and one which requires addressing for equitable use of the BHI in further research and future clinical contexts. The work herein made use of MNI templates, which are standard templates within the field, and yet are not always suitable for use for participants who have diverse ancestries [[63], [64], [65];66]. Alternative templates can easily be input to the BHI pipeline and should be considered for use in future studies.
5. Conclusions
BHI is linked to several known risk factors for cognitive decline, and shows association with various cognitive tests, showing promise for its clinical utility. Further work is required to robustly investigate longitudinal changes in the BHI and its relationship with oft-used cognitive tests over a longer period of time.
CRediT authorship contribution statement
Jodi K. Watt: Conceptualization, Methodology, Investigation, Software, Formal analysis, Visualization, Data curation, Writing – original draft, Writing – review & editing. David Alexander Dickie: Conceptualization, Methodology, Writing – review & editing. Frederick K. Ho: Formal analysis, Writing – review & editing. Donald M. Lyall: Methodology, Writing – review & editing. Jesse Dawson: Conceptualization, Methodology, Supervision, Writing – review & editing. Terence J. Quinn: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This work used data and/or samples from the EPAD project which received support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking EPAD grant agreement n° 115736 and an Alzheimer's Association Grant (SG21-818099-EPAD). JKW was funded for the work herein by a Chief Scientist Office Research Grant (Reference: TCS/19/31).
Funding
This work used data and/or samples from the EPAD project which received support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking EPAD grant agreement n° 115736 and an Alzheimer's Association Grant (SG21-818099-EPAD). JKW was funded for the work herein by a Chief Scientist Office Research Grant (Reference: TCS/19/31).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cccb.2024.100214.
Appendix. Supplementary materials
References
- 1.Pini L., Pievani M., Bocchetta M., Altomare D., Bosco P., Cavedo E., Galluzzi S., Marizzoni M., Frisoni G.B. Brain atrophy in Alzheimer's disease and aging. Ageing Res. Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y., Wardlaw J.M. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc. Neurol. 2016;1(3) doi: 10.1136/svn-2016-000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sweeney M.D., Kisler K., Montagne A., Toga A.W., Zlokovic B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018;21(10):1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debette S., Schilling S., Duperron M.G., Larsson S.C., Markus H.S. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMa Neurol. 2019;76(1):81–94. doi: 10.1001/jamaneurol.2018.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickie D.A., Valdés Hernández M.D.C., Makin S.D., Staals J., Wiseman S.J., Bastin M.E., Wardlaw J.M. The brain health index: towards a combined measure of neurovascular and neurodegenerative structural brain injury. Int. J. Stroke. 2018;13(8):849–856. doi: 10.1177/1747493018770222. [DOI] [PubMed] [Google Scholar]
- 6.Watt J.K., Dickie D.A., Lyall D.M., Ward J., Ho F.K., Dawson J., Quinn T.J. Normative values of the brain health index in UK biobank. Neuroimage. 2023;3(3) doi: 10.1016/j.ynirp.2023.100176. [DOI] [Google Scholar]
- 7.Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 8.Hartley T., Bird C.M., Chan D., Cipolotti L., Husain M., Vargha-Khadem F., Burgess N. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17(1):34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randolph C. Psychological Corporation; San Antonio, TX: 1998. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [Google Scholar]
- 10.Randolph C., Tierney M.C., Mohr E., Chase T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 11.Vandenbroucke J.P., Elm E.V., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J., Poole C., Schlesselman J.J., Egger M., Initiative Strobe. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann. Intern. Med. 2007;147(8) doi: 10.7326/0003-4819-147-8-200710160-00010-w1. W-163. [DOI] [PubMed] [Google Scholar]
