Summary
Advances in radiation techniques have enabled the precise delivery of higher doses of radiotherapy to tumours, while sparing surrounding healthy tissues. Consequently, the incidence of radiation toxicities has declined, and will likely continue to improve as radiotherapy further evolves. Nonetheless, ionizing radiation elicits tissue-specific toxicities that gradually develop into radiation-induced fibrosis, a common long-term side-effect of radiotherapy. Radiation fibrosis is characterized by an aberrant wound repair process, which promotes the deposition of extensive scar tissue, clinically manifesting as a loss of elasticity, tissue thickening, and organ-specific functional consequences. In addition to improving the existing technologies and guidelines directing the administration of radiotherapy, understanding the pathogenesis underlying radiation fibrosis is essential for the success of cancer treatments. This review integrates the principles for radiotherapy dosimetry to minimize off-target effects, the tissue-specific clinical manifestations, the key cellular and molecular drivers of radiation fibrosis, and emerging therapeutic opportunities for both prevention and treatment.
Keywords: Radiation fibrosis, Radiotherapy, Extracellular matrix, Experimental therapeutics, Dosimetry constraints
Search strategy and selection criteria.
Data for this review were identified by searches of PubMed and the Web of Science Database, as well as references from relevant articles using the search terms: “radiation fibrosis” or “radiation induced fibrosis” or “fibrosis” or “radiotherapy” or “inflammation” or “clinical manifestations” or “pathogenesis” or “injury” or “toxicity” or “treatment” or “therapy”. Only articles published in English up to February 29, 2024 were included.
Introduction
Radiation fibrosis is a long-term consequence of radiotherapy characterized by an aberrant wound repair process, which gives rise to an accumulation of fibrotic tissues.1 Amongst patients treated with radiotherapy, 30–70% develop radiation fibrosis that significantly impairs quality of life post-treatment.2 The activation of myofibroblasts leading to excess collagen deposition, and dysregulation of extracellular matrix (ECM) remodelling are all hallmarks of radiation fibrosis.3 These events ultimately result in the symptomatic presentation of tissue thickening, loss of elasticity, and subsequent parenchymal atrophy coupled with impaired organ function. While a general cascade of cellular and molecular events precedes the development of fibrosis, individual tissues manifest unique pathologies that vary in symptomatic severity.3,4
Precision is crucial in radiation treatments to spare healthy tissues from unintended damage and toxicities, such as fibrosis. Advances in radiotherapy delivery have allowed for better visualization of tumours in 4-dimensional space (over time), and delivery of high doses of radiation with fine precision, even when malignant targets are prone to movement or possess irregular morphology.5, 6, 7 Although advances in radiotherapy have increased the success of treatment in managing initial disease, a significant number of cancer survivors will develop long-term toxicities and complications such as radiation fibrosis.8 This review highlights the importance of developing radiotherapy dosimetric constraints to reduce the risk of fibrosis development, as well as the tissue-specific clinical characteristics when fibrosis occurs. The underlying cellular and molecular alterations in the ECM and signal transduction will be discussed, in addition to the efficacy of conventional and experimental fibrosis treatments.
Radiotherapy dosimetry constraints are the first means to prevent severe fibrosis
Advances in radiation techniques, such as intensity-modulated radiotherapy (IMRT) and stereotactic body radiotherapy, have enabled the precise delivery of higher doses of radiotherapy to tumours while sparing surrounding healthy tissues. The incidence of radiation toxicities has consequently declined, and will likely continue to improve as radiotherapy evolves.1,9 Nonetheless, current radiation techniques continue to elicit tissue-specific acute toxicities that may gradually develop into radiation-induced fibrosis over the span of months and years. The risk of developing complications is dependent on several factors, including radiation dose, fractionation schedule, irradiated volume, and tissue-specific radiosensitivity.1
Dosimetry constraints have been thoroughly investigated with the aim of establishing general guidelines to minimize the risk of complications. In 1991, Emami et al. ascribed tolerance doses based on irradiation of one-third, two-thirds, or the whole volume of various organs.10 At that time, high-quality clinical data was sparse, hence the authors established those doses based on the consensus of clinical experience and opinions. Normal tissue complication probability (NTCP) models were developed using the Emami et al. dosimetry constraints to extrapolate the constraints of any uniform or non-uniform dose distribution; however, these models have limitations based on their underlying assumptions of organ structure and function.11 Subsequent clinical data and reports were compiled in the quantitative analyses of normal tissue effects in the clinic (QUANTEC) studies to summarize the dose, volume, and outcomes in the scope of irradiated organ-specific risks.11,12 While these reports focused on conventional fractionated radiotherapy, subsequent studies such as the high dose per fraction, or hypofractionated treatment effects in the clinic (HyTEC) repeated this process and refined specific ranges of dosimetry constraints for these high-dose-per-fraction regimens.12
Findings from dosimetry studies underscore the diversity of organ-specific sensitivities. Some organs, such as the lung, have well-documented dosimetric constraints in the literature while others, such as the skin, are less frequently described, despite the significant proportion of patients who develop dermal radiation toxicities.13, 14, 15, 16 The tolerance of lung tissues has been investigated extensively, with corresponding dosimetric threshold constraints being developed, given the necessity of preserving the functional substructure of airways and alveoli for efficient gas exchange and avoiding fatal toxicities (e.g. pneumonitis). For example, the general dose tolerance of pulmonary tissues was estimated to be a mean of 18–20 Gy with conventionally fractionated radiation therapies with <20% risk of symptomatic pneumonitis.13 The HyTEC pulmonary fibrosis analysis considered 97 studies employing SBRT in hypofractionated radiotherapy for lung cancer, exploring the rate of symptomatic pneumonitis as an endpoint, and reported that the rate was less than 10%–15% when radiotherapy was administered with a mean lung dose (MLD) of ≤8 Gy in 3–5 fractions, and when the percent of the combined lung volume receiving more than 20 Gy (V20) was less than 10–15%.14 This was in contrast to the QUANTEC report which explored symptomatic pneumonitis as an endpoint using conventional 3D conformal radiotherapy (CRT), but similarly emphasized the importance of the MLD (a range of 7–27 Gy was associated with increasing risk from 5 to 40%, respectively) and V20 (≤30% associated with <20% risk) to reduce the development of severe pulmonary toxicities.15
The severity of radiotherapy-induced fibrosis is dependent on the radiotherapy prescription parameters (e.g. dose, dose fractionation, volume, technique), use of combined modality therapy (e.g. concurrent systemic therapy), and/or host factors (e.g. genetic, lifestyle, baseline compromised organ function).1 From the radiotherapy perspective, one could consider optimization of the radiotherapy prescription parameters. The impact of dose and dose fractionation on fibrosis has been well described in QUANTEC15 and HyTEC12 papers. Fractionation allows for increase in radiation tolerance of normal tissues with the increasing overall treatment time and allowance for repair.17,18 Volume and technique have been examined in prior studies comparing 3D CRT versus modern IMRT techniques. A randomized controlled trial of these techniques in head and neck cancer have demonstrated decreased grade 2+ subcutaneous head and neck fibrosis (40–65% vs. <20%, measured at multiple timepoints, p < 0.01) with IMRT.19 Similarly, the “PARCER” randomized controlled trial in pelvic radiotherapy showed reductions in grade 2+ pelvic fibrosis (3% vs. 0%, p = 0.03),6 bowel obstruction (5% vs. 0.6%, p = 0.01),6 and grade 2+ vaginal stenosis (5% vs. 1%, p = 0.06) with IMRT.20 In general however, fibrosis of major organs is rarely investigated as the specific endpoint in existing radiation dosimetry literature. Further studies should be conducted to determine specific constraints to limit the likelihood of fibrosis development. The accessibility to high-quality clinical data detailing radiotherapy dose, volumes, and outcomes remains a significant hurdle in ongoing and future studies.11,12 Improvements in this domain would support clinical decision-making when selecting and administering radiotherapy regimens in a patient-specific manner.
Clinical manifestations of radiation fibrosis
Patients who develop fibrosis post-radiation present with certain clinical hallmarks irrespective of the affected site, including tissue thickening, loss of elasticity, and subsequent parenchymal atrophy coupled with impaired function.21 However, fibrotic development additionally induces unique clinical manifestations that vary depending on the affected tissue, as well as the type and dose of radiotherapy administered (Table 1).3,4
Table 1.
The clinical manifestations of radiation injury, the prevalence of late, fibrosis-related radiation injuries, and frequently associated cancers.
| Site of fibrosis | Clinical features | Prevalence of late, fibrosis-related radiation injuriesa | Frequently associated cancer types | References |
|---|---|---|---|---|
| Skin | Oedema, Alopecia, Dermatitis, Dermal Contraction, Dermal Thickening, Impaired Wound Healing, Ulceration |
|
Breast, HNC, Sarcomas, Skin | 22,23 |
| Lungs | Coughing, Dyspnoea, Chest Pain, Impaired O2 Delivery, Interstitial Oedema |
|
Lymphoma, Breast, Lung, Mesothelioma, Oesophageal, Thymic | 13,24, 25, 26 |
| Heart/vasculature | Angina, Radiation-Induced Heart Disease, Vessel Stenosis, Valvular Disease, Myocardial Infarction, Stroke, Right/Left Ventricular Dysfunction, Conduction Abnormalities, Arrhythmias, Pericardial Disease |
|
Breast, Lymphoma, Lung | 27, 28, 29, 30, 31 |
| Musculature | Muscle Weakness/Atrophy, Limited Range of Motion, Asymmetric Neuropathies |
|
General Consequence of Muscle Irradiation and/or Peripheral Nerve Damage | 27,32, 33, 34 |
| Nervous system | Neuropathic Pain, Sensory Loss, Impaired Muscle Control, Weakness | HNC, HL, Nasopharyngeal, Direct Radiation-Induced Damage | ||
| Gastrointestinal tract (oesophagus, small and large bowel, liver) | Dysphagia, Nausea, Abdominal Pain, Dysmotility, Constipation, Diarrhoea, Strictures, Fistulas, Proctitis, Faecal Incontinence |
|
GI (Stomach, Small and Large Bowel, Rectal, Anal, Liver), GU (Prostate, Bladder), Gynaecologic (Cervical, Uterine) | 27,35, 36, 37 |
| Genitourinary tract | Haematuria, Cystitis, Urinary Incontinence, Reproductive Dysfunction, Urinary Strictures, Fistulae, Strictures |
|
GI, GU, Gynaecologic | 27,38,39 |
| Gynaecologic | Vaginal Narrowing/Shortening, Pain, Dryness, Vaginal Stenosis (VS), Ulceration, Necrosis, Atrophy |
|
Cervical, Vaginal | 40,41 |
Prevalence of fibrosis-related radiation injuries may potentially be related to fibrosis prevalence, but there is no clear demarcation between late radiation toxicity and radiation fibrosis statistics in most studies.
Superficial fibrosis of the skin and its underlying fascia are most common, developing as early as 3 months post-irradiation or over the span of several years (Table 1).42 Patients often initially present with radiation dermatitis, irritated or inflamed skin as an acute toxicity. Over time, the skin may harden and lose elasticity as collagen is deposited in the dermis, while hyperplasia of the epidermis, loss of skin appendages, and hyperkeratosis occur as fibrosis progresses. Palpable fibrotic sequelae may be detected at the site of irradiation after substantial collagen has accumulated in the dermis.27 In rare instances, fibrosis may result in ulceration and necrosis among superficial tissues.
