Summary
Background
Matrix metalloproteinases (MMPs) are implied in blood–brain barrier degradation and haemorrhagic transformation following ischaemic stroke, but their local relevance in the hyperacute disease phase is unknown. We aimed to examine ultra-early MMP-9 and MMP-2 release into collateral blood vessels, and to assess its prognostic value before therapeutic recanalisation by endovascular thrombectomy (EVT).
Methods
We report a cross-sectional proof-of-concept study including patients undergoing EVT for large-vessel ischaemic stroke at the University Hospital Würzburg, Germany. We obtained liquid biopsies from the collateral circulation before recanalisation, and systemic control samples. Laboratory workup included quantification of MMP-9 and MMP-2 plasma concentrations by cytometric bead array, immunohistochemical analyses of cellular MMP-9 and MMP-2 expression, and detection of proteolytic activity by gelatine zymography. The clinical impact of MMP concentrations was assessed by stratification according to intracranial haemorrhagic lesions on postinterventional computed tomography (Heidelberg Bleeding Classification, HBC) and early functional outcome (modified Rankin Scale, mRS). We used multivariable logistic regression, receiver-operating-characteristic (ROC) curves, and fixed-level estimates of test accuracy measures to study the prognostic value of MMP-9 concentrations.
Findings
Between August 3, 2018, and September 16, 2021, 264 matched samples from 132 patients (86 [65.2%] women, 46 [34.8%] men, aged 40–94 years) were obtained. Median (interquartile range, IQR) MMP-9 (279.7 [IQR 126.4–569.6] vs 441 [IQR 223.4–731.5] ng/ml, p < 0.0001) but not MMP-2 concentrations were increased within collateral blood vessels. The median MMP-9 expression level of invading neutrophils was elevated (fluorescence intensity, arbitrary unit: 2276 [IQR 1007–5086] vs 3078 [IQR 1108–7963], p = 0.0018). Gelatine zymography experiments indicated the locally confined proteolytic activity of MMP-9 but not of MMP-2. Pretherapeutic MMP-9 release into stroke-affected brain regions predicted the degree of intracerebral haemorrhages and clinical stroke severity after recanalisation, and independently increased the odds of space-occupying parenchymal haematomas (HBC1c–3a) by 1.54 times, and the odds of severe disability or death (mRS ≥5 at hospital discharge) by 2.33 times per 1000 ng/ml increase. Excessive concentrations of MMP-9 indicated impending parenchymal haematomas and severe disability or death with high specificity.
Interpretation
Measurement of MMP-9 within collateral blood vessels is feasible and identifies patients with stroke at risk of major intracerebral haemorrhages and poor outcome before therapeutic recanalisation by EVT, thereby providing evidence of the concept validity of ultra-early local stroke biomarkers.
Funding
This work was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) and the Interdisciplinary Centre for Clinical Research (IZKF) at the University of Würzburg.
Keywords: Stroke, Ischaemic stroke, Large-vessel occlusion, Matrix metalloproteinases, MMP-9, MMP-2, Biomarker, Haemorrhage, Outcome, Mechanical thrombectomy, Endovascular therapy
Research in context.
Evidence before this study
Matrix metalloproteinase (MMP)-9 and MMP-2 have long been implicated in haemorrhagic transformation and neurological impairment following ischaemic stroke, but no human studies exist on hyperacute release into stroke-affected brain regions and its prognostic significance in a therapeutic context.
Added value of this study
This study investigates MMP-9 and MMP-2, and their prognostic relevance in cerebral liquid biopsies from collateral blood vessels of patients with large-vessel ischaemic stroke before therapeutic recanalisation by endovascular thrombectomy (EVT). We analysed 264 samples of 132 consecutive patients, indicating the locally confined release of enzymatically active MMP-9 but not of MMP-2 into an occluded arterial territory. On single-cell level, invading neutrophils were identified as intravascular source of MMP-9. Local MMP-9 release independently increased the odds of space-occupying parenchymal haematomas, and severe disability or death in the early clinical course after therapeutic recanalisation by EVT. Additionally, our data suggest that excessive local MMP-9 concentrations may add considerable value for predicting these events.
Implications of all the available evidence
Our results posit MMP-9 within collateral blood vessels as pathophysiologically relevant biomarker to identify the clinically most relevant high-risk groups for deleterious outcome among EVT candidates, i.e., before recanalisation of the occluded vessel and reperfusion of the target territory. Although further external validation is warranted, these findings have far-reaching implications for future preclinical and clinical stroke research including trial design and clinical management as they provide the proof-of-concept for the potential prognostic utility of an ultra-early local biomarker before therapeutic recanalisation.
Introduction
Endovascular thrombectomy (EVT) represents the most effective treatment for large-vessel ischaemic stroke, achieving complete independence in activities of daily living in a large proportion of patients.1, 2, 3, 4, 5 Yet there is a trade-off between the indisputable overall benefit conferred by EVT, and the subgroup of patients which presents with poorer prognosis due to critical infarct progression either before or after clot removal.6 In these patients, the clinical consequences may comprise intracranial haemorrhagic complications and neurological impairment in a wide variety of manifestations.4,7,8 Although overall factors associated with such outcomes have been well-delineated in post-hoc analyses of randomised controlled trials (RCTs) of EVT,7,8 individual-level factors to accurately identify patients at risk for the most severe ends of the spectra still remain to be elucidated to establish the best practice for clinical management.9,10 Hence, identifying distinct risk biomarkers and implementing non-traditional clinical trial designs in large-vessel ischaemic stroke (e.g., biomarker-adaptive) could allow for adjustment of individual treatment decisions in high-risk groups.
Disintegration of the neurovascular unit (NVU) including degradation of the endothelial and parenchymal basal lamina is a major pathophysiological substrate of cerebral infarction, its clinical course, and haemorrhagic events.11,12 Principal effector molecules comprise matrix metalloproteinases (MMPs), a group of endopeptidases which, when activated upon cleavage, are capable of destructing all components of the extracellular matrix.11 Among 24 mammalian MMPs, MMP-9 and MMP-2 stand out due to their implication in stroke-induced blood–brain barrier (BBB) breakdown, vasogenic oedema, and haemorrhagic transformation.11,13 However, conflicting clinical data exist addressing the association of MMPs with stroke treatment and prognosis.14, 15, 16, 17 These variations in prognostic value can be explained by the fact that sample material was restricted to systemic sites, which are prone to dilution effects,18,19 and largely obtained at late time points at which infarct progression and MMP release by various resident brain cells have already occurred.11
Recent evidence suggests that large-vessel ischaemic stroke elicits a rapidly evolving intravascular inflammatory response, which is carried into the ischaemic territory by retrograde collateral supply through pial anastomoses, and dominated by neutrophils.18, 19, 20 Retrogradely invading immune cells such as neutrophils represent a potential source of MMPs.11 We therefore aimed to investigate whether MMP-9 and/or MMP-2 are released into collateral blood vessels at ultra-early time points and predict the formation of space-occupying parenchymal haematomas, and severe disability or death before therapeutic recanalisation by EVT. For this purpose, we took advantage of timed and systematic endovascular sampling of liquid biopsies from the occluded cerebral vasculature before the final decision of recanalisation by clot retrieval was made.18,19,21