- 12.Solomon A., Kivipelto M., Molinuevo J.L., Tom B., Ritchie C.W. European prevention of Alzheimer's dementia longitudinal cohort study (EPAD LCS): study protocol. BMJ Open. 2018;8(12) doi: 10.1136/bmjopen-2017-021017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzini L., Ingala S., Wink A.M., Kuijer J.P., Wottschel V., Dijsselhof M., Sudre C.H., Haller S., Molinuevo J.L., Gispert J.D., Cash D.M. The Open-Access European Prevention of Alzheimer's Dementia (EPAD) MRI dataset and processing workflow. NeuroImage. 2022;35 doi: 10.1016/j.nicl.2022.103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avants B.B., Tustison N.J., Stauffer M., Song G., Wu B., Gee J.C. The Insight ToolKit image registration framework. Front. Neuroinform. 2014;8:44. doi: 10.3389/fninf.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., Gee J.C. N4ITK: improved N3 bias correction. IEEe Trans. Med. ImAging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richard E., Jongstra S., Soininen H., Brayne C., van Charante E.P.M., Meiller Y., van der Groep B., Beishuizen C.R., Mangialasche F., Barbera M., Ngandu T. Healthy ageing through internet counselling in the elderly: the HATICE randomised controlled trial for the prevention of cardiovascular disease and cognitive impairment. BMJ Open. 2016;6(6) doi: 10.1136/bmjopen-2015-010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tombaugh T.N., McIntyre N.J. The mini-mental state examination: a comprehensive review. J. Am. Geriatr. Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 19.Arevalo-Rodriguez I., Smailagic N., Roqué-Figuls M., Ciapponi A., Sanchez-Perez E., Giannakou A., Pedraza O.L., Cosp X.B., Cullum S. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI) Cochrane Database Syst. Rev. 2021;(7) doi: 10.1002/14651858.CD010783.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan D., Gallaher L.M., Moodley K., Minati L., Burgess N., Hartley T. The 4 mountains test: a short test of spatial memory with high sensitivity for the diagnosis of pre-dementia Alzheimer's disease. JoVE. 2016;(116):e54454. doi: 10.3791/54454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bettio L.E., Rajendran L., Gil-Mohapel J. The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 2017;79:66–86. doi: 10.1016/j.neubiorev.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Karantzoulis S., Novitski J., Gold M., Randolph C. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): utility in detection and characterization of mild cognitive impairment due to Alzheimer's disease. Arch. Clin. Neuropsychol. 2013;28(8):837–844. doi: 10.1093/arclin/act057. [DOI] [PubMed] [Google Scholar]
- 23.Harris C.R., Millman K.J., Van Der Walt S.J., Gommers R., Virtanen P., Cournapeau D., Wieser E., Taylor J., Berg S., Smith N.J., Kern R. Array programming with NumPy. Nature. 2020;585(7825):357–362. doi: 10.1038/s41586-020-2649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virtanen P., Gommers R., Oliphant T.E., Haberland M., Reddy T., Cournapeau D., Burovski E., Peterson P., Weckesser W., Bright J., Van Der Walt S.J. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods. 2020;17(3):261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinney W. Proceedings of the 9th Python in Science Conference. Vol. 445. 2010. Data structures for statistical computing in python; pp. 51–56. [Google Scholar]
- 26.Hunter J.D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 2007;9(03):90–95. https://doi.ieeecomputersociety.org/10.1109/MCSE.2007.55 [Google Scholar]
- 27.Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., Blondel M., Prettenhofer P., Weiss R., Dubourg V., Vanderplas J. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 2011;12:2825–2830. [Google Scholar]
- 28.Seabold S., Perktold J. Proceedings of the 9th Python in Science Conference. Vol. 57. 2010. Statsmodels: Econometric and statistical modeling with python; pp. 10–25080. [Google Scholar]
- 29.Kluyver T., Ragan-Kelley B., Pérez F., Granger B.E., Bussonnier M., Frederic J., Kelley K., Hamrick J.B., Grout J., Corlay S., Ivanov P. Jupyter Notebooks-a publishing format for reproducible computational workflows. Elpub. 2016;2016:87–90. [Google Scholar]