Radiation-induced lung injury (RILI) and toxicity are among the most thoroughly documented in the literature (Table 1).43,44 Patients are usually diagnosed with radiation pneumonitis during the first six months of fibrosis initiation and development.45 Radiation pneumonitis may require symptomatic management with antibiotics, steroids, oxygen or airway intervention in severe cases. Fatal pneumonitis is possible, particularly if baseline lung function is compromised. If symptoms persist or worsen after resolution of acute pneumonitis, RILI is then classified as radiation-induced lung fibrosis. Patients who received thoracic radiotherapy may develop coughing, chest pain, dyspnoea, pulmonary hypertension, diminished lung function, a restrictive lung defect, increased lung stiffening, and airway obstruction.46,47 These symptoms may be a consequence of airway wall thickening, particularly of the bronchioles and alveoli. Impaired ventilation can be detected as a reduction in systemic oxygen delivery, accompanied by compensatory tachypnoea, or cyanosis in the extremities.27 CT monitoring may be used as a diagnostic tool for lung fibrosis which manifests as thickened airways, as well as a ground-glass appearance of the lungs.47 The Common Terminology Criteria for Adverse Events (CTCAE) is a well-known system for classifying treatment toxicity by clinicians. Pulmonary fibrosis can be categorized from Grade 1 to 5, based on percentage of lung fibrosis on imaging studies, combined with clinical extent of hypoxia, pulmonary hypertension, heart failure or even death (Grade 5).
Radiotherapy may induce a plethora of complications within the circulatory system, at both the level of cardiac structures and the vasculature, which have been associated with poor long-term clinical outcomes thereby necessitating additional clinical management (Table 1).27,28 Patients with symptoms of cardiomyopathies may present with dyspnoea and chest pain, brady–or tachycardia, fluctuations in blood pressure, or fluid retention.27,48 Other severe long-term complications include radiation-induced heart disease, with the development of cardiac fibrosis, valvular disease, conduction abnormalities, pericardial disease, and the accumulation of fibrotic material in the tunica media of vessels causing luminal obstruction and/or plaque ruptures; all of which may develop over the span of months to years.28,29 Plaques resulting from radiation injury are reportedly more prone to causing cerebrovascular accidents or myocardial infarctions than those developing from other sources.30,49,50
Radiation-induced neural injuries often occur indirectly as a consequence of nerve compression or vasa nervorum disruption by fibrotic tissues.32 Patients may present with neuropathic pain, sensory loss, or diminished muscular control. Muscular fibrosis can develop either concurrently or independently of neural injuries.27 For example, if an innervating nerve is damaged in the radiation field, weakness and atrophy can result in the innervated muscles (Table 1). Spinal metastases are often treated with conventional radiotherapy and SBRT, but their adjacency to the spinal cord risks unintended toxicities in the irradiation field.51, 52, 53 Thus, patients may present with several toxicities including vertebral compression fractures and radiation myelopathy, ranging from minor sensory and motor impediments to significant neuromuscular dysfunction. Direct muscular injury can initially present as spasms, which transition to chronic contractions accompanied by loss of mobility due to sclerosis.27,32 Loss of elasticity and restricted range of motion have been similarly reported in irradiated tendons and ligaments due to the onset of fibrosis and sclerosis.33 Radiation may also increase the risk of osteopenia, osteoporosis, and osteoradionecrosis; however, these complications are more typically caused by direct radiation-injury as opposed to ensuing fibrosis.33,54
Symptoms of radiation fibrosis in the digestive system may manifest after a latency period spanning anywhere from six months to three years post-radiation, with delayed enteropathy occasionally reported even 30 years later (Table 1).35 Acute radiation esophagitis presents as odynophagia and dysphagia, with more long-term consequences including dysmotility, oesophageal strictures, and fistula formation.55 Nausea, abdominal pain, and dysmotility may all result from damage to the lower GI tract or thickened luminal walls.27 Bowel fibrosis, resulting from pelvic radiotherapy, can lead to development of adhesions, small bowel obstruction, faecal incontinence, strictures, and increased risk of tissue ischemia, fistulae, and tissue necrosis.27 Radiation toxicities can manifest in the liver either as a direct target of radiotherapy, or given its large size and adjacency, a by-product of irradiating other GI tract components.36 The liver possesses numerous critical functions, including nutrient metabolism, elimination of waste, and protein synthesis.56 Both classic and non-classic radiation-induced liver disease (RILD) may develop weeks to months following radiation, each manifesting with unique symptoms representative of compromised liver function.36,56 Following radiation injury, the activation of hepatic stellate cells and their differentiation towards a myofibroblast phenotype are major contributors to liver fibrosis, exacerbating preceding symptoms of RILD and leading to accumulation of scar tissue.
Renal fibrosis is the end-stage complication of radiation-induced nephrotoxicity, typically preceded by a six-month asymptomatic latent period, followed by progressive chronic radiation nephropathy characterized by oedema, azotaemia, proteinuria, hypertension, anaemia, albuminuria, and chronic kidney disease (CKD).57 Vascular and glomerular complications, loss of nephron mass, and an increase in renal interstitial fibrosis may all be detectable histologically during the late stages of renal nephropathy. Chronic inflammation and cellular senescence post-irradiation are major driving factors in fibrosis development, especially when coupled with CKD, which reduces the regenerative capacity of the organ and remodelling of renal tissues, ultimately compromising renal function.57,58
Pelvic radiotherapy may also increase the risk of fibrosis in the genitourinary (GU) tract, with accumulation of fibrotic tissues impairing both excretory and reproductive function (Table 1).27 In prostate cancer patients, bladder damage and radiation cystitis are not infrequently reported.38 Patients may present with urinary incontinence, haematuria, and the presence of urinary plasminogen activator inhibitor 1, tissue inhibitor of metalloproteinase 1 (TIMP-1), and TIMP-2. Similar sequelae may be experienced by cervix cancer patients treated with radiotherapy.39 Obstructions and strictures have been observed; however, it can be difficult to differentiate the aetiology between radiation treatment and destruction from the pre-existing cancer. Given the adjacencies of the GU and lower GI tracts, symptoms may present in both regions as a consequence of fibrosis.27 Vaginal stenosis (VS) is one significant gynaecological complication frequently associated with pelvic radiotherapy and brachytherapy, through which the accumulation of fibrotic tissue manifests in the abnormal tightening and shortening of the vagina.40,59 VS typically manifests at least three months after receiving radiotherapy and the combined fibro-atrophic pathology manifests clinically as telangiectasias, mucosal pallor, loss of elasticity, dryness, occlusion, and fragility of the vaginal canal.40,59
Overall, the diversity of complications arising from radiotherapy necessitates different clinical management, depending on the site of irradiation, to adequately treat patient symptoms and improve quality of life post-radiation. In addition to existing therapeutics and symptomatic management, improved knowledge of the pathogenesis of fibrosis continues to guide the development of targeted, experimental therapies.
Biological processes underlying radiation fibrosis
Dysregulated extracellular matrix deposition and remodelling
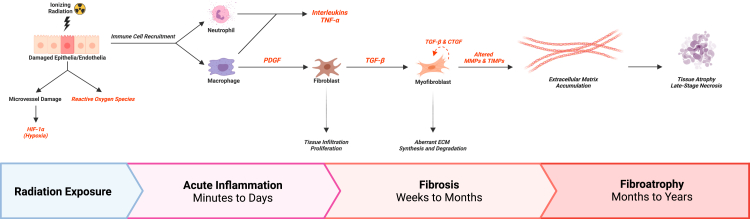
The ECM is a dynamic milieu of proteins, glycoproteins, glycosaminoglycans (GAGs), proteoglycans (PGs), and other macromolecules that form an intricate network imparting unique mechanical properties to connective tissues.60 In addition to providing physical support and points of anchorage for cells, the ECM is also a reservoir for numerous cytokines, growth factors, and bioactive molecules which regulate cellular behaviours and developmental processes.3,60 During fibrosis, ECM synthesis and turnover become dysregulated, resulting in excessive deposition of ECM components and scar tissue accumulation (Fig. 1).
Fig. 1.
An integrated summary of radiation fibrosis pathogenesis. Radiation exposure causes immediate tissue damage in both target and healthy tissues, thereby initiating acute inflammation. Over the span of several days, immune cells, such as macrophages and neutrophils, are recruited to the site of radiation and commence cytokine secretion. Neutrophils produce inflammatory cytokines, including various interleukins and tumour necrosis factor α (TNF-α). Macrophages are a major source of platelet-derived growth factor (PDGF) and transforming growth factor β (TGF-β), which stimulate the proliferation of fibroblasts and differentiation into abnormal myofibroblasts, respectively. Sustained upregulation of TGF-β and connective tissue growth factor (CTGF) positively feedback on myofibroblast fibrogenic activities. Consequently, ECM homeostasis is perturbed, and molecules such as collagen and proteoglycans begin to accumulate over weeks and months. These sustained myofibroblast activities, coupled with aberrant expression of ECM regulators such as matrix metalloproteinases (MMPs) and their inhibitors (TIMPs), lead to extensive scar tissue accumulation, evidenced through tissue thickening, loss of elasticity, and increasing induration or firmness. In late-stage fibrosis, months to years after radiotherapy, extensive ECM deposition and microvasculature damage gives rise to hypoxic environments which may promote parenchymal cell atrophy or tissue necrosis. Created with BioRender.com.
Collagens are collectively the most abundant proteins found in the human body, which are secreted into the extracellular space, then assembled into unique supramolecular structures.61 The production, maintenance, and degradation of collagen is not a static process.16 Rather, a dynamic crosstalk takes place between intracellular molecules and the ECM which influences gene expression and/or facilitates remodelling of ECM components (Table 2). Cultured irradiated skin fibroblasts demonstrate elevated collagen synthesis compared to unirradiated cells, which is further increased upon addition of transforming growth factor β (TGF-β).62 Chronic upregulation of collagen production and deposition, particularly types I and III, by myofibroblasts is a major driver in the development of fibrotic tissues.3 Matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) further regulate collagen turnover and maintenance in the ECM.63,64 Given the extensive size of collagen fibrils, cleavage by MMPs is essential for cellular endocytosis and lysosomal degradation. Dysregulation of both MMP and TIMP activities promotes significant collagen accumulation in the fibrotic microenvironment (Table 2).3
Table 2.
An overview of altered ECM components in fibrosis, and their biological significances.