Methods
Study design and participants
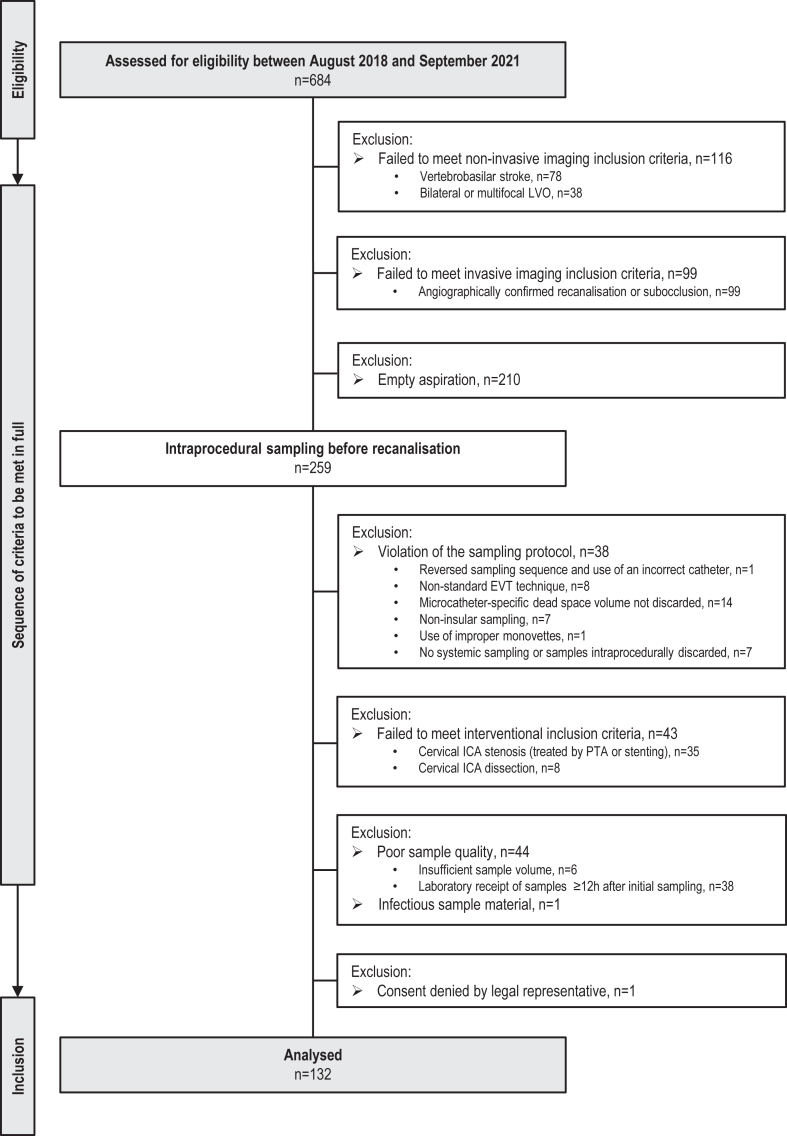
Standardised sampling of liquid biopsies from the human collateral circulation was attempted during EVT for cerebral large-vessel occlusion in a prospective cross-sectional proof-of-concept study of consecutive patients with stroke presenting at the Departments of Neuroradiology and Neurology, University Hospital Würzburg (Würzburg, Germany) between August 3, 2018, and September 16, 2021.18,19 Indication for EVT was given by disabling acute stroke and non-invasive imaging qualifying for stroke intervention according to current guidelines and consensus recommendations.9 Patients with occlusion locations homologous to standard experimental middle cerebral artery (MCA) occlusion models were included (i.e., terminal internal carotid artery (ICA), MCA–M1, and proximal MCA–M2 segment, respectively).6,22 The final target population comprised all patients undergoing EVT for invasively confirmed total vascular occlusion in whom intraprocedural aspiration and processing of 1 ml of citrate–phosphate–dextrose–adenine (CPDA)–anticoagulated local ischaemic and systemic arterial whole blood was successfully performed according to the protocol previously published by our group.18,19 Key exclusion criteria were proven bilateral or multifocal large-vessel occlusion; occlusion locations other than terminal ICA, MCA–M1, and MCA–M2 segment; antegrade cerebral blood flow; large-vessel occlusion in conjunction with either ≥50% cervical ICA stenosis or ICA dissection with or without necessitating intraprocedural cervical ICA percutaneous transluminal angioplasty (PTA) or stent implantation19; and any deviation from the endovascular, sampling or sample pre-processing procedures (see below). The complete flow of patients without missing cases is presented in Fig. 1.
Fig. 1.
Study population. EVT, endovascular thrombectomy; ICA, internal carotid artery; LVO, large-vessel occlusion; PTA, percutaneous transluminal angioplasty.
Sex, reported as male or female, was determined by checking the patients’ identification data and through observation of physiological characteristics; current gender identity was not recorded.
Informed consent was obtained from patients or their legal representatives. Ethical approval was provided by the Ethics Committee of the University of Würzburg (approval number 135/17-me). The study was conducted in conformity with the Declaration of Helsinki, and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.23
Procedures
In brief, endovascular procedures were carried out by certified neurointerventionalists or supervised neurointerventional fellows on biplane angiographic systems (Artis icono or ArtisQ, Siemens Healthineers, Erlangen, Germany). All patients underwent EVT under general anaesthesia according to in-house standard operating procedures. Recanalisation of the predefined target occlusion by stent-embolus retrieval was preceded by microcatheter navigation into the MCA–M2 segment to obtain samples from within the collateral circulation during persistent large-vessel occlusion. After microcatheter (NeuroSlider 27 or 21; Acandis, Pforzheim, Germany) navigation to the target sampling location and discarding the microcatheter-specific dead space volume, sampling of 1 ml ischaemic blood was attempted in every consecutive case. These cerebral target samples were compared to intraindividual control samples taken from the ipsilateral cervical ICA immediately after EVT (physiological forward-flow condition).
All samples were transferred into CPDA monovettes (#04.1938.001, Sarstedt, Nümbrecht, Germany) and further processed. Slides stained with May-Grünwald-Giemsa were used for neutrophil and monocyte quantification. Samples were centrifuged (1000 ×g, 10 min) and plasma was aliquoted, and frozen at −20 °C until analysis.18,19
Plasma concentrations of MMP-9 and MMP-2 were quantified by flow cytometric analysis (BD FACSLyric™ Flow Cytometer, BD Biosciences, RRID:SCR_013311) using fluorescent bead immunoassays according to the manufacturers’ instructions (#740565 and #740559, BioLegend, RRID:SCR_001134).
For immunofluorescent staining of MMP-9 and MMP-2, we used 96 available air-dried cell smear slides, comprising 48 matched pairs of systemic vs ischaemic samples, with 24 subjects for each MMP-9 and MMP-2 analysis. All slides were fixed with acetone for 10 min at −20 °C and blocked for 1 h at room temperature with phosphate-buffered saline (PBS; 1.0 M, pH 7.4) containing 0.2% Triton X-100 (#T8787, Sigma–Aldrich, RRID:SCR_008988), and 5% bovine serum albumin (BSA; #SC-2323, Santa Cruz Biotechnology, RRID:SCR_008987). The slides were incubated over night at 4 °C with a mouse monoclonal MMP-9 antibody (#819701, BioLegend, RRID:AB_2564833; isotype IgG2a, dilution 1:100) or a mouse monoclonal MMP-2 antibody (#436000, Thermo Fisher Scientific, RRID:AB_2532214; isotype IgG1, dilution 1:100) in PBS containing 1% BSA. For MMP-9, an Alexa 647-labelled secondary polyclonal goat anti-mouse antibody (#115-605-003, Jackson ImmunoResearch, RRID:AB_2338902; whole IgG, dilution 1:100) and, for MMP-2, an Alexa 555-labelled secondary polyclonal goat anti-mouse antibody (#A-21422, Thermo Fisher Scientific, RRID: AB_2535844; whole IgG, dilution 1:200) was applied for 1 h at room temperature in PBS containing 1% BSA. Finally, slides were embedded in Gold Antifade Mountant with DAPI (4,6-diamidino-2-phenylindole dihydrochloride) dye for counterstaining of cell nuclei (#P36931, Thermo Fisher Scientific, RRID:SCR_008452).
For single-plane images, the slides were examined at 40× magnification on an inverted Leica DMi8 (Leica Microsystems, RRID:SCR_008960) epifluorescence microscope equipped with a DFC 3000 G camera (exposure time 12.2 ms, gain 1.0). Quantification of MMP-9 and MMP-2 fluorescence intensity at single-neutrophil level was performed using the Fiji24 distribution (Fiji, RRID:SCR_002285) of ImageJ software. The corrected total cellular fluorescence (CTCF)25 was calculated (arbitrary unit) for ten consecutive neutrophils present within at least three fields of view per slide, and for 960 neutrophils in total. The integrated fluorescence density, area, and mean grey value of nuclei were assessed for each cell applying the following formula: CTCF = integrated fluorescence density of the area of the neutrophil—(the area of the neutrophil × mean fluorescence of three background readings).
The residual plasma volumes were retained for further analysis of MMP isoenzymes by gelatine zymography as described previously.26
Non-contrast computed tomography (CT; Somatom Definition AS+, Siemens Healthineers, Erlangen, Germany) images revealing the greatest lesion extent within two to seven days following EVT were employed for radiological assessment of structural tissue damage, with presumed 100% specificity for the presence of bleeding in areas of postinterventional cerebral hyperintensity.27, 28, 29, 30 The Alberta Stroke Program Early CT Score (ASPECTS) was used for assessing structural tissue damage in the absence of bleeding events.27 Intracranial bleeding events were categorized anatomically using the Heidelberg Bleeding Classification (HBC): HBC1a, haemorrhagic infarction (HI1)—scattered small petechiae; HBC1b, HI2—confluent petechiae; HBC1c, parenchymal haematoma (PH1)—haematoma within infarcted tissue, occupying <30%; HBC 2, PH2—haematoma occupying ≥30% of the infarcted tissue; HBC 3a—PH remote from infarcted brain tissue; HBC 3b—intraventricular haemorrhage; HBC 3c—subarachnoid haemorrhage; and HBC 3d—subdural haemorrhage.28,29 These events were further stratified as absent, minor (petechial) intracerebral haemorrhage (combined HBC classes 1a and 1b), major intracerebral haemorrhage (parenchymal haematomas: combined HBC classes 1c, 2, and 3a), and intracranial-extracerebral haemorrhage (combined HBC classes 3b, 3c, and 3d).29,31 All CT images were consensus-read by two neuroradiologists who were blinded to laboratory and clinical data (AGM and MS).