- 30.Lu H., Xu F., Rodrigue K.M., Kennedy K.M., Cheng Y., Flicker B., Hebrank A.C., Uh J., Park D.C. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb. Cortex. 2011;21(6):1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eliot L., Ahmed A., Khan H., Patel J. Dump the “dimorphism”: comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci. Biobehav. Rev. 2021;125:667–697. doi: 10.1016/j.neubiorev.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 32.Strachan M.W., Reynolds R.M., Marioni R.E., Price J.F. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat. Rev. Endocrinol. 2011;7(2):108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 33.Sierra C. Hypertension and the risk of dementia. Front. Cardiovasc. Med. 2020;7:5. doi: 10.3389/fcvm.2020.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McFarlane O., Kędziora-Kornatowska K. Cholesterol and dementia: a long and complicated relationship. Curr. Aging Sci. 2020;13(1):42–51. doi: 10.2174/1874609812666190917155400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuźma E., Lourida I., Moore S.F., Levine D.A., Ukoumunne O.C., Llewellyn D.J. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimer's Dement. 2018;14(11):1416–1426. doi: 10.1016/j.jalz.2018.06.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith C.D., Van Eldik L.J., Jicha G.A., Schmitt F.A., Nelson P.T., Abner E.L., Kryscio R.J., Murphy R.R., Andersen A.H. Brain structure changes over time in normal and mildly impaired aged persons. AIMS. Neurosci. 2020;7(2):120. doi: 10.3934/Neuroscience.2020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamer M., Batty G.D. Association of body mass index and waist-to-hip ratio with brain structure: UK Biobank study. Neurology. 2019;92(6):e594–e600. doi: 10.1212/WNL.0000000000006879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linli Z., Feng J., Zhao W., Guo S. Associations between smoking and accelerated brain ageing. Progr. Neuro-Psychopharmacol. Biol. Psychiatry. 2022;113 doi: 10.1016/j.pnpbp.2021.110471. [DOI] [PubMed] [Google Scholar]
- 39.Topiwala A., Ebmeier K.P., Maullin-Sapey T., Nichols T.E. No safe level of alcohol consumption for brain health: observational cohort study of 25,378 UK Biobank participants. medRxiv. 2021 doi: 10.1101/2021.05.10.21256931. 2021-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C.H., Giuliani F. The role of inflammation in depression and fatigue. Front. Immunol. 2019;10:1696. doi: 10.3389/fimmu.2019.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Troubat R., Barone P., Leman S., Desmidt T., Cressant A., Atanasova B., Brizard B., El Hage W., Surget A., Belzung C., Camus V. Neuroinflammation and depression: a review. Eur. J. Neurosci. 2021;53(1):151–171. doi: 10.1111/ejn.14720. [DOI] [PubMed] [Google Scholar]
- 42.Dotson V.M., Davatzikos C., Kraut M.A., Resnick S.M. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J. Psychiatry Neurosci. 2009;34(5):367–375. [PMC free article] [PubMed] [Google Scholar]
- 43.Harris M.A., Cox S.R., De Nooij L., Barbu M.C., Adams M.J., Shen X., Deary I.J., Lawrie S.M., McIntosh A.M., Whalley H.C. Structural neuroimaging measures and lifetime depression across levels of phenotyping in UK biobank. Transl. Psychiatry. 2022;12(1):157. doi: 10.1038/s41398-022-01926-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheline Y.I. Depression and the hippocampus: cause or effect? Biol. Psychiatry. 2011;70(4):308–309. doi: 10.1016/j.biopsych.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banasr M., Dwyer J.M., Duman R.S. Cell atrophy and loss in depression: reversal by antidepressant treatment. Curr. Opin. Cell Biol. 2011;23(6):730–737. doi: 10.1016/j.ceb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jamieson A., Goodwill A.M., Termine M., Campbell S., Szoeke C. Depression related cerebral pathology and its relationship with cognitive functioning: a systematic review. J. Affect. Disord. 2019;250:410–418. doi: 10.1016/j.jad.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 47.Cullen B., Smith D.J., Deary I.J., Pell J.P., Keyes K.M., Evans J.J. Understanding cognitive impairment in mood disorders: mediation analyses in the UK Biobank cohort. Br. J. Psychiatry. 2019;215(5):683–690. doi: 10.1192/bjp.2019.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyall D.M., Quinn T., Lyall L.M., Ward J., Anderson J.J., Smith D.J., Stewart W., Strawbridge R.J., Bailey M.E., Cullen B. Quantifying bias in psychological and physical health in the UK Biobank imaging sub-sample. Brain Commun. 2022;4(3):119. doi: 10.1093/braincomms/fcac119. fcac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marvel, C.L. and Paradiso, S., 2004. Cognitive and neurological impairment in mood disorders. Psychiatric Clin., 27(1), pp.19-36. 10.1016/S0193-953X(03)00106-0. [DOI] [PMC free article] [PubMed]