| ECM component | Biological significance in fibrosisa | Reference(s) |
|---|---|---|
| Collagen | Provides tissues with tensile strength by forming extensive fibrils or networks within the extracellular matrix (ECM). Collagen types I and III are the most strongly implicated in radiation fibrosis. Collagen accumulation impinges upon adjacent tissues potentially causing thickening, loss of elasticity, atrophy, hypoxia, or compromised function. | 61, 62, 63 |
| Matrix metalloproteinases | Facilitates remodelling and breakdown of ECM components, particularly collagen through collagenase and gelatinase activities. While some MMPs are downregulated in fibrosis, promoting ECM accumulation; others are upregulated and may facilitate the remodelling of the ECM towards a fibrotic microenvironment. | 3,63,64 |
| MMP-1 | Downregulated by TGF-β/Smad signalling in murine dermal fibroblasts post-irradiation accompanied by a simultaneous increase in TIMP-1 and collagen expression. These alterations promote scar tissue accumulation. | 65 |
| MMP-2 | Upregulated in cultured human lung epithelia, and murine dermal fibroblasts post-irradiation. MMP-2 gelatinase is involved in the remodelling of stroma and basement membranes which may mediate disruption of endo- and epithelial barriers during the initial stages of fibrosis. | 65,66 |
| MMP-8 | Upregulated and secreted by macrophages during hepatic fibrosis development, promoting the trans-differentiation of hepatic stellate cells into active myofibroblasts. Expression of MMP-8 is also upregulated in mononuclear phagocytes and airway epithelium in idiopathic pulmonary fibrosis. | 37,67,68 |
| MMP-9 | Upregulated in parenchymal and inflammatory lung cells in a murine model post-irradiation. Coupled with MMP-2, these gelatinases may compromise structural integrity of lung tissues by targeting basement membranes. In transgenic mice overexpressing TGF-β, MMP-9 activity was diminished. | 69 |
| MMP-13 | Upregulated in fibrosis and may promote remodelling of collagen fibrils. Loss of MMP-13 reduced inflammation and fibrotic development in a pulmonary fibrosis murine model. In a hepatic fibrosis murine model, MMP-13 cleaves and activates connective tissue growth factor (CTGF) exacerbating fibrosis. | 70,71 |
| MT1-MMP (MMP-14) | Functions vary broadly in a tissue-specific context. MT1-MMP facilitates collagen remodelling and homeostasis, and promotes activation of other MMPs in adult skin when expressed by cutaneous fibroblasts. MT1-MMP may cooperate with Kras (G12D) to exacerbate pancreatic fibrosis through TGF-β signalling. Conversely, loss of MT1-MMP in a bleomycin-induced pulmonary fibrosis model exacerbated symptoms. | 72, 73, 74 |
| Fibronectin | Contributes to haemostasis, cell adhesion, and cell migration. Fibronectin is associated with abnormal wound healing, stiff matrices, and fibrosis when excessively deposited. It may also function as a chemoattractant for fibroblasts and increase fibrosis-associated myofibroblast activation. | 75,76 |
| ED-A FN Splice Variants | Upregulated in wound healing and repair processes, including fibrosis, and has also been reported to bind latent-TGF-β-binding protein-1, sequestering latent TGF-β in the ECM. ED-A FN-null mice are protected from bleomycin-induced pulmonary fibrosis, albeit at the expense of dysregulated wound repair. | 77 |
| Glycosaminoglycans and proteoglycans | Participates in the wound repair process, binds other ECM components, including TGF-β, and sequesters water molecules and cations in tissues through ionic interactions. | 78 |
| Hyaluronan | Upregulated in wound healing and repair processes. Promotes immune cell recruitment, enhances fibroblast motility and tissue invasion. Accumulates before the onset of fibrosis and thus may serve as a biomarker. | 79 |
| Syndecan-1 | Augments type II pneumocyte cellular activity towards a fibrotic phenotype via TGF-β signalling in idiopathic pulmonary fibrosis models. | 80 |
| Decorin | Binds TGF-β and modulates its bioactivity in vitro and in vivo. In a hepatic fibrosis model, Decorin bound TGF-β with high affinity, thus limiting profibrotic signalling and activation of hepatic stellate cells. | 78,81,82 |
| Biglycan | Binds TGF-β and modulates its bioactivity in vitro, however given its proximity to the pericellular space, it may paradoxically promote profibrotic signalling in vivo by sequestering TGF-β in the vicinity of its receptor. | 78,82 |
| Perlecan | Promotes fibrosis by upregulating collagen fibrillogenesis and growth factor signalling, altering cell adhesion, and preventing fibroblast apoptosis. | 83 |
Some studies investigating ECM components in other fibrotic pathologies are included, in addition to radiation-induced fibrosis models, to highlight potential areas of future investigation for radiation fibrosis molecular biology.
Other ECM constituents, such as fibronectin, GAGs, and PGs, are also dysregulated in fibrosis (Table 2). Fibronectin is a dimeric multi-modular glycoprotein and its deposition is elevated in fibrotic states.84 Notably, the Hep2 binding-site (modules III12–III15) of fibronectin is known to bind a variety of growth factors including TGF-β, hepatocyte growth factor (HGF), connective tissue growth factor (CTGF), and platelet-derived growth factor (PDGF), all of which have been implicated in fibrosis development.84 In addition, the ED-A-containing fibronectin splice variants are upregulated in wound healing, bind to latent-TGF-β-binding protein-1, thereby sequestering latent TGF-β in the ECM.77 GAGs are long anionic polymers of repeating disaccharide subunits, including hyaluronan, chondroitin sulphate, heparin sulphate, and keratan sulphate.85 PGs are large extracellular complexes comprised of a core protein covalently linked to GAG chains through a linker tetrasaccharide.86 Diverse assemblies of GAGs and PGs can be generated in the ECM, and as with fibronectin, aberrant production and deposition of GAGs and PGs will also contribute to the onset of tissue fibrosis (Table 2).78 Highlighted in this review are only a selection of the most prominent molecules altered in fibrotic pathologies. Further investigation of fibrotic ECM alterations could provide novel insight on tissue-specific manifestations of fibrosis and potential therapeutic targets.
Myofibroblasts act in concert with immune populations to orchestrate radiation fibrosis
Fibroblasts are the dominant cell type in connective tissues and are responsible for the synthesis and turnover of major ECM components.87 While various fibroblast differentiation subtypes may exist in a given tissue, atypical myofibroblasts are a crucial hallmark in radiation fibrosis, which terminally differentiate from several resident cell populations in the irradiated field in response to profibrotic signalling, including fibroblasts, epithelial and endothelial cells, mesenchymal stem cells, and smooth muscle cells through mechanisms such as epithelial- and endothelial–mesenchymal transitions (EMT and EndoMT, respectively) (Fig. 1).3,88,89 In healthy tissues, myofibroblasts function in tissue repair, but ultimately undergo apoptosis. In radiation fibrosis however, these cells fail to apoptose, and maintain long-term production of collagen through TGF-β/Smad signalling and CTGF.1,3 Abnormal myofibroblasts can be identified by the expression of α-smooth muscle actin (α-SMA), which regulates the expression of both collagen and other profibrotic signalling molecules, and is also pivotal for the necessary contractile forces during wound contracture.87 The persistence of myofibroblasts in irradiated tissues is thus a critical driver for the development of chronic fibrosis.
The acute inflammatory stage of radiation fibrosis is orchestrated by a number of immune cell populations including monocytes/macrophages, neutrophils, eosinophils, mast cells, and lymphocytes.3 While these immune cells are essential for the induction of inflammation, debris clearance, and wound healing, they have also been reported to modulate ECM deposition.3,89 Radiation exposure has been associated with increased expression of chemokines, which are potent mediators of innate immunity and increase local immune cell infiltration.3 Neutrophils rapidly migrate to sites of radiation tissue injury and produce inflammatory cytokines including IL-1, IL-6, and tumour necrosis factor α (TNF-α), promoting the inflammatory microenvironment and reactive oxygen species (ROS) generation.9,90 Subsequent lymphocyte and monocyte recruitment and interactions lead to the classical activation of M1 macrophages, which are associated with inflammatory cytokine production in the acute stages of fibrosis.91 The progression from acute inflammation to chronic fibrosis is accompanied by a transition towards the alternatively-activated M2 macrophage phenotype.9,90 M2 macrophages secrete profibrotic signalling molecules such as TGF-β, PDGF, fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF), potent mediators of fibroblast proliferation and differentiation into myofibroblasts, which culminate in ECM accumulation during fibrotic remodelling.
The combined effects of radiation exposure, abnormal fibroblasts, and immune populations collectively impact on both the microvasculature and tissue parenchyma in an adverse manner. Cell stress imposed by radiation and extensive ROS generation can directly induce tissue cell death. Compromised endothelial and epithelial barrier function can initially elicit microvascular injury and loss of vascular density post-irradiation, a phenomenon which has been well-documented in pulmonary and cardiac tissues.47,48 These complications may precede or occur concurrently with the acute inflammatory stages of fibrosis, also giving rise to the differentiation of abnormal myofibroblasts that exacerbate the chronic fibrotic state.3,92 Over time, the extensive deposition of ECM further impinges upon the parenchyma of the affected organ(s), which induces loss of elasticity and thickening, leading to tissue atrophy.47 Given that the parenchymal cells comprise the major functional units of any organ, atrophy impairs organ function; manifested as unique clinical symptomology depending on the tissues affected.27
Fibrosis is driven by a complex landscape of proinflammatory and profibrotic molecules
The TGF-β cytokine family consists of three isoforms (β1 to β3) which are essential for survival, influencing cell growth, immune regulation, and ECM deposition.93 TGF-β1 is the principal isoform secreted by inflammatory and endothelial cells which has been critically linked to the pathogenesis of radiation fibrosis (Fig. 1).3 TGF-β molecules are secreted within a complex known as the large latent complex (LLC) and require dissociation for activation, a process which can be mediated by proteases, interactions with other extracellular factors, and ROS. Activated TGF-β binds to the dimeric TGF-β-receptor II (TGFBR2) on the cell surface which then co-localizes with the dimeric TGFBR1 to form a functional heterotetrameric receptor complex.94 The TGFBR complex functions as a serine/threonine kinase to phosphorylate specific members of the Smad family (in the canonical pathway, Smad2/3 which then complex with Smad4) which act as transcription factors promoting the expression of profibrotic genes.
In a murine model, TGF-β expression was significantly upregulated several days post-irradiation, occurring concurrently with immune cell infiltration at the site of irradiation.95 Initially, the latent reservoir of TGF-β within the ECM is likely the most significant source of increased TGF-β activity, given that these molecules are readily activated by the ROS generated by radiation exposure.96 The resulting signal transduction promotes myofibroblast differentiation and profibrotic gene expression, including collagen, fibronectin, and CTGF.97 CTGF is a matricellular protein secreted by fibroblasts.98 Numerous molecules and pathways including fibronectins, TGF-β signalling, hypoxia-inducible factor 1α (HIF-1α), and Rho/ROCK signalling, converge upon CTGF.90,98 Notably, CTGF expression was observed to be absolutely essential for TGF-β-initiated fibrosis, and its inhibition reversed ECM remodelling.98,99 Furthermore, CTGF enhances myofibroblast differentiation and fibrogenic activities, and importantly, has a positive feedback on TGF-β1 expression (Fig. 1).3 This provides a mechanism through which CTGF can perpetuate TGF-β1 expression (and in turn, its own expression) once the initial inflammatory environment transitions into fibrosis.
Other profibrotic molecules implicated in fibrosis pathogenesis include PDGF, TNF-α, HIF-1α, and NADPH oxidases (NOX) (Table 3). PDGFs elicit mitogenic functions by binding to the PDGF α and β receptor (PDGFR) tyrosine kinases on mesenchymal-derived cell surfaces.127 PDGF expression is upregulated during the acute inflammatory stage post-irradiation, and subsequent PDGF-dependent signalling has been implicated in fibroblast profibrotic molecular mechanisms involving TGF-β and TNF-α.101 TNF-α is an inflammatory cytokine that is upregulated post-irradiation and induces changes in gene expression, including TGF-β upregulation, through intermediates such as activator protein 1 (AP-1) and nuclear factor κB (NFκB).104,105,128 HIF-1α has been implicated in fibrosis pathogenesis in several organ systems suggesting that the hypoxic fibrotic environment may be a common contributor to the development of fibrosis.107, 108, 109 Indeed, HIF-1α expression is significantly elevated post-radiation in response to oxidative stress and microvascular damage, and severe hypoxic development post-irradiation was associated with elevated fibrotic activities in a murine model.129 Both TGF-β and CTGF are also transcriptionally regulated by HIF-1α, suggesting that HIF-1α may also perpetuate the expression of these key molecular drivers in fibrosis.108 Members of the NOX enzyme family have been reported to be upregulated post-irradiation and contribute to chronic tissue oxidative stress and radiation toxicity.112 NOX enzymes produce ROS in response to profibrotic signalling molecules, such as TGF-β, and modulate endothelial dysfunction, MMP fibrotic activities, and collagen remodelling.112,113 Taken together, NOX enzymes critically contribute to immune cell infiltration, cellular senescence, apoptosis, and fibrosis in irradiated tissues.
Table 3.