Early functional outcome was assessed by the modified Rankin Scale (mRS) at hospital discharge by certified stroke neurologists (CV and FE) who were blinded to laboratory data and image analyses.32
Statistical analysis
Gaussian distribution was tested by the D'Agostino-Pearson normality test.33 Data were summarized using frequency (percentage) for categorical variables and median (interquartile range, IQR) for continuous variables. The χ2, the Wilcoxon matched-pairs signed rank test, the Mann–Whitney test, and the Kruskal–Wallis test with Benjamini, Krieger and Yekutieli correction (false discovery rate of 5%)34 were used for group comparisons, as appropriate. Spearman's rank correlation coefficient was applied to determine associations between MMP concentrations and leukocyte numbers. Crude univariate (unadjusted) and multivariable-adjusted logistic regression using stepwise-backwards selection with a cut-off point of p > 0.1 were applied to analyse the association of MMP concentrations with major intracerebral bleedings (combined HBC classes 1c–3a) and severe disability or death at hospital discharge (mRS ≥5). Covariate selection (i.e., age, sex, baseline antiplatelet treatment and anticoagulant use, baseline National Institutes of Health Stroke Scale (NIHSS) score, baseline ASPECTS, systolic and diastolic blood pressure before recanalisation, alteplase administration, onset-to-puncture time, occlusion location, number of stent-retrieval manoeuvres, reperfusion status, onset-to-final-recanalisation time, and sampling-to-sampling time) was based on χ2 procedures of clinical characteristics (p ≈ 0.1), literature review of reported risk factors, and methodological considerations regarding sequential intraprocedural sampling.11,35 Multicollinearity was assessed by examination of the correlation matrix and variables with associations ≥0.8 were omitted (Figure S1).36,37 Results from logistic regression are given as adjusted Odds ratios (OR) with 95% confidence interval (CI). Receiver-operating-characteristic (ROC) analysis was performed to further evaluate the logistic models and the diagnostic performance of systemic vs ischaemic MMP-9 concentrations as potential biomarker of major intracerebral haemorrhages and severe disability or death. We calculated and compared the respective areas under the curve (AUC),38 and used the maximized Youden J index (overall correct classification rate minus one) to provide a summary measure of cut-off values. Fixed-level estimates of test accuracy measures including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% CI were calculated for 80%, 90%, 95%, 97.5%, and 99% sensitivity and specificity, respectively, to provide further thresholds for potential clinical decision making on individual patient-level. All estimates were given along with corresponding MMP-9 concentrations. Subgroup analyses were based on specified research questions. Analyses used GraphPad Prism (version 10.1.0, GraphPad Software, RRID:SCR_002798) and MedCalc (version 22.013, MedCalc Software, RRID:SCR_015044).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit it for publication.
Results
Between August 3, 2018, and September 16, 2021, 684 patients with large-vessel ischaemic stroke requiring emergency EVT were screened for eligibility (Fig. 1). Of 132 consecutive patients included in the study, 86 (65.2%) were women, and the median age was 79 (IQR 70–83) years. Table 1 shows demographic, clinical, and radiological characteristics at presentation and over the course of treatment. According to imaging after EVT, 67 (50.8%) subjects were classified as having no intracranial bleeding event (absent), 40 (30.3%) subjects were classified as having a minor intracerebral haemorrhage (HBC1a + b), 18 (13.6%) subjects were classified as having a major intracerebral haemorrhage (HBC1c–3a), and 7 (5.3%) subjects were classified as having an intracranial-extracerebral haemorrhage (HBC3b–d; full listing provided in Table S1). The median NIHSS score at admission was highest in patients developing intracerebral haemorrhagic lesions (HBC1a + b: 15 [IQR 12–18], HBC1c–3a: 16.5 [IQR 13–20.5], Kruskal–Wallis test p = 0.0025), with no significant difference between patients with minor and major intracerebral haemorrhages (two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli p = 0.33). Median infarct extent on ASPECTS at admission was low in patients without intracranial bleeding events and moderate in patients with any type of subsequent intracerebral haemorrhage (absent: 9 [IQR 8–9] vs HBC1a + b: 7 [IQR 6–8] vs HBC1c–3a: 7 [IQR 6–8], Kruskal–Wallis test p < 0.0001), again with no significant difference regarding bleeding severity (two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli p = 0.98). MCA–M2 occlusions were seen less frequently (absent: 32.8% [22 of 67] vs HBC1a + b: 7.5% [3 of 40] vs HBC1c–3a: 16.7% [3 of 18], χ2 test p = 0.008) in patients who later developed haemorrhagic lesions. Functional outcome was significantly worse for patients with haemorrhagic lesions when compared to absence of bleeding events (median mRS; absent: 2 [IQR 1–5] vs HBC1a + b: 5 [IQR 3–5] vs HBC1c–3a: 6 [IQR 5–6], Kruskal–Wallis test p < 0.0001), with major intracerebral haemorrhages resulting in high death rates during hospitalisation (55.6% [10 of 18]). All other demographic, clinical, or treatment-related characteristics including procedural measures were balanced and did not show any significant difference between groups.
Table 1.
Patient characteristics by occurrence and type of intracranial bleeding event after EVT.
| Absenta (n = 67) | HBC class 1a + ba (n = 40) | HBC class 1c–3aa (n = 18) | HBC class 3b–da (n = 7) | pb value | Overall (n = 132) | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age, median (IQR), y | 79 (68–83) | 80 (66.5–84) | 76.5 (71.5–85.3) | 71 (70–81) | 0.7 | 79 (70–83) |
| Sex | ||||||
| Male, n (%) | 22 (32.8) | 14 (35) | 6 (33.3) | 4 (57.1) | 0.97 | 46 (34.8) |
| Female, n (%) | 45 (67.2) | 26 (65) | 12 (66.7) | 3 (42.9) | 0.97 | 86 (65.2) |
| Medical | ||||||
| Hypertension, n (%) | 57 (85.1) | 36 (90) | 14 (77.8) | 7 (100) | 0.46 | 114 (86.4) |
| Diabetes mellitus, n (%) | 15 (22.4) | 8 (20) | 3 (16.7) | 1 (14.3) | 0.86 | 27 (20.5) |
| Hyperlipidemia, n (%) | 22 (32.8) | 16 (40) | 8 (44.4) | 1 (14.3) | 0.58 | 47 (35.6) |
| Atrial fibrillation, n (%) | 38 (56.7) | 23 (57.5) | 8 (44.4) | 4 (57.1) | 0.61 | 73 (55.3) |
| Smoking history, n (%) | 16 (23.9) | 10 (25) | 2 (11.1) | 2 (28.6) | 0.46 | 31 (23.5) |
| Baseline medication | ||||||
| Antithrombotic medication, n (%) | 37 (55.2) | 23 (57.5) | 14 (77.8) | 3 (42.9) | 0.21 | 77 (58.3) |
| Antiplatelet drugs, n (%) | 18 (26.9) | 12 (30) | 10 (55.6) | 0 (0) | 0.06 | 40 (30.3) |
| Anticoagulants, n (%) | 21 (31.3) | 11 (27.5) | 5 (27.8) | 3 (42.9) | 0.9 | 40 (30.3) |
| Antihypertensive drugs, n (%) | 56 (83.6) | 30 (75) | 12 (66.7) | 4 (57.1) | 0.25 | 102 (77.3) |
| Clinical | ||||||
| NIHSS at presentation, median (IQR) | 12 (8–16) | 15 (12–18) | 16.5 (13–20.5) | 9 (6–15) | 0.0025 | 14 (9.3–17) |
| Unknown onset, n (%) | 20 (29.9) | 15 (37.5) | 7 (38.9) | 3 (42.9) | 0.63 | 45 (34.1) |
| ASPECTS at presentation, median (IQR) | 9 (8–9) | 7 (6–8) | 7 (6–8) | 9 (8–10) | <0.0001 | 8 (7–9) |
| Systolic blood pressure, median (IQR), mmHg | 157 (136–180) | 160 (144.5–186) | 160 (155–183.8) | 157 (97.5–164) | 0.31 | 159 (141–180) |
| Diastolic blood pressure, median (IQR), mmHg | 86 (69–96) | 80 (73–98) | 89 (78–102.3) | 65 (57.5–76.5) | 0.11 | 85 (72–98) |
| Heart rate, median (IQR), min−1 | 81 (70–98) | 78 (69.8–89.8) | 82.5 (70.5–102.3) | 77 (61–98) | 0.75 | 80 (70–97.8) |
| Treatment | ||||||
| Thrombolysis | ||||||
| Intravenous alteplase treatment, n (%) | 26 (38.8) | 15 (37.5) | 9 (50) | 2 (28.6) | 0.64 | 52 (39.4) |
| Intervention | ||||||
| Onset-to-puncture, median (IQR), min | 235 (153–325) | 230 (156–280) | 257 (195–322) | 291.5 (147.8–355.8) | 0.82 | 237 (165–316) |
| Angiographic occlusion locationc | ||||||
| M1, n (%) | 40 (59.7) | 29 (72.5) | 11 (61.1) | 4 (57.1) | 0.4 | 84 (63.6) |
| M2, n (%) | 22 (32.8) | 3 (7.5) | 3 (16.7) | 3 (42.9) | 0.008 | 31 (23.5) |
| ICA, n (%) | 8 (11.9) | 10 (25) | 4 (22.2) | 0 (0) | 0.2 | 22 (16.7) |
| Puncture-to-first-pass, median (IQR), min | 42.5 (30–51) | 38.5 (29–57) | 42 (29.5–53.8) | 40.5 (35.8–58) | 0.92 | 41 (30–53.3) |
| Stent-retrieval manoeuvres, n (IQR) | 1 (1–3) | 2 (1–3) | 1 (1–3.5) | 2.5 (1–4) | 0.49 | 2 (1–3) |
| Successful recanalisationd, n (%) | 58 (86.6) | 34 (85) | 14 (77.8) | 6 (85.7) | 0.65 | 112 (84.9) |
| Duration of MT procedure, median (IQR), min | 70 (52–104) | 89 (62.8–138.3) | 74 (53.5–119) | 98 (56–154.8) | 0.23 | 75 (56–118) |
| Onset-to-final-recanalisation, median (IQR), min | 307 (244–425) | 325 (264.5–379.5) | 319 (280–449) | 419 (250–460) | 0.74 | 325 (251.5–408) |
| Sampling | ||||||
| Onset-to-cerebral sampling, median (IQR), min | 274 (191–375) | 285 (192.5–356) | 303 (242–442) | 243.5 (158.3–355) | 0.79 | 285 (198–367) |
| Onset-to-carotid sampling, median (IQR), min | 319 (211–405) | 342 (277.5–390) | 338 (297–460) | 366.5 (245.8–475.3) | 0.78 | 336 (263–405) |
| Outcome | ||||||
| mRS at discharge, median (IQR) | 2 (1–5) | 5 (3–5) | 6 (5–6) | 3 (1–5) | <0.0001 | 4 (1–5) |
| Death during hospital stay, n (%) | 3 (4.5) | 6 (15) | 10 (55.6) | 1 (14.3) | <0.0001 | 20 (15.2) |
ASPECTS, Alberta Stroke Program Early CT Score; EVT, endovascular thrombectomy; HBC, Heidelberg Bleeding Classification; ICA, internal carotid artery; IQR, interquartile range; M1/M2, middle cerebral artery segment; mmHg, millimetres of mercury; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SD; standard deviation; y, years.