- 50.Beaudreau S.A., O'Hara R. Late-life anxiety and cognitive impairment: a review. Am. J. Geriatr. Psychiatry. 2008;16(10):790–803. doi: 10.1097/JGP.0b013e31817945c3. [DOI] [PubMed] [Google Scholar]
- 51.Rock P.L., Roiser J.P., Riedel W.J., Blackwell A. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol. Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- 52.Liew T.M. Subjective cognitive decline, anxiety symptoms, and the risk of mild cognitive impairment and dementia. Alzheimers. Res. Ther. 2020;12:1–9. doi: 10.1186/s13195-020-00673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maestre G., Ottman R., Stern Y., Gurland B., Chun M., Tang M.X., Shelanski M., Tycko B., Mayeux R. Apolipoprotein E and Alzheimer's disease: ethnic variation in genotypic risks. Ann. Neurol. 1995;37(2):254–259. doi: 10.1002/ana.410370217. [DOI] [PubMed] [Google Scholar]
- 54.Evans D.A., Bennett D.A., Wilson R.S., Bienias J.L., Morris M.C., Scherr P.A., Hebert L.E., Aggarwal N., Beckett L.A., Joglekar R., Berry-Kravis E. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch. Neurol. 2003;60(2):185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 55.Rajan K.B., McAninch E.A., Wilson R.S., Weuve J., Barnes L.L., Evans D.A. Race, APOE ɛ4, and long-term cognitive trajectories in a biracial population sample. J. Alzheimer's Dis. 2019;72(1):45–53. doi: 10.3233/JAD-190538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belloy M.E., Andrews S.J., Le Guen Y., Cuccaro M., Farrer L.A., Napolioni V., Greicius M.D. APOE genotype and Alzheimer disease risk across age, sex, and population ancestry. JAMa Neurol. 2023 doi: 10.1001/jamaneurol.2023.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiepers O.J.G., Harris S.E., Gow A.J., Pattie A., Brett C.E., Starr J.M., Deary I.J. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol. Psychiatry. 2012;17(3):315–324. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- 58.Bonham L.W., Geier E.G., Fan C.C., Leong J.K., Besser L., Kukull W.A., Kornak J., Andreassen O.A., Schellenberg G.D., Rosen H.J., Dillon W.P. Age-dependent effects of APOE ε4 in preclinical Alzheimer's disease. Ann. Clin. Transl. Neurol. 2016;3(9):668–677. doi: 10.1002/acn3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palmer J.M., Huentelman M., Ryan L. More than just risk for Alzheimer's disease: APOE ε4′s impact on the aging brain. Trends. Neurosci. 2023 doi: 10.1016/j.tins.2023.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Wisniewski T., Drummond E. APOE-amyloid interaction: therapeutic targets. Neurobiol. Dis. 2020;138 doi: 10.1016/j.nbd.2020.104784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorber S.C., Tremblay M.S. The Objective Monitoring of Physical Activity: Contributions of Accelerometry to Epidemiology, Exercise Science and Rehabilitation. 2016. Self-report and direct measures of health: bias and implications; pp. 369–376. [Google Scholar]
- 62.Ricard J.A., Parker T.C., Dhamala E., Kwasa J., Allsop A., Holmes A.J. Confronting racially exclusionary practices in the acquisition and analyses of neuroimaging data. Nat. Neurosci. 2023;26(1):4–11. doi: 10.1038/s41593-022-01218-y. [DOI] [PubMed] [Google Scholar]
- 63.Rao N.P., Jeelani H., Achalia R., Achalia G., Jacob A., dawn Bharath R., Varambally S., Venkatasubramanian G., Yalavarthy P.K. Population differences in brain morphology: need for population specific brain template. Psychiatry Res. 2017;265:1–8. doi: 10.1016/j.pscychresns.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 64.Bhalerao G.V., Parlikar R., Agrawal R., Shivakumar V., Kalmady S.V., Rao N.P., Agarwal S.M., Narayanaswamy J.C., Reddy Y.J., Venkatasubramanian G. Construction of population-specific Indian MRI brain template: Morphometric comparison with Chinese and Caucasian templates. Asian J. Psychiatr. 2018;35:93–100. doi: 10.1016/j.ajp.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 65.Pai P.P., Mandal P.K., Punjabi K., Shukla D., Goel A., Joon S., Roy S., Sandal K., Mishra R., Lahoti R. BRAHMA: Population specific T1, T2, and FLAIR weighted brain templates and their impact in structural and functional imaging studies. Magn. Reson. Imaging. 2020;70:5–21. doi: 10.1016/j.mri.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Yang G., Zhou S., Bozek J., Dong H.M., Han M., Zuo X.N., Liu H., Gao J.H. Sample sizes and population differences in brain template construction. Neuroimage. 2020;206 doi: 10.1016/j.neuroimage.2019.116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.