Profibrotic molecular alterations implicated in radiation fibrosis onset and development.
| Molecule | Biological significance in fibrosisa | Reference(s) |
|---|---|---|
| TGF-β | TGF-β, and particularly TGF-β1, is the key driver of radiation-induced fibrosis which has been critically linked with numerous pathogenetic mechanisms. TGF-β is upregulated post-irradiation, and the canonical signalling pathway drives the expression of many profibrotic genes such as collagen, fibronectin, and CTGF. Both CTGF and HIF-1α can feedback on TGF-β to maintain its expression. | 3,93, 94, 95, 96, 97 |
| CTGF | CTGF is upregulated in radiation-induced fibrosis, and its expression can be stimulated by TGF-β, HIF-1α, Rho/ROCK signalling, or in an autocrine manner in the absence of these molecules. CTGF interacts with numerous ECM constituents and growth factors to influence aberrant ECM deposition, remodelling, and myofibroblast activity. CTGF expression is essential for TGF-β-initiated fibrosis. | 90,98, 99, 100 |
| PDGF | PDGF is upregulated in radiation-induced fibrosis and was implicated in profibrotic mechanisms including TGF-β and TNF-α. PDGF also promotes fibroblast proliferation and infiltration, exacerbating initial responses to radiation injury. | 101, 102, 103 |
| TNF-α | TNF-α is upregulated in radiation-induced fibrosis models, particularly during acute inflammatory responses, and induces TGF-β expression. TNF-α may function in tandem with NFκB to sustain myofibroblast survival and proliferation in the fibrotic environment. | 104, 105, 106 |
| HIF-1 α | HIF-1α mediates cellular responses to microvasculature damage within hypoxic environments by heterodimerizing with HIF-1β to form the HIF-1 transcription factor, which binds hypoxia-response elements. HIF-1α also regulates the gene expression of molecules contributing to fibrotic pathologies, such as TGF-β and CTGF, and permits the accumulation of collagen and fibronectin. | 107, 108, 109, 110, 111 |
| NADPH oxidase family | NADPH oxidase (NOX) enzymes are key mediators of oxidative stress and ROS production in irradiated tissues. NOX enzymes have been implicated in numerous fibrosis-related mechanisms including cellular senescence, apoptosis, endothelial dysfunction, collagen remodelling, and MMP profibrotic activities. In pulmonary radiation fibrosis, the NOX1 isoform was identified as a specific regulator of endothelial dysfunction and fibroblastic changes, whereas the NOX1, NOX2, and NOX4 isoforms were implicated in cardiac remodelling and fibrosis development. | 112, 113, 114 |
| Interleukins (ILs) | ||
| Interleukin-1 family | IL-1α and IL-1β are upregulated in dermal keratinocytes post-irradiation. Minimal superficial fibrosis developed in an IL-1β knockout model. IL-1α overexpression promoted other dermal radiation toxicities. Conversely, some studies report that IL-1 may act as an antifibrotic molecule in conjunction with IL-12 to favour repair processes over fibrosis. | 115 |
| Interleukin-2 | IL-2 is upregulated in myofibroblasts post-irradiation and was associated with expression of specific cell adhesion markers such as CD44, a molecule which may facilitate growth and migration on hyaluronan-rich matrixes. | 116 |
| Interleukin-4 | IL-4 is upregulated in IPF patients, and its knockout in a murine model protected animals from bleomycin-induced fibrosis. IL-4 has been proposed to act in conjunction with IL-13 to promote fibrotic processes over repair. | 3 |
| Interleukin-5 | IL-5 is upregulated in lung tissues post-irradiation and modulates T cell activity. In IL-5 deficient mice, increased production of IFN-γ results in a significant reduction of liver fibrosis. Eosinophil recruitment was also significantly reduced in liver granulomas. | 117,118 |
| Interleukin-6 | IL-6 expression is upregulated in keloids and is associated with increased scarring. In a bleomycin-induced pulmonary fibrosis model, IL-6 expression was biphasic, with the initial upregulation slowing fibrosis while the second phase promoted fibrotic development. | 119,120 |
| Interleukin-8 | Plasma IL-8 levels in patients receiving radiotherapy for non-small cell lung cancer were negatively correlated with risk of radiation toxicity. Lower levels of IL-8 predisposed patients to a higher risk of developing radiation-induced lung toxicities. In addition, IL-8 production by mesenchymal progenitor cells recruits macrophages to fibrotic sites, and promotes self-renewal and proliferation of mesenchymal cells. | 121,122 |
| Interleukin-10 | IL-10 viral-vector mediated ectopic expression in an IPF murine model was associated with improved survival and significant reduction in TGF-β expression, immune cell infiltration, and fibrosis development. | 123 |
| Interleukin-12 | IL-12 administration attenuates bleomycin-induced fibrosis by increasing expression of antifibrotic IFN-γ. Neutralizing IFN-γ eliminated the antifibrotic effects of IL-12. | 124 |
| Interleukin-13 | IL-13 upregulates TGF-β production and secretion by macrophages, while inhibiting the expression of latent-TGF-β-binding-protein. Both alterations are profibrotic. IL-13 knockout in a murine model protected against fibrosis. | 3,125 |
| Interleukin-21 | IL-21 was essential for CD8+ T cell differentiation into IL-13-producing cells in a pulmonary fibrosis model. IL-21 deficient mice are resistant to bleomycin-induced fibrosis. The combination of IL-4 and IL-21 promotes autocrine stimulation of IL-21, further driving IL-13 production. | 126 |
| Interleukin-33 | IL-33 is produced by radiation-compromised endothelia and facilitates immune cell migration and cytokine signalling, particularly through eosinophil activity. Eosinophils are among the first immune cells which respond to IL-33 and stimulate collagen synthesis by producing TGF-β. | 125 |
Some studies investigating molecular alterations in other fibrotic pathologies are included to highlight potential mechanisms of action in radiation-induced fibrosis.
Numerous interleukins (ILs) have been implicated in the inflammatory processes dominating the acute response to radiation injuries.3 Given the broad repertoire of these molecules, their targets, and functions, specific molecules will not be extensively explored in this review; however, a summary of the findings in the literature is noted in Table 3. Some conflicting findings have also been observed, thereby necessitating further investigation of these molecules and their effects to elucidate the mechanisms through which specific ILs may facilitate the development of chronic fibrosis.
Senescence as a contributor to fibrosis
Radiation and other types of DNA damage can induce premature cellular senescence in normal tissues, a permanent non-replicative growth arrest. Senescence in normal tissues after irradiation can cause parenchymal depletion through loss of replicative potential of adult normal tissue stem cells.114 In addition, senescent cells produce a complex mixture of cytokines, immunomodulatory molecules, and mitogenic compounds called the senescence associated secretory phenotype (SASP). Many of the SASP molecules have been implicated in radiation fibrosis, such as TGF-β, epidermal growth factor (EGF), IL-1, and IL-6.130 The SASP can cause secondary senescence, stimulate fibroblasts to produce collagen matrix, and impact inflammation.131 Clearance of senescent cells or prevention of senescence have been shown to mitigate radiation fibrosis.132
An integrated summary of molecular, cellular, and ECM alterations underlying radiation fibrosis
Radiation-induced fibrosis has been proposed to occur through a three-stage model including an initial inflammatory stage, a subsequent generalized fibrosis stage, concluding to a fibro-atrophic stage (Fig. 1).3,42,133 Acute radiation tissue injury occurs through both direct and indirect mechanisms.3 Cell stress imposed by radiation and NOX enzymes can directly induce tissue cell death. The initial injury promotes an inflammatory response in the local tissue environment and is also capable of affecting other tissues through the bystander effect. Local chemoattractant production facilitates immune cell chemotaxis and recruitment of neutrophils and macrophages.90,134 Neutrophils and M1 macrophages produce proinflammatory cytokines, while over the long-term, M2 macrophages act as a major source of both PDGF and TGF-β. PDGF promotes the infiltration and proliferation of fibroblasts in the injured tissue, while TGF-β promotes differentiation into atypical myofibroblasts.112,134 The latent reservoir of TGF-β, activated by ROS produced in the irradiated field, is a potent source of TGF-β particularly during the acute phase following radiotherapy. Taken together, abnormally elevated TGF-β and CTGF cause myofibroblasts to overproduce and deposit ECM components such as collagen, fibronectin and PGs.135 Concurrently, some MMPs are repressed through the stimulation of TIMPs which exacerbates ECM deposition over time.3 Fibrogenic activities are postulated to be maintained through upregulation of CTGF, even during a reduction of TGF-β expression in the later stages of fibrosis. As fibrosis continues to develop over months and years, the tissue loses elasticity and thickens, leading to impaired function.3,89 Microvascular damage resulting from compromised endothelial function causes tissue ischemia, simultaneously promoting HIF-1α-mediated transcription of profibrotic genes, and ultimately resulting in tissue atrophy and necrosis.
Therapeutic opportunities for radiation fibrosis – conventional and experimental
Conventional therapeutic approaches for radiation fibrosis target broad pathways of radiation injury, and include anti-inflammatory agents, antioxidant treatments, and vascular therapies (Table 4).1 While these approaches have demonstrated some potential to ameliorate fibrosis onset and development in vitro and in vivo, results from clinical studies have been mixed. The conflicting efficacies of single conventional treatments have prompted a shift in focus to multimodal therapies. For example, antioxidant agents have been tested in combination with vascular therapies with some success, such as a combination of pentoxifylline and Vitamin E; however, these trials are limited by small cohort sizes, with conflicting clinical results.1,162, 163, 164, 165
Table 4.
Conventional and experimental therapeutic opportunities for radiation fibrosis.