Intracranial haemorrhages were anatomically categorised according to the Heidelberg Bleeding Classification (HBC: 1a, haemorrhagic infarction (HI1)—scattered small petechiae; 1b, HI2—confluent petechiae; 1c, parenchymal haematoma (PH1)—haematoma within infarcted tissue, occupying <30%; 2, PH2—haematoma occupying ≥30% of the infarcted tissue; 3a—PH remote from infarcted brain tissue; 3b—intraventricular haemorrhage; 3c—subarachnoid haemorrhage; 3d—subdural haemorrhage) and grouped with respect to the type of bleeding (HBC classes 1a + b, minor [petechial] intracerebral haemorrhage; HBC classes 1c–3a, major intracerebral haemorrhage [parenchymal haematoma]; HBC classes 3b–d, intracranial-extracerebral haemorrhage).
The p values for binary variables are based on χ2 test (data for analysis included the following groups: absent, HBC1a + b, and HBC1c–3a). The p values of all other variables are based on Kruskal–Wallis test or one-way ANOVA, as appropriate.
Including multiple sites in the target downstream territory.
Defined as eTICI ≥ 2b50.
Freezing and storage had no impact on MMP stability, as group-stratified plasma levels (see below) were consistent for different storage times.
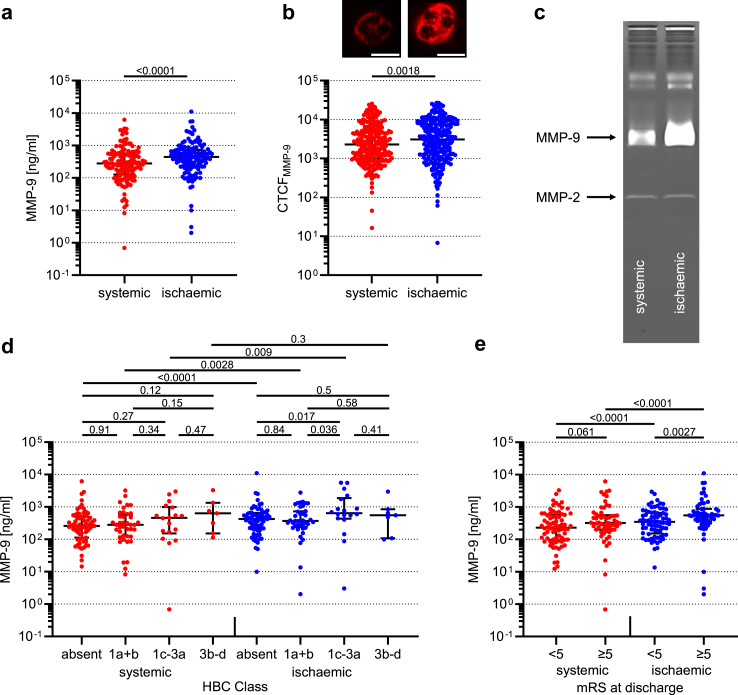
The overall median MMP-9 concentration within collateral blood vessels was increased by 57.7% as compared to systemic control level (279.7 [IQR 126.4–569.6] vs 441 [IQR 223.4–731.5] ng/ml, Wilcoxon matched-pairs signed rank test p < 0.0001; Fig. 2a). By contrast, no significant differences were observed for overall median MMP-2 concentrations (Figure S2a). Gelatine zymography of pooled plasma samples detected higher local MMP-9 but no MMP-2 activity (Fig. 2c). To assess which cell type might represent the dominant intravascular source of MMP-9, we conducted both correlation analyses with corresponding numbers of neutrophils and monocytes, and analysed MMP-9 and MMP-2 on cellular level by immunohistochemistry. Local overall MMP-9 concentrations were best correlated with the number of invading neutrophils (Spearman r = 0.35, p < 0.0001; Figure S3a and c), whereas no correlation with monocyte numbers was found (Figure S3b and d). Consistently, fluorescence microscopy of individual cells showed that large-vessel occlusion induced a 1.35-fold increase of MMP-9 expression in neutrophils in affected brain regions (CTCF: 2276 [IQR 1007–5086] vs 3078 [IQR 1108–7963], Wilcoxon matched-pairs signed rank test p = 0.0018; Fig. 2b). There were no significant differences in cellular MMP-2 expression among neutrophils sampled from the different sites (Figure S2b).
Fig. 2.
Regional MMP-9 plasma concentrations, neutrophil MMP-9 expression, and MMP activity with plasma concentrations stratified by type of intracranial bleeding and functional outcome category. Semi-log plots of MMP-9 plasma concentrations (a, d, and e), fluorescence intensity of neutrophils stained for MMP-9 (b; presented with examples at single-cell level, scale bar in white = 10 μm), and gelatine zymography experiments (c) in arterial non-occlusive control samples at cervical level of the internal carotid artery (systemic) vs samples from centrally within the cerebral collateral circulation (ischaemic) in human large-vessel ischaemic stroke. Zymography bands (c) were obtained from n = 10 pooled plasma samples. Zymographic intensity of 86 kDa MMP-9 and 72 kDa MMP-2, respectively. Intracranial bleedings (d) were anatomically categorized according to the Heidelberg Bleeding Classification (HBC: 1a, haemorrhagic infarction (HI1)—scattered small petechiae; 1b, HI2—confluent petechiae; 1c, parenchymal haematoma (PH1)—haematoma within infarcted tissue, occupying <30%; 2, PH2—haematoma occupying ≥30% of the infarcted tissue; 3a—PH remote from infarcted brain tissue; 3b—intraventricular haemorrhage; 3c—subarachnoid haemorrhage; 3d—subdural haemorrhage) and grouped with respect to the type of bleeding (HBC classes 1a + b, minor [petechial] intracerebral haemorrhage; HBC classes 1c–3a, major intracerebral haemorrhage [parenchymal haematoma]; HBC classes 3b–d, intracranial-extracerebral haemorrhage). Functional outcome (e) was assessed by the modified Rankin Scale (mRS: mRS≥5, severe disability or death at hospital discharge). The long horizontal bar shows the median value, and the short error bar shows the interquartile range. Data were analysed using Wilcoxon matched-pairs signed rank test (a, b, d, and e), Kruskal–Wallis test with Benjamini, Krieger and Yekutieli post hoc analysis (d), and Mann–Whitney test (e). Individual p values are given for p < 0.05. CTCF, corrected total cellular fluorescence; HBC, Heidelberg Bleeding Classification; kDa, kilo Dalton; MMP-9/-2, Matrix Metalloproteinase-9/2; mRS, modified Rankin Scale; ng/ml, nanograms per millilitre; ns, not significant.
After stratifying for intracranial bleeding events, an association between median MMP-9 concentrations within collateral blood vessels and type of intracerebral bleeding was observed, with highest levels in patients with parenchymal haematomas (HBC1a + b: 368.3 [IQR 238–731.5] vs HBC1c–3a: 643 [IQR 434.8–1873] ng/ml, two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli p = 0.036; Fig. 2d). Equally stratified systemic MMP-9 concentrations were similar in pattern but did not significantly differ between groups. Stratification of MMP-9 but not MMP-2 plasma levels by neurological outcome category (i.e., mRS <5 vs ≥ 5) and sampling location revealed higher ischaemic MMP-9 concentrations in patients with early poor neurological outcome (mRS <5: 347.6 [IQR 152.7–654.5] vs mRS ≥5: 552.2 [IQR 360.2–872.5] ng/ml, Mann–Whitney test p = 0.0027; Fig. 2e). There was no association of MMP-2 concentrations with occurrence and type of bleeding, or clinical outcome (Figure S2c and d).
Further analysis of the subgroup of patients with no haemorrhagic event showed no significant difference in MMP-9 concentrations between patients with and without excellent ASPECTS (≥8) values (Figure S4). Median ischaemic MMP-9 levels were increased by 20.5% among alteplase-treated patients (401.2 [IQR 216.1–691] vs 483.6 [IQR 268.1–762.1] ng/ml, Mann–Whitney test p = 0.32; Figure S5). There was no evidence of any sex-specific differences in systemic or ischaemic MMP-9 and MMP-2 concentrations (Figure S6).
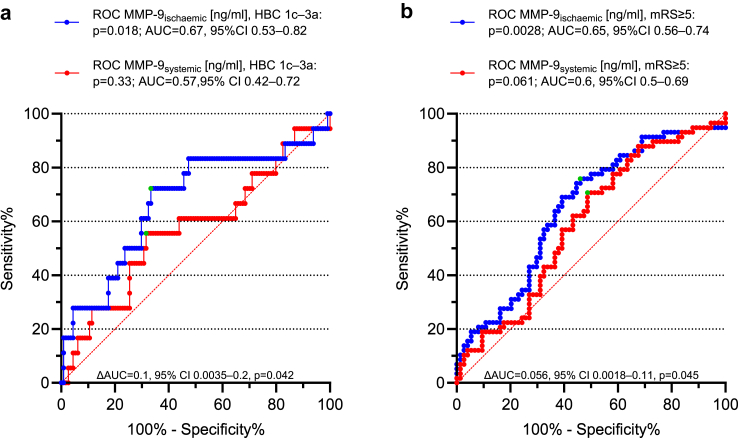
In multivariable-adjusted logistic regression analysis, local MMP-9 concentrations, baseline antiplatelet medication, baseline NIHSS, and baseline ASPECTS were predictive of HBC classes 1c, 2, and 3a haemorrhages (overall model fit: Nagelkerke R2 = 0.32, −2 log likelihood ratio χ2 test p = 0.0004; AUC = 0.84 [95% CI 0.76–0.9]; Table 2; univariate data given in Table S2). Using the same model, MMP-9 concentrations within collateral blood vessels, systolic blood pressure at admission, baseline NIHSS, baseline ASPECTS, and non-successful reperfusion were associated with early poor neurological outcome following EVT (overall model fit: Nagelkerke R2 = 0.35, −2 log likelihood ratio χ2 test p < 0.0001; AUC = 0.8 [95% CI 0.71–0.87]; Table 2). MMP-9 concentrations in collateral blood vessels yielded a significantly larger AUC than the systemic values to discriminate between occurrence or non-occurrence of both major intracerebral haemorrhages (AUC = 0.57 [95% CI 0.42–0.72]; AUC = 0.67 [95% CI 0.53–0.82]; ΔAUC = 0.1 [95% CI 0.0035–0.2], DeLong test p = 0.042; Fig. 3a), and severe disability or death (AUC = 0.6 [95% CI 0.5–0.69]; AUC = 0.65 [95% CI 0.56–0.74]; ΔAUC = 0.056 [95% CI 0.0018–0.11], DeLong test p = 0.045; Fig. 3b). Accordingly, parenchymal haematomas were indicated with 72.2% sensitivity and 66.7% specificity (PPV 25.5%; NPV 93.8%) at a cut-off value (Youden J index) of 552.9 ng/ml (p = 0.018), and mRS scores of 5–6 were indicated with 75.9% sensitivity and 54.1% specificity (PPV 56.4%; NPV 74.1%) at a cut-off value of 363.3 ng/ml (p = 0.0028). A comprehensive set fixed-level estimates of test accuracy measures including sensitivity, specificity, PPV, and NPV derived from MMP-9 ROC analyses is given in Table 3. Excessive local MMP-9 release predicted space-occupying parenchymal haematomas as well as severe disability or death with 99% specificity (HBC1c–3a: PPV of 72.5% for 2928 ng/ml; mRS ≥5: PPV of 84.4% for 2826.9 ng/ml).
Table 2.
Multivariable logistic regression model of predictors of major intracerebral haemorrhages and severe disability or death.
|
HBC class 1c–3a |
mRS ≥5 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor variablea | Coefficient | SE | Odds ratiob | 95% CI | p value | Predictor variablea | Coefficient | SE | Odds ratioc | 95% CI | p value |
| Constant | −2.52 | 1.62 | NA | NA | 0.12 | Constant | −3.63 | 2.1 | NA | NA | 0.083 |
| MMP-9ischaemic | 0.43 | 0.18 | 1.54 | 1.073–2.2 | 0.019 | MMP-9ischaemic | 0.85 | 0.4 | 2.33 | 1.068–5.1 | 0.034 |
| Baseline NIHSS score | 0.14 | 0.048 | 1.15 | 1.045–1.26 | 0.0042 | Baseline NIHSS score | 0.1 | 0.038 | 1.11 | 1.028–1.19 | 0.0072 |
| Baseline ASPECTS | −0.37 | 0.19 | 0.69 | 0.47–1.0065 | 0.054 | Baseline ASPECTS | −0.27 | 0.14 | 0.77 | 0.58–1.015 | 0.064 |
| Baseline antiplatelet treatment | 1.32 | 0.68 | 3.76 | 1.0024–14.13 | 0.049 | Systolic blood pressure at admission | 0.019 | 0.0083 | 1.019 | 1.0028–1.036 | 0.022 |
| No reperfusion | 1.75 | 0.63 | 5.75 | 1.66–19.86 | 0.0058 | ||||||
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; AUC, area under curve; CI, Confidence interval; HBC, Heidelberg Bleeding Classification; MMP-9, Matrix Metalloproteinase-9; mmHg, millimetres of mercury; mRS, modified Rankin Scale; ng/ml, nanograms per millilitre; NIHSS, National Institutes of Health Stroke Scale; NA, not applicable; SE, standard error.
Odds ratios for continuous variables are reported per unit of increment: MMP-9ischaemic [1000 ng/ml]; baseline NIHSS score [1-point NIHSS score increase]; baseline ASPECTS [1-point ASPECTS decrease]; systolic blood pressure at admission [1 mmHg].
Data represent adjusted odds ratios with 95% CI for major intracerebral bleeding events equivalent to the combined Heidelberg Bleeding Classification (HBC) classes 1c, 2, and 3a (categorisation corresponding to Table 1). Overall model fit: Nagelkerke R2 = 0.32, −2 log likelihood ratio χ2 test p = 0.0004; AUC = 0.84 [95% CI 0.76–0.9].
Data represent adjusted odds ratios with 95% CI for severe disability or death as defined by a modified Rankin Scale (mRS) ≥5 at hospital discharge. Overall model fit: Nagelkerke R2 = 0.35, −2 log likelihood ratio χ2 test p < 0.0001; AUC = 0.8 [95% CI 0.71–0.87].
Fig. 3.
ROC analysis of MMP-9 plasma concentrations for discrimination between patients at low and high risk of major intracerebral haemorrhages, and severe disability or death. ROC curves of regional ischaemic (blue) and systemic (red) MMP-9 plasma concentrations in prediction of HBC1c-3a bleedings (a), and mRS ≥5 at hospital discharge (b) in large-vessel ischaemic stroke; categorisation corresponding to Fig. 2. Green dots represent values of maximised balances between sensitivity and specificity. Reported p values for the differences in AUCs were derived from DeLong's test. Youden index, HBC1c–3a: MMP-9ischaemic 552.9 ng/ml, 72.2% sensitivity (95% CI 46.5–90.3%), 66.7% specificity (95% CI 57.2–75.2%); MMP-9systemic 448.9 ng/ml, 55.6% sensitivity (95% CI 30.8–78.5%), 68.4% specificity (95% CI 59.1–76.8%); Youden index, mRS ≥5: MMP-9ischaemic 363.3 ng/ml, 75.9% sensitivity (95% CI 62.8–86.1%), 54.1% specificity (95% CI 42.1–65.7%); MMP-9systemic 235.5 ng/ml, 70.7% sensitivity (95% CI 57.3–81.9), 51.4% specificity (95% CI 39.4–63.1). AUC, area under curve; HBC, Heidelberg Bleeding Classification; MMP-9, Matrix Metalloproteinase-9; ng/ml, nanograms per millilitre; mRS, modified Rankin Scale; ROC, receiver operating characteristic.