| Conventional therapeutics | Clinical significance | Reference(s) |
|---|---|---|
| Anti-inflammatory agents | Both steroidal and non-steroidal anti-inflammatory agents have been administered in studies conducted in vitro and in vivo to ameliorate the acute inflammatory stage preceding fibrosis. Their ability to mitigate established fibrosis has yet to be conclusively documented. These agents may also be constrained by early administration to prophylactically prevent fibrosis onset, and their use could be limited by potential toxicities after prolonged administration. | 1,3,136 |
| Antioxidant agents | Antioxidants mitigate oxidative stress and the profibrotic effects of ROS generated after radiation. Superoxide dismutases (SODs) and other antioxidants like tocopherol/vitamin E demonstrated radioprotective effects and attenuation of profibrotic activities in vitro and in vivo; however, results from clinical studies have been mixed. | 1,136, 137, 138, 139, 140 |
| Vascular therapies | Hyperbaric oxygen is administered in pathologies associated with ischemia, hypoxia, and impaired oxygen delivery to promote wound healing and vessel regrowth. Few studies have examined its success in the clinical treatment of radiation fibrosis, and its efficacy as a standalone therapeutic agent remains controversial. Novel findings from the “HONEY” randomized controlled trial suggest that completion of a hyperbaric oxygen therapy regimen (30–40 therapeutic sessions over 6–8 weeks) may reduce pain and fibrosis in breast cancer patients with late radiation toxicities. | 54,141,142 |
| Pentoxifylline (PTX) is an anti-inflammatory, anti-fibrotic, and anti-coagulating agent that increases blood flow. Among its various effects, PTX is known to interfere with the production and signalling of inflammatory cytokines, such as TNF-α, and has also been associated with alterations in fibrotic gene expression, and improved outcomes in studies of radiation fibrosis patients. | 43,143 | |
| Experimental therapeutics | ||
| TGF-β-related therapeutics | Neutralizing antibodies, gene therapies, and the administration of recombinant soluble TGFBR2 receptors targeting TGF-β signalling have all been examined in fibrosis models and demonstrated success in limiting the amount of active TGF-β in situ or reducing downstream signal transduction; however, given the pleiotropic role of TGF-β, novel systemic therapies must be well-tolerated with respect to off-target side-effects. | 1,144, 145, 146, 147 |
| CTGF-related therapeutics | CTGF blockade using the Pamrevlumab monoclonal antibody attenuated pulmonary remodelling and improved median survival in an animal model. In a Phase 2 randomized trial for IPF, it also decelerated the decline of lung function and disease progression, while being relatively well-tolerated. | 148,149 |
| Statins | Statin-mediated inhibition of Rho/ROCK signalling reduced histopathological changes post-irradiation, including reductions in collagen synthesis, CTGF deposition, and overall fibrosis development. The “PRAVACUR” Phase 2 trial investigated the efficacy of Pravastatin in HNC patients, which demonstrated the potential to reduce the thickness and severity of fibrotic tissues. | 150, 151, 152 |
| RTKI | RTKIs target PDGFR-dependent signalling, thus reducing the proliferation of fibroblasts as well as downstream profibrotic signalling. Imatinib mesylate was found to attenuate radiation fibrosis in animal models. Similar findings were observed using Nintedanib in a pulmonary radiation fibrosis animal model. | 102,153,154 |
| ACE inhibitors | ACE inhibitors suppress the effects of the renin-angiotensin-aldosterone system, including matrix remodelling events. In an animal model, Captopril reduced matrix remodelling and preserved organ function, however radiation toxicities still occurred. Enalapril was well-tolerated in a Phase 2 clinical trial, though patients demonstrated an increased incidence of fibrosis. The clinical role of ACE inhibitors, if any, requires further investigation. | 155, 156, 157 |
| Stem cell therapies | ADSCs are a source of antifibrotic HGF and have been tested for their ability to mitigate cutaneous, muscular, and skeletal fibrosis. Their extracellular vesicles reduce profibrotic gene expression. Stem cells may also be pertinent in regenerating fibrotic tissues affected by atrophy or necrosis, though future studies are necessary. | 158,159 |
| Cellular reprogramming | Cellular reprogramming provides a modality through which abnormal cells, such as myofibroblasts, may be ushered back to a normal phenotype to drive the regression of fibrotic tissue. In a murine liver fibrosis model, viral vector-mediated reprogramming converted myofibroblasts to a hepatocyte-like state and was associated with a decrease in fibrosis. In addition, cellular reprogramming may be utilized to preferentially drive myofibroblast FAO metabolism in conjunction with ECM catabolism to counteract fibrosis. | 160,161 |
Given its central role in fibrosis, TGF-β signalling is an attractive target in the investigation of experimental therapies in various fibrosis models (Table 4); such strategies, including include neutralizing antibodies, gene therapies, and the administration of recombinant soluble TGFBR2 receptors, have all been examined in animal fibrosis models targeting TGF-β signalling, and demonstrated success in limiting the amount of active TGF-β in situ, or reduced downstream signal transduction.1,144,166 Fresolimumab, for example, is a neutralizing antibody effective against all TGF-β isoforms, which has been tested in several fibrosis models and observed to significantly reduce TGF-β-mediated gene expression and myofibroblast infiltration in systemic sclerosis.145 An antisense small interfering RNA (siRNA) for Smad3, a molecule downstream of TGF-β in the canonical signalling pathway, administered as a topical gel also successfully suppressed Smad3, and reduced collagen deposition and epidermal thickening in a murine radiation fibrosis model.146 While such agents need to be well-tolerated, the pleiotropic role of TGF-β presents the risk of off-target effects during systemic therapy. Targeting CTGF activities downstream of TGF-β may be an alternate approach to circumvent this potential limitation. For example, Pamrevlumab is a monoclonal antibody specific to CTGF which successfully attenuated pulmonary remodelling, and improved overall health and median survival in a murine radiation model.167 Pamrevlumab has been subjected to a Phase 2 randomized trial for interstitial pulmonary fibrosis (IPF), and decelerated the decline of lung function (p = 0.033) and disease progression (p = 0.013) while being relatively well-tolerated, suggesting its potential for radiation fibrosis therapy.148
Other pharmacological agents currently being investigated in fibrosis include statins, receptor tyrosine kinase inhibitors (RTKIs), and angiotensin-converting enzyme (ACE) inhibitors (Table 4). Statin-mediated inhibition of Rho/ROCK signalling reduced histopathological changes post-irradiation, including reduction in collagen synthesis, CTGF deposition, and overall fibrosis development.150,151 The “PRAVACUR” Phase 2 trial investigated the efficacy of Pravastatin in head & neck cancer patients (n = 60) with established cutaneous and subcutaneous fibrosis of varying severity.152 Patients received 40 mg/day over 12-months, and amongst the 42 patients who completed the study, 35.7% of patients (95% confidence interval (CI): 21.6–52.0%) demonstrated a reduction of ≥30% fibrosis thickness, while 50% (95% CI: 34.2–65.8%) showed reduced fibrosis severity. RTKIs, such as Imatinib mesylate, have successfully inhibited fibroblast proliferation and profibrotic signalling in murine models when applied either during the early inflammatory phase or later in fibrosis development.102,153 PDGFR-dependent signalling was implicated as one such target of RTKIs, though clinical studies assessing efficacy in humans remain to be conducted.101 Nintedanib, an FDA-approved RTKI used in the treatment of IPF, demonstrated the ability to suppress fibroblast proliferation and ECM deposition, while exerting anti-inflammatory and anti-fibrotic effects.168 A murine model demonstrated that these beneficial effects were maintained in pulmonary radiation fibrosis, notably decreasing oedema and fibrotic tissue deposition.154
Experimental data examining Captopril, an ACE inhibitor (Table 4), demonstrated its potential to reduce matrix remodelling and preserve organ function in pulmonary and cardiac radiation fibrosis murine models.155,169 Both the control and treatment groups however, developed pulmonary vasculature damage from radiation toxicity, indicating that Captopril may not impart sufficient radioprotection as monotherapy.155 A Phase 2 clinical trial of Enalapril (NCT01754909), another ACE inhibitor, investigated the incidence of radiation fibrosis in patients receiving radiotherapy for lung cancer.92 While the drug was well-tolerated, the treatment group (n = 20) demonstrated an increased incidence of fibrosis development, as determined using CT scans, compared to the placebo group (n = 23) (40% vs. 17.4%). Hence, future clinical investigations of ACE inhibitors would be therefore necessary to determine their clinical role, if any, in the prevention of radiation fibrosis.
Pirfenidone is an alternative FDA-approved broad-spectrum antifibrotic molecule for IPF therapy, although unlike Nintedanib, it inhibits fibrotic growth factors and ECM molecular activities through an unclear mechanism.170 In a pulmonary radiation fibrosis model, Pirfenidone decreased TGF-β expression and Smad3 signalling, and prolonged median survival (p < 0.01).170 Similarly, in an intestinal radiation fibrosis model, Pirfenidone reduced myofibroblast development and suppressed canonical TGF-β1 signalling in conjunction with CTGF.171 A small pilot study of patients with established radiation fibrosis (n = 7) reported that patients’ range of motion was improved by at least ∼25% following treatment with Pirfenidone.172
Other novel experimental approaches to counter radiation fibrosis include stem cell therapies and cellular reprogramming (Table 4). Adipose-derived stem cells (ADSCs) have been previously identified as a source of antifibrotic HGF, and tested for their ability to mitigate cutaneous, muscular, and skeletal fibrosis through inhibition of TGF-β and other profibrotic molecules.16,158 In addition, the extracellular nanovesicles generated by ADSCs have also been shown to reduce the expression of profibrotic genes, providing the possibility of a cell-free therapeutic approach.159 Stem cells may also enable the regeneration of healthy tissues when fibrosis results in parenchymal atrophy or tissue necrosis.1 Cellular reprogramming provides a modality through which abnormal cells, such as myofibroblasts, may be ushered back to a normal phenotype to drive the regression of fibrotic tissue.173 In a murine liver fibrosis model, the viral vector-mediated ectopic expression of four transcription factors converted myofibroblasts to a hepatocyte-like state and was associated with a decrease in fibrosis.160 In addition, metabolic dysregulation has been reported in myofibroblasts wherein upregulation of fatty acid oxidation (FAO) promotes ECM catabolism, while downregulation of FAO and upregulation of glycolysis promotes ECM anabolism; cellular reprogramming may hence be utilized to preferentially drive myofibroblast FAO metabolism in conjunction with ECM catabolism to counteract fibrosis.161
Taken together, therapeutic approaches targeting specific pathways are still in their infancy and require investigation in both human cell/tissue models and subsequent clinical trials. While certain treatments may prove successful in vitro or in animal models, the complexity of the fibrotic cascade necessitates caution when predicting efficacy in patients.
Conclusion
Radiation fibrosis is a long-term complication of radiotherapy resulting from an aberrant wound repair process. It is clear that fibrosis initiation and progression are enabled by a plethora of complex biological alterations. The aggregate activities of these pathways promote the differentiation and maintenance of abnormal myofibroblasts which excessively deposit ECM leading to scar tissue accumulation. Unfortunately, there is currently no cure for radiation fibrosis and existing therapies employed in the clinical setting have demonstrated limited success. Future successful clinical management of radiation fibrosis will be incumbent on additional research in fibrosis pathogenesis, advances in radiotherapy techniques, and the development of novel therapeutics, as well as learning from other causes of fibrosis. This review summarizes the current state of knowledge in this domain, but with increasing research efforts in the broad biomedical community, the future is very exciting on being able to prevent, mitigate and cure radiation fibrosis, thereby significantly improving the quality of life for cancer survivors around the world.
Outstanding questions
While no cure currently exists for radiation fibrosis, ongoing developments in radiation techniques, the understanding of fibrosis molecular pathogenesis, and therapeutic modalities may reduce its prevalence or severity over the long-term. To that end, future research should address multiple outstanding questions on the complex clinical and molecular landscape of radiation fibrosis. Access to high-quality clinical data detailing radiotherapy dose, volumes, and outcomes remains a significant hurdle in current radiation fibrosis clinical studies. Upon successfully bridging this gap, novel radiotherapy dosimetry constraints may be investigated with fibrosis as an endpoint, to prevent long-term complications and improve patient quality of life. At present, the epidemiology of radiation fibrosis is most frequently described in the literature with respect to pulmonary and cardiac complications; however, even these data vary between studies. Future studies should therefore build upon the existing literature, as well as elucidating epidemiological characteristics of radiation fibrosis in other major sites prone to developing complications. Finally, in addition to prevention through the development of dosimetric constraints, improvements in clinical management and treatment require further investigation in radiation fibrosis models and subsequent clinical trials. Highlighted in this review are major classes of experimental therapies described in the literature, though as the biomedical knowledge of fibrosis pathogenesis continues to evolve, novel therapeutic targets may also emerge warranting clinical investigation.
Contributors
Conceptualization: Fijardo M, Bissey P-A, Yip KW, Liu FF; Methodology: Fijardo M, Bissey P-A, Yip KW, Liu FF; Validation: All authors; Investigation: All authors; Data Analysis & Interpretation: All authors; Writing–Original Draft: All authors; Writing–Review and Editing: All authors; Supervision: Fijardo M, Yip KW, Liu FF; Project Administration: Fijardo M, Yip KW, Liu FF; Funding Acquisition: Yip KW, Liu FF. All authors have approved the final version of this manuscript.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by The Canadian Institutes of Health Research, the University of Toronto Temerty Faculty of Medicine, Princess Margaret Cancer Centre, The Princess Margaret Cancer Foundation, and the Ontario Ministry of Health, Canada. The funding sources did not have any role in writing the manuscript or the decision to submit it for publication. Authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication.