Table 3.
Fixed-level estimates of diagnostic test accuracy measures for MMP-9 cut-off concentrations in prediction of major intracerebral haemorrhages and severe disability or death.
|
HBC class 1c–3a |
mRS ≥5 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | 95% CI | PPV (%) | 95% CI | NPV (%) | 95% CI | MMP-9ischaemic[ng/ml] | Sensitivity (%) | Specificity (%) | 95% CI | PPV (%) | 95% CI | NPV (%) | 95% CI | MMP-9ischaemic[ng/ml] |
| 80 | 52.6 | 4.4–73.9 | 21.1 | 16.5–26.5 | 94.3 | 86.7–97.7 | >405.1 | 80 | 41.9 | 21.6–60.8 | 51.9 | 46.1–57.7 | 72.8 | 59.9–82.7 | >284.7 |
| 90 | 6.1 | 0.9–52.6 | 13.2 | 11.4–15.1 | 79.5 | 45–94.9 | >85.6 | 90 | 31.1 | 0–54.2 | 50.6 | 46.2–55 | 79.9 | 63.1–90.2 | >207.4 |
| 95 | 0.9 | 0.9–1.8 | 13.1 | 12–14.4 | 52.7 | 6.3–94.8 | >3 | 95 | 0 | 0–14.9 | NA | NA | NA | NA | >9.3 |
| 97.5 | 0.9 | 0.9–1.8 | 13.4 | 12.6–14.4 | 69 | 6.4–98.6 | >2.5 | 97.5 | 0 | 0–17.6 | NA | NA | NA | NA | >2.5 |
| 99 | 0.9 | 0.9–1.8 | 13.6 | 13.1–14.2 | 84.8 | 3.7–99.9 | >2.2 | 99 | 0 | 0–8.1 | NA | NA | NA | NA | >2 |
| Specificity (%) | Sensitivity (%) | Specificity (%) | Sensitivity (%) | ||||||||||||
| 80 | 38.9 | 11.1–66.7 | 23.5 | 13.4–37.9 | 89.2 | 85–92.4 | >776.3 | 80 | 27.6 | 12.1–44.8 | 52 | 36.8–66.7 | 58.5 | 53.7–63.2 | >768.1 |
| 90 | 27.8 | 11.1–50 | 30.5 | 14.8–52.6 | 88.8 | 85.5–91.4 | >1246.4 | 90 | 20.7 | 8.6–32.8 | 61.7 | 41–79.1 | 59.2 | 55.4–62.8 | >1064.5 |
| 95 | 27.8 | 11.1–61.1 | 46.7 | 22.7–72.4 | 89.3 | 86.2–91.8 | >1496.9 | 95 | 15.5 | 5.2–27.6 | 70.9 | 43.3–88.6 | 58.9 | 55.9–61.9 | >1345.7 |
| 97.5 | 16.7 | 0–0 | 51.3 | 18.4–83.1 | 88.1 | 85.7–90.1 | >2682.8 | 97.5 | 10.3 | 0–0 | 76.4 | 39.3–942 | 58.1 | 55.8–60.4 | >2274 |
| 99 | 16.7 | 0–0 | 72.5 | 24.4–95.6 | 88.3 | 85.9–90.3 | >2928 | 99 | 6.9 | 0–0 | 84.4 | 31.7–98.4 | 57.6 | 55.8–59.4 | >2826.9 |
|
Sensitivity (%) |
Specificity (%) |
95% CI |
PPV (%) |
95% CI |
NPV (%) |
95% CI |
MMP-9systemic[ng/ml] |
Sensitivity (%) |
Specificity (%) |
95% CI |
PPV (%) |
95% CI |
NPV (%) |
95% CI |
MMP-9systemic[ng/ml] |
| 80 | 20.2 | 7–36 | 13.7 | 11–16.9 | 86.5 | 70.3–94.5 | >102.6 | 80 | 36.5 | 16.3–50 | 49.7 | 44.3–55.1 | 67 | 56.2–80.9 | >168.3 |
| 90 | 13.2 | 4.4–20.2 | 14.1 | 12.1–16.2 | 89.3 | 65.9–97.3 | >76.3 | 90 | 17.6 | 0–36.5 | 46.1 | 42.8–49.5 | 69.2 | 47.3–84.9 | >83.5 |
| 95 | 0 | 4.4–4.4 | NA | NA | NA | NA | >0.7 | 95 | 5.4 | 0–20.3 | 44.1 | 42.1–46 | 58 | 24.1–85.7 | >22.2 |
| 97.5 | 0 | 36–36 | NA | NA | NA | NA | >0.7 | 97.5 | 0 | 0–5.4 | NA | NA | NA | NA | >4.1 |
| 99 | 0 | 4.4–4.4 | NA | NA | NA | NA | >0.7 | 99 | 0 | 0–0 | NA | NA | NA | NA | >0.7 |
| Specificity (%) | Sensitivity (%) | Specificity (%) | Sensitivity (%) | ||||||||||||
| 80 | 27.8 | 11.1–55.6 | 18 | 8.7–33.5 | 87.5 | 83.9–90.5 | >634.3 | 80 | 22.4 | 12.1–41.4 | 46.8 | 31.2–63 | 56.8 | 52.4–61.1 | >634.3 |
| 90 | 16.7 | 0–33.3 | 20.8 | 7.6–45.9 | 87.3 | 84.7–89.5 | >1122.1 | 90 | 19 | 10.3–32.8 | 59.8 | 38.5–78 | 58.6 | 55.1–62.1 | >929.8 |
| 95 | 11.1 | 0–45.6 | 26 | 7.1–61.9 | 87.1 | 85.1–88.9 | >1763.4 | 95 | 12.1 | 5.2–27 | 65.4 | 36–86.4 | 58 | 55.3–60.6 | >1363.9 |
| 97.5 | 0 | 0–0 | NA | NA | NA | NA | >3034.2 | 97.5 | 6.9 | 0–0 | 68.4 | 28.2–92.3 | 57.2 | 55.3–59.1 | >2444.4 |
| 99 | 0 | 0–0 | NA | NA | NA | NA | >3289.7 | 99 | 1.7 | 0–0 | 44.1 | 43.1–45.1 | 68.7 | 9.1–98 | >3251.9 |
Major intracerebral haemorrhages (combined Heidelberg Bleeding Classification [HBC] classes 1c, 2, and 3a), as per Table 1 categorisation. Functional outcome as assessed by the modified Rankin Scale at hospital discharge (mRS: mRS ≥5, severe disability or death).
CI, Confidence interval; HBC, Heidelberg Bleeding Classification; MMP-9, Matrix Metalloproteinase-9; mRS, modified Rankin Scale; ng/ml, nanograms per millilitre; NPV, negative predictive value; NA, not applicable; PPV, positive predictive value.
Discussion
Our most important finding is that MMP-9 but not MMP-2 was strongly increased within the collateral circulation during large-vessel ischaemic stroke. Moreover, we show that the extent of MMP-9 release into collateral blood vessels reflected the degree of intracerebral haemorrhages and clinical stroke severity after recanalisation, and independently predicted poorest outcome of either endpoint. These data provide the proof-of-concept for the potential prognostic utility of an ultra-early local biomarker before the final decision of recanalisation by EVT is made.
The most likely explanation for these findings lies in the fact that cerebral ischaemia, both in clinical and experimental context, elicits an immediate intravascular inflammatory response, which is dominated by neutrophils,18,20 and known to contribute to infarct progression under vascular occlusion, as well as during ischaemia/reperfusion injury.6,39 Recently, high quantities of the platelet-derived neutrophil-activating chemokine CXC motif ligand (CXCL) 4, and the neutrophil attractant CXCL7 have been detected in human pial blood.40 Therefore, our current findings support a sequence of intravascular events by which platelet activation and alpha-granule release drives neutrophil degranulation and release of MMP-9 with ensuing disturbance of the BBB.11,12,20 These inflammatory processes may perpetuate into the recanalisation phase, thereby explaining the potential prognostic utility of ultra-early local MMP-9 concentrations. This may have multiple implications for research in acute ischaemic stroke.
For decades, researchers have aimed for diagnosis, etiological differentiation, and prognostication of stroke gleaned from blood-based biomarkers which are typically measured in venous samples and often lack temporal, spatial, or functional relevance in ischaemia-induced structural brain damage.11,12,41 Nowadays, as evidence for streamlined decision-making for EVT based on widely available non-invasive imaging techniques grows,3, 4, 5,42 it is reasonable to assume that the near-future use of blood-based biomarkers in the setting of large-vessel occlusion may serve the identification of patients which will likely benefit from timely adjustment of endovascular treatment or medical care thereafter.9,10 Hence, the conceptually new approach of ultra-early local biomarkers prior to EVT appears promising to realise a more tailored stroke care.21 When choosing biomarker candidates, MMPs inherently possess prognostic value as their production is temporally limited, spatially confined through specific regulation at transcriptional level, and causative for a deleterious pathophysiological phenotype.11,13,43
However, available literature on the prognostic significance of MMPs in human stroke is inconsistent. A prospective single-centre study of 255 patients with stroke recruited within 48 h of symptom onset found no association of venous MMP-9 concentrations with either haemorrhagic transformation or functional outcome at three months.16 Several explanations addressing these discrepancies might be discussed. First, we utilised a microcatheter-based technique for direct probing from within the centre of an occluded cerebral vascular territory which previously revealed that non-local sample material does not accurately reflect absolute concentrations, cellular composition, or the degree of activation of blood components in the ischaemic brain region.18,19,44,45 Second, local sample material was obtained within a median time interval of 4 h and 45 min after symptom onset, which is expected to have a beneficial effect on temporal homogeneity in that an early peak of MMP-9 expression may not have been missed.11,15,17 Third, a set of strict protocol requirements were in place to avoid observational biases which might result from efforts not requiring, e.g., mandatory vascular imaging or uniform methods to obtain representative sample material of a sufficiently narrow defined target patient population.18,19 Fourth, outcome variables were chosen to reflect a relevant proportion of patients who are in greater need and capacity for optimisation of treatment at the same time.9,16
Other studies utilising peripheral blood samples have reported an association between MMP-9 concentrations and prognosis of stroke.14,15,17 Two prospective studies strongly suggested that MMP-9 levels in sample material obtained within 3 h of stroke onset predict the occurrence of space-occupying parenchymal haematomas after alteplase treatment.15,17 Consistently, a dose–response association of MMP-9 concentrations and major disability or death at 3 months was reported for samples obtained within 24 h of hospital admission.14
At present, uncertainty exists concerning the cellular origin of MMPs, and the time point at which they exert their effect. Our study now permitted the testing of previously inaccessible sample material, revealing that MMP-9 was locally cleaved into its active form during vascular occlusion.13 Using direct immunofluorescence microscopy, we identified invading neutrophils as intravascular source of MMP-9 (+35.2% expression). Our data accord with experimental findings indicating that leukocytes recruited to the brain serve as a significant cellular source of MMP-9 following transient focal stroke,46 and are further supported through analyses of human ex-vivo specimens revealing that MMP-9-positive neutrophil infiltration surrounding the brain microvasculature is related to BBB breakdown.43 It has been shown that circulating neutrophils from patients treated with alteplase release more MMP-9 after ex-vivo stimulation,16 and that alteplase enters the collateral circulation in-vivo.35 Together, these findings challenge the concept that mainly brain-derived MMP-9 is involved in massive BBB opening and drive attention to regional neutrophil heterogeneity, and an early mode of action from the luminal side of the occluded vascular territory.11,20
Analyses by multivariable logistic regression revealed that regional MMP-9 release prior to EVT independently increased the odds of parenchymal haematomas by 1.54 times, and the odds of severe disability or death by 2.33 times per 1000 ng/ml increase, respectively. Consistently, AUC values indicated no effective discriminatory ability for systemic MMP-9 concentrations in identifying at-risk patients (parenchymal haematomas: AUC = 0.57; severe disability or death: AUC = 0.6) who showed non-distinct demographic, clinical, or treatment-related features, while ischaemic MMP-9 concentrations achieved a significantly higher, albeit moderate, AUC for outcome prognostication across all thresholds (parenchymal haematomas: AUC = 0.67; severe disability or death: AUC = 0.65). ROC analyses were further conducted to provide a more detailed evaluation of predictive thresholds at various MMP-9 concentration levels. Due to the lack of comparability with previously published data on MMP-9 concentrations, we employed the Youden J index to derive potential data-driven cut-off concentrations, which, however, may result in overly optimistic estimates of sensitivity and specificity.47 While this approach yielded lower sensitivity and specificity than expected, it also prioritised a balance between these test measures, potentially neglecting relevant clinical factors such as disease severity and the implications of false results on patient management.47 Therefore, we additionally assessed a range of fixed-level estimates of test accuracy measures, ranging from 80% to 99% sensitivity and specificity, respectively. These analyses aimed to identify candidate diagnostic target concentrations for clinical decision-making, indicating that excessive release occurring in a particular group of patients is specific to deleterious outcome.
The major strength of the study is its standardised and biologically plausible approach which resulted in the definition of a local blood-based biomarker to identify the clinically most relevant high-risk groups among EVT candidates. However, our study is limited by its single-centre design reflecting in-house interventional and intensive care standards, and the small number of patients who developed space-occupying parenchymal haematomas after EVT. Thus, our findings will require verification in a larger multi-centre population to increase generalizability. Furthermore, the measured plasma concentrations and immunofluorescence intensity of MMP-9 do not necessarily indicate activated MMP-9. Nevertheless, zymography experiments provided evidence for the locally confined proteolytic activity of MMP-9. Finally, we recruited patients in whom aspiration of cerebral liquid biopsies succeeded, meaning that we did not have access to outcome prognostication in patients in whom aspiration was attempted but failed.
In conclusion, this study posits MMP-9 concentrations within collateral blood vessels as local biomarker to identify patients at high risk for space-occupying parenchymal haematomas and severe disability or death before therapeutic recanalisation by EVT is achieved. Hence, we hypothesise that the use of point-of-care assays for sample material from the collateral circulation might be an effective addition to future trial designs of EVT to address, e.g., the extensiveness of endovascular treatment, adjunct MMP inhibition, concepts of blood pressure management, and different antiplatelet, and/or antiinflammatory regimens in herewith identified groups of interest.
Contributors
Conceptualization: AMK, MP, GS, and MKS; Data Curation: AMK, AGM, JF, MLV, MS, CV, FE, LZ, and MKS; Formal Analysis: AMK and MKS; Funding Acquisition: AMK, MP, CV, FE, HN, GS, and MKS; Investigation: AMK, MP, AGM, JF, MLV, YX, MS, CV, FE, HN, LZ, and MKS; Methodology: AMK, MP, and MKS; Project Administration: AMK and MKS; Resources: AMK, MP, GS, and MKS; Software: AMK and AGM; Supervision: AMK and MKS; Visualization: AMK and MKS; Writing—original draft: AMK; Writing—review and editing: AMK, MP, AGM, HN, KGH, CH, LZ, GS, and MKS. AMK and MKS have directly accessed and verified the underlying data reported in the manuscript. All authors confirm that they read and approved the final version of the manuscript.
Data sharing statement
Data collected for the study and data from sample analyses will be made available upon reasonable request to the corresponding authors.
Declaration of interests
MP has received speaker honoraria from Bayer and Merck Serono; unrelated to the present study. KGH has received honoraria for acting as an advisor/speaker for Boehringer Ingelheim; unrelated to the present study. MKS declares a grant from CSL Behring, funding from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) project No. 424778381—CRC/TR 295, and is a co-holder of patent PCT/EP2020/068464; all unrelated to the present study. All other authors have nothing to disclose.
Acknowledgements
This study was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) project No. 374031971—CRC/TR 240 (MP, HN, GS, and MKS; Gerok position held by AMK), DFG project No. 413657723—Clinician Scientist programme UNION CVD (AMK, CV, and FE), and the Interdisciplinary Centre for Clinical Research (IZKF) at the University of Würzburg project No. T-516 (AMK and MKS). We thank Julian Kunz and all neurointerventionalists, and radiology technicians of the Department of Neuroradiology for their support in data acquisition and patient handling. We also thank the CSF-laboratory team of the Department of Neurology for excellent technical support.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105095.
Contributor Information
Alexander M. Kollikowski, Email: Kollikowsk_A@ukw.de.
Mirko Pham, Email: Pham_M@ukw.de.
Alexander G. März, Email: Maerz_A@ukw.de.
Jörn Feick, Email: Feick_J@ukw.de.
Marius L. Vogt, Email: Vogt_M2@ukw.de.
Yanyan Xiong, Email: Xiong_Y@ukw.de.
Marc Strinitz, Email: Marc.Strinitz@tum.de.
Christoph Vollmuth, Email: Vollmuth_C1@ukw.de.
Fabian Essig, Email: Essig_F@ukw.de.
Hermann Neugebauer, Email: Neugebauer_H@ukw.de.
Karl Georg Haeusler, Email: Haeusler_K@ukw.de.
Christian Hametner, Email: Hametner_C@ukw.de.
Lena Zimmermann, Email: Papp_L@ukw.de.