References
- 1.Westbury C.B., Yarnold J.R. Radiation fibrosis--current clinical and therapeutic perspectives. Clin Oncol (R Coll Radiol) 2012;24:657–672. doi: 10.1016/j.clon.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Stone H.B., Coleman C.N., Anscher M.S., McBride W.H. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 3.Yarnold J., Brotons M.-C.V. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2010;97:149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Clark M. Minimizing risk of cancer therapeutics. Phys Med Rehabil Clin N Am. 2018;29:701–719. doi: 10.1016/j.pmr.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Linda A., Trovo M., Bradley J.D. Radiation injury of the lung after stereotactic body radiation therapy (SBRT) for lung cancer: a timeline and pattern of CT changes. Eur J Radiol. 2011;79:147–154. doi: 10.1016/j.ejrad.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Hanania A.N., Mainwaring W., Ghebre Y.T., Hanania N.A., Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156:150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinmeier T., Schulze Schleithoff S., Timmermann B. Evolving radiotherapy techniques in paediatric oncology. Clin Oncol. 2019;31:142–150. doi: 10.1016/j.clon.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Anscher M.S. The irreversibility of radiation-induced fibrosis: fact or folklore? J Clin Oncol. 2005;23:8551–8552. doi: 10.1200/JCO.2005.03.6194. [DOI] [PubMed] [Google Scholar]
- 9.Straub J.M., New J., Hamilton C.D., Lominska C., Shnayder Y., Thomas S.M. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141:1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emami B., Lyman J., Brown A., et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 11.Bentzen S.M., Constine L.S., Deasy J.O., et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3–S9. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimm J., Marks L.B., Jackson A., Kavanagh B.D., Xue J., Yorke E. High dose per fraction, hypofractionated treatment effects in the clinic (HyTEC): an overview. Int J Radiat Oncol Biol Phys. 2021;110:1–10. doi: 10.1016/j.ijrobp.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S., Salerno K.E., Citrin D.E. Biology of radiation-induced lung injury. Semin Radiat Oncol. 2021;31:155–161. doi: 10.1016/j.semradonc.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong F.-M.S., Moiseenko V., Zhao J., et al. Organs at risk considerations for thoracic stereotactic body radiation therapy: what is safe for lung parenchyma? Int J Radiat Oncol Biol Phys. 2021;110:172–187. doi: 10.1016/j.ijrobp.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks L.B., Yorke E.D., Jackson A., et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ejaz A., Greenberger J.S., Rubin P.J. Understanding the mechanism of radiation induced fibrosis and therapy options. Pharmacol Ther. 2019;204 doi: 10.1016/j.pharmthera.2019.107399. [DOI] [PubMed] [Google Scholar]
- 17.Nyman J., Turesson I. Does the interval between fractions matter in the range of 4-8 h in radiotherapy? A study of acute and late human skin reactions. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1995;34:171–178. doi: 10.1016/0167-8140(95)01525-l. [DOI] [PubMed] [Google Scholar]
- 18.Brand D.H., Kirby A.M., Yarnold J.R., Somaiah N. How low can you go? The radiobiology of hypofractionation. Clin Oncol. 2022;34:280–287. doi: 10.1016/j.clon.2022.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Gupta T., Sinha S., Ghosh-Laskar S., et al. Intensity-modulated radiation therapy versus three-dimensional conformal radiotherapy in head and neck squamous cell carcinoma: long-term and mature outcomes of a prospective randomized trial. Radiat Oncol Lond Engl. 2020;15:218. doi: 10.1186/s13014-020-01666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chopra S., Gupta S., Kannan S., et al. Late toxicity after adjuvant conventional radiation versus image-guided intensity-modulated radiotherapy for cervical cancer (PARCER): a randomized controlled trial. J Clin Oncol. 2021;39:3682–3692. doi: 10.1200/JCO.20.02530. [DOI] [PubMed] [Google Scholar]
- 21.Stubblefield M.D. Clinical evaluation and management of radiation fibrosis syndrome. Phys Med Rehabil Clin N Am. 2017;28:89–100. doi: 10.1016/j.pmr.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Borrelli M.R., Shen A.H., Lee G.K., Momeni A., Longaker M.T., Wan D.C. Radiation-induced skin fibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83 doi: 10.1097/SAP.0000000000002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei J., Meng L., Hou X., et al. Radiation-induced skin reactions: mechanism and treatment. Cancer Manag Res. 2018;11:167–177. doi: 10.2147/CMAR.S188655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Käsmann L., Dietrich A., Staab-Weijnitz C.A., et al. Radiation-induced lung toxicity – cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat Oncol. 2020;15:214. doi: 10.1186/s13014-020-01654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarzebska N., Karetnikova E.S., Markov A.G., Kasper M., Rodionov R.N., Spieth P.M. Scarred lung. An update on radiation-induced pulmonary fibrosis. Front Med. 2021;7:1100. doi: 10.3389/fmed.2020.585756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z., Wu Z., Ning W. Advances in molecular mechanisms and treatment of radiation-induced pulmonary fibrosis. Transl Oncol. 2019;12:162–169. doi: 10.1016/j.tranon.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiFrancesco T., Khanna A., Stubblefield M.D. Clinical evaluation and management of cancer survivors with radiation fibrosis syndrome. Semin Oncol Nurs. 2020;36 doi: 10.1016/j.soncn.2019.150982. [DOI] [PubMed] [Google Scholar]
- 28.Belzile-Dugas E., Eisenberg M.J. Radiation-induced cardiovascular disease: review of an underrecognized pathology. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taunk N.K., Haffty B.G., Kostis J.B., Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol. 2015;5:39. doi: 10.3389/fonc.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuomo J.R., Sharma G.K., Conger P.D., Weintraub N.L. Novel concepts in radiation-induced cardiovascular disease. World J Cardiol. 2016;8:504–519. doi: 10.4330/wjc.v8.i9.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caron J., Nohria A. Cardiac toxicity from breast cancer treatment: can we avoid this? Curr Oncol Rep. 2018;20:61. doi: 10.1007/s11912-018-0710-1. [DOI] [PubMed] [Google Scholar]
- 32.Stubblefield M.D. Neuromuscular complications of radiation therapy. Muscle Nerve. 2017;56:1031–1040. doi: 10.1002/mus.25778. [DOI] [PubMed] [Google Scholar]
- 33.Stubblefield M.D. Radiation fibrosis syndrome: neuromuscular and musculoskeletal complications in cancer survivors. PM R. 2011;3:1041–1054. doi: 10.1016/j.pmrj.2011.08.535. [DOI] [PubMed] [Google Scholar]
- 34.Azzam P., Mroueh M., Francis M., Daher A.A., Zeidan Y.H. Radiation-induced neuropathies in head and neck cancer: prevention and treatment modalities. ecancermedicalscience. 2020;14:1133. doi: 10.3332/ecancer.2020.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauer-Jensen M., Wang J., Boerma M., Fu Q., Denham J.W. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care. 2007;1:23–29. doi: 10.1097/SPC.0b013e3281108014. [DOI] [PubMed] [Google Scholar]
- 36.Kim J., Jung Y. Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med. 2017;49:e359. doi: 10.1038/emm.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naim A., Baig M.S. Matrix metalloproteinase-8 (MMP-8) regulates the activation of hepatic stellate cells (HSCs) through the ERK-mediated pathway. Mol Cell Biochem. 2020;467:107–116. doi: 10.1007/s11010-020-03705-x. [DOI] [PubMed] [Google Scholar]
- 38.Zwaans B.M.M., Nicolai H.E., Chancellor M.B., Lamb L.E. Prostate cancer survivors with symptoms of radiation cystitis have elevated fibrotic and vascular proteins in urine. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marks L.B., Carroll P.R., Dugan T.C., Anscher M.S. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol. 1995;31:1257–1280. doi: 10.1016/0360-3016(94)00431-J. [DOI] [PubMed] [Google Scholar]
- 40.Morris L., Do V., Chard J., Brand A.H. Radiation-induced vaginal stenosis: current perspectives. Int J Womens Health. 2017;9:273–279. doi: 10.2147/IJWH.S106796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varytė G., Bartkevičienė D. Pelvic radiation therapy induced vaginal stenosis: a review of current modalities and recent treatment advances. Medicina (Mex) 2021;57:336. doi: 10.3390/medicina57040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purkayastha A., Sharma N., Sarin A., et al. Radiation fibrosis syndrome: the evergreen menace of radiation therapy. Asia Pac J Oncol Nurs. 2019;6:238–245. doi: 10.4103/apjon.apjon_71_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giridhar P., Mallick S., Rath G.K., Julka P.K. Radiation induced lung injury: prediction, assessment and management. Asian Pac J Cancer Prev APJCP. 2015;16:2613–2617. doi: 10.7314/apjcp.2015.16.7.2613. [DOI] [PubMed] [Google Scholar]
- 44.Abratt R.P., Morgan G.W., Silvestri G., Willcox P. Pulmonary complications of radiation therapy. Clin Chest Med. 2004;25:167–177. doi: 10.1016/S0272-5231(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 45.Bledsoe T.J., Nath S.K., Decker R.H. Radiation pneumonitis. Clin Chest Med. 2017;38:201–208. doi: 10.1016/j.ccm.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Abratt R.P., Morgan G.W. Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer Amst Neth. 2002;35:103–109. doi: 10.1016/s0169-5002(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 47.Giuranno L., Ient J., De Ruysscher D., Vooijs M.A. Radiation-induced lung injury (RILI) Front Oncol. 2019;9:877. doi: 10.3389/fonc.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang S., Che J., Chu Q., Zhang P. The role of NLRP3 inflammasome in radiation-induced cardiovascular injury. Front Cell Dev Biol. 2020;8:140. doi: 10.3389/fcell.2020.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X., Yu Y., Yang K., et al. Clinical outcomes of radiation-induced carotid stenosis: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.104929. [DOI] [PubMed] [Google Scholar]
- 50.Xu J., Cao Y. Radiation-induced carotid artery stenosis: a comprehensive review of the literature. Interv Neurol. 2014;2:183–192. doi: 10.1159/000363068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greco C., Pares O., Pimentel N., et al. Spinal metastases: from conventional fractionated radiotherapy to single-dose SBRT. Rep Pract Oncol Radiother. 2015;20:454–463. doi: 10.1016/j.rpor.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng K.L., Tseng C.-L., Soliman H., Weiss Y., Sahgal A., Myrehaug S. Stereotactic body radiotherapy (SBRT) for oligometastatic spine metastases: an overview. Front Oncol. 2019;9:337. doi: 10.3389/fonc.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahgal A., Chang J.H., Ma L., et al. Spinal cord dose tolerance to stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2021;110:124–136. doi: 10.1016/j.ijrobp.2019.09.038. [DOI] [PubMed] [Google Scholar]
- 54.Rice N., Polyzois I., Ekanayake K., Omer O., Stassen L.F.A. The management of osteoradionecrosis of the jaws – a review. Surgeon. 2015;13:101–109. doi: 10.1016/j.surge.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Murro D., Jakate S. Radiation esophagitis. Arch Pathol Lab Med. 2015;139:827–830. doi: 10.5858/arpa.2014-0111-RS. [DOI] [PubMed] [Google Scholar]
- 56.Pan C.C., Kavanagh B.D., Dawson L.A., et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94–S100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klaus R., Niyazi M., Lange-Sperandio B. Radiation-induced kidney toxicity: molecular and cellular pathogenesis. Radiat Oncol Lond Engl. 2021;16:43. doi: 10.1186/s13014-021-01764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mylonas K.J., O’Sullivan E.D., Humphries D., et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abb0203. [DOI] [PubMed] [Google Scholar]
- 59.Delishaj D., Barcellini A., D’Amico R., et al. Vaginal toxicity after high-dose-rate endovaginal brachytherapy: 20 years of results. J Contemp Brachytherapy. 2018;10:559–566. doi: 10.5114/jcb.2018.79713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23:S20–S23. doi: 10.1097/IJG.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Illsley M.C., Peacock J.H., McAnulty R.J., Yarnold J.R. Increased collagen production in fibroblasts cultured from irradiated skin and effect of TGF beta(1) - clinical study. Br J Cancer. 2000;83:650–654. doi: 10.1054/bjoc.2000.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagase H., Woessner J.F. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 64.Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases - structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 65.Lee J.W., Zoumalan R.A., Valenzuela C.D., et al. Regulators and mediators of radiation-induced fibrosis: gene expression profiles and a rationale for Smad3 inhibition. Otolaryngol Head Neck Surg. 2010;143:525–530. doi: 10.1016/j.otohns.2010.06.912. [DOI] [PubMed] [Google Scholar]
- 66.Araya J., Maruyama M., Sassa K., et al. Ionizing radiation enhances matrix metalloproteinase-2 production in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L30–L38. doi: 10.1152/ajplung.2001.280.1.L30. [DOI] [PubMed] [Google Scholar]
- 67.Wu Y., Li Z., Xiu A.-Y., Meng D.-X., Wang S.-N., Zhang C.-Q. Carvedilol attenuates carbon tetrachloride-induced liver fibrosis and hepatic sinusoidal capillarization in mice. Drug Des Devel Ther. 2019;13:2667–2676. doi: 10.2147/DDDT.S210797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Craig V.J., Polverino F., Laucho-Contreras M.E., et al. Mononuclear phagocytes and airway epithelial cells: novel sources of matrix metalloproteinase-8 (MMP-8) in patients with idiopathic pulmonary fibrosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang K., Palm J., König J., et al. Matrix-Metallo-Proteinases and their tissue inhibitors in radiation-induced lung injury. Int J Radiat Biol. 2007;83:665–676. doi: 10.1080/09553000701558977. [DOI] [PubMed] [Google Scholar]
- 70.Flechsig P., Hartenstein B., Teurich S., et al. Loss of matrix metalloproteinase-13 attenuates murine radiation-induced pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 2010;77:582–590. doi: 10.1016/j.ijrobp.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 71.George J., Tsutsumi M., Tsuchishima M. MMP-13 deletion decreases profibrogenic molecules and attenuates N-nitrosodimethylamine-induced liver injury and fibrosis in mice. J Cell Mol Med. 2017;21:3821–3835. doi: 10.1111/jcmm.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zigrino P., Brinckmann J., Niehoff A., et al. Fibroblast-derived MMP-14 regulates collagen homeostasis in adult skin. J Invest Dermatol. 2016;136:1575–1583. doi: 10.1016/j.jid.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krantz S.B., Shields M.A., Dangi-Garimella S., et al. MT1-MMP cooperates with Kras(G12D) to promote pancreatic fibrosis through increased TGF-β signaling. Mol Cancer Res MCR. 2011;9:1294–1304. doi: 10.1158/1541-7786.MCR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Placido L., Romero Y., Maldonado M., et al. Loss of MT1-MMP in alveolar epithelial cells exacerbates pulmonary fibrosis. Int J Mol Sci. 2021;22:2923. doi: 10.3390/ijms22062923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patten J., Wang K. Fibronectin in development and wound healing. Adv Drug Deliv Rev. 2021;170:353–368. doi: 10.1016/j.addr.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Maurer L.M., Ma W., Mosher D.F. Dynamic structure of plasma fibronectin. Crit Rev Biochem Mol Biol. 2015;51:213–227. doi: 10.1080/10409238.2016.1184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klingberg F., Chau G., Walraven M., et al. The fibronectin ED-A domain enhances recruitment of latent TGF-β-binding protein-1 to the fibroblast matrix. J Cell Sci. 2018;131 doi: 10.1242/jcs.201293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iozzo R.V., Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol J Int Soc Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albeiroti S., Soroosh A., de la Motte C.A. Hyaluronan’s role in fibrosis: a pathogenic factor or a passive player? BioMed Res Int. 2015;2015 doi: 10.1155/2015/790203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parimon T., Yao C., Habiel D.M., et al. Syndecan-1 promotes lung fibrosis by regulating epithelial reprogramming through extracellular vesicles. JCI Insight. 2020;4 doi: 10.1172/jci.insight.129359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baghy K., Iozzo R.V., Kovalszky I. Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem. 2012;60:262–268. doi: 10.1369/0022155412438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kolb M., Margetts P.J., Sime P.J., Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-β-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1327–L1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 83.Lord M.S., Tang F., Rnjak-Kovacina J., Smith J.G.W., Melrose J., Whitelock J.M. The multifaceted roles of perlecan in fibrosis. Matrix Biol. 2018;68–69:150–166. doi: 10.1016/j.matbio.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 84.Mezzenga R., Mitsi M. The molecular dance of fibronectin: conformational flexibility leads to functional versatility. Biomacromolecules. 2019;20:55–72. doi: 10.1021/acs.biomac.8b01258. [DOI] [PubMed] [Google Scholar]
- 85.Yang J., Chi L. Characterization of structural motifs for interactions between glycosaminoglycans and proteins. Carbohydr Res. 2017;452:54–63. doi: 10.1016/j.carres.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Dick G., Akslen-Hoel L.K., Grøndahl F., Kjos I., Prydz K. Proteoglycan synthesis and Golgi organization in polarized epithelial cells. J Histochem Cytochem Off J Histochem Soc. 2012;60:926–935. doi: 10.1369/0022155412461256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phan S.H. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herskind C., Johansen J., Bentzen S.M., et al. Fibroblast differentiation in subcutaneous fibrosis after postmastectomy radiotherapy. Acta Oncol Stockh Swed. 2000;39:383–388. doi: 10.1080/028418600750013159. [DOI] [PubMed] [Google Scholar]
- 89.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang B., Wei J., Meng L., et al. Advances in pathogenic mechanisms and management of radiation-induced fibrosis. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109560. [DOI] [PubMed] [Google Scholar]
- 91.Duru N., Wolfson B., Zhou Q. Mechanisms of the alternative activation of macrophages and non-coding RNAs in the development of radiation-induced lung fibrosis. World J Biol Chem. 2016;7:231–239. doi: 10.4331/wjbc.v7.i4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Citrin D.E., Prasanna P.G.S., Walker A.J., et al. Radiation-induced fibrosis: mechanisms and opportunities to mitigate. Report of an NCI Workshop, September 19, 2016. Radiat Res. 2017;188:1–20. doi: 10.1667/RR14784.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martin M., Lefaix J., Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 94.Roberts A.B., Russo A., Felici A., Flanders K.C. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann N Y Acad Sci. 2003;995:1–10. doi: 10.1111/j.1749-6632.2003.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 95.Yi E.S., Bedoya A., Lee H., et al. Radiation-induced lung injury in vivo: expression of transforming growth factor-beta precedes fibrosis. Inflammation. 1996;20:339–352. doi: 10.1007/BF01486737. [DOI] [PubMed] [Google Scholar]
- 96.Jobling M.F., Mott J.D., Finnegan M.T., et al. Isoform-specific activation of latent transforming growth factor β (LTGF-β) by reactive oxygen species. Radiat Res. 2006;166:839–848. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 97.Finkelstein J., Johnston C., Baggs R., Rubin P. Early alterations in extracellular-matrix and transforming growth-factor-beta gene-expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994;28:621–631. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 98.Lipson K.E., Wong C., Teng Y., Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5 doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toda N., Mukoyama M., Yanagita M., Yokoi H. CTGF in kidney fibrosis and glomerulonephritis. Inflamm Regen. 2018;38:14. doi: 10.1186/s41232-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frazier K., Williams S., Kothapalli D., Klapper H., Grotendorst G.R. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- 101.Abdollahi A., Li M., Ping G., et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201:925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li M., Abdollahi A., Gröne H.-J., Lipson K.E., Belka C., Huber P.E. Late treatment with imatinib mesylate ameliorates radiation-induced lung fibrosis in a mouse model. Radiat Oncol Lond Engl. 2009;4:66. doi: 10.1186/1748-717X-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borkham-Kamphorst E., Weiskirchen R. The PDGF system and its antagonists in liver fibrosis. Cytokine Growth Factor Rev. 2016;28:53–61. doi: 10.1016/j.cytogfr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Staroslawska E., Czarnocki K.J., Koziol-Montewka M., Donica H., Magrys A. Effect of infliximab on the levels of TNF-alpha and TGF-beta in the whole blood cultures of irradiated patients. Folia Histochem Cytobiol. 2008;46:291–297. doi: 10.2478/v10042-008-0050-3. [DOI] [PubMed] [Google Scholar]
- 105.Johnston C.J., Piedboeuf B., Rubin P., Williams J.P., Baggs R., Finkelstein J.N. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145:762–767. [PubMed] [Google Scholar]
- 106.Epstein Shochet G., Brook E., Israeli-Shani L., Edelstein E., Shitrit D. Fibroblast paracrine TNF-α signaling elevates integrin A5 expression in idiopathic pulmonary fibrosis (IPF) Respir Res. 2017;18:122. doi: 10.1186/s12931-017-0606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Y., Kudo K., Abe Y., et al. Inhibition of transforming growth factor-beta, hypoxia-inducible factor-1alpha and vascular endothelial growth factor reduced late rectal injury induced by irradiation. J Radiat Res. 2009;50:233–239. doi: 10.1269/jrr.08112. [DOI] [PubMed] [Google Scholar]
- 108.Rabbani Z.N., Mi J., Zhang Y., et al. Hypoxia inducible factor 1 alpha signaling in fractionated radiation-induced lung injury: role of oxidative stress and tissue hypoxia. Radiat Res. 2010;173:165–174. doi: 10.1667/RR1816.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mesarwi O.A., Shin M.-K., Bevans-Fonti S., Schlesinger C., Shaw J., Polotsky V.Y. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choudhry H., Harris A.L. Advances in hypoxia-inducible factor biology. Cell Metab. 2018;27:281–298. doi: 10.1016/j.cmet.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 111.Liu L., Zhang P., Bai M., et al. p53 upregulated by HIF-1α promotes hypoxia-induced G2/M arrest and renal fibrosis in vitro and in vivo. J Mol Cell Biol. 2019;11:371–382. doi: 10.1093/jmcb/mjy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang R., Tan C., Najafi M. Cardiac inflammation and fibrosis following chemo/radiation therapy: mechanisms and therapeutic agents. Inflammopharmacology. 2022;30:73–89. doi: 10.1007/s10787-021-00894-9. [DOI] [PubMed] [Google Scholar]
- 113.Choi S.-H., Kim M., Lee H.-J., Kim E.-H., Kim C.-H., Lee Y.-J. Effects of NOX1 on fibroblastic changes of endothelial cells in radiation-induced pulmonary fibrosis. Mol Med Rep. 2016;13:4135–4142. doi: 10.3892/mmr.2016.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Citrin D.E., Shankavaram U., Horton J.A., et al. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst. 2013;105:1474–1484. doi: 10.1093/jnci/djt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu W.M., Ding I., Chen K.Q., et al. Interleukin 1 beta (IL1B) signaling is a critical component of radiation-induced skin fibrosis. Radiat Res. 2006;165:181–191. doi: 10.1667/rr3478.1. [DOI] [PubMed] [Google Scholar]
- 116.Alileche A., Han D., Plaisance S., et al. IL-2 production by myofibroblasts from post-radiation fibrosis in breast cancer patients. Int Immunol. 1994;6:1585–1591. doi: 10.1093/intimm/6.10.1585. [DOI] [PubMed] [Google Scholar]
- 117.Xiong S., Guo R., Yang Z., et al. Treg depletion attenuates irradiation-induced pulmonary fibrosis by reducing fibrocyte accumulation, inducing Th17 response, and shifting IFN-γ, IL-12/IL-4, IL-5 balance. Immunobiology. 2015;220:1284–1291. doi: 10.1016/j.imbio.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Reiman R.M., Thompson R.W., Feng C.G., et al. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74:1471–1479. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berman B., Maderal A., Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43(Suppl 1):S3–S18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 120.Kobayashi T., Tanaka K., Fujita T., et al. Bidirectional role of IL-6 signal in pathogenesis of lung fibrosis. Respir Res. 2015;16:99. doi: 10.1186/s12931-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang S., Campbell J., Stenmark M.H., et al. Plasma levels of IL-8 and TGF-β1 predict radiation-induced lung toxicity in non-small cell lung cancer: a validation study. Int J Radiat Oncol Biol Phys. 2017;98:615–621. doi: 10.1016/j.ijrobp.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang L., Herrera J., Gilbertsen A., et al. IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity. Am J Physiol Lung Cell Mol Physiol. 2018;314:L127–L136. doi: 10.1152/ajplung.00200.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kurosaki F., Uchibori R., Sehara Y., et al. AAV6-Mediated IL-10 expression in the lung ameliorates bleomycin-induced pulmonary fibrosis in mice. Hum Gene Ther. 2018;29:1242–1251. doi: 10.1089/hum.2018.024. [DOI] [PubMed] [Google Scholar]