Guido Stoll, Email: Stoll_G@ukw.de.
Michael K. Schuhmann, Email: Schuhmann_M@ukw.de.
Appendix A. Supplementary data
References
- 1.Goyal M., Menon B.K., van Zwam W.H., et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 2.Jovin T.G., Nogueira R.G., Lansberg M.G., et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet. 2022;399:249–258. doi: 10.1016/S0140-6736(21)01341-6. [DOI] [PubMed] [Google Scholar]
- 3.Sarraj A., Hassan A.E., Abraham M.G., et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023;388:1259–1271. doi: 10.1056/NEJMoa2214403. [DOI] [PubMed] [Google Scholar]
- 4.Huo X., Ma G., Tong X., et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023;388:1272–1283. doi: 10.1056/NEJMoa2213379. [DOI] [PubMed] [Google Scholar]
- 5.Bendszus M., Fiehler J., Subtil F., et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: multicentre, open-label, randomised trial. Lancet. 2023;402:1753–1763. doi: 10.1016/S0140-6736(23)02032-9. [DOI] [PubMed] [Google Scholar]
- 6.Stoll G., Pham M. Beyond recanalization - a call for action in acute stroke. Nat Rev Neurol. 2020;16:591–592. doi: 10.1038/s41582-020-00417-0. [DOI] [PubMed] [Google Scholar]
- 7.van Kranendonk K.R., Treurniet K.M., Boers A.M.M., et al. Hemorrhagic transformation is associated with poor functional outcome in patients with acute ischemic stroke due to a large vessel occlusion. J Neurointerv Surg. 2019;11:464–468. doi: 10.1136/neurintsurg-2018-014141. [DOI] [PubMed] [Google Scholar]
- 8.Ospel J.M., Qiu W., Menon B.K., et al. Radiologic patterns of intracranial hemorrhage and clinical outcome after endovascular treatment in acute ischemic stroke: results from the ESCAPE-NA1 trial. Radiology. 2021;300:402–409. doi: 10.1148/radiol.2021204560. [DOI] [PubMed] [Google Scholar]
- 9.Powers W.J., Rabinstein A.A., Ackerson T., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke. Stroke. 2019;50:E344–E418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 10.Mazighi M., Richard S., Lapergue B., et al. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP-TARGET): a multicentre, open-label, randomised controlled trial. Lancet Neurol. 2021;20:265–274. doi: 10.1016/S1474-4422(20)30483-X. [DOI] [PubMed] [Google Scholar]
- 11.Jickling G.C., Liu D., Stamova B., et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34:185–199. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ra H.-J., Parks W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong C., Yang J., Xu T., et al. Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology. 2017;89:805–812. doi: 10.1212/WNL.0000000000004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montaner J., Molina C.A., Monasterio J., et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 16.Maestrini I., Tagzirt M., Gautier S., et al. Analysis of the association of MPO and MMP-9 with stroke severity and outcome: cohort study. Neurology. 2020;95:e97–e108. doi: 10.1212/WNL.0000000000009179. [DOI] [PubMed] [Google Scholar]
- 17.Castellanos M., Sobrino T., Millán M., et al. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke. 2007;38:1855–1859. doi: 10.1161/STROKEAHA.106.481556. [DOI] [PubMed] [Google Scholar]
- 18.Kollikowski A.M., Schuhmann M.K., Nieswandt B., Müllges W., Stoll G., Pham M. Local leukocyte invasion during hyperacute human ischemic stroke. Ann Neurol. 2020;87:466–479. doi: 10.1002/ana.25665. [DOI] [PubMed] [Google Scholar]
- 19.Strinitz M., Pham M., März A.G., et al. Immune cells invade the collateral circulation during human stroke: prospective replication and extension. Int J Mol Sci. 2021;22:9161. doi: 10.3390/ijms22179161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoll G., Schuhmann M.K., Nieswandt B., Kollikowski A.M., Pham M. An intravascular perspective on hyper-acute neutrophil, T-cell and platelet responses: similarities between human and experimental stroke. J Cereb Blood Flow Metab. 2022;42:1561–1567. doi: 10.1177/0271678X221105764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dagonnier M., Donnan G.A., Davis S.M., Dewey H.M., Howells D.W. Acute stroke biomarkers: are we there yet? Front Neurol. 2021;12 doi: 10.3389/fneur.2021.619721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer C.J. Ischemic stroke: experimental models and reality. Acta Neuropathol. 2017;133:245–261. doi: 10.1007/s00401-017-1667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 24.Schindelin J., Arganda-Carreras I., Frise E., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ives A., Nomura J., Martinon F., et al. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat Commun. 2015;6:6555. doi: 10.1038/ncomms7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuhmann M.K., Bittner S., Meuth S.G., Kleinschnitz C., Fluri F. Fingolimod (FTY720-P) does not stabilize the blood-brain barrier under inflammatory conditions in an in vitro model. Int J Mol Sci. 2015;16:29454–29466. doi: 10.3390/ijms161226177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kollikowski A.M., Cattus F., Haag J., et al. Progression of cerebral infarction before and after thrombectomy is modified by prehospital pathways. J Neurointerv Surg. 2022;14:485–489. doi: 10.1136/neurintsurg-2020-017155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hacke W., Kaste M., Bluhmki E., et al. Thrombolysis with Alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 29.von Kummer R., Broderick J.P., Campbell B.C.V., et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 30.Dekeyzer S., Nikoubashman O., Lutin B., et al. Distinction between contrast staining and hemorrhage after endovascular stroke treatment: one CT is not enough. J Neurointerv Surg. 2017;9:394–398. doi: 10.1136/neurintsurg-2016-012290. [DOI] [PubMed] [Google Scholar]
- 31.Mazya M.V., Ahmed N., Ford G.A., et al. Remote or extraischemic intracerebral hemorrhage--an uncommon complication of stroke thrombolysis: results from the safe implementation of treatments in stroke-international stroke thrombolysis register. Stroke. 2014;45:1657–1663. doi: 10.1161/STROKEAHA.114.004923. [DOI] [PubMed] [Google Scholar]
- 32.Ovbiagele B., Saver J.L. Day-90 acute ischemic stroke outcomes can be derived from early functional activity level. Cerebrovasc Dis. 2010;29:50–56. doi: 10.1159/000255974. [DOI] [PubMed] [Google Scholar]
- 33.D'Agostino R.B., Pearson E.S. Tests for departure from normality. Empirical results for the distributions of b2 and √b1. Biometrika. 1973;60:613–622. [Google Scholar]
- 34.Benjamini Y., Krieger A.M., Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. [Google Scholar]
- 35.Essig F., Kollikowski A.M., Müllges W., et al. Local cerebral recombinant tissue plasminogen activator concentrations during acute stroke. JAMA Neurol. 2021;78:615–617. doi: 10.1001/jamaneurol.2021.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrestha N. Detecting multicollinearity in regression analysis. Am J Appl Math Stat. 2020;8:39–42. [Google Scholar]
- 37.May R.B., Masson M.E.J., Hunter M.A. 1990. Application of statistics in behavioral research. New Jork: Harper & Row. [Google Scholar]
- 38.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 39.Schuhmann M.K., Bieber M., Franke M., et al. Platelets and lymphocytes drive progressive penumbral tissue loss during middle cerebral artery occlusion in mice. J Neuroinflammation. 2021;18:46. doi: 10.1186/s12974-021-02095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kollikowski A.M., Pham M., März A.G., et al. Platelet activation and chemokine release are related to local neutrophil-dominant inflammation during hyperacute human stroke. Transl Stroke Res. 2022;13:364–369. doi: 10.1007/s12975-021-00938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montellano F.A., Ungethüm K., Ramiro L., et al. Role of blood-based biomarkers in ischemic stroke prognosis: a systematic review. Stroke. 2021;52:543–551. doi: 10.1161/STROKEAHA.120.029232. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen T.N., Abdalkader M., Nagel S., et al. Noncontrast computed tomography vs computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol. 2022;79:22–31. doi: 10.1001/jamaneurol.2021.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosell A., Cuadrado E., Ortega-Aznar A., Hernández-Guillamon M., Lo E.H., Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann L., Pham M., März A.G., Kollikowski A.M., Stoll G., Schuhmann M.K. Defining cerebral leukocyte populations in local ischemic blood samples from patients with hyperacute stroke. J Cereb Blood Flow Metab. 2022;42:901–904. doi: 10.1177/0271678X221078617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feick J., Pham M., März A.G., et al. Distinct alterations in oxygenation, ion composition and acid-base balance in cerebral collaterals during large-vessel occlusion stroke. Clin Neuroradiol. 2023;33:973–984. doi: 10.1007/s00062-023-01296-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gidday J.M., Gasche Y.G., Copin J.-C., et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 47.Janssens A.C.J.W., Martens F.K. Reflection on modern methods: revisiting the area under the ROC curve. Int J Epidemiol. 2020;49:1397–1403. doi: 10.1093/ije/dyz274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.