- 124.Keane M.P., Belperio J.A., Burdick M.D., Strieter R.M. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2001;281:L92–L97. doi: 10.1152/ajplung.2001.281.1.L92. [DOI] [PubMed] [Google Scholar]
- 125.Lee E.-J., Kim J.W., Yoo H., et al. Single high-dose irradiation aggravates eosinophil-mediated fibrosis through IL-33 secreted from impaired vessels in the skin compared to fractionated irradiation. Biochem Biophys Res Commun. 2015;464:20–26. doi: 10.1016/j.bbrc.2015.05.081. [DOI] [PubMed] [Google Scholar]
- 126.Brodeur T.Y., Robidoux T.E., Weinstein J.S., Craft J., Swain S.L., Marshak-Rothstein A. IL-21 promotes pulmonary fibrosis through the induction of profibrotic CD8+ T cells. J Immunol. 2015;195:5251–5260. doi: 10.4049/jimmunol.1500777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fredriksson L., Li H., Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 128.Idriss H.T., Naismith J.H. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc Res Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 129.Vujaskovic Z., Anscher M.S., Feng Q.-F., et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol. 2001;50:851–855. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]
- 130.Coppé J.-P., Desprez P.-Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chung E.J., Kwon S., Reedy J.L., et al. IGF-1 receptor signaling regulates type II pneumocyte senescence and resulting macrophage polarization in lung fibrosis. Int J Radiat Oncol Biol Phys. 2021;110:526–538. doi: 10.1016/j.ijrobp.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.He Y., Thummuri D., Zheng G., et al. Cellular senescence and radiation-induced pulmonary fibrosis. Transl Res J Lab Clin Med. 2019;209:14–21. doi: 10.1016/j.trsl.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vallée A., Lecarpentier Y., Guillevin R., Vallée J.-N. Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis. Oncotarget. 2017;8:90579–90604. doi: 10.18632/oncotarget.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ying H., Fang M., Hang Q.Q., Chen Y., Qian X., Chen M. Pirfenidone modulates macrophage polarization and ameliorates radiation-induced lung fibrosis by inhibiting the TGF-β1/Smad3 pathway. J Cell Mol Med. 2021;25:8662–8675. doi: 10.1111/jcmm.16821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Farhood B., Khodamoradi E., Hoseini-Ghahfarokhi M., et al. TGF-β in radiotherapy: mechanisms of tumor resistance and normal tissues injury. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104745. [DOI] [PubMed] [Google Scholar]
- 136.Delanian S., Lefaix J.-L. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73:119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 137.Delanian S., Baillet F., Huart J., Lefaix J.L., Maulard C., Housset M. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1994;32:12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 138.Delanian S., Martin M., Bravard A., Luccioni C., Lefaix J.L. Cu/Zn superoxide dismutase modulates phenotypic changes in cultured fibroblasts from human skin with chronic radiotherapy damage. Radiother Oncol. 2001;58:325–331. doi: 10.1016/s0167-8140(00)00332-7. [DOI] [PubMed] [Google Scholar]
- 139.Landeen K.C., Spanos W.C., Gromer L. Topical superoxide dismutase in posttreatment fibrosis in patients with head and neck cancer. Head Neck. 2018;40:1400–1405. doi: 10.1002/hed.25119. [DOI] [PubMed] [Google Scholar]
- 140.Epperly M.W., Travis E.L., Sikora C., Greenberger J.S. Manganese [correction of Magnesium] superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: modulation of irradiation-induced mRNA for IL-I, TNF-alpha, and TGF-beta correlates with delay of organizing alveolitis/fibrosis. Biol Blood Marrow Transplant. 1999;5:204–214. doi: 10.1053/bbmt.1999.v5.pm10465100. [DOI] [PubMed] [Google Scholar]
- 141.Moen I., Stuhr L.E.B. Hyperbaric oxygen therapy and cancer--a review. Target Oncol. 2012;7:233–242. doi: 10.1007/s11523-012-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mink Van Der Molen D.R., Batenburg M.C.T., Maarse W., et al. Hyperbaric oxygen therapy and late local toxic effects in patients with irradiated breast cancer: a randomized clinical trial. JAMA Oncol. 2024 doi: 10.1001/jamaoncol.2023.6776. published online Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Okunieff P., Augustine E., Hicks J.E., et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J Clin Oncol. 2004;22:2207–2213. doi: 10.1200/JCO.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 144.Shi N., Wang Z., Zhu H., et al. Research progress on drugs targeting the TGF-β signaling pathway in fibrotic diseases. Immunol Res. 2022;70:276–288. doi: 10.1007/s12026-022-09267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rice L.M., Padilla C.M., McLaughlin S.R., et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125:2795–2807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee J.W., Tutela J.P., Zoumalan R.A., et al. Inhibition of Smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch Otolaryngol Head Neck Surg. 2010;136:714–719. doi: 10.1001/archoto.2010.107. [DOI] [PubMed] [Google Scholar]
- 147.Anscher M.S., Thrasher B., Zgonjanin L., et al. Small molecular inhibitor of transforming growth factor-β protects against development of radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2008;71:829–837. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 148.Richeldi L., Fernández Pérez E.R., Costabel U., et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2020;8:25–33. doi: 10.1016/S2213-2600(19)30262-0. [DOI] [PubMed] [Google Scholar]
- 149.Moustafa M., Lipson K.E., Akbarpour M., et al. Late intervention with radiation-induced lung fibrosis - comparing Pamrevlumab anti-CTGF therapy vs. Pirfenidone vs. Nintedanib as mono-, dual- and triple-therapy combinations. Int J Radiat Oncol Biol Phys. 2020;108 [Google Scholar]
- 150.Haydont V., Bourgier C., Pocard M., et al. Pravastatin Inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13:5331–5340. doi: 10.1158/1078-0432.CCR-07-0625. [DOI] [PubMed] [Google Scholar]
- 151.Monceau V., Pasinetti N., Schupp C., Pouzoulet F., Opolon P., Vozenin M.-C. Modulation of the Rho/ROCK pathway in heart and lung after thorax irradiation reveals targets to improve normal tissue toxicity. Curr Drug Targets. 2010;11:1395–1404. doi: 10.2174/1389450111009011395. [DOI] [PubMed] [Google Scholar]
- 152.Bourgier C., Auperin A., Rivera S., et al. Pravastatin reverses established radiation-induced cutaneous and subcutaneous fibrosis in patients with head and neck cancer: results of the biology-driven phase 2 clinical trial pravacur. Int J Radiat Oncol Biol Phys. 2019;104:365–373. doi: 10.1016/j.ijrobp.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 153.Horton J.A., Chung E.J., Hudak K.E., et al. Inhibition of radiation-induced skin fibrosis with imatinib. Int J Radiat Biol. 2013;89:162–170. doi: 10.3109/09553002.2013.741281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.De Ruysscher D., Granton P.V., Lieuwes N.G., et al. Nintedanib reduces radiation-induced microscopic lung fibrosis but this cannot be monitored by CT imaging: a preclinical study with a high precision image-guided irradiator. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2017;124:482–487. doi: 10.1016/j.radonc.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 155.van der Veen S.J., Ghobadi G., de Boer R.A., et al. ACE inhibition attenuates radiation-induced cardiopulmonary damage. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2015;114:96–103. doi: 10.1016/j.radonc.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 156.Patel S., Rauf A., Khan H., Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother. 2017;94:317–325. doi: 10.1016/j.biopha.2017.07.091. [DOI] [PubMed] [Google Scholar]
- 157.Molteni A., Wolfe L.F., Ward W.F., et al. Effect of an angiotensin II receptor blocker and two angiotensin converting enzyme inhibitors on transforming growth factor-beta (TGF-beta) and alpha-actomyosin (alpha SMA), important mediators of radiation-induced pneumopathy and lung fibrosis. Curr Pharm Des. 2007;13:1307–1316. doi: 10.2174/138161207780618777. [DOI] [PubMed] [Google Scholar]
- 158.Ejaz A., Epperly M.W., Hou W., Greenberger J.S., Rubin J.P. Adipose-derived stem cell therapy ameliorates ionizing irradiation fibrosis via hepatocyte growth factor-mediated transforming growth factor-β downregulation and recruitment of bone marrow cells. Stem Cells Dayt Ohio. 2019;37:791–802. doi: 10.1002/stem.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Han H.S., Lee H., You D., et al. Human adipose stem cell-derived extracellular nanovesicles for treatment of chronic liver fibrosis. J Control Release. 2020;320:328–336. doi: 10.1016/j.jconrel.2020.01.042. [DOI] [PubMed] [Google Scholar]
- 160.Song G., Pacher M., Balakrishnan A., et al. Direct reprogramming of hepatic myofibroblasts into hepatocytes in vivo attenuates liver fibrosis. Cell Stem Cell. 2016;18:797–808. doi: 10.1016/j.stem.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 161.Zhao X., Kwan J.Y.Y., Yip K., Liu P.P., Liu F.-F. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020;19:57–75. doi: 10.1038/s41573-019-0040-5. [DOI] [PubMed] [Google Scholar]
- 162.Magnusson M., Höglund P., Johansson K., et al. Pentoxifylline and vitamin E treatment for prevention of radiation-induced side-effects in women with breast cancer: a phase two, double-blind, placebo-controlled randomised clinical trial (Ptx-5) Eur J Cancer. 2009;45:2488–2495. doi: 10.1016/j.ejca.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 163.Gothard L., Cornes P., Brooker S., et al. Phase II study of vitamin E and pentoxifylline in patients with late side effects of pelvic radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2005;75:334–341. doi: 10.1016/j.radonc.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 164.Delanian S., Porcher R., Balla-Mekias S., Lefaix J.-L. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21:2545–2550. doi: 10.1200/JCO.2003.06.064. [DOI] [PubMed] [Google Scholar]
- 165.Jacobson G., Bhatia S., Smith B.J., Button A.M., Bodeker K., Buatti J. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys. 2013;85:604–608. doi: 10.1016/j.ijrobp.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 166.Anscher M.S. Targeting the TGF-β1 pathway to prevent normal tissue injury after cancer therapy. Oncologist. 2010;15:350–359. doi: 10.1634/theoncologist.2009-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bickelhaupt S., Erbel C., Timke C., et al. Effects of CTGF blockade on attenuation and reversal of radiation-induced pulmonary fibrosis. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw339. [DOI] [PubMed] [Google Scholar]
- 168.Wollin L., Wex E., Pautsch A., et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Medhora M., Gao F., Jacobs E.R., Moulder J.E. Radiation damage to the lung: mitigation by angiotensin-converting enzyme (ACE) inhibitors. Respirol Carlton Vic. 2012;17:66–71. doi: 10.1111/j.1440-1843.2011.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qin W., Liu B., Yi M., et al. Antifibrotic agent pirfenidone protects against development of radiation-induced pulmonary fibrosis in a murine model. Radiat Res. 2018;190:396–403. doi: 10.1667/RR15017.1. [DOI] [PubMed] [Google Scholar]
- 171.Sun Y.-W., Zhang Y.-Y., Ke X.-J., Wu X.-J., Chen Z.-F., Chi P. Pirfenidone prevents radiation-induced intestinal fibrosis in rats by inhibiting fibroblast proliferation and differentiation and suppressing the TGF-β1/Smad/CTGF signaling pathway. Eur J Pharmacol. 2018;822:199–206. doi: 10.1016/j.ejphar.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 172.Simone N.L., Soule B.P., Gerber L., et al. Oral pirfenidone in patients with chronic fibrosis resulting from radiotherapy: a pilot study. Radiat Oncol Lond Engl. 2007;2:19. doi: 10.1186/1748-717X-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aydin B., Mazzoni E.O. Cell reprogramming: the many roads to success. Annu Rev Cell Dev Biol. 2019;35:433–452. doi: 10.1146/annurev-cellbio-100818-125127. [DOI] [PubMed] [Google Scholar]